
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 08 March 2021
Sec. Clinical Diabetes
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.624423
This article is part of the Research Topic The Changing Panorama of Diabetes Outcomes: Novel Complications and Novelties in Classical Complication View all 9 articles
 Dana Abdelrahim1†‡
Dana Abdelrahim1†‡ MoezAlIslam E. Faris2*†‡
MoezAlIslam E. Faris2*†‡ Mohamed Hassanein3
Mohamed Hassanein3 Ayman Z. Shakir2†
Ayman Z. Shakir2† Ayesha M. Yusuf2†
Ayesha M. Yusuf2† Aljohara S. Almeneessier4†
Aljohara S. Almeneessier4† Ahmed S. BaHammam5*†
Ahmed S. BaHammam5*†Ramadan is the 9th month of the lunar calendar during which Muslims abstain from food and drink between dawn and sunset for 30 consecutive days. Ramadan fasting is observed by all healthy Muslim adults, as well many Muslims with type 2 diabetes (T2DM). Hypoglycemic events (HE) are a serious complication associated with diabetes management and are associated with increased cardiovascular disease risk. Conflicting results have been reported concerning the incidence of HE among people with T2DM observing Ramadan fasting. This review summarizes available scientific evidence on the occurrence of HE and the effects of different moderators on the incidence of HE among patients with T2DM during Ramadan. We conducted a systematic review of available observational studies and randomized controlled trials (RCTs) for patients with T2DM who fasted during Ramadan, with HE as the primary outcome. Ten databases were searched for relevant studies from inception until October 31, 2020. In total, 68 studies (35 RCTs and 33 observational studies) met the inclusion criteria. Non-sulfonylureas hypoglycemic medications showed superior effects in lowering the incidence of HE over sulfonylureas hypoglycemic medications. Variable moderators were associated with experiencing HE during Ramadan in both observational studies and RCTs, including sex, geographical location, body anthropometric indicators, season, dietary behaviors, fasting duration, time since diagnosis, and pre-fasting education. This comprehensive systematic review covered the largest number of observational and clinical studies investigating the impact of Ramadan on HE among patients with T2DM. The study highlights the significance of different moderators that influence the effect of Ramadan fasting on HE, including dietary behaviors, fasting time duration, sex, season, country, pre-fasting education, age, and time since diagnosis. The study also highlighted the impact of different hypoglycemic medications on HE and noted the superiority of non-sulfonylureas over sulfonylureas hypoglycemic medications in lowering the risk for hypoglycemia in people with T2DM during Ramadan fasting.
Ramadan is the 9th holy month of the Islamic lunar calendar. Fasting during Ramadan is an obligatory duty for all healthy adult Muslims, as stated in the Holy Quran where ALLAH says, “O you who believe, fasting is prescribed for you as it was prescribed for those before you, that you may develop God-consciousness” (Surat Al-Baqarah 2:183). However, the Holy Quran exempts those who are sick, medically unfit, or traveling from fasting during the holy month: “Yet if one among you is sick or is on a journey, [such a person shall then fast] the same number of other days” (Surat Al-Baqarah, 2:185). A majority of Muslim people with diabetes see this fast as a deeply meaningful, spiritual experience; therefore, most still participate, even against medical advice (1). Fasting during Ramadan dictates complete abstinence from food and drink (including water) from dawn to sunset. This encompasses a period of 10–21 h (2) depending on the geographical location and solar season that crosses with the lunar month and continues for 29–30 consecutive days. This abstinence also extends to medications used by patients who choose to fast during the holy month. This may require changes in the timing and possibly medication dose according to the dawn-to-sunset fasting time.
There are around 1.9 billion Muslims worldwide distributed across more than 200 countries and territories and accounting for 25% of the world’s population (3). The global diabetes prevalence in 2019 was estimated at 9.3% (463 million people) (4). Besides, the population-based Epidemiology of Diabetes and Ramadan 2001 study, which included 12,243 patients with diabetes from 13 Islamic countries, estimated that about 79% of patients with type 2 diabetes (T2DM) and about 43% of patients with type 1 diabetes (T1DM) fast during Ramadan (5). This means that about 70 million (50–90 million) people with diabetes worldwide may practice fasting during Ramadan. A retrospective observational study including 3,394 evaluable diabetes cases from 13 countries, found that 64% of patients reported fasting every day, and 94.2% fasted for at least 15 days (6). Given that many patients with diabetes observe fasting during Ramadan, glycemic control management for those patients is a critically important health challenge worldwide (7).
During the last few decades, medical doctors, endocrinologists, and diabetologists observing Muslim patients with diabetes have accumulated growing knowledge and clinical experience. Available research has considered various aspects of fasting during Ramadan. Research topics include clinical (8–10) and metabolic (11, 12) complications, the impact of patient education and pre-fasting preparation (13–19), medication adjustment and insulin dosing (14, 20, 21), and dietary/lifestyle modifications (21, 22) during Ramadan and how these changes impact the outcomes at the end of the fasting month.
Clinical management of intermittent fasting in patients with diabetes is receiving increased attention, including non-religious intermittent fasting practiced by these patients (23). This is because fasting in this population is associated with various risks, including hypoglycemia events (HE) or episodes, postural hypotension, dehydration, increased viscosity of the blood leading to diabetic ketoacidosis, and thrombosis (particularly in T1DM) (24, 25). HE is among the most serious changes patients with diabetes may face during fasting, and are the most challenging complications experienced in T1DM/T2DM management (26, 27). HE refers to an abnormally low concentration of glucose in the blood caused by excessive exercise, insufficient food intake, or overdosage with oral hypoglycemic agents (OHGAs) or insulin (28, 29). HE may lead to impairment of the counter-regulatory system, increased cardiovascular events (even death), and other detrimental effects. These harmful effects are more pronounced in severe HE. A prospective cohort study including 1,066 adults with T2DM aged 60–75 years reported the odds of suffering a macrovascular event were higher in patients with a history of severe HE (30). Blood glucose monitoring, recognition of HE risk factors, educational programs for healthcare professionals and patients with diabetes, and selection of appropriate regimens are key strategies to optimize glycemic control, prevent long-term complications, and minimize the risk for HE (31).
Compared with the preceding months, observation of Ramadan diurnal intermittent fasting (RDIF) by patients with T2DM has been associated with a 7.5-fold increased risk for severe HE (defined as requiring hospitalization) (5). This was supported by another study (32) that found that switching anti-hyperglycemic treatment from sulfonylureas (SU) agents to sitagliptin for Muslim patients with T2DM who fasted during Ramadan was associated with a 50% reduction in the risk for symptomatic HE. That study also reported that the incidence of symptomatic HE was as high as 20% during Ramadan in those with T2DM receiving SU treatment. In contrast, other studies did not report any adverse metabolic effects (including HE) among patients with diabetes observing RDIF (33, 34). The present systematic review explored current medical evidence on the prevalence of HE among patients with diabetes observing RDIF. This systematic review will guide physicians in their clinical decision-making, delivery of care, and developing policies regarding Ramadan fasting by patients with diabetes (35).
Two authors (MF and DA) conducted an electronic search of 10 databases: EBSCOhost, CINAHL, Cochrane, EMBASE, PubMed/MEDLINE, Scopus, Google Scholar, ProQuest Medical, ScienceDirect, and Web of Science from 1950 until October 31, 2020. Key search terms included: “Ramadan fasting” OR “Islamic fasting” OR “Ramadan intermittent fasting” OR “Ramadan diurnal fasting” OR “Ramadan model of intermittent fasting” OR “Intermittent prolonged fasting during Ramadan” OR “Ramadan fast” OR “Recurrent circadian fasting” AND “Hypoglycemia” OR “Hypoglycaemia” OR “Hypoglycemic events” OR “Hypoglycemic episodes” AND “Diabetes mellitus” OR “Type 2 diabetes mellitus” OR “T2DM.” Additional articles were obtained by searching relevant systematic reviews and reviews, as well as hand-searching the reference lists of relevant studies. Corresponding and other authors were contacted via email or Research Gate to obtain missing full-text articles. As necessary, authors were contacted to obtain relevant articles and reviews and ensure that all related publications were included in this review.
The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies was used to identify data sources (36). Papers were first subdivided into observational studies or clinical interventional trials involving patients with T2DM who decided to observe Ramadan fasting. The inclusion criteria for the selected observational and clinical studies were papers that: 1) were published as a primary research paper in a peer-reviewed journal; 2) included cohorts of patients with T2DM that opted to practice intermittent fasting during Ramadan; 3) reported the primary outcome of hypoglycemia or HE before, during (as the primary outcome), or after Ramadan fasting month; and 4) included male or female patients with diabetes. We included studies involving all patients with T2DM that intended to fast during Ramadan and were taking insulin or non-insulin antidiabetic agents. These agents included metformin (MTF), SU agents, meglitinides, thiazolidinediones, glucagon-like peptide (GLP)-1 receptor analogs, alpha-glucosidase inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium-glucose co-transport 2 inhibitors (SGLT2-I). The frequency of documented or symptomatic HE during Ramadan fasting was reported as the primary endpoint where available. HE or episodes were defined as patients’ subjective symptoms or documented blood glucose <70 mg/dl (3.9 mmol/L). Severe HE was defined as an episode of HE requiring third-party assistance or hospitalization (29).
Of the 3,389 studies initially retrieved, 68 studies that investigated the effects of RDIF on HE among patients with diabetes observing RDIF met the inclusion criteria. Figure 1 presents the stages of evaluation and exclusion of the identified studies. Table 1 summarizes the general characteristics of these studies. The extracted information included the year of publication, country/city, the season of the fasting month, fasting duration (hours), study design, number of participants and percentage of male participants, type of diabetes studied, age of participants, duration since diabetes diagnosis, type of intervention (fasting alone or with other medical/dietary/educational interventions), tested outcome(s), and results. All included studies used a pre-post model to report changes in HE.
Out of the 68 selected papers, there were 35 randomized controlled trials (RCTs) and 33 observational studies. Because of the nature of Ramadan month and its connection with the lunar calendar, Ramadan fell in different solar seasons in the included studies. The majority of the studies were conducted during the summer season, especially those conducted during 2010–2018. There was wide variation in the age of study participants, ranging from 17 to 94 years. Also, there was variation in the duration of fasting (9–17 h/day) associated with different geographical locations and seasons. Participants in the included studies were examined for HE and cardiometabolic risk factor outcomes.
