
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 22 May 2020
Sec. Neuroendocrine Science
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00335
This article is part of the Research Topic The Oxytocin System in Fear, Stress, Anguish, and Pain View all 8 articles
Background: Although the default mode network (DMN) is a core network essential for brain functioning, little is known about its developmental trajectory, particularly on factors associated with its coherence into a functional network. In light of adult studies indicating DMN's susceptibility to stress-related conditions, we examined links between variability on oxytocin-pathway genes and DMN connectivity in youth exposed to chronic war-related trauma
Methods: Following a cohort of war-exposed children from early childhood, we imaged the brains of 74 preadolescents (age 11–13 years; 39 war-exposed) during rest using magnetoencephalography (MEG). A cumulative risk index on oxytocin-pathway genes was constructed by combining single nucleotide polymorphisms on five genes previously linked with social deficits and psychopathology; OXTR rs1042778, OXTR rs2254298, OXTRrs53576, CD38 rs3796863, and AVPR1A RS3. Avoidant response to trauma reminders in early childhood and anxiety disorders in late childhood were assessed as predictors of disruptions to DMN theta connectivity.
Results: Higher vulnerability on oxytocin-pathway genes predicted greater disruptions to DMN theta connectivity. Avoidant symptoms in early childhood and generalized anxiety disorder in later childhood were related to impaired DMN connectivity. In combination, stress exposure, oxytocin-pathway genes, and stress-related symptoms explained 24.6% of the variance in DMN connectivity, highlighting the significant effect of stress on the maturing brain.
Conclusions: Findings are the first to link the oxytocin system and maturation of the DMN, a core system sustaining autobiographical memories, alteration of intrinsic and extrinsic attention, mentalization, and sense of self. Results suggest that oxytocin may buffer the effects of chronic early stress on the DMN, particularly theta rhythms that typify the developing brain.
The default mode network (DMN) is a cortical network that becomes more active and synchronized during rest and deactivates when one is engaged in a goal-oriented task (1). The DMN comprises seven brain regions, including the left and right angular gyrus, ventro- and dorso-medial pre-frontal cortex, posterior cingulate cortex/pre-cuneus, right medial pre-frontal cortex, and left inferior temporal gyrus (2), and its organization and functioning during rest is considered to define the personal “fingerprint” of the brain (3). In adults, the DMN is thought to play a key role in sustaining the sense of self and in organizing the individual's experience of the world; it supports self-referential thinking, introspection (4), internal narratives (4), mentalizing activity (5), the autobiographical self and self-projection (6). Highly synchronized and smooth activity of the DMN is critical for a sense of well-being, personal identity, and resilience (7–11).
The importance of the DMN for proper brain functioning is widely-accepted. The DMN is involved in overall brain activity as well as in attentive, cognitive, and social processes, and associations have been repeatedly shown between DMN dysfunction and a host of psychiatric disorders in adults (12–16). In contrast, very little is currently known about the development and functional connectivity of the DMN in children and particularly lacking are data related to DMN maturation under high-risk conditions, with even more limited knowledge on how structures of the DMN cohere in children reared under various high-risk conditions. A study utilizing resting state functional connectivity MRI and graph theory methods to characterize the maturation of the DMN found that in contrast to the strong interhemispheric connectivity between homolog brain regions in adult, DMN connectivity in children is sparse and becomes more integrated with development (17). The authors interpret their findings in relation to the ever-maturing functions sustained by the DMN, such as consolidation of the sense of self that is still immature in children. For example, the ability to encode and retrieve stored memories is fully functioning at an early age but episodic memory continues to improve with age. This improvement does not stem from the mere development of encoding and retrieval but from the ability to incorporate more complex strategies to encode self-relevant information, which is supported by the progressive integration of the DMN into a unified network. These notions are supported by a study indicating that DMN connectivity in children and adolescents is related to the quality of reconstruction of past experiences and the ability to imagine the future (18).
Very few studies tested the maturation of DMN connectivity across childhood and adolescence. A longitudinal fMRI study found significant changes in DMN connectivity between the ages 10 and 13, suggesting that it continues to consolidate throughout development (19). This is consistent with cross-sectional studies that compared DMN connectivity in children, adolescents, and adults (17, 20–23). Notably, the findings that age-related differences are observed even within the narrow age-range of 10–13 lends further support to the hypothesis that early adolescence may be a particularly important time-window for the integration of the DMN into a coherent network.
While very little research has tested DMN connectivity using magnetoencephalography (MEG), MEG has unique benefits for the study of DMN functionality due to its high temporal resolution which enables the decomposing of neural signals into frequency bands (24). Using MEG/EEG studies it was found that whereas in adults, the strongest activity in the DMN during rest is in the alpha band (8–12 Hz), during childhood the dominant frequency is the theta band (4–7 Hz), which gradually shifts to alpha as children grow (25–28). In a previous longitudinal study we utilized MEG to investigate the effect of early life stress (ELS) on DMN connectivity in preadolescents and their mothers and found that ELS decreased theta DMN connectivity in adolescents and alpha DMN connectivity in mothers (29); hence, we focused here on theta connectivity in youth by assessing Phase Locking Value [PLV; Lachaux et al. (30)], the most commonly used index to measure phase coupling in EEG/MEG experiments (31, 32). PLV quantifies the degree to which signals rise and fall together over time in absolute values; high PLV (values closer to 1) implies more synchronized signals while low PLV (values closer to 0) denotes lower synchrony. We found that the experience of anxious, intrusive parenting across the first decade and higher cortisol levels predicted DMN connectivity impairment, indicating that neurobiological, clinical, and relational factors that index stress longitudinally shape DMN connectivity in children.
The neuropeptide oxytocin (OT) has been repeatedly implicated in attachment, bonding, and sociality across mammalian species (33, 34). In addition to its involvement in bond formation, OT plays a critical role in stress regulation. In response to emotional, physical, or pharmacological stress, OT is released into the bloodstream as well as within hypothalamic and extrahypothalamic limbic regions where it regulates HPA activity and modulates response to stress (35–37). Since the DMN is highly sensitive to stress, its functionality is probably impacted by the OT system and evidence points to the association between OT and DMN activity in adults (38–40). One study describes associations between allelic variability on the oxytocin receptor gene (OXTR) and DMN connectivity (40). Following research indicating that 40% of the variance in DMN connectivity is heritable, Wang et al. (40) found that compared with individuals with OXTRrs2254298 GG genotype, individuals carrying the AA/AG genotypes displayed lower DMN connectivity.
