
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 08 September 2020
Sec. Reproduction
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00621
Background: Polycystic ovary syndrome (PCOS) is a heterogeneous endocrine disorder that is influenced by both genetic and environmental factors. However, the etiology of PCOS remains unclear.
Methods: We conducted a two-sample Mendelian randomization (MR) analysis to assess the causal effects of genetically determined metabolites (GDMs) on the risk of PCOS. We used summary level data of a genome-wide association study (GWAS) on 486 metabolites (n = 7,824) as exposure and a PCOS GWAS consisting of 4,138 cases and 20,129 controls as the outcome. Both datasets were obtained from publicly published databases. For each metabolite, a genetic instrumental variable was generated to assess the relationship between the metabolite and PCOS. For MR analysis, we primarily used the standard inverse variance weighted (IVW) method, while three additional methods—the MR-Egger, weighted median, and MR-PRESSO (pleiotropy residual sum and outlier) methods—were performed as sensitivity analyses.
Results: Using genetic variants as predictors, we observed a robust relationship between epiandrosterone sulfate (EPIA-S) and PCOS (PIVW = 0.0186, PMR−Egger = 0.0111; PWeighted−median = 0.0154, and PMR−PRESSO = 0.0290). Similarly, 3-dehydrocarnitine, 4-hydroxyhippurate, hexadecanedioate, and β-hydroxyisovalerate may also have causal effects on PCOS development.
Conclusions: We identified metabolites that might have causal effects on PCOS development. Our study emphasizes the role of genetic factors underlying the causal relationships between metabolites and PCOS and provides novel insights through the integration of metabolomics and genomics to better understand the mechanisms involved in human disease pathogenesis.
Polycystic ovary syndrome (PCOS) is the leading cause of female infertility worldwide, affecting 6–20% of the female population of reproductive age (1, 2). Hyperandrogenism (HA), ovulatory dysfunction (OD), and polycystic ovarian morphology (PCOM) are common clinical manifestations of PCOS (3). Furthermore, women with PCOS usually exhibit a wide range of endocrine-metabolic disturbances, including insulin resistance, hyperinsulinemia, obesity, and adipose tissue dysfunction, which eventually result in type 2 diabetes mellitus and cardiovascular disease (4–6). Although the latest advances have suggested that PCOS is a complex disease influenced by both genetic and environmental factors, its etiology and the underlying biological processes still need to be researched.
Modern omics-based technologies, including genomics, transcriptomics, proteomics, and metabolomics, have greatly advanced our understanding of the pathophysiological process of human complex diseases and successfully identified a series of biomarkers that could lead to earlier diagnosis of diseases or therapeutic targets for disorders (7). Therefore, these approaches provide a systematic readout of the inherent genetic architecture, the dynamics of physiological and biochemical indicators, and the environmental exposure for individuals (8). Metabolomics characterize downstream gene regulation and protein activity and are considered to be more representative of clinical phenotypes. During the past decade, advances in metabolomics have led to considerable achievements in detecting chemical components that contribute to the occurrence of PCOS. Zhao et al. assessed the metabolic profiles of 217 cases and 48 controls and identified a series of carbohydrate, lipid, and amino acid metabolism in PCOS (9). Chang et al. performed a combinative analysis of non-targeted and targeted metabolomics on obese women and found specific amino acid elevations in PCOS (10). Zhang et al. recruited 286 subjects and investigated the disturbed metabolic profiles for specific pathogenic characteristics of PCOS patients, such as HA and insulin resistance (11). However, these studies typically had small sample sizes and provided limited information about pathophysiological mechanisms. A comprehensive analysis of genomics and metabolomics could provide novel insights into understanding the underlying mechanism of genetic and metabolic interactions in the pathogenesis of PCOS. Recently, a database of genotype-dependent metabolic phenotypes [called genetically determined metabolites (GDMs)] has been developed using a genome-wide association study (GWAS) with non-targeted metabolomics. The established GDMs provide functional intermediates to facilitate understanding of the potential relevance of human serum metabolites and related genetic variants in the pathogenesis of complex diseases (12–15).
Mendelian randomization (MR) is a novel genetic epidemiological approach that uses genetic variants as instrumental variables to assess the causality of an agent on clinical outcomes of interest (16). The basic principle of the MR study design uses instrumental variables rather than only exposure to infer causality of exposures on clinical outcomes. This primarily requires the assumption that the generated instrumental variable (usually genetic variants) is reliably associated with the exposure and acts on the outcome directly through exposure of interest. Unlike traditional metabolomic approaches, MR can provide unbiased detection of causal effects, considering the fact that genetic variants are less susceptible to environmental factors (17, 18). In the past decade, MR has been widely applied to infer causal relationships using publicly available GWAS summary statistics (19–21). Taking advantage of GDMs and GWAS findings for PCOS, we conducted this two-sample (exposure and outcome measured in different samples) MR study to (i) assess the causal effects of 486 serum metabolites on the risk of developing PCOS and (ii) investigate the genetic variants that determine the variation of the metabolites, which also contribute to the development of PCOS.
