- 1School of Nursing and Rehabilitation, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Orthopedic Surgery, The First Affiliated Hospital of Shandong First Medical University, Jinan, China
- 3Department of Cardiology at the First Hospital of Jilin University, Changchun, China
- 4Department of Cardiology at the First Hospital of China Medical University, and Department of Cardiology at the People’s Hospital of Liaoning Province, Shenyang, China
Fibroblast growth factor 21 (FGF21), is an emerging metabolic regulator mediates multiple beneficial effects in the treatment of metabolic disorders and related complications. Recent studies showed that FGF21 acts as an important inhibitor in the onset and progression of cardiovascular complications of diabetes mellitus (DM). Furthermore, evidences discussed so far demonstrate that epigenetic modifications exert a crucial role in the initiation and development of DM-related cardiovascular complications. Thus, epigenetic modifications may involve in the function of FGF21 on DM-induced cardiovascular complications. Therefore, this review mainly interprets and delineates the recent advances of role of FGF21 in DM cardiovascular complications. Then, the possible changes of epigenetics related to the role of FGF21 on DM-induced cardiovascular complications are discussed. Thus, this article not only implies deeper understanding of the pathological mechanism of DM-related cardiovascular complications, but also provides the possible novel therapeutic strategy for DM-induced cardiovascular complications by targeting FGF21 and related epigenetic mechanism.
Introduction
Fibroblast growth factor 21 (FGF21), belongs to the FGF family, is mainly expressed in the liver, adipose tissues and skeletal muscle (1, 2). FGF21 action is mediated by FGF receptors (FGFRs) and β-klotho (a single-pass transmembrane protein, known as a co-receptor for cellular responsiveness to FGF21 action) (1, 2). Endogenous FGF21 has been proposed to be a hormone to maintain lipid and glucose metabolism under both physiological and pathological conditions (2–6). In addition, FGF21 also plays a critical role in the treatment of cardiovascular diseases (7, 8). For example, high serum level of endogenous FGF21 is considered as a compensatory response to ameliorate atherosclerotic diseases or represents the resistant state of FGF21 (7, 9), while treatment with exogenous FGF21 could protect against atherosclerosis (10). Particularly, several lines of evidences indicate a close and complicated relationship between DM-induced cardiovascular complications and FGF21 (11–13). Studies have shown that the early compensatory serum high level of FGF21 is responsive to the occurrence and development of DM-induced cardiovascular complications (14, 15). While the deletion of FGF21 could aggravate the DM-induced cardiovascular injury (16, 17). Furthermore, exogenous FGF21 has been shown could improve DM-induced cardiovascular injury in rodents (11, 18). The cardiovascular protective effect of exogenous FGF21 is mainly mediated by the anti-oxidative stress (11), anti-inflammatory (19), anti-apoptosis (20) and lipid-lowering effects (21). However, despite as a biomarker and diagnostic indicator of DM-related cardiovascular diseases in clinic (14, 22), the clinical implementation of FGF21 still has some obstacles due to its complex pharmacokinetic and biophysical characteristics (23).
To date, accumulating evidences have demonstrated that hyperglycemia could result in continuous cardiovascular complications despite achievement of glycemic control, which is called “metabolic memory” (24–26). Metabolic memory is related to the epigenetic modifications without the change of DNA sequence, including modifications of chromatin histone, methylation of DNA, and gene regulations by non-coding RNAs (25). Thus, a deep study of the epigenetic modifications and formulation of corresponding treatment strategies are beneficial to the prevention and treatment of diabetic cardiovascular complications. Moreover, the role of epigenetic mechanism related to FGF21 in the treatment of DM and associated complications has attracted extensive attention of researchers. For example, it is reported that the inhibition of histone deacetylase 3 (HDAC3) could up-regulate Fgf21 gene transcription to ameliorate DM-induced vascular injury (27). It has also been shown that exogenous FGF21 treatment might increase microRNA (miRNA)-155-3p and miRNA-1968-5p to control hepatic energy metabolism in the state of insulin resistance (28). In addition, according to a recent study, administration of FGF21 in an obese mice model could improve hepatic steatosis and autophagy through upregulating autophagy genes via demethylation of lysine 27 on histone 3 (H3K27) (29). Therefore, the purpose of this review is to analyze the effects and related mechanisms of FGF21 on DM-related cardiovascular complications. Moreover, the possible epigenetic changes that may be related to the function of FGF21 on DM-associated cardiovascular complications is discussed, so as to provide reference for further studies.
FGF21 and Diabetic Cardiomyopathy
Diabetic cardiomyopathy (DCM) is defined as a chronic myocardial disorder caused by DM, and its onset is not related to hypertension, coronary artery disease, and valvular heart disease (30, 31). Hyper-glycaemia (32), insulin resistance (32), micro-vascular lesions (33) and calcium overload in cardiomyocytes (31) were reported to be involved in this disorder. Mechanisms such as oxidative stress (34), lipid metabolism imbalance (35), inflammatory response (36), autophagy suppression (37), as well as myocardial cell apoptosis (37) are key factors to facilitate the progression of DCM. Recently, a growing body of evidence demonstrates that FGF21 may be an effective drug for the treatment of DCM, especially in the aspects of reducing oxidative stress (19), inflammatory (19), apoptosis (20) and lowering lipid (21) in the myocardium. For instance, Wu et al. demonstrated FGF21 reduces inflammation in cardiomyocytes by upregulating adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK)/paraoxonase 1 (PON1) signaling (19). Zhang et al. have shown that FGF21 alleviates DM-related cardiac apoptosis via activating the extracellular signal-regulated kinase 1/2 (ERK1/2)/mitogen-activated protein kinase 14 (p38 MAPK)/AMPK pathway in a mice model of type 1 DM (T1DM) (20). Besides, a previous study demonstrated that FGF21 also exerts lipid-lowering and anti-oxidative effect through activating AMPK/acetyl-CoA carboxylase (ACC)/carnitine palmitoyltransferase-1 (CPT-1) pathway and AMPK/protein kinase B (Akt2)/glycogen synthase kinase-3β (GSK3β)/Fyn/nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway in a mice model of type 2 DM (T2DM) (21). Furthermore, long-term treatment of FGF21 could improve cardiac mitochondrial redox homoeostasis and structural changes by activating ERK1/2/peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α)/CPT-1 lipid-lowering pathway and phosphatidylinositol-3-kinase (PI3K)/Akt2/B-cell lymphoma-2 (Bcl-2)/Bcl-2-associated X protein (Bax)/Caspase 3 anti-apoptotic pathways in an obese, insulin-resistant rat model with FGF21 resistance (18). In addition, it is reported that administration of FGF21 could increase serum level of adiponectin (a kind of hormone has been proved to exert cardioprotective effect (38)), suggesting the cardioprotective effect of FGF21 on DCM may be regulated by increasing adiponectin level in serum (18). However, the existence of FGFR1 and β-klotho in the myocardium indicates that FGF21 may also directly protect the heart against DM (39, 40). Indeed, previous study proved FGF21 strongly improved high-glucose (HG)-induced oxidative stress and fibrosis in primary mouse cardiomyocytes, and these protective effects of FGF21 were markedly weakened by genetic blockage of β-klotho (19), suggesting FGF21 ameliorates DCM may be mediated by its direct action on the heart. Therefore, FGF21 is considered as a promising candidate for the therapy of DCM. The mechanisms of exogenous FGF21 action in DCM treatment is presented in Figure 1.