Patients with diabetes in the included studies used different types of medications during the fasting month. This included insulin-treated patients with T2DM that used an insulin pump, multiple daily injections, insulin lispro, insulin glargine, soluble human insulin, insulin detemir (Levemir), and biphasic insulin. Different types of OHGAs were also reported, such as biguanides (MTF), SU (gliclazide, glipizide, glimepiride, glibenclamide, or glyburide), DPP-4 inhibitors (sitagliptin, vildagliptin), thiazolidinediones (pioglitazone), SGLT2-I (dapagliflozin, canagliflozin), incretin mimetics, and GLP-1 receptor agonists (lixisenatide injection, liraglutide).
The included studies were conducted across 33 countries in four continents. This included six African countries: Algeria (n=6), Egypt (n=14), Libya (n=1), Morocco (n=8), South Africa (n=3), and Tunisia (n=5). There were also studies from 19 Asian and Middle Eastern countries: Bangladesh (n=4), India (n=17), Indonesia (n=6), Malaysia (n=13), Pakistan (n=11), Singapore (n=4), Thailand (n=1), China (n=1), Iran (n=3), Iraq (n=1), Jordan (n=7), Kuwait (n=12), Lebanon (n=12), Oman (n=2), Qatar (n=3), Saudi Arabia (n=19), Turkey (n=9), Israel (n=7), and the United Arab Emirates (UAE) (n=20). There were studies from seven countries in Europe: France (n=4), Germany (n=3), Sweden (n=1), the UK (n=8), Spain (n=1), Russia (n=1), and Denmark (n=1). Finally, one study from North America was located (Canada: n=1). Eleven countries that were represented in this review were from the Middle East region (the UAE, Saudi Arabia, Jordan, Lebanon, Kuwait, Qatar, Oman, Turkey, Israel, Iraq, and Iran); these countries include about one-fifth of the global Muslim population.
In total, there were 26,706 participants in the included studies, of which 48.6% were male (n=12,988). Three studies (n=218 participants) did not mention the percentage of males in the study population, which was a major drawback in those studies. Most studies were conducted in places where Ramadan fell in summer (n=50). Nine studies were conducted in autumn, one in winter, and one in spring. Besides, seven studies considered participants in countries across different continents; therefore, they reported varied seasons. The minimum fasting duration (hours) was 8.88 h, and the maximum was 16.5 h, with average fasting hours of 13.22 h. The youngest mean age of participants was 24.5 years, and the oldest mean age was 62.1 years (age range: 17–94 years). Similarly, the shortest mean duration since diabetes diagnosis was 2.97 years, and the longest was 15 years (range: 0.3–32.9 years). Table 2 summarizes the HE outcomes reported in the experimental studies (RCTs) involving patients with T2DM observed during Ramadan fasting month. HE reported in the observational studies involving patients with T2DM observed during Ramadan fasting month are summarized in Table 3.
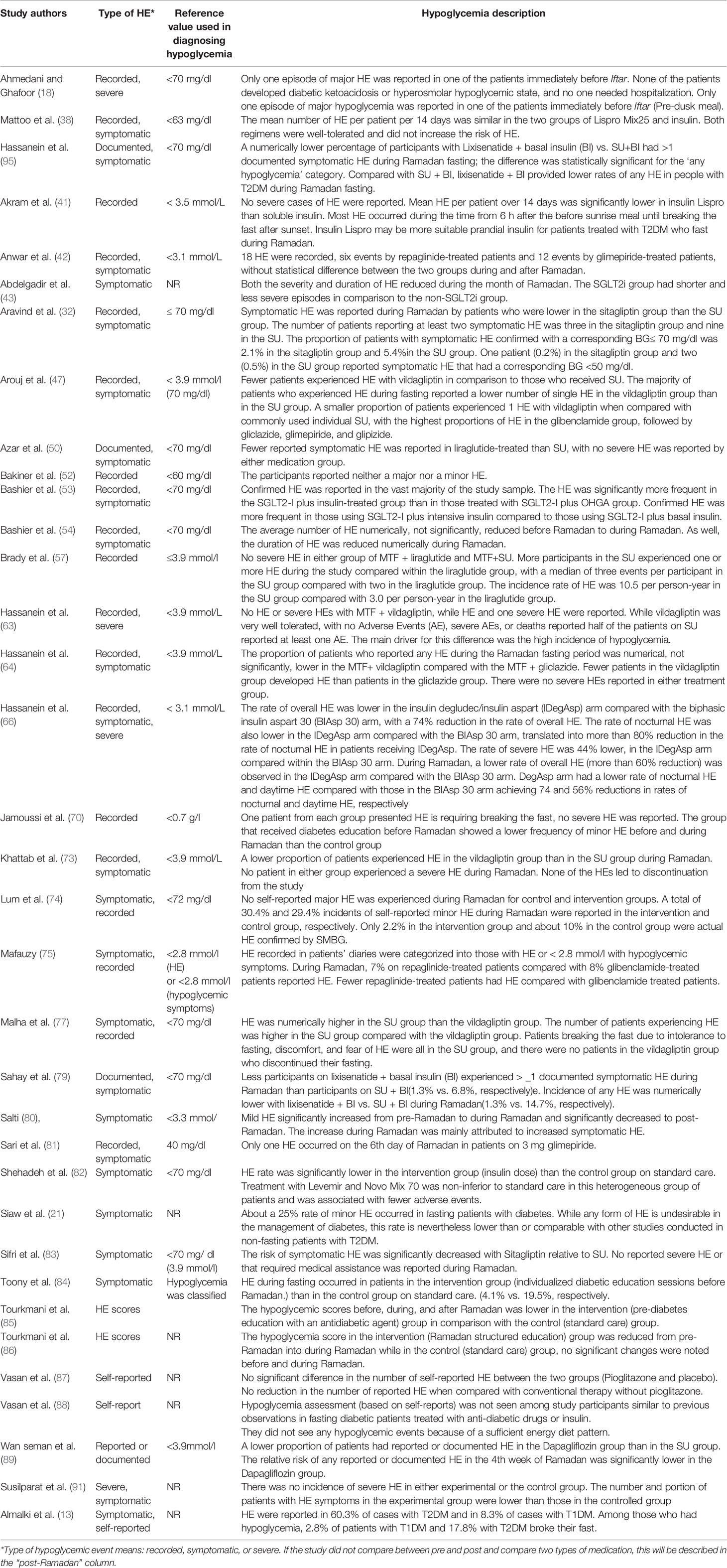
Table 2 Description of the experimental studies regarding hypoglycemia and hypoglycemic events during Ramadan fasting among patients with T2DM.
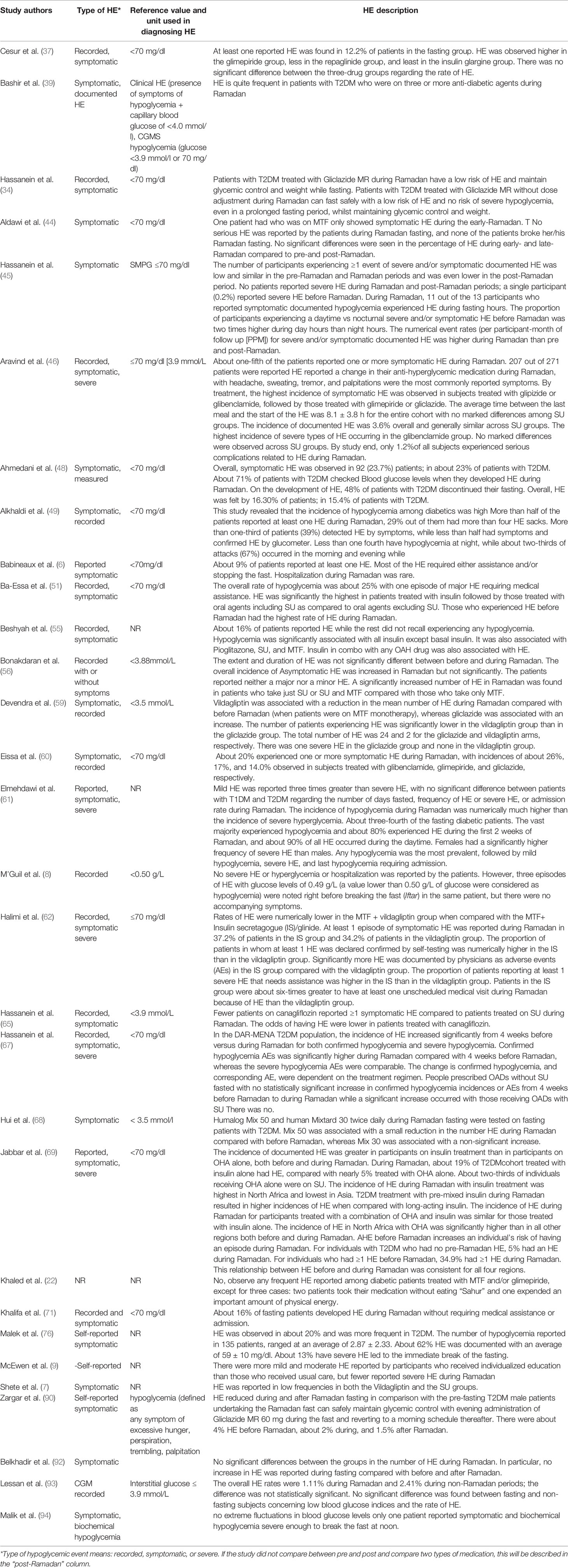
Table 3 Description of the observational studies regarding hypoglycemia and hypoglycemic events during Ramadan fasting among patients with T2DM.
The Ramadan and Diabetes Practical Guidelines (96) classifies people with diabetes that had severe hypoglycemia within the 3 months before Ramadan, a history of recurrent hypoglycemia, or a history of hypoglycemia unawareness as a very high-risk group. The guidelines, therefore, recommend that these people should not observe Ramadan fasting.
The patient’s awareness and understanding of the complication associated with fasting, including the possibility of developing HE, is highly important. Several reports have highlighted the positive effect of Ramadan-focused diabetes counseling and education for patients with diabetes before Ramadan on lowering the incidence and severity of HE during fasting (17, 84–86, 97, 98). A recent systematic review by Tourkmani and colleagues explored the impact of Ramadan-focused diabetes education on HE risk and metabolic control for patients with T2DM and highlighted the crucial impact of education and awareness (99). That review found a significant reduction in HE risk (by 81%) for fasting patients in intervention groups that received Ramadan-focused education compared with patients receiving conventional care. Furthermore, HbA1c was significantly improved among patients who received a Ramadan-focused diabetes education intervention compared with those receiving conventional care (99).
