- 1Department of Gastroenterology, National Clinical Research Center for Digestive Disease, Beijing Friendship Hospital, Capital Medical University, Beijing Digestive Disease Center, Beijing Key Laboratory for Precancerous Lesion of Digestive Disease, Beijing, China
- 2Clinical Epidemiology and EBM Unit, National Clinical Research Center for Digestive Disease, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 3Department of Emergencies and Critical Care, Oslo University Hospital, Oslo, Norway
- 4Department of Cardiology, Kailuan General Hospital, Tangshan, China
Objective: Obesity has been demonstrated to show a consistent link with the increased possibility of nonalcoholic fatty liver disease (NAFLD). Since both serum uric acid (SUA) and obesity are essential components of metabolic syndrome (MetS), it is uncertain whether the incidence of NAFLD results from serum uric acid, obesity, or other potential factors based on previous studies.
Patients and methods: This study enrolled 16,839 participants with no history of alcohol consumption and no fatty liver disease in 2010. All participants completed a survey which included health and lifestyle questionnaires, and underwent physical examination, ultrasonography, and laboratory examinations of blood samples. After the four-year follow up, 5,104 (30.31%) participants were diagnosed with NAFLD. The associations between SUA, BMI or obesity, and incident NAFLD were assessed by multivariate linear regression, logistic regression analysis, and mediation analysis, respectively.
Results: By adjusting demographic and serum characteristics, linear correlation coefficients between obesity and SUA were 20.26 [95% confidence interval (CI)]: 15.74, 24.77), 13.31 (95% CI: 6.63, 19.99) and 22.21 (95% CI: 16.41, 28.02) in the total population, and in the female and male groups, respectively. The odds ratios were 2.49 (95% CI: 1.61, 3.87) in the total population, 5.71 (95% CI: 2.25, 14.45) in the female group and 1.99 (95% CI: 1.15, 3.45) in the male group for the correlation between obesity and incident NAFLD. The mediation analysis showed that SUA contributed to 10.03%, 0.58%, and 12.54% of obesity-related NAFLD development in the total population, females and males, respectively.
Conclusion: The findings showed mediation linkages of both obesity and SUA with the incident NAFLD. The role of SUA as a mediator constitutes clinical significance that should be recognized and considered.
Introduction
Nonalcoholic fatty liver disease (NAFLD) refers to the presence of hepatic steatosis without any other causes for secondary hepatic fat accumulation such as heavy alcohol consumption. NAFLD is now subdivided into nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH); the boundary is whether there is evidence of significant inflammation (1). Strong relationships between NAFLD and insulin resistance (IR), type 2 diabetes mellitus (T2DM), metabolic syndrome (MetS), cardiovascular disease, and other chronic diseases have been proven (2). Previous studies have showed that more than one third of diabetic patients have NAFLD (3, 4), and bidirectionally, NAFLD is associated with a two-fold risk of incident T2DM and MetS (5). Besides, NAFLD conferred an OR of 1.64 ([95% confidence interval (CI)]: 1.26, 2.13) for cardiovascular disease (6), which showed that NAFLD is also associated with an increased risk of cardiovascular disease (7).
Serum uric acid (SUA) levels are the result of purine levels as well as alterations due to lipid metabolism and glucose (8, 9). Several pieces of evidence have shown that SUA levels are correlated with NAFLD onset and progression (10). At the same time, serum levels of uric acid and the prevalence of NAFLD dramatically increase with measures of obesity. Although the incidence of NAFLD and high uric acid is related to body mass index (BMI), it is still unknown whether uric acid affects the incidence of BMI-related NAFLD through some potential pathways. Previous study has also suggested a significant role of elevated SUA in the pathogenesis of NAFLD (8). There is also no evidence available on the possible pathways through which obesity, SUA, and NAFLD interact. From a hemodynamic perspective, reductions in weight and BMI may have a significant impact on SUA, and renal urate excretion suggests that changes in weight may play a role in the regulation of SUA levels. SUA levels may increase blood pressure, triglyceride, and insulin levels while also decreasing cholesterol levels, which could lead to the onset of NAFLD (10). In this context, researchers have hypothesized that SUA mediates the positive association between obesity and NAFLD. Consistent with this hypothesis, a recent review illustrated the mechanisms linking obesity, SUA, and NAFLD based on clinical studies (11). However, this hypothesis has not been tested in large-scale prospective cohort studies. Elucidating the associations among obesity, SUA, and NAFLD is significant because obesity-related hyperuricemia leads to increased societal and economic burdens of obesity-associated NAFLD and mortality. Thus, we used multivariate analyses to confirm the connections among obesity, SUA, and NAFLD. Furthermore, mediation methods were used to examine the connections and associations among SUA, obesity, and NAFLD within the context of a large community-based cohort that included 16,839 adults.
Material and Methods
Patient Enrollment and Setting
The Kailuan Longitudinal Study is an ongoing epidemiologic prospective cohort (trial registration number: ChiCTR-TNC-11001489, registration site: http://www.chictr.org.cn/index.aspx) (12–14) of all employees (≥18 years of age, including those who retired) who received the Kailuan Group’s detailed and thorough medical examination at Tangshan City, China. The employees underwent questionnaire investigations and clinical and laboratory examinations every two years. The data collected from the Kailuan Longitudinal Study started in 2010.