We obtained data for genetic factors that influence human blood metabolites from the study of Shin et al. (15). They conducted genome-wide association scans using the metabolome as a phenotype. The study comprised 7,824 adult individuals from two European population studies. Metabolic profiling was performed on fasting serum using ultrahigh-performance liquid-phase chromatography and gas chromatography coupled with tandem mass spectrometry (13, 22). A standardized process of identification and relative quantification, data reduction, and quality assurance was performed using Metabolon, Inc. (https://www.metabolon.com/). A total of 486 metabolites, assigned to eight broad metabolic groups (amino acids, carbohydrates, cofactors and vitamins, energy, lipids, nucleotides, peptides, and xenobiotic metabolism), were included in the final GWAS analysis. Among these, 196 (37%) were classified as “unknown,” which meant that their chemical identity had not been clearly determined thus far. We included these “unknown” metabolites in our study as they still attracted attention from other researchers and might provide further useful information in the future (22). After genotyping, imputation, and quality control (QC), ~2.1 million single-nucleotide polymorphisms (SNPs) were identified in the final GWAS meta-analysis. The complete GWAS summary statistics are publicly available through the Metabolomics GWAS server at http://metabolomics.helmholtz-muenchen.de/gwas/.
In order to satisfy the primary conditions for preforming MR, we implemented strict procedures to select the genetic instruments of these 486 metabolites. First, we screened out the genetic variants that were strongly (P < 1 × 10−5) associated with specific metabolites to ensure that the generated instrument could explain a larger variance in the corresponding metabolite. Next, we selected independent SNPs (r2 < 0.1 within ± 500 kb) to generate the instrumental variable using a clumping procedure with the European 1,000G as reference panel. We further tested whether these genetic instruments could explain the variation of the corresponding metabolites to avoid instruments with a weak first stage. The proportion of variability (R2) and F statistic was calculated to assess the strength of these instrumental variables (23). F statistic > 10 was considered for selection of strong instrumental variables (24).
Genetic associations with PCOS were obtained from a recent large GWAS meta-analysis, with 4,138 cases and 20,129 controls collated from six European cohorts (25). The included samples were either diagnosed according to the National Institutes of Health (NIH) (require HA and OD) or Rotterdam criteria (requires at least two traits of HA, OD, and PCOM) (26). All data involved in the GWAS analysis had been approved by the authors' Institutional Review Board (IRB). Written informed consent was also obtained from all participants. Summary-level results were obtained from these studies, and QC procedures were performed according to the EasyQC pipeline (27). The genome-wide association analysis was performed using a fixed-effect, inverse variance weighted (IVW) meta-analysis using METAL (28).
To calculate causal estimates, we used the standard IVW method for the two-sample MR analysis of the summarized datasets of the serum metabolites and PCOS (16). The IVW approach was employed with the fundamental assumption that all genetic variants referred to valid instruments, and it thus provided efficient and consistent casual estimates. Specifically, the IVW estimate could be equivalently interpreted as a liner regression with SNP–exposure associations as the independent variable and SNP–outcome associations as the dependent variable, setting the intercept term to zero. The P-value was calculated from a standard normal cumulative distribution function of the ratio of the combined causal effects and its standard error. The results were considered statistically significant at the threshold of P < 0.05.
The IVW approach referred to the primary MR analysis and successfully aided in inferring the causality of an exposure for outcome. However, there were still several concerns. One important concern was the existence of horizontal pleiotropy. Horizontal pleiotropy occurs when any variants were invalid instruments and acted on the outcome through other ways (not through the concerned exposure). To control for horizontal pleiotropy, we next applied additional MR methods for sensitivity analyses: the weighted median method, which allowed a subset of genetic variants (<50%) to be invalid instrumental variables (29); MR-Egger, which worked even when up to 50% of the variants came from invalid instrumental variables (30); and MR-PRESSO (pleiotropy residual sum and outlier), which could provide a pleiotropy residual sum and outlier test by identifying and discarding horizontal pleiotropic outliers (31). Further, we detected the presence of horizontal pleiotropy through the MR-PRESSO Global test. All MR analyses were carried out using the R package “MendelianRandomisation” as well as the MR-PRESSO software (https://github.com/rondolab/MR-PRESSO).
Following standard procedures, we obtained the instrumental variables containing 3–675 independent SNPs for the 486 metabolites. The variables explained 0.8–83.5% (median 4.7%) of the variance for their corresponding metabolites. The minimum F statistic for representing the strength of the predictive instrumental variable was 20.33. All instrumental variables for the 486 metabolites were sufficiently informative for MR analysis.
