- 1Department of Epidemiology, Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, China
- 2Department of Chronic Communicable Disease, Center for Disease Control and Prevention of Jiangsu Province, Nanjing, China
Background: Periodontitis and metabolic syndrome (MetS) are two major global health problems that are widely prevalent in the world, although the former is a common infection in developing countries and the latter is a non-infectious but prevalent disease in developed countries. This study aims to provide an updated review on the existence and magnitude of the relationship between periodontal disease and the risk of MetS.
Methods: We searched the PubMed, Web of Science, ScienceDirect, Chinese National Knowledge Infrastructure, and Wanfang databases for original studies assessing the association between periodontitis and MetS published before August 2019. We calculated the pooled crude and adjusted odds ratios (ORs) together with the 95% confidence intervals (95% CIs) to estimate the strength of this association. Subgroup analysis was performed by considering the diagnostic method or the country where the studies were performed.
Results: We identified 43 potentially eligible articles for this systematic review, including 32 cross-sectional studies, eight case–control studies, and three cohort studies. Among them, 39 articles presented enough information to be included in the meta-analysis. The pooled crude and adjusted ORs were 1.99 (95% CI: 1.75–2.25) and 1.46 (95% CI: 1.31–1.61), respectively. Subgroup analysis showed a consistent relation stratified by either the diagnostic method or the country where the studies were performed. The pooled OR was 1.68 (95% CI: 1.41–2.00) for Japan, 1.75 (95% CI: 1.31–2.34) for the USA, 1.81 (95% CI: 1.35–2.42) for Korea, and 2.29 (95% CI: 1.53–3.41) for China.
Conclusion: Our results provide compelling evidence for the association between periodontitis and MetS. Patients with periodontal disease are a critical screening population for MetS. We also recommend that people exhibiting components of MetS should receive a periodontal check-up and pay attention to their oral health.
Introduction
Oral health is an undervalued parameter of global health and has been considered inferior on the agendas of policymakers (1). There has been increasing scientific interest regarding the interactions between oral health and systemic diseases. In 2007, the World Health Organization (WHO) called on the integration of health policies regarding oral and general health (1). The European Union and the USA have also emphasized the importance of oral health in overall health (2). Numerous studies have shown a relationship between deficiently poor oral hygiene and different systemic disorders, such as cardiovascular disease (CVD) and metabolic syndrome (MetS). A variety of theories have been proposed, of which a bulk of them hypothesize the mediation of the inflammatory response (3). Periodontitis is one of the most common chronic oral diseases and is characterized by the pathological loss of the periodontal ligament and adjacent supporting alveolar bone. It can also be described as a bacteria-induced complex chronic inflammatory disease (4). It is estimated that approximately 20 to 60% of the world's population may have some degree of periodontal disease (5). Periodontitis is not merely a consequence of plaque accumulation; it is also affected by host factors (6). If discovered in the initial stage, it can be managed successfully without causing much morbidity.
MetS is a prevalent and multifactorial disorder that consists of a cluster of several clinical physical conditions and biological abnormalities that increase the risk of mortality; MetS is affected by insulin resistance and promotes cardiovascular diseases (7). These conditions/diseases include glucose intolerance/insulin resistance/hyperglycemia, hypertension, visceral obesity, and dyslipidemia (8). MetS has an estimated prevalence of 17–32% in the general population (9), which suggests that nearly one-quarter of adults throughout the world are affected (10).
The association between periodontitis and MetS has gained research interest in the scientific literature. There is variation in the reported degree of association, which may be due to the different definitions of MetS, the methodology used to assess periodontitis, and criteria for subject enrollment (11). In addition, there are several different approaches to determine the association between these two diseases/conditions. The American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP) have emphasized that more studies are needed to assess the association between periodontitis and various systemic conditions, including MetS (12). This study aims to provide an updated review on the existence and magnitude of the relationship between periodontal disease and the risk of MetS. A better understanding of periodontal diseases in the development of MetS and vice versa is required for medical and dental professionals to provide the general population with appropriate care.
Methods
Literature Search and Study Selection
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were used (Supplementary Table 1) (13). We searched the PubMed, ScienceDirect, Web of Science (WOS), Chinese National Knowledge Infrastructure (CNKI), and Wanfang databases for studies that reported an association between periodontitis and MetS in adults up to August 2019. Studies were limited to human studies and those published in either English or Chinese. Related keywords used for PubMed were as follows: Periodontal disease OR periodontitis OR periodontal pocket OR clinical attachment loss AND metabolic syndrome OR metabolic disease OR syndrome X OR Reaven's syndrome OR MetS. PubMed MeSH terms were as follows: Metabolic Syndrome/Syndrome X (MeSH term Metabolic Syndrome X for both) and Periodontal Diseases, Periodontitis, Pocket Depth (No MeSH term), Periodontal Pocket, Periodontal Pocketing (MeSH term Periodontal Pocket), Attachment Loss (MeSH term Periodontal Attachment Loss), and Clinical Attachment Loss (MeSH term Tooth Mobility). Keywords for WOS and ScienceDirect searches were metabolic syndrome/periodontal disease, metabolic syndrome/periodontitis, metabolic syndrome/pocket depth, and metabolic syndrome/periodontal pocket. The terms we used were explored to ensure the retrieval of all the items needed concerning the specific search terms. Titles and abstracts of selected studies were examined for their possible relevance to the association between periodontitis and MetS. Studies were considered eligible using the following criteria: original epidemiological studies; observational studies including a cross-sectional, case–control, or cohort design; adult samples; having at least one diagnostic standard for periodontitis that was clearly defined; and having clear criteria for the diagnosis of MetS. Studies matching the eligibility criteria were considered for this systematic review. Abstracts and reviews were excluded; however, references in reviews were used for a supplementary literature search. In total, 43 studies (38 published in English and five published in Chinese) met the inclusion criteria for the systematic review with a total sample size of 114,181 participants. Among them, 39 studies had enough information to be included in the meta-analysis. A random-effect model was conducted to estimate the pooled odds ratios (ORs) together with 95% confidence intervals (95% CIs) to establish the strength of association.
Data Extraction
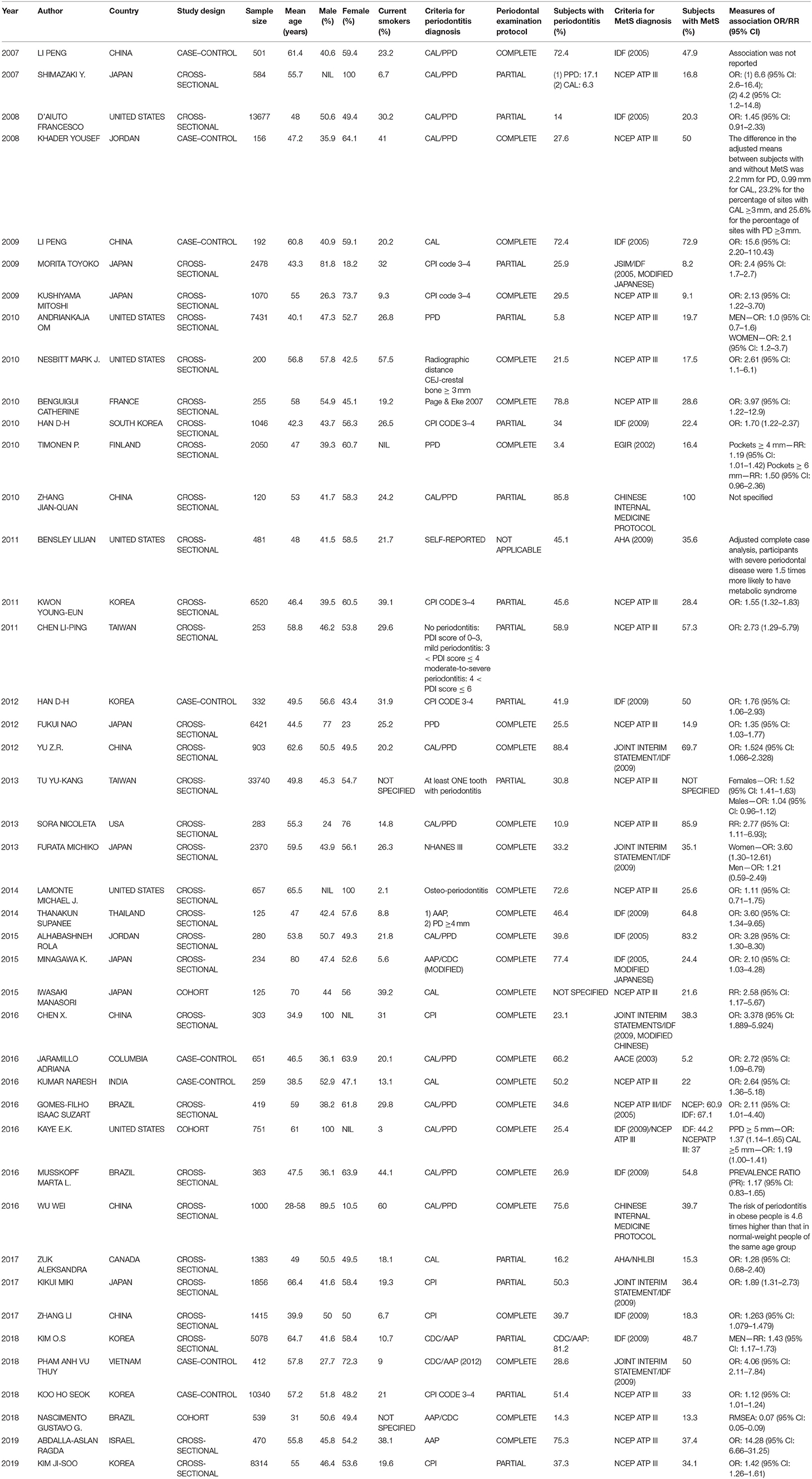
Two reviewers independently identified eligible studies on the above topic, assessed the publication validity, and subsequently extracted data. The discrepancies were discussed and resolved, and the studies were concluded after consultation with the supervisor of this research. We collected data on the year of publication, the first author, study country, study design, sample size, mean age, sex, current tobacco smoking status, criteria for periodontitis diagnosis, periodontal examination protocol, the number of subjects diagnosed with periodontitis, criteria for MetS diagnosis, the number of subjects diagnosed with MetS, and the strength of the association [OR, relative risk (RR) and 95% CI].