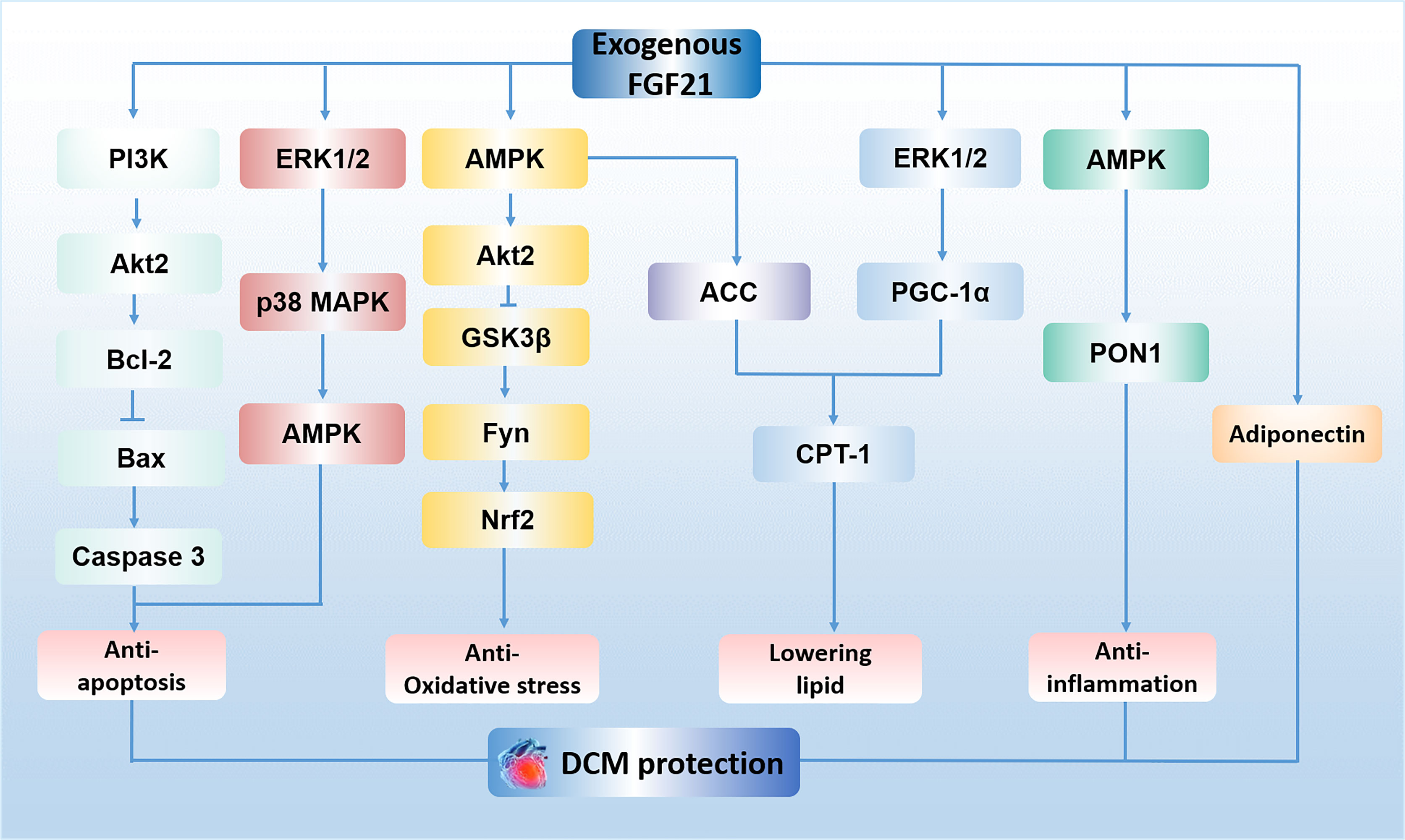
Figure 1 The mechanisms of exogenous FGF21 action in DCM treatment. FGF21 plays an anti-apoptosis role in cardiomyocytes by activating PI3K/Akt2/Bcl-2/Bax/Caspase 3 pathway and ERK1/2/p38 MAPK/AMPK pathway. FGF21 protects against oxidative stress by activating AMPK/Akt2/GSK3β/Fyn/Nrf2 pathway. FGF21 lowers lipid accumulation via activating AMPK/ACC/CPT-1 pathway and ERK1/2/PGC-1α/CPT-1 pathway in the myocardium. FGF21 reduces inflammation in cardiomyocytes by upregulating AMPK/PON1 signaling. The effect of FGF21 on the treatment of DCM is also regulated by adiponectin. PI3K, phosphatidylinositol-3-kinase; Akt2, protein kinase B; Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X protein; ERK1/2, extracellular signal-regulated kinase 1/2; p38 MAPK, mitogen-activated protein kinase 14; AMPK, adenosine 5′-monophosphate (AMP)-activated protein kinase; GSK3β, glycogen synthase kinase-3β; Nrf2, nuclear factor (erythroid-derived 2)-like 2; ACC, acetyl-CoA carboxylase; CPT-1, carnitine palmitoyltransferase 1; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; PON1, paraoxonase 1; DCM, diabetic cardiomyopathy.
Epigenetic Regulation Related to FGF21 in Diabetic Cardiac Complications
Histone Modifications
Epigenetic changes of histones, such as methylation, phosphorylation, acetylation, and ubiquitination, are key factors contribute to the development of chronic diabetic complications (25). Histone deacetylases (HDACs) are one of the vital cellular regulators that could regulate histone deacetylation (41). Xu et al. reported that activity of HDAC3 is significantly enhanced in the heart of diabetic mice. They further found that HDAC3 inhibition suppresses DM-induced oxidative stress and inflammation to improve cardiac dysfunction and remodeling in the diabetic mice (42). Actually, endogenous FGF21 could also be regulated by HDAC3 (43–45). Remarkably, a previous study has demonstrated that HDAC3 inhibition could result in FGF21 secretion and then lead to the reduction of aortic fibrosis and inflammation in a diabetic mice model, mechanistically, inhibition of HDAC3 may promote Nrf2 activity by the up-regulation of miRNA-200a expression with a down-regulation of kelch-like ECH-associated protein 1 (Keap1) to preserve expression of hepatic FGF21 (27). However, whether attenuation of DCM is regulated by FGF21/HDAC3 requires further exploration.
Non-Coding RNAs
Except for histone modifications, non-coding RNAs (ncRNAs) also have been implicated as new participants in the pathogenesis of DM-associated complications. In general, ncRNAs, including miRNAs, circular RNAs (cirRNAs), and long non-coding RNAs (lncRNAs) (46), act as important regulators in controlling gene transcription and protein expression. Of note, miRNAs could base pair with specific target mRNAs to control expression of gene and regulate a series of biological functions (47–49). The aberrantly expression of miRNAs in human diseases showing the therapeutic potential by targeting miRNAs (25). Recently, Costantino et al. showed that the up-regulation of miRNA-34 and miRNA-218 induced by hyperglycaemia in the heart leads to persistent oxidative stress, while inhibition of these miRNAs reduces oxidative stress and restores left ventricular dysfunction (50). Furthermore, it is reported that obesity-induced elevated miRNA-34a could suppress sirtuin 1 (SIRT1) function and adipocyte FGF21, while downregulation of miRNA-34a could improve hepatic FGF21 signaling to alleviate adiposity (51). In addition, Zhang et al. reported that activated FGF21/SIRT1 pathway by fenofibrate could increase cardiac autophagy to improve fibrosis and inflammation induced by DM (52). Thus, it is possible that downregulation of miRNA-34a may increase the activation of FGF21/SIRT1 to improve DCM. In terms of FGF21 regulating miRNAs, Li et al. reported that FGF21 improves ischemic arrhythmias via inhibiting miRNA-143/early growth response protein 1 pathway in a myocardial infarction model (53), yet, the effect of FGF21/miRNA-143 in regulating DCM needs further investigation.
DNA Methylation
DNA methylation always leads to gene silencing, which mainly occurs on the cytosine ring of ‘CpG islands’ in the 5′ regulatory regions of genes. Besides, DNA methylation is regulated by DNA methyltransferases (Dnmts) (54). You et al. have shown that Fgf21 gene is a key target of Dnmt3a in the occurrence of adipose insulin resistance. Expression of endogenous FGF21 is decreased by Dnmt3a-mediated DNA methylation at the Fgf21 promoter, while exogenous FGF21 can restore Dnmt3a overexpression-induced insulin resistance (55). A previous report illustrated that DNA demethylation of Fgf21 gene induced by peroxisome proliferator activate receptor α (PPARα) during the postnatal period could increase hepatic FGF21 expression, which may partly attenuate diet-induced obesity in adulthood (56). However, the involvement of Dnmt3a and Fgf21 gene methylation in diabetic cardiac complications is not fully studied.