With the use of genetic variants as proxies, the IVW identified 24 metabolites with causal effects on PCOS, among which 13 (54.2%) were known metabolites while the remaining 11 belonged to the “unknown” subgroup (Figure 1, Supplemental Table 1). We focused on the 13 known metabolites, which included nine lipids, a xenobiotic, a peptide, an amino acid, and a nucleotide. 3-Dehydrocarnitine was the most significant chemical compound with predicted causal effects on PCOS (P = 0.0007). The risk of developing PCOS increased 5-fold for a 1-s.d. increase in the level of 3-dehydrocarnitine (OR = 6.72; 95% CI 2.22–20.32). Two other carnitines with causal associations with PCOS were hexanoylcarnitine (OR = 2.65; 95% CI 1.35–5.19; P = 0.0045) and 2-tetradecenoyl carnitine (OR = 0.52; 95% CI 0.30–0.90; P = 0.0193). Notably, 2-tetradecenoyl carnitine had an inverse association with PCOS, unlike 3-dehydrocarnitine and hexanoylcarnitine. 4-Hydroxyhippurate, a xenobiotic, was associated with an increased risk of developing PCOS. β-Hydroxyisovalerate, classified as an amino acid, also appeared to be a pathogenic risk factor for PCOS (OR = 2.84; 95% CI 1.20–6.76; P = 0.0179).
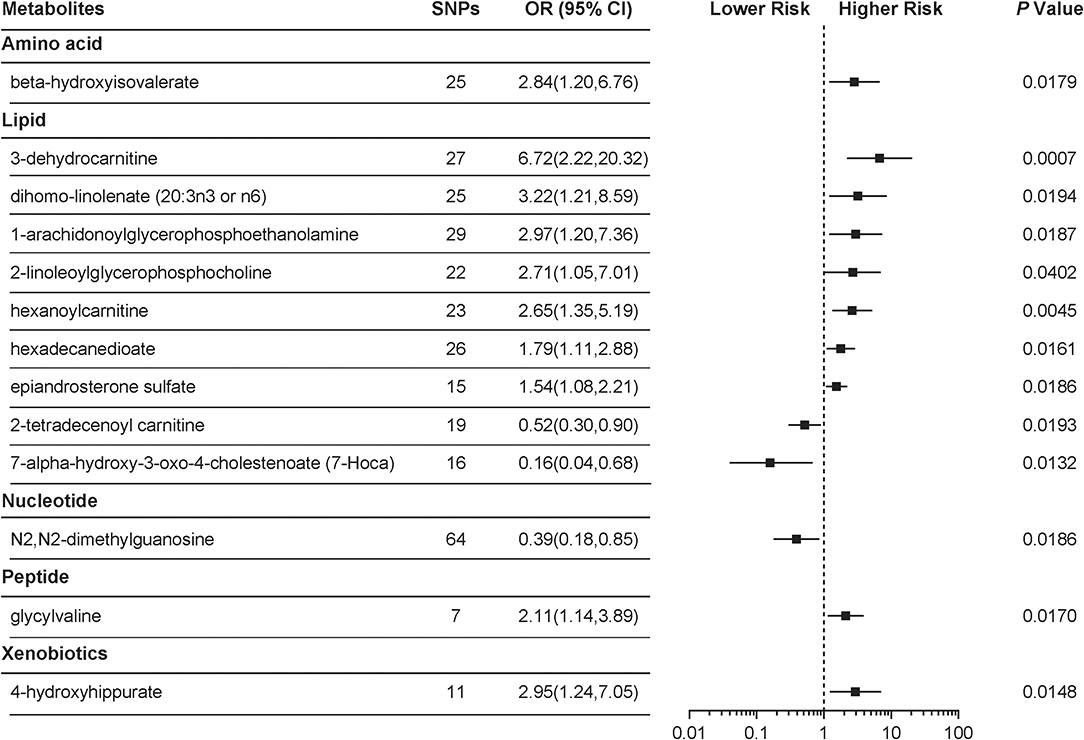
Figure 1. Mendelian randomization (MR) association of serum metabolites on the risk of polycystic ovary syndrome (PCOS). Causal estimates are obtained using metabolite-related single-nucleotide polymorphisms (SNPs) as instrumental variables using the inverse variance weighted (IVW) method. Odds ratio (OR) and 95% confidence intervals (95% CIs) are provided for PCOS per 1-s.d. higher level of specific metabolite.
