- Key Laboratory of Reproductive Genetics (Ministry of Education) and Department of Reproductive Endocrinology, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Capsule: Oligo/amenorrhea is an independent risk factor of low ovarian response but not high ovarian response, particularly in women with low AMH levels.
Objective: To investigate the association of menstrual cycle length (MCL) with anti-Müllerian hormone (AMH) and ovarian response.
Methods: This was a retrospective cohort study. A total of 7471 women who underwent ovarian stimulation and oocyte retrieval were enrolled. The main outcome was the number of oocytes retrieved.
Main Results: A total of 5734 patients were eligible for analysis. In women without polycystic ovary syndrome (PCOS), serum AMH levels and antral follicle count were significantly lower in women with short cycles and higher in women with oligo/amenorrhea than those with a normal menstrual cycle. In women with PCOS, compared to women with a normal menstrual cycle, women with short cycles and women with oligo/amenorrhea showed higher antral follicle count and higher serum AMH levels. Compared with the 0-25th range group of AMH levels, 75-100th percentile groups showed a significantly increased rate of oligo/amenorrhea in women with and without PCOS [adjusted odds ratio (OR) =1.9 (1.04, 3.46), 2.4 (1.70, 3.35)]. In women without PCOS, the low ovarian response was more common in women with short cycles and less common in women with oligo/amenorrhea compared to women with normal cycles [OR=3.0 (2.38, 3.78), 0.7 (0.55, 0.96), respectively]. When adjusted for AMH levels, both short cycles and oligo/amenorrhea were associated with an increased risk of low response [adjusted OR=1.3 (1.02, 1.75), 1.3 (0.93, 1.86), respectively]. In women without PCOS and with low AMH levels, the low ovarian response was more common in women with short cycles as well as in women with oligo/amenorrhea [OR=1.5 (1.08, 1.98), 1.7 (1.08, 2.69), adjusted OR=1.2 (0.86, 1.74), 2.2 (1.31, 3.82), respectively].
Conclusion: AMH levels are significantly associated with increased risk of oligo/amenorrhea in women with and without PCOS. AMH is an indispensable confounder in the association between MCL and ovarian response in women without PCOS. Oligo/amenorrhea is an independent risk factor associated with a low ovarian response in women without PCOS, particularly those with low AMH levels.
Introduction
Infertility is defined as the inability of a couple to get pregnant after one year of regular unprotected intercourse (1). Infertility is also a prevalent medical condition affecting 10% to 20% of couples across different countries (2, 3). In vitro fertilization and embryo transfer (IVF-ET) has allowed millions of infertile couples worldwide to successfully conceive since 1978 (4). Gonadotropins are regularly used to stimulate ovarian follicle growth in IVF procedures, and ovarian response to gonadotropins can largely influence the chance of success in an IVF cycle (5). A good marker to predict ovarian response may help clinicians to manage the doses of gonadotropins and other procedures in an IVF cycle.
In females, serum AMH arises mainly from pre-antral and small antral follicles (6, 7), and serum AMH levels are proportional to the number of growing follicles in the ovaries (6, 8). Many studies indicate that serum AMH levels can be used to predict IVF cycle success (9–11). Currently, serum AMH levels are considered as the best available measure of ovarian reserve under a variety of clinical conditions, including infertility treatment, the forecasting of reproductive lifespan, ovarian surgery, and gonadotoxic cancer therapy (12–14). Additionally, emerging evidence shows that serum AMH levels can be used to predict ovarian response to exogenous gonadotropins (7, 14–16).
Menstruation is cyclic in response to the interactions of hormones secreted from the hypothalamus, pituitary, and ovaries. The menstrual cycle length (MCL) is the time frame from the first day of menstrual bleeding of one cycle to the onset of menses of the next cycle. The majority of MCL is between 25 to 35 days in women (17). MCL can be affected by several diseases, such as polycystic ovarian syndrome (PCOS), endometriosis, hyperprolactinemia, gynecological cancer, etc. Recent studies indicate that serum AMH levels were higher in PCOS women with menstrual disturbance when compared to PCOS women with regular cycles (18). Serum AMH levels are positively associated with MCL in healthy women, which puts forth a new notion of utilizing MCL to predict possible AMH associated outcomes, including ovarian response (19–21). We hypothesize that the various MCL in women may reflect some AMH-related clinical characteristics. The primary aim of our study is to explore the association of MCL with AMH and ovarian response in women undergoing ovarian stimulation.
Materials and Methods
This study was approved by the Ethics Committee of Women’s Hospital of Zhejiang University (reference: IRB-20200016-R) and written informed consent was obtained from each participant. Patients undergoing their first ovarian stimulation cycle from June 2017 to September 2019 in Women’s Hospital of Zhejiang University were enrolled in this study. Patients were excluded if they had a history of oophorectomy, or if they had been previously diagnosed with endometriosis or hyperprolactinemia or any other reasons except polycystic ovarian syndrome (PCOS) that might cause irregular menstrual cycle, or if their menstrual cycle was maintained by drugs, or if they were not measured with serum AMH levels, or if their MCL data, or oocyte retrieval data were not clearly recorded in the dataset. In most cases, the most commonly reported MCL in the year preceding the treatment cycle was recorded in the dataset. The average MCL was used in several women who had different lengths of the cycle (for example, 28 days in this cycle and 29 days in the next cycle). Cycles with MCL < 26 days were defined as short cycles and MCL > 35 days was defined as oligo/amenorrhea according to previous studies (18, 22, 23). A cycle length of 26-35 days was considered as a normal cycle in this study.
