
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 15 March 2021
Sec. Clinical Diabetes
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.627395
This article is part of the Research Topic Advances in the Research of Diabetic Retinopathy View all 20 articles
Objective: Growth differentiation factor 15 (GDF-15) is a member of the TGF-β superfamily that has anti-inflammatory properties. The objective of this study was to evaluate the relationship between circulating GDF-15 levels and diabetic retinopathy (DR) in patients with type 2 diabetes.
Materials/Methods: A case–control study was performed in which 402 patients with type 2 diabetes were enrolled. Of these, 171 patients had DR and the remaining 231 patients without DR acted as controls. The plasma GDF-15 levels were measured using ELISA, while DR was diagnosed using the canon ophthalmic digital imaging system and the Canon EOS 10D digital camera (Canon, Tokyo, Japan) through a non-pharmacologically dilated pupil.
Results: The levels of GDF-15 were significantly higher in patients with DR [168.9 (112.9–228.3) pg/ml vs. 127.8 (96.1–202.8) pg/ml, P < 0.001] compared to controls. Results of the Spearman correlation analysis showed that the GDF-15 levels were positively associated with the duration of diabetes morbidity, fasting plasma glucose, systolic blood pressure, albumin/creatinine ratio, creatinine, and liver enzymes, but negatively associated with eGFR (both P < 0.001). The participants in the highest GDF-15 quartile had a significantly increased risk for DR (OR = 2.15, 95% CI 1.53–3.02) after adjusting for potential cofounders.
Conclusions: The circulating GDF-15 levels are positively associated with DR independent of potential cofounders.
The prevalence of diabetes in China has become a major public health concern with ~9.7% of all adults affected (1). Diabetic retinopathy (DR) is a microvascular complication of diabetes and is the leading cause of blindness among adults of working-age around the world (2). Although DR is initially asymptomatic, its progression is characterized by damage to the retinal microvasculature due to inflammation and oxidative stress caused by chronic hyperglycemia. Recently, studies have focused on inflammatory biomarkers and risk factors for endothelial dysfunction, such as C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α) that are considered to be prognostic factors for the development of DR. These studies have also shown that some cytokines may be associated with the development of DR (3–5).
Growth differentiation factor 15 (GDF-15), also known as macrophage inhibitory cytokine-1(MIC-1) (6), placental transformation growth factor-b (PTGF-b) (7–9), prostate derived factor (PDF) (10), placental bone morphogenetic protein (PLAB) (11, 12), NSAID activated gene-1 (NAG-1) (13, 14), and PL74 (15), are divergent members of the transforming growth factor-β (TGF-β) superfamily with anti-inflammatory properties. GDF-15 is highly expressed in cardiomyocytes, adipocytes, macrophages, endothelial cells, and vascular smooth muscle cells during tissue injury and inflammatory states. It plays a crucial role in the development and progression of cardiovascular diseases such as heart failure, coronary artery diseases, atrial fibrillation, diabetes, cancer, and cognitive impairment (16, 17). In type 2 diabetes, GDF-15 predicts the development of proteinuria in patients with diabetic nephropathy, suggesting that GDF-15 may be a part of an anti-inflammatory response to microvascular damages (18). Moreover, GDF-15 is associated with a number of circulating proangiogenic endothelial progenitor cells in patients with type 2 diabetes (19). Furthermore, GDF-15 expression is markedly increased before the onset of type 2 diabetes (20), which suggests that GDF-15 is a potential biomarker of DR (21).
Therefore, the current study aimed to investigate the relationship between plasma GDF-15 levels and the risk of DR in a large cohort of type 2 diabetic patients.
This was a cohort study aimed at assessing the risk factors associated with the development of diabetic complications. Study participants were type 2 diabetes patients recruited from the Department of Endocrinology at Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine between 2013 and 2014. Diabetes was defined according to the 2008 American Diabetes Association diagnostic criteria (22).
Individuals with the following conditions were excluded from the study: cancer, acquired immune deficiency syndrome, severe psychological disorders, clinical signs or symptoms of inborn errors of metabolism, a history of vitreal surgery, senile dementia, tuberculosis, a cataract on examination, or any other communicable disease. A total of 402 participants with type 2 diabetes were included in this study. Written informed consent was obtained from all participants. The Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine approved this study.
Anthropometric measurements, questionnaire, physical examination, and laboratory measurements were performed. The waist circumference was defined as the midway level between the costal margins and the iliac crests. Blood pressure was assessed twice on the right arm after a 15-min rest in a sitting position using a standard mercury sphygmomanometer. BMI was calculated as the weight in kilograms divided by the square height in meters (kg/m2). Age, alcohol consumption (yes/no, history of alcohol consumption was defined as “yes”, lack of history was defined as “no”), smoking (yes/no, history of smoking was defined as “yes”; lack of history was defined as “no”), education status, and duration of diabetes morbidity were assessed using interviews. Based on the International Physical Activity Questionnaire scoring protocol, the physical activity level was classified as low, moderate, or high level.
The A1c was measured using high-performance liquid chromatography (BIO-RAD, D10, CA), while fasting plasma glucose levels were tested using the glucose oxidase method (ADVIA-1650 Chemistry System, Bayer, Leverkusen, Germany). Lipid profiles, liver enzyme profiles, and creatinine (Cr) were determined using a Hitachi 7080 analyzer (Hitachi 7080; Tokyo, Japan), while fasting insulin was determined using the radioimmunoassay method (Linco Research, St. Charles, MO). The albumin/creatinine ratio (ACR) was calculated as milligrams of urinary albumin excretion per gram of urinary creatinine. Glomerular filtration rate (eGFR) was estimated using a simplified Modification of Diet in Renal Disease formula recalibrated for the Chinese: 186 × (serum creatinine × 0.011)−1.154 × (age)−0.203 × (0.742 if female) × 1.233 (23). The homoeostasis model assessment (HOMA) value for insulin resistance (HOMA-IR) was evaluated with the following formula: fasting insulin × fasting glucose/22.5. The HOMA-β was calculated using the formula described by Matthews et al. (24).
