- The State Key Lab of Reproductive, Department of Urology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Pyrethroids may be related to male reproductive system damage. However, the results of many previous studies are contradictory and uncertain. Therefore, a systematic review and a meta-analysis were performed to assess the relationship between pyrethroid exposure and male reproductive system damage. A total of 72 articles were identified, among which 57 were selected for meta-analysis, and 15 were selected for qualitative analysis. Pyrethroid exposure affected sperm count (SMD= -2.0424; 95% CI, -2.4699 to -1.6149), sperm motility (SMD=-3.606; 95% CI, -4.5172 to -2.6948), sperm morphology (SMD=2.686; 95% CI, 1.9744 to 3.3976), testis weight (SMD=-1.1591; 95% CI, -1.6145 to -0.7038), epididymal weight (SMD=-1.1576; 95% CI, -1.7455 to -0.5697), and serum testosterone level (SMD=-1.9194; 95% CI, -2.4589 to -1.3798) in the studies of rats. We found that gestational and lactational exposure to pyrethroids can reduce sperm count (SMD=1.8469; 95% CI, -2.9010 to -0.7927), sperm motility (SMD=-2.7151; 95% CI, -3.9574 to -1.4728), testis weight (SMD=-1.4361; 95% CI, -1.8873 to -0.9848), and epididymal weight (SMD=-0.6639; 95% CI, -0.9544 to -0.3733) of F1 offspring. Exposure to pyrethroids can increase malondialdehyde (SMD=3.3451; 95% CI 1.9914 to 4.6988) oxide in testes and can reduce the activities of glutathione (SMD=-2.075; 95% CI -3.0651 to -1.0848), superoxide dismutase (SMD=-2.4856; 95% CI -3.9612 to -1.0100), and catalase (SMD=-2.7564; 95% CI -3.9788 to -1.5340). Pyrethroid exposure and oxidative stress could damage male sperm quality. Gestational and lactational pyrethroid exposure affects the reproductive system of F1 offspring.
Introduction
Synthetic pyrethroids (SPs) are among the most extensively used pesticides worldwide. They are derived from pyrethrins, which are found in the flowers of Chrysanthemum cinerariaefolium (1). In 1949, allethrin was identified as the first pyrethroid pesticide (2). With the continuous progress of science and technology, the number of SPs that have been developed has increased, some of which include: cypermethrin, deltamethrin, fenvalerate, permethrin, pyrethrin, resmethrin, and sumithrin (3). Pyrethroids are endocrine-disrupting chemicals (EDCs) that are responsible for male reproductive impairment (4). According to their chemical structure, pyrethroids consist of two types. Type I pyrethroids do not have a cyano moiety at the α-position (e.g., permethrin and allethrin), whereas Type II pyrethroids, such as cypermethrin, have α-cyano moiety (5). With the phasing out of the varieties of organochlorine and organophosphorus pesticides, pyrethroids have been widely applied to agricultural production, household use, and public health places (6). Pyrethroids often show more advantages than traditional insecticides due to their increased environmental stability and toxicity toward insects (7).
In the past two decades, people have been increasingly exposed to SPs in the environment and homes and through their diet due to the extensive use of these SPs. Acute toxic doses of type I pyrethroids can cause hyperexcitation, ataxia, tremor, and paralysis, whereas type II pyrethroids can lead to hypersensitivity, salivation, and choreoathetosis (8). In recent years, especially since pyrethroids have been listed as direct or indirect endocrine disruptors, an increasing number of studies have focused on their reproductive and endocrine risks. A variety of pyrethroids and their metabolites may disrupt hormone receptors and further interfere with the endocrine reproductive system (9, 10). A recent study showed that workers exposed to pyrethroids had poor semen quality (11).
Many studies have been conducted on mammals. Exposure to cypermethrin was associated with decreased levels of endocrine hormones, such as testosterone, luteinizing hormone, and follicle-stimulating hormone, as well as the testis and epididymis weights of rats (12). E. Kilian et al. focused on the effect of deltamethrin and phytoestrogens on rat reproductive parameters, and the results suggested that they both influenced sperm count and testis mass; the authors hypothesized that deltamethrin may have estrogenic effects (13, 14).
We conducted a systematic review and aggregated the available published data on the effect of SPs on semen parameters using a meta-analysis. Our study aims to determine the influence of SPs on the reproductive parameters of humans and rats and potential toxic effects on offspring.
Materials and Methods
Search Strategy
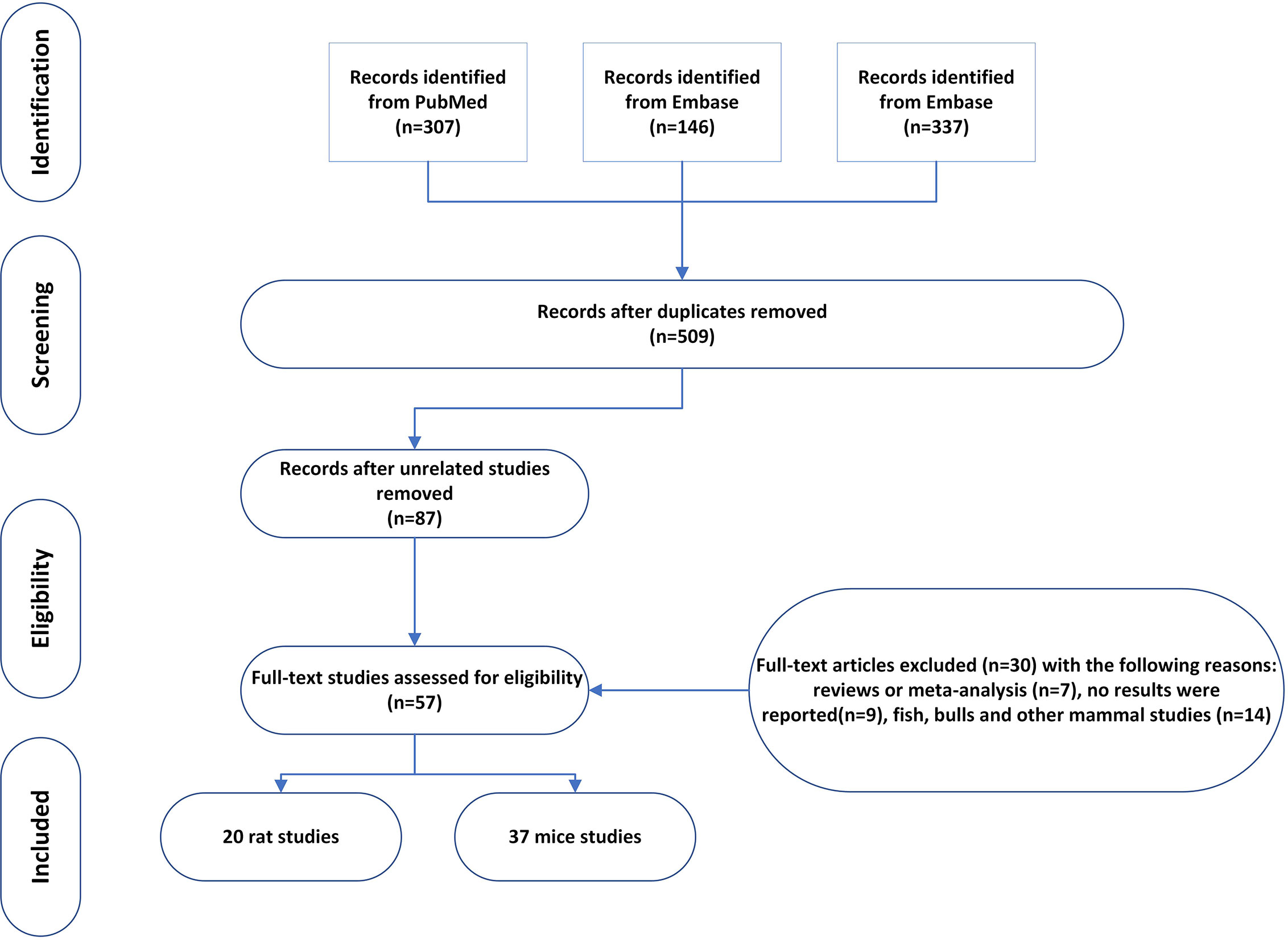
On March 24, 2021, we conducted systematic searches in PubMed, EMBASE, and Web of Science to identify all relevant studies published from 1990 to March 2021 using the following search terms: ((((((((((((((((((((((((((pyrethrin) OR pyrethroid*) OR allethrin stereoisomers) OR bifenthrin) OR beta-cyfluthrin) OR cyfluthrin) OR cyphenothrin) OR deltamethrin) OR esfenvalerate) OR etofenprox) OR fenpropathrin) OR taufluvalinate) OR lambda cyhalothrin) OR gamma cyhalothrin) OR imiprothrin) OR 1RS cispermethrin) OR permethrin) OR prallethrin) OR resmethrin) OR sumithrin) OR d-phenothrin) OR tefluthrin) OR tralomethrin) OR zeta-cypermethrin) OR cypermethrin) OR tetramethrin) AND ((((((((((((((sperm count) OR (sperm concentration)) OR (sperm morphology)) OR (sperm motility)) OR (sperm energy metabolism)) OR (sperm viability)) OR (sperm fertilization capacity)) OR (sperm capacitation reaction)) OR (sperm acrosome reaction)) OR (follicle stimulating hormone)) OR (luteinizing hormone)) OR (testosterone, estrogen)) OR (testis size)) OR (testis weight)) OR (sexual drive). A total of 307, 146, and 337 results were respectively retrieved in PubMed, EMBASE, and Web of Science. After removing duplicates, we were left with 509 potentially relevant articles.
The retrieved titles and abstracts were initially screened. Full texts of selected abstracts matching the inclusion criteria were obtained.