Ovarian stimulation was induced either in the long or short GnRH agonist or GnRH antagonist protocol. In the long protocol, GnRH agonist was administered in the mid-luteal phase of the cycle preceding ovarian stimulation. In the short protocol, the GnRH agonist was administered on day 2 of the ovarian stimulation cycle. In the GnRH antagonist protocol, GnRH antagonist was administered according to the follicle development and serum estradiol levels (around 5 or 6 days after Gn treatment). Recombinant follicle stimulation hormone (FSH) (Gonafen, Pricon) and/or human menopausal gonadotrophin (Livzon, China) was commenced on day 2 of the cycle at a doses of 75-225 IU, and the doses were adjusted according to the ovarian response (follicle count under ultrasound and/or serum E2 levels). When the size of at least two leading follicles reached 18 mm, ovulation was triggered by the administration of recombinant HCG (6500 IU; Livzon, China); then, the oocyte was retrieved 36–38 h later. Low, moderate, and high ovarian response in this study were defined as oocyte retrieval number ≤ 3, 3-15, and ≥16, respectively, according to a previous study (24).
Serum AMH, FSH, luteinizing hormone (LH), progesterone, and estradiol levels were measured by electrochemiluminescence on menstrual cycle day 2 or day 3 within 12 months before the treatment cycle (Sandhofer Strasse 116,68305 Mannheim, Germany). The assay kit for AMH measurement could detect a range from 0.01 to 23 ng/ml. In some cases (less than ten) that the test value would beyond this range, we chose the value of 0.01 or 23 ng/ml for substitution (0.01 ng/ml for “< 0.01 ng/ml” and 23 ng/ml for “> 23 ng/ml”). The intra- and inter-assay coefficients of variation were all less than 10%.
Comparison between groups was performed using the independent sample t-test, ANOVA test, Chi-square test (χ2), and non-parametric test as appropriate. Logistic regression analysis was used to determine the risk of factors and the results were expressed with the odds ratio (OR) or adjusted OR (aOR) with 95% confidence intervals (CI). All statistical procedures were run on SPSS version 22.0. P < 0.05 was considered a statistically significant difference.
Results
Characteristics of Women With Short Cycles, Normal MCL and Oligo/Amenorrhea
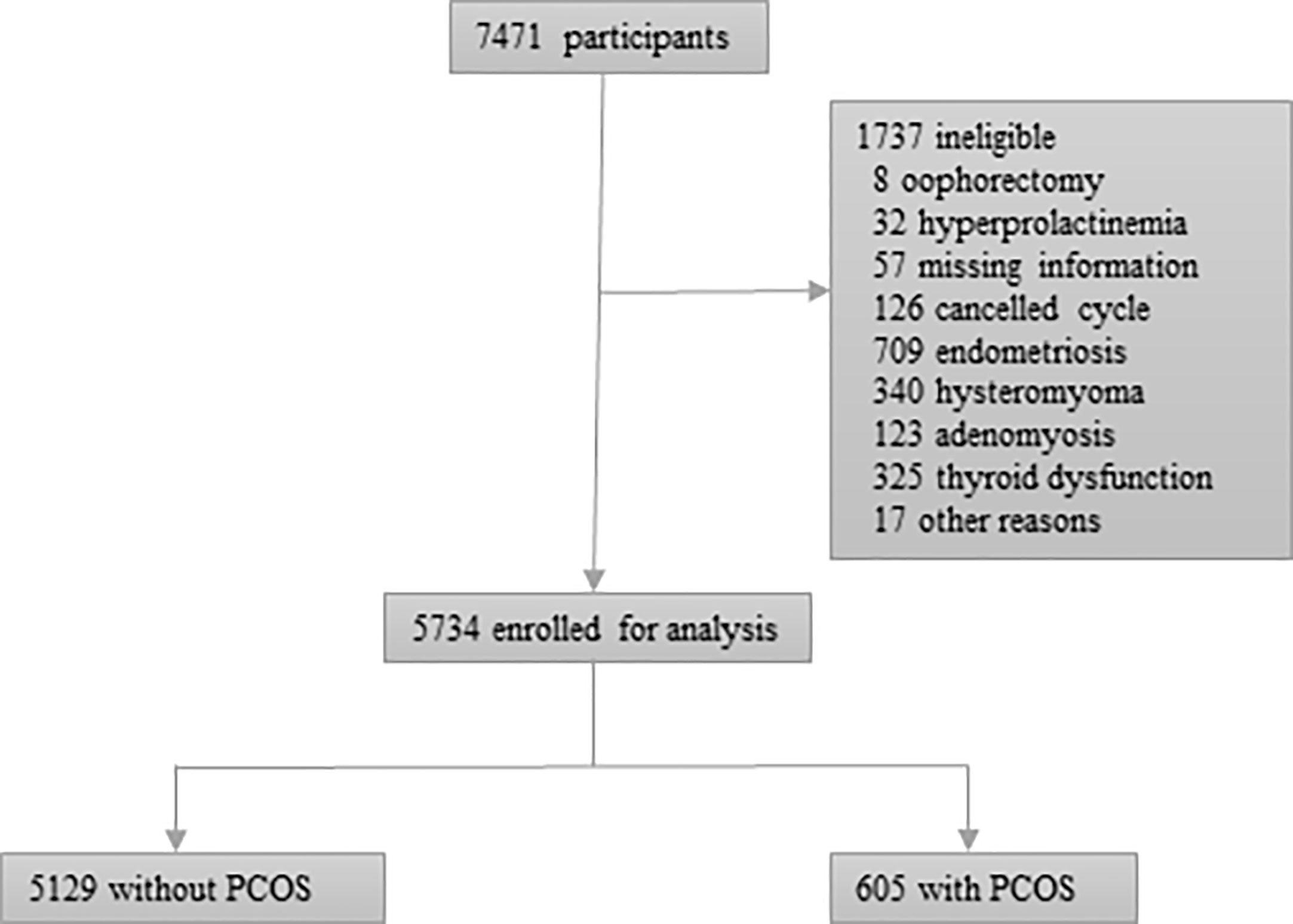
7471 women met the inclusion criteria and 5734 women were eligible for analysis. The reason for excluded women could be seen in Figure 1. 5129 women did not meet the diagnosing criteria of PCOS and 605 women had a diagnosis of PCOS.
