- 1Dipartimento di Malattie Endocrine e del Metabolismo, IRCCS Istituto Auxologico Italiano IRCCS, Milan, Italy
- 2Dipartimento di Biotecnologie Mediche e Medicina Traslazionale, Università degli Studi di Milano - LITA, Segrate, Italy
The mechanisms underlying thyroid gland development have a central interest in biology and this review is aimed to provide an update on the recent advancements on the early steps of thyroid differentiation that were obtained in the zebrafish, because this teleost fish revealed to be a suitable organism to study the early developmental stages. Physiologically, the thyroid precursors fate is delineated by the appearance among the endoderm cells of the foregut of a restricted cell population expressing specific transcription factors, including pax2a, nkx2.4b, and hhex. The committed thyroid primordium first appears as a thickening of the pharyngeal floor of the anterior endoderm, that subsequently detaches from the floor and migrates to its final location where it gives rise to the thyroid hormone-producing follicles. At variance with mammalian models, thyroid precursor differentiation in zebrafish occurs early during the developmental process before the dislocation to the eutopic positioning of thyroid follicles. Several pathways have been implicated in these early events and nowadays there is evidence of a complex crosstalk between intrinsic (coming from the endoderm and thyroid precursors) and extrinsic factors (coming from surrounding tissues, as the cardiac mesoderm) whose organization in time and space is probably required for the proper thyroid development. In particular, Notch, Shh, Fgf, Bmp, and Wnt signaling seems to be required for the commitment of endodermal cells to a thyroid fate at specific developmental windows of zebrafish embryo. Here, we summarize the recent findings produced in the various zebrafish experimental models with the aim to define a comprehensive picture of such complicated puzzle.
Introduction
In all vertebrates, the thyroid gland provides the production of thyroid hormones (TH), thyroxine (T4) and its active metabolite triiodothyronine (T3), which exerts multiple roles during embryonic growth and organ development, and influencing several physiological mechanisms, including neurological development, metabolism, reproduction, thermoregulation, cardiac function, and tissue repair (1).
Following the fundamental steps of embryonic development, the establishment of a complex network intrinsic and extrinsic signals from surrounding tissues is the prerequisite for the acquisition of cell competence and the subsequent organogenesis of the thyroid gland (2).
In mammals, the development of the thyroid gland begins with the evagination of the primordium from the thyroid diverticulum located on the midline of the pharyngeal floor. The undifferentiated precursors then migrate to reach a position deep in the cervical mesenchyme where they will differentiate into hormone-producing follicles (3). In teleost fish, such as zebrafish, the thyroid gland differs from that in other vertebrates: a) the endodermal precursors start to differentiate before the relocalization; b) the thyroid follicles do not form a compact gland but remain loosely dispersed along the pharyngeal midline (4). However, despite differences in size, shape, or anatomical position, the capacity of thyroid follicles to face the physiological demand of thyroid hormone (TH), as a key prerequisite for normal thyroid function, is well conserved across species (1–3, 5, 6).
In humans, the vast majority of cases with congenital hypothyroidism (CH) is due to thyroid dysgenesis (TD), resulting from defects during embryonic thyroid development. Although next-generation sequencing techniques allow the identification of new candidate genes, TD is frequently reported to be sporadic and causative gene variations has been identified in less than 10% of cases (7). This is, in part, due to our still limited knowledge on the morphogenetic events underlying thyroid organogenesis (2, 6).
Especially, while core aspects of thyroid development have been extensively studied in various species, many significant questions are still open: the intrinsic factors responsible for the regional specification of the endoderm, and the role of extrinsic signals that regulate foregut endoderm patterning, remain to be fully clarified. Our current knowledge about these events derives from a patchwork of different observations obtained through variable experimental approaches in diverse species. For this reason, it is difficult to generate a strong hypothesis about the specific role of each signal during thyroid development, as well as their individual contribution to TD pathogenesis (1, 6, 8–16).
In the last decades, the zebrafish embryo has been extensively used as a model system for thyroid development (1, 5, 8, 10, 13, 17), but it is a relative newcomer among other model organisms to study early endoderm formation and induction (18–20). However, the zebrafish embryo offers many advantages that could overcome some limitations of mammalian models. For instance, the cellular dynamics of mammalian thyroid organogenesis are poorly studied due to the inaccessibility and opacity of the fetal tissues. In contrast, the optical clarity and the external fertilization of zebrafish make the embryos accessible to live monitoring and external manipulation. Moreover, the availability of molecular tools for the genetic manipulation of zebrafish allows the generation of several targeted-genetic mutants and transgenic zebrafish lines expressing fluorescent proteins in specific cell types (12). Noteworthy, the high conservation of the morphogenetic events of zebrafish embryogenesis, including thyroid organogenesis, makes zebrafish a valuable model for investigating the very early phases of thyroid development.
In this review, we attempt to summarize both classical and recent findings of thyroid development in the zebrafish embryo, with a particular focus on the early morphogenetic events that regulate endoderm formation and endodermal cells induction into thyroid precursors.
Key Features of Thyroid Development in Zebrafish
The analysis of zebrafish thyroid tissue during embryogenesis is particularly difficult due to its small size. However, thanks to the optical clarity of zebrafish embryos, the expression of the different thyroid markers was initially studied using whole mount in situ hybridization or immunofluorescence on embryos fixed at the desired developmental stage (5, 6, 8, 21). More recently, the generation of transgenic lines that express the fluorescent reporter under the control of a specific thyroid promoter gene (e.g., thyroglobulin, tg) allows the real-time and in vivo analysis of gene expression, cell morphology and thyroid anatomy (12, 14, 22). Moreover, the combination of double reporter lines [e.g., tg(tg:mCherry;myl7:EGFP)] permits the study of the anatomical relationship between cardiovascular remodeling and thyroid development (22). Very recently, Haerlingen et al. (16) performed an accurate analysis of the expression of the thyroid transcription factors (TTFs) by performing a cell counting on entire thyroid primordia isolated from immunostained zebrafish embryos and reporter lines (16).
These recent works describe that thyroid organogenesis begins around 20 hours post fertilization (hpf) with the expression of pax2a at the level of the foregut endoderm. Around 24 hpf, a monolayer of committed endodermal cells expressing the TTFs nkx2.4b and hhex, also known as thyroid anlage, are detectable at the level of the pharyngeal floor of the primitive pharynx, close to the apical pole of the primitive heart tube (8, 12, 21–23). The expression of pax2a in a relatively large domain of foregut endoderm before the expression of nkx2.4b suggests that thyroid specification occurs in two steps: endodermal specification of thyroid precursors expressing pax2a, and commitment of precursors to the thyroid fate upon nkx2.4b activation (16). Around 28-30 hpf, the thyroid anlage also starts to express pax8 and foxe1, but the functional roles of these TTFs for zebrafish thyroid cell differentiation remain unclear. Around 36-40 hpf, the thyroid anlage expands forming the so-called thyroid placode, a multilayer of thyroid precursors protruding into the underlying mesenchyme and budding from the pharyngeal epithelium in a rostro-ventral direction and starts to express the differentiated thyroid marker tg. The first follicular cell (TFC) is formed by 55 hpf and express all of the functional genes like thyroperoxidase (tpo), Na/I symporter (slc5a5), and thyroid stimulating hormone receptor (tshr) required for the establishment of competence for TH synthesis. During the later stages of embryonic development and larval transition (55-120 hpf), the thyroid tissue proliferates doubling the number of TFCs and migrates posteriorly forming distinct follicular units scattered along the pharyngeal midline, moving close and along the ventral aorta (12, 14, 16, 22).
Loss of function experiments indicate that zebrafish TTFs have similar roles to their mammalian homologues NKX2-1, PAX8, and HHEX, respectively. Zebrafish models with defective expression of zebrafish TTFs exhibit defects in early thyroid morphogenesis resulting in severe thyroid hypoplasia or athyreosis. The morpholino-mediated knockdown of nkx2.4b, pax2a, or hhex cause a failure of thyroid primordium specification, whereas the abrogation of hhex results in a reduced formation of follicles (4, 8, 13, 24). More recently, athyreosis or thyroid hypoplasia are observed in about the 50% of somatic mutants of nkx2.4b or pax2a generated using the CRISPR/Cas9 technology (14).
Early Events of Zebrafish Thyroid Development
As a general concept, the complete development of endoderm-derived organs is a multistep process: 1) induction of endoderm, 2) endoderm reorganization into specific regions, 3) start of organogenesis, with differentiation of endoderm precursors. Notably, the differentiation of the endoderm-derived organs, such as thyroid, liver, and pancreas, in the zebrafish embryo starts within the endodermal tissue (8, 25, 26).
Although the main morphogenetic events of thyroid development have been outlined in various model organisms, the precise contribution and interplay of the different molecular mechanisms regulating endodermal cell fate and tissue dynamics processes accounting for the early steps of thyroid development remains to be fully dissected. Moreover, it has been demonstrated that for several organs, including thyroid, the number of precursors that are initially committed from the endodermal layer will determine the final size of the organ (27–29).
Therefore, the understanding of the origin and nature of signals that directly or indirectly drive the very early phases of thyroid morphogenesis is particularly important in thyroid research.
Endoderm Formation and Cell-Fate Decision
The repertoire of structures derived from zebrafish endoderm is similar to those of mammals, with the exception of the intestinal tube instead of stomach and duodenum, and the swim bladder rather than lungs (30–32).