The circulating levels of GDF-15 were determined in duplicate using the Duoset kit for ELISA (DY805; R&D Systems, Minneapolis, MN) according to the instructions of the manufacturer. The ELISA system had an intra-assay coefficient of variation of 3 to 9% and an inter-assay coefficient of variation of 4 to 10.2%.
The circulating adiponectin and C-reactive protein (CRP) were measured using ELISA kits (DY1065 and DY1707, respectively; R&D Systems, Minneapolis, MN) according to the instructions of the manufacturer.
Dilated ophthalmic eye examinations including fundus photography were performed by an experienced ophthalmologist. DR was classified as cases without diabetic retinopathy (non-DR), mild non-proliferative DR (NPDR), moderate NPDR and vision-threatening DR (VTDR).
Fundus photography was performed using digital non-mydriatic camera (CR6-45NM; Canon, Lake Success, NY) according to the International Classification of Diabetic Retinopathy (25). The severity of DR was classified as 1) non-DR; 2) mild non-proliferative DR (NPDR); 3) moderate NPDR; 4) severe NPDR; and 5) proliferative DR (PDR). Due to the limited number of study participants with PDR (n = 2), PDR cases were combined with severe NPDR cases to give the vision-threatening DR (VTDR) group. When binocular DR was present and unequal, we used the more advanced DR measurement for analyses. Patients with ungradable retinal fundus photographs of both eyes were excluded from the study.
Continuous variables with normal distribution were shown as means with SDs, whereas variables with skewed distribution were presented as median with interquartile range. For comparisons between groups, continuous variables were compared using Student t tests or Mann–Whitney U tests. Categorical variables were expressed as proportions and compared across groups using X2 tests. Spearman correlation analysis was used to calculate the correlation coefficients between GDF-15 and metabolic parameters. Multivariate logistic regression models were performed to determine the potential relationship between GDF-15 levels and the risk of DR. To minimize the potential confounding factors, covariates were selected based on biologic interest, well established risk factors for DR, or associated exposures and outcomes. Variables showing P < 0.05 in the univariable regression were entered into the multivariable model. All statistical analyses were performed with the SPSS software (version 25.0). A two-sided P < 0.05 was considered to be statistically significant.
Out of a study population of 402 participants, 171 individuals (42.5%) had DR. The plasma GDF-15 levels were significantly higher in patients with DR (168.9 [112.9–228.3] pg/ml vs. 127.8 [96.1–202.8] pg/ml, P < 0.001) compared to controls. Moreover, there was an increasing trend in the median (inter-quartile range) of GDF-15 concentrations from the patients with no DR, mild NPDR, moderate NPDR, to VTDR (P < 0.001 for trend) (Figure 1). The clinical characteristics of the study participants are shown in Table 1.
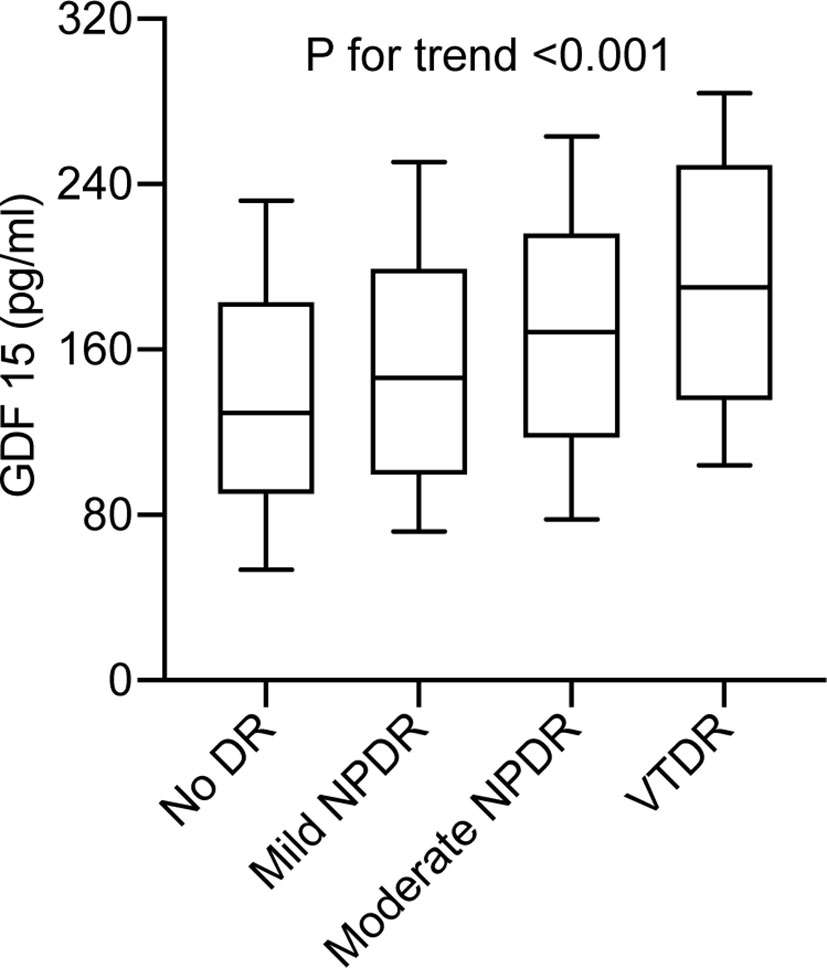
Figure 1 Plasma GDF-15 levels in type 2 diabetes without DR compared with patients with mild non-proliferative DR (NPDR), moderate NPDR, or vision-threatening DR (VTDR). Boxes represent the inter-quartile range, with the median superimposed as a horizontal line. Error bars indicate ranges of plasma GDF-15 levels. P < 0.001 for trend.