A recent evidence demonstrated that DM-induced cardiac fibrosis and remodeling could be ameliorated by Salvianolic acid B via enhancing DNA methylation on the promoter of insulin-like growth factor-binding protein 3 (IGFBP3). Mechanistically, the suppression of IGFBP3 could increase the phosphorylation of ERK and AKT activities, which ultimately lead to the improvement of left ventricular dysfunction of diabetic mice (57). As we have mentioned that FGF21 could improve DCM by the activation of ERK (20) and AKT (21) pathway, thus, whether FGF21 could ameliorate DCM through inducing DNA methylation on IGFBP3 requires further investigation.
In conclusion, all these findings suggest that expression of endogenous FGF21 is closely related to epigenetic modification in multiple pathological conditions. However, lots of work remain to be done to fully understand the relationship between epigenetic regulation and FGF21 in DM-induced cardiac complications.
FGF21 and Diabetic Vascular Complications
FGF21 and Endothelial Dysfunction Induced by DM
The HG environment could reduce the ratio of Ser1177/Thr495 phosphorylation of endothelial nitric oxide synthase (eNOS) and increase inflammatory response to destroy endothelial function in diabetic mice (58). This dysfunction of endothelial cells (ECs) is not only considered as a well-accepted marker, but also a starting point for angiopathy in DM (59). Endogenous FGF21 and β-klotho could be upregulated by HG, while administration of FGF21 could prevent HG-induced cellular damage and eNOS dysfunction in ECs (60). Moreover, previous study demonstrated that exogenous FGF21 inhibits oxidative stress and apoptosis induced by HG in ECs through the activation of PI3K/Akt/forkhead-box type O 3a signaling pathway (61). Ying et al. suggested FGF21 could directly suppress oxidative stress and enhance endothelium-dependent vasorelaxation of aorta through the activation of calcium/calmodulin-dependent protein kinase kinase 2/AMPKα pathway in both T1DM and T2DM mice model, and that is independent of its glucose-lowering and insulin-sensitizing effects. Additionally, the FGFR and β-klotho has been proved to be expressed in ECs, and accordingly the protective effect of FGF21 on inhibiting oxidative stress could be blocked by FGFR antagonist (11). These above findings suggest that FGF21 could be a promising therapeutic drug for vascular complications induced by DM.
FGF21 and Atherosclerosis Induced by DM
Atherosclerosis, a chronic and progressive disease in the large-sized arteries, is characterized by the accumulation of lipids in the artery (62). Compared with non-DM patients, DM significantly enhances brachial–ankle pulse wave velocity, increases vascular intima-medial thickness, and formats a mass of plaques in the artery. These pathologic changes are postulated to be mechanisms for the subclinical atherosclerosis (initiation stage of atherosclerosis) (63). It is reported that elevated serum level of FGF21 was related to atherosclerosis in subjects with DM (12, 14, 15, 64), suggesting FGF21 resistance and/or a compensatory mechanism in response to DM. Yan et al. found that deletion of FGF21 in diabetic mice could worsen DM-induced cell apoptosis and aortic remodeling by aggravating aortic oxidative stress and inflammation, while the pathologic changes caused by FGF21 knockout was reversed by exogenous FGF21 administration in diabetic mice (17). Recently, Kim et al. also demonstrated that FGF21 combined with glucagon-like peptide-1 analogue could strongly ameliorate atherosclerosis-related process induced by T2DM in a mice model (65). Therefore, the above results indicate that FGF21 may act as not only a biomarker but also a promising therapeutic agent for atherosclerosis induced by DM.
Moreover, neointima hyperplasia, as the pathological base of atherosclerosis, has been found to be related to DM. Wei et al. have shown FGF21 could significantly prohibit neointima hyperplasia possibly through the inhibition of spleen tyrosine kinase (Syk)/leucine-rich repeat (LRR)-containing protein 3 (NLRP3) inflammasome pathway in diabetic mice (13). Given that FGFR1 is highly-expressed in aorta (66), Wei et al. further found that the inhibiting effect of FGF21 on Syk and NLRP3 inflammasome activity is abolished by FGFR1 inhibitor in vascular smooth muscle cells (VSMCs) (13), which indicates that FGF21 may directly act on VSMCs to improve atherosclerosis.
Perivascular adipose tissue (PVAT), a vessel-supporting connective tissue (67), could protect against blood vessels injury induced by DM (68). Chang et al. found that the activation of PVAT could attenuate atherosclerosis (69). Berti et al. demonstrated that exogenous FGF21 may protect against atherosclerosis by inducing PVAT to release omentin 1 (70). Herein, FGF21-medieted PVAT activation is indicated to be an effective method for the treatment of DM-related atherosclerosis. Expression of endogenous FGF21 and its possible function in different diabetic atherosclerosis is described in Table 1.
FGF21 and Vascular Calcification Induced by DM
Vascular calcification (VC) is considered as an important complication induced by DM (71). The main pathological changes in VC including the decreased compliance of the vascular wall and increased stiffness, which easily lead to a multiple of adverse cardiovascular events (72). It is reported that DM may promote VC by enhancing the expression of inflammatory cytokines, activating bone morphogenetic proteins pathway and receptor activator of nuclear factor-kβ (RANK)/RANK ligand pathway (73). Recently, Gan et al. demonstrated that lower baseline level of FGF21 in serum could predict a better long-term prognosis in patients with both DM and coronary artery calcification (22). But it has been proved that the application of FGF21 could resist to the calcification of VSMCs by the activation of FGFR1/3/β-klotho/P38/MAPK/runt-related transcription factor 2 signaling pathway (72). Furthermore, Shi et al. indicated that exogenous FGF21 could perform an anti-calcifying effect by inhibiting endoplasmic reticulum stress-induced apoptosis in a rat model (74). However, the anti-calcifying role of FGF21 in the treatment of DM-induced vascular calcification and its related pathway needs further investigation.
Epigenetic Regulation Related to FGF21 in Diabetic Vascular Complications
Histone Modifications
In recent years, emerging evidences have shown that histone modifications are related to vascular dysfunction triggered by DM (75, 76). Several evidences demonstrated that histone methylation of the Fgf21 promoter may involve in the development of diabetic vascular complications. Claycombe et al. showed that expression of histone methyltransferase G9a is increased and transcription of Fgf21 gene is decreased in an obesity and insulin resistance rat model (77). Also, it is reported that the transcriptional repression of Fgf21 could be mediated by histone methyltransferase G9a through increasing dimethylation at lysine 9 on histone 3 (H3K9-me2) of the Fgf21 promoter during refeeding (78). Based on the above studies, it appears that down-regulation of histone methylation of the Fgf21 promoter by decreasing the expression of histone methyltransferase G9a may be a potential therapeutic strategy to improve vascular complications related to DM.
HDAC inhibition has been found to be successful for improving diabetic vascular complications (27, 76). Besides, several pieces of evidences converge to suggest that the inhibition of HDAC may increase the transcription of Fgf21 gene to protect against vascular complications induced by DM. Our previous study has shown that HDAC3 inhibition may promote hepatic FGF21 synthesis and elevate serum protein level of FGF21, which contribute to improve DM-induced aortic inflammation and associated pathologies (27). Moreover, it is reported that NaB could increase Fgf21 gene transcription by inhibiting the activity of HDAC3 to improve fatty acid oxidation and stimulate ketone body production in a dietary obese mice model (45). In addition, sodium butyrate (NaB), has been shown to attenuate aortic endothelial dysfunction induced by DM via inhibiting HDAC3 activity (76). Also, grape seed procyanidin extract, as a strong inducer of Fgf21, could indirectly increase expression of Fgf21 gene and protein by the inhibition of HDAC and subsequent activation of PPARα, thereby, exert therapeutic effect on hypertriglyceridemia (79). All these findings indicate that blocking the activity of HDAC could increase Fgf21 gene expression, which may lead to a recovery in DM-induced vascular complications.