Study Selection and Eligibility Criteria
We included animal and human studies from which primary data were gathered. The titles and abstracts of retrieved articles were independently screened by at least three of the authors (XZ, TZ, and XR). Articles deemed potentially eligible by either reviewer were retrieved for full-text review. The following inclusion and exclusion criteria were used for the meta-analysis. (1) The article should have been published between January 1990 and June 2020. (2) Pyrethroid pesticide should be the only insecticide used in the experiment, which did not include other insecticides, such as dichlorodiphenyltrichloroethane (DDT) and parathion. (3) Animal studies that involved mice or rats were included. (4) Studies involving the combination of pyrethroid pesticide and other insecticide were also excluded. (5) The paper should report the reproductive parameters, such as sperm count, sperm motility, sperm morphology, or serum testosterone. (6) Appropriate result data must be included, and the mean values and standard deviation (Mean ± SD) were used to perform quantitative analysis (standard error is transformed into standard deviation). We identified 57 studies that met our inclusion criteria (15–71).
Data Extraction and Quality Assessment
To minimize bias and improve reliability, three researchers (XZ, TZ, and XR) independently extracted the data and resolved disagreements by discussion. In addition to the data on the means and standard deviations of relative sperm and testis parameters with and without pyrethroid pesticide exposure, the following data were also extracted: first author, dates of publication, the country of publication, published year, strains of rats, exposure periods, and dose. We used Engauge Digitizer to extract information from figures if no other statistical estimates were available. After the extraction of data, they were checked by the authors for discrepancies to minimize the possibility of errors.
Data Synthesis and Analysis
All statistical analyses were carried out in R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). Pooled standardized mean difference (SMD) between comparison groups were calculated to determine the effect size. Both fixed effects models and random effects models (REMs) were fitted to assess the model types that were most suited to the data. Heterogeneity was evaluated using the Q test and the I2 statistic. Statistical significance was set at a p value <0.05. Publication bias was assessed using funnel plots for direct comparisons with 10 or more studies (Appendix B in Supplementary Material). Sensitivity analysis was conducted to evaluate the influence of individual studies on the summary effect estimate.
Results
Study Base
A total of 57 studies were included in the meta-analysis, including 20 rat studies and 37 mice studies. The number of studies included in each meta-analysis varied according to the sperm parameters reported, as follows: 31 studies provided data on sperm count; 25 studies provided data on motility; 28 studies provided data on morphology; 21 provided data on testis weight; 22 provided data on epididymis weight; and 22 provided data on serum testosterone. In the following sections, we first present the results of the quantitative meta-analysis. Next, we review papers that did not report results.
Global Assessment: Reproductive Toxicity of All Pyrethroid Insecticides in Rats and Mice
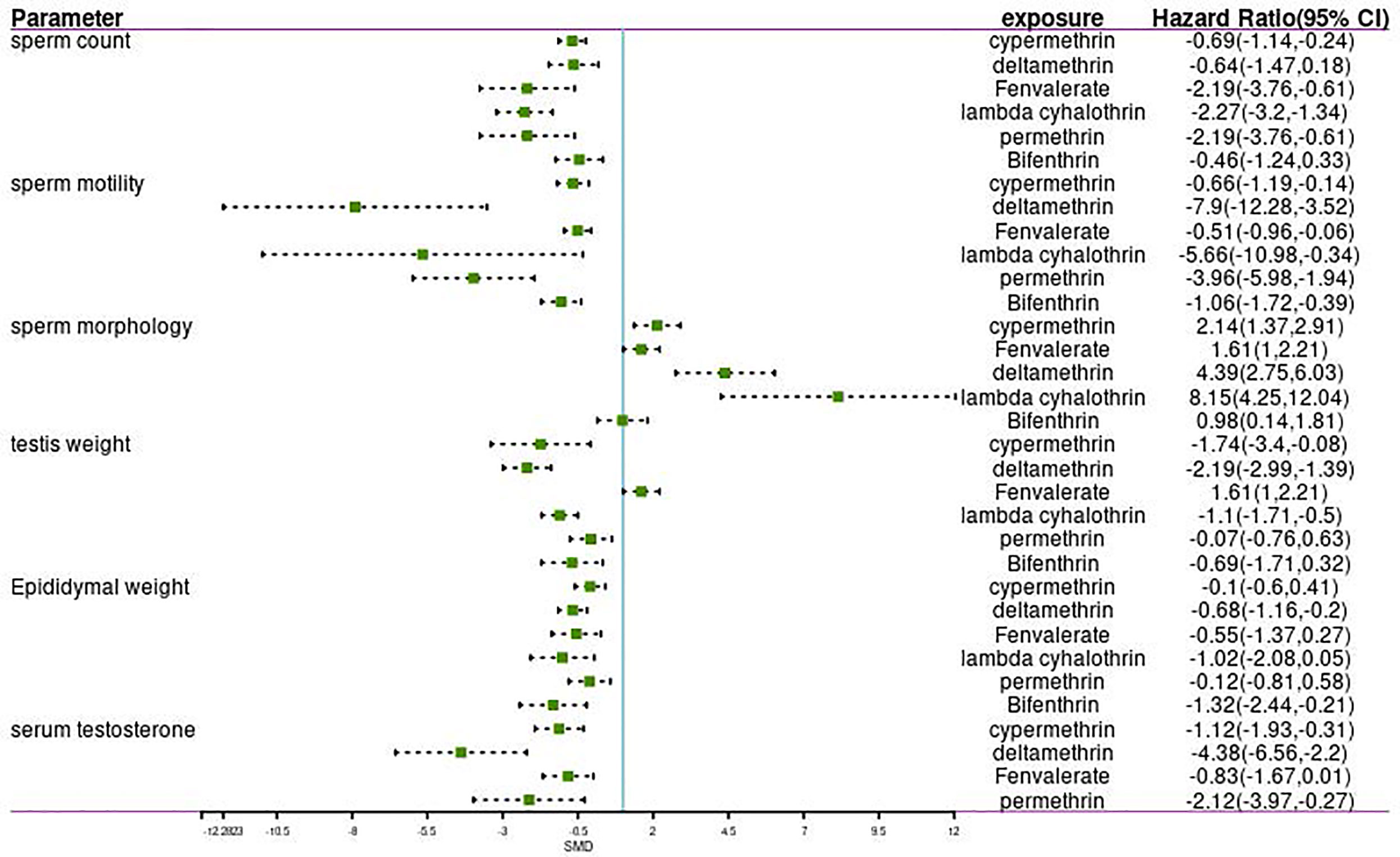