Quality Assessment
We used the Newcastle–Ottawa scale to evaluate the quality of the studies. A “star system” was used to judge the study in the following three contexts: the selection of the study groups, the comparability of the groups, and the ascertainment of either the exposure or the outcome of interest. The results varied across the selected studies, which is shown in Supplementary Table 1. The scale has a score of 0–9 stars for each article. A higher number of stars indicates a higher quality of the study (14).
Statistical Analysis
The associations between periodontitis and MetS were assessed by the pooled ORs with the corresponding 95% CIs. Data from 39 studies were collected individually, and a crude OR was calculated, followed by the random-effect model. Then, the second analysis, consisting of the pooled adjusted OR, was derived from the given adjusted ORs from each study. I2 was also used to test the heterogeneity among the included studies. Consequently, subgroup analysis was first performed on the method used to diagnose periodontitis, that is, either partial-mouth periodontal examination or full-mouth periodontal examination. The second subgroup analysis was on the criteria for the diagnosis of MetS, which are the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria, the 2005 International Diabetes Federation (IDF, 2005) criteria, or the 2009 IDF criteria. Finally, the subgroup analysis was performed by country, namely, China, Japan, the USA, and Korea, the countries where most of the published studies have been conducted. The subgroup analysis was performed using both the crude and the adjusted ORs separately. Stata version 15.0 (StataCorp, College Station, Texas, USA) was used to analyze the data.
Results
Description of Studies
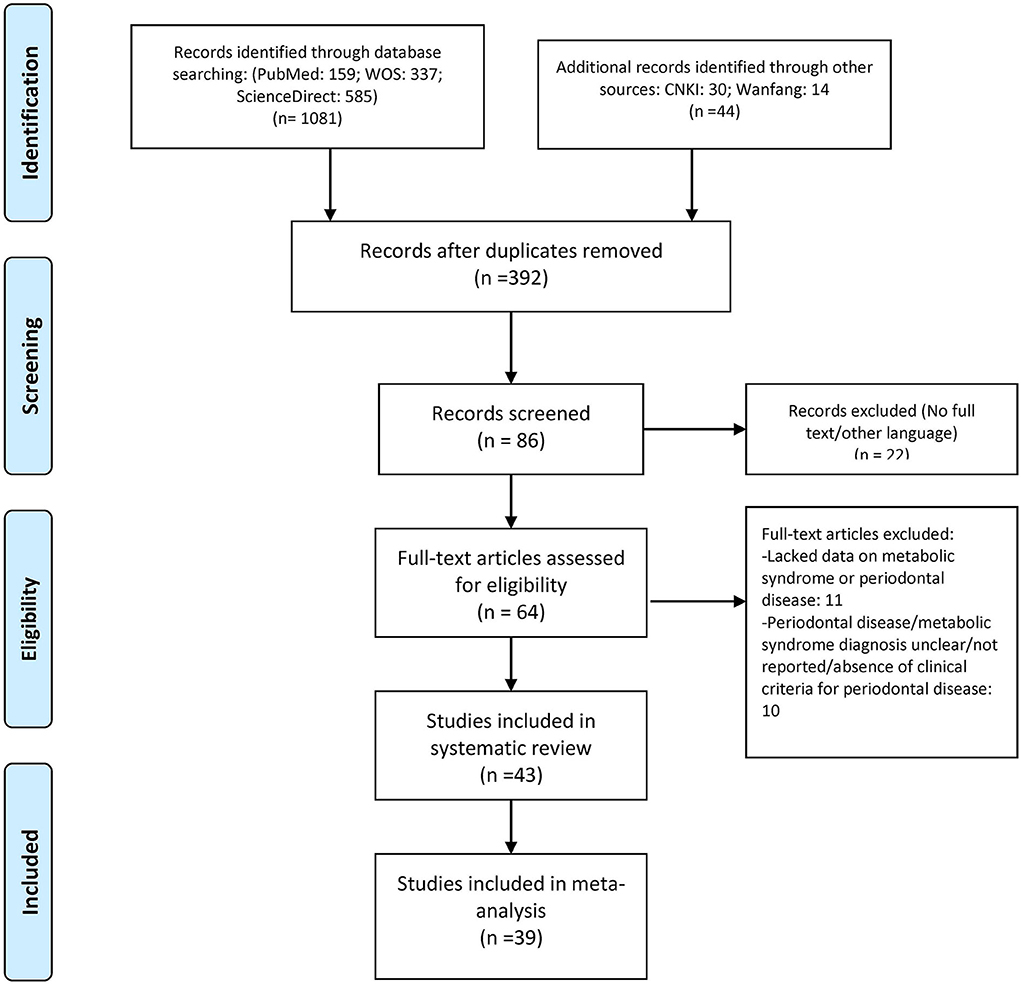
During the literature search, 1,125 articles were selected, and to find other associated studies, articles listed in their references were also screened. Duplicates and other non-related articles were not considered for the systematic review. Then, among these, 86 of the articles were retrieved, and their titles, abstracts, and full texts were scrutinized for possible relevance. Finally, 43 articles were selected for the systematic review, and 39 were enrolled in this meta-analysis where the crude ORs were calculated individually (Figure 1). Additionally, 35 studies with adjusted ORs were used from the specified studies (6, 10, 12, 15–54).
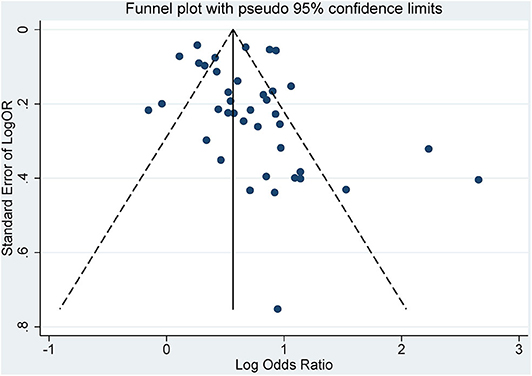
The characteristics of each study are listed in Table 1. Forty-three studies published from 2007 to 2019 were analyzed. There were studies from eastern Asia as well as western Asia, European countries, and North and South American countries. Thirty-eight were published in English, while 5 of them were published in Chinese. The sample size ranged from 120 (27) to 33,740. (33) Of the studies meeting the inclusion criteria for analysis, three were cohort studies (40, 45, 53), 32 were cross-sectional studies, and 8 were case–control studies. The criteria used for the diagnosis of periodontitis were different in these studies. Some used the CPI index, while others used the CAL or PPD. Twenty-seven studies had complete full-mouth examination, 15 had partial-mouth examination, and one study (28) was self-reported periodontitis. The proportion of periodontitis ranged from 3.4 to 88.4% among the mentioned studies. For the diagnosis of MetS, 21 studies used NCEP ATP III criteria, 5 used the 2005 IDF criteria, 13 used the 2009 IDF criteria, and the remaining studies used different methods. The percentage range of patients with MetS ranged from 5.2 to 100% among the studies. The funnel plot for the investigation of publication bias was drawn by plotting the standard error of the logOR against the logOR. As shown in the plot, the risk of publication bias was not significant (Figure 2).