The incidence of HE before Ramadan was associated with the incidence of HE during Ramadan among fasting patients with T2DM, as revealed in the CREED study (69). This was supported by another study that reported HE before Ramadan was associated with an increased risk for experiencing HE during Ramadan (51). Among individuals with one or more HE before Ramadan, 34.9% had one or more HE during Ramadan. This relationship between HE before and during Ramadan was consistent across all studies from four regions (Middle East, Europe, North Africa, and Asia). Another factor associated with HE among fasting patients with diabetes was the use of insulin therapy. Insulin therapy is associated with HE among patients with diabetes (100), and a significant correlation was found between the occurrence of HE during Ramadan and the use of insulin therapy before and during Ramadan (69).
A key reason for the variability in reporting HE among patients with diabetes fasting during Ramadan was differences in the biochemical reference ranges used for a blood glucose level. For example, some studies used <3.1 mmol/L to assess hypoglycemia, others (i.e., the majority) used 3.9 mmol/L, and still, others used 4.4 mmol/L. Another reason for this heterogeneity was variation in defining HE. For example, some authors reported any HE, some reported only severe episodes, and others reported: “any hypoglycemia” (mild or severe) reported, documented, or biochemically measured among participants. Other possible reasons for the variability in reporting HE as the primary outcome across different studies included: variations in study design, intervention type (e.g., insulin titration, mixing different types of OHGAs, combining insulin or OHGA therapy with/without patient education about fasting), and type of dietary modifications and whether participants were adhering to healthy balanced diet during the feasting/eating night hours. Differences in the method of detecting hypoglycemia across the studies (e.g., using a diary, office visits, mobile application, or other methods) also contributed to this variation.
Variation in geographical locations and subsequent differences in the time and duration of fasting, climatic conditions, socioeconomic status, cultural/socio-cultural backgrounds, and dietary and lifestyle behaviors may all influence diabetic complications related to glycemic control during Ramadan, especially HE. For example, one study found that a lower proportion of Malaysian patients reported symptomatic HE compared with those from India (32). A multicenter study found the highest rate of symptomatic HE was reported among patients from Israel, followed by Malaysia, the UAE, India, and Saudi Arabia (46). Further, the CREED study reported patients with T2DM fasting during Ramadan that were resident in North Africa and Europe had higher odds of HE than those in the Middle East (69). That study also noted the overall incidence was significantly different (P < 0.0001) across regions, ranging from 4.1% in the Middle East to 13% in North Africa. This finding highlighted the effects of geographical location and dietary/cultural background in influencing glycemic control during Ramadan fasting. Further, the degree of strictness in practicing fasting by patients with diabetes varies among countries, which also affects the extent to which diabetic complications (including hypoglycemia) will occur. Salti and colleagues (80) found that the risk for HE during Ramadan was higher in Indonesia, Malaysia, Saudi Arabia than in Egypt, Morocco, and Tunisia.
It has been repeatedly reported that HE often occurred during the last 4–6 h before the end of fasting (43). SGLT2-I treatment showed a superior effect in lowering both the severity and duration of HE during Ramadan month, which may partly be explained by pre-Ramadan dose adjustment by treating physicians or patients’ self-titration after receiving Ramadan-focused education. The main reason behind these episodes was reported to be missing the pre-fasting pre-dawn meal (i.e., suhoor) while taking the prescribed dose of insulin (18).
Dietary intakes and eating behaviors differ among fasting people during Ramadan, especially in adherence to eating suhoor. This meal is important as it helps people with diabetes prevent anticipated hypoglycemia during fasting hours. This was reported among patients with diabetes in Malaysia, where eating suhoor reduced the risk for HE by more than two-fold compared with missing this meal (101). The length of fasting duration during Ramadan is another variable that impacted the occurrence of hypoglycemia and HE among fasting patients with diabetes during Ramadan. This is supported by repeated reports that most HE occurred in the daytime hours before iftar (sunset “breakfast” meal) among patients with diabetes observing Ramadan fasting (8, 45, 49, 51, 54, 61, 71, 102–104).
Vasan and colleagues (88) indicated that most fasting patients reported that consuming large quantities of food that yielded sufficient energy could help them sustain fasting and prevent hypoglycemia. Increased consumption of dietary fat, especially saturated fats, has been reported among Muslims during fasting periods. Although binge eating of high-calorie food is contrary to prophetic guidance and religious preaching, the main concern among fasting patients with diabetes was an interruption of continuous 30-day fasting due to hypoglycemia (88). Norouzy et al. highlighted the significance of dietary management in mitigating and ameliorating metabolic abnormalities during Ramadan fasting by patients with diabetes (78). That study found that deterioration in glycemic control during Ramadan was less prevalent among diet-controlled patients than those who used OHGAs (78).
The lack of proper education before Ramadan fasting may lead to adverse metabolic complications among fasting patients with diabetes. A qualitative study showed that some fasting patients experienced daily hypoglycemia in the afternoon because they had not adjusted their treatment schedule adequately [102]. That study also reported that other patients continued to inject short-acting insulin analogs at noon during fasting because they had not been told to stop their treatment or had not been made aware of the risk for hypoglycemia associated with not stopping. In addition, one patient erroneously doubled his SU dosage at noon assuming that would improve his glycemic control during night hours (102). Skipping the pre-dawn meal (suhoor) and taking SU medication before sunrise exposed fasting patients to a high risk for hypoglycemia. Furthermore, many patients insisted on not taking anything orally despite daytime hypoglycemia and did not seek any medical care if general malaise occurred as they did not wish to break their fast (102).
Body anthropometrics for fasting people with diabetes may affect the risk of developing HE during Ramadan. For example, Salti et al. (80) found that lower weight and smaller waist circumference were associated with increased risk for HE among fasting people during Ramadan. This could be explained by the fact that thin people have fewer glycogen stores than obese people, as obesity is associated with higher glycogen synthase enzyme expression and glycogen stores in adipose tissue (105).
Sex was a variable that was expected to affect the occurrence of hypoglycemia and HE among fasting people with diabetes during Ramadan month. One study reported that females had a significantly higher frequency of severe HE and a lower mean number of fasting days than males during Ramadan month (61). This was supported by Masood et al. (97), who found that females with diabetes experienced hypoglycemia at a higher rate than males during their Ramadan fasting. This sex-based variation in hypoglycemia and HE could be attributable to differing habitual daily activities and routine work performed by males and females during Ramadan. For example, females (especially those taking care of their families) may spend more time and effort in housekeeping and working or child care, cooking, and worshipping, whereas males tend to spend more time in relaxation and sleeping during fasting days (106).
The duration since diabetes diagnosis is another factor that contributes to differences in hypoglycemia and subsequent HE among people with diabetes fasting during Ramadan. Early reports by Khalifa et al. (71) indicated that patients with a longer duration of diabetes had significantly higher frequencies of hypoglycemia during Ramadan (107). That study found that diabetes complications, including hypoglycemia, were significantly more represented among patients who had diabetes for more than 10 years compared with those that had diabetes for less than 10 years (32.2% vs. 12.1%) (107). This could be explained by reduced attention to the disease and its complications over time decreased effectiveness of medications used over a long time, or other social or socioeconomic factors affecting glycemic control among patients with diabetes.
It is noteworthy that the majority of the identified studies were conducted during the summer season (studies published from 2010 to 2018). During summer, the duration of fasting is 12–21 h/day; these longer daytime hours mean that fasting during the summer season is expected to be associated with more HE than other seasons. Therefore, the season should be considered by patients when choosing to fast and by doctors in managing the patient’s blood sugar. Further, this seasonal variation in Ramadan fasting could be a subject for meta-regression and subgroup analysis. Such research would complement previous systematic reviews with meta- and subgroup-analyses that explored the impact of fasting duration as a significant moderator for the effect of RDIF on body weight and metabolic syndrome components in healthy people (108, 109).
The included studies suggested that SU treatment was an inferior option in terms of the ability to maintain glycemic control and minimize the risk for hypoglycemia and subsequent HE during Ramadan fasting among patients with diabetes. SU agents have previously been associated with a higher risk for HE during Ramadan fasting than other OHGAs in patients with T2DM (32, 42, 50, 56, 65, 73, 83, 110). Further, SU use has been connected with patients breaking their fast because of intolerance to fasting, discomfort, and fear of hypoglycemia, which was not seen in patients using other OHGAs (e.g., vildagliptin) (77). However, one study (44) did not associate SU use (including gliclazide) with higher rates of HE compared with DPP-4 inhibitors as reported in some observational studies (64, 111). This could be attributed to the provision of individualized patient advice and education about dose adjustments used in these studies. Besides, another study supported the safety and effectiveness of SU treatment in lowering the risk for HE among adult males with T2DM. Zargar and colleagues (90) reported male patients under glycemic control with gliclazide MR 60 mg monotherapy could safely maintain the same degree of control by switching to an evening dose schedule during Ramadan, with a low rate of HE reported among these patients.
Conversely, other OHGAs showed superior effects, including MTF, SGLT2-I (that inhibit renal glucose reabsorption and increase renal glucose excretion), and repaglinide (short-acting insulin secretagogue that acts by binding to β-cells in the pancreas to stimulate insulin release). A recent expert panel statement on the use of SGL2-I highlighted the significance of the use of these hypoglycemic agents by patients with diabetes observing Ramadan. The expert panel discussed the efficacy and safety of SGLT2-I based on outcomes of recent clinical trials with SGLT2-I across the Middle East and Africa (MEA) region during Ramadan, with emphasis on hypoglycemia as a serious complication during Ramadan fasting. This statement aided physicians in the MEA region (which includes about one-fifth of the world’s Muslim population) regarding appropriate decision-making for patients during Ramadan (112). Repaglinide was found to effectively reduce the frequency of HE among patients during Ramadan fasting (81).
MTF is the preferred agent for managing patients with T2DM. MTF is associated with a 1%–2% reduction in HbA1c and carries a low risk for HE. Therefore, MTF is an attractive therapy for the majority of patients who decide to undergo fasting (113). If a patient is taking MTF three times daily, the midday dose may be omitted, and a larger dose may be taken in the evening (113–115). Those on a twice-daily regimen do not need to change administration times. However, patients who experience gastrointestinal side effects or HE symptoms should have their dose reduced. Overall, MTF has a low (but not negligible) risk for promoting HE (2).