The meta-analysis gives equal weight to each of the xenobiotic exposure indicators, including allethrin, bifenthrin, cypermethrin, deltamethrin, fenvalerate, lambda-cyhalothrin, and permethrin. Statistics were obtained, and analysis was performed on rats and mice, respectively (Tables 1, 2 and Figures 1–3). The funnel plots showed that the study had a risk of bias (Appendix B in the Supplementary Material).
Sperm Count
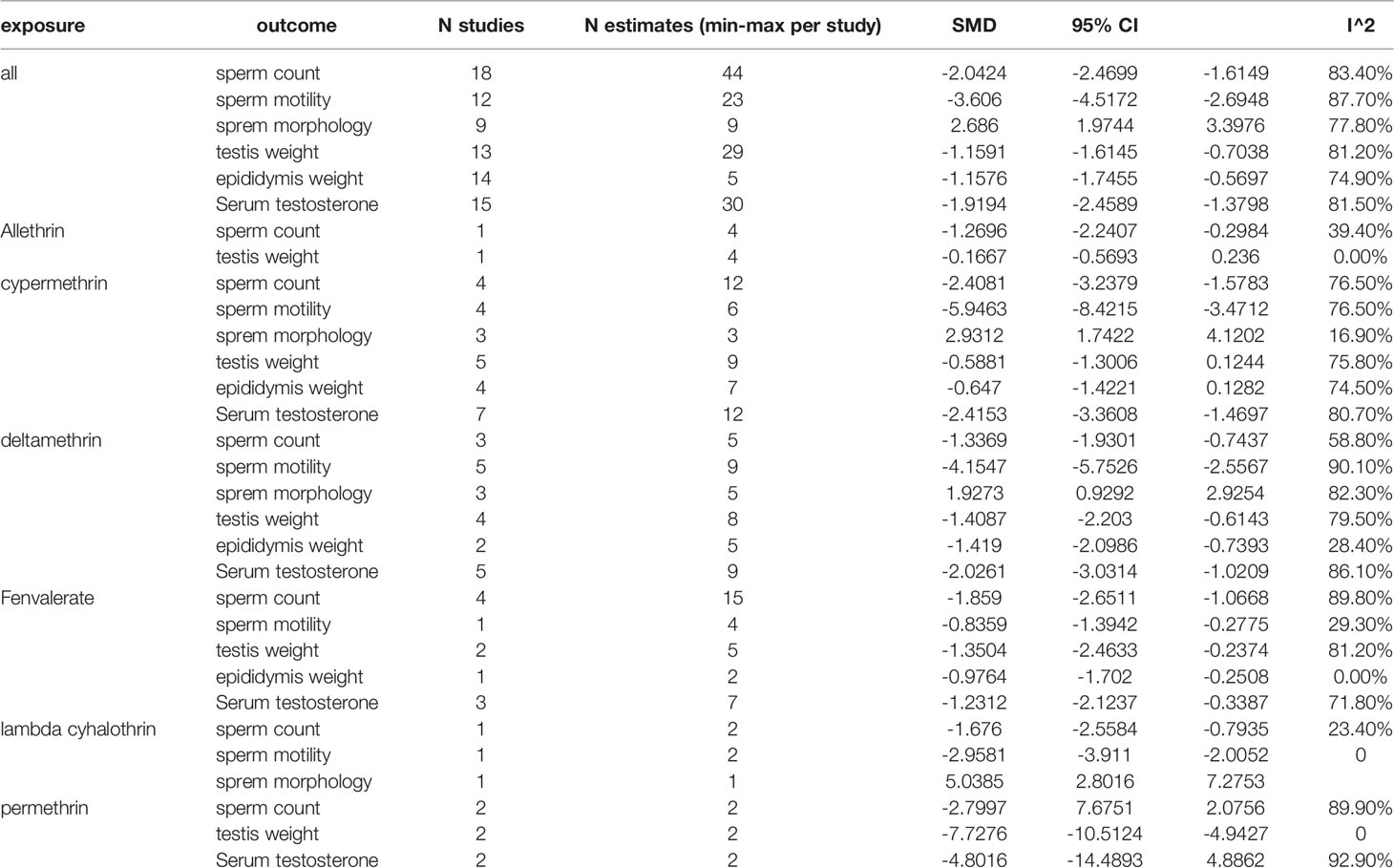
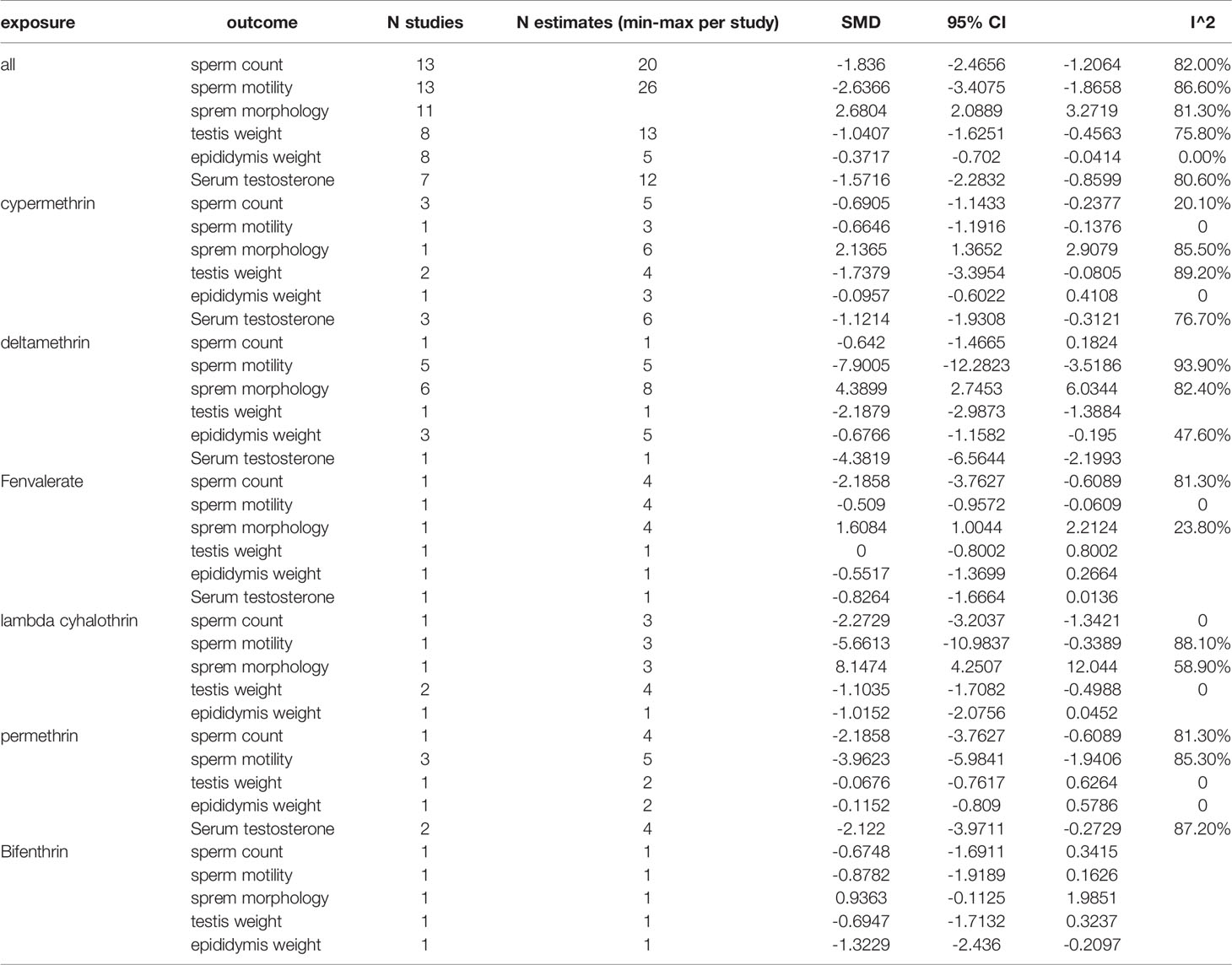
Among the works that involved rats, 18 studies and 44 estimates evaluated the effects of pyrethroids on sperm count (Tables 1 and 2). Pooled results indicated that sperm count was lower in pyrethroid exposure groups (REM SMD = -2.0424; 95% CI, -2.4699 to -1.6149; p<0.0001). In the works involving mice, 13 studies demonstrated the effects of pyrethroids on sperm count. Sperm count was significantly affected by pyrethroids (REM SMD = -1.836; 95% CI, -2.4656 to -1.2064; p<0.0001). Both sensitivity analyses demonstrated that the observed pooled effect size was not affected by the removal of any of the studies.
Sperm Motility
Twelve and thirteen studies were conducted on the toxic effects of pyrethroids on rats and mice, respectively (Tables 1 and 2). Sperm motility was lower in the pyrethroid exposure groups than in the control groups, and the pooled SMDs by REM were -3.606 (95% CI, -4.5172 to -2.6948; p < 0.0001) and -2.6366 (95% CI, -3.4075 to -1.8658 p < 0.0001), respectively. When any of the other studies were removed, the observed pooled effect size was not affected.
Sperm Morphology
Nine rat studies and eleven mice studies observed an abnormal rate in sperm morphology (Tables 1 and 2). The pooled results indicated that pyrethroid exposure was a risk factor of impaired morphology, and the SMD by REM was 2.686 (95% CI, 1.9744 to 3.3976; p < 0.0001) and 2.6804 (95% CI, 2.0889 to 3.2719; p < 0.0001) in rat and mice studies, respectively. Each sensitivity analysis was not affected by the removal of any of the studies.
Testis Weight and Epididymal Weight
In the studies of rats, the pooled SMD by REM of testis weight was -1.1591 (95% CI, -1.6145 to -0.7038 p < 0.0001), and the epididymal weight was also lower in the pyrethroid exposure groups than in the control groups. The pooled SMD by REM was -1.1576 (95% CI, -1.7455 to -0.5697; p = 0.0001). Similarly, in studies on mice, the pooled SMDs by REM of testis and epididymal weight were -1.0407 (95% CI, -1.6251 to -0.4563; p = 0.0005) and -0.3717 (95% CI, -0.7020 to -0.0414; p = 0.0274), respectively. Overall, pyrethroids were a risk factor for reduced testis weight and epididymal weight. In the studies on mice epididymal weight, sensitivity analyses indicated that the paper of Jing-Yi Hu et al. showed a slight increase in the SMD to -1.07 (Tables 1 and 2).
Serum Testosterone
Fifteen and seven studies detected the content of serum testosterone in pyrethroid exposure and control groups of rats and mice, respectively (Tables 1 and 2). Overall, the serum testosterone level was significantly affected by pyrethroids, and the pooled SMDs by REM were -1.9194 (95% CI, -2.4589 to -1.3798; p < 0.0001) and -1.5716 (95% CI, -2.2832 to -0.8599; p < 0.0001).
Subgroup Analysis for the Effect of Different Pyrethroids on Reproductive Parameters
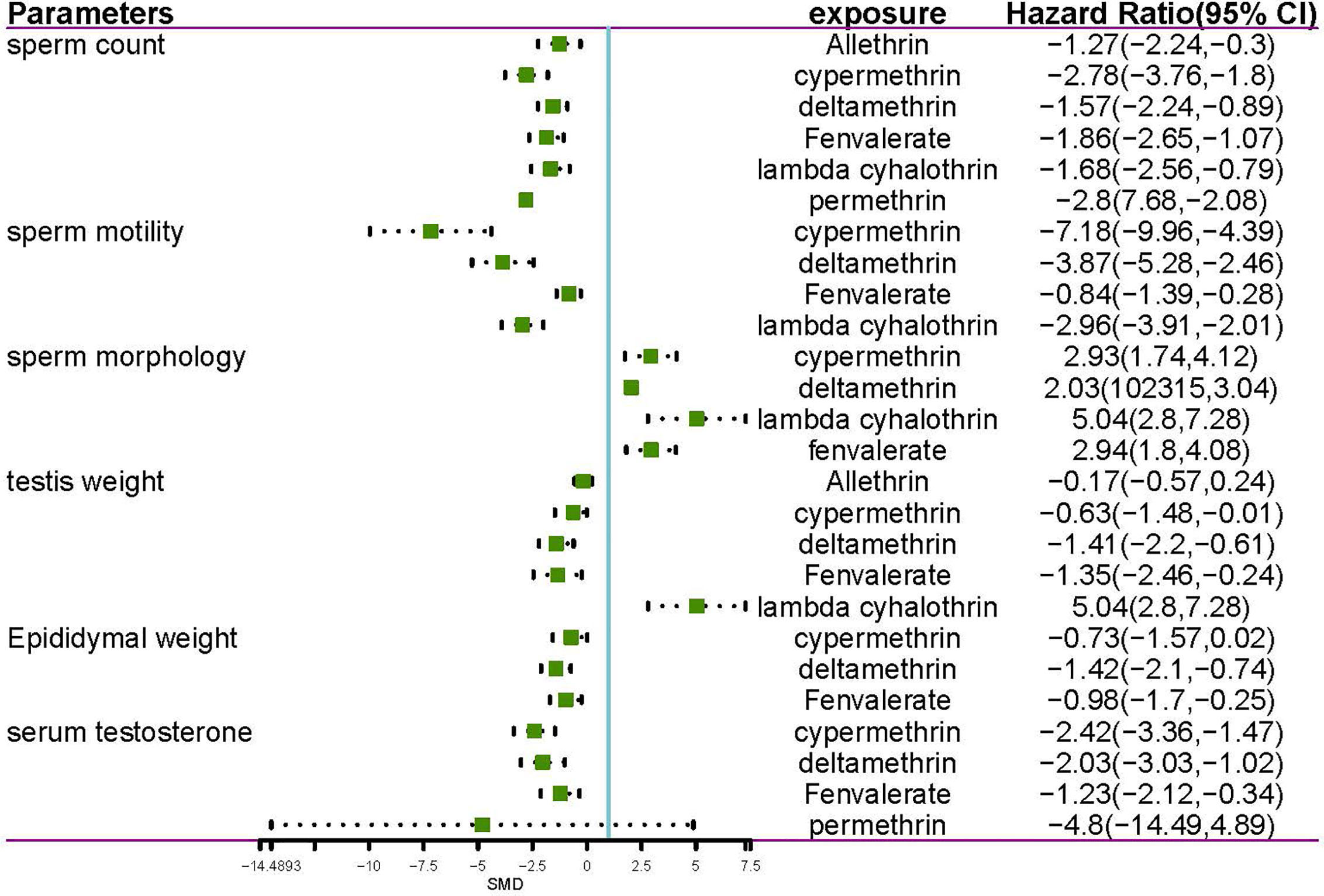
Data were available for substance-specific analyses of any of the reproductive parameters for the most frequently examined pyrethroids (allethrin, bifenthrin, cypermethrin, deltamethrin, fenvalerate, lambda-cyhalothrin, and permethrin). The results are summarized in Tables 1 and 2. Figures 2 and 3 show the forest plot.