Meta-Analysis
From the data extracted, 39 of the studies showed an association of MetS and periodontitis, with a crude pooled OR of 1.99 (95% CI: 1.75–2.25). The heterogeneity test showed that I2 = 87.7% and P < 0.001 (Figure 3). We further summarized the adjusted ORs, which were mentioned in 32 studies, and showed a pooled adjusted OR of 1.45 (CI: 1.31–1.60). The heterogeneity test showed that I2 = 73.5% and P < 0.001 (Figure 4).
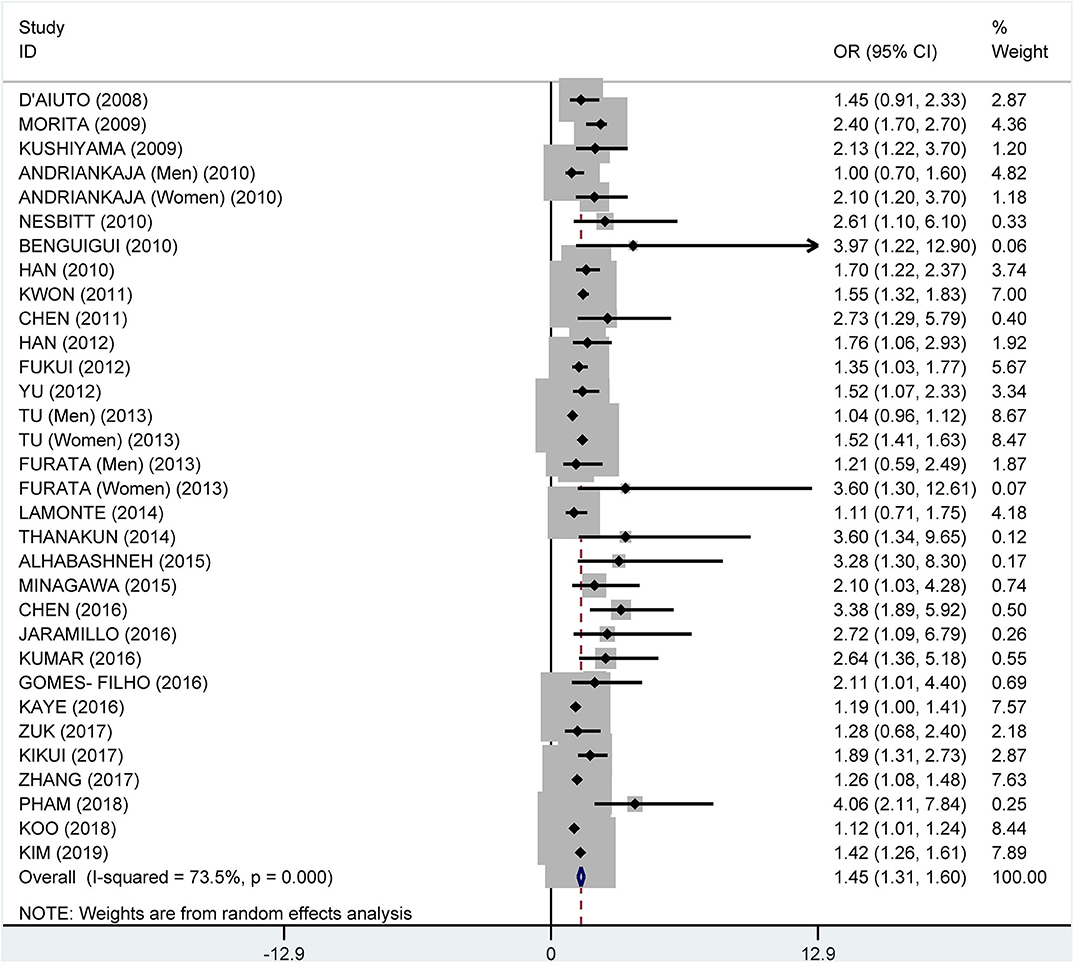
Figure 4. Pooled adjusted odds ratios of the association between periodontitis and metabolic syndrome.
Subgroup Analysis
Subgroup analysis on the tooth examination used to diagnose periodontitis showed a crude OR of 1.91 (95% CI: 1.58–2.31, I2 = 94.0%, P < 0.001) for partial periodontal examination and a crude OR of 2.11 (95% CI: 1.74–2.55, I2 = 78.3%, P < 0.001) for complete periodontal examination (Figure 5). The pooled adjusted OR was 1.38 (95% CI: 1.18, 1.57), I2 = 24.3%, P = 0.180) for the complete periodontal examination and 1.47 (95% CI: 1.27–1.66, I2 = 86.4%, P < 0.001) for the partial periodontal examination (Figure 6).
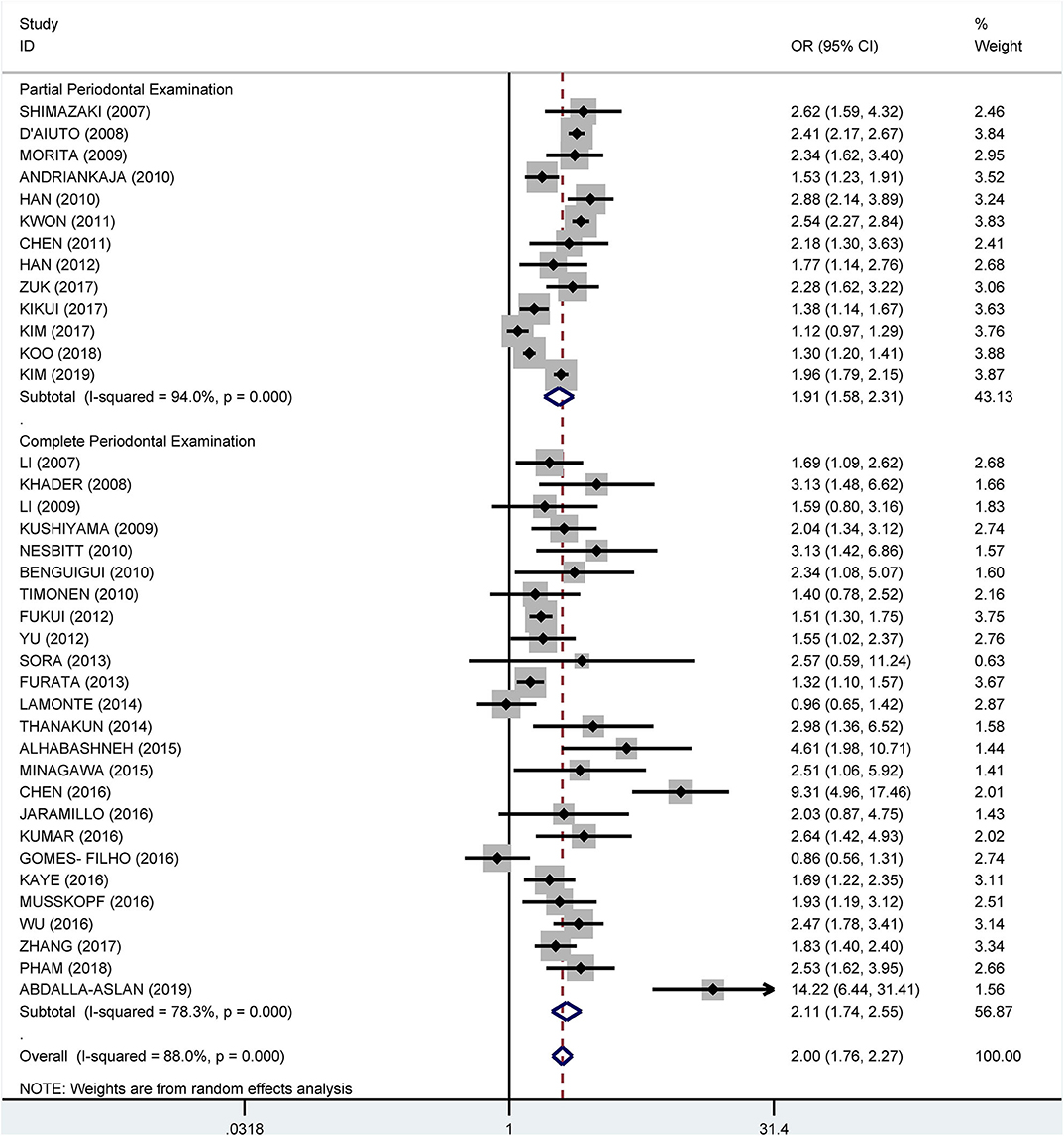
Figure 5. Subgroup analysis of pooled crude odds ratios of the association between periodontitis and metabolic syndrome by the method of examination used to diagnose periodontitis.