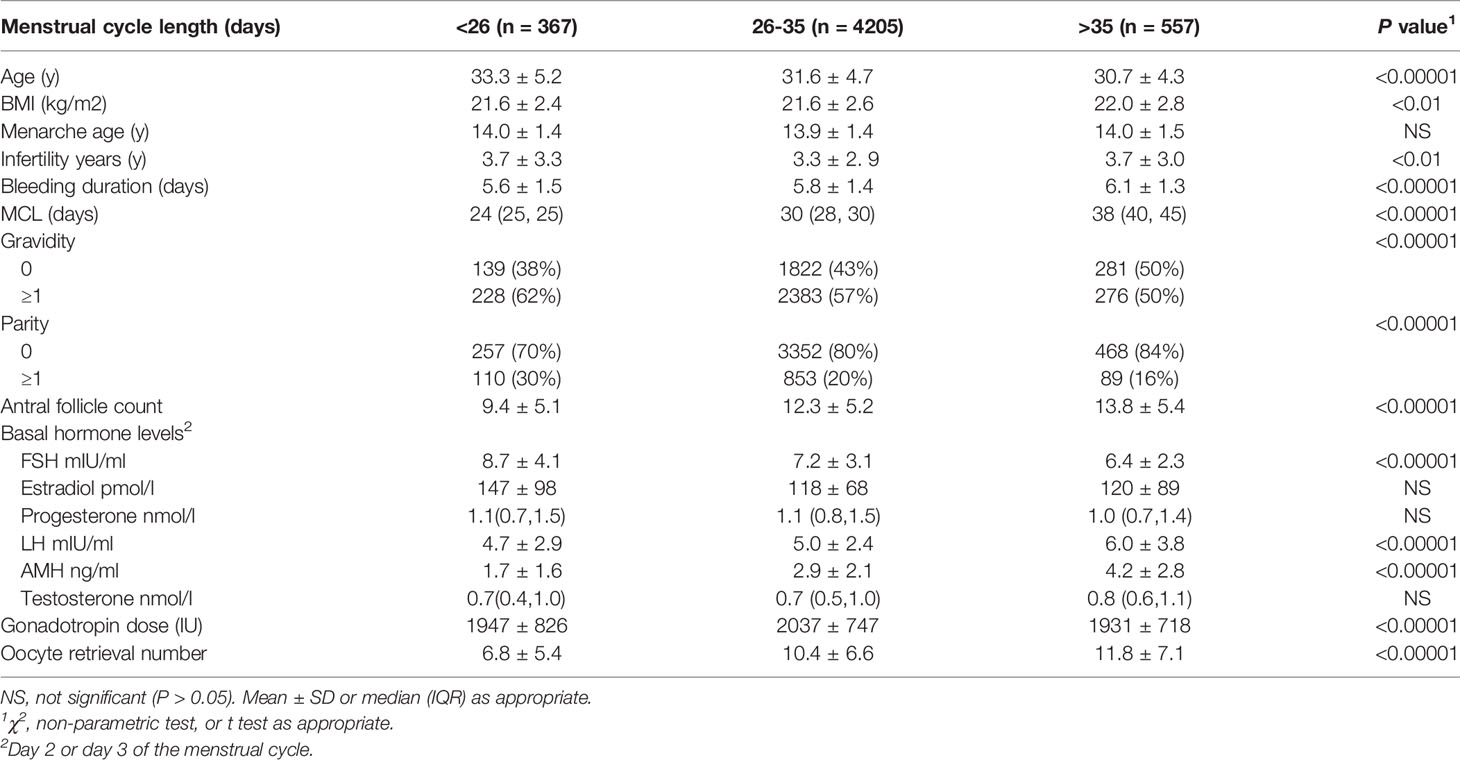
In women without PCOS, compared to women with a cycle length of 26-35 days, women with short cycles had higher average age, a longer infertility time, a higher rate of gravidity and parity, and a shorter mean bleeding duration. Women with oligo/amenorrhea showed a lower average age, a higher average BMI, a longer infertility time, a lower rate of gravidity and parity, and a longer mean bleeding duration (Table 1). Additionally, women with short cycles showed higher basal FSH levels and lower LH levels, whereas women with oligo/amenorrhea showed lower basal FSH levels and higher LH levels. Serum AMH levels, antral follicle count, and oocyte retrieval numbers were significantly lower in women with short cycles and higher in women with oligo/amenorrhea than those with a normal menstrual cycle. The total gonadotropin doses were significantly lower in women with oligo/amenorrhea (Table 1). The P value for each parameter comparison could be seen in Table 1.
In women with PCOS, compared to women with a cycle length of 26-35 days, women with short cycles and women with oligo/amenorrhea showed higher antral follicle count and higher serum AMH levels (Supplemental Table 1). Age, BMI, infertile age, bleeding duration, parity, gravidity, FSH levels, and LH levels were not statistically significant between the three groups. The parameter comparisons could be seen in Supplemental Table 1.
Association Between Serum AMH Levels and MCL
To investigate whether elevated AMH levels were associated with oligo/amenorrhea, we stratified AMH levels by 0-25th, 25-50th, 50-75th, 75-100th percentile.