To further understand the association between genetic variability on the OXTR and stress-related conditions, studies have suggested that a cumulative genetic risk index—a measure combining multiple single nucleotide polymorphisms (SNPs) on oxytocin-pathway genes linked with social dysfunction—provides a better risk estimate than the study of individual SNPs (41–44). Three OXTR SNPs have been typically included in this cumulative index, the rs2254298, rs53576, and rs1042778 alleles. In addition to these three alleles, CD38, a multifactorial molecule implicated in cell proliferation, differentiation, and migration that plays a critical role in regulating OT release (45) and vasopressin AVPR1a, a closely linked neuropeptide to OT that is considered to evolve from the same ancient vasotocin molecule (46), have been incorporated into the oxytocin-pathway genes cumulative index. This cumulative risk index, combining the three OXTR SNPs, CD38, and AVPR1a, has been shown to predict PTSD chronicity in children exposed to chronic trauma (47) and to correlate with empathic failure among new romantic partners (48). Furthermore, the cumulative risk approach is more statistically powerful and enables the evaluation of a continuum of genetic risk in relation to stress-related conditions.
In light of the above, the current study utilized a unique cohort of stress-exposed children living in Sderot, Israel, a small town located on the Gaza boarder which has been subjected to continuous, life-threatening trauma for nearly two decades. Sderot has been the target of continuous rocket attacks since 2001, with six periods of exacerbations when the town was under attack by dozens of daily missiles for several days to several weeks. During attacks, citizens hear a siren warning, leaving them only 15 s to reach shelters before an explosion. Children were followed at several time-points from early childhood and at the transition to adolescence (11–13 years) underwent MEG scanning to assess DMN connectivity. Our goal was to examine the cumulative contributions of genetic susceptibility on the OT system and anxiety-related symptoms, which are known to jointly impact neural responses (49, 50), to the development of DMN connectivity in children growing in the context of chronic trauma. We thus examined stress-related factors across childhood and hypothesized that cumulatively these factors may place children at risk for maturation of the DMN. Specially, we considered genetic variability on the OT system, avoidant manifestations in early childhood, and the consolidation of a full-blown anxiety disorder in late childhood as factors which, when occurring together across development, may tip children toward a risk trajectory. Our conceptual model is presented in Figure 1.
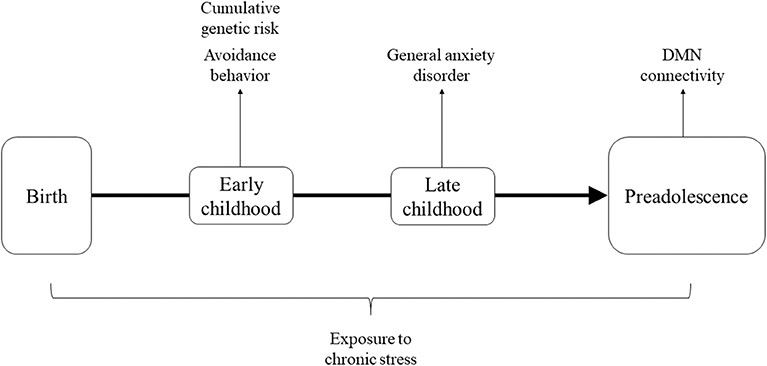
Figure 1. Theoretical framework describing factors across development hypothesized to cumulatively impact maturation of DMN connectivity.
In early childhood, we focused on children's avoidant responses to trauma reminders. Avoidance is a core symptom cluster in the diagnosis of PTSD and has shown to predict PTSD chronicity in children and adults (47, 51). The diagnosis of PTSD in early childhood, before children can verbally recount the trauma, requires clinical observation of the child's behavioral response to trauma reminders [DC: 0–3R; Zero to Three (52)]. Young children's behavioral response to trauma reminders is characterized by four types of responses; regressive-clinging, aggressive, hyper-anxious, and avoidant (53), of which the avoidant type is the most detrimental for later development (47). In terms of anxiety disorders, longitudinal studies has shown that temperamental avoidance and reticence in early childhood increase the risk for the consolidation of anxiety disorders by late childhood and adolescence, and only those children who showed both reticence in early childhood and anxiety disorders later on exhibited alterations in neural functioning (54–57). We thus examined the joint contribution of early behavioral avoidance and later anxiety disorders to DMN connectivity at the transition to adolescence.
Several hypotheses were formulated. First, we expected that greater cumulative risk index on oxytocin-pathway genes would be associated with reduced connectivity of the DMN (hypothesis 1). We also expected that avoidant response to trauma reminders in early childhood would be linked with impaired DMN connectivity (hypothesis 2). Similarly, the existence of a full-blown anxiety disorder, which increases the likelihood of a chronic course of PTSD (51), was expected to predict lower DMN connectivity (hypothesis 3). Finally, these factors in combination—genetic risk, avoidant response, and anxiety disorder, were expected to jointly explain variance in DMN connectivity in preadolescence (hypothesis 4).
The original sample included 232 children in two groups (war-exposed and control) who were observed four times. In the current study we used data collected in early childhood (T1), late childhood (T3), and early preadolescence (T4). Details of the first (T1) second assessment (T2) appear elsewhere (53, 58).
In early childhood (time 1 [T1]), the initial sample (n = 232) included 47.6% boys and 47.1% firstborns (M = 2.76 years, SD = 0.91). The war-exposed group included 148 families living in the same neighborhoods in Sderot, Israel, located 10 km from the Gaza border. The control group included 84 non-exposed families from comparable towns in the Tel-Aviv area matched to the exposed group by age, gender, birth order, parental age and education, maternal employment, and marital status and subsequently screened for trauma.
At late childhood (M = 9.3 years, SD = 1.41), 177 families were revisited: 101 war-exposed and 76 control families. Attrition was mainly related to inability to locate families or families relocating to another city.