To study the induction of thyroid organogenesis, it is important to know “how”, “where”, and “when” the differentiation of endoderm begins. In zebrafish, the establishment of the three primary germ layers occurs between the blastula and the gastrula stages (2.5-10 hpf) of embryonic development. The future ectoderm originates from cells located in the animal pole of early blastula, whereas cells of the marginal region are bipotential for mesodermal and endodermal fates, forming the so-called mesendodermal layer (Figure 1A).
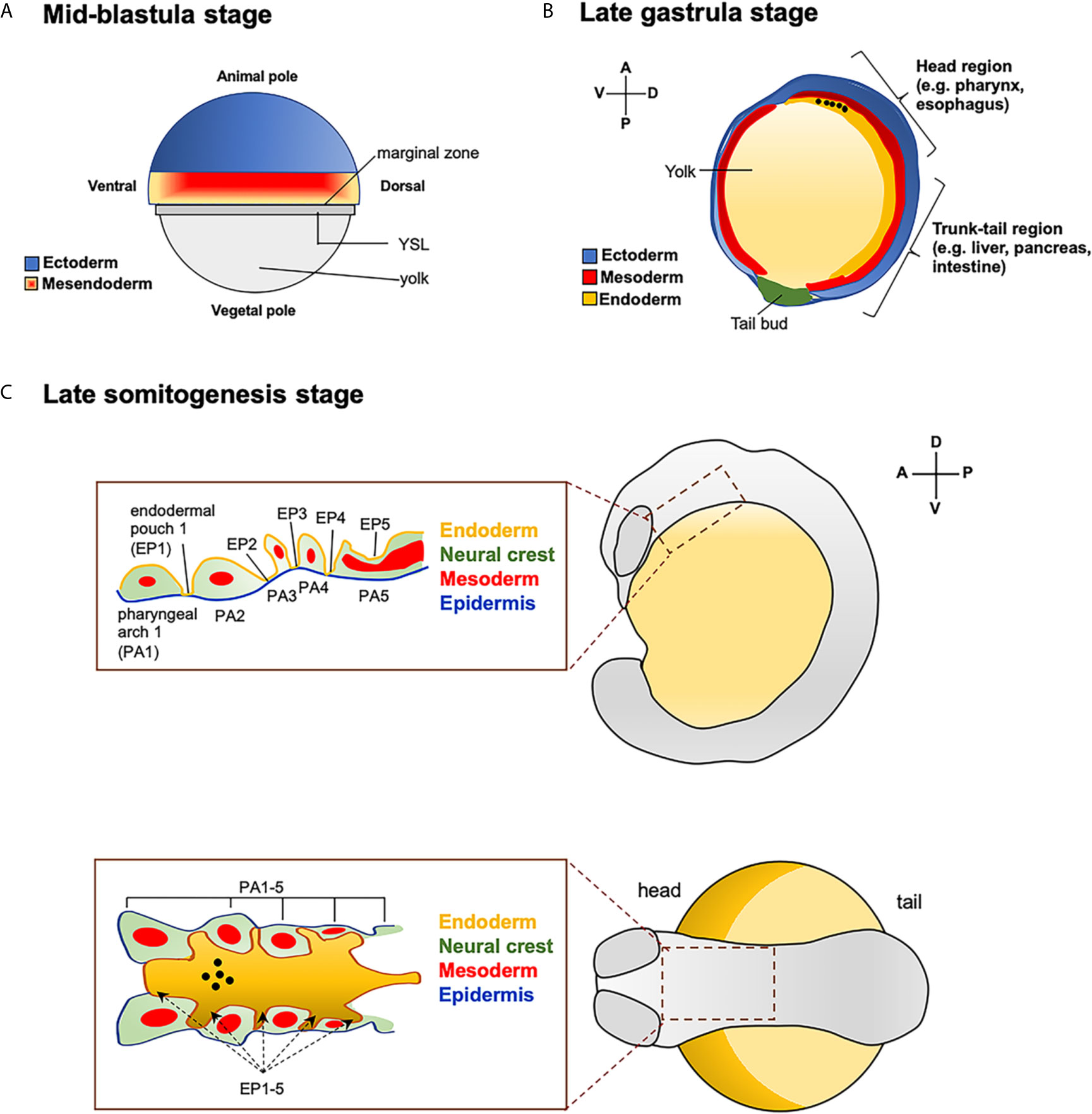
Figure 1 Fate map of zebrafish endoderm. (A) Representation of zebrafish blastula in surface view. Position of ectoderm (in blue) and mesendoderm (mesoderm + endoderm pluripotent cells, in red and orange mash-up) at the mid-blastula stage. YLS: yolk syncytial layer. (B) Zebrafish embryo at late gastrula stage. Antero-posterior and dorso-ventral movements of the three germ-layers. The presumptive location of endodermal cells that will give rise to the head (e.g., pharynx, esophagus) and trunk-tail (e.g., liver, pancreas and intestine) organs are also reported. The black dots indicate the position of the endodermal cell that contribute to the pharynx, as a site of origin of the thyroid anlage. (C) Close up of the pharyngeal endodermal region in embryos at the late somitogenesis (in lateral and dorsal view). Pharyngeal endoderm is surrounded by mesoderm, and neural crest and epidermis (ectoderm). The black dots indicate the position of the future thyroid anlage that will originate from the pharyngeal floor.
A restricted fate of marginal cells is acquired soon after the onset of gastrulation (33). In fact, when marginal cells from gastrulae at 50%-epiboly stage (5.3 hpf) are transplanted into animal blastomeres, they predominantly differentiate into endodermal cells. In contrast, transplanted cells from blastulae at 40% epiboly stage (5 hpf) mostly contribute to neuroectoderm formation (34, 35).
During gastrulation, involution and convergent-extension movements drive the migration of the diverse cell populations into their final locations. At early gastrulation (5.7-6 hpf), both mesodermal and endodermal cells lying the marginal zone start to involute, where the endodermal cells rapidly disperse throughout a “random walk” mechanism. At mid-gastrula stage (8-9 hpf), the endodermal cells converge to form a distinctive flattened morphology and start to express typical endodermal makers (18, 36). At the end of gastrulation (10 hpf), the endodermal cells extend along the antero-posterior axes and differentiate into the various precursor cells that will give rise to the endoderm-derived organs. The endodermal cells located in the dorsal-anterior direction will contribute to the head organs, like pharynx (site of origin of thyroid anlage) and esophagus, while the most ventral-posterior located endoderm will contribute to organs of trunk-tail region, such as liver, pancreas and intestine (Figure 1B) (35, 37, 38). At the end of somitogenesis (14-16 hpf) the dorsal-ventral and anterior-posterior axis are completely formed, and both anterior and posterior endodermal cells start to differentiate into their various organ progenitors.
At the level of the pharyngeal region, the endoderm is surrounded by mesoderm, neural crest and epidermis (ectoderm). Five endodermal pouches separate the pharyngeal arches, whose development involves tissues from all three germ layers (Figure 1C) (39, 40).
Critical Aspects of Endoderm Formation
All of the events that shape the developing embryos are regulated by key signaling molecules, also termed morphogens (41). Any pathway relevant to endoderm induction and specification of the pharyngeal endoderm could play a role in thyroid specification. The study of earlier steps of thyroid organogenesis cannot be done in murine models, since mouse embryos with targeted inactivation of genes required for endoderm patterning die in stages prior the definition of the thyroid anlage (42).
Therefore, our knowledge of the morphogenetic events underlie endoderm formation is mostly derived from studies in Xenopus and zebrafish (18, 43). Zebrafish has the advantage to combine features of both mammalian and amphibian development. In particular, while mammalian development largely depends on the crosstalk of embryonic and extraembryonic tissues, the induction of early morphogenetic events in amphibians is controlled by maternal factors (33, 44).
In zebrafish, the induction and patterning of the three germ layers rely on signals derived from extraembryonic tissues (yolk and yolk syncytial layer, YLS) and maternal factors initially deposited within the oocyte (41, 45–47). For instance, Nodal/TGFβ ligands, morphogens with critical roles in mesoderm and endoderm specification, are initially expressed under maternal control and then secreted by the extraembryonic YSL to pattern the embryonic blastoderm (48).
The presence of maternal factors during the first hours of development appears to contribute to the survival of embryos wherein their corresponding zygotic genes are inactivated, permitting the analysis of thyroid morphogenesis in later stages (49, 50).
Additionally, before the development of a functional cardiovascular system (around 24 hpf), the zebrafish embryos receive adequate oxygen supply by simple diffusion and can survive for days without blood circulation or a functional heart. In fact, cardiovascular defects are usually tolerated, and the embryos remain viable and develop relatively normally (51, 52). In light of the close relationship between thyroid and heart development, this quality is of particular importance for the study of genes that affect both endoderm and mesoderm development (8, 22).
Morphogens Involved in Endoderm Induction and Thyroid Specification
The Nodal Pathway
The generation of several zebrafish models with targeted genetic inactivation or overexpression facilitates the study of the various morphogenetic pathways involved in endoderm development.