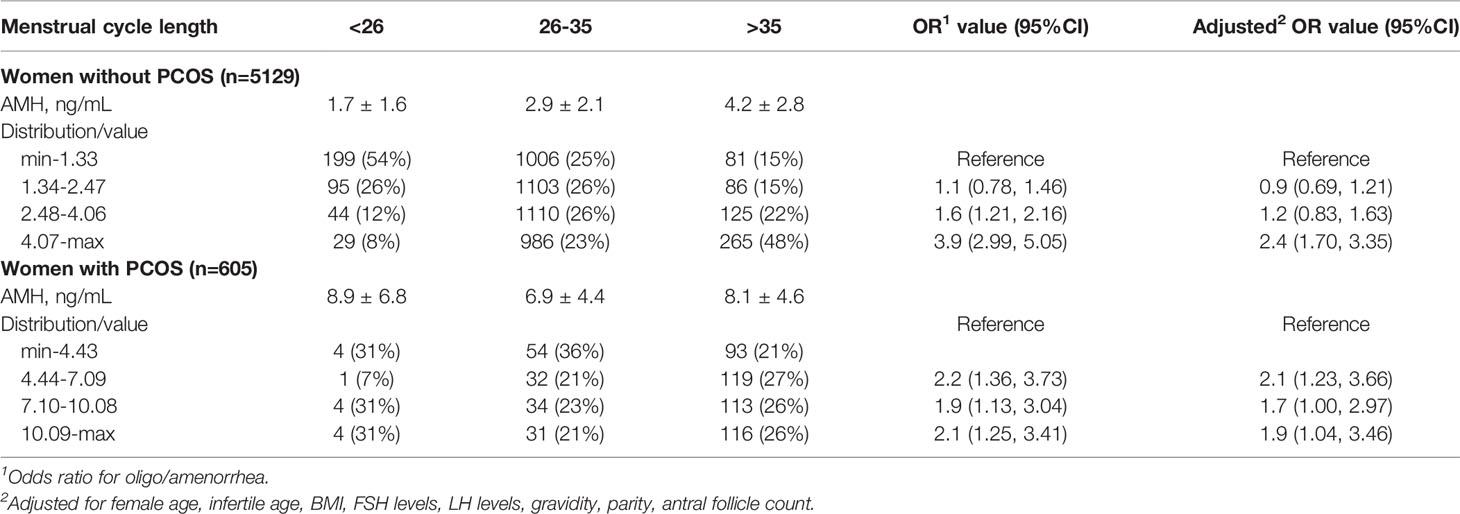
The range of AMH values in women without PCOS were min-1.33, 1.34-2.47, 2.48-4.06, and 4.07-max, respectively. Compared with the 0-25th range group, 75-100th percentile groups showed a significantly increased rate of oligo/amenorrhea [crude OR = 3.9 (2.99, 5.05), adjusted OR = 2.4 (1.70, 3.35)] (Table 2).
The range of AMH values in women with PCOS were min-4.43, 4.44-7.09, 7.10-10.08, and 10.09-max, respectively. Compared with the 0-25th range group, 25-50th, 50-70th, 75-100th percentile groups showed a significantly increased rate of oligo/amenorrhea [adjusted OR = 2.1 (1.23, 3.66), 1.7 (1.00, 2.97), 1.9 (1.04, 3.46), respectively] (Table 2).
Characteristics of Patients With High, Moderate, and Low Ovarian Response
In the 5129 women without PCOS, there were 805 women with a low response, 3305 with a moderate response, and 1019 with a high response. Women with low response showed a higher average age and BMI, a lower MCL, a higher rate of gravidity and parity, higher serum FSH levels and estradiol levels, and lower AMH levels and antral follicle count. Other variables comparison could be seen in Table 3.
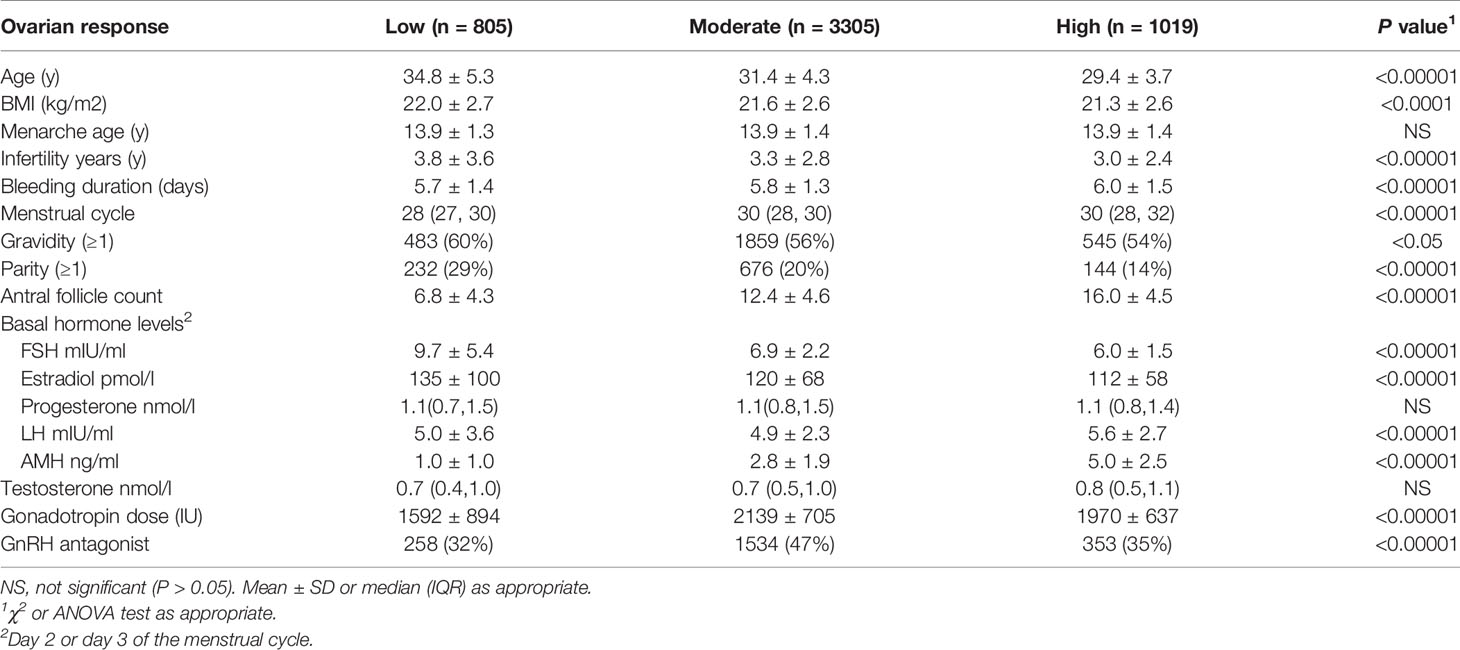
Table 3 Characteristics of women without PCOS and with the low, moderate, and high ovarian response (n=5129).
