- Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
Background: Hip structural analysis (HSA) is a method for evaluating bone geometry reflecting bone structural and biomechanical properties. However, tissue-selective estrogen complex (TSEC) treatment effects on HSA have not been investigated.
Objective: This study was performed to evaluate the effect of TSEC treatment on hip geometry in postmenopausal Korean women. The treatment was given for 12 months, and hip geometry was measured by HSA.
Materials and Methods: A total of 40 postmenopausal women who received TSEC containing conjugated estrogen 0.45 mg and bazedoxifene 20 mg for treating vasomotor symptoms were included in this retrospective cohort study. The changes in bone mineral density and parameters of HSA including the outer diameter, cross-sectional area, cross-sectional moment of inertia, cortical thickness, section modulus, and buckling ratio as determined by dual-energy X-ray absorptiometry were compared before and after 12 months of TSEC treatment.
Results: Mean age and years since menopause were 55.1 and 4.5 years, respectively. Total hip bone mineral density significantly increased by 0.74% after treatment (P=0.011). The changes in HSA were mainly demonstrated in the narrow femoral neck: cross-sectional area (P=0.003) and cortical thickness (P<0.001) increased significantly. For the shaft region, only SM decreased significantly after treatment (P=0.009). However, most parameters did not change significantly with TSEC treatment in the intertrochanteric and shaft regions.
Conclusions: Our findings demonstrate that 12 months of TSEC treatment could improve bone geometry as measured by HSA. The findings suggest that TSEC might be an interesting option for the prevention of fracture as well as osteoporosis in postmenopausal women.
Introduction
Menopausal hormone therapy (MHT), estrogen alone, or estrogen-progestin combination, is effective for the prevention of osteoporosis and fractures in postmenopausal women (1, 2). However, occurrence of adverse side effects and concerns about risks associated with MHT have deterred use of MHT for this purpose. Experimental evidence suggests that most of the side effects and risks are related to the progestogen component, and a combined therapy of conjugated estrogen (CE) and bazedoxifene was developed recently and used worldwide as an alternative to conventional MHT. This new progestin-free tissue-selective estrogen complex (TSEC) would be a useful MHT option especially in women who cannot tolerate the side effects related to progestogens or who have a higher risk for breast cancer (1), and also showed beneficial effects on bone mineral density (BMD) in previous studies (3–5).
Estrogen regulates bone metabolism via effects on osteocytes, osteoblast, and osteoclasts, and its main effect is inhibition of bone remodeling (6). MHT reduced all major osteoporotic fractures including hip and non-vertebral fractures (7, 8), and bazedoxifene reduced spinal fractures and even prevented hip fractures in postmenopausal women at higher risk of hip fracture (9). Therefore, TSEC, a combination of these two regimens, can also be expected to have beneficial effects on fracture risk. However, fracture risk has not been adequately assessed for low doses of CE (< 0.625 mg), although the effect of estrogen on bone is dose-dependent. Moreover, the effect of TSEC on fracture risk has never been evaluated because the study duration was short (12–24 months) and the subjects were young (subject mean age was in their 50s) in previous studies (3–5).
Hip structural analysis (HSA) is a method for evaluating bone geometry reflecting bone structural and biomechanical properties using dual energy X-ray absorptiometry (DXA) scan of the proximal femur. HSA is a software method to extract information, a line of pixel values across the proximal femur bone axis, from image data of DXA bone mass (10). The profile of a mass projection could be used to estimate geometric relevance. In spite of several limitations such as high correlation with BMD or 2D nature (11, 12), HSA has been applied to assess bone mechanical strength, and therefore, to evaluate the effect of osteoporosis treatment on fracture risk (13–15). However, TSEC treatment effects on HSA have not been investigated.
Therefore, this study evaluated the change in HSA after 12 months of TSEC treatment in postmenopausal Korean women.
Materials and Methods
Study Population
All postmenopausal women who received MHT for relieving vasomotor symptoms at the Menopause Clinic at Samsung Medical Center were considered for inclusion. Menopause was diagnosed when women had no spontaneous menstruation over 12 months without any specific cause for amenorrhea.