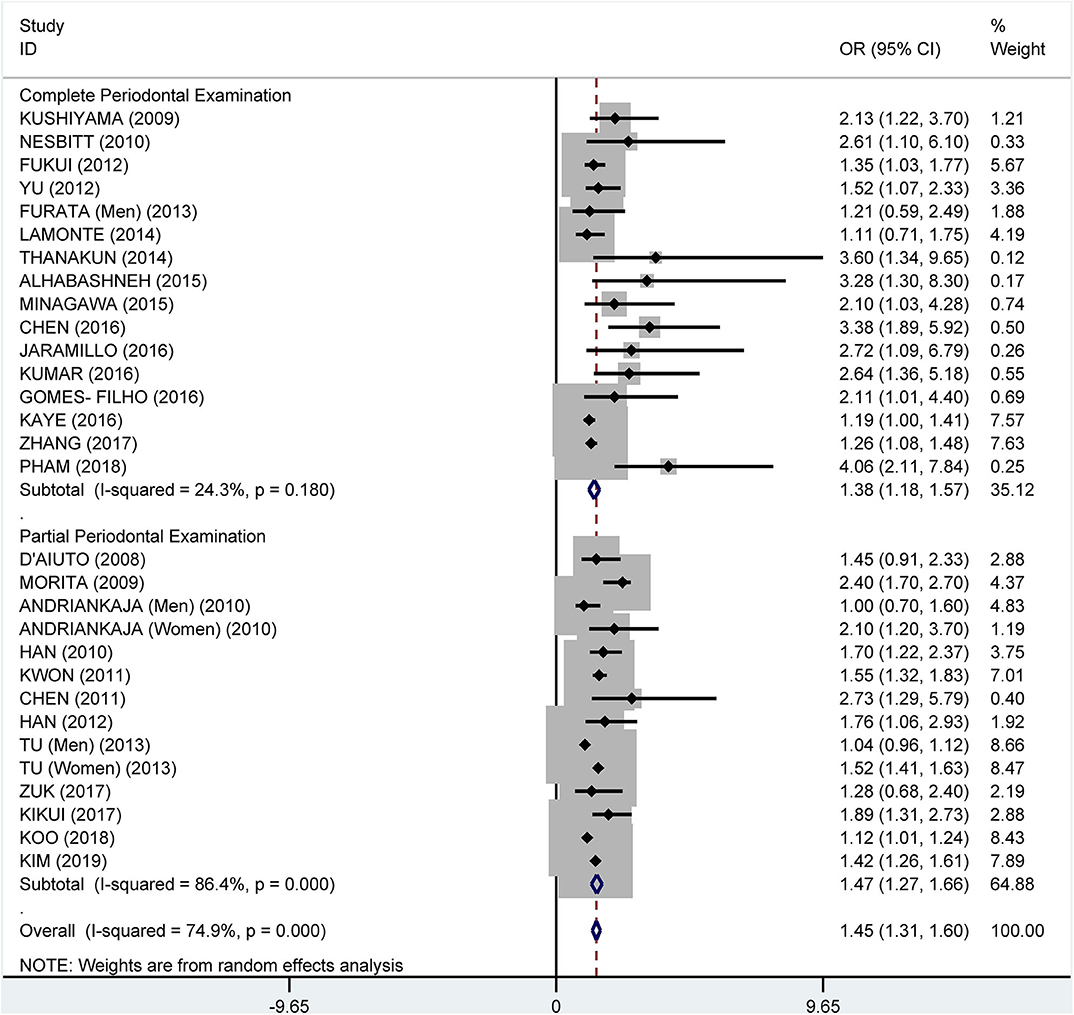
Figure 6. Subgroup analysis of pooled adjusted odds ratios of the association between periodontitis and metabolic syndrome by the method of examination used to diagnose periodontitis.
Subgroup analysis by the diagnostic criteria of MetS showed that the crude OR was 1.83 (95%: 1.45–2.30, I2 = 86.6%, P < 0.001) for the 2009 IDF criteria, 2.08 (95% CI: 1.69-2.55, I2 = 90.7%, P < 0.001) for the NCEP ATP III criteria, and 2.18 (95% CI: 1.62–2.93, I2 = 25.9%, P = 0.249) for 2005 IDF criteria (Figure 7). The pooled adjusted OR was 1.34 (95% CI: 1.16, 1.52, I2 = 83.5%, P < 0.001) for the NCEP ATP III criteria, 1.48 (95% CI: 1.24–1.72, I2 = 38.0%, P = 0.088) for the 2009 IDF criteria, and 2.39 (95% CI: 1.92–2.86, I2 = 0.0%, P = 0.830) for the 2005 IDF criteria (Figure 8).
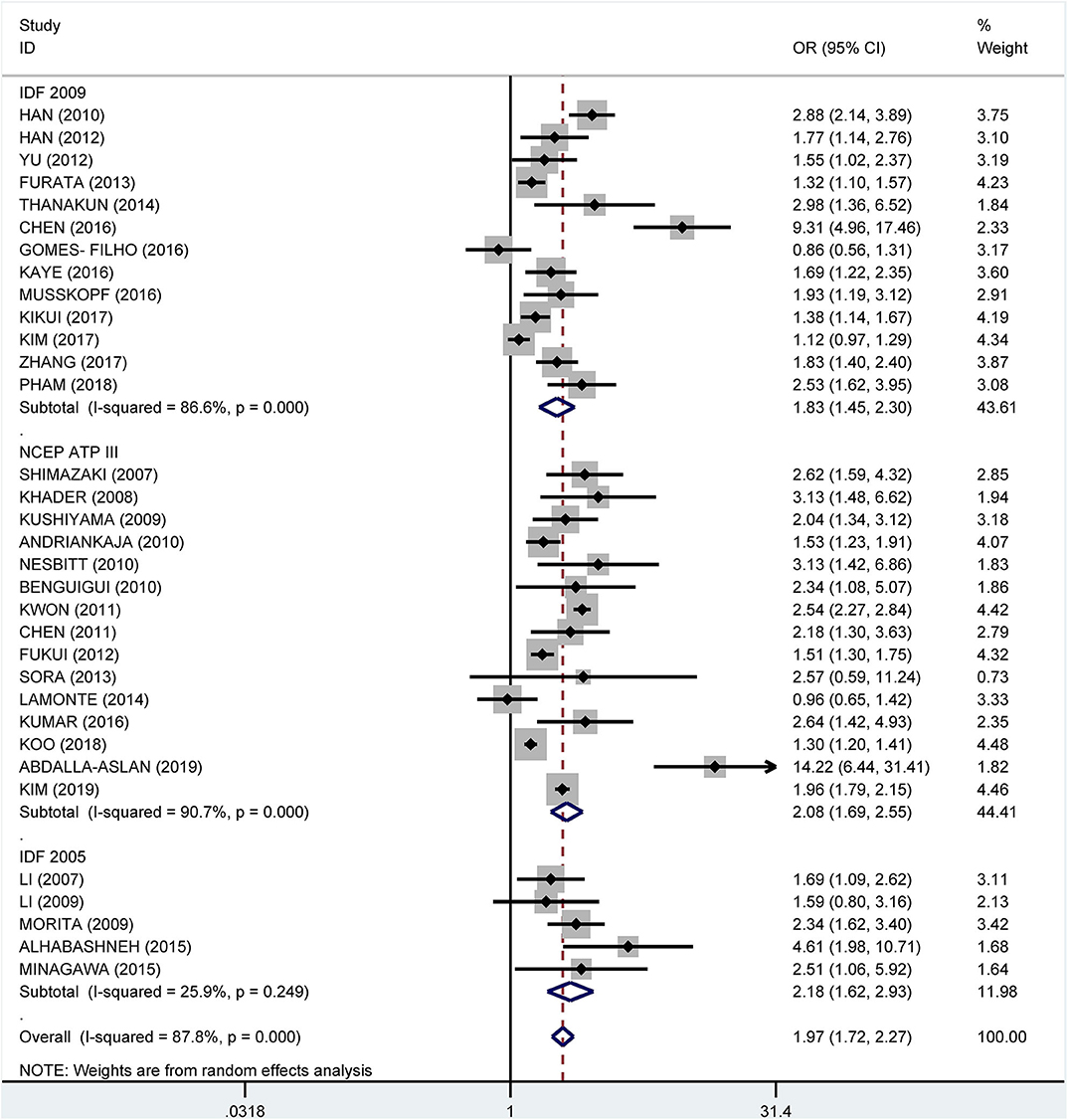
Figure 7. Subgroup analysis of pooled crude odds ratios of the association between periodontitis and metabolic syndrome by diagnostic criteria for metabolic syndrome.