Moreover, SIRT1, belongs to HDACs, has also been found to improve hyperglycemia-induced endothelial dysfunction by deacetylating histone 3 (H3) at the p66Shc promoter (80). It is well-documented that the protective effect of exogenous FGF21 on obesity and T2DM is closely depends on the activation of SIRT1 and subsequently leads to the deacetylation of its downstream targets, PGC-1α and H3 in human adipocytes (81). Of note, previous study has demonstrated that the protective effect of exogenous FGF21 on diabetic heart is strongly correlated with SIRT1 activity, and the increasement of FGF21 may promote SIRT1-mediated autophagy to prevent pathological and functional abnormalities of heart induced by T1DM (52). These findings implicated that SIRT1 may act as an important factor in mediating the protective effect of exogenous FGF21 on the DM-related vascular complications, however, the related specific epigenetic mechanisms need to be further explored in the future.
Non-Coding RNAs
Up to now, the role of ncRNAs in DM-mediated vascular injury has been widely explored. lncRNAs, a kind of ncRNAs that more than 200 nucleotides in length (82), have been identified as crucial epigenetic regulators in a variety of biological processes, including act as molecular sponges or scaffolds for certain molecules (83). Zhang et al. have shown that lncRNAs participate in the treatment of diabetic vascular complication. They demonstrated that the overexpression of lncRNA MEG3 may inhibit the expression of TGF-β1 and VEGF to ameliorate diabetic retinopathy, which suggests the up-regulation of the lncR MEG3 as a promising therapy for diabetic vascular complications (84). However, Wan et al. reported that overexpression of lncR AK005401 exacerbates hippocampal injury induced by acute ischemia/reperfusion through significantly increasing the expressions of Yin Yang 1 and decreasing expression of FGF21 to result in reactive oxygen species (ROS) generation, cell apoptosis and mitochondria injury (85). Whether endogenous FGF21 could be inhibited by lncRNAs AK005401 in the diabetic vascular complications needs to be further explored.
The elevated expression of miRNA-34a could lead to diabetic endothelial dysfunction by downregulation of SIRT1 in diabetic mice (86). Also, it is reported that inhibition of miRNA-34a is able to prevent HG-mediated impaired angiogenesis in mouse microvascular ECs by increasing the expression of SIRT1 (87). As we have known that the upregulation of miRNA-34a in obesity restrains fat browning partly by the suppression of FGF21 signaling and SIRT1, while down-regulation of miRNA-34a could upregulate the expression of FGFR1, β-klotho and SIRT1 function to reduce adiposity (51), thus it is convincible that miRNA-34a inhibition may attenuate diabetic vascular complications by improving hepatic FGF21 signaling.
It has been found that endogenous FGF21 expression is downregulated, but sterol regulatory element-binding protein 2 (SREBP2) expression is upregulated in a rat experimental atherosclerosis model (88). Xue et al. reported that FGF21 and glucagon-like polypeptide 1 improve lipid metabolism in diabetic mice by downregulating the expression of the SREBP1/2 genes (89). Moreover, Lin et al. has shown that replenishment of FGF21 could reduce cholesterol synthesis and attenuate hypercholesterolemia in apolipoprotein E–/– mice by the inhibition of SREBP2 hepatic expression (10). The above results indicate that FGF21 could prevent atherosclerosis via downregulating expression of SREBP2. Moreover, it is reported that miRNA-33 could interact with SREBPs to aggravate atherosclerosis by affecting macrophage actions (90). Therefore, it is believed that FGF21 prevents atherosclerosis may be mediated by inhibiting the expression of miRNA-33 to repress SREBP2 hepatic expression (91). In addition, although no directly evidence showed that miRNA-33 was involved in the treatment of DM-induced vascular dysfunction so far. Yang et al. has shown that miRNA-33 acts as a key regulator in gestational DM of pregnancy (92). Thus, it is still worth for further exploration on the role of miRNA-33 in the effect of FGF21 on DM-induced atherosclerosis.
Under normal conditions, cirRNAs are key regulators of multiple biological processes by being translated themselves or by regulating protein function, by acting as microRNA or protein inhibitors (93). Moreover, cirRNAs are crucial regulators in the pathogenesis of many metabolic diseases. Of note, it is reported cirRNAs are related to the regulation of β-cell activity in the development of DM (94). In addition, microarray profiling of cirRNA revealed a total of 95 differentially expressed cirRNAs in human ECs under hyperglycaemic conditions, which confirmed the key regulatory role of cirRNAs in DM (95). However, whether cirRNAs involve in the protective effect of FGF21 on diabetic vascular complications is still unknown.
DNA Methylation
A couple of studies proposed that DNA methylation is closely related to the development of diabetic vascular complications (96, 97), while the role of Fgf21 methylation in diabetic vascular complications remains largely unknown. It has been shown that DNA methylation at the Fgf21 locus was increased in human DM subjects, which is mediated by Dnmt3a and ultimately lead to insulin resistance (55). Moreover, Yuan et al. demonstrated that the PPARα-dependent Fgf21 demethylation occurs in the liver during the postnatal period, also, they propose that Fgf21 methylation, as a form of epigenetic memory, could persist into adulthood and play a key role in the developmental progress of obesity (56). These above studies suggest that targeting endogenous Fgf21 gene methylation could also be a potential method for the treatment of DM and related vascular complications. However, whether exogenous FGF21 could ameliorate DM-induced vascular complications by altering the DNA methylation patterns of specific genes remains to be further studied (54). Possible epigenetic modifications targeting FGF21 in diseases related to DM are presented in Figure 2.
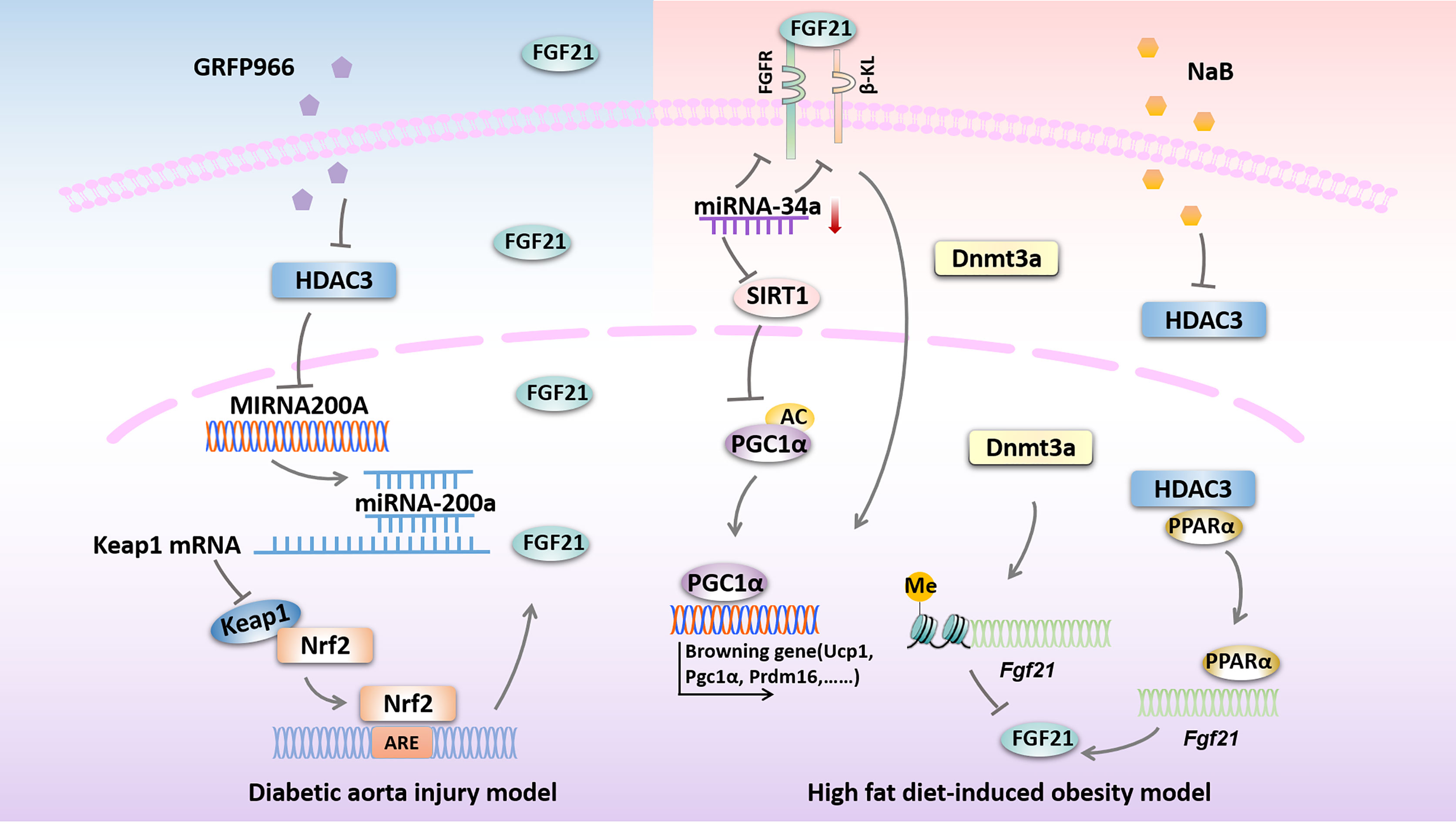
Figure 2 Possible epigenetic modifications targeting FGF21 in diseases related to DM. HDAC3 inhibition by GRFP966 promotes Nrf2 activity via the up-regulation of miRNA-200a expression with down-regulation of keap1 to preserve expression of hepatic FGF21 in diabetic aorta injury model. In high fat diet-induced obesity model, down-regulation of miRNA-34a improves hepatic expression of FGF21 signaling and SIRT1 to promote browning fat formation. Expression of FGF21 is reduced by Dnmt3a-mediated DNA methylation at the Fgf21 promoter. NaB increases Fgf21 gene transcription by inhibiting HDAC3 interacts with PPARα in the Fgf21 promoter. HDAC3, histone deacetylase 3; miRNA-200a, microRNA-200a; Keap1, kelch-like ECH-associated protein 1; Nrf2, Nuclear factor (erythroid-derived 2)-like 2; ARE, AU-rich element; FGFR, fibroblast growth factor receptor; β-KL, β-klotho; miRNA-34a, microRNA-34a; SIRT1, sirtuin 1; PGC1α, peroxisome proliferator-activated receptorγcoactivator-1 α; Ucp1, uncoupled protein 1; Prdm16, PRD1-BF1 and RIZ1 homeodomain protein 16; Dnmt3a, DNA methyltransferase 3a; NaB, sodium butyrate; PPARα, peroxisome proliferator activate receptor α.