One study noted that the highest incidence of symptomatic HE during Ramadan was observed in T2DM patients treated with glipizide or glibenclamide, followed by those treated with glimepiride or gliclazide, with wide variation in the incidence of HE among those using different SU agents during Ramadan fasting (46). Further, the total number of breaking-the-fast events reported was highest among patients with T2DM treated with glibenclamide, with the highest incidence of severe HE (requiring medical assistance or not) occurring in this group (46). This finding contradicts the previous report by Belkhadir et al. (92), who found that glibenclamide was safe and effective in preventing hypoglycemia for patients with T2DM who fasted during Ramadan. Another study reported that the proportion of patients with at least one symptomatic HE was highest in the glimepiride group, followed by the glibenclamide group, and lastly, the gliclazide group treated with SU during Ramadan fasting (32). Similar findings were also reported for patients with T2DM fasting during Ramadan, where fewer patients on vildagliptin experienced one or more HE compared with commonly used individual SU agents (47). The highest proportion of HE was in the glibenclamide group, followed by gliclazide, glimepiride, and glipizide (47). Another study found that MTF with vildagliptin was efficient in lowering the incidence of hypoglycemia and severe HE among patients with diabetes treated with MTF and SU glinide (62) or MTF and SU gliclazide (59, 63).
The positive effect of vildagliptin in lowering HE is a known characteristic of this medication, with hypoglycemia rarely observed in clinical trials among patients with diabetes (116). The mechanism of sustained glucagon secretion during hypoglycemia by vildagliptin could be attributed to the ability of gastric inhibitory polypeptide triggered by vildagliptin that stimulates glucagon secretion during hypoglycemia (117). Dapagliflozin and liraglutide showed a superior effect in lowering HE, especially when combined with MTF compared with SU and MTF (57, 89). Further, the superiority of DPP-4 inhibitors (sitagliptin and vildagliptin) in lowering the HE was apparent among fasting patients with T2DM compared with those receiving SU treatment (7, 32, 47, 60, 73, 77). Similarly, liraglutide showed superiority in lowering HE among fasting patients with T2DM compared with SU treatment during Ramadan, suggesting liraglutide is a safe, effective, and well-tolerated choice when used as glucose-lowering therapy in patients with T2DM who choose to fast during Ramadan (50).
Vildagliptin is an attractive treatment option for patients with T2DM who are fasting during Ramadan (7). Vildagliptin can improve glycemic control by inducing glucose-dependent insulin secretion through inhibiting the DPP-4 enzyme, thereby enhancing the sensitivity and responsiveness of β- and α-cells to glucose (116, 118). The ability of vildagliptin to lower the risk for HE may be because suppression of both glucagon secretion and meal-dependent insulin secretion is glucose-dependent (119, 120). This favorable outcome observed during fasting is attributable to the suppression of inappropriate glucagon secretion during hyperglycemia and the enhancement of the glucose-dependent insulinotropic polypeptide (GIP)-mediated effect on glucagon, which results in a protection against hypoglycemia. The levels of both GLP-1 and GIP remain high during the inter-meal and overnight periods when hypoglycemia is more likely to occur (73). Sitagliptin, another DPP-4 inhibitor, also showed a superior effect in lowering symptomatic HE during Ramadan compared with SU agents in Muslim patients with T2DM (83).
The protective effect of canagliflozin (an SGL2-I) against HE among fasting patients with diabetes was further elaborated in the Canagliflozin in Ramadan Tolerance Observational Study. That study evaluated the tolerability of canagliflozin combined with MTF with or without DPP-4 inhibitors for the treatment of T2DM in patients fasting during Ramadan. The results showed that during Ramadan, patients on canagliflozin experienced fewer symptomatic HE compared with the SU group. Adjustment for medication was not needed for those on canagliflozin, whereas about 9% of those treated with SU adjusted their medication dose near the beginning of Ramadan (65).
Patients with T2DM treated with insulin showed variable hypoglycemia outcomes during their Ramadan fasting. While hypoglycemia may be predicted by insulin therapy (55), different reports indicated that combining basal insulin with incretin mimetics (which act like GLP-1 receptor agonists) such as lixisenatide injection, showed better glycemic control and lower frequency and severity of hypoglycemia than combining insulin therapy with SU (79, 95). However, combining insulin glargine with the SU glimepiride was helpful in patients with T2DM who wished to fast during Ramadan, provided glimepiride was given at the time of breaking the fast and insulin glargine titrated to provide FBG >6.7 mmol/L (80). However, both rapid and long-acting insulin were significantly associated with hypoglycemic attacks (49), with HE highest in patients treated with insulin, followed by those treated with OHGAs with SU when compared with OHGAs without SU (51). A comparison between intensive insulin and basal insulin showed that the average number of HE was significantly higher in intensive insulin therapy than basal insulin therapy (54).
It is not recommended for patients with poorly controlled T2DM to fast because of the risk for dehydration associated with hyperglycemia. Clinical experience supports that patients with diabetes controlled by diet alone can safely fast. This is because fasting, especially the intermittent pattern followed during Ramadan, has many beneficial effects including metabolic and glucoregulatory mechanisms. Patients treated with OHGAs may fast if allowed by their physician; however, their regimen will need to be adjusted to reflect lifestyle changes. MTF and thiazolidinediones are the preferred hypoglycemic drugs because they are associated with a lower risk for HE compared with insulin secretagogues. Short-acting insulin secretagogues are preferable to SU. Insulin-treated patients may be treated similarly to patients with T1DM using a basal-bolus method or other regimens as recommended by their physician. Close follow-up and frequent monitoring are essential. Attention should be paid to the sunset fast-breaking meal, particularly in excessive intake of traditional sweets and sweetened drinks, as this can lead to uncontrolled postprandial hyperglycemia (28).
The variation in HE outcomes at the end of Ramadan could be attributed to various influencing factors that act simultaneously on shaping the effect of RDIF on HE and the related cardiometabolic risk factors. These factors could be classified into internal (patient-centered) factors and external (Ramadan month-related) factors. Among the internal factors, patients’ age, sex, time since diabetes diagnosis, pre-fasting control of blood sugar, commitment to the prescribed medications before and during Ramadan month, and adherence to prescribed diet and lifestyle modifications before Ramadan month. The external factors include fasting duration, climate conditions, working conditions, social activities, practicing religious rituals of Ramadan month, and the quantity and quality of foods consumed during the night hours (especially the pre-fasting meal or suhoor), with emphasis on the last time this meal was consumed before starting fasting. Other lifestyle changes accompanying Ramadan fasting month are thought to impact the extent to which RDIF affects cardiometabolic risk factors, including changes in sleep pattern/duration, diurnal exercise, changes in work schedule, nocturnal activities, and nocturnal light exposure (121). Further, the discrepancies in reported results concerning the effect of RDIF on HE could also be attributable to differing experimental and study designs. For example, RCTs allow control of confounding factors and minimize the risk of bias, therefore giving more robust and accurate measures for the targeted outcomes. However, observational designs, owing to their inherent limitations do not have such controls, which may result in less accurate outcome estimations (122).
During Ramadan, medications used by patients with diabetes may have to be adjusted. Patients who observe Ramadan fasting have to consider potential diabetic complications such as hyperglycemia, HE, dehydration, and ketoacidosis. Risk factors associated with fasting include the presence of co-morbidities and diabetic complications, advanced age, frailty, the risk for HE or a history of impaired HE awareness, living alone, T1DM, and pregnancy (2). Certain classes of glucose-lowering medications are associated with an increased risk for HE, such as SU agents and insulin. Besides, the largest dose of these medications should be taken in the evening, along with the large meal that is usually consumed at that time (iftar).
SU and insulin secretagogues are widely used during Ramadan. However, recent studies have highlighted an increased risk for HE during fasting among patients treated with insulin secretagogues (32, 42, 110). The risk for HE increases exponentially in older patients and patients with renal failure and medical conditions treated with SU (113). Long-acting agents such as glibenclamide (glyburide) should be avoided. The morning dose of shorter-acting preparations such as glipizide or gliclazide can be halved, and the evening dose kept the same. One study tried switching the timing of SU administration from morning to evening and found no effect on the rate of HE. A five-country observational study on SU treatment during Ramadan reported a 20% prevalence of HE. SU agents have a moderate to high risk for HE as they promote insulin secretion that is not glucose-dependent.
The variable effect of RDIF on the occurrence of HE among patients with diabetes could also be attributed to the different types of medications used in the management of diabetic patients fasting during Ramadan. Vildagliptin and gliclazide are among the commonly used medications that were effective in lowering the occurrence of HE among patients with T2DM fasting during Ramadan (64). Vildagliptin is an oral anti-hyperglycemic agent of the DPP-4 inhibitor class of drugs used for adults with T2DM. Vildagliptin enhances pancreatic islet cell responsiveness to glucose (123). Vildagliptin is a combination tablet containing MTF, whereas gliclazide is a SU insulin secretagogue that helps the pancreas make more insulin. The superiority of vildagliptin over other OHGAs was reviewed by Aziz (124), who found that in comparison with the OHGAs and SU agents (which carry a higher and significant risk for HE), DPP-4 inhibitors (e.g., vildagliptin) demonstrated a lower risk for HE during Ramadan fasting, with better patient compliance. This effect is supported by findings from many clinical observational and clinical studies in different locations around the world.
Decreased food intake is a well-known risk factor for HE among patients with diabetes (125). There are no reliable estimates concerning the contribution of HE to mortality in T2DM; however, it is thought that HE is an infrequent cause of death in this population (125). Rates of HE are lower in patients with T2DM when compared with T1DM (5), and are even lower in patients with T2DM treated with oral agents (5). Loke and colleagues investigated the effect of various risk factors on HE in patients with diabetes who fasted during Ramadan, and reported the rate of HE was 1.6 times higher during fasting compared with non-fasting periods (101). They observed that good metabolic control (<8%) and old age (>60 years) increased the risk ratio more than twice, and eating a meal before fasting reduced the risk ratio to less than half. However, the difference in that study was smaller than indicated in the EPIDAR study (5), which showed that fasting during Ramadan increased the risk for severe HE (defined as hospitalization due to HE) by 4.7-fold among patients with T1DM (from 3 to 14 events/100 people/month) and 7.5-fold in patients with T2DM (from 0.4 to 3 events/100 people/month). Severe HE was more frequent in patients in whom the OHGA or insulin dosage was changed and in those who reported significant lifestyle changes (5, 125).