At the top of these mechanisms lies Nodal signaling, one of the major contributor of mesoderm and endoderm cell commitment from the bipotential mesendodermal layer, which also plays a role in defining antero-posterior and left-right axes, and neural patterning (53). Nodal signaling is first described in mice with the identification of the activin-like members of the transforming growth factor β (TGF-β) family (54). In zebrafish, cyclops (cyc) and squint (sqt), two nodal-related ligands are necessary to initiate endoderm specification (49). Cyc and sqt bind the TARAM-A and the one-eyed pinhead (oep) receptors inducing a signalling cascade mediated by Smad proteins, which culminates with the expression of GATA-binding protein 5 (gata5), and MIX-like (bon, mezzo) transcription factors (55–57). Gata5, bon, and mezzo act in parallel, and in a partially redundant manner, to induce the expression of the transcription factor sox32 (formerly called casanova or cas), which is essential for the transcription of other endoderm-related transcription factors, like sox17 and foxA2 (18, 55).
Thanks to the stability and diffusion of its ligands, Nodal signaling is able to act in a long-range and in a dose-dependent manner. For instance, endoderm specification requires higher levels of Nodal signal than mesoderm (58). Moreover, mechanisms involving the Nodal antagonist lefty and the proteolytic inactivation of its ligands finely regulate the spatiotemporal activity of Nodal signaling (53).
Significant knowledge on the role of Nodal pathway during the early steps of thyroid development has been described in the zebrafish model by Elsalini and Rohr (59). The oeptz57 mutant embryos display a complete failure mesendodermal cells commitment that results in the loss of endoderm specification during gastrulation, and absence of thyroid primordium in later stages (59, 60).
Moreover, the disrupted expression of cyclops in cycm294 mutant leads to an incomplete development of pharyngeal endoderm and reduced number of functional thyroid follicles, whereas the deletion of both cyc and hhex genes in cycb216 mutants results in a complete absence of thyroid primordium and no T4-positive follicles in later stages (24, 61). These data suggest that the number of cells initially committed to a thyroid fate restricts the final size, and that a proper thyroid development requires both endoderm specification and expression of early TTFs (24, 62).
Also, the genetic inactivation of the downstream effectors of Nodal signaling bon, gata5 and sox32 perturbs endoderm organization, specifically leading to an absent or reduced expression of sox17 and foxA2, which prevents the specification of thyroid primordium (24, 53, 57, 63, 64).
However, when the inhibition of Nodal/TGFβ signaling is temporally restricted treating the embryos with chemicals from 50%-epiboly to early somitogenesis, the thyroid specification is only modestly affected despite a marked alteration of anterior endoderm (9). In this developmental window, the development of endodermal progenitors with thyroid commitment appeared to be mostly independent of Nodal/TGFβ signaling. Interestingly, the administration of inhibitors at high dosage results in the formation of ectopic clusters of nkx2.4b-positive cells in posterior pharyngeal region, suggesting a possible posteriorizing activity of Nodal signaling during the antero-posterior patterning of the endoderm (9).
Notch Signaling
In general, the endoderm of zebrafish gastrula is characterized by a “salt-and-pepper” distribution along the marginal zone of the two endodermal markers sox17 and foxA2, which are under the control of the Nodal transcription factors bon, gata5, and mezzo (65). Since only a portion of marginal cells expressing the Nodal genes become endoderm, it seems reasonable that additional genetic programs are implicated in the endoderm-fate restriction of marginal cells (66). Several studies described the key role of the Notch pathway during endoderm development. It has been demonstrated that the Notch signal acts restricting the commitment of endodermal cells through the lateral inhibition of neighboring cells, preventing them to undergo the same fate (67–70).
In vertebrates, there are five Notch ligands, three δ-like (DLL1, DLL3, DLL4) and two Jagged (JAG1 and JAG2), and four Notch receptors (NOTCH1–NOTCH4), with mostly non redundant functions. After the interaction with the ligand, the C-terminal region of Notch receptor, called Notch Intracellular domain (NICD), is released into the cytoplasm and translocates into the nucleus where it induces the expression of the transcriptional repressors belonging to the Hairy and Enhancer of Split (Hes) family (70).
In zebrafish, the importance of Notch during embryonic growth and specification of several organs and tissues has been extensively reported. In particular, Notch signaling appears fundamental for the differentiation of primary neuron and oligodendrocyte, the decision between hypochord and notochord cell fates, and the determination of pancreas identity (71–76).
Concerning early endoderm induction, it has been shown that the overexpression of Notch signaling is associated with a reduced number of endodermal cells expressing sox17 and foxA2 in zebrafish gastrula. In contrast, the overexpression of bon, which acts upstream of sox17 and foxA2, results in a diminished activation of Notch signal. Moreover, bon, gata5, and mezzo are expressed in the same region as deltaC, deltaD, and notch1 in the marginal zone (orthologues of mammalian DLL1, DLL3, and NOTCH1, respectively), and oep morphants display no expression of Notch receptors (66). Altogether these findings indicate that Notch pathway is directly dependent upon Nodal activity, and that the activation of Notch signaling during early embryonic growth restricts the number of endodermal cells, likely pushing the bipotential mesendodermal cells to become mesoderm (66).
Regarding thyroid development, our research group reported that the hyperactivation or the inactivation of the Notch signaling from about 20 hpf, just before the onset of thyroid primordium, is associated with the reduction or increment of the number of thyroid precursors, respectively (77). The overexpression of NICD in conditional activated zebrafish line abolishes the expression of nkx2.4b, together with a significant reduction of mature thyroid follicles. Conversely, the chemical or genetic inhibition of Notch signaling (DAPT or Mibta52b mutant) result in an increased expression of nkx2.4b and number of tg-positive cells at later stages. Given that the constitutive lack of Notch signaling (Mib mutants) or its time-controlled inhibition with DAPT at 20 hpf show similar thyroid phenotypes, we assumed that Notch signaling acts just before the endodermal specification of thyroid precursors.
Additionally, we demonstrated the Notch ligands jag1a and jag1b (homologous of the human JAG1) are detectable in the same territories of tg but with distinctive roles during thyroid development. Jag1b appears to be important to define the number of thyroid precursors that differentiates from pharyngeal endoderm, whereas jag1a acts in later stages regulating the renewal and displacement of follicles along the pharyngeal tube (77).
In the light of these findings, Notch signaling appears to act at multiple levels during thyroid development: controlling the differentiation of the primitive endoderm, restricting the number of endodermal cells committed to a thyroid fate, and regulating the expansion and migration of the fully functional thyroid follicles. However, given the roles of Notch in mesoderm induction and differentiation of mesoderm-derived structures, it is also conceivable that thyroid development is influenced by permissive factors from the surrounding tissues (e.g., lateral plate mesoderm) (15, 22, 77).
Haerlingen et al. (9) recently performed an extensive screening of thyroid defects in zebrafish embryos obtained after time-restricted administration of several chemical inhibitors of different morphogenic pathway, including the Notch signaling. The authors described that the treatment with two new Notch inhibitors has no major effects on thyroid development (9). The reasons of conflicting findings to those previously discussed are currently unknown, but differences in experimental condition or compounds activity in the different studies should be also taken into account. However, it is also possible that cardiac and pharyngeal vessels abnormalities observed in DAPT-treated embryos and Mib mutants account for the onset of the most severe thyroid phenotype. This view is also supported by the existence of a common pathway regulating thyroid and heart development (22). Nevertheless, the involvement of Jag1-Notch signaling in thyroid organogenesis is also sustained by the enrichment in JAG1 pathogenic variants among patients with CH, and in particular in syndromic cases with thyroid dysgenesis and associated cardiac defects, as well as by the occurrence of thyroid dysfunction among young patients with Alagille syndrome (13, 77, 78).
Sonic Hedgehog Pathway
In vertebrates, Sonic Hedgehog (SHH) is known to be an important regulator of embryogenesis, during left-right determination of body axis and organogenesis, and it is also implicated in the development of the pharyngeal apparatus (79–81).
The activation of SHH signaling starts with the binding of SHH to the transmembrane receptor PTCH1 that releases its inhibitory effect on the neighbor receptor SMO. As a result, SMO binds SUFU into the cytosol, negatively regulating its binding to the GLI transcription factors. Once released, GLI translocates into the nucleus and interacts with specific GLI-responsive elements located into the promoter region of target genes, thus activating or repressing their expression (82). Genes of the SHH signaling are expressed during the early developmental stages of zebrafish embryonic development (8). Targeted inactivation of shh results in abnormal thyroid migration with absent bifurcation and mislocalization of follicles, likely due to the asymmetrical development of the pharyngeal arches of shh mutants (23, 24). Similarly, Haerlingen et al. (9) described that chemical perturbation of SHH pathway with cyclopamine only has minor effects during endoderm formation and specification of thyroid anlage but appears to induce thyroid abnormalities in later stages, likely due to impaired cardiovascular development (9). Moreover, since migration of follicles follows the anatomy of the dorsal aorta, SHH signaling seems to be important for the definition of the foregut territories that sustains thyroid development (6).
However, we recently described a novel implication of SHH signaling in zebrafish thyroid specification (83, 84). The genetic screening in cohorts of patients with syndromic or isolated CH have associated mutations in GLIS3, a downstream effector of SHH, and defects in thyroid development (7, 85, 86). In zebrafish, glis3 is expressed in the pharyngeal endoderm at 22-24 hpf, and knockdown embryos for glis3 present a reduced number of thyroid precursors expressing nkx2.4b and pax2a, and low T4-producing follicles at the larval stage. Conversely, the overexpression glis3 mRNA results in an enlargement of thyroid precursor population and increased number of mature follicles. Interestingly, cyclopamine abolishes the expression of glis3, but the exogenous expression glis3 is able to rescue the thyroid phenotype of cyclopamine treated embryos. Since, both endoderm and cardiovascular development appear unaffected in glis3 knockdown embryos, these findings suggest a possible direct role of glis3, belonging the SHH pathway, in regulating the amount of endodermal precursors that will differentiate into thyroid cells (83). A similar mechanism for Glis3 has been described in mice pancreas development, where Glis3 appears fundamental for β-cell lineage specification and insulin expression (87). Altogether these data highlight the causative role of GLIS3 biallelic mutations in a complex human phenotype including thyroid dysgenesis together with neonatal type-1 diabetes (NDH syndrome) (85, 86).