Conclusion
Existing evidences have proved the strong protective effects of FGF21 on diabetic cardiovascular complications, such as inhibition of fibrosis and anti-oxidative stress, as well as reduction of apoptosis and inflammation levels in different diabetic cardiovascular complications models. Remarkably, a body of evidence indicating that epigenetic changes are closely involved in FGF21 and cardiovascular complications of DM, including modification of histone, ncRNAs and DNA methylation, which often occur simultaneously and work together in cardiovascular complications of DM. However, related mechanisms remain not fully elucidated. In particular, the discussion about the relationship between DNA methylation and FGF21 in the diabetic cardiovascular complications still in an early stage. Therefore, deeper research must to be carried out in the future.
Author Contributions
MX, JW (4th author), JW (5th author), and YG performed the systematic search, did data extraction, interpreted the data and drafted the review. YT, SW, and JZ contributed to the discussion. JG supervised and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Qilu Young Scholar’s Program of Shandong University (21330089963007), National Natural Science Foundation of China (81770375) and Jilin Science and Technology Department (20200801061GH).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Staiger H, Keuper M, Berti L, Hrabe de Angelis M, Haring HU. Fibroblast Growth Factor 21-Metabolic Role in Mice and Men. Endocr Rev (2017) 38:468–88. doi: 10.1210/er.2017-00016
2. Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev Physiol (2016) 78:223–41. doi: 10.1146/annurev-physiol-021115-105339
3. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARalpha and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab (2007) 5:426–37. doi: 10.1016/j.cmet.2007.05.002
4. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine Regulation of the Fasting Response by PPARalpha-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab (2007) 5:415–25. doi: 10.1016/j.cmet.2007.05.003
5. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a Novel Metabolic Regulator. J Clin Invest (2005) 115:1627–35. doi: 10.1172/jci23606
6. Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, et al. Thermogenic Activation Induces FGF21 Expression and Release in Brown Adipose Tissue. J Biol Chem (2011) 286:12983–90. doi: 10.1074/jbc.M110.215889
7. Wu L, Qian L, Zhang L, Zhang J, Zhou J, Li Y, et al. Fibroblast Growth Factor 21 Is Related to Atherosclerosis Independent of Nonalcoholic Fatty Liver Disease and Predicts Atherosclerotic Cardiovascular Events. J Am Heart Assoc (2020) 9:e015226. doi: 10.1161/JAHA.119.015226
8. Katsiki N, Mantzoros C. Fibroblast Growth Factor 21: A Role in Cardiometabolic Disorders and Cardiovascular Risk Prediction? Metabolism (2019) 93:iii–v. doi: 10.1016/j.metabol.2019.01.005
9. Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, et al. Serum Fibroblast Growth Factor-21 Levels Are Associated With Carotid Atherosclerosis Independent of Established Cardiovascular Risk Factors. Arterioscler Thromb Vasc Biol (2013) 33:2454–9. doi: 10.1161/ATVBAHA.113.301599
10. Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, et al. Fibroblast Growth Factor 21 Prevents Atherosclerosis by Suppression of Hepatic Sterol Regulatory Element-Binding Protein-2 and Induction of Adiponectin in Mice. Circulation (2015) 131:1861–71. doi: 10.1161/CIRCULATIONAHA.115.015308
11. Ying L, Li N, He Z, Zeng X, Nan Y, Chen J, et al. Fibroblast Growth Factor 21 Ameliorates Diabetes-Induced Endothelial Dysfunction in Mouse Aorta via Activation of the CaMKK2/AMPKalpha Signaling Pathway. Cell Death Dis (2019) 10:665. doi: 10.1038/s41419-019-1893-6
12. Yafei S, Elsewy F, Youssef E, Ayman M, El-Shafei M. Fibroblast Growth Factor 21 Association With Subclinical Atherosclerosis and Arterial Stiffness in Type 2 Diabetes. Diabetes Metab Syndr (2019) 13:882–8. doi: 10.1016/j.dsx.2018.12.007
13. Wei W, Li XX, Xu M. Inhibition of Vascular Neointima Hyperplasia by FGF21 Associated With FGFR1/Syk/NLRP3 Inflammasome Pathway in Diabetic Mice. Atherosclerosis (2019) 289:132–42. doi: 10.1016/j.atherosclerosis.2019.08.017
14. Xiao Y, Liu L, Xu A, Zhou P, Long Z, Tu Y, et al. Serum Fibroblast Growth Factor 21 Levels Are Related to Subclinical Atherosclerosis in Patients With Type 2 Diabetes. Cardiovasc Diabetol (2015) 14:72. doi: 10.1186/s12933-015-0229-9
15. An SY, Lee MS, Yi SA, Ha ES, Han SJ, Kim HJ, et al. Serum Fibroblast Growth Factor 21 Was Elevated in Subjects With Type 2 Diabetes Mellitus and Was Associated With the Presence of Carotid Artery Plaques. Diabetes Res Clin Pract (2012) 96:196–203. doi: 10.1016/j.diabres.2012.01.004
16. Yan X, Chen J, Zhang C, Zhou S, Zhang Z, Chen J, et al. FGF21 Deletion Exacerbates Diabetic Cardiomyopathy by Aggravating Cardiac Lipid Accumulation. J Cell Mol Med (2015) 19:1557–68. doi: 10.1111/jcmm.12530
17. Yan X, Chen J, Zhang C, Zeng J, Zhou S, Zhang Z, et al. Fibroblast Growth Factor 21 Deletion Aggravates Diabetes-Induced Pathogenic Changes in the Aorta in Type 1 Diabetic Mice. Cardiovasc Diabetol (2015) 14:77. doi: 10.1186/s12933-015-0241-0
18. Tanajak P, Sa-Nguanmoo P, Wang X, Liang G, Li X, Jiang C, et al. Fibroblast Growth Factor 21 (FGF21) Therapy Attenuates Left Ventricular Dysfunction and Metabolic Disturbance by Improving FGF21 Sensitivity, Cardiac Mitochondrial Redox Homoeostasis and Structural Changes in Pre-Diabetic Rats. Acta Physiol (Oxf) (2016) 217:287–99. doi: 10.1111/apha.12698
19. Wu F, Wang B, Zhang S, Shi L, Wang Y, Xiong R, et al. FGF21 Ameliorates Diabetic Cardiomyopathy by Activating the AMPK-Paraoxonase 1 Signaling Axis in Mice. Clin Sci (Lond) (2017) 131:1877–93. doi: 10.1042/CS20170271
20. Zhang C, Huang Z, Gu J, Yan X, Lu X, Zhou S, et al. Fibroblast Growth Factor 21 Protects the Heart From Apoptosis in a Diabetic Mouse Model via Extracellular Signal-Regulated Kinase 1/2-Dependent Signalling Pathway. Diabetologia (2015) 58:1937–48. doi: 10.1007/s00125-015-3630-8