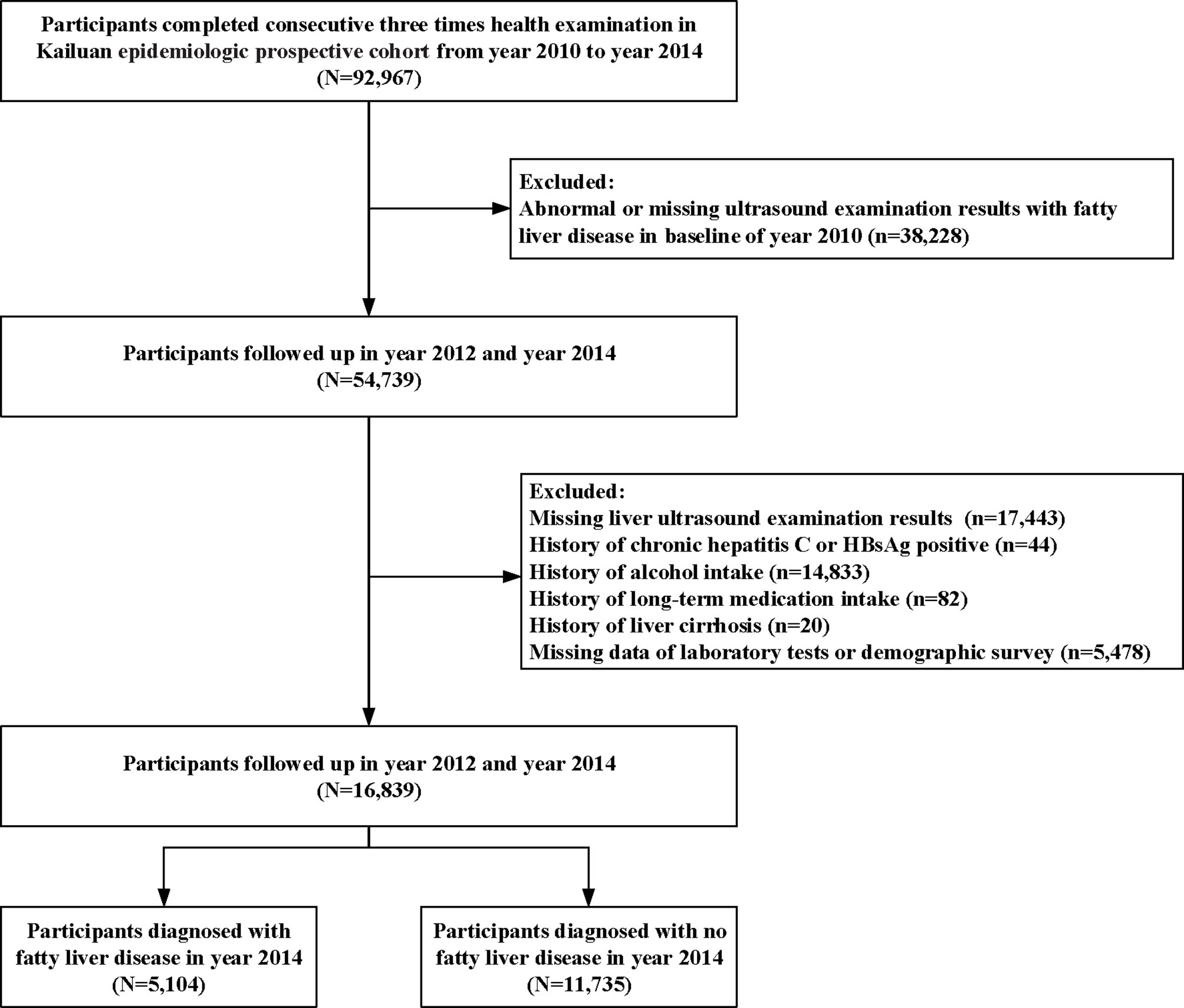
For this study, 92,967 participants completed the three consecutive surveys between 2010 and 2014. All the participants received questionnaire surveys, clinical and laboratory examinations in 2010, 2012, and 2014, respectively. During the four-year period, the demographic and clinical characteristics showed limited changes, within 15%, in both participants with obesity and without obesity (Supplementary Figure 1). The results showed the smoking rates were 30-31% in the obesity group and 26-27% in the non-obesity group over four years, which also showed stable trends.
Briefly, the present study excluded participants who fulfilled the following exclusion criteria: (i) abnormal or missing ultrasound examination results with fatty liver disease at baseline in 2010; (ii) missing liver ultrasound examination results in the follow-up; (iii) history of chronic hepatitis C or HBsAg positivity, alcohol intake, long-term medication intake, or clinical medical diagnostic history of liver cirrhosis; and (iv) missing laboratory data or demographic survey data. The completed survey included health and lifestyle questionnaires, physical examination, ultrasonography, and laboratory examinations of blood samples. A standardized self-administered questionnaire surveyed the medical history and lifestyle factors of all participants. In cases in which some participants had difficulties in completing the questionnaire, a nurse provided assistance.
The study was approved by the Ethics Committees of Kailuan Hospital (ethical approval number: 2006-5) in compliance with the Declaration of Helsinki. All participants signed written informed consent.
Clinical Variables Collection
Participants underwent their clinical examination in the morning after an overnight fast. The examination included blood pressure assessment, anthropometry, and physical examination by a physician. Demographic data regarding gender, age, smoking and alcohol use, physical activity, and medical history (e.g., diabetes and hypertension) were collected using a self-reported questionnaire (12, 14–16). There were also clinical and detailed measurements of height and weight with a stadiometer and balance beam scale. Additionally, BMI was calculated by dividing weight in kilos by height squared in meters (kg/m2) (17). Obesity was defined as BMI ≥ 28 kg/m2 (18). Three measurements of systolic blood pressure (SBP) were then assessed at five-minute intervals after a five-minute rest using a standardized sphygmometer in the seated position; we used the average of three measurements.
Lab Testing
All participants had no food intake for more than eight hours before the physical examination. Elbow venous blood was taken in the early morning to determine the levels of SUA, serum creatinine (Cr), triglyceride (TG), fasting blood glucose (FBG), total cholesterol (TC), and C-reactive protein (CRP). Venous blood samples were also collected after an overnight eight-hour timeframe, followed by transfer of the samples into EDTA-containing vacuum tubes. The endpoint test method was used to measure total TC levels, while the glycerol phosphate oxidase method was used for TG level measurements. For FBG levels, the hexokinase/glucose-6-phosphate dehydrogenase method was used for the measurements. Moreover, an autoanalyzer (Hitachi 747; Hitachi, Tokyo, Japan) at the central laboratory of Kailuan General Hospital analyzed the samples (15, 19, 20).
Outcome Definition
The outcome was determined by analyzing images obtained from abdominal realtime ultrasonography (Philips HD-15, Bothell, WA) with a transducer frequency of 2.50–3.50 MHz by trained technicians; these images provided the basis for the measurement of fatty liver disease. After an overnight fast, the participants were examined in a supine position or a left lateral position with the right upper quadrant of the abdomen exposed. An image server was used to store all the ultrasonographic images. Two doctors examined the liver morphology, size, envelope, boundary, internal echo spot, distribution, liver contour display, and intrahepatic tubular structure and finally recorded the results of the examination.
According to the ultrasonographic diagnostic criteria of NAFLD of the Study Group of Liver and Metabolism, Chinese Society of Endocrinology, signs or evidence of at least two of the following factors served as the basis of the diagnosis of fatty liver: (1) hypoechogenicity in the far field of the liver; (2) hyperechogenicity in the near field of the liver or bright liver, as well as signs of it being stronger than the kidney cortex; and (3) a blurry intrahepatic tubular structure (9). The diagnostic criteria for NAFLD were as follows: (a) a lack of evidence such as alcohol consumption and long-term use of medication and (b) ultrasound findings of fatty liver disease. Alcohol intake in this study was defined as any form of alcohol intake (e.g., beer, white spirit with a high or low content of aromatics, and wine) (21).
Statistical Methods
Continuous variables, such as age and BMI, were described as the mean ± standard deviation (SD), followed by a comparison using Student’s t test. Other continuous variables were described as medians (interquartile ranges, IQRs) and compared by the Mann-Whitney U-test. Categorical variables, such as gender and education, were described as percentages and were compared using the χ2 test. Multivariate linear regression analysis was used to assess the association between SUA, BMI, and obesity. The covariates of age, gender, work type, education level, physical exercise, serum Cr, TG, FBG, cholesterol and CRP were adjusted. The β value and 95% CI were estimated. Multivariate logistic regression analysis was further used to assess the association between the incidence of NAFLD and BMI or obesity, respectively. Covariates such as age, gender, work type, education level, physical exercise, serum Cr, TG, FBG, cholesterol and CRP were included in this analysis. The odds ratio (OR) and 95% confidence interval (CI) were estimated.