The inclusion criteria were: postmenopausal women (1) with an intact uterus, (2) who received TSEC, (3) who were over 40 years old, (4) who were diagnosed with osteopenia at the femoral neck or total hip using DXA, and (5) who had results of bone densitometry and geometry before and after 12 months of TSEC use.
Exclusion criteria were: (1) use of MHT other than TSEC; (2) use of any medication that could affect bone metabolism, e.g., bisphosphonate, glucocorticoids, anti-convulsants, or heparin; (3) having a history of any disease that could affect bone metabolism, e.g., hyperthyroidism or hyperparathyroidism; (4) having been lost to follow-up before 12 months of TSEC treatment; and (5) having a history of hip fracture.
A total of 40 postmenopausal women were included for the analysis in this retrospective cohort study. The study protocol was approved by the Institutional Review Board of Samsung Medical Center, Seoul, Korea. Informed consent from participants was exempted by the Institutional Review Board because of the retrospective nature of the analysis.
Treatment and Measurement
Women received TSEC (Duavive®, Pfizer Ireland Pharmaceuticals, Newbridge, Ireland) for relief of vasomotor symptoms based on the preferences of both patients and doctors. Each TSEC tablet contained CE 0.45 mg and bazedoxifene 20 mg.
The baseline characteristics of the study population, i.e., age, body mass index, years since menopause, and parity, were obtained from medical records. In addition, the results of bone densitometry and hip geometry were also obtained.
BMD was measured at the hip using DXA (Delphi Q; Hologic Inc., Bedford, MA, USA). The in vivo coefficient of variation was 1.0% for the hip. For the evaluation of hip bone geometry, the HSA™ program was used for three regions, the femoral narrow neck, intertrochanter, and shaft. These were displayed on the DXA image. At each region, the HSA™ program produced geometric parameters including the outer diameter (OD), cross-sectional area (CSA), cross-sectional moment of inertia (CSMI), cortical thickness (CT), section modulus (SM), and buckling ratio (BR).
Statistical Analysis
All statistical analyses were performed using SPSS Statistics 25 software (SPSS Inc., Chicago, IL). Data are presented as mean ± standard error of the mean or number (percent). The normality assumption of the data was confirmed using the Levene’s test for homogeneity of variances before statistical analysis. Changes in BMD and various parameters of HSA were compared before and after treatment using paired t tests. In addition, differences in HSA parameters between responders (no change or increase in BMD from baseline at 12 months) and non-responders (decrease in BMD from baseline at 12 months) were compared by t test or Mann–Whitney test. P < 0.05 was considered statistically significant.
Results
Table 1 demonstrates the baseline characteristics of the study participants. The mean age and years since menopause were 55.1 and 4.5 years, respectively. The mean body index was 21.5 kg/m2, and only two women were obese.
Figure 1 depicts the change in BMD after 12 months of TSEC treatment. Both BMDs at the femoral neck (from 0.624 to 0.631 g/cm2, P = 0.022) and total hip (from 0.770 to 0.776 g/cm2, P = 0.011) increased significantly by 1.26% and 0.74%, respectively. In addition, T-score also improved significantly at the femoral neck (from -1.68 to -1.59, P = 0.004) and total hip (from -0.72 to -0.65, P = 0.006).