Effects of Pyrethroid Exposure on Gestational and Lactational Aspects of Reproductive System
Eight studies that included 410 rats were included in this analysis (Figure 4). The studies reported various parameters, including sperm count, sperm motility, serum testosterone, testis weight, and epididymal weight, which were affected by gestational and lactational exposure to pyrethroids. In general, gestational and lactational pyrethroid exposure and F1 reproductive system were related, and the meta-analysis showed that the sperm count of F1 rats or mice decreased in the exposed groups (the pooled SMDs by REM was -1.8469; 95% CI, -2.9010 to -0.7927; p = 0.0006). Two studies examined the F1 sperm motility after gestational and lactational exposure to pyrethroids, and the SMD by REM was -2.7151 (95% CI, -3.9574 to -1.4728; p<0.0001). In addition, eight studies reported the decrease in testis weight, and the SMD by REM was -1.4361 (95% CI, -1.8873 to -0.9848; p<0.0001). Moreover, the epididymal weight showed a decreasing trend (REM SMD: -0.6639; 95% CI, -0.9544 to -0.3733; p<0.0001). However, the changes of serum testosterone were not significant (REM SMD: -0.3608; 95% CI, -0.9135 to 0.1919; p=0.2007).
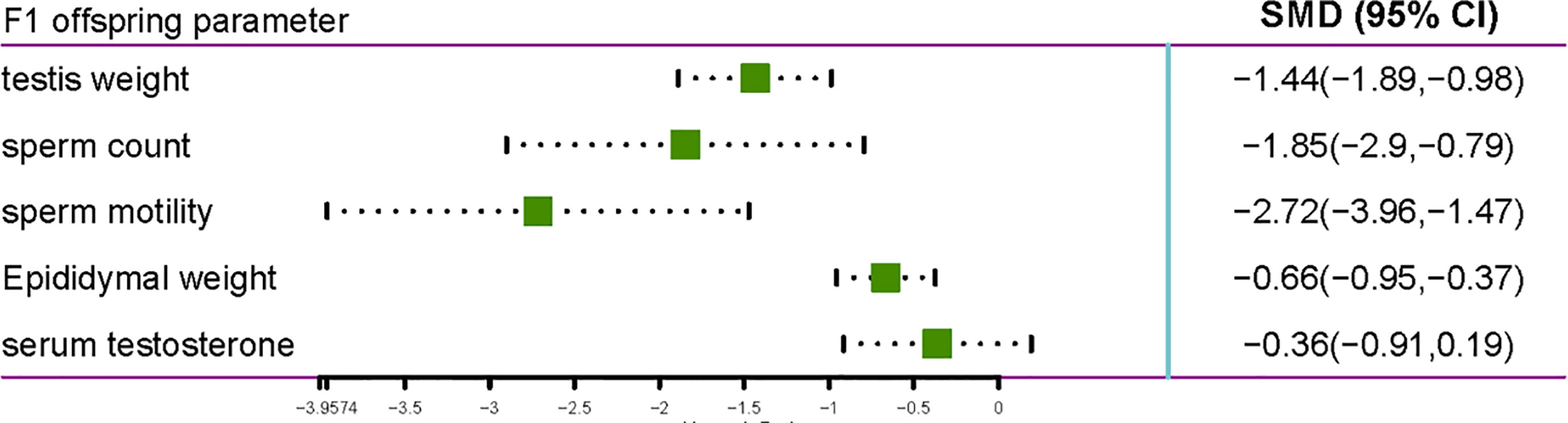
Figure 4 Forest plot showing the effect of pyrethroid exposure on the F1 offspring of rats and mice.
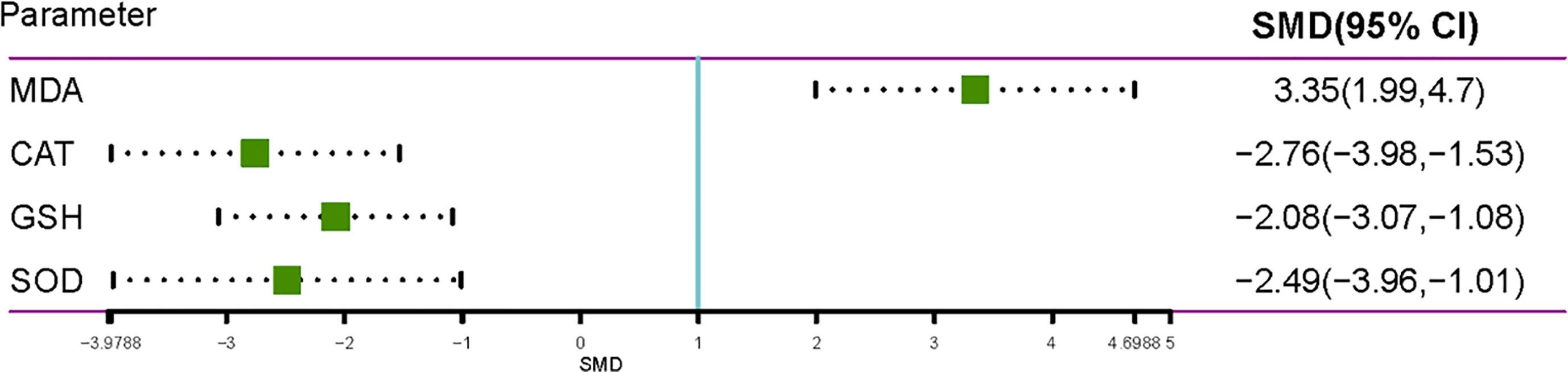
Pyrethroids Oxidative Stress Induction on Rats and Mice
Nine studies reported the presence of changes in the level of MDA, which is a product of lipid peroxidation, and testicular enzymatic activities (glutathione, GSH; superoxide dismutase, SOD; and catalase, CAT) of both the control and treated animals (Figure 5). Pyrethroids exposure significantly decreased the SOD, CAT, and GSH activities in the testis of treated animals compared with those of control animals, and the SMDs by REM were -2.4856 (95% CI -3.9612 to -1.0100), -2.7564 (95% CI -3.9788 to -1.5340), and -2.075 (95% CI -3.0651 to -1.0848), respectively. The MDA level increased compared with control animals, and the SMD by REM was 3.3451 (95% CI 1.9914 to 4.6988) (Figure 5).
Human Research on the Effect of SPs About the Male Reproductive System
A total of 17 human studies were included in the analysis (Table 3), among which 15 studies evaluated the effect of pyrethroids on sperm quality. The details of these 17 studies are summarized in Table 3. Some urinary pyrethroid metabolites are 3-phenoxybenzoic acid (3-PBA) and cis- and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (CDCCA and TDCCA). 3-PBA is common in several pyrethroids, including cyhalothrin, cypermethrin, deltamethrin, fenvalerate, and permethrin, whereas CDCCA and TDCCA are metabolites of cis-permethrin and trans-permethrin, respectively.