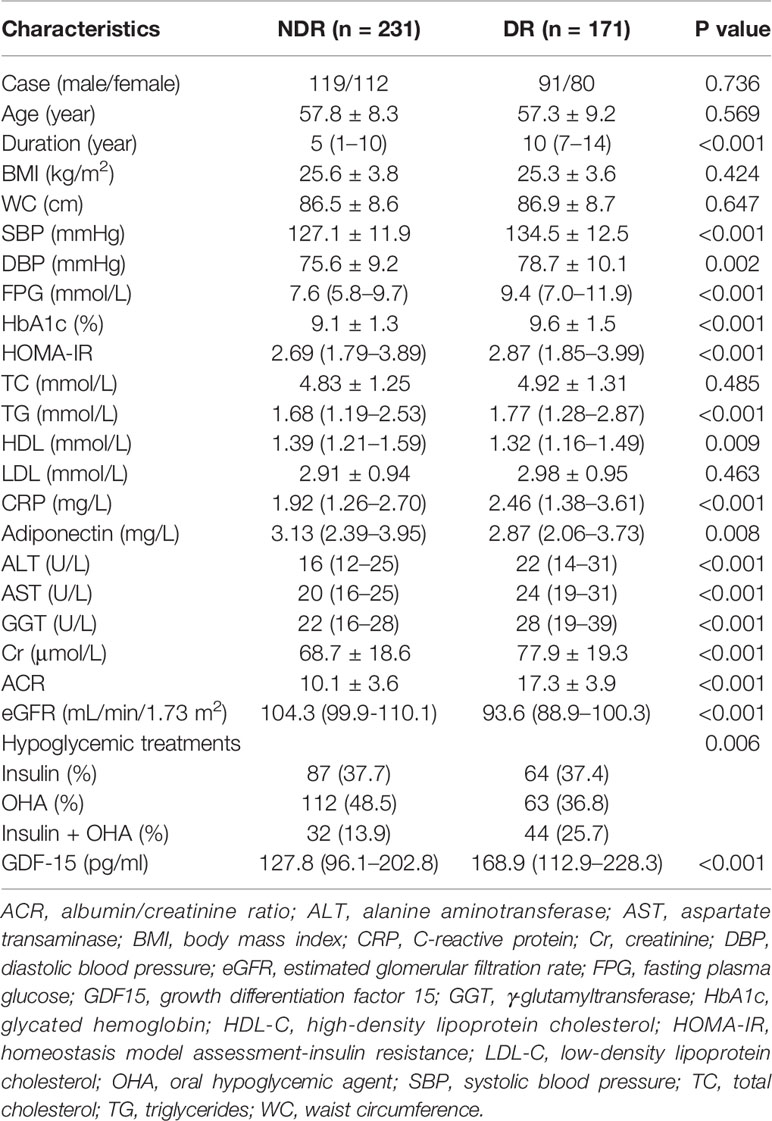
Table 1 Characteristics of subjects according to the presence or absence of diabetic retinopathy (DR) (n = 402).
Results of the Spearman correlation analysis showed that GDF-15 was positively correlated with age (r = 0.14, P < 0.0001), duration of morbidity (r = 0.40, P < 0.0001), fasting plasma glucose (r = 0.13, P < 0.0001), systolic blood pressure (r = 0.15, P < 0.0001), Cr (r = 0.21, P < 0.0001), and ACR (r = 0.13, P < 0.0001), but was negatively correlated with eGFR (r = −0.21, P < 0.0001) (Figure 2). Multiple regression analysis with a stepwise model was used to assess the independent variables that affect the GDF-15 plasma levels. The variables entered in the model were as follows: age, gender, CRP, BMI, WC, SBP, DBP, ALT, AST, GGT, HbA1c, fasting plasma glucose, fasting plasma insulin, triglycerides, total cholesterol, LDL-c and HDL-c. The main determinants of GDF-15 were age (β = 0.304, P < 0.001), ALT (β = 0.153, P < 0.001) and Cr (β = 0.152, P < 0.001).