In the 605 women without PCOS, there were 35 women with a low response, 293 with a moderate response, and 277 with a high response. Women with low response showed a higher average age, higher serum FSH levels, lower AMH levels, and a lower antral follicle count. Other variables comparison could be seen in Supplemental Table 2.
Association Between MCL and Ovarian Response
In women without PCOS, the low ovarian response was more common in women with short cycles and less common in women with oligo/amenorrhea compared to women with normal cycles [OR=3.0 (2.38, 3.78), 0.7 (0.55, 0.96), respectively]. When adjusted for AMH levels, both short cycles and oligo/amenorrhea were associated with an increased risk of low response [adjusted OR=1.3 (1.02, 1.75), 1.3 (0.93, 1.86), respectively]. The result was similar when adjusted for AMH levels and other potential confounders [adjusted OR=1.2 (0.87, 1.55), 1.5 (1.05, 2.22), respectively] (Table 4).
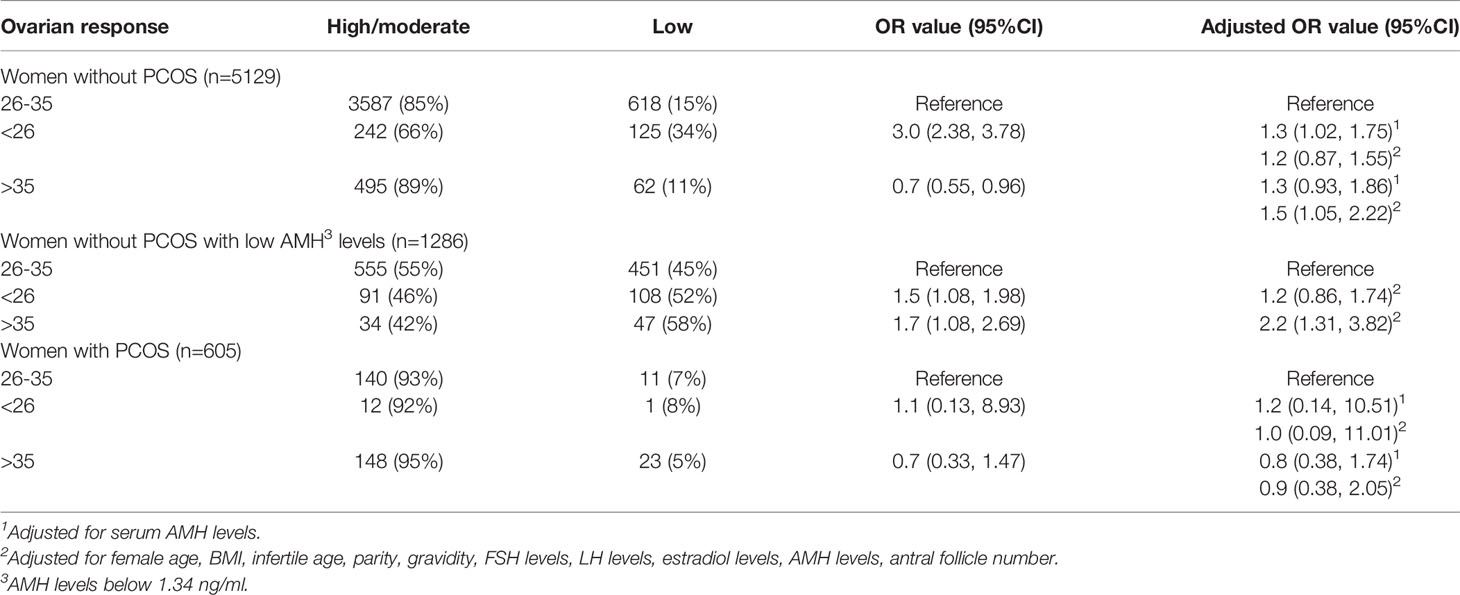
Table 4 The association of oligo/amenorrhea with low ovarian response in women with and without PCOS.
In women without PCOS and with low AMH levels, the low ovarian response was more common in women with short cycles as well as in women with oligo/amenorrhea compared to women with normal cycles [OR=1.5 (1.08, 1.98), 1.7 (1.08, 2.69), adjusted OR=1.2 (0.86, 1.74), 2.2 (1.31, 3.82), respectively] (Table 4).
In women with PCOS, there was no statistically significant difference of the low ovarian response rate in women with short cycles, normal cycles, and oligo/amenorrhea (Table 4).
Discussion
In the present study, we show that elevated AMH levels are significantly associated with increased risk of oligo/amenorrhea in women with and without PCOS. AMH is an indispensable confounder in the association between MCL and ovarian in women without PCOSresponse. Oligo/amenorrhea is an independent risk factor associated with a low ovarian response, particularly in women with low AMH levels.