Considering the impairment of thyroid specification in glis3 knockdown embryos and the mislocalization of thyroid follicles observed in previous models, shh signaling seems to be required during early embryonic development for the commitment of thyroid precursors, and later, likely involving different downstream effectors, to control thyroid cell migration along the pharyngeal vessels (6, 9, 83, 88).
Fibroblast Growth Factors
As already mentioned, the proper development of the endoderm-derived organs needs a source of permissive signals from the surrounding mesoderm. Experiments conducted in primary cell cultures explanted from mouse embryos demonstrated that the inhibition of FGF signal from the cardiac mesoderm prevents the specification of thyroid progenitors, whereas FGF2 induces thyroid primordium specification from the anterior foregut endoderm (89–91). Although FGF signaling is well described in the mesoderm context, only few studies have addressed the role of FGF in endoderm patterning and thyroid development in the zebrafish (35, 56, 92, 93). Wendl and colleagues (2007) reported that, during zebrafish somitogenesis, fgf8 is detectable in cardiac mesoderm, adjacent the developing anterior foregut endoderm, and that fgf8 aceti282a mutants exhibit thyroid dysgenesis (93). Additional findings derived from the observation of embryos with abolished expression of hand2, encoding for a bHLH transcription factor expressed in the neural crest mesenchyme of the pharyngeal arches, branchial arteries, and heart tube, and at low levels in the pharyngeal endoderm (94). Zebrafish hand2s6 mutants display defects in thyroid primordium specification in presence of normal endoderm formation. Similarly, in hand2c99 mutants the thyroid primordium is absent, concurrent with defects on pharynx, heart, and fin development (93). Since the endoderm-derived thyroid precursors originate from the pharyngeal region proximal to the hand2-expressing mesoderm cells (e.g., cardiac mesoderm), it is conceivable that hand2 drives the expression of mesodermal factors with a permissive action in thyroid specification. Intriguingly, the ectopic overexpression of FGF rescues the thyroid defects of hand2s6 mutants, suggesting that FGF acts in parallel or downstream the hand2 signaling (93).
Furthermore, it has been reported that the overexpression of FGFs or FGF receptor strongly interfere with endoderm formation, whereas the inactivation of FGF signaling through the microinjection of antisense morpholinos is associated with an increased number of endodermal precursors. Interestingly, FGF inhibition is able to partially recover both endoderm induction and thyroid defects of casta56 mutants, indicating that the proper endoderm formation in response to Nodal signaling is negative regulated by FGF (56).
In addition to those previously observed, Haerlingen and colleagues (9) described that the chemical inhibition of FGF during gastrula stages is associated with an enhanced expression of nkx2.4b in the thyroid primordium at 28 hpf, whereas the administration of the same compounds during somitogenesis completely abolish the specification of thyroid primordium, suggesting that these permissive factors act during critical developmental windows (9).
Bone Morphogenic Proteins
The role of BMPs regulating mesoderm and ectoderm specification is well documented but only few studies explain its function during endoderm patterning (95). In mice, BMP signaling is required for definitive endoderm formation and migration of the anterior visceral endoderm, as evidenced by alterations of endoderm morphogenesis after depletion of Bmp1a (96, 97). Tiso and colleagues (2002) found that, in zebrafish, BMP contributes the dorsal-ventral patterning of the embryo. Interestingly, increased levels of BMP by bmp2 mRNA microinjection have no consequences on the number of endoderm precursors but affect the expression of her5, a critical factor for antero-posterior patterning. A gradient of BMP signaling seems to define the extension of her5 territory during gastrulation and thus, the allocation of anterior endodermal precursors (98). By contrast, Poulain et al. (56) described that the overexpression of a cocktail of bmp2, bmp4 and bmp7 strongly diminishes the number of endoderm precursors, whereas they are only slightly increased after the inhibition of BMP by the overexpression of noggin mRNA. Interestingly, the simultaneous inhibition of BMP and FGF signaling results in a massive increment of endodermal precursors (56). These studies give us new information about role for BMP in controlling endoderm induction and highlight that both positive (by Nodal) and negative (by BMP and FGF) signals are required for proper endoderm patterning.
Haerlingen et al. (9) described that the pharmacological inhibition of BMP during gastrulation severely affects thyroid specification, a phenotype that is difficult to explain given the posteriorizing BMP activity during endoderm formation (9). However, recent studies demonstrate that BMP is also important to induce ventralization of the foregut endoderm, and for that reason it might alters the specification of the thyroid bud that will later develop form that endodermal region (99). Intriguingly, these data suggest that the potential of the endodermal cells to became thyroid cells might be assigned already during gastrulation, and in this context, BMP activity seems to be required for such dorso-ventral patterning (9).
Moreover, similarly to FGF (see section 5.4), the chemical inhibition of BMP during somitogenesis impairs thyroid development by completely blocking the expression of early thyroid markers in the future thyroid region. Associated thyroid and cardiac defects are reported in embryos treated with antagonists of FGF (fgfr1) or BMP (alk1, 2, 3 and 6) receptors (9). Although in contrast to those observed in previous works that described the negative regulation of FGF and BMP signaling on thyroid specification, these results would add new pieces of this complex puzzle that will help the comprehension of the early molecular events driving thyroid development. It is plausible that the block FGF or BMP signaling during somitogenesis can perturb cardiac mesoderm development or its positioning relative to the endoderm, which in turn affects the induction of endodermal thyroid precursors as a consequence of an alteration in local signal activities.
Very recently, using the zebrafish reporter lines Tg(dusp6:d2EGFP) and Tg(BRE:dmKO2), Haerlingen et al. (16) mapped the activity of FGF and BMP in the foregut endoderm during early thyroid development (16). FGF signal is detectable around 18/19 hpf and co-localizes with the pax2a+ thyroid precursors, whereas BMP co-localizes with nkx2.4b in the thyroid precursors at 22-24 hpf, near the heart tube. This suggests a possible correlation between FGF and pax2a, and BMP and nkx2.4b activation, and further supports the cardiac origins of FGF and BMP signals in regulating thyroid development (15, 56). To further dissect the dynamic activity of FGF and BMP, the authors also performed a 3-hours treatment with FGF or BMP inhibitors at early, middle, and late segmentation stages (16). In contrast to the strong reduction nkx2.4b and tg expression after FGF inhibition during the whole segmentation stage (9), the short-treatment with an FGF inhibitor do not significant alters the expression of TTFs.
However, the BMP inhibition of embryos at early and late segmentation stages leads to the absence of thyroid progenitors, similar to that observed after BMP inhibition during the entire segmentation stage. During the early and late segmentation periods the lack of BMP activity is associated with the absence or strong reduction of TTFs expression, whereas TTFs levels appear largely unaffected at the mid-segmentation period. These new findings indicate that FGF activity is required during the whole segmentation period, whereas BMP could play a biphasic role during zebrafish segmentation (16).
Interestingly, the overexpression of fgfr1 between the middle and late stages of somitogenesis results in an increment of thyroid progenitors expressing pax2a, but not nkx2.4b. In parallel, the overexpression of bmp2b during early segmentation is associated to a corresponding increment of nkx2.4b+ precursors and thyroid enlargement that abnormally elongates along the AP axis, likely due to major left-right asymmetry defects. Since pax2a is expressed earlier than nkx2.4b during thyroid specification, these data suggest that a coordinate activity of FGF and BMP governs early zebrafish thyroid development, where BMP signal during early segmentation acts upstream to FGF (16).
The importance of FGF and BMP signaling in thyroid fate decision has been also described in stem cell-based experiments by the Kotton group. Longmire et al. (90) established a protocol to efficiently isolate Nkx2.1+ lung and thyroid lineages derived from induced pluripotent stem cells (iPSCs) in culture supplemented with FGF, BMP and Wnt (90). Kurmann and colleagues (2015) confirmed that the combinatorial effect of FGF and BMP is sufficient for Nkx2.1+ endodermal-derived lineage differentiation with thyroid potential from embryonic stem cells (ESCs). Also, iPSCs can be fully reprogrammed into endodermal progenitors with thyroid potential by exposure to FGF2 and BMP4. Interestingly, the sorting of iPSCs expressing both Nkx2.1+ and Pax8+ give rise to thyroid epithelial cells expressing Nkx2.1, Pax8, Foxe1, Hhex, and Tg. Also, the treatment of iPSCs with BMP (Dorsomorphin) or FGF (LY) inhibitors reduces their capacity to express mature thyroid markers (89). Additional findings from Serra et al. (100) demonstrated that Wnt and BMP signaling are required to specify lung progenitors, whereas FGF and BMP signaling appear to be necessary to promote the specification of the thyroid lineage (100).
The ability to specifically isolate lung or thyroid competent lineages with ability to differentiate and reorganize into organ-specific functional units (bronchial tree/alveoli and thyroid follicles, respectively) makes a strong basis for further studies in developmental biology and regenerative medicine (100).