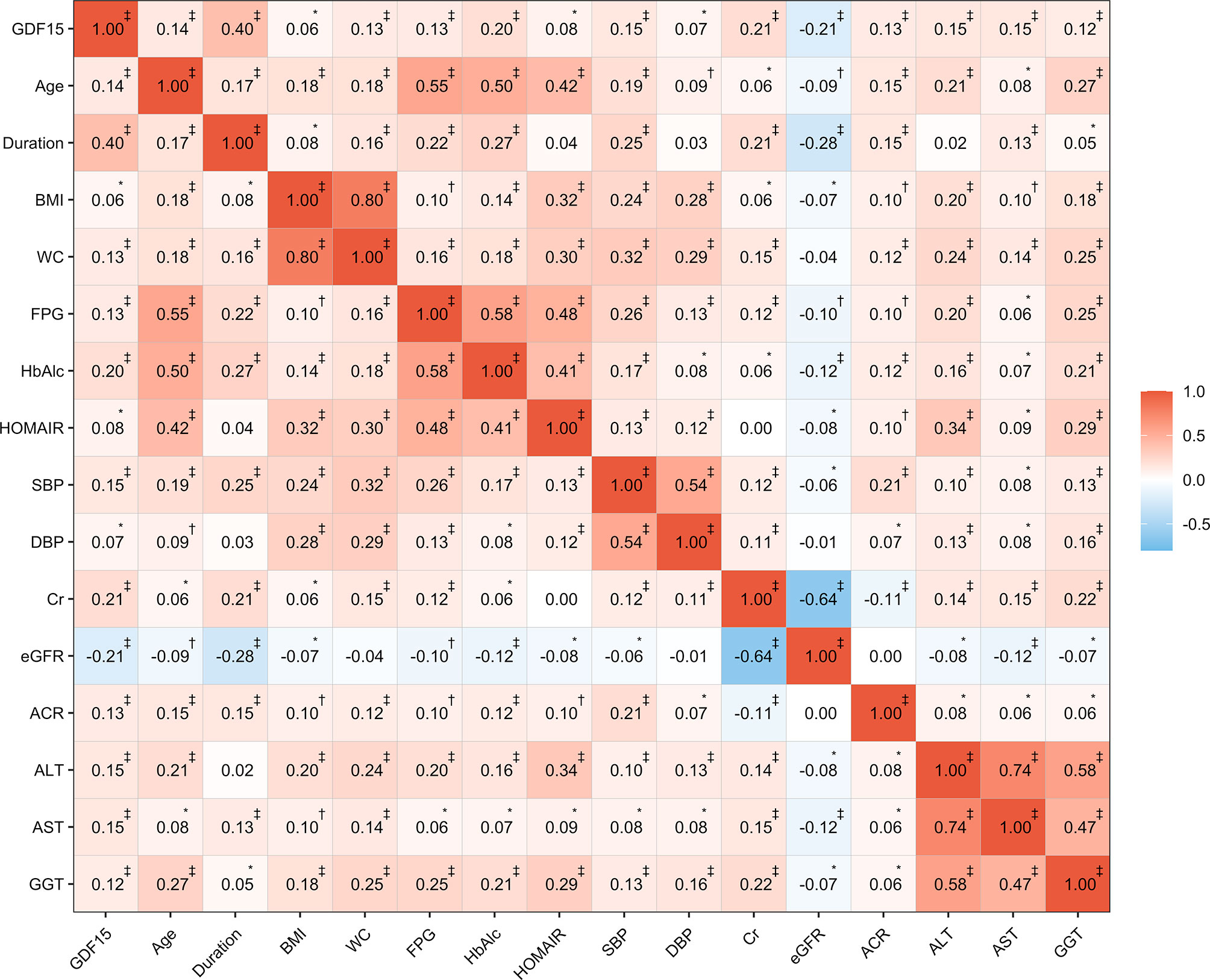
Figure 2 Correlations between GDF-15 and clinical characteristics. The values in the cell represent the correlation coefficients based on spearman correlation. *P < 0.05; †P < 0.001; ‡P < 0.0001.
Table 2 displays the odds ratios (ORs) for DR based on the GDF-15 quartiles. As expected, there was an increase in the ORs for DR from the 1st to the 4th GDF-15 quartiles in the study cohort (P < 0.001 for trend). In the highest GDF-15 quartile, the adjusted OR of DR was 2.15 [95% confidence interval (CI) 1.53–3.02] after adjusting for age, gender, smoking, alcohol consumption, education status, physical activity, BMI, waist circumference, CRP, adiponectin, HOMA-IR, liver enzymes, diabetes duration, FPG, eGFR, Cr, and ACR. A positive linear dose–response relationship was evident in the cubic spline regression model (Figure 3, P for non-linearity > 0.1).
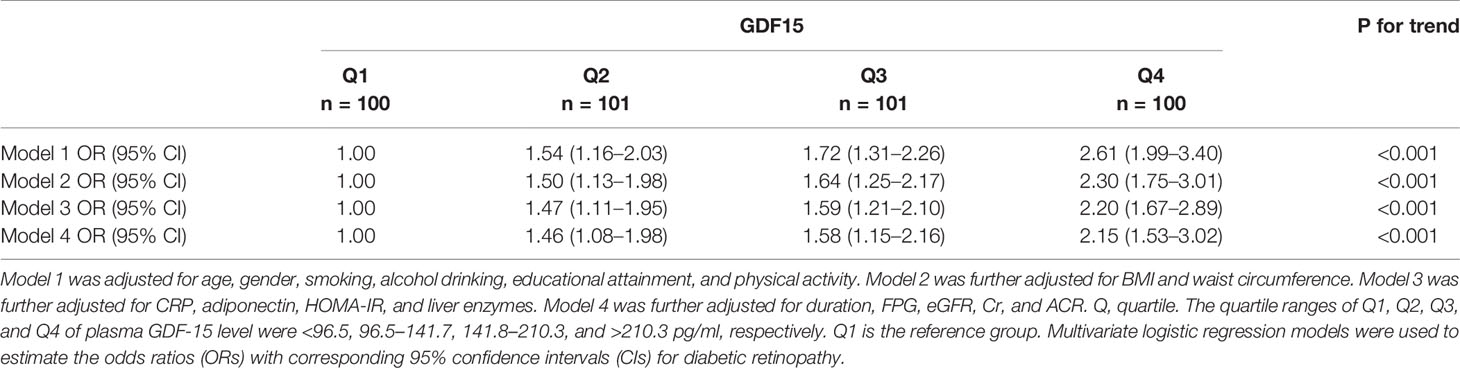
Table 2 Adjusted odds ratios (ORs) of diabetic retinopathy according to quartiles of plasma growth differentiation factor 15 (GDF-15) levels.