AMH is predominantly known for its roles in the ovary. Recent studies have uncovered the neuroendocrine functions of AMH in the hypothalamus and pituitary (25). AMHR2 is broadly expressed in various brain area and cell types involved in central control of reproduction, including the hypothalamic gonadotropin-releasing hormone (GnRH) neurons (26). Peripheral administration of AMH can stimulate GnRH secretion and subsequent LH secretion to the levels equivalent to generate an ovulatory surge (26). Additionally, AMH directly and specifically regulates female FSH secretion in the pituitary (27). These studies indicate that AMH may participate in the interaction of hormones involved in the regulation of MCL. It is likely that elevated AMH levels may regulate the frequency of GnRH/LH pulse and affect the process of ovulation and menstrual cycle through the hypothalamus-pituitary-ovary axis (26, 28). Our result is consistent with previous findings that patients with higher AMH levels have longer MCL (18–21). MCL in women with the irregular menstrual cycle is gradually normalized with aging (29), but this has not been well explained. Our study shows that MCL is significantly associated with serum AMH levels when adjusted for potential confounding factors. Increased female age is significantly associated with a reduced risk of oligo/amenorrhea but it is insignificant in AMH adjusted model. It is likely that the gradually normalized MCL with aging can be explained by the progressively decreased circulating AMH levels (8).
The convenience of testing AMH any time throughout the menstrual cycle makes it a good marker for the prediction of ovarian reserve and ovarian response to gonadotropin stimulation (16). Considering that elevated serum AMH levels were significantly associated with an increased rate of oligo/amenorrhea, we hypothesize that MCL can be used to predict ovarian response. A previous retrospective observational study shows that the high value of MCL can increase the risk of ovarian hyperstimulation syndrome and MCL shortening is a risk factor of ovarian aging and poor ovarian response in normo-ovulatory infertile women (30). There are two limitations in this study for the least. The sample size is not large and potential confounders, including AMH and female age, are not adjusted (30). A recent meta-analysis also highlights that MCL shortening in women with regular menstrual cycles is associated with reduced ovarian response (31). Our study is consistent with these results showing short cycles is a risk factor for low ovarian response independent of age and AMH levels in women without PCOS. Interestingly, we find the low ovarian response is less common in women with oligo/amenorrhea and the trend can be reversed when adjusted for AMH levels in women without PCOS, indicating that AMH may be a key confounding factor when MCL is used to predict AMH associated outcomes. Then we are interested in whether oligo/amenorrhea is associated with low ovarian response in a certain range of AMH levels. We found oligo/amenorrhea is a risk factor in women without PCOS and with AMH levels in the 0-25th percentile but not in the 25-50th, 50-75th, 75-100th percentile (data not shown for 25-50th, 50-75th, 75-100th percentile). We define low ovarian response as oocyte retrieval number ≤ 3 in this study, and these women are unlikely to have a good pregnancy outcome (32). A recent study indicates that women with very low AMH values can have in vitro fertilization success (33). Our study shows that women with low AMH values are more likely to have very few oocytes collected if they show oligo/amenorrhea compared to those with the normal menstrual cycle. Therefore, it worths attention to the potential causes of oligo/amenorrhea in women undergoing ovarian stimulation and oocyte retrieval, particularly in women without PCOS and with low AMH levels. Probably due to the small number of women diagnosed with PCOS, we did not find oligo/amenorrhea is associated with low ovarian response in these women. Future studies investigating oligo/amenorrhea and outcomes in women diagnosed with PCOS will be interesting because oligo/amenorrhea is common in these women and it will provide good guidance for clinical practice.
The main limitation of this study is its retrospective study design. Although we adjusted our statistical analysis for a number of known and suspected confounders, some potential confounding factors may have an impact on the result. Oligo/amenorrhea can be caused by a variety of physiopathological factors, such as endometriosis, hyperprolactinemia, thyroid dysfunction, chemoradiotherapy, etc. Even we excluded potential conditions that may cause an irregular menstrual cycle, it is difficult to rule out all patients with at least one of these conditions. Furthermore, the recorded MCL of some patients in the dataset may not accurately be the real MCL in patients. For example, patients may report 2 months (60 days) if their true MCL is 58 days or 62 days. Previous studies indicate that cycles with lengths of 30 to 31 days have the highest fecundity, and conceptions after shorter and longer cycles are more likely to be spontaneously aborted (34). Additionally, Chinese women with menstrual cycle lengths >29 days are less likely to get pregnant (35). Because the pregnancy outcome in many of the enrolled women is not available, we did not analyze the association of MCL with pregnancy outcome in this study but it is promising for future investigations.