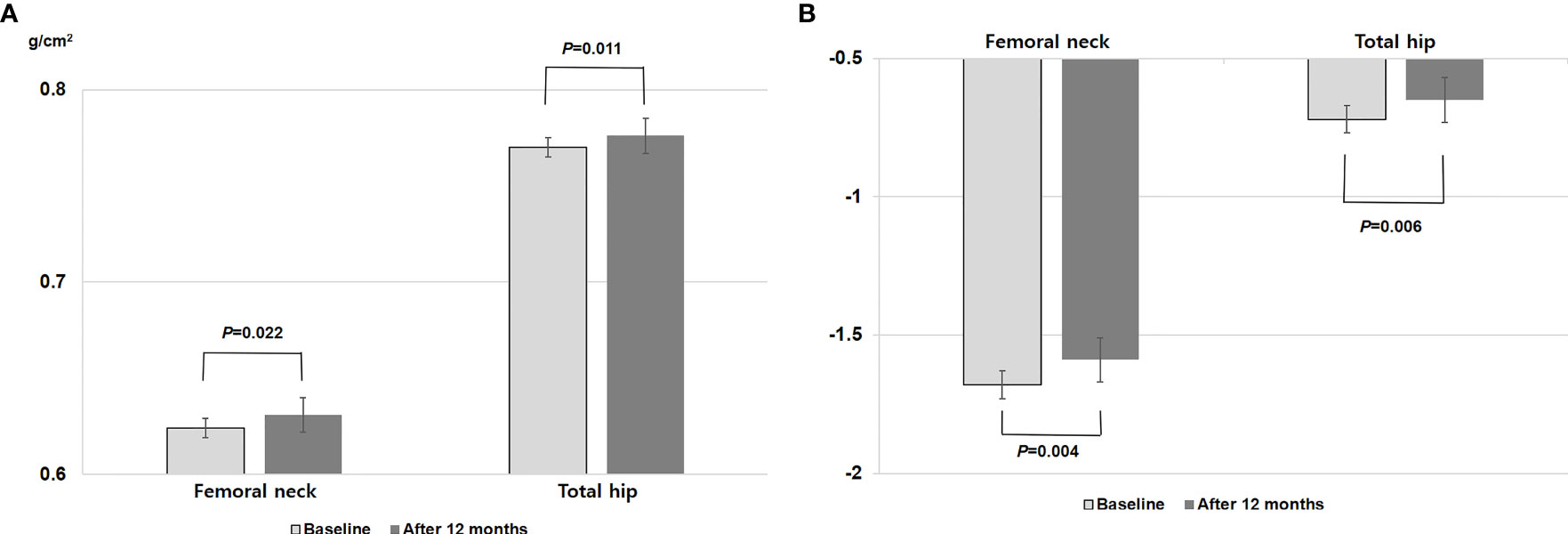
Figure 1 Changes in (A) bone mineral density and (B) T-score at the femoral neck and total hip after 12 months of TSEC treatment. P value by paired t test.
Table 2 shows the changes in various parameters of HSA after 12 months of TSEC treatment. For the narrow neck of the femur, CSA (P = 0.003) and CT (P<0.001) increased significantly. In addition, although not statistically significant, CSMI and SM tended to increase and BR tended to decrease. However, OD did not change. For the intertrochanteric region, women had a wider diameter after treatment (P = 0.041). However, other parameters such as CSA, CSMI, SM, CT, and BR did not change significantly after treatment. For the shaft region, only SM decreased significantly after treatment (P = 0.009), and OD, CSA, CSMI, CT, and BR did not differ.
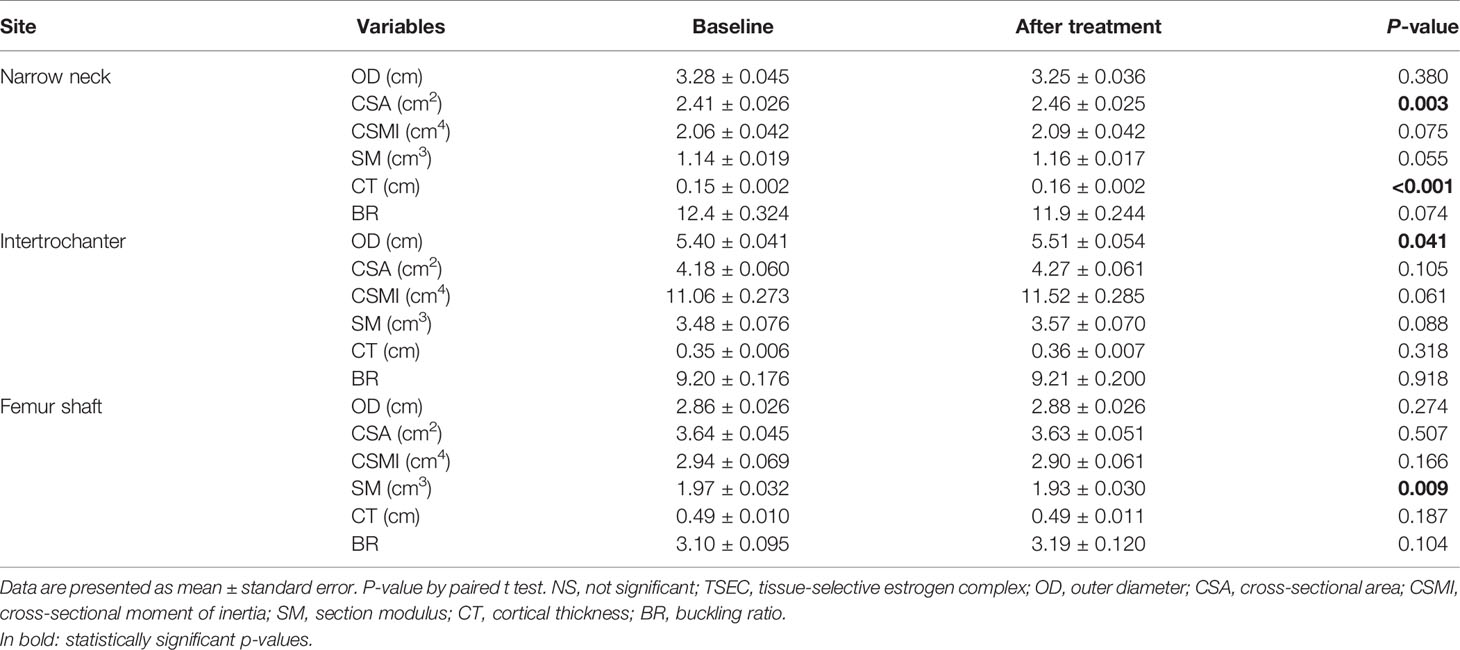
Table 2 Changes in parameters of hip structural analysis at three regions after 1 year of TSEC treatment.
Table 3 presents the changes in HSA according to BMD response. Responders were women with no change or increase in total hip BMD after treatment. Because the number of each group was relatively small, some parameters in Table 3 did not meet the normality assumption. In these contexts, t test or Mann–Whitney test were used where indicated. For the narrow femoral neck, percent changes in OD, CSA, and CSMI were significantly higher in responders than in non-responders. Values were significantly decreased in OD and CSMI or only slightly increased in CSA in non-responders after treatment. Although not statistically significant, BR also decreased in responders in contrast to a minimal increase in non-responders. For the intertrochanteric region, OD and BR decreased in responders and increased in non-responders, and the changes were significantly different between responders and non-responders (P = 0.031 for OD and P=0.026 for BR). For the shaft region, CSA and CT increased in responders and decreased in non-responders after treatment, showing a significant difference of percent changes between the two groups (P = 0.005 for CSA and P = 0.042 for CT).