Given the associations between SUA, obesity, and incident NAFLD, a mediation analysis was conducted to examine how SUA as a mediator impacted the association between obesity (the independent variable) and incident NAFLD (the outcome variable). The total effect (the association between BMI or obesity and NAFLD), direct effect (the total effect without the influence of SUA), and indirect effect (the effect of BMI or obesity on the outcome of NAFLD attributable to SUA) were assessed with adjusted variables of age, gender, work type, education level, physical exercise, serum Cr, TG, FBG, cholesterol, and CRP. Percent mediation was calculated by dividing the indirect effect by the total effect and represented the proportion of the total effect attributable to the mediator (22). The statistical analyses and tests adopted a two-tailed P value of 0.05 or less as an indication of statistical significance. All statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
The Baseline Information and Outcomes of NAFLD
Tables 1 and 2 show the baseline information of the participants in the study divided by gender and NAFLD. A total of 16,839 individuals (5,418 females and 11,421 males) who met the inclusion criteria were included in the analysis (Figure 1). After four years of follow-up, 5,104 participants were diagnosed with nonalcoholic fatty liver disease. The male group showed a higher percentage of incident NAFLD than the female group (31.41% vs. 28.00%) after four years (P<0.01).
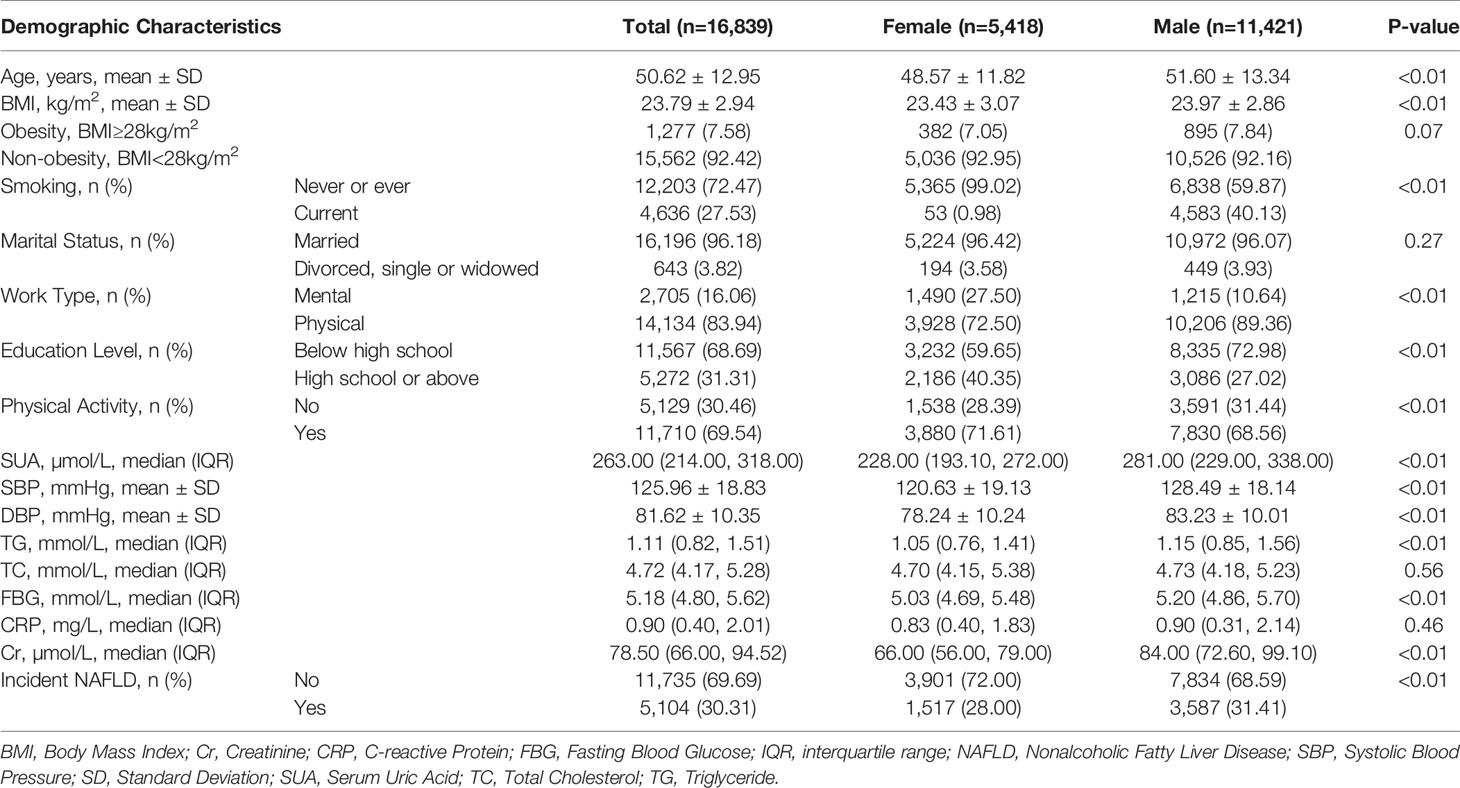
Table 1 Demographic and serum biomarker information of the participants with no NAFLD in baseline in the Kailuan cohort study.