Wnt Pathway
Wnt pathway is a highly conserved signal transduction pathway involved in critical events of embryogenesis, such as cell fate decision, cell differentiation, and body axis determination during embryonic development (101). The Wnt cascade integrates signals from other pathways, including FGF and BMP (102, 103). Wnt ligands are secreted glycoproteins that bind to the N-terminal extracellular domain of the Frizzled receptors, forming a large surface complex with the co-receptor LPR5/6. The Wnt receptor complex activates the canonical Wnt/ß-catenin pathway, or via the Wnt/Ca2+ or Wnt/planar cell polarity signaling pathways (104, 105). The functions of Wnt/ß-catenin pathway have been extensively studied in heart development, where it promotes precardiac mesoderm specification in cardiomyocyte precursors in zebrafish blastula. Intriguingly, Wnt inhibits cardiomyocyte differentiation at the gastrula stage, highlighting the importance of temporal activity of Wnt signaling during embryonic development (105). Regarding endoderm specification, it has been reported that in Xenopus, an antero-posterior gradient of Wnt activity seems to be necessary for anterior patterning of liver, lung, and thyroid, and posterior endoderm patterning of intestine (106). In zebrafish, a source of Wnt signal is required for proliferation of liver progenitors (107).
Very recently, the group of Costagliola reported that the overactivation of Wnt during early zebrafish development results in an impaired specification of both cardiac and thyroid tissues (9, 15). Embryos treated with BIO and AZA (Wnt up-regulator) at the thyroid anlage stage (28 hpf) show a reduction of nkx2.4b expression in the prospective thyroid region, in a dose-dependent manner. Embryos treated in later stage (55 hpf) exhibit a tiny thyroid primordium with few tg-positive cells. However, when the timing of drug treatment is shifted at early developmental stages, the thyroid defects primarily arise during gastrula and early somitogenesis. The block of cardiomyocyte differentiation by a specific morpholino (mef2c/d-MO) produces a similar thyroid phenotype, further supporting the view that altered signaling between cardiac mesoderm and endodermal thyroid precursors is associated with impaired thyroid specification. The thyroid defect is partially restored by BMP overactivation in these models, thus confirming that a source of BMP from cardiac mesoderm is critical for thyroid specification (15).
Discussion
The huge effort of thyroid research in the last few decades successfully identified several genes and signaling pathways involved in intrinsic or extrinsic control of thyroid development. However, a very limited number of human CH cases with TH can be explained by mutations in these genes.
Targeted inactivation of genes involved in foregut development usually leads to growth arrest or premature death in mice (42). Thus, the availability of additional models is mandatory for study the early events that govern thyroid organogenesis.
As summarized in the present review, our knowledge of the earliest events regulating thyroid development, in particular from the initial regionalization and induction of the endoderm to thyroid anlage specification, has been moved forward by studies in zebrafish. What appeared clear from these findings is that the onset of the thyroid gland is more complex than initially expected. Several intrinsic and extrinsic signals appear to act in synergy to drive the cellular fate of the differentiating endoderm. Moreover, some signaling pathways (e.g., Notch and Sonic Hedgehog) seem to play roles during different phases of thyroid development: restricting the number of endodermal cells committed to thyroid fate and, subsequently, controlling differentiation and migration of thyroid follicles. Notably, such direct effects on thyroid specification seems to occur when Notch receptors bind the jag1 ligands (expressed in thyroid tissue) or Shh signal cascade culminates in the activation of glis3 (expressed in pharyngeal endoderm) (77, 83).
Several recent works have demonstrated the crucial role of surrounding tissues (e.g., lateral plate mesoderm) in the control of the initial steps of endoderm formation, as well as in the acquisition of thyroid competence of endodermal progenitors (1, 2, 9, 15, 18, 33). Noteworthy, the detailed mapping of spatial and temporal expression of TTFs and FGF/BMP described by Costagliola group has significantly increased our comprehension about the fine regulation and crosstalk between mesoderm and endoderm. Of particular interest, the colocalization of FGF and pax2a signals in cells located in the foregut endoderm at 18-20 hpf, would help the identification of endodermal precursors with a thyroid competence before the onset of the thyroid primordium, thus allowing a more targeted study of thyroid precursors (16).
However, one of the major problems hampering a complete understanding of the specific contribution of each signaling pathway during the early events of thyroid development are the presence of discordant phenotypes observed in numerous zebrafish models generated by different experimental protocols. For instance, Poulain et al. (56) describe that the inhibition of FGF signaling by microinjection of morpholinos results to an increase of endodermal precursors, whereas Haerlingen et al. (9) show that the chemical inhibition of FGF during somitogenesis completely abolish the specification of thyroid primordium (9, 56). Although it is difficult to compare models produced with different methodology, the analysis of their similarities and differences would be informative in terms of gene dosage, spatial and temporal activity, as well as maternal and zygotic contribution of a gene of interest. Nevertheless, different thyroid phenotypes are described after the treatment of zebrafish embryos with the same inhibitor, as in the case of DAPT (9, 77) or cyclopamine (9, 84). Indeed, an additional effort should be made to optimize the experimental protocols and understand possible unknown off-target effects of such inhibitors. In Table 1, we summarize all of the available zebrafish models generated for studying endoderm induction and thyroid specification.
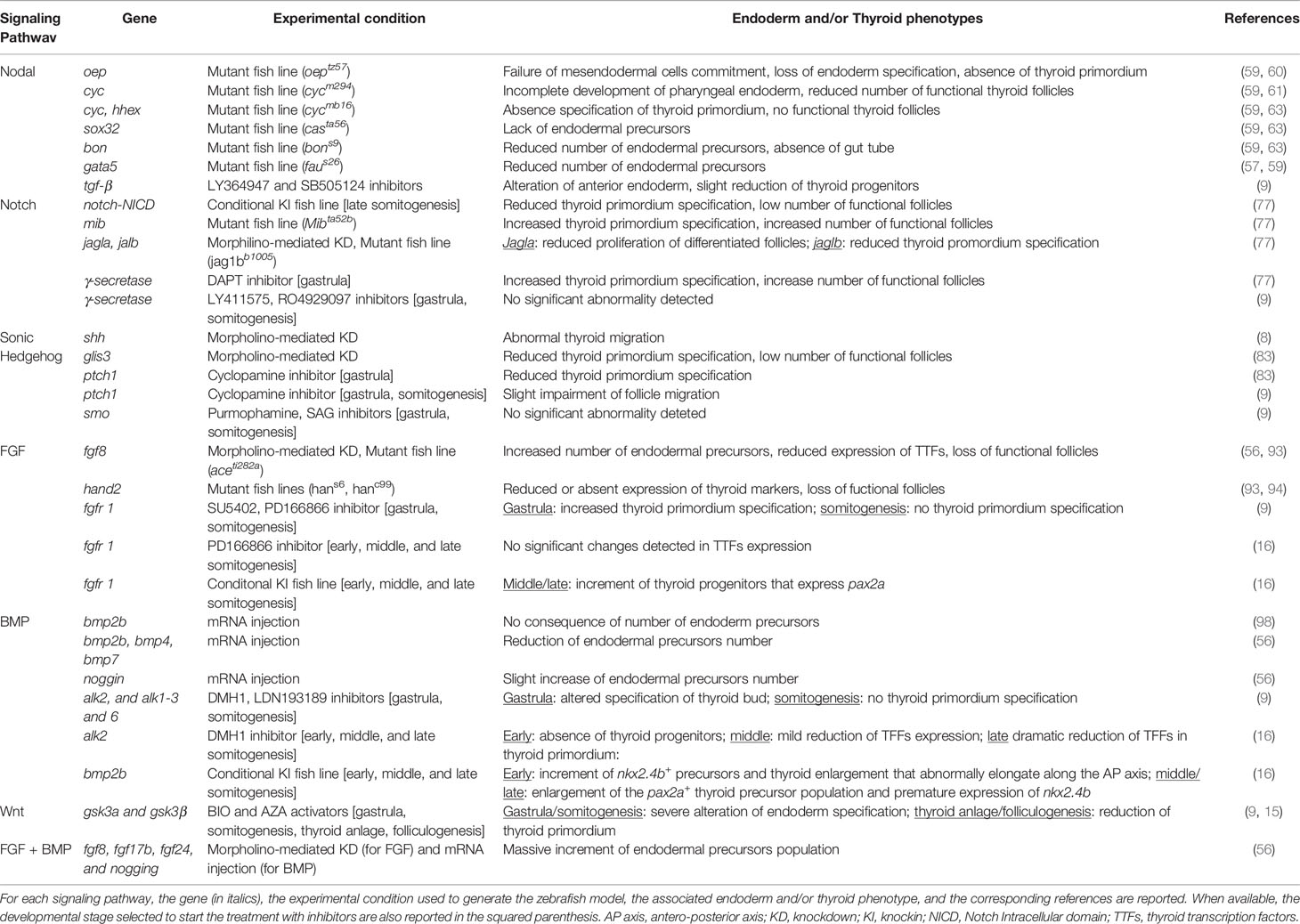
Table 1 Morphogenetic pathways involved in endoderm induction and specification of thyroid primordium.
Altogether, this work demonstrates that zebrafish is a valuable model for the investigation of the complex crosstalk between the several, and possibly redundant, factors that act through the expression of specific endodermal or mesodermal genes that guide the proper thyroid specification.