21. Yang H, Feng A, Lin S, Yu L, Lin X, Yan X, et al. Fibroblast Growth Factor-21 Prevents Diabetic Cardiomyopathy via AMPK-Mediated Antioxidation and Lipid-Lowering Effects in the Heart. Cell Death Dis (2018) 9:227. doi: 10.1038/s41419-018-0307-5
22. Gan F, Huang J, Dai T, Li M, Liu J. Serum Level of Fibroblast Growth Factor 21 Predicts Long-Term Prognosis in Patients With Both Diabetes Mellitus and Coronary Artery Calcification. Ann Palliat Med (2020) 9:368–74. doi: 10.21037/apm.2020.03.28
23. Geng L, Lam KSL, Xu A. The Therapeutic Potential of FGF21 in Metabolic Diseases: From Bench to Clinic. Nat Rev Endocrinol (2020) 16:654–67. doi: 10.1038/s41574-020-0386-0
24. Reddy MA, Zhang E, Natarajan R. Epigenetic Mechanisms in Diabetic Complications and Metabolic Memory. Diabetologia (2015) 58:443–55. doi: 10.1007/s00125-014-3462-y
25. Jin J, Wang X, Zhi X, Meng D. Epigenetic Regulation in Diabetic Vascular Complications. J Mol Endocrinol (2019) 63:R103–15. doi: 10.1530/JME-19-0170
26. Singh K, Pal D, Sinha M, Ghatak S, Gnyawali SC, Khanna S, et al. Epigenetic Modification of MicroRNA-200b Contributes to Diabetic Vasculopathy. Mol Ther (2017) 25:2689–704. doi: 10.1016/j.ymthe.2017.09.009
27. Zhang J, Xu Z, Gu J, Jiang S, Liu Q, Zheng Y, et al. HDAC3 Inhibition in Diabetic Mice May Activate Nrf2 Preventing Diabetes-Induced Liver Damage and FGF21 Synthesis and Secretion Leading to Aortic Protection. Am J Physiol Endocrinol Metab (2018) 315:E150–e162. doi: 10.1152/ajpendo.00465.2017
28. Hochreuter MY, Altıntaş A, Garde C, Emanuelli B, Kahn CR, Zierath JR, et al. Identification of Two microRNA Nodes as Potential Cooperative Modulators of Liver Metabolism. Hepatol Res (2019) 49:1451–65. doi: 10.1111/hepr.13419
29. Byun S, Seok S, Kim YC, Zhang Y, Yau P, Iwamori N, et al. Fasting-Induced FGF21 Signaling Activates Hepatic Autophagy and Lipid Degradation via JMJD3 Histone Demethylase. Nat Commun (2020) 11:807. doi: 10.1038/s41467-020-14384-z
30. Boudina S, Abel ED. Diabetic Cardiomyopathy, Causes and Effects. Rev Endocr Metab Disord (2010) 11:31–9. doi: 10.1007/s11154-010-9131-7
31. Zhang X, Yang L, Xu X, Tang F, Yi P, Qiu B, et al. A Review of Fibroblast Growth Factor 21 in Diabetic Cardiomyopathy. Heart Fail Rev (2019) 24:1005–17. doi: 10.1007/s10741-019-09809-x
32. Jia G, Whaley-Connell A, Sowers JR. Diabetic Cardiomyopathy: A Hyperglycaemia- and Insulin-Resistance-Induced Heart Disease. Diabetologia (2018) 61:21–8. doi: 10.1007/s00125-017-4390-4
33. Alonso N, Moliner P, Mauricio D. Pathogenesis, Clinical Features and Treatment of Diabetic Cardiomyopathy. Adv Exp Med Biol (2018) 1067:197–217. doi: 10.1007/5584_2017_105
34. Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, et al. Inhibition of Superoxide Generation and Associated Nitrosative Damage is Involved in Metallothionein Prevention of Diabetic Cardiomyopathy. Diabetes (2005) 54:1829–37. doi: 10.2337/diabetes.54.6.1829
35. Zheng Z, Ma T, Guo H, Kim KS, Kim KT, Bi L, et al. 4-O-Methylhonokiol Protects Against Diabetic Cardiomyopathy in Type 2 Diabetic Mice by Activation of AMPK-Mediated Cardiac Lipid Metabolism Improvement. J Cell Mol Med (2019) 23:5771–81. doi: 10.1111/jcmm.14493
36. Wang Y, Luo W, Han J, Khan ZA, Fang Q, Jin Y, et al. MD2 Activation by Direct AGE Interaction Drives Inflammatory Diabetic Cardiomyopathy. Nat Commun (2020) 11:2148. doi: 10.1038/s41467-020-15978-3
37. Yao Q, Ke ZQ, Guo S, Yang XS, Zhang FX, Liu XF, et al. Curcumin Protects Against Diabetic Cardiomyopathy by Promoting Autophagy and Alleviating Apoptosis. J Mol Cell Cardiol (2018) 124:26–34. doi: 10.1016/j.yjmcc.2018.10.004
38. Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, et al. Receptor-Mediated Activation of Ceramidase Activity Initiates the Pleiotropic Actions of Adiponectin. Nat Med (2011) 17:55–63. doi: 10.1038/nm.2277
39. Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, et al. Fibroblast Growth Factor 21 Protects Against Cardiac Hypertrophy in Mice. Nat Commun (2013) 4:2019. doi: 10.1038/ncomms3019
40. Planavila A, Redondo-Angulo I, Villarroya F. FGF21 and Cardiac Physiopathology. Front Endocrinol (Lausanne) (2015) 6:133. doi: 10.3389/fendo.2015.00133
41. Hildmann C, Riester D, Schwienhorst A. Histone Deacetylases–an Important Class of Cellular Regulators With a Variety of Functions. Appl Microbiol Biotechnol (2007) 75:487–97. doi: 10.1007/s00253-007-0911-2
42. Xu Z, Tong Q, Zhang Z, Wang S, Zheng Y, Liu Q, et al. Inhibition of HDAC3 Prevents Diabetic Cardiomyopathy in OVE26 Mice via Epigenetic Regulation of DUSP5-ERK1/2 Pathway. Clin Sci (Lond) (2017) 131:1841–57. doi: 10.1042/CS20170064
43. Feigenson M, Shull LC, Taylor EL, Camilleri ET, Riester SM, van Wijnen AJ, et al. Histone Deacetylase 3 Deletion in Mesenchymal Progenitor Cells Hinders Long Bone Development. J Bone Miner Res (2017) 32:2453–65. doi: 10.1002/jbmr.3236
44. Leng Y, Wang J, Wang Z, Liao HM, Wei M, Leeds P, et al. Valproic Acid and Other HDAC Inhibitors Upregulate FGF21 Gene Expression and Promote Process Elongation in Glia by Inhibiting HDAC2 and 3. Int J Neuropsychopharmacol (2016) 19 :1–13. doi: 10.1093/ijnp/pyw035
45. Li H, Gao Z, Zhang J, Ye X, Xu A, Ye J, et al. Sodium Butyrate Stimulates Expression of Fibroblast Growth Factor 21 in Liver by Inhibition of Histone Deacetylase 3. Diabetes (2012) 61:797–806. doi: 10.2337/db11-0846
46. Li Y, Li G, Guo X, Yao H, Wang G, Li C. Non-Coding RNA in Bladder Cancer. Cancer Lett (2020) 485:38–44. doi: 10.1016/j.canlet.2020.04.023
47. Ameres SL, Zamore PD. Diversifying microRNA Sequence and Function. Nat Rev Mol Cell Biol (2013) 14:475–88. doi: 10.1038/nrm3611
48. Catalanotto C, Cogoni C, Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Function. Int J Mol Sci (2016) 17:1–17. doi: 10.3390/ijms17101712
49. Vasudevan S. Posttranscriptional Upregulation by microRNAs. Wiley Interdiscip Rev RNA (2012) 3:311–30. doi: 10.1002/wrna.121