Table 1 shows the sensitivity analysis results for assessing the robustness of our IVW estimates. Epiandrosterone sulfate (EPIA-S) was the only metabolite with robust associations across all additional MR methods (PIVW = 0.0186; PMR−Egger = 0.0111; PWeighted−median = 0.0154; and PMR−PRESSO = 0.0290), and there was no evidence of horizontal pleiotropy (PGlobal = 0.5190). Using 15 genetic predictors as instrumental variables (variance explained = 8.0%; F statistic = 45.53), we observed a 50% higher risk of developing PCOS for each 1-s.d. increase in the level of EPIA-S (Figure 2A). There were also several metabolites that passed some of the additional sensitivity tests, such as 3-dehydrocarnitine (PMR−Egger = 0.0727; PWeighted−median = 0.0017; and PMR−PRESSO = 0.0023, Figure 3A), 4-hydroxyhippurate (PMR−Egger = 0.2294; PWeighted−median = 0.0318; and PMR−PRESSO = 0.0351, Figure 3B), hexadecanedioate (PMR−Egger = 0.0320; PWeighted−median = 0.1272; and PMR−PRESSO = 0.0238, Figure 3C), and β-hydroxyisovalerate (PMR−Egger = 0.2096; PWeighted−median = 0.0300; and PMR−PRESSO = 0.0051, Figure 3D). These metabolites may have plausible effects on PCOS because the MR-Egger and weighted median method are based on assumptions that might be incorrect. The relationship between hexadecanedioate and PCOS should be carefully investigated as the MR-Egger method yielded an inverse association compared to the other MR methods.
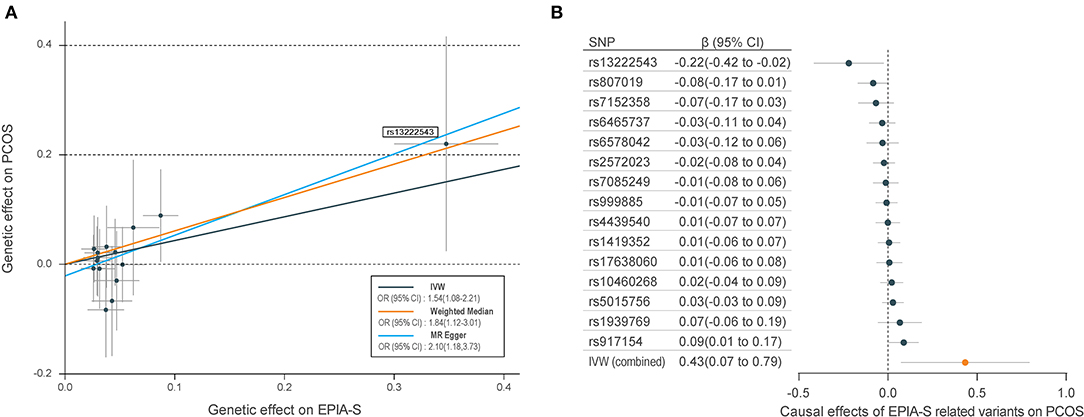
Figure 2. Mendelian randomization (MR) plots for relationship of epiandrosterone sulfate (EPIA-S) with polycystic ovary syndrome (PCOS). (A) Scatter plot of potential effects of single-nucleotide polymorphisms (SNPs) on EPIA-S vs. PCOS, with the slope of each line corresponding to the estimated MR effect per method. SNPs showing negative signals with EPIA-S are plotted after orientation to the exposure-increasing allele. SNPs with higher effects on both metabolites and PCOS are marked on the plots. (B) Forest plot of individual and combined effects of EPIA-S related SNPs on PCOS. Data are expressed as raw β values with 95% confidence interval (CI).