A total of 166 families, including 111 children, were revisited at preadolescence. Out of the 111 children revisited, 74 children (43 girls), containing 39 war-exposed children, participated in MEG scanning (M = 11.81 years, SD = 1.24). Table 1 presents demographic and background information. Of 111 children initially recruited at T4, 36 did not yield MEG data: 18 were MEG incompatible (mostly owing to metal implants), 6 refused MEG scan, and 12 did not complete the experiment.
The study was approved by local institutional review board, and written informed consent was obtained from parents after receiving a complete description of the study.
During the home visits (which lasted 3.5 h on average), DNA samples were collected from the children. In addition, trained clinicians interviewed mothers about their children and children were observed during trauma reminders, to diagnose PTSD [detailed in Feldman and Vengrober (53)]. Home visits included other procedures which exceed the scope of the current study and which are described elsewhere (53, 59).
DNA of child was extracted from 20 ml of mouthwash samples using Master Pure kit (Epicenter, Madison WI). All genotype frequencies of OXTR, CD38, and AVPR1A SNPs were in Hardy-Weinberg equilibrium [for full details on genotyping see references (48, 60–65)]. A cumulative genetic risk factor was computed for each child by summing the number of genetic risk variations associated with psychiatric risk or social difficulties (Table 2). These included the OXTR rs1042778 TT genotype, associated with greater risk for autism (65) and lower empathy in healthy adults (66); OXTR rs2254298 GG genotype, associated with smaller amygdala volume in two independent samples (67, 68) and risk for major depression (60); the A allele (AA or AG) on the OXTRrs53576, related to risk for autism (69), lower empathy (70), and non-optimal parenting (62); the CC genotype on the CD38 rs3796863, linked with greater risk for autism in two independent samples (71, 72) and non-optimal parenting (63, 64); and the 327 bp allele on the AVPR1A RS3, associated with lower altruism and non-optimal mothering (61, 73, 74). Risk scores ranged from 0 (no risk) to 5 (risk on all 5 SNPs). In total DNA was extracted from 231 children which include the final sample of 74 children conducting the MEG scan.
During the maternal PTSD interview, which lasted ~1 h, the clinician probed the trauma in detail while children were present in the room and mother and child's behavior during the trauma reminders were videotaped. The child's emotions and behaviors to trauma reminders were coded offline by raters blinded to child psychiatric information along 15 scales, each rated from 1 (low) to 5 (high). Coding of trauma response was based on the Coding Interactive Behavior [CIB, Feldman (75)] and described in detail elsewhere (53). The CIB is a well-validated coding system for adult-child interaction and child behavioral response that has shown good psychometric properties and sensitivity to adult and child interactive behavior related to age, culture, biological and social-emotional risk conditions, and the effects of intervention (76–79). Interrater reliability was conducted for 46 interactions and reliability averaged 93%, intraclass r = 0.91. Child avoidance behavior included child gaze aversion, emotional withdrawal, moving away from mother's arms' reach, and increased and inappropriate preoccupation with objects (α = 0.81).
During the 3 h home visit, general anxiety disorder in children was assessed via interviewing mothers. Home visits included other procedures which exceed the scope of the current study and which are described elsewhere (58).
The developmental and Well-Being Assessment was used to diagnose child Axis-I disorders, including GAD. The DAWBA is a structured interview generating ICD-10 and DSM-IV psychiatric diagnoses in 5–17-years-old children (80). The DAWBA, administered to mothers, is well-validated, including a large epidemiological study in Israel (81). The DAWBA was administered by clinicians and supervised by child psychiatrist, blind to any other information, with reliability > 85% and cases conferred every few weeks. GAD scores ranged between 0 (i.e., lack of GAD symptoms) to 17 (i.e., severe GAD).
Children visited with their mothers the MEG unit at Bar Ilan University where they underwent a magnetoencephalography (MEG) scan. MEG scans included recordings of brain activity during rest (data from this stage is used here) as well as an empathy task used elsewhere (82).
To avoid discomfort among participants and to minimize artifacts due to restlessness, spontaneous brain activity was measured using MEG while participants rested with open eyes in a dimmed light room for 2 min. Ongoing brain activity was recorded using a whole-head 248-channel magnetometer array (Magnes 3600WH; 4-D Neuroimaging, San Diego, CA) in supine position inside a magnetically shielded room. Data were sampled online at 1017.23 Hz with bandpass of 0.1–400 Hz. Reference coils located ~30 cm above the head oriented by the x-, y-, and z-axes were used to remove environmental noise. Five coils were attached to participants' scalp for recording the head position relative to the 248-sensor array. External noise and heartbeat artifacts were removed using a predesigned algorithm (83). Further data processing and analysis were performed using MATLAB and the FieldTrip toolbox (84). Data were segmented into 1,000 ms epochs (with 500 ms overlap between neighboring epochs). Segments containing muscle artifacts and signal jumps were excluded from further analysis by visual inspection. Data were then filtered in the 1–100-Hz range with 10 s padding (to avoid distortion of the real signal at the ends of segments). To clean eye blinks, eye movements, and leftover heartbeats, spatial component analysis was applied.
DMN seed coordinates were predefined based on prior research (2) in which resting-state MEG data linked with the DMN network defined in functional magnetic resonance imaging studies to detect the equivalent MEG seed coordinates to functional magnetic resonance imaging studies [seed coordinates appear in Zeev-Wolf et al. (29)]. Because children's brain anatomy differs from that of adults, we used different brain templates for children and mothers (Montreal Neurological Institute). The templates were modified to fit each participant's digitized head shape using SPM8 (Wellcome Department of Imaging Neuroscience University College London; http://www.fil.ion.ucl.ac.uk). The head shape was manually digitized (FASTRAK digitizer; Polhemus, Colchester, VT), and the participant's brain volume was divided into a regular grid. Grid positions were obtained by linear transformation in a canonical 1-cm grid. This procedure facilitates group analysis because no spatial interpolation of the volumes of reconstructed activity is required. For each grid position, spatial filters were reconstructed to record activity from location of interest while suppressing other activity.