To summarize, our study shows that elevated AMH levels are significantly associated with increased risk of oligo/amenorrhea in women with and without PCOS. Serum AMH is an important confounder for MCL to predict ovarian response. Oligo/amenorrhea is an independent risk factor associated with the low ovarian response in women without PCOS, particularly those with low AMH levels. Future studies using MCL to predict other AMH associated outcomes are promising.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Women’s Hospital of Zhejiang University (reference: IRB-20200016-R). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
K-LH reviewed the literature, designed the study, analyzed the data, wrote the manuscript and designed the tables. All authors participated in analysis or interpretation of data for the work. KG and DZ provided some key ideas for this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by the National Key Research and Development Program of China (2018YFC1005003, 2017YFC1001003), the National Natural Science Foundation of China (No. 81974224, 81771535), the Fundamental Research Funds for the Central Universities, the Natural Science Foundation of Zhejiang Province (No. LZ18H040001), Zhejiang University Scholarship for Outstanding Doctoral Candidates, and Zhejiang University Education Foundation Global Partnership Fund.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.612042/full#supplementary-material
References
1. Medicine PCoASfR. Definitions of Infertility and Recurrent Pregnancy Loss: A Committee Opinion. Fertil Steril (2013) 99:63. doi: 10.1016/j.fertnstert.2012.09.023
2. Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of Infertility in the United States as Estimated by the Current Duration Approach and a Traditional Constructed Approach. Fertil Steril (2013) 99:1324–31.e1. doi: 10.1016/j.fertnstert.2012.11.037
3. Zhou Z, Zheng D, Wu H, Li R, Xu S, Kang Y, et al. Epidemiology of Infertility in China: A Population-Based Study. Bjog (2018) 125:432–41. doi: 10.1111/1471-0528.14966
4. Kupka MS, Ferraretti AP, de Mouzon J, Erb K, D’Hooghe T, Castilla JA, et al. Assisted Reproductive Technology in Europe, 2010: Results Generated From European Registers by Eshredagger. Hum Reprod (2014) 29:2099–113. doi: 10.1093/humrep/dew151
5. Hendriks DJ, te Velde ER, Looman CW, Bancsi LF, Broekmans FJ. Expected Poor Ovarian Response in Predicting Cumulative Pregnancy Rates: A Powerful Tool. Reprod BioMed Online (2008) 17:727–36. doi: 10.1016/S1472-6483(10)60323-9
6. Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions Between Androgens, FSH, Anti-Müllerian Hormone and Estradiol During Folliculogenesis in the Human Normal and Polycystic Ovary. Hum Reprod Update (2016) 22:709–24. doi: 10.1093/humupd/dmw027
7. Iliodromiti S, Anderson RA, Nelson SM. Technical and Performance Characteristics of Anti-Mullerian Hormone and Antral Follicle Count as Biomarkers of Ovarian Response. Hum Reprod Update (2015) 21:698–710. doi: 10.1093/humupd/dmu062
8. Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The Physiology and Clinical Utility of Anti-Mullerian Hormone in Women. Hum Reprod Update (2014) 20:370–85. doi: 10.1093/humupd/dmt062
9. Wang S, Zhang Y, Mensah V, Huber WJ 3rd, Huang YT, Alvero R. Discordant Anti-Müllerian Hormone (AMH) and Follicle Stimulating Hormone (FSH) Among Women Undergoing In Vitro Fertilization (IVF): Which One Is the Better Predictor for Live Birth? J Ovarian Res (2018) 11:60. doi: 10.1186/s13048-018-0430-z
10. Lukaszuk K, Liss J, Kunicki M, Jakiel G, Wasniewski T, Woclawek-Potocka I, et al. Anti-Müllerian Hormone (AMH) Is a Strong Predictor of Live Birth in Women Undergoing Assisted Reproductive Technology. Reprod Biol (2014) 14:176–81. doi: 10.1016/j.repbio.2014.03.004
11. Hu KL, Liu FT, Xu H, Li R, Qiao J. Association of Serum anti-Müllerian Hormone and Other Factors With Cumulative Live Birth Rate Following IVF. Reprod BioMed Online (2020) 40(5):675–83. doi: 10.1016/j.rbmo.2020.01.024
12. Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Mullerian Hormone: Ovarian Reserve Testing and Its Potential Clinical Implications. Hum Reprod Update (2014) 20:688–701. doi: 10.1093/humupd/dmu020
13. Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, et al. Association Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age. JAMA (2017) 318:1367–76. doi: 10.1001/jama.2017.14588
14. Peluso C, Fonseca FL, Rodart IF, Cavalcanti V, Gastaldo G, Christofolini DM, et al. AMH: An Ovarian Reserve Biomarker in Assisted Reproduction. Clin Chim Acta (2014) 437:175–82. doi: 10.1016/j.cca.2014.07.029
15. Penarrubia J, Fabregues F, Manau D, Creus M, Casals G, Casamitjana R, et al. Basal and Stimulation Day 5 Anti-Mullerian Hormone Serum Concentrations as Predictors of Ovarian Response and Pregnancy in Assisted Reproductive Technology Cycles Stimulated With Gonadotropin-Releasing Hormone Agonist–Gonadotropin Treatment. Hum Reprod (2005) 20:915–22. doi: 10.1093/humrep/deh718
16. Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing Ovarian Response: Antral Follicle Count Versus Anti-Mullerian Hormone. Reprod BioMed Online (2015) 31:486–96. doi: 10.1016/j.rbmo.2015.06.015
17. Reed BG, Carr BR, Feingold KR, Anawalt B, Boyce A, George Chrousos G, et al. The Normal Menstrual Cycle and the Control of Ovulation. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, editors. Endotext. South Dartmouth, MA: MDText.com, Inc. (2000).