In conclusion, the described zebrafish models open new perspectives in understanding the early stages of thyroid development. These data represent a practical basis to set protocols for the generation of functional thyroid follicles from stem cells and will hopefully translate in an improved understanding of the pathogenesis of thyroid dysgenesis, the most frequent congenital endocrine defect and preventable cause of intellectual disability.
Author Contributions
FM and GR performed literature search and drafted the manuscript and figures. LP revised and finalized the manuscript for submission. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by Ricerca Corrente funds of Istituto Auxologico Italiano, IRCCS (project Zebratir, # 05C102_2011).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Nilsson M, Fagman H. Development of the Thyroid Gland. Development (2017) 144(12):2123–40. doi: 10.1242/dev.145615
2. De Felice M, Di Lauro R. Minireview: Intrinsic and Extrinsic Factors in Thyroid Gland Development: An Update. Endocrinology (2011) 152(8):2948–56. doi: 10.1210/en.2011-0204
3. De Felice M, Di Lauro R. Thyroid Development and its Disorders: Genetics and Molecular Mechanisms. Endocr Rev (2004) 25(5):722–46. doi: 10.1210/er.2003-0028
4. Wendl T, Lun K, Mione M, Favor J, Brand M, Wilson SW, et al. Pax2.1 is Required for the Development of Thyroid Follicles in Zebrafish. Development (2002) 129(15):3751–60. doi: 10.1242/dev.129.15.3751
5. Fagman H, Nilsson M. Morphogenesis of the Thyroid Gland. Mol Cell Endocrinol (2010) 323(1):35–54. doi: 10.1016/j.mce.2009.12.008
6. Fagman H, Nilsson M. Morphogenetics of Early Thyroid Development. J Mol Endocrinol (2011) 46(1):R33–42. doi: 10.1677/JME-10-0084
7. de Filippis T, Gelmini G, Paraboschi E, Vigone MC, Di Frenna M, Marelli F, et al. A Frequent Oligogenic Involvement in Congenital Hypothyroidism. Hum Mol Genet (2017) 26(13):2507–14. doi: 10.1093/hmg/ddx145
8. Alt B, Reibe S, Feitosa NM, Elsalini OA, Wendl T, Rohr KB. Analysis of Origin and Growth of the Thyroid Gland in Zebrafish. Dev Dyn (2006) 235(7):1872–83. doi: 10.1002/dvdy.20831
9. Haerlingen B, Opitz R, Vandernoot I, Trubiroha A, Gillotay P, Giusti N, et al. Small-Molecule Screening in Zebrafish Embryos Identifies Signaling Pathways Regulating Early Thyroid Development. Thyroid (2019) 29(11):1683–703. doi: 10.1089/thy.2019.0122
10. Larrivée-Vanier S, Deladoey J. Zebrafish Embryo: A New Model for Studying Thyroidmorphogenesis. Curr Opin Endocrine Metab Res (2018) 2:3–9. doi: 10.1016/j.coemr.2018.01.005
11. Marelli F, Persani L. How Zebrafish Research has Helped in Understanding Thyroid Diseases. F1000Res (2017) 6:2137. doi: 10.12688/f1000research.12142.1
12. Opitz R, Antonica F, Costagliola S. New Model Systems to Illuminate Thyroid Organogenesis. Part I: An Update on the Zebrafish Toolbox. Eur Thyroid J (2013) 2(4):229–42. doi: 10.1159/000357079
13. Porazzi P, Calebiro D, Benato F, Tiso N, Persani L. Thyroid Gland Development and Function in the Zebrafish Model. Mol Cell Endocrinol (2009) 312(1-2):14–23. doi: 10.1016/j.mce.2009.05.011
14. Trubiroha A, Gillotay P, Giusti N, Gacquer D, Libert F, Lefort A, et al. A Rapid CRISPR/Cas-Based Mutagenesis Assay in Zebrafish for Identification of Genes Involved in Thyroid Morphogenesis and Function. Sci Rep (2018) 8(1):5647. doi: 10.1038/s41598-018-24036-4
15. Vandernoot I, Haerlingen B, Gillotay P, Trubiroha A, Janssens V, Opitz R, et al. Enhanced Canonical Wnt Signaling During Early Zebrafish Development Perturbs the Interaction of Cardiac Mesoderm and Pharyngeal Endoderm and Causes Thyroid Specification Defects. Thyroid (2021) 31(3):420–38. doi: 10.1089/thy.2019.0828
16. Haerlingen B, Opitz R, Vandernoot I, Molinaro A, Shankar M, Gillotay P, et al. Timed Mesodermal FGF and BMP Govern the Multistep Thyroid Specification. bioRxiv (2020). doi: 10.1101/2020.08.13.249540
17. Opitz R, Maquet E, Zoenen M, Dadhich R, Costagliola S. TSH Receptor Function is Required for Normal Thyroid Differentiation in Zebrafish. Mol Endocrinol (2011) 25(9):1579–99. doi: 10.1210/me.2011-0046
18. Alexander J, Stainier DY. A Molecular Pathway Leading to Endoderm Formation in Zebrafish. Curr Biol (1999) 9(20):1147–57. doi: 10.1016/S0960-9822(00)80016-0
19. Li N, Wei C, Olena AF, Patton JG. Regulation of Endoderm Formation and Left-Right Asymmetry by miR-92 During Early Zebrafish Development. Development (2011) 138(9):1817–26. doi: 10.1242/dev.056697
20. Ober EA, Field HA, Stainier DY. From Endoderm Formation to Liver and Pancreas Development in Zebrafish. Mech Dev (2003) 120(1):5–18. doi: 10.1016/S0925-4773(02)00327-1
21. Rohr KB, Concha ML. Expression of nk2.1a During Early Development of the Thyroid Gland in Zebrafish. Mech Dev (2000) 95(1-2):267–70. doi: 10.1016/S0925-4773(00)00345-2
22. Opitz R, Maquet E, Huisken J, Antonica F, Trubiroha A, Pottier G, et al. Transgenic Zebrafish Illuminate the Dynamics of Thyroid Morphogenesis and its Relationship to Cardiovascular Development. Dev Biol (2012) 372(2):203–16. doi: 10.1016/j.ydbio.2012.09.011
23. Alt B, Elsalini OA, Schrumpf P, Haufs N, Lawson ND, Schwabe GC, et al. Arteries Define the Position of the Thyroid Gland During its Developmental Relocalisation. Development (2006) 133(19):3797–804. doi: 10.1242/dev.02550
24. Elsalini OA, von Gartzen J, Cramer M, Rohr KB. Zebrafish Hhex, nk2.1a, and pax2.1 Regulate Thyroid Growth and Differentiation Downstream of Nodal-dependent Transcription Factors. Dev Biol (2003) 263(1):67–80. doi: 10.1016/S0012-1606(03)00436-6
25. Tehrani Z, Lin S. Endocrine Pancreas Development in Zebrafish. Cell Cycle (2011) 10(20):3466–72. doi: 10.4161/cc.10.20.17764
26. Zaret KS. Regulatory Phases of Early Liver Development: Paradigms of Organogenesis. Nat Rev Genet (2002) 3(7):499–512. doi: 10.1038/nrg837
27. Stanger BZ. Organ Size Determination and the Limits of Regulation. Cell Cycle (2008) 7(3):318–24. doi: 10.4161/cc.7.3.5348
28. Stanger BZ, Tanaka AJ, Melton DA. Organ Size is Limited by the Number of Embryonic Progenitor Cells in the Pancreas But Not the Liver. Nature (2007) 445(7130):886–91. doi: 10.1038/nature05537
29. Lania G, Zhang Z, Huynh T, Caprio C, Moon AM, Vitelli F, et al. Early Thyroid Development Requires a Tbx1-Fgf8 Pathway. Dev Biol (2009) 328(1):109–17. doi: 10.1016/j.ydbio.2009.01.014
30. Perry SF, Wilson RJ, Straus C, Harris MB, Remmers JE. Which Came First, the Lung or the Breath? Comp Biochem Physiol A Mol Integr Physiol (2001) 129(1):37–47. doi: 10.1016/S1095-6433(01)00304-X
31. Brugman S. The Zebrafish as a Model to Study Intestinal Inflammation. Dev Comp Immunol (2016) 64:82–92. doi: 10.1016/j.dci.2016.02.020
32. Longo S, Riccio M, McCune AR. Homology of Lungs and Gas Bladders: Insights From Arterial Vasculature. J Morphol (2013) 274(6):687–703. doi: 10.1002/jmor.20128
33. Fukuda K, Kikuchi Y. Endoderm Development in Vertebrates: Fate Mapping, Induction and Regional Specification. Dev Growth Differ (2005) 47(6):343–55. doi: 10.1111/j.1440-169X.2005.00815.x
34. Melby AE, Warga RM, Kimmel CB. Specification of Cell Fates at the Dorsal Margin of the Zebrafish Gastrula. Development (1996) 122(7):2225–37. doi: 10.1242/dev.122.7.2225
35. Warga RM, Nusslein-Volhard C. Origin and Development of the Zebrafish Endoderm. Development (1999) 126(4):827–38. doi: 10.1242/dev.126.4.827