About 1.5 billion Muslims globally are expected to observe Ramadan and refrain from food and drink from dawn to sunset. Even with the relatively large number of studies investigating RDIF and diabetes, the impact of RDIF on these patients is not fully understood. In particular, the effect of fasting during Ramadan on rates of HE in patients with diabetes is not known with certainty. There are a limited number of studies in this area, which warrants further longitudinal, controlled clinical studies. To our knowledge, this is the first systematic review exploring the impacts of RDIF on HE and related cardiometabolic risk factors in patients with diabetes. Although this systematic review could not reach definite conclusions because of conflicting findings and lack of control for confounding factors, we found no evidence suggesting a negative impact of observing RDIF on HE among patients with diabetes or other overt harms. Nonetheless, the authors emphasize the need to conduct further studies using larger datasets and controlled for more confounders, including meta-analyses of obtained data, and adjusted for different covariates using sub-group analyses.
The present review did not conduct a meta-analysis for the outcome of using different OHGA and insulin therapies in preventing HE during Ramadan. Nonetheless, the present findings regarding the positive effect of non-SU hypoglycemic medications during Ramadan (e.g., DPP-4 inhibitors) in lowering the incidence of HE among fasting people with diabetes mirrors the findings of a previous systematic review and network meta-analysis (126). That meta-analysis showed that DPP-4 inhibitors were associated with reducing the development of HE during Ramadan in both observational and experimental (RCT) studies. Further, the findings highlighted the importance of pre-Ramadan education as a beneficial tool in reducing HE during Ramadan.
The present systematic review entailed imitations related to the limitations of the sources studied. Differences in definitions and reporting of outcomes also made comparisons across studies difficult. Owing to the nature of Ramadan fasting, the studies were conducted in different geographical locations where daylight hours and climatic conditions differed, and fasting was observed under different conditions. The variable study designs and different demographic characteristics of study participants were further limitations that might have significantly impacted the accuracy of the conclusions from this systematic review. There were few studies with different patient subgroups and many studies used small sample sizes, which limited our ability to draw firm conclusions.
Patients with T2DM may experience safer fasting and have a lower risk for acute complications if they comply with necessary adjustments in the timing and dosage of their medication. Better outcomes may be achieved if medication changes are accompanied by appropriate dietary and lifestyle changes. Hypoglycemia and HE are preventable for patients with diabetes observing Ramadan fasting. Mounting evidence suggests that combining MTF with OHGAs (e.g., a DPP-4 inhibitor and SGLT2-I with less reliance on SU) showed inferior effects in preventing HE among patients with diabetes that decided to fast during Ramadan. Ramadan fasting does not necessarily increase the burden of acute complications and cardiometabolic deteriorations unless the fasting patient does not comply with established medical and dietary guidelines for fasting during Ramadan. Patients with diabetes who decide to fast during Ramadan should be counseled regarding the importance of strict adherence to the adjusted dose and timing of their medication, adhere to healthy diet and lifestyle behaviors during Ramadan fasting, and be closely clinically monitored.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Conceptualization: MF and AB. Methodology: MF. Validation: MF and DA. Investigation: MF and DA. Resources: MF. Data curation: MF, DA, AY, and AS. Writing – original draft: MF, DA, and AB. Writing – review and editing: AB, AA, and MH. Visualization: MF, AB, AA, and MH. Supervision: MF. Project administration: MF and AB. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number ISKSURP-340.
HE, hypoglycemic event; MTF, metformin; OHGAs, oral hypoglycemic agents; RDIF, Ramadan diurnal intermittent fasting; SGLT2-I, sodium-glucose co-transporter inhibitors; SU, sulfonylureas; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
1. Hassanein M, Al-Arouj M, Ben-Nakhi A, Jabbar A, Hanif W, Al-Madani A. Diabetes and Ramadan: practical guidelines International Diabetes Federation (IDF) in collaboration with the Diabetes and Ramadan (DAR) International Alliance. Diabetes Res Clin Practice (2016) 126:303–16. doi: 10.1016/j.diabres.2017.03.003
2. Gilani A, Davies M, Khunti K. Religious fasting, Ramadan and hypoglycemia in people with diabetes. Diabetic Hypoglycemia (2014) 7(1):15–9.
4. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
5. Salti I, Bénard E, Detournay B, Bianchi-Biscay M, Le Brigand C, Voinet C, et al. A population-based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care (2004) 27(10):2306–11. doi: 10.2337/diacare.27.10.2306
6. Babineaux SM, Toaima D, Boye KS, Zagar A, Tahbaz A, Jabbar A, et al. Multi-country retrospective observational study of the management and outcomes of patients with Type 2 diabetes during Ramadan in 2010 (CREED). Diabetic Med (2015) 32(6):819–28. doi: 10.1111/dme.12685
7. Shete A, Shaikh A, Nayeem KJ, Rodrigues L, Ali MSS, Shah P, et al. Vildagliptin vs sulfonylurea in Indian Muslim diabetes patients fasting during Ramadan. World J Diabetes (2013) 4(6):358–64. doi: 10.4239/wjd.v4.i6.358
8. M’guil M, Ragala M, El Guessabi L, Fellat S, Chraibi A, Chebraoui L, et al. Is Ramadan fasting safe in type 2 diabetic patients in view of the lack of significant effect of fasting on clinical and biochemical parameters, blood pressure, and glycemic control? Clin Exp hypertension (2008) 30(5):339–57. doi: 10.1080/10641960802272442
9. McEwen LN, Ibrahim M, Ali NM, Assaad-Khalil SH, Tantawi HR, Nasr G, et al. Impact of an individualized type 2 diabetes education program on clinical outcomes during Ramadan. BMJ Open Diabetes Res Care (2015) 3(1):e000111. doi: 10.1136/bmjdrc-2015-000111
10. Sadikot S, Jothydev K, Zargar AH, Ahmad J, Arvind SR, Saboo B. Clinical practice points for diabetes management during RAMADAN fast. Diabetes Metab Syndrome: Clin Res Rev (2017) 11:S811–S9. doi: 10.1016/j.dsx.2017.06.003
11. Laajam M. Ramadan fasting and non-insulin-dependent diabetes: effect on metabolic control. East Afr Med J (1990) 67(10):732–6.
12. Benaji B, Mounib N, Roky R, Aadil N, Houti I, Moussamih S, et al. Diabetes and Ramadan: review of the literature. Diabetes Res Clin Pract (2006) 73(2):117–25. doi: 10.1016/j.diabres.2005.10.028
13. Almalki MH, Hussen I, Khan SA, Almaghamsi A, Alshahrani F. Assessment of Ramadan education and knowledge among diabetic patients. Clin Med Insights: Endocrinol Diabetes (2018) 11:1179551417751611. doi: 10.1177/1179551417751611
14. Hassanein M, Abdelgadir E, Bashier A, Rashid F, Al Saeed M, Khalifa A, et al. The role of optimum diabetes care in form of Ramadan focused diabetes education, flash glucose monitoring system and pre-Ramadan dose adjustments in the safety of Ramadan fasting in high risk patients with diabetes. Diabetes Res Clin Pract (2019) 150:288–95. doi: 10.1016/j.diabres.2018.12.013
15. Zainudin SB, Abu Bakar KNB, Abdullah SB, Hussain AB. Diabetes education and medication adjustment in Ramadan (DEAR) program prepares for self-management during fasting with tele-health support from pre-Ramadan to post-Ramadan. Ther Adv Endocrinol Metab (2018) 9(8):231–40. doi: 10.1177/2042018818781669
16. Mustafa HE, Hashim T, Beshyah SA, Amin R, Eissa R, Tommy M, et al. The effect of “Targeted Diabetes Education” on glycemic control during Ramadan fasting. Ibnosina J Med Biomed Sci (2012) 4(6):242–8. doi: 10.4103/1947-489X.210781
17. Bravis V, Hui E, Salih S, Mehar S, Hassanein M, Devendra D. Ramadan Education and Awareness in Diabetes (READ) programme for Muslims with Type 2 diabetes who fast during Ramadan. Diabetic Med (2010) 27(3):327–31. doi: 10.1111/j.1464-5491.2010.02948.x
18. Ahmedani MY, Ghafoor E. Achieving safer Ramadan fasting by keeping flexible glycemic targets during the day and tighter targets during the night in insulin treated people with type 2 diabetes. Diabetes Res Clin Pract (2020) 165:108234. doi: 10.1016/j.diabres.2020.108234
19. Gad H, Al-Muhannadi H, Purra H, Mussleman P, Malik RA. The effect of Ramadan focused education on patients with Type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract (2020) 162:108–22. doi: 10.1016/j.diabres.2020.108122
20. Kobeissy A, Zantout MS, Azar ST. Suggested insulin regimens for patients with type 1 diabetes mellitus who wish to fast during the month of Ramadan. Clin Ther (2008) 30(8):1408–15. doi: 10.1016/j.clinthera.2008.08.007
21. Siaw MYL, Chew DEK, Dalan R, Abdul Shakoor SAKK, Othman N, Choo CH, et al. Evaluating the effect of Ramadan fasting on Muslim patients with diabetes in relation to use of medication and lifestyle patterns: a prospective study. Int J Endocrinol (2014) 2014:308546. doi: 10.1155/2014/308546
22. Khaled BM, Belbraouet S. Effect of Ramadan fasting on anthropometric parameters and food consumption in 276 type 2 diabetic obese women. Int J Diabetes Dev Ctries (2009) 29(2):62–8. doi: 10.4103/0973-3930.53122
23. Grajower MM, Horne BD. Clinical management of intermittent fasting in patients with diabetes mellitus. Nutrients (2019) 11(4):873. doi: 10.3390/nu11040873
24. Pakkir Maideen NM, Jumale A, Alatrash JI. Management of diabetes mellitus in Islamic fasting. J Nutrition Fasting Health (2019) 7(1):26–36. doi: 10.22038/jnfh.2019.37514.1164
25. Darko N, Dallosso H, Hadjiconstantinou M, Hulley K, Khunti K, Davies M. Qualitative evaluation of A Safer Ramadan, a structured education programme that addresses the safer observance of Ramadan for Muslims with Type 2 diabetes. Diabetes Res Clin Pract (2020) 160:107979. doi: 10.1016/j.diabres.2019.107979
26. Cryer P. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia (2002) 45(7):937–48. doi: 10.1007/s00125-002-0822-9
27. Cryer PE. Hypoglycemia in type 1 diabetes mellitus. Endocrinol Metab Clin North Am (2010) 39(3):641–54. doi: 10.1016/j.ecl.2010.05.003
28. WHO. Regional Office for the Eastern Mediterranean. Guidelines for the prevention, management and care of diabetes mellitus (2006). Available at: https://apps.who.int/iris/handle/10665/119799.