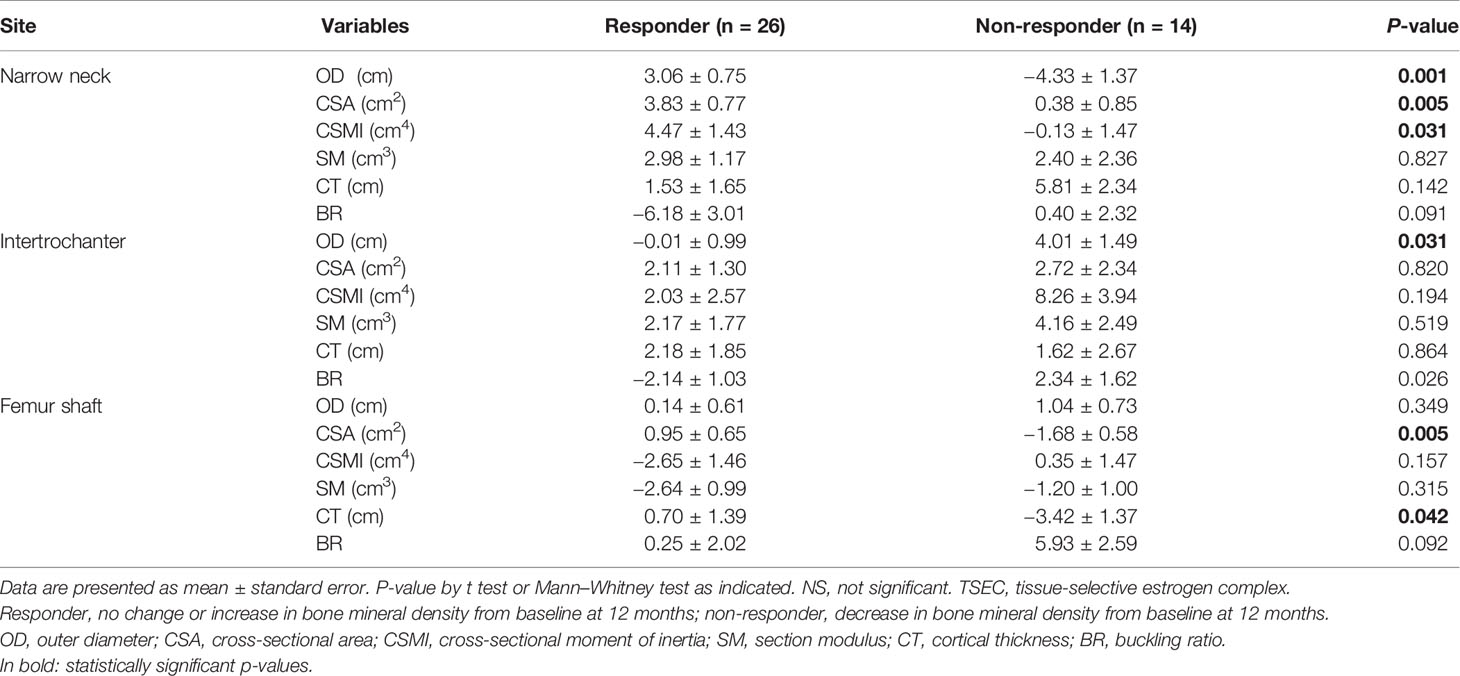
Table 3 Percent changes in parameters of hip structural analysis at three regions after 1 year of TSEC treatment, according to the response of bone mineral density at the total hip.
Discussion
This study evaluated the changes in BMD and HSA after 12 months of TSEC treatment in postmenopausal Korean women. This study demonstrated that, with TSEC treatment, hip BMD significantly increased, and some parameters of HSA were improved with TSEC treatment.
In the present study, total hip BMD significantly increased by 0.7% after treatment. This finding is similar to previous studies that demonstrated an approximate 1% (SMART-1), 0.84% (SMART-4), and 0.5% (SMART-5) increase of total hip BMD after 12 months of TSEC treatment (3–5). Since our study population was osteopenic postmenopausal women who were at low risk for bone loss or fracture, this amount of BMD increase could maintain women’s bone health (16). In addition, the percentage of total hip BMD responders was 65% in the present study. This is comparable to the 61.5% of SMART-4 (4) and 70% of SMART-1 responders (3). The amount of total hip BMD increase in the current study was similar to that seen with bazedoxifene alone (9). This suggests that the combination of bazedoxifene 20 mg and CE 0.45 mg did not produce an additional BMD gain by adding the estrogen component. One possible explanation is that, since both CE and bazedoxifene bind to the estrogen receptor and CE has a more potent effect, addition of bazedoxifene to CE may attenuate the BMD response (3, 5).