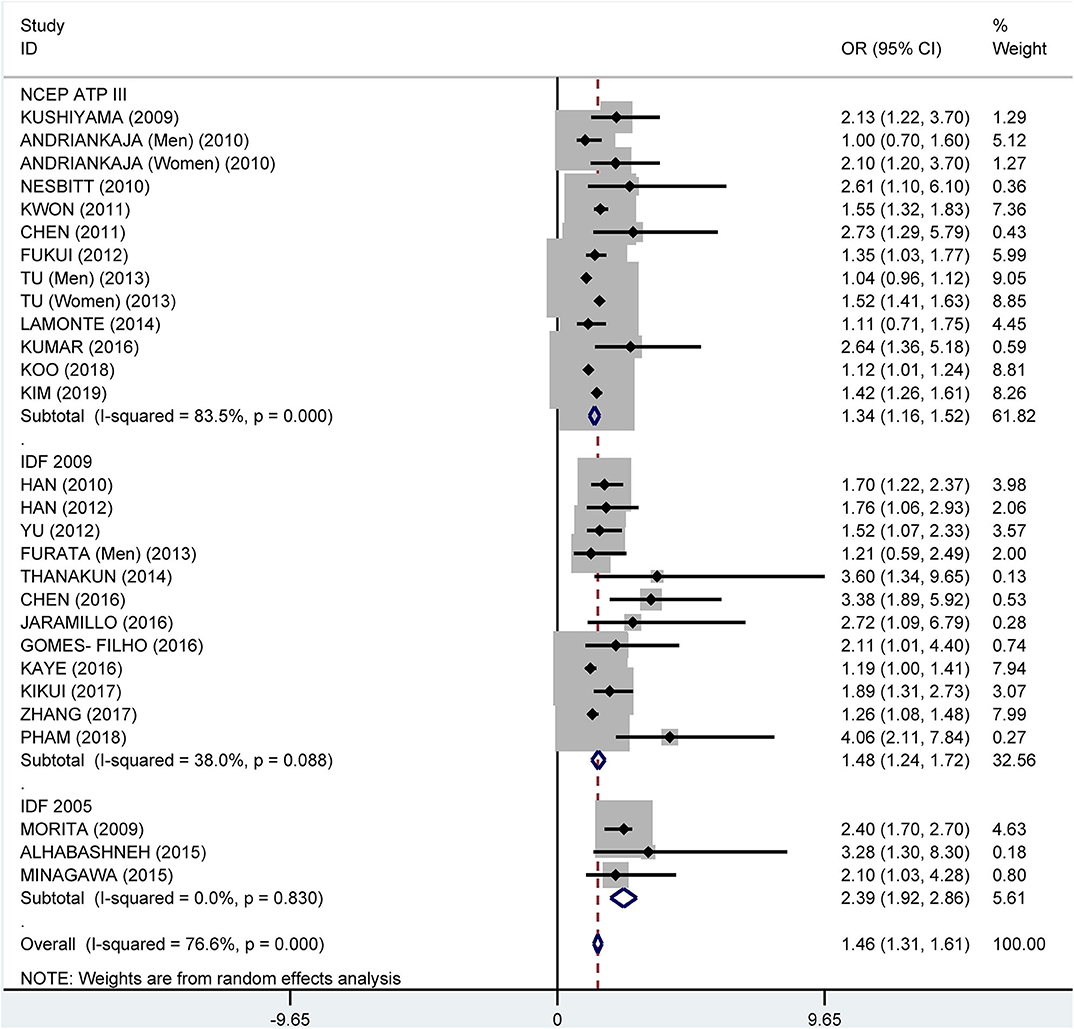
Figure 8. Subgroup analysis of pooled adjusted odds ratios of the association between periodontitis and metabolic syndrome by diagnostic criteria for metabolic syndrome.
Subgroup analysis by country showed crude ORs of 1.68 (95% CI: 1.41–2.00, I2 = 63.5%, P = 0.011) for Japan, 1.75 (95% CI: 1.31–2.34, I2 = 81.9%, P < 0.001) for the USA, 1.81 (95% CI: 1.35–2.42, I2 = 96.6%, P < 0.001) for Korea, and 2.29 (95% CI: 1.53–3.41, I2 = 81.5%, P < 0.001) for China (Figure 9). The adjusted OR was 1.19 (95% CI: 1.02–1.36, I2 = 0.0%, P = 0.471) for the USA, 1.41 (95% CI: 1.17–1.66, I2 = 76.7%, P = 0.002) for Korea, 1.51 (95% CI: 0.93–2.08, I2 = 57.3%, P = 0.096) for China, and 1.81 (95% CI: 1.33–2.29, I2 = 61.2%, P = 0.024) for Japan, respectively (Figure 10).
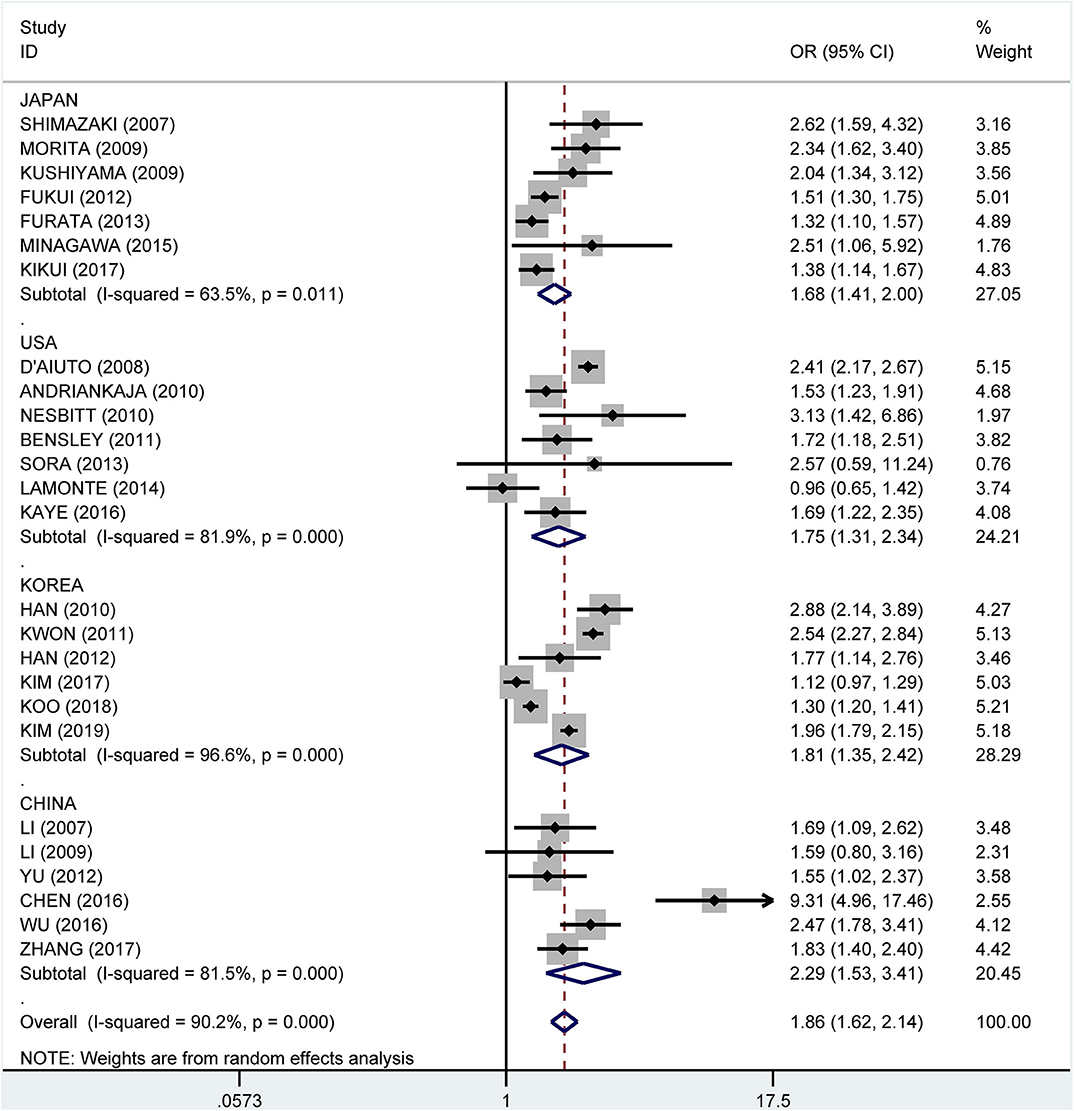
Figure 9. Subgroup analysis of pooled crude odds ratios of the association between periodontitis and metabolic syndrome by country.