50. Costantino S, Paneni F, Mitchell K, Mohammed SA, Hussain S, Gkolfos C, et al. Hyperglycaemia-Induced Epigenetic Changes Drive Persistent Cardiac Dysfunction via the Adaptor P66(Shc). Int J Cardiol (2018) 268:179–86. doi: 10.1016/j.ijcard.2018.04.082
51. Fu T, Seok S, Choi S, Huang Z, Suino-Powell K, Xu HE, et al. MicroRNA 34a Inhibits Beige and Brown Fat Formation in Obesity in Part by Suppressing Adipocyte Fibroblast Growth Factor 21 Signaling and SIRT1 Function. Mol Cell Biol (2014) 34:4130–42. doi: 10.1128/MCB.00596-14
52. Zhang J, Cheng Y, Gu J, Wang S, Zhou S, Wang Y, et al. Fenofibrate Increases Cardiac Autophagy via FGF21/SIRT1 and Prevents Fibrosis and Inflammation in the Hearts of Type 1 Diabetic Mice. Clin Sci (Lond) (2016) 130:625–41. doi: 10.1042/CS20150623
53. Li J, Xu C, Liu Y, Li Y, Du S, Zhang R, et al. Fibroblast Growth Factor 21 Inhibited Ischemic Arrhythmias via Targeting miR-143/EGR1 Axis. Basic Res Cardiol (2020) 115:9. doi: 10.1007/s00395-019-0768-4
54. Hu X, Bai T, Xu Z, Liu Q, Zheng Y, Cai L. Pathophysiological Fundamentals of Diabetic Cardiomyopathy. Compr Physiol (2017) 7:693–711. doi: 10.1002/cphy.c160021
55. You D, Nilsson E, Tenen DE, Lyubetskaya A, Lo JC, Jiang R, et al. Dnmt3a is an Epigenetic Mediator of Adipose Insulin Resistance. Elife (2017) 6:1–20. doi: 10.7554/eLife.30766
56. Yuan X, Tsujimoto K, Hashimoto K, Kawahori K, Hanzawa N, Hamaguchi M, et al. Epigenetic Modulation of Fgf21 in the Perinatal Mouse Liver Ameliorates Diet-Induced Obesity in Adulthood. Nat Commun (2018) 9:636. doi: 10.1038/s41467-018-03038-w
57. Li CL, Liu B, Wang ZY, Xie F, Qiao W, Cheng J, et al. Salvianolic Acid B Improves Myocardial Function in Diabetic Cardiomyopathy by Suppressing IGFBP3. J Mol Cell Cardiol (2020) 139:98–112. doi: 10.1016/j.yjmcc.2020.01.009
58. Matsumoto S, Shimabukuro M, Fukuda D, Soeki T, Yamakawa K, Masuzaki H, et al. Azilsartan, an Angiotensin II Type 1 Receptor Blocker, Restores Endothelial Function by Reducing Vascular Inflammation and by Increasing the Phosphorylation Ratio Ser(1177)/Thr(497) of Endothelial Nitric Oxide Synthase in Diabetic Mice. Cardiovasc Diabetol (2014) 13:30. doi: 10.1186/1475-2840-13-30
59. Vita JA, Keaney JF Jr. Endothelial Function: A Barometer for Cardiovascular Risk? Circulation (2002) 106:640–2. doi: 10.1161/01.cir.0000028581.07992.56
60. Wang XM, Song SS, Xiao H, Gao P, Li XJ, Si LY. Fibroblast Growth Factor 21 Protects Against High Glucose Induced Cellular Damage and Dysfunction of Endothelial Nitric-Oxide Synthase in Endothelial Cells. Cell Physiol Biochem (2014) 34:658–71. doi: 10.1159/000363031
61. Guo D, Xiao L, Hu H, Liu M, Yang L, Lin X. FGF21 Protects Human Umbilical Vein Endothelial Cells Against High Glucose-Induced Apoptosis via PI3K/Akt/Fox3a Signaling Pathway. J Diabetes Complications (2018) 32:729–36. doi: 10.1016/j.jdiacomp.2018.05.012
63. Won KB, Chang HJ, Kim HC, Jeon K, Lee H, Shin S, et al. Differential Impact of Metabolic Syndrome on Subclinical Atherosclerosis According to the Presence of Diabetes. Cardiovasc Diabetol (2013) 12:41. doi: 10.1186/1475-2840-12-41
64. Zhang X, Hu Y, Zeng H, Li L, Zhao J, Zhao J, et al. Serum Fibroblast Growth Factor 21 Levels is Associated With Lower Extremity Atherosclerotic Disease in Chinese Female Diabetic Patients. Cardiovasc Diabetol (2015) 14:32. doi: 10.1186/s12933-015-0190-7
65. Kim JH, Lee GY, Maeng HJ, Kim H, Bae JH, Kim KM, et al. Effects of Glucagon-Like Peptide-1 Analogue and Fibroblast Growth Factor 21 Combination on the Atherosclerosis-Related Process in a Type 2 Diabetes Mouse Model. Endocrinol Metab (Seoul) (2021) 36:157–70. doi: 10.3803/EnM.2020.781
66. Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, et al. Research Resource: Comprehensive Expression Atlas of the Fibroblast Growth Factor System in Adult Mouse. Mol Endocrinol (2010) 24:2050–64. doi: 10.1210/me.2010-0142
67. Hildebrand S, Stümer J, Pfeifer A. PVAT and Its Relation to Brown, Beige, and White Adipose Tissue in Development and Function. Front Physiol (2018) 9:70. doi: 10.3389/fphys.2018.00070
68. Li T, Liu X, Ni L, Wang Z, Wang W, Shi T, et al. Perivascular Adipose Tissue Alleviates Inflammatory Factors and Stenosis in Diabetic Blood Vessels. Biochem Biophys Res Commun (2016) 480:147–52. doi: 10.1016/j.bbrc.2016.09.106
69. Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, et al. Loss of Perivascular Adipose Tissue on Peroxisome Proliferator-Activated Receptor-γ Deletion in Smooth Muscle Cells Impairs Intravascular Thermoregulation and Enhances Atherosclerosis. Circulation (2012) 126:1067–78. doi: 10.1161/circulationaha.112.104489
70. Berti L, Hartwig S, Irmler M, Radle B, Siegel-Axel D, Beckers J, et al. Impact of Fibroblast Growth Factor 21 on the Secretome of Human Perivascular Preadipocytes and Adipocytes: A Targeted Proteomics Approach. Arch Physiol Biochem (2016) 122:281–8. doi: 10.1080/13813455.2016.1212898
71. Stabley JN, Towler DA. Arterial Calcification in Diabetes Mellitus: Preclinical Models and Translational Implications. Arterioscler Thromb Vasc Biol (2017) 37:205–17. doi: 10.1161/ATVBAHA.116.306258
72. Cao F, Wang S, Cao X, Liu X, Fu K, Hao P, et al. Fibroblast Growth Factor 21 Attenuates Calcification of Vascular Smooth Muscle Cells In Vitro. J Pharm Pharmacol (2017) 69:1802–16. doi: 10.1111/jphp.12826
73. Ghosh S, Luo D, He W, Chen J, Su X, Huang H. Diabetes and Calcification: The Potential Role of Anti-Diabetic Drugs on Vascular Calcification Regression. Pharmacol Res (2020) 158:104861. doi: 10.1016/j.phrs.2020.104861
74. Shi Y, Wang S, Peng H, Lv Y, Li W, Cheng S, et al. Fibroblast Growth Factor 21 Attenuates Vascular Calcification by Alleviating Endoplasmic Reticulum Stress Mediated Apoptosis in Rats. Int J Biol Sci (2019) 15:138–47. doi: 10.7150/ijbs.28873
75. Manea SA, Antonescu ML, Fenyo IM, Raicu M, Simionescu M, Manea A. Epigenetic Regulation of Vascular NADPH Oxidase Expression and Reactive Oxygen Species Production by Histone Deacetylase-Dependent Mechanisms in Experimental Diabetes. Redox Biol (2018) 16:332–43. doi: 10.1016/j.redox.2018.03.011