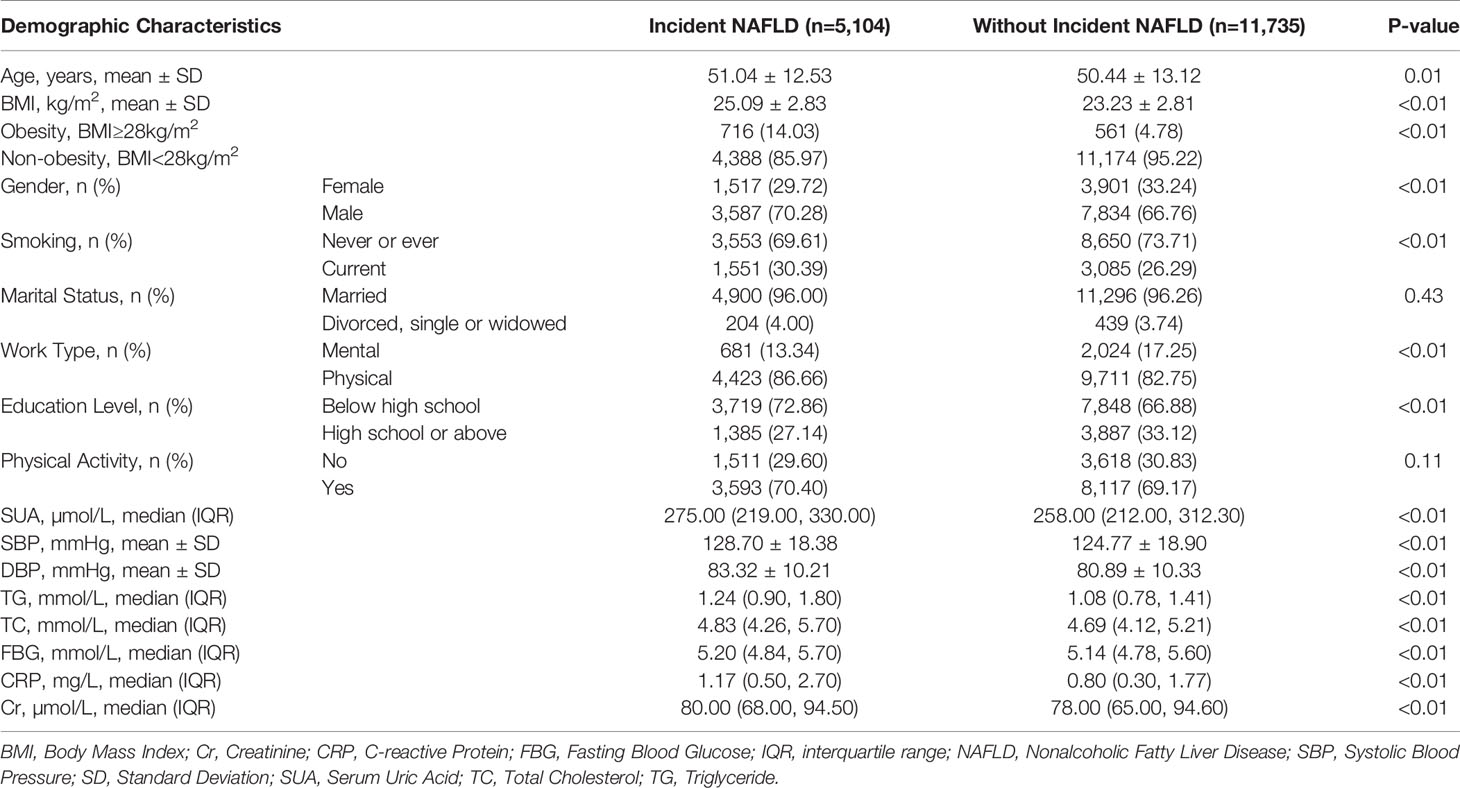
Table 2 Baseline demographic and serum biomarker information of the NAFLD patients and normal participants in the Kailuan cohort study.
Compared with females, the included males were three years older (51.60 ± 13.34 years vs. 48.57 ± 11.82 years, P<0.01). In males, the BMI was 23.97 ± 2.86 kg/m2, versus 23.43 ± 3.07 kg/m2 in females (P<0.01). The smoking rate (40.13% vs. 0.98%) and physical work rate (89.36% vs. 72.50%) were higher in males than in females (P<0.01). Conversely, the physical activity rate among females was 71.61%, which was higher than that among males (68.56%, P<0.01).
In addition to the differences in demographic information, males were almost significantly higher than females in all serum biomarkers, including SUA, SBP, TC, FBG, and Cr (P<0.01). The serum levels of TC and CRP were shown to be insignificant (P=0.56 and P=0.46). Due to the different values of demographic and serum information, the following analyses were all divided by gender to determine whether there were different trends in the incidence of NAFLD.
As shown in Table 2, almost all the baseline demographic and serum biomarkers showed significant differences between participants with incident NAFLD and those without incident NAFLD (all P<0.05), in addition to marital status (P=0.43) and physical activity (P=0.11). Participants with incident NAFLD after the four-year follow-up showed a higher BMI (25.09 ± 2.83kg/m2 vs. 23.23 ± 2.81kg/m2) and higher SUA levels (275 µmol/L vs. 258 µmol/L) than participants without NAFLD (P<0.01). Other serum biomarkers, such as TG, TC, and FBG of participants with incident NAFLD showed higher levels than those of participants without NAFLD (all P<0.01).
Multivariate Linear Regression Between Obesity and SUA in Males and Females
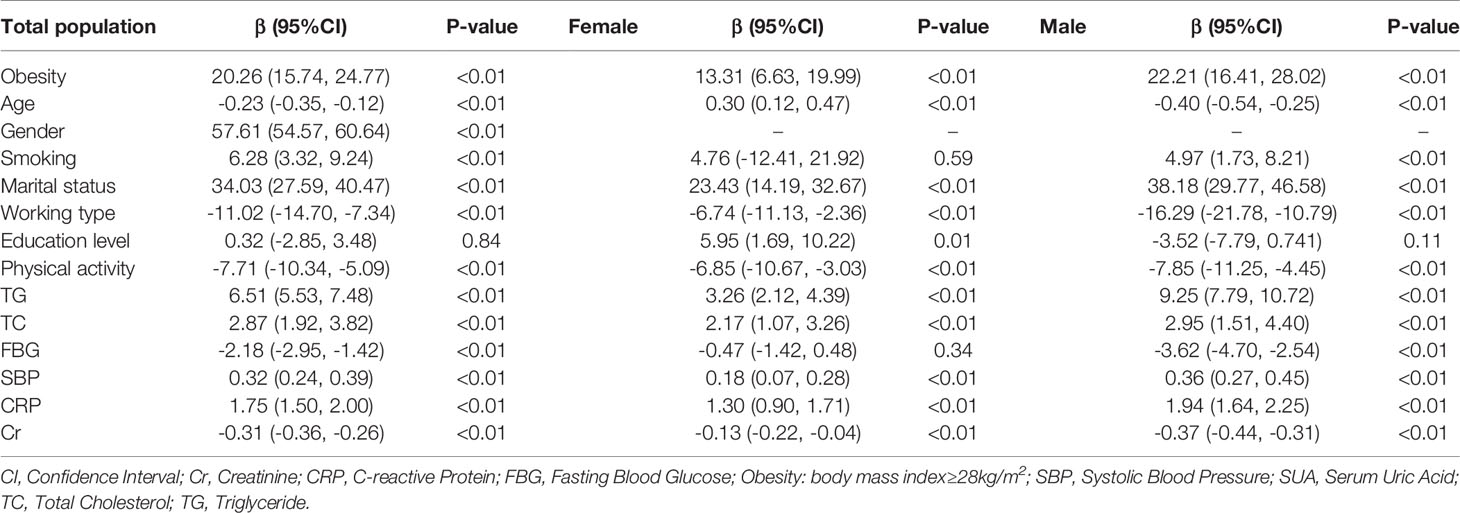
Attempts were made to show the relationship between obesity (BMI ≥ 28 kg/m2) and SUA across different genders. As shown in the multivariate linear regression analyses in Table 3, by adjusting for age, gender, smoking, marital status, work type, education level, physical activity, TG, TC, FBG, SBP, CRP and Cr, the correlation coefficient between obesity and SUA was 20.26 (95% CI: 15.74, 24.77) in the total population. The correlation of obesity and SUA was higher in the male group (22.21, 95% CI: 16.41-28.02) than in the female group (13.31, 95% CI: 6.63-19.99). The Supplementary Table 1 shows the results of linear regression between BMI and SUA in males and females.