36. Pezeron G, Mourrain P, Courty S, Ghislain J, Becker TS, Rosa FM, et al. Live Analysis of Endodermal Layer Formation Identifies Random Walk as a Novel Gastrulation Movement. Curr Biol (2008) 18(4):276–81. doi: 10.1016/j.cub.2008.01.028
37. Grapin-Botton A, Constam D. Evolution of the Mechanisms and Molecular Control of Endoderm Formation. Mech Dev (2007) 124(4):253–78. doi: 10.1016/j.mod.2007.01.001
38. Stainier DY. A Glimpse Into the Molecular Entrails of Endoderm Formation. Genes Dev (2002) 16(8):893–907. doi: 10.1101/gad.974902
39. Kopinke D, Sasine J, Swift J, Stephens WZ, Piotrowski T. Retinoic Acid is Required for Endodermal Pouch Morphogenesis and Not for Pharyngeal Endoderm Specification. Dev Dyn (2006) 235(10):2695–709. doi: 10.1002/dvdy.20905
40. Okada K, Takada S. The Second Pharyngeal Pouch is Generated by Dynamic Remodeling of Endodermal Epithelium in Zebrafish. Development (2020) 147(24). doi: 10.1242/dev.194738
41. Pinheiro D, Heisenberg CP. Zebrafish Gastrulation: Putting Fate in Motion. Curr Top Dev Biol (2020) 136:343–75. doi: 10.1016/bs.ctdb.2019.10.009
42. Felice M, Lauro RD. Murine Models for the Study of Thyroid Gland Development. Endocr Dev (2007) 10:1–14. doi: 10.1159/000106814
43. Horb ME, Slack JM. Endoderm Specification and Differentiation in Xenopus Embryos. Dev Biol (2001) 236(2):330–43. doi: 10.1006/dbio.2001.0347
44. Langdon YG, Mullins MC. Maternal and Zygotic Control of Zebrafish Dorsoventral Axial Patterning. Annu Rev Genet (2011) 45:357–77. doi: 10.1146/annurev-genet-110410-132517
45. Carvalho L, Heisenberg CP. The Yolk Syncytial Layer in Early Zebrafish Development. Trends Cell Biol (2010) 20(10):586–92. doi: 10.1016/j.tcb.2010.06.009
46. Marlow FL. Setting Up for Gastrulation in Zebrafish. Curr Top Dev Biol (2020) 136:33–83. doi: 10.1016/bs.ctdb.2019.08.002
47. Stern CD, Downs KM. The Hypoblast (Visceral Endoderm): An Evo-Devo Perspective. Development (2012) 139(6):1059–69. doi: 10.1242/dev.070730
48. Fan X, Hagos EG, Xu B, Sias C, Kawakami K, Burdine RD, et al. Nodal Signals Mediate Interactions Between the Extra-Embryonic and Embryonic Tissues in Zebrafish. Dev Biol (2007) 310(2):363–78. doi: 10.1016/j.ydbio.2007.08.008
49. Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, et al. Zebrafish Organizer Development and Germ-Layer Formation Require Nodal-Related Signals. Nature (1998) 395(6698):181–5. doi: 10.1038/26013
50. Schier AF, Talbot WS. Molecular Genetics of Axis Formation in Zebrafish. Annu Rev Genet (2005) 39:561–613. doi: 10.1146/annurev.genet.37.110801.143752
51. Gut P, Reischauer S, Stainier DYR, Arnaout R. Little Fish, Big Data: Zebrafish as a Model for Cardiovascular and Metabolic Disease. Physiol Rev (2017) 97(3):889–938. doi: 10.1152/physrev.00038.2016
52. Stainier DY. Zebrafish Genetics and Vertebrate Heart Formation. Nat Rev Genet (2001) 2(1):39–48. doi: 10.1038/35047564
53. Schier AF. Nodal Morphogens. Cold Spring Harb Perspect Biol (2009) 1(5):a003459. doi: 10.1101/cshperspect.a003459
54. Zhou X, Sasaki H, Lowe L, Hogan BL, Kuehn MR. Nodal is a Novel TGF-beta-like Gene Expressed in the Mouse Node During Gastrulation. Nature (1993) 361(6412):543–7. doi: 10.1038/361543a0
55. Aoki TO, Mathieu J, Saint-Etienne L, Rebagliati MR, Peyrieras N, Rosa FM. Regulation of Nodal Signalling and Mesendoderm Formation by TARAM-A, a TGFbeta-related Type I Receptor. Dev Biol (2002) 241(2):273–88. doi: 10.1006/dbio.2001.0510
56. Poulain M, Furthauer M, Thisse B, Thisse C, Lepage T. Zebrafish Endoderm Formation is Regulated by Combinatorial Nodal, FGF and BMP Signalling. Development (2006) 133(11):2189–200. doi: 10.1242/dev.02387
57. Reiter JF, Kikuchi Y, Stainier DY. Multiple Roles for Gata5 in Zebrafish Endoderm Formation. Development (2001) 128(1):125–35. doi: 10.1242/dev.128.1.125
58. Rodaway A, Takeda H, Koshida S, Broadbent J, Price B, Smith JC, et al. Induction of the Mesendoderm in the Zebrafish Germ Ring by Yolk Cell-Derived TGF-Beta Family Signals and Discrimination of Mesoderm and Endoderm by FGF. Development (1999) 126(14):3067–78. doi: 10.1242/dev.126.14.3067
59. Elsalini OA, Rohr KB. Phenylthiourea Disrupts Thyroid Function in Developing Zebrafish. Dev Genes Evol (2003) 212(12):593–8. doi: 10.1007/s00427-002-0279-3
60. Schier AF, Neuhauss SC, Helde KA, Talbot WS, Driever W. The One-Eyed Pinhead Gene Functions in Mesoderm and Endoderm Formation in Zebrafish and Interacts With No Tail. Development (1997) 124(2):327–42. doi: 10.1242/dev.124.2.327
61. Talbot WS, Egan ES, Gates MA, Walker C, Ullmann B, Neuhauss SC, et al. Genetic Analysis of Chromosomal Rearrangements in the Cyclops Region of the Zebrafish Genome. Genetics (1998) 148(1):373–80. doi: 10.1093/genetics/148.1.373
62. Hatta K, Kimmel CB, Ho RK, Walker C. The Cyclops Mutation Blocks Specification of the Floor Plate of the Zebrafish Central Nervous System. Nature (1991) 350(6316):339–41. doi: 10.1038/350339a0
63. Kikuchi Y, Agathon A, Alexander J, Thisse C, Waldron S, Yelon D, et al. Casanova Encodes a Novel Sox-Related Protein Necessary and Sufficient for Early Endoderm Formation in Zebrafish. Genes Dev (2001) 15(12):1493–505. doi: 10.1101/gad.892301
64. Kikuchi Y, Trinh LA, Reiter JF, Alexander J, Yelon D, Stainier DY. The Zebrafish Bonnie and Clyde Gene Encodes a Mix Family Homeodomain Protein That Regulates the Generation of Endodermal Precursors. Genes Dev (2000) 14(10):1279–89. doi: 10.1101/gad.14.10.1279
65. Hagos EG, Dougan ST. Time-Dependent Patterning of the Mesoderm and Endoderm by Nodal Signals in Zebrafish. BMC Dev Biol (2007) 7:22. doi: 10.1186/1471-213X-7-22
66. Kikuchi Y, Verkade H, Reiter JF, Kim CH, Chitnis AB, Kuroiwa A, et al. Notch Signaling can Regulate Endoderm Formation in Zebrafish. Dev Dyn (2004) 229(4):756–62. doi: 10.1002/dvdy.10483
67. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch Signaling: Cell Fate Control and Signal Integration in Development. Science (1999) 284(5415):770–6. doi: 10.1126/science.284.5415.770
68. Bolos V, Grego-Bessa J, de la Pompa JL. Notch Signaling in Development and Cancer. Endocr Rev (2007) 28(3):339–63. doi: 10.1210/er.2006-0046
69. Bray SJ. Notch Signalling: A Simple Pathway Becomes Complex. Nat Rev Mol Cell Biol (2006) 7(9):678–89. doi: 10.1038/nrm2009
70. Fiuza UM, Arias AM. Cell and Molecular Biology of Notch. J Endocrinol (2007) 194(3):459–74. doi: 10.1677/JOE-07-0242
71. Appel B, Eisen JS. Regulation of Neuronal Specification in the Zebrafish Spinal Cord by Delta Function. Development (1998) 125(3):371–80. doi: 10.1242/dev.125.3.371
72. Appel B, Givan LA, Eisen JS. Delta-Notch Signaling and Lateral Inhibition in Zebrafish Spinal Cord Development. BMC Dev Biol (2001) 1:13. doi: 10.1186/1471-213X-1-13
73. Li XY, Zhai WJ, Teng CB. Notch Signaling in Pancreatic Development. Int J Mol Sci (2015) 17(1):1748. doi: 10.3390/ijms17010048
74. Nakhai H, Siveke JT, Klein B, Mendoza-Torres L, Mazur PK, Algul H, et al. Conditional Ablation of Notch Signaling in Pancreatic Development. Development (2008) 135(16):2757–65. doi: 10.1242/dev.013722
75. Popko B. Notch Signaling: A Rheostat Regulating Oligodendrocyte Differentiation? Dev Cell (2003) 5(5):668–9. doi: 10.1016/S1534-5807(03)00331-9
76. Ware M, Hamdi-Roze H, Dupe V. Notch Signaling and Proneural Genes Work Together to Control the Neural Building Blocks for the Initial Scaffold in the Hypothalamus. Front Neuroanat (2014) 8:140. doi: 10.3389/fnana.2014.00140