76. Wu J, Jiang Z, Zhang H, Liang W, Huang W, Zhang H, et al. Sodium Butyrate Attenuates Diabetes-Induced Aortic Endothelial Dysfunction via P300-Mediated Transcriptional Activation of Nrf2. Free Radic Biol Med (2018) 124:454–65. doi: 10.1016/j.freeradbiomed.2018.06.034
77. Claycombe KJ, Vomhof-DeKrey EE, Garcia R, Johnson WT, Uthus E, Roemmich JN. Decreased Beige Adipocyte Number and Mitochondrial Respiration Coincide With Increased Histone Methyl Transferase (G9a) and Reduced FGF21 Gene Expression in Sprague-Dawley Rats Fed Prenatal Low Protein and Postnatal High-Fat Diets. J Nutr Biochem (2016) 31:113–21. doi: 10.1016/j.jnutbio.2016.01.008
78. Tong X, Zhang D, Buelow K, Guha A, Arthurs B, Brady HJ, et al. Recruitment of Histone Methyltransferase G9a Mediates Transcriptional Repression of Fgf21 Gene by E4BP4 Protein. J Biol Chem (2013) 288:5417–25. doi: 10.1074/jbc.M112.433482
79. Downing LE, Ferguson BS, Rodriguez K, Ricketts ML. A Grape Seed Procyanidin Extract Inhibits HDAC Activity Leading to Increased Pparalpha Phosphorylation and Target-Gene Expression. Mol Nutr Food Res (2017) 61:1–7. doi: 10.1002/mnfr.201600347
80. Zhou S, Chen HZ, Wan YZ, Zhang QJ, Wei YS, Huang S, et al. Repression of P66Shc Expression By SIRT1 Contributes to the Prevention of Hyperglycemia-Induced Endothelial Dysfunction. Circ Res (2011) 109:639–48. doi: 10.1161/CIRCRESAHA.111.243592
81. Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast Growth Factor 21 Regulates Energy Metabolism by Activating the AMPK-SIRT1-PGC-1alpha Pathway. Proc Natl Acad Sci U S A (2010) 107:12553–8. doi: 10.1073/pnas.1006962107
82. Sun X, Wong D. Long non-Coding RNA-Mediated Regulation of Glucose Homeostasis and Diabetes. Am J Cardiovasc Dis (2016) 6:17–25.
83. Biswas S, Thomas AA, Chakrabarti S. LncRNAs: Proverbial Genomic “Jun” or Key Epigenetic Regulators During Cardiac Fibrosis in Diabetes? Front Cardiovasc Med (2018) 5:28. doi: 10.3389/fcvm.2018.00028
84. Zhang D, Qin H, Leng Y, Li X, Zhang L, Bai D, et al. LncRNA MEG3 Overexpression Inhibits the Development of Diabetic Retinopathy by Regulating TGF-Beta1 and VEGF. Exp Ther Med (2018) 16:2337–42. doi: 10.3892/etm.2018.6451
85. Wan H, Yang Y, Li M, Liu X, Sun Y, Wang K, et al. Activation of AK005401 Aggravates Acute Ischemia/Reperfusion Mediated Hippocampal Injury by Directly Targeting YY1/FGF21. Aging (Albany NY) (2019) 11:5108–23. doi: 10.18632/aging.102106
86. Li Q, Kim YR, Vikram A, Kumar S, Kassan M, Gabani M, et al. P66Shc-Induced MicroRNA-34a Causes Diabetic Endothelial Dysfunction by Downregulating Sirtuin1. Arterioscler Thromb Vasc Biol (2016) 36:2394–403. doi: 10.1161/ATVBAHA.116.308321
87. Arunachalam G, Lakshmanan AP, Samuel SM, Triggle CR, Ding H. Molecular Interplay Between microRNA-34a and Sirtuin1 in Hyperglycemia-Mediated Impaired Angiogenesis in Endothelial Cells: Effects of Metformin. J Pharmacol Exp Ther (2016) 356:314–23. doi: 10.1124/jpet.115.226894
88. Li Q, Wang H, Zhang C, Tong R, Chen H, Qie R. Ethyl Acetate Extract of Sappanwood Alleviates Experimental Atherosclerosis in Rats Through Changes in FGF21 and SREBP-2 Expression. Int J Clin Exp Pathol (2020) 13:220–9.
89. Xue B, Xiao X, Yu T, Xiao X, Xie J, Ji Q, et al. Mesenchymal Stem Cells Modified by FGF21 and GLP1 Ameliorate Lipid Metabolism While Reducing Blood Glucose in Type 2 Diabetic Mice. Stem Cell Res Ther (2021) 12:133. doi: 10.1186/s13287-021-02205-z
90. Rosenson RS, Brewer HB Jr., Davidson WS, Fayad ZA, Fuster V, Goldstein J, et al. Cholesterol Efflux and Atheroprotection: Advancing the Concept of Reverse Cholesterol Transport. Circulation (2012) 125:1905–19. doi: 10.1161/CIRCULATIONAHA.111.066589
91. Guo Y, Luo F, Yi Y, Xu D. Fibroblast Growth Factor 21 Potentially Inhibits microRNA-33 Expression to Affect Macrophage Actions. Lipids Health Dis (2016) 15:208. doi: 10.1186/s12944-016-0381-6
92. Yang S, Si L, Fan L, Jian W, Pei H, Lin R. Polysaccharide IV From Lycium Barbarum L. Improves Lipid Profiles of Gestational Diabetes Mellitus of Pregnancy by Upregulating ABCA1 and Downregulating Sterol Regulatory Element-Binding Transcription 1 via miR-33. Front Endocrinol (Lausanne) (2018) 9:49. doi: 10.3389/fendo.2018.00049
93. Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The Biogenesis, Biology and Characterization of Circular RNA. Nat Rev Genet (2019) 20:675–91. doi: 10.1038/s41576-019-0158-7
94. Stoll L, Sobel J, Rodriguez-Trejo A, Guay C, Lee K, Veno MT, et al. Circular RNAs as Novel Regulators of Beta-Cell Functions in Normal and Disease Conditions. Mol Metab (2018) 9:69–83. doi: 10.1016/j.molmet.2018.01.010
95. Shang FF, Luo S, Liang X, Xia Y. Alterations of Circular RNAs in Hyperglycemic Human Endothelial Cells. Biochem Biophys Res Commun (2018) 499:551–5. doi: 10.1016/j.bbrc.2018.03.187
96. Duraisamy AJ, Mishra M, Kowluru RA. Crosstalk Between Histone and DNA Methylation in Regulation of Retinal Matrix Metalloproteinase-9 in Diabetes. Invest Ophthalmol Vis Sci (2017) 58:6440–8. doi: 10.1167/iovs.17-22706
Keywords: FGF21, epigenetic, diabetes, cardiovascular complications, mechanisms
Citation: Xiao M, Tang Y, Wang S, Wang J, Wang J, Guo Y, Zhang J and Gu J (2021) The Role of Fibroblast Growth Factor 21 in Diabetic Cardiovascular Complications and Related Epigenetic Mechanisms. Front. Endocrinol. 12:598008. doi: 10.3389/fendo.2021.598008
Received: 23 August 2020; Accepted: 17 June 2021;
Published: 19 July 2021.
Edited by:
Guy A. Rutter, Imperial College London, United KingdomReviewed by:
Xavier Prieur, INSERM U1087 L’unité de recherche de l’institut du thorax, FranceWeiping Jia, Shanghai Sixth People’s Hospital, China
Copyright © 2021 Xiao, Tang, Wang, Wang, Wang, Guo, Zhang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junlian Gu, anVubGlhbl9ndUBzZHUuZWR1LmNu
 Mengjie Xiao1
Mengjie Xiao1 Junlian Gu
Junlian Gu