To calculate DMN theta connectivity, we extracted time series from the activation seeds by applying a linear-constrained minimum-variance beamformer in a predefined frequency band (4–7 Hz). Next, we computed the PLV between all seven seed pairs (21 pairs). The PLV measures the phase difference between two recorded signals to quantify the consistency of the phase difference over time (30). See Figure 2 for an illustration of the DMN.
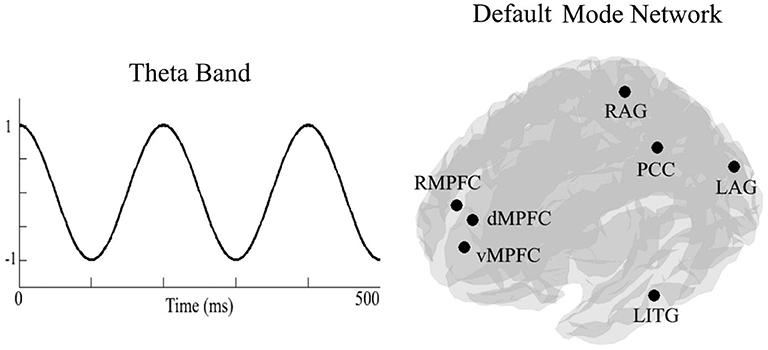
Figure 2. On the left side an, illustration of the theta frequency band (4–7 Hz). On the right side, an illustration of locations of the seven seeds of the default mode network. RMPFC, right medial pre-frontal cortex; dMPFC, dorsomedial pre-frontal cortex; vMPFC, ventromedial pre-frontal cortex; RAG, right angular gyrus; LAG, left angular gyrus; PCC, posterior cingulate cortex/precuneus; LITG, left inferior temporal gyrus.
In order to test the hypotheses stated above, we run three types of analyses: (a) three independent t-tests between children with high and low genetic risk, high and low avoidant behavior, and high and low anxiety disorders on DMN connectivity, (b) correlations between all variables to understand the inter-relationships between them, and (c) a regression analysis to test the additive contribution of exposure, genetic risk, avoidant behavior, and anxiety disorder to the variability of DMN connectivity.
As a first step, we measured DMN connectivity in war-exposed and control children. War-exposed children had lower theta band PLV (M = 0.54, SD = 0.15) compared to controls (M = 0.6, SD = 0.11), t(73) = 1.99, p = 0.05, Cohen's d = 0.456, 95% CI [0, 0.121], indicating that exposure to chronic early stress negatively impacts maturation of the DMN. Following, children were divided into high and low groups once, according to the median of cumulative genetic risk from the entire sample (high ≥ 3), once according to the median of avoidance (high ≥ 2), and once according to the median of GAD (high ≥ 7) scores. Three independent t-test analyses comparing DMN connectivity (i.e., PLV) in the theta band between children with high (n = 32) and low (n = 42) cumulative genetic risk, high (n = 36) and low (n = 38) avoidance, and high (n = 42) and low (n = 30) GAD scores were conducted. Levene's test indicated that variances were equal between groups in all three analyses. Results show that children with low cumulative genetic risk (M = 0.601, SD = 0.134) had higher PLV than children with high cumulative genetic risk (M = 0.526, SD = 0.149), t(73) = 2.515, p = 0.014, Cohen's d = 0.529, 95% CI [0.001, 0.141] (see Figure 3A). A similar trend was found for both avoidance and GAD scores, indicating that children with low avoidance (M = 0.601, SD = 0.134) had higher PLV than children with high avoidance (M = 0.543, SD = 0.141), t(72) = 1.786, p = 0.078, Cohen's d = 0.422, 95% CI [−0.006, 0.122] (see Figure 3B); and that children with low GAD scores (M = 0.598, SD = 0.127) had higher PLV than children with high GAD scores (M = 0.538, SD = 0.153), t(70) = 1.81, p = 0.075, Cohen's d = 0.427, 95% CI [−0.006, 0.126] (see Figure 3C), though note that the latter two effects did not reach significance. There were no significant interactions between exposure and the other variables: F(1, 73) = 0.101, p = 0.752 for the interaction with genetic risk; F(1, 73) = 1.169, p = 0.283 for the interaction with avoidance behavior; F(1, 71) = 0.573, p = 0.452 for the interaction with GAD.
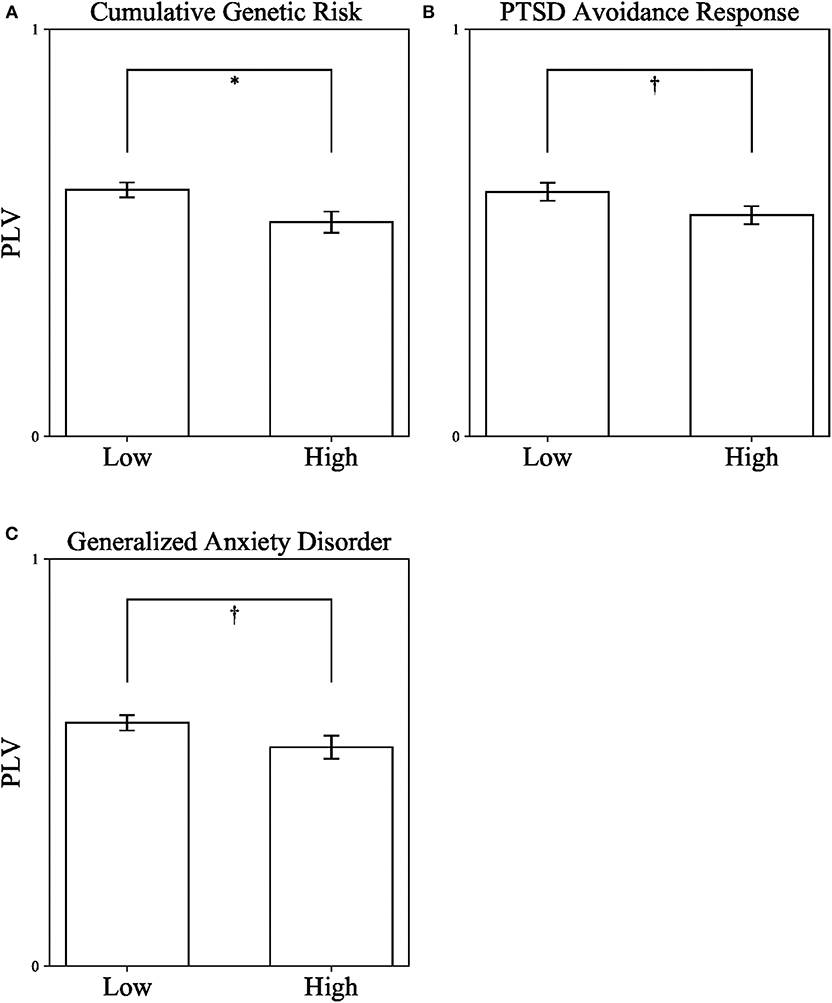
Figure 3. Differences in DMN connectivity (PLV) from a series of independent t-tests between children with high and low Cumulative Genetic Risk on Oxytocin-Pathway Genes (A), PTSD Avoidance Response in Early Childhood (B), and Generalized Anxiety Disorder in Late Childhood (C). PLV, Phase locking value. *p < 0.05; †p < 0.1. Error bars represent standard errors.