Multivariate Logistic Regression Between Obesity, SUA and NAFLDin Males and Females
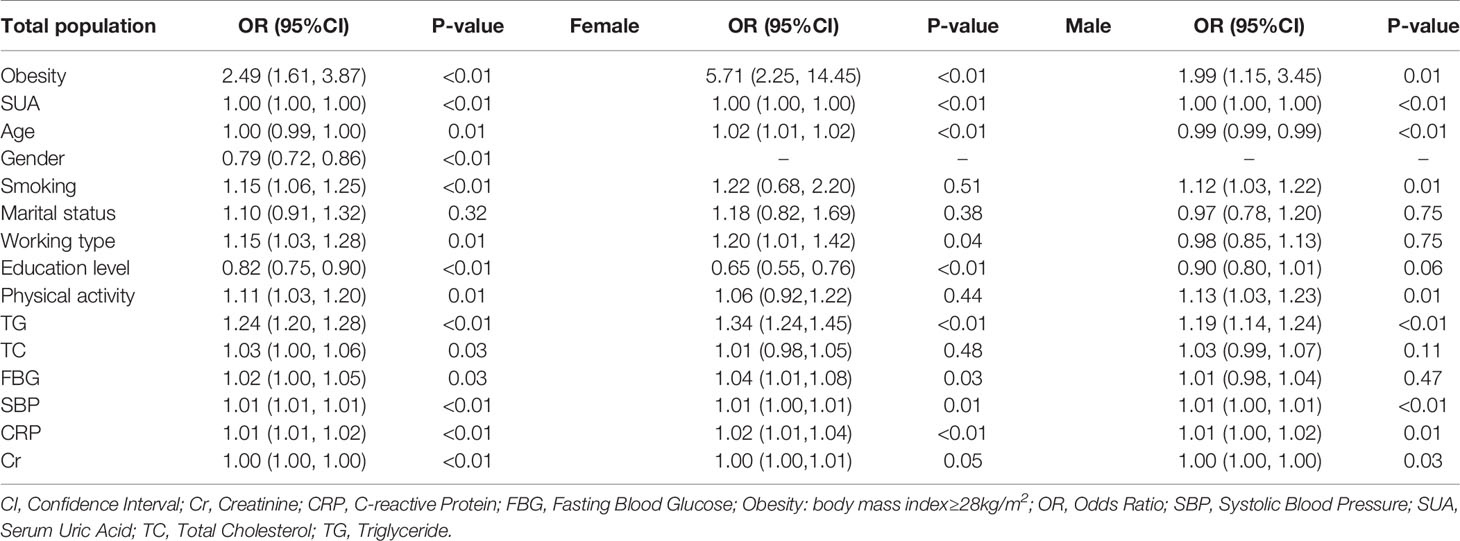
Table 4 shows the relationship between obesity, SUA, and incident NAFLD according to gender-specific groups. Both obesity and SUA showed a significant correlation with incident NAFLD. The ORs for obesity were 2.49 (95% CI: 1.61, 3.87) in the total population, 5.71 (95% CI: 2.25, 14.45) in the female group and 1.99 (95% CI: 1.15, 3.45) in the male group for the correlation between obesity and incident NAFLD. The ORs for SUA were all significant (P<0.01) in the total population, the females and the males, respectively. The Supplementary Table 2 shows the results of logistic regression between BMI, SUA and NAFLD in males and females.
SUA Mediated Obesity-Related NAFLD
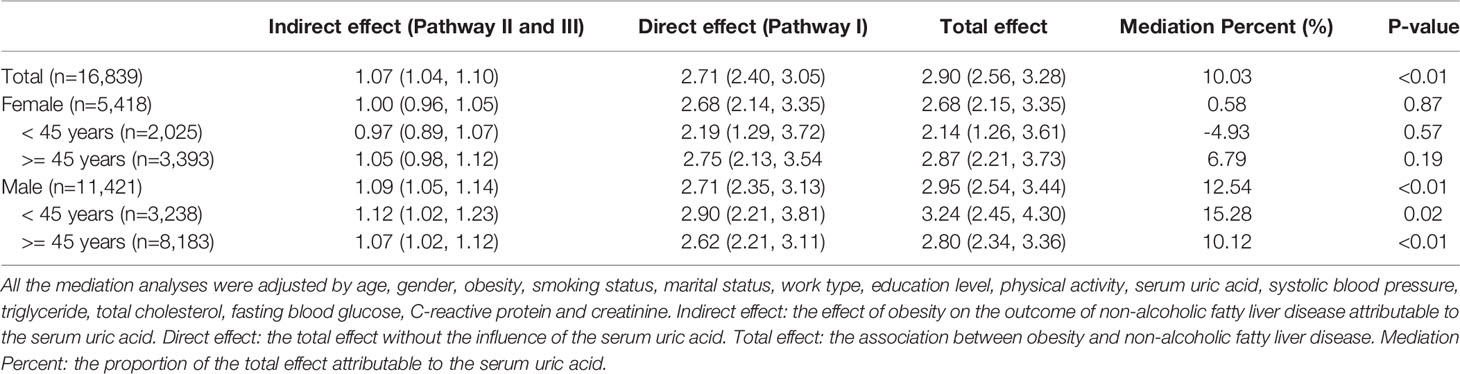
To characterize the effects of obesity on NAFLD that could be explained by a concomitant increase in serum uric acid, mediation analysis was performed to better understand the extent of the interactions. Table 5 was obtained after performing and drawing a relationship triangle through the mediation analysis (Figure 2). The indirect effect is the effect that obesity has on nonalcoholic fatty liver through the mediator SUA. The direct effect is the amount of effect directly exerted on nonalcoholic fatty liver, irrespective of the mediator. Pathway I in Figure 2 represents the direct effect, while pathways II and III together represent the indirect effect. Mediation analysis showed that SUA contributed to 10.03% of obesity-related NAFLD development. In the male group older than 45 years old, SUA accounted for 10.12% of the risk for obesity-related NAFLD development with significance (P<0.01). In contrast, in the female group, although SUA accounted for 6.79% of obesity-related NAFLD, the mediation effects seemed to be insignificant (P=0.19). In males, SUA mediated in different age groups. The mediation effect of SUA was significant in males, whether the age was older than 45 (15.28%) or less than 45 years old (10.12%) (P=0.12 and P<0.01). In females, the mediation effects were not significant both in older group (more than 45 years, P=0.19) and younger group (younger than 45 years, P=0.57).