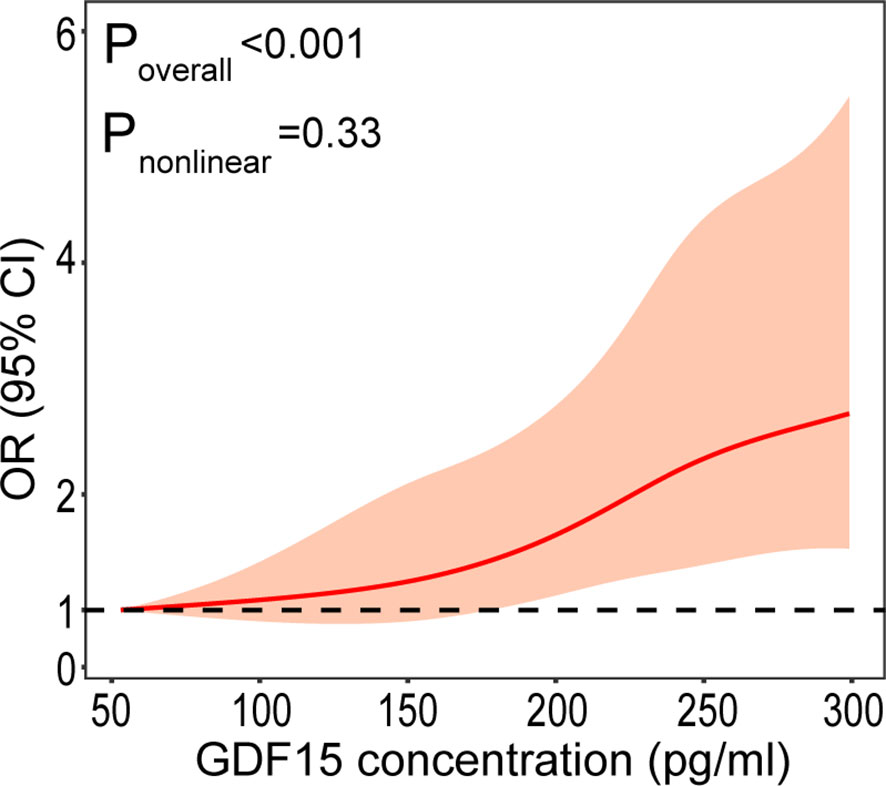
Figure 3 Plasma GDF-15 levels on a continuous scale and the presence of DR. The solid line represents the odds ratio (OR) and the gray area represents the 95% confidence interval (CI). Model was adjusted for age, gender, smoking, drinking, physical activity, educational attainment, BMI, waist circumference, CRP, adiponectin, HOMA-IR, liver enzymes, duration, FPG, eGFR, Cr, and ACR.
Subgroup analyses were performed to examine potential effect modifiers, stratified by age (<65 versus ≥65 years), sex (male versus female), SBP (<140 versus ≥140 mmHg), waist circumference (<90 versus ≥90 cm for men and <85 versus ≥85 cm for women and), CRP (<3.0 versus ≥3.0 mg/L), and ACR (<30 versus ≥30 mg/g). Results of stratified analyses showed that the positive associations between GDF-15 levels and the presence of DR remained consistent across all subgroups (Figure 4). No interaction was observed with any of the variables (all P for interaction > 0.1).
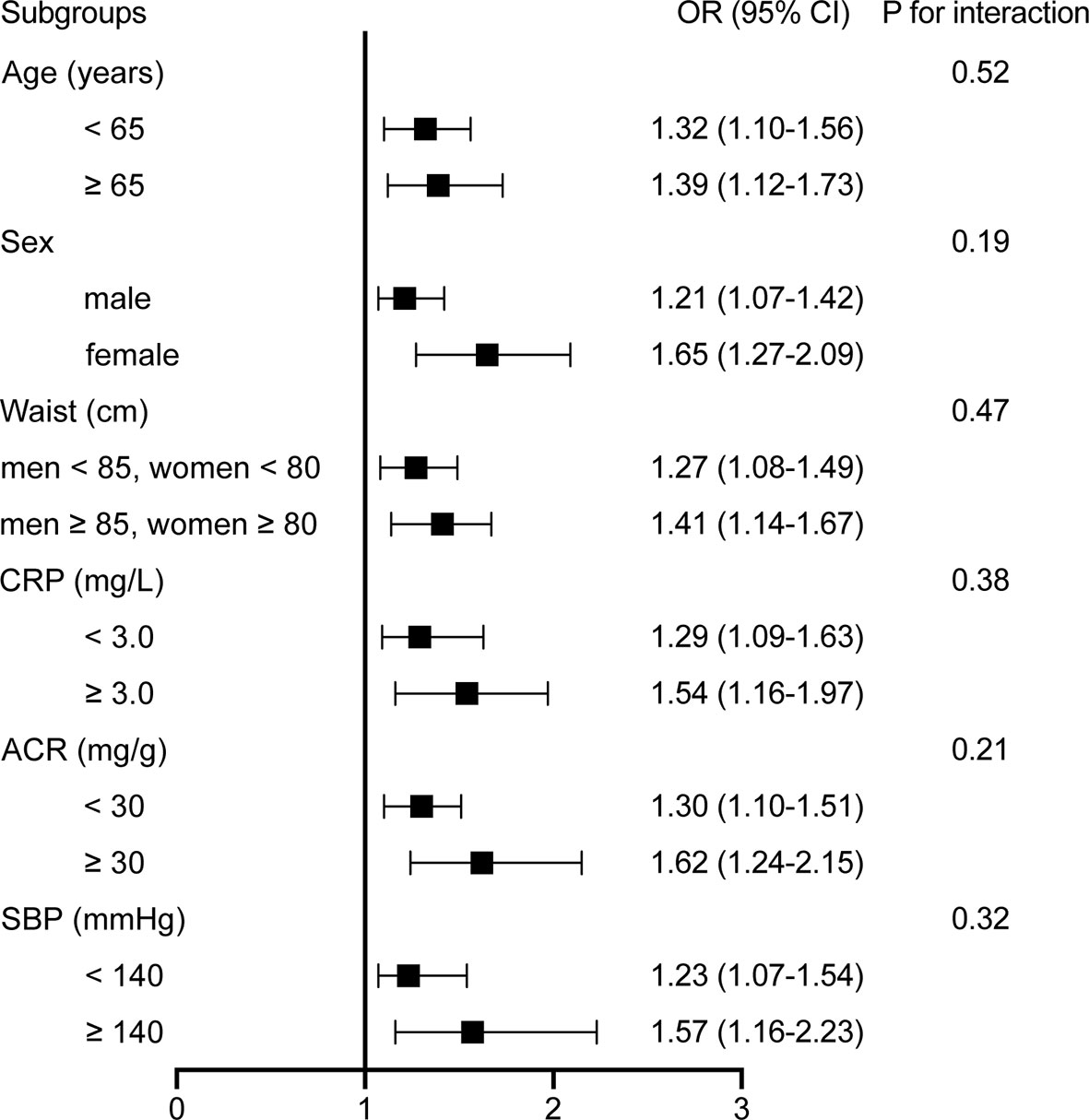
Figure 4 Stratified analyses of the associations [odds ratio (95% confidence interval)] between plasma GDF-15 levels (per 50 pg/ml increment) and DR. Model was adjusted for age, gender, smoking, drinking, physical activity, education status, BMI, waist circumference, CRP, adiponectin, HOMA-IR, liver enzymes, duration, FPG, eGFR, Cr, and ACR. Subgroup variable was excluded from the model.