We ad-hoc also looked at differences in cumulative genetic risk, avoidance behavior, and GAD scores between war-exposed and control children. We expected to find differences for GAD scores and avoidance behavior between the groups but not for cumulative genetic risk. Results partly supported our assumptions; avoidance behavior was higher in the exposed children (M = 1.653, SD = 0.944) compared to controls (M = 1.231, SD = 0.553), t(198.663) = 3.89, p < 0.001, Cohen's d = 0.525, 95% CI [0.165, 0.679] (equal variances not assumed) and cumulative genetic risk did not differ between the groups [t(229) = 1.475, p = 0.142]. However, there was also no difference in the prevalence of GAD between groups [t(172) = 1.485, p = 0.139].
As can be seen in Table 1, there was a gender difference between war-exposed and control children. However, gender was ruled out as an alternative explanation for DMN connectivity since no gender-by-gender or gender-by-exposure effects were found [F(1, 73) = 0.001, p = 0.99; F(1, 71) < 0.001, p = 0.99, respectively]. Similarly, while there was a gender difference between children with high and low GAD scores (χ2 = 5.634, p = 0.018; 8/22 boys/girls in the high GAD group and 19/23 boys/girls in the low GAD group), there was no gender-by-GAD effect [F(1, 72) = 2.67, p = 1.07]. Furthermore, there were no gender differences between children with high and low genetic risk (χ2 = 0.303, p = 0.582), high and low avoidance behaviors (χ2 = 0.071, p = 0.79) or high and low GAD scores (χ2 = 0.303, p = 0.582).
Next, Pearson correlation analysis was conducted between all five variables across all participants (for full results see Table 3). It was found that DMN connectivity (i.e., PLV) is significantly negatively correlated with cumulative genetic risk (r = −0.359, p = 0.002), avoidance (r = −0.327, p = 0.008), and GAD scores (r = −0.308, p = 0.009), indicating that greater the genetic risk, the more avoidance the child exhibits to trauma reminders and the more severe his/her GAD, the less connectivity the child has in the theta band in the DMN (i.e., lower PLV). Moreover, cumulative genetic risk was significantly positively correlated with GAD scores (r = 0.218, p = 0.004), indicating that the more genetic risk factors the child has the more severe his/her GAD. In addition, exposure was significantly positively correlated with avoidance (r = 0.229, p = 0.001), indicating that exposed children are more likely to exhibit avoidance behavior. For scatter plots see Figure 4.
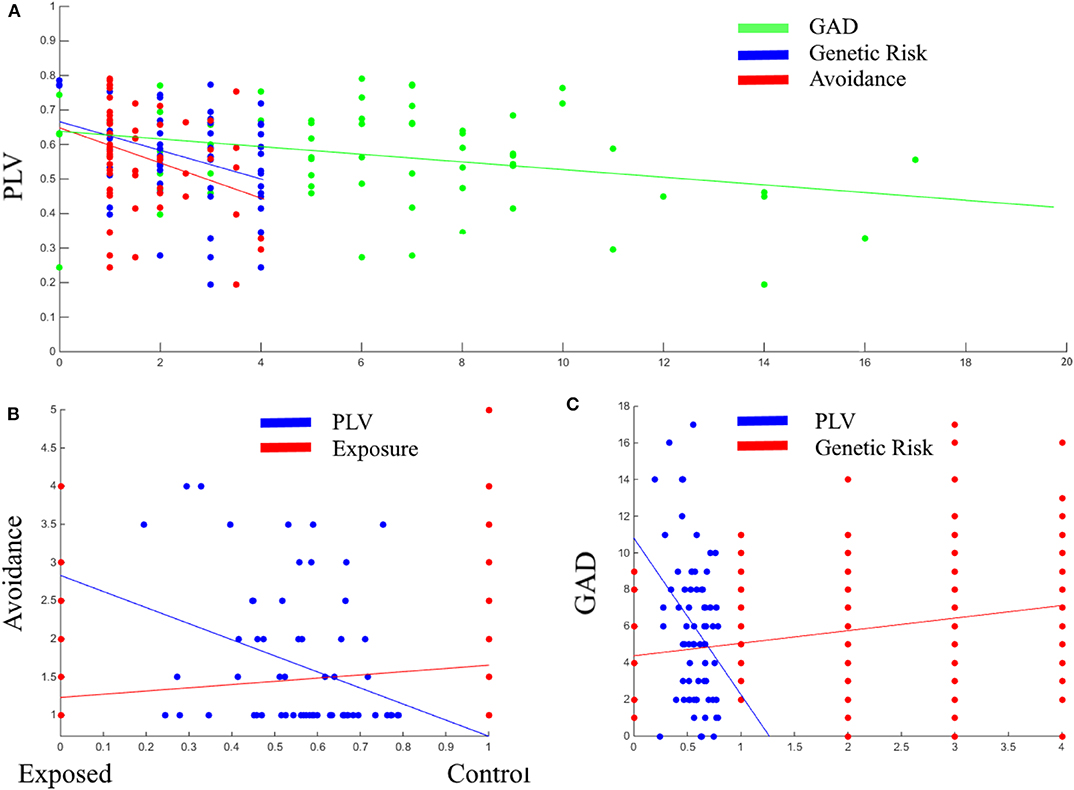
Figure 4. Scatter plots between PLV, GAD, genetic risk, and avoidance (A); Between avoidance, PLV, and exposure (B); Between GAD, PLV, and genetic risk (C). PLV, Phase locking value; GAD, General anxiety disorder scores; Genetic Risk, cumulative genetic risk. Linear lines are regression lines (between the variable on axis Y and the variable in the same color as the regression line).