18. Abbara A, Eng PC, Phylactou M, Clarke SA, Hunjan T, Roberts R, et al. Anti-Mullerian Hormone (AMH) in the Diagnosis of Menstrual Disturbance Due to Polycystic Ovarian Syndrome. Front Endocrinol (Lausanne) (2019) 10:656. doi: 10.3389/fendo.2019.00656
19. Zhu R, Lee BH, Huang Z, Indran IR, Li J, Shen L, et al. Antimullerian Hormone, Antral Follicle Count and Ovarian Volume Predict Menstrual Cycle Length in Healthy Women. Clin Endocrinol (Oxf) (2016) 84:870–7. doi: 10.1111/cen.12984
20. Kristensen SL, Ramlau-Hansen CH, Andersen CY, Ernst E, Olsen SF, Bonde JP, et al. The Association Between Circulating Levels of Antimullerian Hormone and Follicle Number, Androgens, and Menstrual Cycle Characteristics in Young Women. Fertil Steril (2012) 97:779–85. doi: 10.1016/j.fertnstert.2011.12.017
21. Guzel Y, Aba YA, Yakin K, Oktem O. Menstrual Cycle Characteristics of Young Females With Occult Primary Ovarian Insufficiency At Initial Diagnosis and One-Year Follow-Up With Serum AMH Level and Antral Follicle Count. PloS One (2017) 12:e0188334. doi: 10.1371/journal.pone.0188334
22. Santulli P, Tran C, Gayet V, Bourdon M, Maignien C, Marcellin L, et al. Oligo-Anovulation Is Not a Rarer Feature in Women With Documented Endometriosis. Fertil Steril (2018) 110:941–8. doi: 10.1016/j.fertnstert.2018.06.012
23. Mumford SL, Steiner AZ, Pollack AZ, Perkins NJ, Filiberto AC, Albert PS, et al. The Utility of Menstrual Cycle Length as an Indicator of Cumulative Hormonal Exposure. J Clin Endocrinol Metab (2012) 97:E1871–9. doi: 10.1210/jc.2012-1350
24. Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, et al. Conventional Ovarian Stimulation and Single Embryo Transfer for IVF/ICSI. How Many Oocytes Do We Need to Maximize Cumulative Live Birth Rates After Utilization of All Fresh and Frozen Embryos? Hum Reprod (2016) 31:370–6. doi: 10.1093/humrep/dev316
25. Barbotin A-L, Peigné M, Malone Samuel A, Giacobini P. Emerging Roles of Anti-Müllerian Hormone in Hypothalamic-Pituitary Function. Neuroendocrinology (2019) 109(3):218–29. doi: 10.1159/000500689
26. Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, et al. Novel Role for Anti-Mullerian Hormone in the Regulation of GnRH Neuron Excitability and Hormone Secretion. Nat Commun (2016) 7:10055. doi: 10.1038/ncomms10055
27. Garrel G, Racine C, L’Hote D, Denoyelle C, Guigon CJ, di Clemente N, et al. Anti-Mullerian Hormone: A New Actor of Sexual Dimorphism in Pituitary Gonadotrope Activity Before Puberty. Sci Rep (2016) 6:23790. doi: 10.1038/srep23790
28. Tata B, Mimouni NEH, Barbotin AL, Malone SA, Loyens A, Pigny P, et al. Elevated Prenatal Anti-Mullerian Hormone Reprograms the Fetus and Induces Polycystic Ovary Syndrome in Adulthood. Nat Med (2018) 24:834–46. doi: 10.1038/s41591-018-0035-5
29. Panidis D, Tziomalos K, Papadakis E, Chatzis P, Kandaraki EA, Tsourdi EA, et al. Associations of Menstrual Cycle Irregularities With Age, Obesity and Phenotype in Patients With Polycystic Ovary Syndrome. Hormones (Athens) (2015) 14:431–7. doi: 10.14310/horm.2002.1593
30. Gizzo S, Andrisani A, Noventa M, Quaranta M, Esposito F, Armanini D, et al. Menstrual Cycle Length: A Surrogate Measure of Reproductive Health Capable of Improving the Accuracy of Biochemical/Sonographical Ovarian Reserve Test in Estimating the Reproductive Chances of Women Referred to ART. Reprod Biol Endocrinol (2015) 13:28. doi: 10.1186/s12958-015-0024-1
31. Younis JS, Iskander R, Fauser B, Izhaki I. Does an Association Exist Between Menstrual Cycle Length Within the Normal Range and Ovarian Reserve Biomarkers During the Reproductive Years? A Systematic Review and Meta-Analysis. Hum Reprod Update (2020) 26(6):904–28. doi: 10.1093/humupd/dmaa013
32. La Marca A, Grisendi V, Giulini S, Sighinolfi G, Tirelli A, Argento C, et al. Live Birth Rates in the Different Combinations of the Bologna Criteria Poor Ovarian Responders: A Validation Study. J Assist Reprod Genet (2015) 32:931–7. doi: 10.1007/s10815-015-0476-4
33. Sefrioui O, Madkour A, Aboulmaouahib S, Kaarouch I, Louanjli N. Women With Extreme Low AMH Values Could Have In Vitro Fertilization Success. Gynecol Endocrinol (2019) 35:170–3. doi: 10.1080/09513590.2018.1505850
34. Small CM, Manatunga AK, Klein M, Feigelson HS, Dominguez CE, McChesney R, et al. Menstrual Cycle Characteristics: Associations With Fertility and Spontaneous Abortion. Epidemiology (2006) 17:52–60. doi: 10.1097/01.ede.0000190540.95748.e6
Keywords: anti-müllerian hormone, menstrual cycle, oligomenorrhea, polycystic ovary syndrome, ovarian response
Citation: Hu K-L, Gan K, Ying Y, Zheng J, Chen R, Xue J, Wu Y, Liu Y, Zhu Y, Xing L and Zhang D (2021) Oligo/Amenorrhea Is an Independent Risk Factor Associated With Low Ovarian Response. Front. Endocrinol. 12:612042. doi: 10.3389/fendo.2021.612042
Received: 30 September 2020; Accepted: 11 May 2021;
Published: 09 June 2021.
Edited by:
Alessandro Conforti, University of Naples Federico II, ItalyReviewed by:
Renato Fraietta, Federal University of São Paulo, BrazilTulay Irez, Yeni Yüzyıl University, Turkey
Copyright © 2021 Hu, Gan, Ying, Zheng, Chen, Xue, Wu, Liu, Zhu, Xing and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Zhang, emhhbmdkYW5Aemp1LmVkdS5jbg==; orcid.org/0000-0003-1295-4795
†These authors have contributed equally to this work
 Kai-Lun Hu
Kai-Lun Hu Kwanghann Gan†
Kwanghann Gan† Dan Zhang
Dan Zhang