In this cohort study, we found a significantly positive association between circulating GDF-15 levels and the risk of DR in individuals with type 2 diabetes. This association remained even after extensively adjusting for potential confounders through the stratification of several potential risk factors that may have an effect on the GDF-15–DR relationship.
In our study, plasma levels of GDF-15 were significantly higher in patients with DR compared to patients without DR. Moreover, results of our study showed that plasma GDF-15 concentrations were associated with progression of DR in individuals with type 2 diabetes, after controlling for confounding risk factors. Glycemia, blood pressure, and duration of diabetes morbidity have been identified as risk factors for DR although there are still some controversies (26, 27). This is consistent with the findings from our study that showed that the duration of diabetes morbidity, HbA1c, CRP, and systolic blood pressure was a risk factor for DR in patients with type 2 diabetes. In line with a previous study (28), we also found that the plasma levels of GDF-15 were significantly associated with renal damage and could predict the development of diabetic retinopathy in patients with type 2 diabetes. Importantly, the association between circulating plasma GDF-15 concentrations and the progression of DR in type 2 diabetic patients in our study was independent of the traditional DR risk factors mentioned above.
GDF-15 was discovered and cloned as a divergent member of the TGF-β superfamily in the late 1990s (6). The GDF-15 gene is composed of two exons and contains one single intron that interrupts the coding sequences at identical positions within the pre-pro-domain of the corresponding proteins (7, 29). It is widely expressed in almost all tissues indicating that it has numerous vital cellular functions such as proliferation, migration, maintenance, and homeostasis (10). This suggests that alterations of serum GDF-15 levels may be associated with various diseases including heart failure, coronary artery diseases, cancer, diabetes, and diabetic renal damage (16–18). Circulating GDF-15 is likely to be secreted by endothelial cells such that the serum levels of GDF-15 are a general indication of endothelial and microvascular damage. This may explain the association between GDF-15 and DR since DR is primarily caused by microvascular injury during diabetes (30).
The mechanism of the microvascular effects of GDF-15 on DR in patients with type 2 diabetes has not been elucidated yet. Previous studies have shown that DR is characterized by a decrease in retinal perfusion caused by the constriction of arterioles, endothelial cell degeneration, microvascular destabilization caused by the retinal pericyte loss, and a release of proangiogenic factors that promote the development of many abnormal new vessels (26, 30). In addition, GDF-15 plays a major role in regulating the recruitment of inflammatory cells by directly interfering with leukocyte integrin activation and inhibiting leukocyte arrest and extravasation on the endothelium (30). Due to the anti-inflammatory role of GDF-15 in the vascular endothelial cell, the plasma levels of GDF-15 tend to rise with the progress of microvascular injury (31).
Alternatively, as a member of the TGF-β family, GDF-15 may signal through an alternate, non-TGF-β receptor mediated mechanism instead of the classical TGF-β signaling pathway through Smad phosphorylation (32–34). It may also play an important role in angiogenesis (34). GDF-15 impairs in vitro angiogenesis by blocking connective tissue growth factor 2 (CCN-2)-mediated tube formation in human umbilical vein endothelial (HUVEC) cells as well as inhibiting the CCN-2-dependent activation of focal adhesion kinase and subsequent decrease in αVβ3 integrin clustering. In the same vein, GDF-15, in combination with BMP-2, has been shown to mediate the inhibition of fenretidine-dependent tumor vessel growth by interfering with endothelial cell growth, migration, and invasion (35). There have been reports that angiogenesis in hypoxic HUVEC cells is promoted by a signaling pathway, which includes hypoxia-inducible factor 1-alpha (HIF-1alpha), VEGF, and p53 (36). Moreover, GDF-15 regulates endothelial cells through altered endothelial caveolar signaling (37). Increased circulating levels of GDF-15 have been attributed, inter alia, to endothelial dysfunction (38). It is possible that the increase in the plasma levels of GDF-15 in the patients with diabetes may cause a counter-regulatory and compensatory mechanism which protects against angiogenesis, but not sufficient to protect against DR. Interestingly, consistent with our hypothesis, we observed that the incidence of DR increased with the plasma GDF-15 quartiles in this study.
This study establishes the association between DR and GDF-15 in type 2 diabetes. There are several limitations to this study. First, due to the cross-sectional nature of the present study, we could not determine whether GDF-15 plays a causal role in the pathogenesis of DR. Accordingly, prospective studies in the future are of vital importance. Second, plasma GDF-15 levels may vary among different populations, and it is unclear how local and ocular factors can influence DR. Third, the sample size was relatively small. Further studies are also required to determine the GDF-15 levels in a larger population. Moreover, VEGF levels in patients with type 2 diabetes may be associated with the development of DR. However, due to the limitations in the study design, we did not measure VEGF levels in the patients. Lastly, given the limited number of study participants with PDR (n = 2), we did not analyze the differences in GDF-15 levels between PDR and NPDR. Based on a previous study (39), PDR was combined with severe NPDR in our study to give the vision-threatening DR (VTDR) group. There is therefore need to investigate the differences between PDR and NPDR.