Lastly, hierarchical regression analysis was conducted to predict DMN theta connectivity (PLV values). Exposure was entered in the first step; cumulative genetic risk was entered in the second step; and avoidance and GAD scores were entered in the third step. This order was chosen to reflect the chronological influence from early to later. Regression statistics are presented in Table 4. The hierarchical regression revealed that at stage one, exposure did not contribute significantly to the regression model, F(1, 60) = 1.28, p = 0.262, as it accounted only for 2.1% of the variation in PLV. However, introducing cumulative genetic risk explained an additional 13.7% in variation of PLV and this change in R2 was found significant, F(1, 59) = 9.621, p = 0.003. Similarly, adding avoidance and GAD scores in the third step explained an additional 8.8% in variation of PLV and this change in R2 was also found significant, F(1, 57) = 3.308, p = 0.044. Together, the four variables explained 24.6% of the variance in PLV. As seen in Table 4, the final model indicates that cumulative risk on oxytocin-pathway genes and child avoidant response to trauma reminders in early childhood each uniquely predicted variability in DMN connectivity at the transition to adolescence.
While the consolidation of the DMN into a unified network marks a developmental achievement critical for proper brain functioning [for a systematic review and meta-analysis see Mak et al. (85)], very little research examined DMN connectivity across childhood and adolescence, with nearly none testing its maturation in relation to stress-related symptoms and markers of risk and resilience. In the current study, we examined the association between genetic variability on the OT system and DMN connectivity at the transition to adolescence. Our goal was to expand knowledge on how ELS and psychological manifestations of stress undermine the DMN's ability to function as a coherent network whereas better innate functionality of the OT system may facilitates DMN maturation. Utilizing a prospective longitudinal study and following children across the first decade of life, we found that exposure to ELS, cumulative genetic risk on oxytocin-pathway genes, avoidant behavioral response to trauma reminders in early childhood, and the diagnosis of an anxiety disorder in late childhood cumulatively explained 25% of the variance in DMN connectivity in preadolescence. Overall, our findings point in four important directions. First, greater inborn functionality of the OT system sustains maturation of the DMN and may serve to buffer the effects of chronic early stress on DMN connectivity. Second, young children's avoidant response to trauma reminders in the context of chronic trauma serves as an important early indicator of difficulties in neural maturation a decade later. Third, the existence of a well-defined anxiety disorder bears negative consequences for the development of the DMN, a core system sustaining the emerging self. Finally, independent of ELS exposure, these factors cumulatively predict DMN connectivity in preadolescence and when appearing together across childhood may chart a risk trajectory for the maturation of the DMN.
Our findings are the first to demonstrate how the interplay of genetic factors and stress-related symptoms as they mutually influence each other over time shapes DMN connectivity at the transition to adolescence. The DMN is known to be involved in processes implicated in stress regulation, such as hypervigilance and alertness, alterations of intrinsic and extrinsic attention, self-referential mental activity, and recollection of personal experiences (1, 86). Studies in adults have shown that following stress, DMN activation is both reduced (87) and less integrated (88), and various ELS conditions, such as a history of poverty reduces DMN connectivity (88). Much further longitudinal research is thus required to tease various forms of ELS and to chart specific developmental trajectories for each condition that may impair the functioning of core neural networks.
DMN connectivity has been repeatedly associated with PTSD symptoms and anxiety disorders in adults. PTSD has been linked with reduced connectivity of the DMN (13, 14, 89) and a history of chronic early trauma has been associated with reduced DMN connectivity in adulthood, with the degree of impairment increasing as a function of the severity of dissociative symptoms (89). Furthermore, mindfulness-based psychotherapy helped increase DMN connectivity in veterans suffering from war-related PTSD and the improvement correlated specifically with reduction in avoidant symptoms (13), consistent with our findings on the specific associations between DMN connectivity and avoidant symptoms. Similarly, PTSD patients exhibited shorter activations of the DMN after exposure to trauma reminders that correlated with the severity of symptoms (90). Trauma-focused cognitive behavior therapy rescued DMN activity duration of activation and brought it back to normality and other stress-related psychopathologies linked with alterations in DMN activation patterns in adults (91, 92). Our findings highlight avoidant response to trauma reminders in early childhood, which is associated with PTSD chronicity in childhood (47), as an early marker of disrupted neural maturation and underscores the need to identify young children with avoidant symptoms and avoidant behavior in contexts of chronic stress and trauma as those who require the most rapid and targeted interventions.
In addition to PTSD, anxiety and stress-related disorders in adults have been linked with disruptions to DMN functionality (91, 92) and our findings are the first to show similar associations in children. Comorbidity between depression and anxiety was associated with reduced connectivity in the anterior regions of the DMN and increased connectivity in the posterior regions. During task, when DMN activity is typically suppressed, psychosomatic patients with anxiety disorders showed reduced activation in selected DMN nodes (92). In adults, disruptions to DMN alpha connectivity were related to anxious and depressive symptoms (29). The current findings indicate that when an anxiety disorder is consolidated by late childhood, this poses a risk for the development of DMN connectivity during the 10–13-years-old period described by Sherman et al. (19) as a sensitive window for the maturation of the DMN.