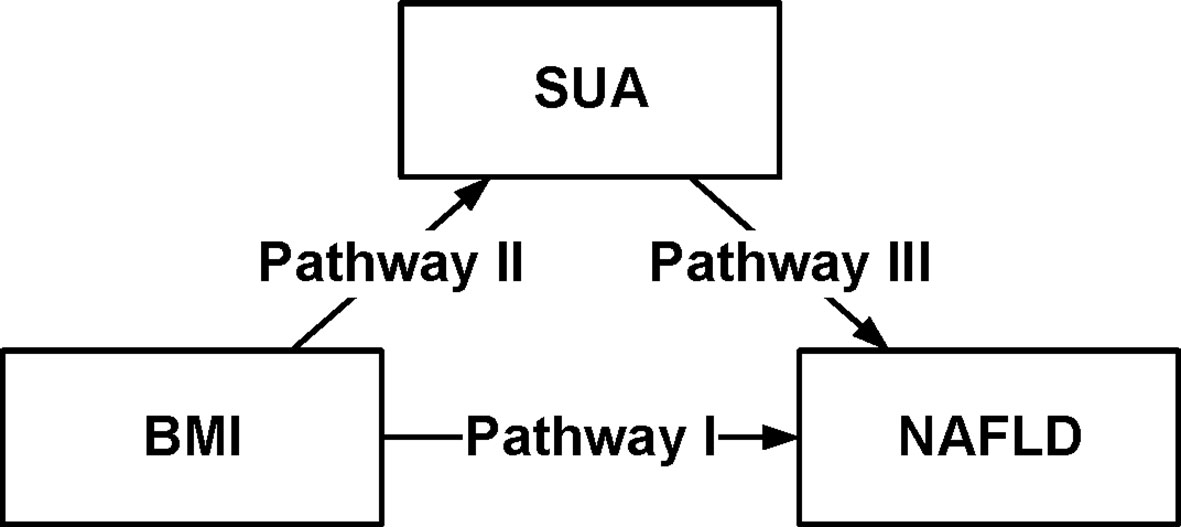
Figure 2 Conceptual version of the model used in this analysis. Serum Uric Acid (SUA) mediates the association between body mass index (BMI) and non-alcoholic fatty liver disease (NAFLD), in 16,839 individuals in the Kailuan Group. Pathway I represented the direct effect, pathway II and pathway III represented the indirect effect.
Discussion
Nonalcoholic fatty liver disease has become increasingly prevalent in recent years in Asia (23). We enrolled 16,839 participants who were free from NAFLD at the baseline examination in 2010. After the four-year follow-up, 5,104 (30.31%) participants were diagnosed with NAFLD. Our results showed that the obesity-related increased threat of NAFLD seemed to be affected by obesity-related changes related to SUA.
In many studies, the incidence of NAFLD increased with obesity, which is consistent with the notion that NAFLD is an obesity-related disorder (24). Consistent with our findings, previous studies have reported that SUA increases with increasing BMI (25) and, intriguingly, is also a risk factor for NAFLD (26). In a study including 166 compensated patients with a histological diagnosis of NAFLD, among which most patients were overweight or obesity, hyperuricemia was found to be associated with histological features of liver disease (27). In addition, a retrospective study of 118 consecutive biopsy-proven NAFLD patients showed that SUA was an independent predictor of NASH and its individual histological lesions, notably including fibrosis (28). Additionally, a meta-analysis of 25 studies found an approximately two-fold increased risk of NAFLD among subjects with hyperuricemia compared with subjects without hyperuricemia (29).
Another study by Zhang et al. showed that both uric acid levels and obesity contributed to the risk of NAFLD (30). In our four-year cohort study, the results coincided with Zhang’s findings. Both obesity (OR=2.49, P<0.01) and SUA (OR=1.00, P<0.01) showed significant correlation with incident NAFLD. The present study further used mediation analysis to evaluate the interactions between obesity, SUA and incident NAFLD in this longitudinal cohort with a large sample size. Mediation analysis showed that SUA contributed to 10% of obesity-related NAFLD development. This is consistent with the notion that increased SUA could be one of the main pathophysiological mechanisms for the increased prevalence of NAFLD in adults with obesity. Biological evidence has also suggested a role for IR, with its links to NAFLD that also showcase the role that IR plays in the accumulation of lipids in the liver (31). Equally important, there have been findings that both IR and hyperuricemia show commonality in certain directional causal impacts. IR induces hyperinsulinemia that could lead to the rise in reabsorption of renal urate through the stimulation of urate-anion exchange; thus, there is a reduction in the excretion of uric acid from the renal tubular cells due to insulin, increasing uric acid impacts pro-inflammatory action on adipocytes as well as stimulates cell proliferation, and the impairments in sensitivity to insulin and effectiveness of glucose of cells because of the impact to glucose indicate transport that is integral in obesity, and MetS (32, 33). From all of these findings, the examination of the links between SUA levels and NAFLD, as well as factors such as levels of insulin and obesity, entails a homeostasis model assessment involving resistance to insulin and levels of glucose that requires investigation and evaluation to expose the mechanisms, dynamics, and processes underlying the SUA and NAFLD relationship (34).
This is a large community-based prospective cohort study with a diagnosis of NAFLD performed by liver ultrasound, which is much more sensitive and specific than biomarkers such as fatty liver index. This study showed the association between incident NAFLD and SUA, which also indicated that obesity and hyperuricemia were linked to NAFLD incidence through mediation analysis. In these multivariate analyses, the confounding factors of obesity and NAFLD necessitate a level of controlled expectation to identify potential manipulable mechanisms. This is an important point because, first, obesity among people with hyperuricemia makes them more prone to increases in uric acid and body weight, leading to the need for these individuals to handle or manage both aspects. Second, another point of emphasis is the way that obesity and hyperuricemia influence the onset of NAFLD because of pathogenic processes. However, the mediation effect is significant in males, but not in females. The main reasons might be as follows: first, the prevalence of NAFLD in men is two times higher than that in women due to the protective effect of estrogen (11). On the other hand, men are more prone to abdominal obesity, which is directly related to the occurrence of NAFLD. Therefore, it can be concluded that SUA contributed to 10-15% of obesity-related NAFLD development in males, and that SUA acts as a mediator of both pathophysiology and clinical connection. When this theory is tested and affirmed, there will be the possibility to manage or treat NAFLD through restoration of the disrupted internal mechanisms within the body. This hypothesis has indicated that obesity-related hyperuricemia is reversible or can be treated with certain pharmacological and lifestyle interventions.
The study has some limitations. This current study was limited to Chinese demographics, specifically, those living in North China. Thus, the results of this study lack generalization to an extent due to different factors, but there is still the uniting or homogeneous aspect of our cohort that could help in reducing the lack of generalization to a more global context. The proportion of females was smaller than the males (32%; n=5,418); however, the large sample size still enabled us to conduct gender-specific analyses. Finally, though the participants enrolled from the epidemiologic prospective cohort, data on aspartate aminotransferase to calculate the scores on either NAFLD fibrosis score or fibrosis-4 score was not available. Nevertheless, participants with the clinical history of liver cirrhosis were excluded by searching for the clinical medical record.