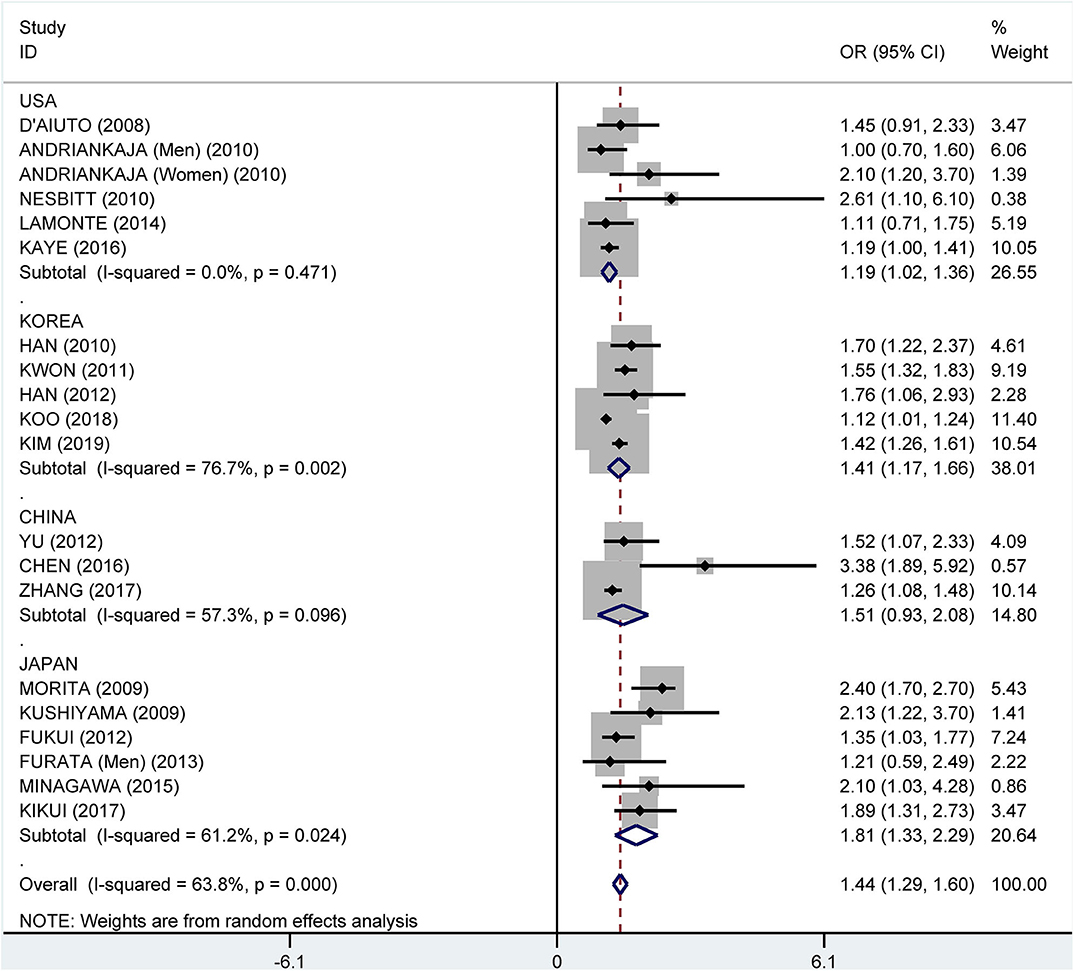
Figure 10. Subgroup analysis of pooled adjusted odds ratios of the association between periodontitis and metabolic syndrome by country.
Discussion
There is undoubtedly a positive association between periodontitis and MetS based upon the studies that were reviewed in this meta-analysis. There are a multitude of variables that can have ramifications in the association between periodontitis and MetS, including study population/subjects as well as ethnicity/geography, which have not been studied. Studies involved in this systematic review were conducted in 15 different countries worldwide, and the majority of the studies demonstrated a higher risk of MetS for patients with periodontal diseases.
The human body has multiple organ systems, and these systems are intimately and mutually dependent on each other. As a result, ailments in some organs or their components may play a contributing factor in the occurrence and evolution of a particular disease in other body locations (55, 56). The influence of oral health on one's general health has been scrutinized for the last decennium by a multitude of surveys (57). A systematic review including 72 studies and data from 291,170 individuals in 37 countries estimated that the global prevalence of severe periodontitis in 2010 was 10.8% (95% CI: 10.1–11.6%), thus making severe periodontitis rank 6th in the Global Burden of Disease (58).
The observation of a relationship between periodontitis and systemic diseases may significantly expand the scope and appreciation of oral health and dental practice. The general population, as well as non-dental medical professionals, should be informed that periodontal diseases play an important role in overall health. Due to a possible etiopathogenic relationship between periodontitis and various systemic diseases, patients with severe periodontal disease should be referred to screen for CVD, diabetes, MetS, and other systemic diseases. Patients prone to or diagnosed with certain systemic diseases may also be referred to dentists to check for and treat periodontal disease. Oral inflammatory lesions have different mechanisms concerning the possible association with systemic diseases (59–62). The oral cavity hosts several cell populations expressing mesenchymal stem cell like-features (63). The human periapical cyst mesenchymal stem cells (hPCy-MSCs) collected from the surgically removed periapical cysts exhibit interesting and valuable potentialities that could be of high impact in the future regenerative medicine applications (63). It is necessary to strengthen the cooperation between different clinical departments and treat the disease with a holistic view.
The main limitation of this particular review is that the analysis and comparison of ORs using the data available may be debatable, as the percentage of patients considered to have been diagnosed with periodontitis and MetS was determined with different diagnostic criteria. The fact that there is no global consensus on one specific set of diagnostic criteria for these two diseases is an enigma. Different researchers use different criteria depending upon the country where the study is being conducted, availability, and the accessibility of resources as well as specialized human resources to conduct the examination part of the survey, and this may affect the results of each study. Furthermore, most of the studies reviewed on this related issue are cross-sectional studies, and they do demonstrate an association of periodontal disease with MetS, but the prevalence ferreted at a single point thereby gives only a transient excerpt or cause–effect cannot be determined. An additional consideration is that many of the definitions of MetS allow the inclusion of subjects who have already been on medication for the treatment of some components of MetS, for instance, hypertension, diabetes, or dyslipidemia. The consequence of these medications on systemic inflammation is concealed, and some might have anti-inflammatory actions; thus, patients might have different periodontal statuses. One study reported by Shimazaki et al. showed that MetS increased the risk of periodontitis, with ORs for greater pocket depth and clinical attachment loss exhibited in 4 or 5 components of 6.6 (95% CI: 2.6–16.4) and 4.2 (95% CI: 1.2–14.8), respectively (16). Due to the lack of longitudinal studies, it is difficult to determine the relative contribution of periodontitis to MetS and the contribution of MetS to periodontitis. However, this does not affect our suggestion that clinicians and the general public should pay attention to the relationship between oral health and systemic disease.
Conclusion
Our results provide compelling evidence for the association between periodontitis and MetS. Patients with periodontal disease are a critical screening population for MetS. We also recommend that people exhibiting components of MetS should receive a periodontal check-up and pay attention to their oral health. Among the golden rules for maintaining good oral hygiene is that one should visit a dentist at least twice a year, brush at least twice daily, floss regularly, and have a healthy diet, thereby avoiding too many sweet and sticky foodstuffs. Particular attention should be given to children, and oral hygiene, as well as healthy lifestyle habits, should be implemented in the educational curriculum so that from an early age, one is made aware of these basics to have a healthier and brighter future generation. Policymakers should invest in the promotion and prevention of these issues so that the financial impact due to the treatment and rehabilitation of these diseases is less.