The current study expands our previous knowledge by linking genetic variability on the extended OT system with functionality of the DMN in children in the context of chronic early life stress. Based on the literature linking OT to stress-related psychopathologies, such as PTSD and anxiety (70), the OT system has been considered as a potential route for prevention and intervention in stress-related disorders (93). Our findings show that cumulative genetic risk on the extended OT system and avoidant symptoms in early childhood were each uniquely predictive of disruptions to DMN connectivity in early adolescence, above and beyond stress exposure, adding to the discussion on the role of the OT system in brain maturation in the context of chronic early stress. These findings suggest that there may be non-circumstantial factors that pose a risk to functionality of the DMN. Even among children reared in low-risk contexts, those born with more risky dispositions and develop avoidant responses that mature into a full-blown anxiety disorder can suffer a protracted maturation of the DMN. We have previously shown that in these children greater salivary oxytocin was associated with better immune biomarkers and both oxytocin and immune-system functionality buffered the effect of trauma on the development of anxiety disorders (94). This suggests that multiple indices of OT-system functionality may serve as indices of resilience and buffer the effects of stress on outcome in children exposed to chronic stress, trauma, and adversity.
Our findings have several clinical implications for children growing up amidst chronically stressed conditions. First, boosting the child's OT system, whether through intranasal administration or via bolstering parent-child synchrony, parental touch, and physical contact which have shown to increase OT production (95, 96), may provide a buffer against stress-related psychopathologies. Thus, early interventions aimed at increasing child-parent synchronization and physical touch or nasally administrating OT may serve as protective factors for brain development. Second, interventions focusing on reducing avoidant behavior in response to ELS may protect children from the long-lasting effects of ELS on the DMN. Indeed, studies show that therapeutic strategies, whether cognitive or emotional focusing on reducing avoidant behavior are highly successful in reducing the severity of GAD in adults (97) and our findings suggest that the same mechanisms may operate in children.
Several study limitations should be taken into consideration in the interpretation of the findings. First, consistent with much prior research we treated the DMN as a unitary system; however, several recent studies have demonstrated that the DMN comprises several interrelated subsystems (1, 98–100). Similarly, we treated the cumulative genetic risk system as a unitary factor in which each gene received equal weight and it is possible that one or more genes are more important in the current context. Future research should address this possibility by statistically determining the importance of each gene across subjects toward the computation of a more nuanced cumulative variable. In addition, we did not target other neural systems associated with the DMN or OT. Further, there are external factors that predict DMN connectivity in children that were not included in current study. For example, maternal depression, anxiety, and post-traumatic symptoms, which we previously found to be linked with DMN maturation (29). Thus, in order to draw a more comprehensive picture, future research should take into account, in addition to child's characteristics, also the child's surrounding envelope. Finally, some of the findings are only marginally significant, such as the difference in DMN connectivity between children high and low in avoidant behavior and children high and low in anxiety disorder, and these findings should be treated with caution. Nevertheless, it is important to note that while these variables were not significant when tested independently, taken together, they had a significant cumulative effect and when appearing on top of each other across development were found to impair the development of the DMN.
Despite the above limitations, the current study expends previous knowledge by demonstrating the long-lasting effect of ELS on the developing brain. This is the first study to longitudinally test the cumulative impact of genetic, behavioral, and clinical factors as they unfold across development on maturation of a core neural system. Our findings highlight the role of the oxytocin system for brain maturation during a sensitive period of the transition to adolescence and may have important implication for both research on the developing brain and the construction of targeted interventions for children exposed to chronic stress, war, and trauma.
The datasets analyzed in this article are not publicly available. Request to access the datasets should be directed toZmVsZG1hbi5ydXRoQGdtYWlsLmNvbQ==.
The studies involving human participants were reviewed and approved by Ethics Committee of Bar Ilan University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
RF designed the longitudinal study and wrote the paper. JL designed and conducted the MEG experiment. MZ-W analyzed the MEG data and wrote the paper. RE conducted the genetic analysis.
This study was supported by a Brain and Behavior (NARSAS) Independent Investigator Award to RF and the Simms/Mann Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Raichle ME. The brain's default mode network. Annu Rev Neurosci. (2015) 38:433–47. doi: 10.1146/annurev-neuro-071013-014030
2. de Pasquale F, Della Penna S, Snyder AZ, Marzetti L, Pizzella V, Romani GL, et al. A cortical core for dynamic integration of functional networks in the resting human brain. Neuron. (2012) 74:753–64. doi: 10.1016/j.neuron.2012.03.031
3. Liu J, Liao X, Xia M, He Y. Chronnectome fingerprinting: Identifying individuals and predicting higher cognitive functions using dynamic brain connectivity patterns. Hum. Brain Mapp. (2018) 39:902–15. doi: 10.1002/hbm.23890
4. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. (2001) 98:4259–64. doi: 10.1073/pnas.071043098
5. Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc B Biol Sci. (2003) 358:459–73. doi: 10.1098/rstb.2002.1218
6. Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. (2007) 11:49–57. doi: 10.1016/j.tics.2006.11.004
7. Doucet GE, Bassett DS, Yao N, Glahn DC, Frangou S. The role of intrinsic brain functional connectivity in vulnerability and resilience to bipolar disorder. Am J Psychiatry. (2017) 174:1214–22. doi: 10.1176/appi.ajp.2017.17010095
8. Du M-Y, Liao W, Lui S, Huang X-Q, Li F, Kuang W-H, et al. Altered functional connectivity in the brain default-mode network of earthquake survivors persists after 2 years despite recovery from anxiety symptoms. Soc Cogn Affect Neurosci. (2015) 10:1497–505. doi: 10.1093/scan/nsv040
9. Hemington KS, Rogachov A, Cheng JC, Bosma RL, Kim JA, Osborne NR, et al. Patients with chronic pain exhibit a complex relationship triad between pain, resilience, and within- and cross-network functional connectivity of the default mode network. Pain. (2018) 159:1621–30. doi: 10.1097/j.pain.0000000000001252
10. Luo Y, Kong F, Qi S, You X, Huang X. Resting-state functional connectivity of the default mode network associated with happiness. Soc Cogn Affect Neurosci. (2016) 11:516–24. doi: 10.1093/scan/nsv132
11. Moran JM, Kelley WM, Heatherton TF. What can the organization of the brain's default mode network tell us about self-knowledge? Front Hum Neurosci. (2013) 7:391. doi: 10.3389/fnhum.2013.00391
12. Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. (2009) 34:187–94.