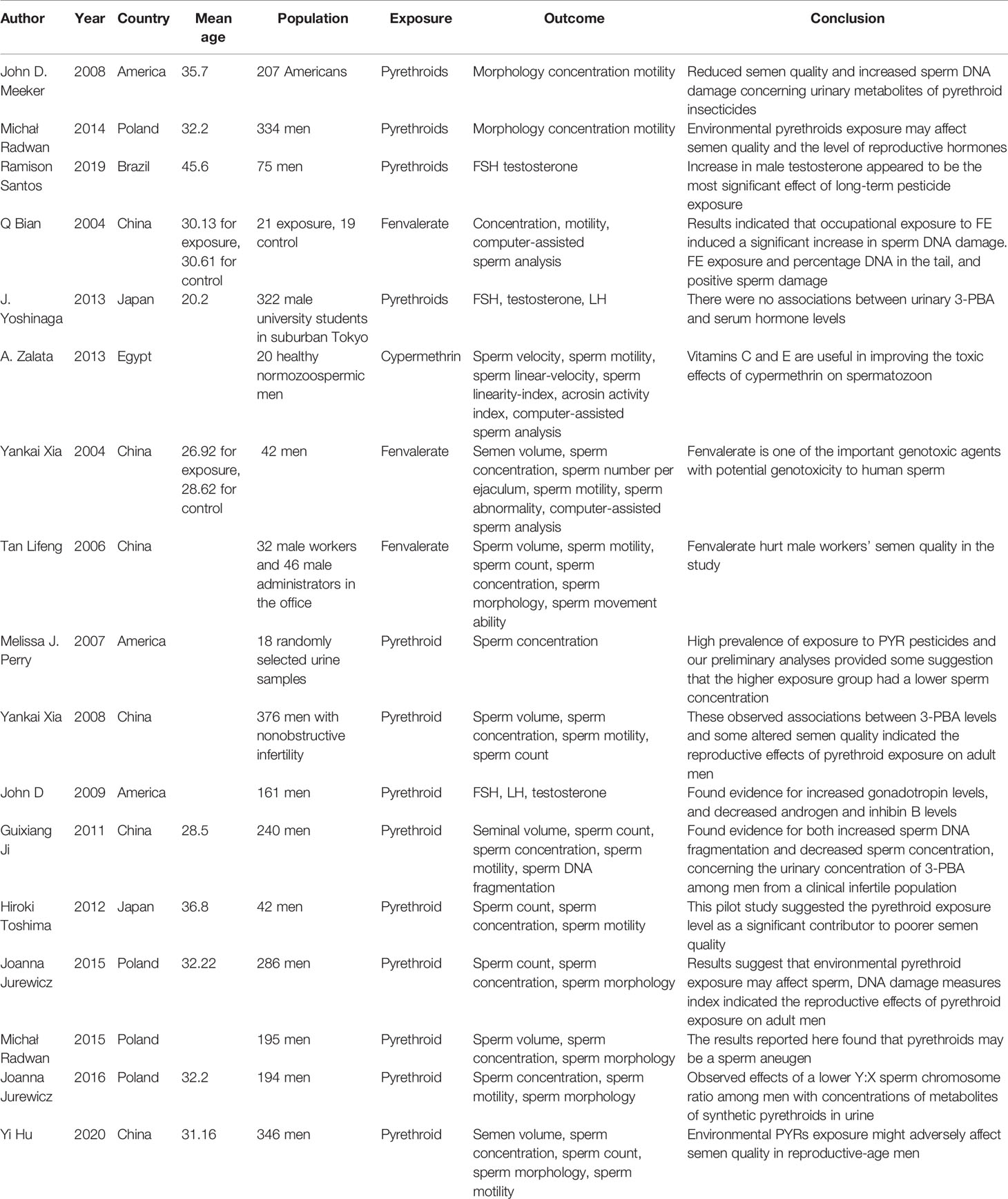
Table 3 Characteristics of included human studies reporting an association between pyrethroid exposure and reproductive parameters.
Several studies evaluated the relationship between the urinary pyrethroid metabolites and sperm quality. John D. Meeker et al. (72) studied the relationship between urinary pyrethroid metabolites and semen quality, sperm motion parameters, and sperm DNA damage in 207 men. The results showed the association between urinary metabolites of pyrethroid insecticides and the decrease in semen quality. In another article, Michał Radwan et al. (73) showed the significant associations between urinary pyrethroids metabolite levels and the decrease in sperm concentration, the level of testosterone, and the semen parameters, as determined by computer-aided analysis. In contrast, J. Yoshinaga (74) conducted an experiment involving 322 male university students and showed that no associations existed between urinary 3-PBA and serum hormone levels. A study of the relationship between the urinary metabolite of pyrethroid insecticides and semen quality conducted on 376 healthy participants focused on sperm quality (volume, motility, number of spermatozoa, and concentration), and results showed the association between urinary metabolite of pyrethroid insecticides and sperm quality (75). Furthermore, a high level of the urinary metabolite of pyrethroid was related to the increase in gonadotropin levels and decrease in androgen and inhibin B levels (76). A recent cross-sectional study of 346 men conducted by Yi Hu et al. (77) showed that the urinary metabolite of pyrethroids was negatively associated with sperm morphology, sperm count, and semen quality.
Only one study focused on pyrethroid exposure and the levels of reproductive hormones. Ramison Santos (78) assessed the association of short-term and long-term exposure to pesticides with circulating levels of reproductive hormones in an agricultural population in the South of Brazil. They found that recent use of lambda-cyhalothrin was associated with increased male luteinizing hormone (LH) levels.
An in vitro study (79) assessed the potential effect of cypermethrin on human spermatozoa and the possible ameliorative effects of vitamins C and E. Semen samples of 20 healthy normozoospermic men were used, and the results indicated that in vitro cypermethrin can alter sperm function and induce DNA damage in spermatozoa, which improved after the administration of vitamins C and E and was maximal when both vitamins were used together.
Several studies have reported the relationship between pyrethroids exposure and genetic or chromosome damage. A study focused on sperm DNA integrity; 240 healthy participants were recruited. Results showed a significant positive correlation between urinary 3-PBA level and sperm DNA fragmentation (80). A study by Yankai Xia et al. (81) investigated the possible association between fenvalerate and spermatozoa DNA damage. Nineteen fenvalerate-exposed workers and 23 non-exposed workers were included. Sperm DNA strands were found to have breaks in fenvalerate-exposed workers using the Comet and TUNEL assays. Another study aimed to assess the relationship between pyrethroid exposure and sperm DNA damage (82). Compared with the reference quartile, the level of 3-PBA in >50th percentile of urine was positively related to the percentage of high DNA fragmentation index. Also, a positive association was observed between the CDCCA 4th percentile and the percentage of medium DNA fragmentation index and the percentage of immature sperms. Only one study particularly focused on sperm aneuploidy. Michał Radwan et al. (83) examined the relationship between exposure to pyrethroids and sperm aneuploidy. With regard to the urinary metabolite of pyrethroids, the levels of TDCCA and CDCCD in urine of the >50th percentile were related to XY disomy and chromosome 18 disomy, respectively. One study focused on the relationship between human sperm sex ratio and environmental endocrine. Joanna Jurewicz et al. (84, 85) found that the level of urinary pyrethroid metabolites was negatively related to Y:X sperm chromosome ratio.
Three studies focused on fecundability after pyrethroid exposure. In the study by Markku Sallmén (86) involving 578 greenhouses workers and their wives, individuals who were exposed to pyrethroids showed decreased fecundability. The exposure to pyrethroids of men working in greenhouses may be associated with reduced fertility. Tan Lifeng et al. (87) conducted a study involving 32 male workers and 22 male administrators. The results showed that high exposure of fenvalerate was associated with the abnormality in sperm quality. Similarity, Hiroki Toshima (88) conducted a study involving 42 Japanese male partners in couples who underwent infertility consultation. A positive association was observed between pyrethroid exposure level and poor semen quality.
Discussion
Various recent studies assessed the influence of pyrethroid exposure on the male reproductive system. To our knowledge, this is the first systematic review with a meta-analysis that rigorously evaluated the relationship between pyrethroid exposure and male reproductive disorders. Our meta-analysis has an advantage in comparison with narrative reviews. In this study, we conducted rat and mice meta-analyses, including the effects of gestational and lactational exposure to pyrethroids on F1 offspring and the effects of direct exposure on the male reproductive system. Next, we observed a significant decrease in sperm motility, sperm count, serum testosterone, testis weight, and epididymal weight with the exposure of pyrethroid in rats and mice. Interestingly, gestational and lactational exposure of pyrethroids demonstrated similar damage to the male reproductive system.
Over the past decades, male infertility has received an increasing amount of attention worldwide (89). In the past 50 years, evidence of the decreased sperm concentration in European and African males had been explored (90, 91). There were many reasons that accounted for male infertility. For example, alcohol, endocrine disruptors, and environmental pollutants may play an important role in the decline in sperm concentration. The damage caused by the increasing exposure of endocrine disruptors, organic pollutants, heavy metals, and pesticides in daily life on the human body has been increasingly studied. One cohort study (92) showed that exposure to BPA was associated with abnormal sperm tail morphology. Recently, SPs have been widely used all over the world due to their highly toxic effects on insects and are presented in numerous commercial insecticide formulations. As one of the most frequently applied EDCs, pyrethroids primarily entered the body through skin contact, inhalation, or food/water ingestion. And the metabolites of pyrethroids have frequently been detected in urine samples collected from the general population (15).
Through systematic review and meta-analysis of the literature, we have explored the damage to the male reproductive system by seven pyrethroids, including allethrin, bifenthrin, cypermethrin, deltamethrin, fenvalerate, lambda-cyhalothrin, and permethrin. Our results suggested that all seven pyrethroids had an overall negative effect on semen parameters. Each of them demonstrated a negative effect on sperm count, sperm motility, sperm morphology, testis weight, epididymal weight, and serum testosterone. Besides, we also found evidence that gestational and lactational pyrethroids exposure can lead to male reproductive damage in F1 offspring. By conducting a subgroup analysis, we received a more in-depth view. Considering each group individually, different and interesting results can be observed. When comparing the rat and mice groups, we found that pyrethroids had a stronger effect on sperm and serum testosterone in rat groups. When considering the results for different pyrethroids, we found that all pyrethroid types damaged the male reproductive system. Further subgroup analysis demonstrated that sperm count, sperm motility, sperm morphology, testis weight epididymis weight, and serum testosterone showed a consistent trend with SP exposure. A low amount of data was found in some subgroups, and some results were not statistically significant. Next, we also performed a systematic review of human research on the effect of SPs about the male reproductive system. Interestingly, the metabolites of SPs can be detected in the urine of almost everyone. Further studies demonstrated that high-risk groups, such as workers who have been exposed to SPs for a long time, were found to have more SPs metabolites in their urine. Besides, the concentration of SPs metabolites in urine was often negatively correlated with sperm quality.