The risk of hip fracture is low in young postmenopausal women who usually take TSEC, and the study duration in previous studies regarding TSEC was too short (≤ 24 months). Therefore, fracture risk related to TSEC use has never really been addressed. In addition, either low-dose CE (0.45 mg) or bazedoxifene therapy has not been proven to reduce the hip fracture risk in postmenopausal women at low risk. In these contexts, HSA was assessed as a possible surrogate marker to evaluate beneficial effects of TSEC on fracture risk in this study.
In the present study, the changes in HSA parameters, indicating geometry-related improvement, were mainly observed in the narrow femoral neck region. The femoral neck is the most common location of fractures. Our findings are consistent with the 3-year retrospective study in postmenopausal women with osteoporosis (mean age: 67.4 years). Those researchers reported that treatment with bazedoxifene was associated with significant increases in CSA and SM in the narrow femoral neck and in CSA in the trochanter region. Also, almost no significant change in the shaft region compared with placebo was demonstrated (17). Besides bazedoxifene results, results from the following three studies regarding MHT are also similar to our study findings. In a prospective study analyzing about 600 current hormone users, CSA and CT increased compared to never users (18). In addition, in the 3-year randomized clinical trial in women over the age of 65 years, MHT using CE 0.625 mg (n = 93) increased CSA and SM in the narrow femoral neck region, and BR decreased after treatment (19). From the Women’s Health Initiative study (20), CSA and CT increased and BR decreased in the narrow neck region at year 1 among hormone users compared with placebo. Favorable changes in HSA parameters in the intertrochanter or shaft region were also not observed. No significant differences in the geometric changes were found between estrogen alone and estrogen-progestin treatment, suggesting that the effect of estrogen was responsible for the changes.