Conclusion
This large community-based longitudinal cohort study, in which potential confounding factors were adjusted for, provides a unique opportunity to understand the relationships of SUA with obesity-related NAFLD. The results suggested that patients living with obesity should pay attention to their uric acid levels to prevent NAFLD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The study was approved by the ethics committees of Kailuan General Hospital (the ethical approval number: 2006-5), in compliance with the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SW and SZ conceived and designed the experiments. SC collected the data. SZ, XS, HS, YJ, and RY conducted the experiments. QZ, JX, and XM analyzed the data. QZ and XM drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the Beijing Natural Science Foundation of China (7204249) and Beijing Talents Fund (2018000021469G198).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all the participants and their relatives and the members of the survey teams in the 11 regional hospitals of Kailuan Medical Group, as well as the project development and management teams in Beijing Friendship Hospital and the Kailuan Group.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.657856/full#supplementary-material
Supplementary Table 1 | Linear regression of the influencing factors for SUA divided by gender. BMI, Body Mass Index; CI, Confidence Interval; Cr, Creatinine; CRP, C-reactive Protein; FBG, Fasting Blood Glucose; SBP, Systolic Blood Pressure; SUA, Serum Uric Acid; TC, Total Cholesterol; TG, Triglyceride.
Supplementary Table 2 | Logistic regression for the association of BMI, SUA and NAFLD divided by gender. BMI, Body Mass Index; CI, Confidence Interval; Cr, Creatinine; CRP, C-reactive Protein; FBG, Fasting Blood Glucose; OR, Odds Ratio; SBP, Systolic Blood Pressure; SUA, Serum Uric Acid; TC, Total Cholesterol; TG, Triglyceride.
Abbreviations
BMI, Body Mass Index; CI, Confidence Interval; Cr, Creatinine; CRP, C-reactive Protein; FBG, Fasting Blood Glucose; IR, Insulin Resistance; MetS, Metabolic Syndrome; NASH, Nonalcoholic Steatohepatitis; NAFL, Nonalcoholic Fatty Liver; NAFLD, Nonalcoholic Fatty Liver Disease; OR, Odds Ratio; SBP, Systolic Blood Pressure; SD, Standard Deviation; SUA, Serum Uric Acid; T2DM, Type 2 Diabetes Mellitus; TC, Total Cholesterol; TG, Triglyceride.
References
1. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance From the American Association for the Study of Liver Diseases. Hepatol (Baltimore Md) (2018) 67:328–57. doi: 10.1002/hep.29367
2. Reccia I, Kumar J, Akladios C, Virdis F, Pai M, Habib N, et al. Non-Alcoholic Fatty Liver Disease: A Sign of Systemic Disease. Metabol: Clin Exp (2017) 72:94–108. doi: 10.1016/j.metabol.2017.04.011
3. Byrne CD, Targher G. NAFLD: A Multisystem Disease. J Hepatol (2015) 62:S47–64. doi: 10.1016/j.jhep.2014.12.012
4. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The Global Epidemiology of NAFLD and NASH in Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis. J Hepatol (2019) 71:793–801. doi: 10.1016/j.jhep.2019.06.021
5. Younossi ZM. Non-Alcoholic Fatty Liver Disease - a Global Public Health Perspective. J Hepatol (2019) 70:531–44. doi: 10.1016/j.jhep.2018.10.033
6. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-Alcoholic Fatty Liver Disease and Risk of Incident Cardiovascular Disease: A Meta-Analysis. J Hepatol (2016) 65:589–600. doi: 10.1016/j.jhep.2016.05.013
7. Targher G, Byrne C, Tilg H. NAFLD and Increased Risk of Cardiovascular Disease: Clinical Associations, Pathophysiological Mechanisms and Pharmacological Implications. Gut (2020) 69:1691–705. doi: 10.1136/gutjnl-2020-320622
8. Kanbay M, Jensen T, Solak Y, Le M, Roncal-Jimenez C, Rivard C, et al. Uric Acid in Metabolic Syndrome: From an Innocent Bystander to a Central Player. Eur J Internal Med (2016) 29:3–8. doi: 10.1016/j.ejim.2015.11.026
9. Gao X, Fan JG. Diagnosis and Management of non-Alcoholic Fatty Liver Disease and Related Metabolic Disorders: Consensus Statement From the Study Group of Liver and Metabolism, Chinese Society of Endocrinology. J Diabetes (2013) 5:406–15. doi: 10.1111/1753-0407.12056
10. Gu Z, Li D, He H, Wang J, Hu X, Zhang P, et al. Body Mass Index, Waist Circumference, and Waist-to-Height Ratio for Prediction of Multiple Metabolic Risk Factors in Chinese Elderly Population. Sci Rep (2018) 8:385. doi: 10.1038/s41598-017-18854-1.
11. Xu K, Zhao X, Fu X, Xu K, Li Z, Miao L, et al. Gender Effect of Hyperuricemia on the Development of Nonalcoholic Fatty Liver Disease (NAFLD): A Clinical Analysis and Mechanistic Study. Biomed Pharmacother (2019) 117:109158. doi: 10.1016/j.biopha.2019.109158
12. Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, et al. Prevalence of Ideal Cardiovascular Health and its Relationship With the 4-Year Cardiovascular Events in a Northern Chinese Industrial City. Circ Cardiovasc Qual Outcomes (2012) 5:487–93. doi: 10.1161/CIRCOUTCOMES.111.963694
13. Wang L, Cui L, Wang Y, Vaidya A, Chen S, Zhang C, et al. Resting Heart Rate and the Risk of Developing Impaired Fasting Glucose and Diabetes: The Kailuan Prospective Study. Int J Epidemiol (2015) 44:689–99. doi: 10.1093/ije/dyv079
14. Jin C, Chen S, Vaidya A, Wu Y, Wu Z, Hu FB, et al. Longitudinal Change in Fasting Blood Glucose and Myocardial Infarction Risk in a Population Without Diabetes. Diabetes Care (2017) 40:1565–72. doi: 10.2337/dc17-0610
15. Huang S, Li J, Shearer GC, Lichtenstein AH, Zheng X, Wu Y, et al. Longitudinal Study of Alcohol Consumption and HDL Concentrations: A Community-Based Study. Am J Clin Nutr (2017) 105:905–12. doi: 10.3945/ajcn.116.144832
16. Li Y, Huang Z, Jin C, Xing A, Liu Y, Huangfu C, et al. Longitudinal Change of Perceived Salt Intake and Stroke Risk in a Chinese Population. Stroke (2018) 49:1332–9. doi: 10.1161/STROKEAHA.117.020277
17. Cecelja M, Chowienczyk P. Dissociation of Aortic Pulse Wave Velocity With Risk Factors for Cardiovascular Disease Other Than Hypertension A Systematic Review. Hypertension (2009) 54:1328–36. doi: 10.1161/HYPERTENSIONAHA.109.137653
18. Hou X, Lu J, Weng J, Ji L, Shan Z, Liu J, et al. Impact of Waist Circumference and Body Mass Index on Risk of Cardiometabolic Disorder and Cardiovascular Disease in Chinese Adults: A National Diabetes and Metabolic Disorders Survey. PloS One (2013) 8:e57319. doi: 10.1371/journal.pone.0057319
19. Li J, Huang Z, Hou J, Sawyer AM, Wu Z, Cai J, et al. Sleep and CKD in Chinese Adults: A Cross-Sectional Study. Clin J Am Soc Nephrol (2017) 12:885–92. doi: 10.2215/CJN.09270816
20. Wu S, Li Y, Jin C, Yang P, Li D, Li H, et al. Intra-Individual Variability of High-Sensitivity C-reactive Protein in Chinese General Population. Int J Cardiol (2012) 157:75–9. doi: 10.1016/j.ijcard.2010.12.019
21. Shen X, Jin C, Wu Y, Zhang Y, Wang X, Huang W, et al. Prospective Study of Perceived Dietary Salt Intake and the Risk of non Alcoholic Fatty Liver Disease. J Hum Nutr Dietetics (2019) 32 :802–9. doi: 10.1111/jhn.12674.