The mechanisms underlying the male reproductive toxicity of pyrethroids are still being explored. One research demonstrated that bifenthrin reduced sperm motility and kinematic parameters by reducing intracellular ATP level (68). Long term exposure to pyrethroid interferes with the expression of genes that govern spermatogenesis, steroidogenesis, apoptosis, and genetic reprogramming of male gametes (69). To further explore the potential associations between SPs and oxidative stress, we evaluated oxidative stress induction on rats and mice exposed to pyrethroids. The results showed that some pyrethroids, such as lambda-cyhalothrin, deltamethrin, cypermethrin, and bifenthrin can increase the level of MDA oxide in testes and reduce the activities of GSH, SOD, and CAT. And the concentration changes of MDA, GSH, SOD, and CAT were observed in parallel to the reduction in the sperm count. Nano selenium, as a potent antioxidant, had been reported to minimize reproductive toxic effects of SPs by decreasing the concentration of MDA (70). Spirulina, which is a microalga rich in antioxidant compounds, had been found to reverse deleterious effects of the reproductive system caused by bifenthrin. Our assessment provided valuable information for exploring the cause of infertile men. And we hope that our research can provide better understanding for male infertility patients.
Conclusion
In this study, we provided interesting results from rodent studies regarding the influence of pyrethroids on male reproduction. Based on the aforementioned data, all seven SPs demonstrated toxicity to the male reproductive system. SP exposure during pregnancy and adolescence can cause damage to male F1 offspring or the male reproductive system. Oxidative stress may be essential in the damage of the male reproductive system. Finally, due to the wide use of SPs, humans will inevitably be widely exposed to SPs. And SP exposure was closely associated with human sperm quality.
Author Contributions
XZ, XC and TZ collected the data and performed the meta-analysis. XR and XZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81972386, 81672531 to CQ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.656106/full#supplementary-material
References
1. Katsuda Y. Progress and Future of Pyrethroids. Top Curr Chem (2012) 314:1–30. doi: 10.1007/128_2011_252
2. Mehrpour O, Karrari P, Zamani N, Tsatsakis AM, Abdollahi M. Occupational Exposure to Pesticides and Consequences on Male Semen and Fertility: A Review. Toxicol Lett (2014) 230(2):146–56. doi: 10.1016/j.toxlet.2014.01.029
3. Saillenfait AM, Ndiaye D, Sabate JP. Pyrethroids: Exposure and Health Effects–an Update. Int J Hygiene Environ Health (2015) 218(3):281–92. doi: 10.1016/j.ijheh.2015.01.002
4. Freemont JA, Littler SW, Hutt OE, Mauger S, Meyer AG, Winkler DA, et al. Molecular Markers for Pyrethrin Autoxidation in Stored Pyrethrum Crop: Analysis and Structure Determination. J Agric Food Chem (2016) 64(38):7134–41. doi: 10.1021/acs.jafc.6b02959
5. Saillenfait AM, Ndiaye D, Sabate JP. The Estrogenic and Androgenic Potential of Pyrethroids In Vitro. Review. Toxicol Vitro Int J Published Assoc BIBRA (2016) 34:321–32. doi: 10.1016/j.tiv.2016.02.020
6. Wang X, Martinez MA, Dai M, Chen D, Ares I, Romero A, et al. Permethrin-Induced Oxidative Stress and Toxicity and Metabolism. A Review. Environ Res (2016) 149:86–104. doi: 10.1016/j.envres.2016.05.003
7. Marettova E, Maretta M, Legath J. Effect of Pyrethroids on Female Genital System. Review. Anim Reprod Sci (2017) 184:132–8. doi: 10.1016/j.anireprosci.2017.07.007
8. Burns CJ, Pastoor TP. Pyrethroid Epidemiology: A Quality-Based Review. Crit Rev Toxicol (2018) 48(4):297–311. doi: 10.1080/10408444.2017.1423463
9. Chrustek A, Holynska-Iwan I, Dziembowska I, Bogusiewicz J, Wroblewski M, Cwynar A, et al. Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina (Kaunas Lithuania) (2018) 54(4). doi: 10.3390/medicina54040061
10. Lu Q, Sun Y, Ares I, Anadon A, Martinez M, Martinez-Larranaga MR, et al. Deltamethrin Toxicity: A Review of Oxidative Stress and Metabolism. Environ Res (2019) 170:260–81. doi: 10.1016/j.envres.2018.12.045
11. Matsuo N. Discovery and Development of Pyrethroid Insecticides. Proc Jpn Acad Ser B Phys Biol Sci (2019) 95(7):378–400. doi: 10.2183/pjab.95.027
12. Navarrete-Meneses MDP, Perez-Vera P. Pyrethroid Pesticide Exposure and Hematological Cancer: Epidemiological, Biological and Molecular Evidence. Rev Environ Health (2019) 34(2):197–210. doi: 10.1515/reveh-2018-0070
13. Ye X, Liu J. Effects of Pyrethroid Insecticides on Hypothalamic-Pituitary-Gonadal Axis: A Reproductive Health Perspective. Environ pollution (Barking Essex 1987) (2019) 245:590–9. doi: 10.1016/j.envpol.2018.11.031
14. Lybrand DB, Xu H, Last RL, Pichersky E. How Plants Synthesize Pyrethrins: Safe and Biodegradable Insecticides. Trends Plant Sci (2020) 25(12):1240–51. doi: 10.1016/j.tplants.2020.06.012
15. Shi X-D, Bi H-J, Fu H-L, Li L-Y, Liu D-K, Li M-J, et al. Effect of Low-Dose Fenvalerate on Semen Quality Capacitation in Adult Mice. Chin Med J (English Edition) (2011) 124(10):1529–33. doi: 10.3760/cma.j.issn.0366-6999.2011.10.017
16. Bhunya SP, Pati PC. Effect of Deltamethrin, a Synthetic Pyrethroid, on the Induction of Chromosome Aberrations, Micronuclei and Sperm Abnormalities in Mice. Mutagenesis (1990) 5(3):229–32. doi: 10.1093/mutage/5.3.229
17. Elbetieha A, Da’as SI, Khamas W, Darmani H. Evaluation of the Toxic Potentials of Cypermethrin Pesticide on Some Reproductive and Fertility Parameters in the Male Rats. Arch Environ Contamination Toxicol (2001) 41(4):522–8. doi: 10.1007/s002440010280
18. Hu J-Y, Wang S-L, Zhao R-C, Yang J, Chen J-H, Song L, et al. [Effects of Fenvalerate on Reproductive and Endocrine Systems of Male Rats]. Zhonghua Nan Ke Xue = Natl J Andrology (2002) 8(1):18–21.
19. Mani U, Islam F, Prasad AK, Kumar P, Suresh Kumar V, Maji BK, et al. Steroidogenic Alterations in Testes and Sera of Rats Exposed to Formulated Fenvalerate by Inhalation. Hum Exp Toxicol (2002) 21(11):593–7. doi: 10.1191/0960327102ht298oa
20. Xu LC, Zhan NY, Liu R, Song L, Wang XR. Joint Action of Phoxim and Fenvalerate on Reproduction in Male Rats. Asian J Andrology (2004) 6(4):337–41.
21. Song L, Wang YB, Sun H, Gu AH, Sun Y, Wang XR. [Fenvalerate Affects Sperm Motility in SD Rats]. Zhonghua Nan Ke Xue = Natl J Andrology (2007) 13(7):588–91.
22. Zhang SY, Ito Y, Yamanoshita O, Yanagiba Y, Kobayashi M, Taya K, et al. Permethrin may Disrupt Testosterone Biosynthesis Via Mitochondrial Membrane Damage of Leydig Cells in Adult Male Mouse. Endocrinology (2007) 148(8):3941–9. doi: 10.1210/en.2006-1497
23. Arena AC, Fernandez CD, Porto EM, Bissacot DZ, Pereira OC, Kempinas WG. Fenvalerate, a Pyrethroid Insecticide, Adversely Affects Sperm Production and Storage in Male Rats. J Toxicol Environ Health Part A (2008) 71(23):1550–8. doi: 10.1080/15287390802392024
24. Hu JY, Wang XR. [Joint Action of Phoxim and Fenvalerate on Spermatogenesis of Male Rats]. Zhonghua Nan Ke Xue = Natl J Andrology (2008) 14(11):968–72.