The beneficial effect of estrogen seems to be associated with increases in cortical thickness and section moduli via direct effects on bone (18). The load sensitivity can be increased by estrogen on bone tissue. In addition, estrogen has a positive effect on muscle strength; this may play an indirect role on bone strength (21). However, we could not conclude that the combination of bazedoxifene and CE had additional benefits on hip structural geometry compared with estrogen or bazedoxifene alone. Differences in treatment (dose, type, duration) and study population (age, baseline BMD, history of fracture, or other risk factors) have a considerable impact on bone health; therefore, the degree of beneficial effects will differ across studies.
This is the first study to evaluate the change in HSA with TSEC treatment in young osteopenic postmenopausal women. Although previous studies showed a significant increase in BMD and significant decreases in bone turnover markers, HSA has never been assessed in TSEC users. In addition, previous studies evaluated the effects of MHT on HSA in older women (over 65 years old); but, from recent guidelines, MHT is no longer recommended for bone health in older women (1, 2). The current main indication for MHT is young postmenopausal women having vasomotor symptoms who are younger than 60 years or within 10 years from menopause onset. This is similar to our study population. Therefore, the current study could provide real world evidence regarding TSEC on hip geometry.
However, this study had several limitations. This was not a prospective clinical trial, and duration of treatment and sample size may not have been adequate to assess the effects of TSEC on HSA. In a post-hoc power analysis, this study had a power of about 30% to detect differences in the changes of HSA parameters with an alpha error of 0.05. This study did not contain a control group who had osteopenia but did not use TSEC. However, it already has been shown that parameters of HSA did not change significantly in placebo group during one year (20). In addition, although intake of calcium and vitamin D was encouraged for all patients and daily supplementation was provided if necessary, we could not ascertain that all patients consumed sufficient calcium and vitamin D for maintaining bone health. Finally, the clinical implication of our findings is not clear in young postmenopausal women at low risk of hip fracture, and therefore, the results should be interpreted with caution.
In conclusion, this pilot study demonstrates that a combination of CE and bazedoxifene could enhance improvements in bone strength evaluated by HSA and suggests that TSEC may be a treatment option for prevention of fracture and osteoporosis in postmenopausal women. Further long-term, large-scale study is necessary to draw a clear conclusion.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Samsung medical center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
BK, SK, D-YL, and DC were responsible for the concept and design of the study, searching for and analyzing data, and the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Baber RJ, Panay N, Fenton A, IMS Writing Group. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric (2016) 19:109–50. doi: 10.3109/13697137.2015.1129166
2. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause (2017) 24:728–53. doi: 10.1097/GME.0000000000000921
3. Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril (2009) 92:1045–52. doi: 10.1016/j.fertnstert.2009.02.093
4. Mirkin S, Komm BS, Pan K, Chines AA. Effects of bazedoxifene/conjugated estrogens on endometrial safety and bone in postmenopausal women. Climacteric (2013) 16:338–46. doi: 10.3109/13697137.2012.717994
5. Pinkerton JV, Harvey JA, Lindsay R, Pan K, Chines AA, Mirkin S, et al. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab (2014) 99:E189–98. doi: 10.1210/jc.2013-1707
6. Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol HSMetab (2012) 23:576–81. doi: 10.1016/j.tem.2012.03.008
7. Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the women’s health initiative randomized trial. J Bone Miner Res (2006) 21:817–28. doi: 10.1359/jbmr.060312
8. Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA (2003) 290:1729–38. doi: 10.1001/jama.290.13.1729
9. Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR, de Villiers TJ, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res (2008) 23:1923–34. doi: 10.1359/jbmr.080710
10. Martin RB, Burr DB. Non-invasive measurement of long bone cross-sectional moment of inertia by photon absorptiometry. J Biomech (1984) 17:195–201. doi: 10.1016/0021-9290(84)90010-1
11. Beck TJ, Broy SB. Measurement of Hip Geometry-Technical Background. J Clin Densitom (2015) 18:331–7. doi: 10.1016/j.jocd.2015.06.006
12. Choi YJ. Dual-Energy X-Ray Absorptiometry: Beyond Bone Mineral Density Determination. Endocrinol Metab (2016) 31:25–30. doi: 10.3803/EnM.2016.31.1.25
13. Rivadeneira F, Zillikens MC, De Laet CE, Hofman A, Uitterlinden AG, Beck TJ, et al. Femoral Neck BMD Is a Strong Predictor of Hip Fracture Susceptibility in Elderly Men and Women Because It Detects Cortical Bone Instability: The Rotterdam Study. J Bone Miner Res (2007) 22:1781–90. doi: 10.1359/jbmr.070712
14. Kaptoge S, Beck TJ, Reeve J, Stone KL, Hillier TA, Cauley JA, et al. Prediction of Incident Hip Fracture Risk by Femur Geometry Variables Measured by Hip Structural Analysis in the Study of Osteoporotic Fractures. J Bone Miner Res (2008) 23:1892–904. doi: 10.1359/jbmr.080802
15. LaCroix AZ, Beck TJ, Cauley JA, Lewis CE, Bassford T, Jackson R, et al. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int (2010) 21:919–29. doi: 10.1007/s00198-009-1056-1
16. Gallagher JC, Palacios S, Ryan KA, Yu CR, Pan K, Kendler DL, et al. Effect of conjugated estrogens/bazedoxifene on postmenopausal bone loss: pooled analysis of two randomized trials. Menopause (2016) 23:1083–91. doi: 10.1097/GME.0000000000000694
17. Beck TJ, Fuerst T, Gaither KW, Sutradhar S, Levine AB, Hines T, et al. The effects of bazedoxifene on bone structural strength evaluated by hip structure analysis. Bone (2015) 77:115–9. doi: 10.1016/j.bone.2015.04.027
18. Beck TJ, Stone KL, Oreskovic TL, Hochberg MC, Nevitt MC, Genant HK, et al. Effects of current and discontinued estrogen replacement therapy on hip structural geometry: the study of osteoporotic fractures. J Bone Miner Res (2001) 16:2103–10. doi: 10.1359/jbmr.2001.16.11.2103
19. Greenspan SL, Beck TJ, Resnick NM, Bhattacharya R, Parker RA. Effect of hormone replacement, alendronate, or combination therapy on hip structural geometry: a 3-year, double-blind, placebo-controlled clinical trial. J Bone Miner Res (2005) 20:1525–32. doi: 10.1359/JBMR.050508
20. Chen Z, Beck TJ, Cauley JA, Lewis CE, LaCroix A, Bassford T, et al. Hormone therapy improves femur geometry among ethnically diverse postmenopausal participants in the Women’s Health Initiative hormone intervention trials. J Bone Miner Res (2008) 23:1935–45. doi: 10.1359/jbmr.080707
Keywords: tissue-selective estrogen complex (TSEC), bone mineral density, hip structural analysis, menopause, hormone therapy (HT)
Citation: Kim BM, Kim SE, Lee D-Y and Choi D (2021) Effect of Tissue-Selective Estrogen Complex on Hip Structural Geometry in Postmenopausal Women: A 12-Month Study. Front. Endocrinol. 12:649952. doi: 10.3389/fendo.2021.649952
Received: 05 January 2021; Accepted: 17 February 2021;
Published: 11 March 2021.
Edited by:
Katherine A. Staines, University of Brighton, United KingdomReviewed by:
Hasmik Jasmine Samvelyan, University of Brighton, United KingdomFiona Saunders, University of Aberdeen, United Kingdom
Copyright © 2021 Kim, Kim, Lee and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Yun Lee, ZG9uZ3l1bjA0MDYubGVlQHNhbXN1bmcuY29t
 Bo Mi Kim
Bo Mi Kim Sung Eun Kim
Sung Eun Kim Dong-Yun Lee
Dong-Yun Lee