29. Tourkmani AM, Alharbi TJ, Rsheed AMB, AlRasheed AN, AlBattal SM, Abdelhay O, et al. Hypoglycemia in Type 2 Diabetes Mellitus patients: A review article. Diabetes Metab Syndrome: Clin Res Rev (2018) 12(5):791–4. doi: 10.1016/j.dsx.2018.04.004
30. Bedenis R, Price AH, Robertson CM, Morling JR, Frier BM, Strachan MWJ, et al. Association Between Severe Hypoglycemia, Adverse Macrovascular Events, and Inflammation in the Edinburgh Type 2 Diabetes Study. Diabetes Care (2014) 37(12):3301–8. doi: 10.2337/dc14-0908
31. Shafiee G, Mohajeri-Tehrani M, Pajouhi M, Larijani B. The importance of hypoglycemia in diabetic patients. J Diabetes Metab Disord (2012) 11(1):17. doi: 10.1186/2251-6581-11-17
32. Aravind S, Ismail SB, Balamurugan R, Gupta JB, Wadhwa T, Loh SM, et al. Hypoglycemia in patients with type 2 diabetes from India and Malaysia treated with sitagliptin or a sulfonylurea during Ramadan: a randomized, pragmatic study. Curr Med Res Opin (2012) 28(8):1289–96. doi: 10.1185/03007995.2012.707119
33. Hawli YM, Zantout MS, Azar ST. Adjusting the basal insulin regimen of patients with type 1 diabetes mellitus receiving insulin pump therapy during the Ramadan fast: a case series in adolescents and adults. Curr Ther Res (2009) 70(1):29–34. doi: 10.1016/j.curtheres.2009.02.001
34. Hassanein M, Al Sifri S, Shaikh S, Raza SA, Akram J, Pranoto A, et al. A real-world study in patients with type 2 diabetes mellitus treated with gliclazide modified-release during fasting: DIA-RAMADAN. Diabetes Res Clin Pract (2020) 163:108154. doi: 10.1016/j.diabres.2020.108154
35. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol (2018) 18(1):1–7. doi: 10.1186/s12874-018-0611-x
36. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol (2009) 62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006
37. Cesur M, Corapcioglu D, Gursoy A, Gonen S, Ozduman M, Emral R, et al. A comparison of glycemic effects of glimepiride, repaglinide, and insulin glargine in type 2 diabetes mellitus during Ramadan fasting. Diabetes Res Clin Pract (2007) 75(2):141–7. doi: 10.1016/j.diabres.2006.05.012
38. Mattoo V, Milicevic Z, Malone J, Schwarzenhofer M, Ekangaki A, Levitt L, et al. A comparison of insulin lispro Mix25™ and human insulin 30/70 in the treatment of Type 2 diabetes during Ramadan. Diabetes Res Clin Pract (2003) 59(2):137–43. doi: 10.1016/S0168-8227(02)00202-4
39. Bashir M, Elhadd T, Ali H, Baagar K, Hakam IAA, Al-Mohanadi DH, et al. A pilot study using flash continuous glucose monitoring in patients with type-2 diabetes on multiple anti-diabetic agents during Ramadan. Diabetes Metab Syndrome: Clin Res Rev (2018) 12(6):965–8. doi: 10.1016/j.dsx.2018.06.005
40. Hassanein M, Rashid F, Elsayed M, Basheir A, Al Saeed M, Abdelgadir E, et al. Assessment of risk of fasting during Ramadan under optimal diabetes care, in high-risk patients with diabetes and coronary heart disease through the use of FreeStyle Libre flash continuous glucose monitor (FSL-CGMS). Diabetes Res Clin Pract (2019) 150:308–14. doi: 10.1016/j.diabres.2019.01.038
41. Akram J, De Verga V, Group TRS. Insulin lispro (Lys (B28), Pro (B29)) in the treatment of diabetes during the fasting month of Ramadan. Diabetic Med (1999) 16(10):867–74. doi: 10.1046/j.1464-5491.1999.00164.x
42. Anwar A, Azmi K, Hamidon B, Khalid B. An open label comparative study of glimepiride versus repaglinide in type 2 diabetes mellitus Muslim subjects during the month of Ramadan. Med J Malaysia (2006) 61(1):28–35. doi: 10.1111/dme.12836
43. Abdelgadir E, Rashid F, Bashier A, Al Saeed M, Khalifa A, Alawadi F, et al. Use of flash glucose monitoring system in assessing safety of the SGLT2 inhibitors during Ramadan fasting in high risk insulin treated patients with type 2 diabetes. Diabetes Metab Syndrome: Clin Res Rev (2019) 13(5):2927–32. doi: 10.1016/j.dsx.2019.07.055
44. Aldawi N, Darwiche G, Abusnana S, Elbagir M, Elgzyri T. Initial increase in glucose variability during Ramadan fasting in non-insulin-treated patients with diabetes type 2 using continuous glucose monitoring. Libyan J Med (2018) 14(1):1535747. doi: 10.1080/19932820.2018.1535747
45. Hassanein M, Buyukbese MA, Malek R, Pilorget V, Naqvi M, Berthou B, et al. Real-world safety and effectiveness of insulin glargine 300 U/mL in participants with type 2 diabetes who fast during Ramadan: the observational ORION study. Diabetes Res Clin Pract (2020). doi: 10.1016/j.diabres.2020.108189
46. Aravind S, Tayeb KA, Ismail SB, Shehadeh N, Kaddaha G, Liu R, et al. Hypoglycaemia in sulphonylurea-treated subjects with type 2 diabetes undergoing Ramadan fasting: a five-country observational study. Curr Med Res Opin (2011) 27(6):1237–42. doi: 10.1185/03007995.2011.578245
47. Al-Arouj M, Hassoun AA, Medlej R, Pathan M, Shaltout I, Chawla MS, et al. The effect of vildagliptin relative to sulphonylureas in Muslim patients with type 2 diabetes fasting during Ramadan: the VIRTUE study. Int J Clin Pract (2013) 67(10):957–63. doi: 10.1111/ijcp.12243
48. Ahmedani MY, Alvi SFD, Fawwad A, Basit A. Implementation of Ramadan-specific diabetes management recommendations: a multi-centered prospective study from Pakistan. J Diabetes Metab Disord (2014) 13(1):37. doi: 10.1186/2251-6581-13-37
49. AlKhaldi YM, AlKhaldi AY, AlQahtani AS, Al-Shahrani BS, Meshawi EA, Albishri BM. Incidence of hypoglycemia and its risk factors among diabetics during Ramadan in Abha city, Aseer Region, KSA. J Family Med Primary Care (2019) 8(9):2793. doi: 10.4103/jfmpc.jfmpc_250_19
50. Azar S, Echtay A, Wan Bebakar W, Al Araj S, Berrah A, Omar M, et al. Efficacy and safety of liraglutide compared to sulphonylurea during R amadan in patients with type 2 diabetes (LIRA-R amadan): a randomized trial. Diabetes Obes Metab (2016) 18(10):1025–33. doi: 10.1111/dom.12733
51. Ba-Essa EM, Hassanein M, Abdulrhman S, Alkhalifa M, Alsafar Z. Attitude and safety of patients with diabetes observing the Ramadan fast. Diabetes Res Clin Pract (2019) 152:177–82. doi: 10.1016/j.diabres.2019.03.031
52. Bakiner O, Ertorer ME, Bozkirli E, Tutuncu NB, Demirag NG. Repaglinide plus single-dose insulin glargine: a safe regimen for low-risk type 2 diabetic patients who insist on fasting in Ramadan. Acta Diabetologica (2009) 46(1):63–5. doi: 10.1007/s00592-008-0062-7
53. Bashier A, Khalifa AA, Abdelgadir EI, Al Saeed MA, Al Qaysi AA, Bayati MBA, et al. Safety of sodium-glucose cotransporter 2 inhibitors (SGLT2-I) during the month of Ramadan in Muslim patients with type 2 diabetes. Oman Med J (2018) 33(2):104. doi: 10.5001/omj.2018.21
54. Bashier AM, Hussain AKB, Alawadi F, Alsayyah F, Alsaeed M, Rashid F, et al. Impact of optimum diabetes care on the safety of fasting in Ramadan in adult patients with type 2 diabetes mellitus on insulin therapy. Diabetes Res Clin Pract (2019) 150:301–7. doi: 10.1016/j.diabres.2019.01.037
55. Beshyah SA, Hassanein M, Ahmedani MY, Shaikh S, Ba-Essa EM, Megallaa MH, et al. Diabetic hypoglycaemia during Ramadan fasting: A trans-national observational real-world study. Diabetes Res Clin Pract (2019) 150:315–21. doi: 10.1016/j.diabres.2019.01.039
56. Bonakdaran S, Khajeh-Dalouie M. The effects of fasting during Ramadan on glycemic excursions detected by continuous glucose monitoring system (CGMS) in patients with type 2 diabetes. Med J Malaysia (2011) 66(5):447–50.