25. Song L, Wang YB, Sun H, Yuan C, Hong X, Qu JH, et al. Effects of Fenvalerate and Cypermethrin on Rat Sperm Motility Patterns In Vitro as Measured by Computer-Assisted Sperm Analysis. J Toxicol Environ Health Part A (2008) 71(5):325–32. doi: 10.1080/15287390701738517
26. Zhang SY, Ueyama J, Ito Y, Yanagiba Y, Okamura A, Kamijima M, et al. Permethrin may Induce Adult Male Mouse Reproductive Toxicity Due to Cis Isomer Not Trans Isomer. Toxicology (2008) 248(2-3):136–41. doi: 10.1016/j.tox.2008.03.018
27. Issam C, Samir H, Zohra H, Monia Z, Hassen BC. Toxic Responses to Deltamethrin (DM) Low Doses on Gonads, Sex Hormones and Lipoperoxidation in Male Rats Following Subcutaneous Treatments. J Toxicol Sci (2009) 34(6):663–70. doi: 10.2131/jts.34.663
28. Wang XZ, Liu SS, Sun Y, Wu JY, Zhou YL, Zhang JH. Beta-Cypermethrin Impairs Reproductive Function in Male Mice by Inducing Oxidative Stress. Theriogenology (2009) 72(5):599–611. doi: 10.1016/j.theriogenology.2009.04.016
29. Abdallah FB, Hamden K, Galeraud-Denis I, El Feki A, Keskes-Ammar L. An In Vitro Study on Reproductive Toxicology of Deltamethrin on Rat Spermatozoa. Andrologia (2010) 42(4):254–9. doi: 10.1111/j.1439-0272.2009.00986.x
30. Abdallah FB, Slima AB, Dammak I, Keskes-Ammar L, Mallek Z. Comparative Effects of Dimethoate and Deltamethrin on Reproductive System in Male Mice. Andrologia (2010) 42(3):182–6. doi: 10.1111/j.1439-0272.2009.00976.x
31. Al-Hamdani NM, Yajurvedi HN. Cypermethrin Reversibly Alters Sperm Count Without Altering Fertility in Mice. Ecotoxicol Environ Saf (2010) 73(5):1092–7. doi: 10.1016/j.ecoenv.2010.04.009
32. Perobelli JE, Martinez MF, da Silva Franchi CA, Fernandez CDB, Viana de Camargo JL, Kempinas WDG. Decreased Sperm Motility in Rats Orally Exposed to Single or Mixed Pesticides. J Toxicol Environ Health-Part a-Current Issues (2010) 73(13-14):991–1002. doi: 10.1080/15287391003751802
33. Prakash N, Vijay KM, Sunilchandra U, Pavithra B, Pawar A. Evaluation of Testicular Toxicity Following Short-Term Exposure to Cypermethrin in Albino Mice. Toxicol Int (2010) 17(1):18–21. doi: 10.4103/0971-6580.68344
34. Wang H, Wang Q, Zhao XF, Liu P, Meng XH, Yu T, et al. Cypermethrin Exposure During Puberty Disrupts Testosterone Synthesis Via Downregulating StAR in Mouse Testes. Arch Toxicol (2010) 84(1):53–61. doi: 10.1007/s00204-009-0479-y
35. Yuan C, Wang C, Gao SQ, Kong TT, Chen L, Li XF, et al. Effects of Permethrin, Cypermethrin and 3-Phenoxybenzoic Acid on Rat Sperm Motility In Vitro Evaluated With Computer-Assisted Sperm Analysis. Toxicol Vitro Int J Published Assoc BIBRA (2010) 24(2):382–6. doi: 10.1016/j.tiv.2009.11.001
36. Zhang H, Wang H, Wang Q, Zhao XF, Liu P, Ji YL, et al. Pubertal and Early Adult Exposure to Fenvalerate Disrupts Steroidogenesis and Spermatogenesis in Mice at Adulthood. J Appl Toxicol JAT (2010) 30(4):369–77. doi: 10.1002/jat.1507
37. Issam C, Zohra H, Monia Z, Hassen BC. Effects of Dermal Sub-Chronic Exposure of Pubescent Male Rats to Permethrin (PRMT) on the Histological Structures of Genital Tract, Testosterone and Lipoperoxidation. Exp Toxicologic Pathol (2011) 63(4):393–400. doi: 10.1016/j.etp.2010.02.016
38. Joshi SC, Bansal B, Jasuja ND. Evaluation of Reproductive and Developmental Toxicity of Cypermethrin in Male Albino Rats. Toxicol Environ Chem (2011) 93(3):593–602. doi: 10.1080/02772248.2010.537441
39. Ben Abdallah F, Fetoui H, Zribi N, Fakhfakh F, Keskes L. Protective Role of Caffeic Acid on Lambda Cyhalothrin-Induced Changes in Sperm Characteristics and Testicular Oxidative Damage in Rats. Toxicol Ind Health (2012) 28(7):639–47. doi: 10.1177/0748233711420470
40. Gao X, Wang Q, Wang J, Wang C, Lu L, Gao R, et al. Expression of Calmodulin in Germ Cells is Associated With Fenvalerate-Induced Male Reproductive Toxicity. Arch Toxicol (2012) 86(9):1443–51. doi: 10.1007/s00204-012-0825-3
41. Li X, Cai D. [Single and Combined Toxic Effects of di-2-ethylhexyl Phthalate and Cypermethrin on Fertility and Development in the the Prepubertal Male Rats]. Wei Sheng Yan Jiu = J Hygiene Res (2012) 41(5):710–6.
42. Oda SS, El-Maddawy Z. Protective Effect of Vitamin E and Selenium Combination on Deltamethrin-Induced Reproductive Toxicity in Male Rats. Exp Toxicologic Pathol Off J Gesellschaft fur Toxikologische Pathologie (2012) 64(7-8):813–9. doi: 10.1016/j.etp.2011.03.001
43. Sakr SA, Al-Amoudi WM. Effect of Ginger Extract on Deltamethrin Induced Histomorphological and Immunohistochemical Changes in Testes of Albino Rats. Life Sci Journal-Acta Zhengzhou Univ Overseas Edition (2012) 9(1):771–8.
44. Wang D, Kamijima M, Okamura A, Ito Y, Yanagiba Y, X-f J, et al. Evidence for Diazinon-Mediated Inhibition of Cis-Permethrin Metabolism and its Effects on Reproductive Toxicity in Adult Male Mice. Reprod Toxicol (2012) 34(4):489–97. doi: 10.1016/j.reprotox.2012.07.007
45. Ben Abdallah F, Fetoui H, Zribi N, Fakhfakh F, Keskes L. Quercetin Attenuates Lambda Cyhalothrin-Induced Reproductive Toxicity in Male Rats. Environ Toxicol (2013) 28(12):673–80. doi: 10.1002/tox.20762
46. Ben Slima A, Ali MB, Barkallah M, Traore AI, Boudawara T, Allouche N, et al. Antioxidant Properties of Pelargonium Graveolens L’Her Essential Oil on the Reproductive Damage Induced by Deltamethrin in Mice as Compared to Alpha-Tocopherol. Lipids Health Dis (2013) 12:30. doi: 10.1186/1476-511X-12-30
47. Hu JX, Li YF, Li J, Pan C, He Z, Dong HY, et al. Toxic Effects of Cypermethrin on the Male Reproductive System: With Emphasis on the Androgen Receptor. J Appl Toxicol JAT (2013) 33(7):576–85. doi: 10.1002/jat.1769
48. Li Yan F, Pan C, Hu Jin X, Li J, Xu Li C. Effects of Cypermethrin on Male Reproductive System in Adult Rats. Biomed Environ Sci (2013) 26(3):201–8. doi: 10.3967/0895-3988.2013.03.007
49. Sharma P, Huq AU, Singh R. Cypermethrin Induced Reproductive Toxicity in Male Wistar Rats: Protective Role of Tribulus Terrestris. J Environ Biol (2013) 34(5):857–62.
50. Al-Sarar AS, Abobakr Y, Bayoumi AE, Hussein HI, Al-Ghothemi M. Reproductive Toxicity and Histopathological Changes Induced by Lambda-Cyhalothrin in Male Mice. Environ Toxicol (2014) 29(7):750–62. doi: 10.1002/tox.21802
51. Ben Halima N, Ben Slima A, Moalla I, Fetoui H, Pichon C, Gdoura R, et al. Protective Effects of Oat Oil on Deltamethrin-Induced Reprotoxicity in Male Mice. Food Funct (2014) 5(9):2070–7. doi: 10.1039/C4FO00190G
52. Madhubabu G, Yenugu S. Allethrin Induced Toxicity in the Male Reproductive Tract of Rats Contributes to Disruption in the Transcription of Genes Involved in Germ Cell Production. Environ Toxicol (2014) 29(11):1330–45. doi: 10.1002/tox.21864
53. Sharma P, Huq AU, Singh R. Cypermethrin-Induced Reproductive Toxicity in the Rat is Prevented by Resveratrol. J Hum Reprod Sci (2014) 7(2):99–106. doi: 10.4103/0974-1208.138867
54. Sharma P, Singh R, Jan M. Dose-Dependent Effect of Deltamethrin in Testis, Liver, and Kidney of Wistar Rats. Toxicol Int (2014) 21(2):131–9. doi: 10.4103/0971-6580.139789
55. Mostafa Hel S, Abd El-Baset SA, Kattaia AA, Zidan RA, Al Sadek MM. Efficacy of Naringenin Against Permethrin-Induced Testicular Toxicity in Rats. Int J Exp Pathol (2016) 97(1):37–49. doi: 10.1111/iep.12168
56. Patrick SM, Bornman MS, Joubert AM, Pitts N, Naidoo V, de Jager C. Effects of Environmental Endocrine Disruptors, Including Insecticides Used for Malaria Vector Control on Reproductive Parameters of Male Rats. Reprod Toxicol (Elmsford NY) (2016) 61:19–27. doi: 10.1016/j.reprotox.2016.02.015
57. El Sayed Mostafa H, El-Baset SAA, Kattaia AAA, Zidan RA, Al Sadek MMA. Efficacy of Naringenin Against Permethrin-Induced Testicular Toxicity in Rats. Int J Exp Pathol (2016) 97(1):37–49. doi: 10.1111/iep.12168
58. Alaa-Eldin EA, El-Shafei DA, Abouhashem NS. Individual and Combined Effect of Chlorpyrifos and Cypermethrin on Reproductive System of Adult Male Albino Rats. Environ Sci Pollution Res Int (2017) 24(2):1532–43. doi: 10.1007/s11356-016-7912-6
59. Khaki A, Khaki AA, Rajabzadeh A. The Effects of Permethrin and Antioxidant Properties of Allium Cepa (Onion) on Testicles Parameters of Male Rats. Toxin Rev (2017) 36(1):1–6. doi: 10.1080/15569543.2016.1235582
60. Madhubabu G, Yenugu S. Allethrin Toxicity Causes Reproductive Dysfunction in Male Rats. Environ Toxicol (2017) 32(6):1701–10. doi: 10.1002/tox.22394