Author Contributions
RG, DT, and JW conceived the study, analyzed the data, and drafted the manuscript. QL participated in the study design and helped refine the manuscript. All authors contributed to the study and have read and approved the final version.
Funding
This study was supported by the National Nature Science Foundation of China (81973103), the National Key R&D Program of China (2017YFC0907000), the Qinglan Project of Jiangsu Province (2019), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00336/full#supplementary-material
Supplementary Table 1. Newcastle-Ottawa quality assessment scale.
References
1. Vieira CL, Caramelli B. The history of dentistry and medicine relationship: could the mouth finally return to the body? Oral Dis. (2009) 15:538–46. doi: 10.1111/j.1601-0825.2009.01589.x
2. Chan S. Pasternak GM, West MJ. The place of periodontal examination and referral in general medicine. Periodontol. (2017) 74:194–9. doi: 10.1111/prd.12199
4. Slots J. Periodontitis: facts, fallacies and the future. Periodontol. (2000) 75:7–23. doi: 10.1111/prd.12221
5. Daudt LD, Musskopf ML, Mendez M, Remonti LLR, Leitao CB, Gross JL, et al. Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. Braz Oral Res. (2018) 32:e35. doi: 10.1590/1807-3107bor-2018.vol32.0035
6. Abdalla-Aslan R, Findler M, Levin L, Zini A, Shay B, Twig G, et al. Where periodontitis meets metabolic syndrome-the role of common health-related risk factors. J Oral Rehabil. (2019) 46:647–56. doi: 10.1111/joor.12798
7. Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. (2004) 33:351–75. doi: 10.1016/j.ecl.2004.03.005
8. Watanabe K, Cho YD. Periodontal disease and metabolic syndrome: a qualitative critical review of their association. Arch Oral Biol. (2014) 59:855–70. doi: 10.1016/j.archoralbio.2014.05.003
9. Nibali L, Tatarakis N, Needleman I, Tu YK, D'Aiuto F, Rizzo M, et al. Clinical review: association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2013) 98:913–20. doi: 10.1210/jc.2012-3552
10. Han DH, Lim S, Paek D, Kim HD. Periodontitis could be related factors on metabolic syndrome among Koreans: a case-control study. J Clin Periodontol. (2012) 39:30–7. doi: 10.1111/j.1600-051X.2011.01806.x
11. Hirotomi T, Kocher T, Yoshihara A, Biffar R, Micheelis W, Hoffmann T, et al. Comparison of periodontal conditions among three elderly populations in Japan and Germany. J Clin Periodontol. (2014) 41:633–42. doi: 10.1111/jcpe.12267
12. Zuk A, Quinonez C, Lebenbaum M, Rosella LC. The association between undiagnosed glycaemic abnormalities and cardiometabolic risk factors with periodontitis: results from. 2007-2009 canadian health measures survey. J Clin Periodontol. (2017) 44:132–41. doi: 10.1111/jcpe.12684
13. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
14. Wells G, Shea BJ, O'Connell D, Peterson J, Welch V, Loses M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Ottawa: The Ottawa Hospital Research Institute (2013). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed May 28, 2020).
15. Li P, Lu YS, Qingxian HE. A survey on periodontal status of patients with metabolic syndrome in a Beijing community. J Pract Stomatol. (2007) 23:434–8. doi: 10.3969/j.issn.1001-3733.2007.03.032
16. Shimazaki Y, Saito T, Yonemoto K, Kiyohara Y, Iida M, Yamashita Y. Relationship of metabolic syndrome to periodontal disease in Japanese women: the Hisayama Study. J Dent Res. (2007) 86:271–5. doi: 10.1177/154405910708600314
17. D'Aiuto F, Sabbah W, Netuveli G, Donos N, Hingorani AD, Deanfield J, et al. Association of the metabolic syndrome with severe periodontitis in a large US. population-based survey. J Clin Endocrinol Metab. (2008) 93:3989–94. doi: 10.1210/jc.2007-2522
18. Khader Y, Khassawneh B, Obeidat B, Hammad M, El-Salem K, Bawadi H, et al. Periodontal status of patients with metabolic syndrome compared to those without metabolic syndrome. J Periodontol. (2008) 79:2048–53. doi: 10.1902/jop.2008.080022
19. Li P, He L, Sha YQ, Luan QX. Relationship of metabolic syndrome to chronic periodontitis. J Periodontol. (2009) 80:541–9. doi: 10.1902/jop.2009.080387
20. Morita T, Ogawa Y, Takada K, Nishinoue N, Sasaki Y, Motohashi M, et al. Association between periodontal disease and metabolic syndrome. J Public Health Dent. (2009) 69:248–53. doi: 10.1111/j.1752-7325.2009.00130.x
21. Kushiyama M, Shimazaki Y, Yamashita Y. Relationship between metabolic syndrome and periodontal disease in Japanese adults. J Periodontol. (2009) 80:1610–5. doi: 10.1902/jop.2009.090218
22. Andriankaja OM, Sreenivasa S, Dunford R, DeNardin E. Association between metabolic syndrome and periodontal disease. Aust Dent J. (2010) 55:252–9. doi: 10.1111/j.1834-7819.2010.01231.x
23. Nesbitt MJ, Reynolds MA, Shiau H, Choe K, Simonsick EM, Ferrucci L. Association of periodontitis and metabolic syndrome in the Baltimore longitudinal study of aging. Aging Clin Exp Res. (2010) 22:238–42. doi: 10.1007/BF03324802
24. Benguigui C, Bongard V, Ruidavets JB, Chamontin B, Sixou M, Ferrieres J, et al. Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. J Clin Periodontol. (2010) 37:601–8. doi: 10.1111/j.1600-051X.2010.01571.x
25. Han DH, Lim SY, Sun BC, Paek D, Kim HD. The association of metabolic syndrome with periodontal disease is confounded by age and smoking in a Korean population: the Shiwha-Banwol environmental health study. J Clin Periodontol. (2010) 37:609–16. doi: 10.1111/j.1600-051X.2010.01580.x
26. Timonen P, Niskanen M, Suominen-Taipale L, Jula A, Knuuttila M, et al. Metabolic syndrome, periodontal infection, and dental caries. J Dent Res. (2010) 89:1068–73. doi: 10.1177/0022034510376542
27. Zhang J, Zhao X, Zhong H, Pan Y, Zhang L, Li M, et al. Survey on periodontal status in metabolic syndrome patients. J Oral Sci Res. (2010) 26:126–9. doi: 10.13701/j.cnki.kqyxyj.2010.01.045
28. Bensley L, vanEenwyk J, Ossiander EM. Associations of self-reported periodontal disease with metabolic syndrome and number of self-reported chronic conditions. Prev Chronic Dis. (2011) 8:A50.