57. Brady E, Davies M, Gray L, Saeed M, Smith D, Hanif W, et al. A randomized controlled trial comparing the GLP-1 receptor agonist liraglutide to a sulphonylurea as add on to metformin in patients with established type 2 diabetes during Ramadan: the Treat 4 Ramadan Trial. Diabetes Obes Metab (2014) 16(6):527–36. doi: 10.1111/dom.12249
58. Dabbous Z, Bashir M, Elzouki A-N, Ahmed MS, Farouk S, Hassanien M, et al. Differential effects of gender and patient background diversity on the changes in metabolic and biophysical profiles in people with type-2 diabetes from different ethnicities who fast during Ramadan (H1439); a prospective study from Qatar. Diabetes Res Clin Pract (2019) 152:171–6. doi: 10.1016/j.diabres.2019.03.032
59. Devendra D, Gohel B, Bravis V, Hui E, Salih S, Mehar S, et al. Vildagliptin therapy and hypoglycaemia in Muslim type 2 diabetes patients during Ramadan. Int J Clin Pract (2009) 63(10):1446–50. doi: 10.1111/j.1742-1241.2009.02171.x
60. Eissa MK, Ezzat MAW, Kamal YM, El Sayed A. Safety and Efficacy of The Available Oral Anti diabetic Drugs In Treating Type II Diabetics During Ramadan Fasting In Sohag Governorate. Sohag Med J (2017) 21(3):383–90. doi: 10.21608/smj.2017.41240
61. Elmehdawi RR, Mukhtad NA, Allaghi NI, Elmajberi SJ. Fasting of Ramadan in peoples with diabetes in Benghazi, Libya: an exploratory study. Libyan J Med (2010) 5(1). doi: 10.3402/ljm.v5i0.5036
62. Halimi S, Levy M, Huet D, Quéré S, Dejager S. Experience with vildagliptin in type 2 diabetic patients fasting during Ramadan in France: insights from the VERDI study. Diabetes Ther (2013) 4(2):385–98. doi: 10.1007/s13300-013-0038-7
63. Hassanein M, Hanif W, Malik W, Kamal A, Geransar P, Lister N, et al. Comparison of the dipeptidyl peptidase-4 inhibitor vildagliptin and the sulphonylurea gliclazide in combination with metformin, in Muslim patients with type 2 diabetes mellitus fasting during Ramadan: results of the VECTOR study. Curr Med Res Opin (2011) 27(7):1367–74. doi: 10.1185/03007995.2011.579951
64. Hassanein M, Abdallah K, Schweizer A. A double-blind, randomized trial, including frequent patient–physician contacts and Ramadan-focused advice, assessing vildagliptin and gliclazide in patients with type 2 diabetes fasting during Ramadan: the STEADFAST study. Vasc Health Risk Manage (2014) 10:319. doi: 10.2147/VHRM.S64038
65. Hassanein M, Echtay A, Hassoun A, Alarouj M, Afandi B, Poladian R, et al. Tolerability of canagliflozin in patients with type 2 diabetes mellitus fasting during Ramadan: Results of the Canagliflozin in Ramadan Tolerance Observational Study (CRATOS). Int J Clin Pract (2017) 71(10):e12991. doi: 10.1111/ijcp.12991
66. Hassanein M, Echtay AS, Malek R, Omar M, Shaikh SS, Ekelund M, et al. Original paper: Efficacy and safety analysis of insulin degludec/insulin aspart compared with biphasic insulin aspart 30: A phase 3, multicentre, international, open-label, randomised, treat-to-target trial in patients with type 2 diabetes fasting during Ramadan. Diabetes Res Clin Pract (2018) 135:218–26. doi: 10.1016/j.diabres.2017.11.027
67. Hassanein M, Al Awadi FF, El Hadidy KES, Ali SS, Echtay A, Djaballah K, et al. The characteristics and pattern of care for the type 2 diabetes mellitus population in the MENA region during Ramadan: An international prospective study (DAR-MENA T2DM). Diabetes Res Clin Pract (2019) 151:275–84. doi: 10.1016/j.diabres.2019.02.020
68. Hui E, Bravis V, Salih S, Hassanein M, Devendra D. Comparison of Humalog Mix 50 with human insulin Mix 30 in type 2 diabetes patients during Ramadan. Int J Clin Pract (2010) 64(8):1095–9. doi: 10.1111/j.1742-1241.2010.02347.x
69. Jabbar A, Hassanein M, Beshyah SA, Boye KS, Yu M, Babineaux SM. CREED study: Hypoglycaemia during Ramadan in individuals with Type 2 diabetes mellitus from three continents. Diabetes Res Clin Pract (2017) 132:19–26. doi: 10.1016/j.diabres.2017.07.014
70. Jamoussi H, Othman RB, Chaabouni S, Gamoudi A, Berriche O, Mahjoub F, et al. Interest of the therapeutic education in patients with type 2 diabetes observing the fast of Ramadan. Alexandria J Med (2017) 53(1):71–5. doi: 10.1016/j.ajme.2016.01.002
71. Khalifa AA, El Rashid AO, Bashier AM. Safety and Efficacy of Liraglutide as an Add-On Therapy to Pre-Existing AntiDiabetic Regimens during Ramadan, A Prospective Observational Trial. J Diabetes Metab (2015) 6(9):2–5. doi: 10.4172/2155-6156.1000590
72. Khatib FA, Shafagoj YA. Metabolic alterations as a result of Ramadan fasting in non-insulin-dependent diabetes mellitus patients in relation to food intake. Saudi Med J (2004) 25(12):1858–63.
73. Khattab M, Mahmoud K, Shaltout I. Effect of vildagliptin versus sulfonylurea in muslim patients with type 2 diabetes fasting during Ramadan in Egypt: results from VIRTUE study. Diabetes Ther (2016) 7(3):551–60. doi: 10.1007/s13300-016-0190-y
74. Lum ZK, Khoo ZR, Toh WYS, Kamaldeen SAK, Shakoor A, Tsou KYK, et al. Efficacy and safety of use of the Fasting Algorithm for Singaporeans With Type 2 Diabetes (FAST) during Ramadan: a prospective, multicenter, randomized controlled trial. Ann Family Med (2020) 18(2):139–47. doi: 10.1370/afm.2500
75. Mafauzy M. Repaglinide versus glibenclamide treatment of Type 2 diabetes during Ramadan fasting. Diabetes Res Clin Pract (2002) 58(1):45–53. doi: 10.1016/S0168-8227(02)00104-3
76. Malek R, Hannat S, Nechadi A, Mekideche FZ, Kaabeche M. Diabetes and Ramadan: a multicenter study in Algerian population. Diabetes Res Clin Pract (2019) 150:322–30. doi: 10.1016/j.diabres.2019.02.008
77. Malha LP, Taan G, Zantout MS, Azar ST. Glycemic effects of vildagliptin in patients with type 2 diabetes before, during and after the period of fasting in Ramadan. Ther Adv Endocrinol Metab (2014) 5(1):3–9. doi: 10.1177/2042018814529062
78. Norouzy A, Mohajeri S, Shakeri S, Yari F, Sabery M, Philippou E, et al. Effect of Ramadan fasting on glycemic control in patients with Type 2 diabetes. J Endocrinol Invest (2012) 35(8):766. doi: 10.3275/8015
79. Sahay R, Hafidh K, Djaballah K, Coudert M, Azar S, Shehadeh N, et al. Safety of lixisenatide plus basal insulin treatment regimen in Indian people with type 2 diabetes mellitus during Ramadan fast: A post hoc analysis of the LixiRam randomized trial. Diabetes Res Clin Pract (2020) 108148. doi: 10.1016/j.diabres.2020.108148
80. Salti I, Diabetes, Group RS. Efficacy and safety of insulin glargine and glimepiride in subjects with Type 2 diabetes before, during and after the period of fasting in Ramadan. Diabetic Med (2009) 26(12):1255–61. doi: 10.1111/j.1464-5491.2009.02836.x
81. Sari R, Balci MK, Akbas SH, Avci B. The effects of diet, sulfonylurea, and Repaglinide therapy on clinical and metabolic parameters in type 2 diabetic patients during Ramadan. Endocr Res (2004) 30(2):169–77. doi: 10.1081/ERC-200027375
82. Shehadeh N, Maor Y, Group RS. Effect of a new insulin treatment regimen on glycaemic control and quality of life of Muslim patients with type 2 diabetes mellitus during Ramadan fast–an open label, controlled, multicentre, cluster randomised study. Int J Clin Pract (2015) 69(11):1281–8. doi: 10.1111/ijcp.12695
83. Al Sifri S, Basiounny A, Echtay A, Al Omari M, Harman-Boehm I, Kaddaha G, et al. The incidence of hypoglycaemia in Muslim patients with type 2 diabetes treated with sitagliptin or a sulphonylurea during Ramadan: a randomised trial. Int J Clin Pract (2011) 65(11):1132–40. doi: 10.1111/j.1742-1241.2011.02797.x
84. El Toony LF, Hamad DA, Omar OM. Outcome of focused pre-Ramadan education on metabolic and glycaemic parameters in patients with type 2 diabetes mellitus. Diabetes Metab Syndrome: Clin Res Rev (2018) 12(5):761–7. doi: 10.1016/j.dsx.2018.04.036
85. Tourkmani AM, Hassali MA, Alharbi TJ, Alkhashan HI, Alobikan AH, Bakhiet AH, et al. Impact of Ramadan focused education program on hypoglycemic risk and metabolic control for patients with type 2 diabetes. Patient preference adherence (2016) 10:1709. doi: 10.2147/PPA.S113324
86. Tourkmani AM, Alharbi TJ, Rsheed AMB, AlRasheed AN, AlBattal SM, Abdelhay O, et al. Impact of Ramadan Focused Education Program on medications adjustment for patients with type 2 diabetes in a primary health care institution in Saudi Arabia. Diabetes Metab Syndrome: Clin Res Rev (2019) 13(1):161–5. doi: 10.1016/j.dsx.2018.07.012
87. Vasan S, Thomas N, Bharani AM, Abraham S, Job V, John B, et al. A double-blind, randomized, multicenter study evaluating the effects of pioglitazone in fasting Muslim subjects during Ramadan. Int J Diabetes Dev Ctries (2006) 26(June):70–6. doi: 10.4103/0973-3930.28276
88. Vasan SK, Karol R, Mahendri N, Arulappan N, Jacob JJ, Thomas N. A prospective assessment of dietary patterns in Muslim subjects with type 2 diabetes who undertake fasting during Ramadan. Indian J Endocrinol Metab (2012) 16(4):552. doi: 10.4103/2230-8210.98009
89. Wan Seman W, Kori N, Rajoo S, Othman H, Mohd Noor N, Wahab N, et al. Switching from sulphonylurea to a sodium-glucose cotransporter2 inhibitor in the fasting month of Ramadan is associated with a reduction in hypoglycaemia. Diabetes Obes Metab (2016) 18(6):628–32. doi: 10.1111/dom.12649
90. Zargar A, Siraj M, Jawa A, Hasan M, Mahtab H. Maintenance of glycaemic control with the evening administration of a long acting sulphonylurea in male type 2 diabetic patients undertaking the Ramadan fast. Int J Clin Pract (2010) 64(8):1090–4. doi: 10.1111/j.1742-1241.2009.02262.x
91. Susilparat P, Pattaraarchachai J, Songchitsomboon S, Ongroongruang S. Effectiveness of contextual education for self-management in Thai Muslims with type 2 diabetes mellitus during Ramadan. J Med Assoc Thai (2014) 97(Suppl 8):S41–9.