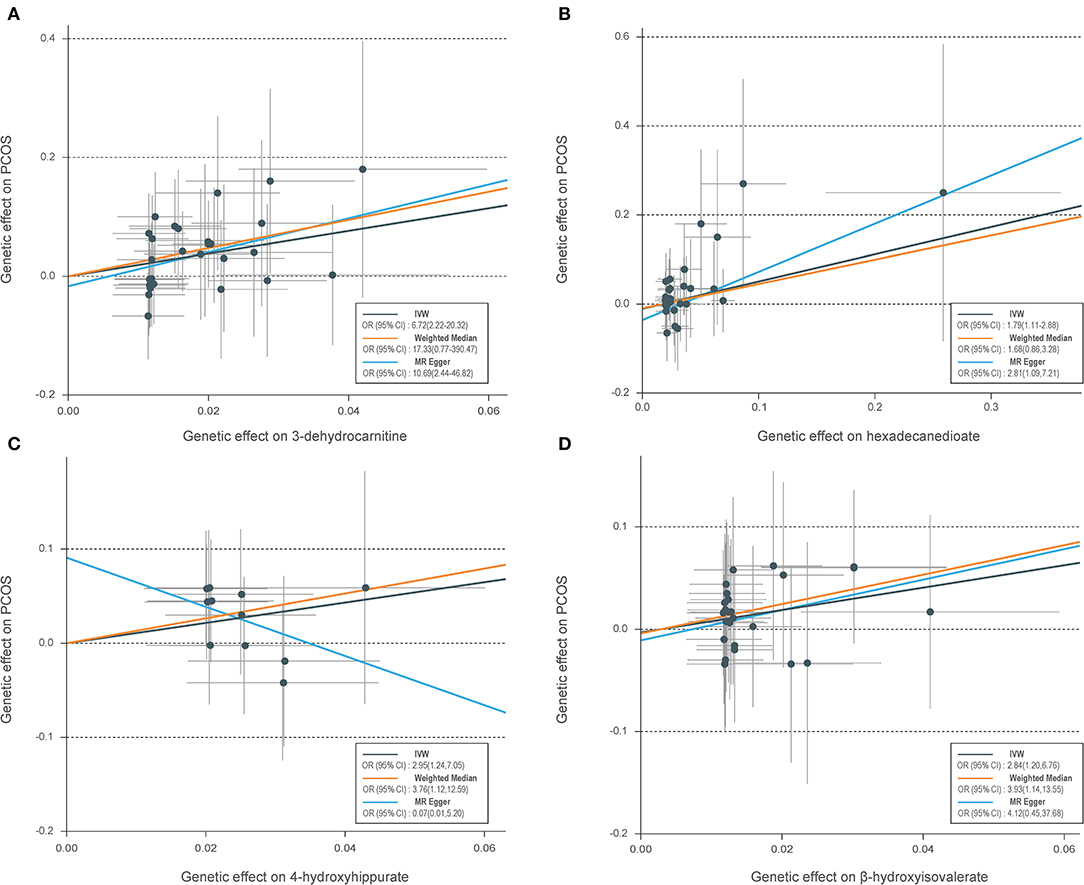
Figure 3. Scatter plots of genetic associations with four suggestive metabolites vs. the associations with polycystic ovary syndrome (PCOS). (A) 3-Dehydrocarnitine; (B) hexadecanedioate; (C) 4-hydroxyhippurate; (D) β-hydroxyisovalerate. Each of the single-nucleotide polymorphisms (SNPs) associated with metabolites are represented by a black dot with the error bar depicting the standard error of its association with metabolite (horizontal) and PCOS (vertical). The slopes of each line represent the causal association for each method. SNPs showing negative signals with metabolites are plotted after orientation to the exposure-increasing allele.
We further reported the potential genetic variants that might have decisive roles in determining the causal relationships between the metabolites and PCOS. Among the 15 SNPs in the instrumental variable of EPIA-S, rs13222543 showed the most significant association signal and the largest association coefficient with EPIA-S (β = −0.347; SE = 0.024; P = 3.31E−47, Table 2). Interestingly, it also showed a strong effect on PCOS (β = −0.220; SE = 0.100; P = 0.033, Figure 2B). Table 2 shows all the leading SNPs for determining the relationships for metabolites with PCOS. Further, we listed all the genetic variants for determining levels of EPAS-S, 3-dehydrocarnitine, 4-hydroxyhippurate, hexadecanedioate, β-hydroxyisovalerate, and the other metabolites in Supplemental Tables 2–7. These generated SNPs could provide important information for revealing potential pathophysiological mechanism or therapeutic targets for PCOS.
We performed a two-sample MR analysis to provide an unbiased detection of potential causal effects of GDMs on PCOS. Using genetic variants as proxies, we observed that genetically determined higher level of EPIA-S is associated with increased risk of developing PCOS. Our study also detected other metabolites that showed significant signals in most MR methods, including 3-dehydrocarnitine, 4-hydroxyhippurate, hexadecanedioate, and β-hydroxyisovalerate. Our findings screened out the potential genetic variants that contribute to the underlying causality of metabolites on PCOS. To the best of our knowledge, this is the first study integrating metabolomics with genomics to reveal the pathophysiological mechanisms of PCOS. Our study provides novel insights into the understanding of the role of interactions between genetic and metabolic factors in the pathogenesis of human diseases.
Based on 15 genetic scores with different degrees of specificity to EPIA-S, we demonstrated that high levels of EPIA-S are genetically associated with a higher risk of developing PCOS. Despite a lack of information on its effects, EPIA-S has recently been proposed as a marker for oral or intramuscular testosterone administration (32, 33). This was also proposed for the administration of other steroid hormones, such as dehydroepiandrosterone (DHEA), 4-androstenedione, and dihydrotestosterone (34). PCOS has long been recognized as a disorder of excessive androgen biosynthesis, use, or metabolism. DHEA sulfate (DHEA-S), DHEA, androstenedione, and testosterone are routinely assessed to identify HA in women with PCOS (35). However, these biochemical indices usually cannot provide a reliable reflection of HA. EPIA-S might be an alternative biomarker in the diagnosis of PCOS, considering its excellent performance in testosterone management. However, the relationship between EPIA-S and PCOS has not been verified by clinical data, and further research is required to understand the potential role of EPIA-S in the diagnosis or treatment of PCOS.