77. Porazzi P, Marelli F, Benato F, de Filippis T, Calebiro D, Argenton F, et al. Disruptions of Global and JAGGED1-mediated Notch Signaling Affect Thyroid Morphogenesis in the Zebrafish. Endocrinology (2012) 153(11):5645–58. doi: 10.1210/en.2011-1888
78. de Filippis T, Marelli F, Nebbia G, Porazzi P, Corbetta S, Fugazzola L, et al. Jag1 Loss-Of-Function Variations as a Novel Predisposing Event in the Pathogenesis of Congenital Thyroid Defects. J Clin Endocrinol Metab (2016) 101(3):861–70. doi: 10.1210/jc.2015-3403
79. Moore-Scott BA, Manley NR. Differential Expression of Sonic Hedgehog Along the Anterior-Posterior Axis Regulates Patterning of Pharyngeal Pouch Endoderm and Pharyngeal Endoderm-Derived Organs. Dev Biol (2005) 278(2):323–35. doi: 10.1016/j.ydbio.2004.10.027
80. Tsukui T, Capdevila J, Tamura K, Ruiz-Lozano P, Rodriguez-Esteban C, Yonei-Tamura S, et al. Multiple Left-Right Asymmetry Defects in Shh(-/-) Mutant Mice Unveil a Convergence of the Shh and Retinoic Acid Pathways in the Control of Lefty-1. Proc Natl Acad Sci USA (1999) 96(20):11376–81. doi: 10.1073/pnas.96.20.11376
81. Westerlund J, Andersson L, Carlsson T, Fagman H, Nilsson M. Misguided Migration of C Cell Precursors to Extra-Thyroidal Locations Related to Defective Pharyngeal Pouch Development in Shh Deficient Mice. Cell Dev Biol (2013) 2(4):129. doi: 10.4172/2168-9296.1000129
82. Varjosalo M, Taipale J. Hedgehog: Functions and Mechanisms. Genes Dev (2008) 22(18):2454–72. doi: 10.1101/gad.1693608
83. Rurale G, Marelli F, Duminuco P, Persani L. Glis3 as a Critical Regulator of Thyroid Primordium Specification. Thyroid (2020) 30(2):277–89. doi: 10.1089/thy.2019.0196
84. Rurale G, Persani L, Marelli F. GLIS3 and Thyroid: A Pleiotropic Candidate Gene for Congenital Hypothyroidism. Front Endocrinol (Lausanne) (2018) 9:730–7. doi: 10.3389/fendo.2018.00730
85. Dimitri P. The Role of GLIS3 in Thyroid Disease as Part of a Multisystem Disorder. Best Pract Res Clin Endocrinol Metab (2017) 31(2):175–82. doi: 10.1016/j.beem.2017.04.007
86. Senee V, Chelala C, Duchatelet S, Feng D, Blanc H, Cossec JC, et al. Mutations in GLIS3 are Responsible for a Rare Syndrome With Neonatal Diabetes Mellitus and Congenital Hypothyroidism. Nat Genet (2006) 38(6):682–7. doi: 10.1038/ng1802
87. Kang HS, Kim YS, ZeRuth G, Beak JY, Gerrish K, Kilic G, et al. Transcription Factor Glis3, a Novel Critical Player in the Regulation of Pancreatic Beta-Cell Development and Insulin Gene Expression. Mol Cell Biol (2009) 29(24):6366–79. doi: 10.1128/MCB.01259-09
88. ZeRuth GT, Yang XP, Jetten AM. Modulation of the Transactivation Function and Stability of Kruppel-Like Zinc Finger Protein Gli-Similar 3 (Glis3) by Suppressor of Fused. J Biol Chem (2011) 286(25):22077–89. doi: 10.1074/jbc.M111.224964
89. Kurmann AA, Serra M, Hawkins F, Rankin SA, Mori M, Astapova I, et al. Regeneration of Thyroid Function by Transplantation of Differentiated Pluripotent Stem Cells. Cell Stem Cell (2015) 17(5):527–42. doi: 10.1016/j.stem.2015.09.004
90. Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, et al. Efficient Derivation of Purified Lung and Thyroid Progenitors From Embryonic Stem Cells. Cell Stem Cell (2012) 10(4):398–411. doi: 10.1016/j.stem.2012.01.019
91. Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different Thresholds of Fibroblast Growth Factors Pattern the Ventral Foregut Into Liver and Lung. Development (2005) 132(1):35–47. doi: 10.1242/dev.01570
92. Wallace KN, Pack M. Unique and Conserved Aspects of Gut Development in Zebrafish. Dev Biol (2003) 255(1):12–29. doi: 10.1016/S0012-1606(02)00034-9
93. Wendl T, Adzic D, Schoenebeck JJ, Scholpp S, Brand M, Yelon D, et al. Early Developmental Specification of the Thyroid Gland Depends on Han-Expressing Surrounding Tissue and on FGF Signals. Development (2007) 134(15):2871–9. doi: 10.1242/dev.02872
94. Yelon D, Ticho B, Halpern ME, Ruvinsky I, Ho RK, Silver LM, et al. The bHLH Transcription Factor Hand2 Plays Parallel Roles in Zebrafish Heart and Pectoral Fin Development. Development (2000) 127(12):2573–82. doi: 10.1242/dev.127.12.2573
95. Lovely CB, Swartz ME, McCarthy N, Norrie JL, Eberhart JK. Bmp Signaling Mediates Endoderm Pouch Morphogenesis by Regulating Fgf Signaling in Zebrafish. Development (2016) 143(11):2000–11. doi: 10.1242/dev.129379
96. Davis S, Miura S, Hill C, Mishina Y, Klingensmith J. BMP Receptor IA is Required in the Mammalian Embryo for Endodermal Morphogenesis and Ectodermal Patterning. Dev Biol (2004) 270(1):47–63. doi: 10.1016/j.ydbio.2004.01.048
97. Miura S, Singh AP, Mishina Y. Bmpr1a is Required for Proper Migration of the AVE Through Regulation of Dkk1 Expression in the Pre-Streak Mouse Embryo. Dev Biol (2010) 341(1):246–54. doi: 10.1016/j.ydbio.2010.02.038
98. Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F. BMP Signalling Regulates Anteroposterior Endoderm Patterning in Zebrafish. Mech Dev (2002) 118(1-2):29–37. doi: 10.1016/S0925-4773(02)00252-6
99. Tucker JA, Mintzer KA, Mullins MC. The BMP Signaling Gradient Patterns Dorsoventral Tissues in a Temporally Progressive Manner Along the Anteroposterior Axis. Dev Cell (2008) 14(1):108–19. doi: 10.1016/j.devcel.2007.11.004
100. Serra M, Alysandratos KD, Hawkins F, McCauley KB, Jacob A, Choi J, et al. Pluripotent Stem Cell Differentiation Reveals Distinct Developmental Pathways Regulating Lung- Versus Thyroid-Lineage Specification. Development (2017) 144(21):3879–93. doi: 10.1242/dev.150193
101. Steinhart Z, Angers S. Wnt Signaling in Development and Tissue Homeostasis. Development (2018) 145(11). doi: 10.1242/dev.146589
102. Hikasa H, Sokol SY. Wnt Signaling in Vertebrate Axis Specification. Cold Spring Harb Perspect Biol (2013) 5(1):a007955. doi: 10.1101/cshperspect.a007955
103. Polevoy H, Gutkovich YE, Michaelov A, Volovik Y, Elkouby YM, Frank D. New Roles for Wnt and BMP Signaling in Neural Anteroposterior Patterning. EMBO Rep (2019) 20(6). doi: 10.15252/embr.201845842
104. Palomer E, Buechler J, Salinas PC. Wnt Signaling Deregulation in the Aging and Alzheimer’s Brain. Front Cell Neurosci (2019) 13:227. doi: 10.3389/fncel.2019.00227
105. Gordon MD, Nusse R. Wnt Signaling: Multiple Pathways, Multiple Receptors, and Multiple Transcription Factors. J Biol Chem (2006) 281(32):22429–33. doi: 10.1074/jbc.R600015200
106. McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin Signaling in the Anterior Endoderm is Essential for Liver and Pancreas Development. Development (2007) 134(12):2207–17. doi: 10.1242/dev.001230
Keywords: congenital hypothyroidism, thyroid development, endoderm, Notch, sonic hedgehog, fibroblast growth factor, bone morphogenic protein, Wnt/b-catenin
Citation: Marelli F, Rurale G and Persani L (2021) From Endoderm to Progenitors: An Update on the Early Steps of Thyroid Morphogenesis in the Zebrafish. Front. Endocrinol. 12:664557. doi: 10.3389/fendo.2021.664557
Received: 05 February 2021; Accepted: 14 May 2021;
Published: 04 June 2021.
Edited by:
Mikael Nilsson, University of Gothenburg, SwedenReviewed by:
Francesco Antonica, University of Trento, ItalyHenrik Fagman, University of Gothenburg, Sweden
Benoit Haerlingen, Université libre de Bruxelles, Belgium
Copyright © 2021 Marelli, Rurale and Persani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federica Marelli, ZmVkZXJpY2EubWFyZWxsaUB1bmltaS5pdA==; Luca Persani, cGVyc2FuaUBhdXhvbG9naWNvLml0
 Federica Marelli
Federica Marelli Giuditta Rurale
Giuditta Rurale Luca Persani
Luca Persani