22. Lamarche F, Agharazii M, Nadeau-Fredette A, Madore F, Goupil R. Central and Brachial Blood Pressures, Statins, and Low-Density Lipoprotein Cholesterol: A Mediation Analysis. Hypertension (Dallas Tex: 1979) (2018) 71:415–21. doi: 10.1161/HYPERTENSIONAHA.117.10476
23. Kim SS, Cho HJ, Kim HJ, Kang DR, Berry JR, Kim JH, et al. Nonalcoholic Fatty Liver Disease as a Sentinel Marker for the Development of Diabetes Mellitus in non-Obese Subjects. Digest Liver Dis: Off J Ital Soc Gastroenterol Ital Assoc Study Liver (2018) 50:370–7. doi: 10.1016/j.dld.2017.12.018
24. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, Incidence, and Outcome of non-Alcoholic Fatty Liver Disease in Asia, 1999-2019: A Systematic Review and Meta-Analysis. Lancet Gastroenterol Hepatol (2019) 4:389–98. doi: 10.1016/S2468-1253(19)30039-1
25. Ishizaka N, Ishizaka Y, Toda A, Tani M, Koike K, Yamakado M, et al. Changes in Waist Circumference and Body Mass Index in Relation to Changes in Serum Uric Acid in Japanese Individuals. J Rheumatol (2010) 37:410–6. doi: 10.3899/jrheum.090736
26. Jaruvongvanich V, Ahuja W, Wirunsawanya K, Wijarnpreecha K, Ungprasert P. Hyperuricemia is Associated With Nonalcoholic Fatty Liver Disease Activity Score in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Eur J Gastroenterol Hepatol (2017) 29:1031–5. doi: 10.1097/MEG.0000000000000931
27. Petta S, Cammà C, Cabibi D, Di Marco V, Craxì A. Hyperuricemia is Associated With Histological Liver Damage in Patients With non-Alcoholic Fatty Liver Disease. Alimentary Pharmacol Ther (2011) 34:757–66. doi: 10.1111/j.1365-2036.2011.04788.x
28. Ballestri S, Nascimbeni F, Romagnoli D, Lonardo A. The Independent Predictors of non-Alcoholic Steatohepatitis and its Individual Histological Features.: Insulin Resistance, Serum Uric Acid, Metabolic Syndrome, Alanine Aminotransferase and Serum Total Cholesterol are a Clue to Pathogenesis and Candidate Targets for Treatment. Hepatol Res: Off J Japan Soc Hepatol (2016) 46:1074–87. doi: 10.1111/hepr.12656
29. Wijarnpreecha K, Panjawatanan P, Lekuthai N, Thongprayoon C, Cheungpasitporn W, Ungprasert P. Hyperuricaemia and Risk of Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Liver Int: Off J Int Assoc Study Liver (2017) 37:906–18. doi: 10.1111/liv.13329
30. Zhang SJ, Du TT, Li MN, Lu HM, Lin X, Yu XF. Combined Effect of Obesity and Uric Acid on Nonalcoholic Fatty Liver Disease and Hypertriglyceridemia. Med (Baltimore) (2017) 96:e6381. doi: 10.1097/MD.0000000000006381
31. Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic Fatty Liver Disease: A Precursor of the Metabolic Syndrome. Digest Liver Dis (2015) 47:181–90. doi: 10.1016/j.dld.2014.09.020
32. Liu ZT, Que SP, Zhou L, Zheng SS. Dose-Response Relationship of Serum Uric Acid With Metabolic Syndrome and Non-alcoholic Fatty Liver Disease Incidence: A Meta-Analysis of Prospective Studies. Sci Rep (2015) 5:14325. doi: 10.1038/srep14325
33. Yuan H, Yu C, Li X, Sun L, Zhu X, Zhao C, et al. Serum Uric Acid Levels and Risk of Metabolic Syndrome: A Dose-Response Meta-Analysis of Prospective Studies. J Clin Endocrinol Metab (2015) 100:4198–207. doi: 10.1210/jc.2015-2527
Keywords: serum uric acid, body mass index, mediation analysis, metabolic syndrome, nonalcoholic fatty liver disease, obesity
Citation: Zhang Q, Ma X, Xing J, Shi H, Yang R, Jiao Y, Chen S, Wu S, Zhang S and Sun X (2021) Serum Uric Acid Is a Mediator of the Association Between Obesity and Incident Nonalcoholic Fatty Liver Disease: A Prospective Cohort Study. Front. Endocrinol. 12:657856. doi: 10.3389/fendo.2021.657856
Received: 01 February 2021; Accepted: 19 April 2021;
Published: 13 May 2021.
Edited by:
Luca Busetto, Università degli Studi di Padova, ItalyReviewed by:
Francesca Battista, University of Padua, ItalyStefano Ballestri, Local Health Unit of Modena, Italy
Copyright © 2021 Zhang, Ma, Xing, Shi, Yang, Jiao, Chen, Wu, Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiujing Sun, sunxiujing@ccmu.edu.cn
 Qian Zhang
Qian Zhang Xiaoqian Ma
Xiaoqian Ma Jie Xing
Jie Xing Haiyun Shi
Haiyun Shi Runkuan Yang
Runkuan Yang Yue Jiao
Yue Jiao Shuohua Chen
Shuohua Chen Shouling Wu3
Shouling Wu3 Shutian Zhang
Shutian Zhang Xiujing Sun
Xiujing Sun