The present study also identified additional metabolites that showed possible association with PCOS, including 3-dehydrocarnitine, 4-hydroxyhippurate, hexadecanedioate, and β-hydroxyisovalerate. 3-Dehydrocarnitine is a member of the carnitine family that is an intermediate in carnitine degradation. Carnitines have long been associated with weight loss, glucose tolerance, insulin function, and fatty acid metabolism (36). A recent study also suggested that 3-dehydrocarnitine is an early biomarker for predicting type 2 diabetes, with applications even prior to the development of insulin resistance (37). Thus, 3-dehydrocarnitine might play a role in abnormal glucose metabolism, which is a common clinical manifestation in PCOS patients. 4-Hydroxyhippuric acid is a microbial end-product derived from polyphenol metabolism by the microflora in the intestine (38). A natural polyphenol, resveratrol, is reported to play a role in inhibiting, androgen production and has been suggested to be a potential therapeutic agent for PCOS (39–41). This might suggest that the polyphenols have a potential value as therapeutic compounds for PCOS. Hexadecanedioate is a candidate biomarker for monitoring organic anion-transporting polypeptide (OATP) function in preclinical species or humans (42). OATP is a group of transporters that are required in DHEA circulation. A previous study also found increased levels of OATP-family transporters in patients with PCOS-endometria, which suggests that OATP plays a functional role in the pathogenesis of PCOS (43). β-Hydroxyisovalerate is a conjugate base of 3-hydroxyisovaleric acid. 3-Hydroxyisovaleric acid was demonstrated to be related to impaired cellular respiration and mitochondrial function (44). Carnitines were also suggested to be involved in the metabolism of 3-hydroxyisovaleric acid (45). In general, the identified metabolites contributed to our understanding of the pathogenesis of PCOS and might also serve as possible therapeutic targets.
We focused on genetic variants that contribute to variation in the target metabolites. The SNP rs13222543, which is located at the intron region of ZCWPW1, was the most significant variant for EPIA-S. ZCWPW1 is a candidate gene for Alzheimer's disease; however, no link had previously been established between ZCWPW1 and EPIA-S (46, 47). Interestingly, enrichment in the male testis for RNA expression of ZCWPW1 has been reported by the Human Protein Atlas database (https://www.proteinatlas.org/), although the expression levels are also high in the thyroid gland, fallopian tube, and ovary (48). This suggests that ZCWPW1 may play a role in steroid hormone metabolism. The SNP rs6691848 is related to 3-dehydrocarnitine and is located on ZMYM6, which is actively expressed in the pituitary gland, and regulates steroid metabolism. Although further evidence is lacking, these connections provide new clues to understand the underlying molecular mechanisms of PCOS.
The present study has several limitations. First, the MR identified causal metabolites associated with the risk of developing PCOS using genetic variants as instrumental variables. Further experimental studies should be conducted to verify these findings. Second, multiple testing was not adjusted, but robustness of the results had been supported by using multiple MR algorithms. Third, the accuracy of MR depends on how well the genetic instruments explain the exposure. The current GWAS analysis on metabolites is based on European populations with a limited sample size. Effort should therefore be made to collect more samples across a broader swath of the population to provide a more accurate assessment of the influence of genetic factors on metabolites. Finally, the findings of our study might be only limited to the European population, not necessarily generalizable to others.
The present study adopted an MR approach to identify PCOS-related metabolites. The MR approach used genetic variants as instrumental variables to provide unconfounded estimates of the causal relationships between serum metabolites and PCOS. EPIA-S was identified as a causal metabolite that was robustly associated with PCOS development. Some other metabolites, such as 3-dehydrocarnitine, 4-hydroxyhippurate, hexadecanedioate, and β-hydroxyisovalerate, may also have causal effects on the development of PCOS. We emphasized the role of genetic factors underlying the causal relationships between metabolites and PCOS. We provided novel insights by integrating metabolomics with genomics to better understand the mechanisms underlying the pathogenesis of human disease.
Publicly available datasets were analyzed in this study. This data can be found here: http://metabolomics.helmholtz-muenchen.de/gwas/, https://doi.org/10.17863/CAM.27720.
XL and SS were responsible for the study conception and study design. MJ, CH, and QZ were involved in data acquisition and study execution. SS and MJ analyzed the data and drafted the manuscript. WS and JS contributed to interpretation and editing of the manuscript. All authors approved the final version of the manuscript.