In conclusion, the results from our study suggest that there is a significant and independent association between the increased plasma levels of GDF-15 and DR. Future prospective studies with greater numbers of patients should be done to provide a link between increased circulating plasma GDF-15 concentration and the severity of DR.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Xinhua Hospital Ethics Committee Affiliated to Shanghai Jiaotong University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Conceived and designed the experiments: ZY and QS. Analyzed the data: YN, WZ, and JS. Contributed reagents/materials/analysis tools: YN, ZY, JS, YL, XL, NL, HZ, and LQ. Wrote the paper: YN, WZ, and JS. All authors contributed to the article and approved the submitted version.
This work was supported by grants from National Key R&D Program of China(2016YFC0901200, 2016YFC0901203), the Shanghai Sailing Program (18YF1415800), the Shanghai Science and Technology Commission (15411953200, 10411956600, 14ZR1427400), National Natural Science Foundation of China (81300667, 81370953, 81370935, 81670743), Shanghai Health System Outstanding Young Talents Training Program (XYQ2013098), Shanghai Education Committee Key Program (14zz110), and State Key Development Program for Basic Research of China (2012CB517501).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Yang SH, Dou KF, Song WJ. Prevalence of diabetes among men and women in China. N Engl J Med (2010) 362(25):2425–6. doi: 10.1056/NEJMc1004671
2. Abougalambou SSI, Abougalambou AS. Risk factors associated with diabetic retinopathy among type 2 diabetes patients at teaching hospital in Malaysia. Diabetes Metab Syndr (2015) 9(2):98–103. doi: 10.1016/j.dsx.2014.04.019
3. Cekić S, Cvetković T, Jovanović I, Jovanović P, Pesić M, Stanković Babić G, et al. C-reactive protein and chitinase 3-like protein 1 as biomarkers of spatial redistribution of retinal blood vessels on digital retinal photography in patients with diabetic retinopathy. Bosn J Basic Med Sci (2014) 14(3):177–84. doi: 10.17305/bjbms.2014.3.21
4. Kuo JZ, Guo X, Klein R, Klein BE, Cui J, Rotter JI, et al. Systemic soluble tumor necrosis factor receptors 1 and 2 are associated with severity of diabetic retinopathy in Hispanics. Ophthalmology (2012) 119(5):1041–6. doi: 10.1016/j.ophtha.2011.10.040
5. Roy MS, Janal MN, Crosby J, Donnelly R. Inflammatory biomarkers and progression of diabetic retinopathy in African Americans with type 1 diabetes. Invest Ophthalmol Vis Sci (2013) 54(8):5471–80. doi: 10.1167/iovs.13-12212
6. Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA (1997) 94(21):11514–9. doi: 10.1073/pnas.94.21.11514
7. Lawton LN, Bonaldo MF, Jelenc PC, Qiu L, Baumes SA, Marcelino RA, et al. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene (1997) 203(1):17–26. doi: 10.1016/S0378-1119(97)00485-X
8. Yokoyama-Kobayashi M, Saeki M, Sekine S, Kato S. Human cDNA encoding a novel TGF-beta superfamily protein highly expressed in placenta. J Biochem (1997) 122(3):622–6. doi: 10.1093/oxfordjournals.jbchem.a021798
9. Tan M, Wang Y, Guan K, Sun Y. PTGF-beta, a type beta transforming growth factor (TGF-beta) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-beta signaling pathway. Proc Natl Acad Sci USA (2000) 97(1):109–14. doi: 10.1073/pnas.97.1.109
10. Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, et al. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J Biol Chem (1998) 273(22):13760–7. doi: 10.1074/jbc.273.22.13760
11. Hromas R, Hufford M, Sutton J, Xu D, Li Y, Lu L. PLAB, a novel placental bone morphogenetic protein. Biochim Biophys Acta (1997) 1354(1):40–4. doi: 10.1016/S0167-4781(97)00122-X
12. Appierto V, Tiberio P, Villani MG, Cavadini E, Formelli F. PLAB induction in fenretinide-induced apoptosis of ovarian cancer cells occurs via a ROS-dependent mechanism involving ER stress and JNK activation. Carcinogenesis (2009) 30(5):824–31. doi: 10.1093/carcin/bgp067
13. Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci USA (2004) 101(50):17468–73. doi: 10.1073/pnas.0406142101
14. Baek SJ, Eling TE. Changes in gene expression contribute to cancer prevention by COX inhibitors. Prog Lipid Res (2006) 45(1):1–16. doi: 10.1016/j.plipres.2005.10.001
15. Li H, Dakour J, Guilbert LJ, Winkler-Lowen B, Lyall F, Morrish DW. PL74, a novel member of the transforming growth factor-beta superfamily, is overexpressed in preeclampsia and causes apoptosis in trophoblast cells. J Clin Endocrinol Metab (2005) 90(5):3045–53. doi: 10.1210/jc.2004-0808
16. Lindahl B. The story of growth differentiation factor 15: another piece of the puzzle. Clin Chem (2013) 59(11):1550–2. doi: 10.1373/clinchem.2013.212811
17. Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation (2014) 130(21):1847–58. doi: 10.1161/CIRCULATIONAHA.114.011204
18. Hellemons ME, Mazagova M, Gansevoort RT, Henning RH, de Zeeuw D, Bakker SJL, et al. Growth-differentiation factor 15 predicts worsening of albuminuria in patients with type 2 diabetes. Diabetes Care (2012) 35(11):2340–6. doi: 10.2337/dc12-0180