92. Belkhadir J, El Ghomari H, Klöcker N, Mikou A, Nasciri M, Sabri M. Muslims with non-insulin dependent diabetes fasting during Ramadan: treatment with glibenclamide. Br Med J (1993) 307(6899):292–5. doi: 10.1136/bmj.307.6899.292
93. Lessan N, Hannoun Z, Hasan H, Barakat M. Glucose excursions and glycaemic control during Ramadan fasting in diabetic patients: insights from continuous glucose monitoring (CGM). Diabetes Metab (2015) 41(1):28–36. doi: 10.1016/j.diabet.2014.11.004
94. Malik U, Mahmood N, Khan KA, Hameed M, Randhawa FA, Salman S, et al. Glycemic control of type 2 diabetic patients during ramazan fasting. J Ayub Med Coll Abbottabad (2017) 29(1):102–6.
95. Hassanein MM, Sahay R, Hafidh K, Djaballah K, Li H, Azar S, et al. Safety of lixisenatide versus sulfonylurea added to basal insulin treatment in people with type 2 diabetes mellitus who elect to fast during Ramadan (LixiRam): An international, randomized, open-label trial. Diabetes Res Clin Pract (2019) 150:331–41. doi: 10.1016/j.diabres.2019.01.035
96. Hassanein M, Al-Arouj M, Hamdy O, Bebakar WMW, Jabbar A, Al-Madani A, et al. Diabetes and Ramadan: Practical guidelines. Diabetes Res Clin Pract (2017) 126:303–16. doi: 10.1016/j.diabres.2017.03.003
97. Masood SN, Masood Y, Mumtaz SN, Maqsood A, Hakim R, Shaikh Z, et al. Ramadan fasting related awareness, practices and experiences of a representative group of Urban Pakistani Diabetics. Pak J Med Sci (2012) 28(3):432–6.
98. Lee J, Lee S, Nasir N, How S, Tan C, Wong C. Diabetes telemonitoring reduces the risk of hypoglycaemia during Ramadan: a pilot randomized controlled study. Diabetic Med (2015) 32(12):1658–61. doi: 10.1111/dme.12836
99. Tourkmani AM, Abdelhay O, Alharbi TJ, Bin Rsheed AM, Azmi Hassali M, Alrasheedy AA, et al. Impact of Ramadan-focused diabetes education on hypoglycemia risk and metabolic control for patients with type 2 diabetes mellitus: A systematic review. Int J Clin Pract (2020) e13817. doi: 10.1111/ijcp.13817
100. McCall AL. Insulin Therapy and Hypoglycemia. Endocrinol Metab Clin North Am (2012) 41(1):57–87. doi: 10.1016/j.ecl.2012.03.001
101. Loke S, Rahim K, Kanesvaran R, Wong T. A prospective cohort study on the effect of various risk factors on hypoglycaemia in diabetics who fast during Ramadan. Med J Malaysia (2010) 65(1):3–6.
102. Gaborit B, Dutour O, Ronsin O, Atlan C, Darmon P, Gharsalli R, et al. Ramadan fasting with diabetes: an interview study of inpatients’ and general practitioners’ attitudes in the South of France. Diabetes Metab (2011) 37(5):395–402. doi: 10.1016/j.diabet.2010.12.010
103. Deeb A, Al Qahtani N, Attia S, Al Suwaidi H, Nagelkerke N. Does reducing basal insulin during Ramadan fasting by children and adolescents with type 1 diabetes decrease the risk of symptomatic hypoglycemia? Diabetes Technol Ther (2016) 18(9):539–42. doi: 10.1089/dia.2016.0197
104. Afandi B, Kaplan W, Majd L, Roubi S. Rate, timing, and severity of hypoglycemia in adolescents with type 1 diabetes during Ramadan fasting: a study with freestyle libre flash glucose monitoring system. Ibnosina J Med Biomed Sci (2018) 10(1):9. doi: 10.4103/ijmbs.ijmbs_73_17
105. Ceperuelo-Mallafré V, Ejarque M, Serena C, Duran X, Montori-Grau M, Rodríguez MA, et al. Adipose tissue glycogen accumulation is associated with obesity-linked inflammation in humans. Mol Metab (2016) 5(1):5–18. doi: 10.1016/j.molmet.2015.10.001
106. Ali Z, Abizari A-R. Ramadan fasting alters food patterns, dietary diversity and body weight among Ghanaian adolescents. Nutr J (2018) 17(1):75. doi: 10.1186/s12937-018-0386-2
107. Cortez DN, Reis IA, Souza DAS, Macedo MML, Torres HDC. Complications and the time of diagnosis of diabetes mellitus in primary care. Acta Paul Enferm (2015) 28(3):250–5. doi: 10.1590/1982-0194201500042
108. Faris M, Jahrami HA, Alsibai J, Obaideen AA. Impact of Ramadan diurnal intermittent fasting on the metabolic syndrome components in healthy, non-athletic Muslim people aged over 15 years: a systematic review and meta-analysis. Br J Nutr (2020) 123(1):1–22. doi: 10.1017/S000711451900254X
109. Jahrami HA, Alsibai J, Clark CCT, Faris ME. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on body weight in healthy subjects aged 16 years and above. Eur J Nutr (2020) 59(6):2291–316. doi: 10.1007/s00394-020-02216-1
110. GLIRA TGiRSG. The Efficacy and Safety of Glimepiride in the Management of Type 2 Diabetes in Muslim Patients During Ramadan. Diabetes Care (2005) 28(2):421–2. doi: 10.2337/diacare.28.2.421
111. Mbanya JC, Al-Sifri S, Abdel-Rahim A, Satman I. Incidence of hypoglycemia in patients with type 2 diabetes treated with gliclazide versus DPP-4 inhibitors during Ramadan: a meta-analytical approach. Diabetes Res Clin Pract (2015) 109(2):226–32. doi: 10.1016/j.diabres.2015.04.030
112. Hassanein M, Bashier A, Randeree H, Abouelmagd M, AlBaker W, Afandi B, et al. Use of SGLT2 Inhibitors During Ramadan: An Expert Panel Statement. Diabetes Res Clin Pract (2020). doi: 10.1016/j.diabres.2020.108465
113. Ibrahim M, Al Magd MA, Annabi FA, Assaad-Khalil S, Ba-Essa EM, Fahdil I, et al. Recommendations for management of diabetes during Ramadan: update 2015. BMJ Open Diabetes Res Care (2015) 3(1):e001248. doi: 10.1136/bmjdrc-2015-000108
114. Hui E, Bravis V, Hassanein M, Hanif W, Malik R, Chowdhury T, et al. Management of people with diabetes wanting to fast during Ramadan. BMJ (2010) 340:c3053. doi: 10.1136/bmj.c3053
115. Al-Arouj M, Assaad-Khalil S, Buse J, Fahdil I, Fahmy M, Hafez S, et al. Recommendations for management of diabetes during Ramadan: update 2010. Diabetes Care (2010) 33(8):1895–902. doi: 10.2337/dc10-0896
116. Ahrén B. Vildagliptin: a DPP-4 inhibitor for the treatment of Type 2 diabetes. Diabetes Manage (2012) 2(5):453. doi: 10.2217/dmt.12.40
117. Christensen M, Vedtofte L, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes (2011) 60(12):3103–9. doi: 10.2337/db11-0979
118. Mari A, Sallas W, He Y, Watson C, Ligueros-Saylan M, Dunning B, et al. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed β-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab (2005) 90(8):4888–94. doi: 10.1210/jc.2004-2460
119. Ahrén B, Schweizer A, Dejager S, Dunning BE, Nilsson PM, Persson M, et al. Vildagliptin enhances islet responsiveness to both hyper-and hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab (2009) 94(4):1236–43. doi: 10.1210/jc.2008-2152
120. Schweizer A, Foley JE, Kothny W, Ahrén B. Clinical evidence and mechanistic basis for vildagliptin’s effect in combination with insulin. Vasc Health Risk Manage (2013) 9:57. doi: 10.2147/VHRM.S40972
121. BaHammam AS, Almeneessier AS. Recent Evidence on the Impact of Ramadan Diurnal Intermittent Fasting, Mealtime, and Circadian Rhythm on Cardiometabolic Risk: A Review. Front Nutr (2020) 7(28). doi: 10.3389/fnut.2020.00028
122. Concato J. Observational versus experimental studies: what’s the evidence for a hierarchy? NeuroRx (2004) 1(3):341–7. doi: 10.1602/neurorx.1.3.341
123. Mathieu C, Degrande E. Vildagliptin: a new oral treatment for type 2 diabetes mellitus. Vasc Health Risk Manage (2008) 4(6):1349–60. doi: 10.2147/VHRM.S3005
124. Aziz KM. Fasting during Ramadan: efficacy, safety, and patient acceptability of vildagliptin in diabetic patients. Diabetes Metab Syndr Obes (2015) 8:207–11. doi: 10.2147/DMSO.S54683
125. Ahmad J, Pathan MF, Jaleel MA, Fathima FN, Raza SA, Khan AA, et al. Diabetic emergencies including hypoglycemia during Ramadan. Indian J Endocrinol Metab (2012) 16(4):512. doi: 10.4103/2230-8210.97996
Keywords: caloric restriction (CR), diet, hypoglycemia, glucose, lifestyle, mealtime
Citation: Abdelrahim D, Faris MAIE, Hassanein M, Shakir AZ, Yusuf AM, Almeneessier AS and BaHammam AS (2021) Impact of Ramadan Diurnal Intermittent Fasting on Hypoglycemic Events in Patients With Type 2 Diabetes: A Systematic Review of Randomized Controlled Trials and Observational Studies. Front. Endocrinol. 12:624423. doi: 10.3389/fendo.2021.624423
Received: 31 October 2020; Accepted: 11 January 2021;
Published: 08 March 2021.
Edited by:
Ernesto Maddaloni, Sapienza University of Rome, ItalyReviewed by:
Mahmoud Ibrahim, Center for Diabetes Education (EDC), United StatesCopyright © 2021 Abdelrahim, Faris, Hassanein, Shakir, Yusuf, Almeneessier and BaHammam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed S. BaHammam, YXNoYW1tYW1Aa3N1LmVkdS5zYQ==; MoezAlIslam E. Faris, bWZhcmlzQHNoYXJqYWguYWMuYWU=; bW9lemZhcmlzQGhvdG1haWwuY29t
†ORCID: Dana N. Abdelrahim, orcid.org/0000-0002-5111-7132; MoezAlIslam E. Faris, orcid.org/0000-0002-7970-2616; Ayman Z. Shakir, orcid.org/0000-0002-3741-3760; Ayesha M. Yusuf, orcid.org/0000-0001-6672-6283; Aljohara S. Almeneessier, orcid.org/0000-0002-6298-1751; Ahmed S. BaHammam, orcid.org/0000-0002-1706-6167
‡These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.