29. Kwon YE, Ha JE, Paik DI, Jin BH, Bae KH. The relationship between periodontitis and metabolic syndrome among a Korean nationally representative sample of adults. J Clin Periodontol. (2011) 38:781–6. doi: 10.1111/j.1600-051X.2011.01756.x
30. Chen LP, Hsu SP, Peng YS, Chiang CK, Hung KY. Periodontal disease is associated with metabolic syndrome in hemodialysis patients. Nephrol Dial Transplant. (2011) 26:4068–73. doi: 10.1093/ndt/gfr209
31. Fukui N, Shimazaki Y, Shinagawa T, Yamashita Y. Periodontal status and metabolic syndrome in middle-aged Japanese. J Periodontol. (2012) 83:1363–71. doi: 10.1902/jop.2012.110605
32. Yu ZR, Liu LS, Luan QX, Wang XY, Li P, Sha YQ, et al. [Correlation between periodontitis and metabolic syndrome of the middle-aged and aged population in Shijingshan community of Beijing]. Beijing Da Xue Xue Bao Yi Xue Ban. (2012) 44:633–8. doi: 10.3969/j.issn.1671-167X.2012.04.031
33. Tu YK, D'Aiuto F, Lin HJ, Chen YW, Chien KL. Relationship between metabolic syndrome and diagnoses of periodontal diseases among participants in a large Taiwanese cohort. J Clin Periodontol. (2013) 40:994–1000. doi: 10.1111/jcpe.12157
34. Sora ND, Marlow NM, Bandyopadhyay D, Leite RS, Slate EH, Fernandes JK. Metabolic syndrome and periodontitis in Gullah African Americans with type 2 diabetes mellitus. J Clin Periodontol. (2013) 40:599–606. doi: 10.1111/jcpe.12104
35. Furuta M, Shimazaki Y, Takeshita T, Shibata Y, Akifusa S, Eshima N, et al. Gender differences in the association between metabolic syndrome and periodontal disease: the Hisayama Study. J Clin Periodontol. (2013) 40:743–52. doi: 10.1111/jcpe.12119
36. LaMonte MJ, Williams AM, Genco RJ, Andrews CA, Hovey KM, Millen AE, et al. Association between metabolic syndrome and periodontal disease measures in postmenopausal women: the Buffalo OsteoPerio study. J Periodontol. (2014) 85:1489–501. doi: 10.1902/jop.2014.140185
37. Thanakun S, Watanabe H, Thaweboon S, Izumi Y. Association of untreated metabolic syndrome with moderate to severe periodontitis in Thai population. J Periodontol. (2014) 85:1502–14. doi: 10.1902/jop.2014.140105
38. Alhabashneh R, Khader Y, Herra Z, Asa'ad F, Assad F. The association between periodontal disease and metabolic syndrome among outpatients with diabetes in Jordan. J Diabetes Metab Disord. (2015) 14:67. doi: 10.1186/s40200-015-0192-8
39. Minagawa K, Iwasaki M, Ogawa H, Yoshihara A, Miyazaki H. Relationship between metabolic syndrome and periodontitis in 80-year-old Japanese subjects. J Periodontal Res. (2015) 50:173–9. doi: 10.1111/jre.12190
40. Iwasaki M, Sato M, Minagawa K, Manz MC, Yoshihara A, Miyazaki H. Longitudinal relationship between metabolic syndrome and periodontal disease among Japanese adults aged ≥70 years: the Niigata Study. J Periodontol. (2015) 86:491–8. doi: 10.1902/jop.2015.140398
41. Chen X, Xie L, Liu Y, Chen D, Yu Q, Gan X, et al. Metabolic syndrome and periodontal disease among civilian pilots. Aerosp Med Hum Perform. (2016) 87:1016–020. doi: 10.3357/AMHP.4654.2016
42. Jaramillo A, Contreras A, Lafaurie GI, Duque A, Ardila CM, Duarte S, et al. Association of metabolic syndrome and chronic periodontitis in Colombians. Clin Oral Investig. (2017) 21:1537–44. doi: 10.1007/s00784-016-1942-9
43. Kumar N, Bhardwaj A, Negi PC, Jhingta PK, Sharma D, Bhardwaj VK. Association of chronic periodontitis with metabolic syndrome: a cross-sectional study. J Indian Soc Periodontol. (2016) 20:324–9. doi: 10.4103/0972-124X.183096
44. Gomes-Filho IS, das Merces MC, de Santana Passos-Soares J, Seixas da Cruz S, Teixeira Ladeia AM, Trindade SC, et al. Severity of periodontitis and metabolic syndrome: is there an association? J Periodontol. (2016) 87:357–66. doi: 10.1902/jop.2015.150367
45. Kaye EK, Chen N, Cabral HJ, Vokonas P, Garcia RI. Metabolic syndrome and periodontal disease progression in men. J Dent Res. (2016) 95:822–8. doi: 10.1177/0022034516641053
46. Musskopf ML, Daudt LD, Weidlich P, Gerchman F, Gross JL, Oppermann RI. Metabolic syndrome as a risk indicator for periodontal disease and tooth loss. Clin Oral Investig. (2017) 21:675–83. doi: 10.1007/s00784-016-1935-8
47. Wu W. Study on the correlation between periodontal disease and metabolic syndrome. Chin J Clin. (2016) 44:71–2. doi: 10.3969/j.issn.2095-8552.2016.01.026
48. Kikui M, Kokubo Y, Ono T, Kida M, Kosaka T, Yamamoto M, et al. Relationship between metabolic syndrome components and periodontal disease in a Japanese general population: the suita study. J Atheroscler Thromb. (2017) 24:495–507. doi: 10.5551/jat.33761
49. Zhang L, Li Y, Sang X, Li S, Hu X, Liu J. Epidemiological survey and risk factors analysis of periodontitis in rural Uygur in Moyu County. J Xinjiang Med University. (2017) 40:557–63. doi: 10.3969/j.issn.1009-5551.2017.05.001
50. Kim OS, Shin MH, Kweon SS, Lee YH, Kim OJ, Kim YJ, et al. Chung: The severity of periodontitis and metabolic syndrome in Korean population: The Dong-gu study. J Periodontal Res. (2018) 53:362–368. doi: 10.1111/jre.12521
51. Pham T. The association between periodontal disease severity and metabolic syndrome in Vietnamese patients. Int J Dent Hyg. (2018) 16:484–91. doi: 10.1111/idh.12350
52. Koo HS, Hong SM. Prevalence and risk factors for periodontitis among patients with metabolic syndrome. Metab Syndr Relat Disord. (2018) 16:375–81. doi: 10.1089/met.2018.0003
53. Nascimento GG, Leite FRM, Peres KG, Demarco FF, Correa MB, Peres MA. Metabolic syndrome and periodontitis: a structural equation modeling approach. J Periodontol. (2019) 90:655–62. doi: 10.1002/JPER.18-0483
54. Kim JS, Kim SY, Byon MJ, Lee JH, Jeong SH, Kim JB. Association between periodontitis and metabolic syndrome in a korean nationally representative sample of adults aged. 35-79 years. Int J Environ Res Public Health. (2019) 16:2930. doi: 10.3390/ijerph16162930
55. Friedewald VE, Kornman KS, Beck JD, Genco R, Goldfine A, Libby P, et al. American Journal of Cardiology and Journal of Periodontology Editors' Consensus: periodontitis and atherosclerotic cardiovascular disease. Am J Cardiol. (2009) 104:59–68. doi: 10.1016/j.amjcard.2009.05.002
56. Barry M, Pearce H, Cross L, Tatullo M, Gaharwar AK. Advances in nanotechnology for the treatment of osteoporosis. Curr Osteoporos Rep. (2016) 14:87–94. doi: 10.1007/s11914-016-0306-3
57. Carramolino-Cuellar E, Tomas I, Jimenez-Soriano Y. Relationship between the oral cavity and cardiovascular diseases and metabolic syndrome. Med Oral Patol Oral Cir Bucal. (2014) 19:e289–94. doi: 10.4317/medoral.19563
58. Papapanou PN, Susin C. Periodontitis epidemiology: is periodontitis under-recognized, over-diagnosed, or both? Periodontol. (2017) 75:45–51. doi: 10.1111/prd.12200
59. Ballini A, Tete S, Scattarella A, Cantore S, Mastrangelo F, Papa F, et al. The role of anti-cyclic citrullinated peptide antibody in periodontal disease. Int J Immunopathol Pharmacol. (2010) 23:677–81. doi: 10.1177/039463201002300234
60. Mori G, Brunetti G, Colucci S, Oranger A, Ciccolella F, Sardone F, et al. Osteoblast apoptosis in periodontal disease: role of TNF-related apoptosis-inducing ligand. Int J Immunopathol Pharmacol. (2009) 22:95–103. doi: 10.1177/039463200902200111
61. Ballini A, Cantore S, Farronato D, Cirulli N, Inchingolo F, Papa F, et al. Periodontal disease and bone pathogenesis: the crosstalk between cytokines and porphyromonas gingivalis. J Biol Regul Homeost Agents. (2015) 29:273–81. doi: 10.1177/0394632015590949
62. Cantore S, Ballini A, Mori G, Dibello V, Marrelli M, Mirgaldi R, et al. Anti-plaque and antimicrobial efficiency of different oral rinses in a 3-day plaque accumulation model. J Biol Regul Homeost Agents. (2016) 30:1173–8.
Keywords: periodontal disease, periodontitis, periodontal pocket, clinical attachment loss, metabolic syndrome, meta-analysis
Citation: Gobin R, Tian D, Liu Q and Wang J (2020) Periodontal Diseases and the Risk of Metabolic Syndrome: An Updated Systematic Review and Meta-Analysis. Front. Endocrinol. 11:336. doi: 10.3389/fendo.2020.00336
Received: 05 January 2020; Accepted: 29 April 2020;
Published: 09 June 2020.
Edited by:
Marco Tatullo, University of Bari Medical School, ItalyReviewed by:
Andrea Ballini, University of Bari Aldo Moro, ItalyOmar Etienne, INSERM Délégation Paris 6, France
Copyright © 2020 Gobin, Tian, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianming Wang, am13YW5nQG5qbXUuZWR1LmNu
†These authors have contributed equally to this work
 Romila Gobin
Romila Gobin Dan Tian
Dan Tian Qiao Liu1,2
Qiao Liu1,2 Jianming Wang
Jianming Wang