The study is funded by General Projects of Social Development in Shaanxi Province (Grant No. 2018SF-247).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the Clinical Research Center of the First Affiliated Hospital of Xi'an Jiaotong University for statistical assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00621/full#supplementary-material
1. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. (2007) 370:685–97. doi: 10.1016/S0140-6736(07)61345-2
2. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. (2010) 25:544–51. doi: 10.1093/humrep/dep399
3. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. (2016) 2:16057. doi: 10.1038/nrdp.2016.57
4. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. (2011) 7:219–31. doi: 10.1038/nrendo.2010.217
5. Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. (2011) 43:55–9. doi: 10.1038/ng.732
6. Kandaraki E, Christakou C, Diamanti-Kandarakis E. Metabolic syndrome and polycystic ovary syndrome and vice versa. Arq Bras Endocrinol Metabol. (2009) 53:227–37. doi: 10.1590/S0004-27302009000200014
7. Joyce AR, Palsson BO. The model organism as a system: integrating 'omics' data sets. Nat Rev Mol Cell Biol. (2006) 7:198–210. doi: 10.1038/nrm1857
8. Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. (2008) 134:714–7. doi: 10.1016/j.cell.2008.08.026
9. Zhao Y, Fu L, Li R, Wang LN, Yang Y, Liu NN, et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC Med. (2012) 10:153. doi: 10.1186/1741-7015-10-153
10. Chang AY, Lalia AZ, Jenkins GD, Dutta T, Carter RE, Singh RJ, et al. Combining a nontargeted and targeted metabolomics approach to identify metabolic pathways significantly altered in polycystic ovary syndrome. Metabolism. (2017) 71:52–63. doi: 10.1016/j.metabol.2017.03.002
11. Zhang Z, Hong Y, Chen M, Tan N, Liu S, Nie X, et al. Serum metabolomics reveals metabolic profiling for women with hyperandrogenism and insulin resistance in polycystic ovary syndrome. Metabolomics. (2020) 16:20. doi: 10.1007/s11306-020-1642-y
12. Gieger C, Geistlinger L, Altmaier E, Hrabe de Angelis M, Kronenberg F, Meitinger T, et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet. (2008) 4:e1000282. doi: 10.1371/journal.pgen.1000282
13. Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wägele B, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. (2011) 477:54–60. doi: 10.1038/nature10354
14. Kettunen J, Tukiainen T, Sarin AP, Ortega-Alonso A, Tikkanen E, Lyytikainen LP, et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet. (2012) 44:269–76. doi: 10.1038/ng.1073
15. Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. (2014) 46:543–50. doi: 10.1038/ng.2982
16. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
17. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
18. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
19. Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Vosa U, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. (2019) 51:600–5. doi: 10.1038/s41588-019-0350-x
20. Telomeres Mendelian Randomization C, Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN, et al. Association between telomere length and risk of cancer and non-neoplastic diseases: a mendelian randomization study. JAMA Oncol. (2017) 3:636–51. doi: 10.1001/jamaoncol.2016.5945
21. White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, et al. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol. (2016) 1:692–9. doi: 10.1001/jamacardio.2016.1884
22. Krumsiek J, Suhre K, Evans AM, Mitchell MW, Mohney RP, Milburn MV, et al. Mining the unknown: a systems approach to metabolite identification combining genetic and metabolic information. PLoS Genet. (2012) 8:e1003005. doi: 10.1371/journal.pgen.1003005
23. Pierce BL, Ahsan H, VanderWeele TJ. Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
24. Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. doi: 10.1093/ije/dyt179
25. Cotsapas C, Day F, Karaderi T, Jones MR, Meun C, He C, et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLOS Genet. (2018) 14:e1007813. doi: 10.1371/journal.pgen.1007813
26. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Grou. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004
27. Winkler TW, Day FR, Croteau-Chonka DC, Wood AR, Locke AE, Magi R, et al. Quality control and conduct of genome-wide association meta-analyses. Nat Protoc. (2014) 9:1192–212. doi: 10.1038/nprot.2014.071
28. Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. (2010) 26:2190–1. doi: 10.1093/bioinformatics/btq340
29. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
30. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. (2016) 45:1961–74. doi: 10.1093/ije/dyw220
31. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
32. Esquivel A, Alechaga E, Monfort N, Ventura R. Sulfate metabolites improve retrospectivity after oral testosterone administration. Drug Test Anal. (2019) 11:392–402. doi: 10.1002/dta.2529
33. Esquivel A, Alechaga E, Monfort N, Yang S, Xing Y, Moutian W, et al. Evaluation of sulfate metabolites as markers of intramuscular testosterone administration in caucasian and Asian populations. Drug Test Anal. (2019) 11:1218–30. doi: 10.1002/dta.2598
34. Piper T, Putz M, Schanzer W, Pop V, McLeod MD, Uduwela DR, et al. Epiandrosterone sulfate prolongs the detectability of testosterone, 4-androstenedione, and dihydrotestosterone misuse by means of carbon isotope ratio mass spectrometry. Drug Test Anal. (2017) 9:1695–703. doi: 10.1002/dta.2291
35. Duskova M, Kolatorova L, Starka L. Androgens in women - critical evaluation of the methods for their determination in diagnostics of endocrine disorders. Physiol Res. (2018) 67 (Suppl. 3):S379–90. doi: 10.33549/physiolres.933964
36. Samimi M, Jamilian M, Ebrahimi FA, Rahimi M, Tajbakhsh B, Asemi Z. Oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol. (2016) 84:851–7. doi: 10.1111/cen.13003
37. Sun L, Liang L, Gao X, Zhang H, Yao P, Hu Y, et al. Early prediction of developing type 2 diabetes by plasma acylcarnitines: a population-based study. Diabetes Care. (2016) 39:1563–70. doi: 10.2337/dc16-0232
38. Jacobs DM, Spiesser L, Garnier M, de Roo N, van Dorsten F, Hollebrands B, et al. SPE-NMR metabolite sub-profiling of urine. Anal Bioanal Chem. (2012) 404:2349–61. doi: 10.1007/s00216-012-6339-2
39. Ortega I, Duleba AJ. Ovarian actions of resveratrol. Ann N Y Acad Sci. (2015) 1348:86–96. doi: 10.1111/nyas.12875
40. Furat Rencber S, Kurnaz Ozbek S, Eraldemir C, Sezer Z, Kum T, Ceylan S, et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. J Ovarian Res. (2018) 11:55. doi: 10.1186/s13048-018-0427-7
41. Banaszewska B, Wrotynska-Barczynska J, Spaczynski RZ, Pawelczyk L, Duleba AJ. Effects of resveratrol on polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. (2016) 101:4322–8. doi: 10.1210/jc.2016-1858
42. Shen H, Chen W, Drexler DM, Mandlekar S, Holenarsipur VK, Shields EE, et al. Comparative evaluation of plasma bile acids, dehydroepiandrosterone sulfate, hexadecanedioate, and tetradecanedioate with coproporphyrins I and III as markers of OATP inhibition in healthy subjects. Drug Metab Dispos. (2017) 45:908–19. doi: 10.1124/dmd.117.075531
43. Plaza-Parrochia F, Poblete C, Gabler F, Carvajal R, Romero C, Valladares L, et al. Expression of steroid sulfated transporters and 3β-HSD activity in endometrium of women having polycystic ovary syndrome. Steroids. (2015) 104:189–95. doi: 10.1016/j.steroids.2015.10.001
44. Mock DM, Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, et al. Urinary excretion of 3-hydroxyisovaleric acid and 3-hydroxyisovaleryl carnitine increases in response to a leucine challenge in marginally biotin-deficient humans. J Nutr. (2011) 141:1925–30. doi: 10.3945/jn.111.146126
45. Maeda Y, Ito T, Ohmi H, Yokoi K, Nakajima Y, Ueta A, et al. Determination of 3-hydroxyisovalerylcarnitine and other acylcarnitine levels using liquid chromatography-tandem mass spectrometry in serum and urine of a patient with multiple carboxylase deficiency. J Chromatogr B Analyt Technol Biomed Life Sci. (2008) 870:154–9. doi: 10.1016/j.jchromb.2007.11.037
46. Wachinger C, Nho K, Saykin AJ, Reuter M, Rieckmann A, Alzheimer's Disease Neuroimaging I. A longitudinal imaging genetics study of neuroanatomical asymmetry in Alzheimer's disease. Biol Psychiatry. (2018) 84:522–30. doi: 10.1016/j.biopsych.2018.04.017
47. Chibnik LB, White CC, Mukherjee S, Raj T, Yu L, Larson EB, et al. Susceptibility to neurofibrillary tangles: role of the PTPRD locus and limited pleiotropy with other neuropathologies. Mol Psychiatry. (2018) 23:1521–9. doi: 10.1038/mp.2017.20
Keywords: genetically determined metabolites, polycystic ovary syndrome, mendelian randomization, epiandrosterone sulfate, rs13222543
Citation: Sun S, Jiao M, Han C, Zhang Q, Shi W, Shi J and Li X (2020) Causal Effects of Genetically Determined Metabolites on Risk of Polycystic Ovary Syndrome: A Mendelian Randomization Study. Front. Endocrinol. 11:621. doi: 10.3389/fendo.2020.00621
Received: 31 January 2020; Accepted: 30 July 2020;
Published: 08 September 2020.
Edited by:
Katja Teerds, Wageningen University, NetherlandsReviewed by:
Yuhua Shi, Shandong University, ChinaCopyright © 2020 Sun, Jiao, Han, Zhang, Shi, Shi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojuan Li, bHhqdWFubkBvdXRsb29rLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.