19. Berezin AE. Circulating Biomarkers in Heart Failure. Adv Exp Med Biol (2018) 1067:89–108. doi: 10.1007/5584_2017_140
20. Carstensen M, Herder C, Brunner EJ, Strassburger K, Tabak AG, Roden M, et al. Macrophage inhibitory cytokine-1 is increased in individuals before type 2 diabetes diagnosis but is not an independent predictor of type 2 diabetes: the Whitehall II study. Eur J Endocrinol (2010) 162(5):913–7. doi: 10.1530/EJE-09-1066
21. Chung JO, Park S-Y, Cho DH, Chung DJ, Chung MY. Relationship between plasma growth differentiation factor-15 levels and diabetic retinopathy in individuals with type 2 diabetes. Sci Rep (2020) 10(1):20568. doi: 10.1038/s41598-020-77584-z
22. Diagnosis and classification of diabetes mellitus. Diabetes Care (2013) 36 Suppl 1:S67–74. doi: 10.2337/dc13-S067
23. Ma Y-C, Zuo L, Chen J-H, Luo Q, Yu X-Q, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol (2006) 17(10):2937–44. doi: 10.1681/ASN.2006040368
24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/BF00280883
25. Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology (2003) 110(9):1677–82. doi: 10.1016/S0161-6420(03)00475-5
26. Lajer M, Jorsal A, Tarnow L, Parving H-H, Rossing P. Plasma growth differentiation factor-15 independently predicts all-cause and cardiovascular mortality as well as deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetes Care (2010) 33(7):1567–72. doi: 10.2337/dc09-2174
27. Dirani M, Xie J, Fenwick E, Benarous R, Rees G, Wong TY, et al. Are obesity and anthropometry risk factors for diabetic retinopathy? The diabetes management project. Invest Ophthalmol Vis Sci (2011) 52(7):4416–21. doi: 10.1167/iovs.11-7208
28. Frimodt-Møller M, von Scholten BJ, Reinhard H, Jacobsen PK, Hansen TW, Persson FI, et al. Growth differentiation factor-15 and fibroblast growth factor-23 are associated with mortality in type 2 diabetes - An observational follow-up study. PloS One (2018) 13(4):e0196634. doi: 10.1371/journal.pone.0196634
29. Böttner M, Suter-Crazzolara C, Schober A, Unsicker K. Expression of a novel member of the TGF-beta superfamily, growth/differentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res (1999) 297(1):103–10. doi: 10.1007/s004410051337
30. Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med (2011) 17(5):581–8. doi: 10.1038/nm.2354
31. Bonfiglio V, Platania CBM, Lazzara F, Conti F, Pizzo C, Reibaldi M, et al. TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy. Int J Mol Sci (2020) 21(24):9558. doi: 10.3390/ijms21249558
32. Heger J, Schiegnitz E, von Waldthausen D, Anwar MM, Piper HM, Euler G. Growth differentiation factor 15 acts anti-apoptotic and pro-hypertrophic in adult cardiomyocytes. J Cell Physiol (2010) 224(1):120–6. doi: 10.1002/jcp.22102
33. Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW-W, Bauskin AR, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med (2007) 13(11):1333–40. doi: 10.1038/nm1677
34. Whitson RJ, Lucia MS, Lambert JR. Growth differentiation factor-15 (GDF-15) suppresses in vitro angiogenesis through a novel interaction with connective tissue growth factor (CCN2). J Cell Biochem (2013) 114(6):1424–33. doi: 10.1002/jcb.24484
35. Ferrari N, Pfeffer U, Dell’Eva R, Ambrosini C, Noonan DM, Albini A. The transforming growth factor-beta family members bone morphogenetic protein-2 and macrophage inhibitory cytokine-1 as mediators of the antiangiogenic activity of N-(4-hydroxyphenyl)retinamide. Clin Cancer Res (2005) 11(12):4610–9. doi: 10.1158/1078-0432.CCR-04-2210
36. Song H, Yin D, Liu Z. GDF-15 promotes angiogenesis through modulating p53/HIF-1α signaling pathway in hypoxic human umbilical vein endothelial cells. Mol Biol Rep (2012) 39(4):4017–22. doi: 10.1007/s11033-011-1182-7
37. Mazagova M, Buikema H, Landheer SW, Vavrinec P, Av B, Henning RH, et al. Growth differentiation factor 15 impairs aortic contractile and relaxing function through altered caveolar signaling of the endothelium. Am J Physiol Heart Circ Physiol (2013) 304(5):H709–18. doi: 10.1152/ajpheart.00543.2012
38. Tchou I, Margeli A, Tsironi M, Skenderi K, Barnet M, Kanaka-Gantenbein C, et al. Growth-differentiation factor-15, endoglin and N-terminal pro-brain natriuretic peptide induction in athletes participating in an ultramarathon foot race. Biomarkers (2009) 14(6):418–22. doi: 10.1080/13547500903062976
Keywords: GDF-15, type 2 diabetes, mild non-proliferative DR, moderate NPDR, vision-threatening DR
Citation: Niu Y, Zhang W, Shi J, Liu Y, Zhang H, Lin N, Li X, Qin L, Yang Z and Su Q (2021) The Relationship Between Circulating Growth Differentiation Factor 15 Levels and Diabetic Retinopathy in Patients With Type 2 Diabetes. Front. Endocrinol. 12:627395. doi: 10.3389/fendo.2021.627395
Received: 09 November 2020; Accepted: 25 February 2021;
Published: 15 March 2021.
Edited by:
Khalid Siddiqui, King Saud University, Saudi ArabiaReviewed by:
Claudio Bucolo, University of Catania, ItalyCopyright © 2021 Niu, Zhang, Shi, Liu, Zhang, Lin, Li, Qin, Yang and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Yang, eWFuZ3poZW5AeGluaHVhbWVkLmNvbS5jbg==; Qing Su, c3VxaW5nQHhpbmh1YW1lZC5jb20uY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.