61. Sharma P, Aslam Khan I, Singh R. Curcumin and Quercetin Ameliorated Cypermethrin and Deltamethrin-Induced Reproductive System Impairment in Male Wistar Rats by Upregulating the Activity of Pituitary-Gonadal Hormones and Steroidogenic Enzymes. Int J Fertility Sterility (2018) 12(1):72–80. doi: 10.1055/s-0034-1382471
62. Zhang J, Hu Y, Guo J, Pan R, Shi R, Tian Y, et al. Fenvalerate Decreases Semen Quality in Puberty Rat Through Germ Cell Apoptosis. Andrologia (2018) 50(9):e13079. doi: 10.1111/and.13079
63. Bagherpour H, Karimpour Malekshah A, Talebpour Amiri F, Azadbakht M. Protective Effect of Green Tea Extract on the Deltamethrin-Induced Toxicity in Mice Testis: An Experimental Study. Int J Reprod Biomed (Yazd Iran) (2019) 17(5):337–48. doi: 10.18502/ijrm.v17i5.4601
64. Hong T, Li R, Sun L-L, Xu J, He M-T, Wang W, et al. Role of the Gene Phlda1 in Fenvalerate-Induced Apoptosis and Testicular Damage in Sprague-Dawley Rats. J Toxicol Environ Health-Part a-Current Issues (2019) 82(15):870–8. doi: 10.1080/15287394.2019.1664584
65. Osama E, Galal AAA, Abdalla H, El-Sheikh SMA. Chlorella Vulgaris Ameliorates Testicular Toxicity Induced by Deltamethrin in Male Rats Via Modulating Oxidative Stress. Andrologia (2019) 51(3):e13214. doi: 10.1111/and.13214
66. Barkallah M, Slima AB, Elleuch F, Fendri I, Pichon C, Abdelkafi S, et al. Protective Role of Spirulina Platensis Against Bifenthrin-Induced Reprotoxicity in Adult Male Mice by Reversing Expression of Altered Histological, Biochemical, and Molecular Markers Including Micrornas. Biomolecules (2020) 10(5). doi: 10.3390/biom10050753
67. Maksoud HA, Mahfouz M, Soliman M, Elharrif MG, Abbass M, El-Badry M. Harmful Effects of Pyrethroid Ester Insecticide on the Male Reproductive System Mainly Through Affecting Testicular Function and Inflammatory Markers. Biocell (2020) 44(1):111–5. doi: 10.32604/biocell.2020.08399
68. Bae JW, Kwon WS. The Deleterious Toxic Effects of Bifenthrin on Male Fertility. Reprod Toxicol (Elmsford NY) (2021) 101:74–80. doi: 10.1016/j.reprotox.2021.03.002
69. Ravula AR, Yenugu S. Effect of Oral Administration of a Mixture of Pyrethroids at Doses Relevant to Human Exposure on the General and Male Reproductive Physiology in the Rat. Ecotoxicol Environ Saf (2021) 208:111714. doi: 10.1016/j.ecoenv.2020.111714
70. Hozyen HF, Khalil HMA, Ghandour RA, Al-Mokaddem AK, Amer MS, Azouz RA. Nano Selenium Protects Against Deltamethrin-Induced Reproductive Toxicity in Male Rats. Toxicol Appl Pharmacol (2020) 408:115274. doi: 10.1016/j.taap.2020.115274
71. Katragadda V, Adem M, Mohammad RA, Sri Bhasyam S, Battini K. Testosterone Recuperates Deteriorated Male Fertility in Cypermethrin Intoxicated Rats. Toxicol Res (2021) 37(1):125–34. doi: 10.1007/s43188-020-00046-1
72. Meeker JD, Barr DB, Hauser R. Human Semen Quality and Sperm DNA Damage in Relation to Urinary Metabolites of Pyrethroid Insecticides. Hum Reprod (2008) 23(8):1932–40. doi: 10.1093/humrep/den242
73. Radwan M, Jurewicz J, Wielgomas B, Sobala W, Piskunowicz M, Radwan P, et al. Semen Quality and the Level of Reproductive Hormones After Environmental Exposure to Pyrethroids. J Occup Environ Med (2014) 56(11):1113–9. doi: 10.1097/JOM.0000000000000297
74. Yoshinaga J, Imai K, Shiraishi H, Nozawa S, Yoshiike M, Mieno MN, et al. Pyrethroid Insecticide Exposure and Reproductive Hormone Levels in Healthy Japanese Male Subjects. Andrology (2014) 2(3):416–20. doi: 10.1111/j.2047-2927.2014.00202.x
75. Xia Y, Han Y, Wu B, Wang S, Gu A, Lu N, et al. The Relation Between Urinary Metabolite of Pyrethroid Insecticides and Semen Quality in Humans. Fertility Sterility (2008) 89(6):1743–50. doi: 10.1016/j.fertnstert.2007.05.049
76. Meeker JD, Barr DB, Hauser R. Pyrethroid Insecticide Metabolites are Associated With Serum Hormone Levels in Adult Men. Reprod Toxicol (Elmsford NY) (2009) 27(2):155–60. doi: 10.1016/j.reprotox.2008.12.012
77. Hu Y, Zhang Y, Vinturache A, Wang Y, Shi R, Chen L, et al. Effects of Environmental Pyrethroids Exposure on Semen Quality in Reproductive-Age Men in Shanghai, China. Chemosphere (2020) 245:125580. doi: 10.1016/j.chemosphere.2019.125580
78. Santos R, Piccoli C, Cremonese C, Freire C. Thyroid and Reproductive Hormones in Relation to Pesticide Use in an Agricultural Population in Southern Brazil. Environ Res (2019) 173:221–31. doi: 10.1016/j.envres.2019.03.050
79. Zalata A, Elhanbly S, Abdalla H, Serria MS, Aziz A, El-Dakrooy SA, et al. In Vitro Study of Cypermethrin on Human Spermatozoa and the Possible Protective Role of Vitamins C, and XXXE. Andrologia (2014) 46(10):1141–7. doi: 10.1111/and.12206
80. Ji G, Xia Y, Gu A, Shi X, Long Y, Song L, et al. Effects of non-Occupational Environmental Exposure to Pyrethroids on Semen Quality and Sperm DNA Integrity in Chinese Men. Reprod Toxicol (Elmsford NY) (2011) 31(2):171–6. doi: 10.1016/j.reprotox.2010.10.005
81. Xia Y, Bian Q, Xu L, Cheng S, Song L, Liu J, et al. Genotoxic Effects on Human Spermatozoa Among Pesticide Factory Workers Exposed to Fenvalerate. Toxicology (2004) 203(1-3):49–60. doi: 10.1016/j.tox.2004.05.018
82. Bian Q, Xu LC, Wang SL, Xia YK, Tan LF, Chen JF, et al. Study on the Relation Between Occupational Fenvalerate Exposure and Spermatozoa DNA Damage of Pesticide Factory Workers. Occup Environ Med (2004) 61(12):999–1005. doi: 10.1136/oem.2004.014597
83. Radwan M, Jurewicz J, Wielgomas B, Piskunowicz M, Sobala W, Radwan P, et al. The Association Between Environmental Exposure to Pyrethroids and Sperm Aneuploidy. Chemosphere (2015) 128:42–8. doi: 10.1016/j.chemosphere.2014.12.077
84. Jurewicz J, Radwan M, Sobala W, Radwan P, Jakubowski L, Wielgomas B, et al. Exposure, to Widespread Environmental Endocrine Disrupting Chemicals and Human Sperm Sex Ratio. Environ Pollution (2016) 213:732–40. doi: 10.1016/j.envpol.2016.02.008
85. Jurewicz J, Radwan M, Wielgomas B, Sobala W, Piskunowicz M, Radwan P, et al. The Effect of Environmental Exposure to Pyrethroids and DNA Damage in Human Sperm. Syst Biol Reprod Med (2015) 61(1):37–43. doi: 10.3109/19396368.2014.981886
86. Sallmén M, Liesivuori J, Taskinen H, Lindbohm ML, Anttila A, Aalto L, et al. Time to Pregnancy Among the Wives of Finnish Greenhouse Workers. Scandinavian J Work Environ Health (2003) 29(2):85–93. doi: 10.5271/sjweh.709
87. Lifeng T, Shoulin W, Junmin J, Xuezhao S, Yannan L, Qianli W, et al. Effects of Fenvalerate Exposure on Semen Quality Among Occupational Workers. Contraception (2006) 73(1):92–6. doi: 10.1016/j.contraception.2005.06.067
88. Toshima H, Suzuki Y, Imai K, Yoshinaga J, Shiraishi H, Mizumoto Y, et al. Endocrine Disrupting Chemicals in Urine of Japanese Male Partners of Subfertile Couples: A Pilot Study on Exposure and Semen Quality. Int J Hygiene Environ Health (2012) 215(5):502–6. doi: 10.1016/j.ijheh.2011.09.005
89. Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent Age-Dependent Declines in Human Semen Quality: A Systematic Review and Meta-Analysis. Ageing Res Rev (2015) 19:22–33. doi: 10.1016/j.arr.2014.10.007
90. Sengupta P, Borges E, Dutta S, Krajewska-Kulak E. Decline in Sperm Count in European Men During the Past 50 Years. Hum Exp Toxicol (2017) 37(3):247–55. doi: 10.1177/0960327117703690
91. Sengupta P, Nwagha U, Dutta S, Krajewska-Kulak E, Izuka E. Evidence for Decreasing Sperm Count in African Population From 1965 to 2015. Afr Health Sci (2017) 17(2):418–27. doi: 10.4314/ahs.v17i2.16
Keywords: pyrethroids, meta-analysis, sperm performance, fertility, male reproduction
Citation: Zhang X, Zhang T, Ren X, Chen X, Wang S and Qin C (2021) Pyrethroids Toxicity to Male Reproductive System and Offspring as a Function of Oxidative Stress Induction: Rodent Studies. Front. Endocrinol. 12:656106. doi: 10.3389/fendo.2021.656106
Received: 21 January 2021; Accepted: 14 April 2021;
Published: 27 May 2021.
Edited by:
Jens Fedder, Odense University Hospital, DenmarkReviewed by:
Özlem Atlı Eklioğlu, Anadolu University, TurkeyLaura Maria Mongioì, University of Catania, Italy
Copyright © 2021 Zhang, Zhang, Ren, Chen, Wang and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Qin, bm11cWluY2hhb0AxNjMuY29t; ShangQian Wang, d3NxQG5qbXUuZWR1LmNu
†These authors have contributed equally to this work
 Xu Zhang
Xu Zhang Tongtong Zhang
Tongtong Zhang Xiaohan Ren
Xiaohan Ren Xinglin Chen†
Xinglin Chen† Chao Qin
Chao Qin