13. King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, et al. Altered default mode network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depress Anxiety. (2016) 33:289–99. doi: 10.1002/da.22481
14. Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Théberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. (2010) 121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x
15. Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques P, Marques F, et al. Stress impact on resting state brain networks. PLoS ONE. (2013) 8:e66500. doi: 10.1371/journal.pone.0066500
16. Soares JM, Sampaio A, Marques P, Ferreira LM, Santos NC, Marques F, et al. Plasticity of resting state brain networks in recovery from stress. Front Hum Neurosci. (2013) 7:919. doi: 10.3389/fnhum.2013.00919
17. Fair DA, Cohen AL, Dosenbach NUF, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci USA. (2008) 105:4028–32. doi: 10.1073/pnas.0800376105
18. Østby Y, Walhovd KB, Tamnes CK, Grydeland H, Westlye LT, Fjell AM. Mental time travel and default-mode network functional connectivity in the developing brain. Proc Natl Acad Sci USA. (2012) 109:16800–4. doi: 10.1073/pnas.1210627109
19. Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev Cogn Neurosci. (2014) 10:148–59. doi: 10.1016/j.dcn.2014.08.002
20. Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. (2007) 104:13507–12. doi: 10.1073/pnas.0705843104
21. Sato JR, Salum GA, Gadelha A, Picon FA, Pan PM, Vieira G, et al. Age effects on the default mode and control networks in typically developing children. J Psychiatr Res. (2014) 58:89–95. doi: 10.1016/j.jpsychires.2014.07.004
22. Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. (2010) 52:290–301. doi: 10.1016/j.neuroimage.2010.04.009
23. Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. (2011) 31:18578–89. doi: 10.1523/JNEUROSCI.4465-11.2011
24. Zeev-Wolf M, Levy J, Jahshan C, Peled A, Levkovitz Y, Grinshpoon A, et al. MEG resting-state oscillations and their relationship to clinical symptoms in schizophrenia. Neuroimage Clin. (2018) 20:753–61. doi: 10.1016/J.NICL.2018.09.007
25. Bell MA. The ontogeny of the EEG during infancy and childhood: implications for cognitive development. In: Neuroimaging in Child Neuropsychiatric Disorders. Berlin; Heidelberg: Springer (1998). p. 97–111. doi: 10.1007/978-3-642-95848-9_9
26. Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clin Neurophysiol. (2001) 112:806–14. doi: 10.1016/S1388-2457(01)00488-6
27. Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. (1999) 29:169–95. doi: 10.1016/S0165-0173(98)00056-3
28. Palva S, Palva JM. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front Psychol. (2011) 2:204. doi: 10.3389/fpsyg.2011.00204
29. Zeev-Wolf M, Levy J, Goldstein A, Zagoory-Sharon O, Feldman R. Chronic early stress impairs default mode network connectivity in preadolescents and their mothers. Biol Psychiatry. (2019) 4:72–80. doi: 10.1016/j.bpsc.2018.09.009
30. Lachaux J-P, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brains signals. Hum Brain Mapp. (1999) 8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C
31. Aydore S, Pantazis D, Leahy RM. A note on the phase locking value and its properties. Neuroimage. (2013) 74:231–44. doi: 10.1016/j.neuroimage.2013.02.008
32. Bruña R, Maestú F, Pereda E. Phase locking value revisited: teaching new tricks to an old dog. J Neural Eng. (2018) 15:056011. doi: 10.1088/1741-2552/aacfe4
33. Feldman R. The neurobiology of mammalian parenting and the biosocial context of human caregiving. Horm Behav. (2016) 77:3–17. doi: 10.1016/j.yhbeh.2015.10.001
34. Feldman R, Braun K, Champagne FA. The neural mechanisms and consequences of paternal caregiving. Nat Rev Neurosci. (2019) 20:205–24. doi: 10.1038/s41583-019-0124-6
35. Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J. Neuroendocrinol. (2008) 20:858–65. doi: 10.1111/j.1365-2826.2008.01726.x
36. Neumann ID, Krömer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. (2000) 96:31–8. doi: 10.1016/S0167-0115(00)00197-X
37. Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. (2008) 586:377–85. doi: 10.1113/jphysiol.2007.145896
38. Brodmann K, Gruber O, Goya-Maldonado R. Intranasal oxytocin selectively modulates large-scale brain networks in humans. Brain Connect. (2017) 7:454–63. doi: 10.1089/brain.2017.0528
39. Riem MME, Van IJzendoorn MH, Tops M, Boksem MAS, Rombouts SARB, Bakermans-Kranenburg MJ. Oxytocin effects on complex brain networks are moderated by experiences of maternal love withdrawal. Eur Neuropsychopharmacol. (2013) 23:1288–95. doi: 10.1016/j.euroneuro.2013.01.011
40. Wang J, Braskie MN, Hafzalla GW, Faskowitz J, McMahon KL, de Zubicaray GI, et al. Relationship of a common OXTR gene variant to brain structure and default mode network function in healthy humans. Neuroimage. (2017) 147:500–6. doi: 10.1016/j.neuroimage.2016.12.062
41. Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol Psychiatry. (2015) 79:174–84. doi: 10.1016/j.biopsych.2015.08.008
42. Hernandez LM, Lawrence KE, Padgaonkar NT, Inada M, Hoekstra JN, Lowe JK, et al. Imaging-genetics of sex differences in ASD: distinct effects of OXTR variants on brain connectivity. Transl Psychiatry. (2020) 10:82. doi: 10.1038/s41398-020-0750-9
43. Kumsta R, Heinrichs M. Oxytocin, stress and social behavior: neurogenetics of the human oxytocin system. Curr Opin Neurobiol. (2013) 23:11–6. doi: 10.1016/j.conb.2012.09.004
44. Tabak BA, Vrshek-Schallhorn S, Zinbarg RE, Prenoveau JM, Mineka S, Redei EE, et al. Interaction of CD38 variant and chronic interpersonal stress prospectively predicts social anxiety and depression symptoms over six years. Clin Psychol Sci. (2016) 4:17–27. doi: 10.1177/2167702615577470
45. Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. (2007) 446:41–5. doi: 10.1038/nature05526
46. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. (2001) 81:629–83. doi: 10.1152/physrev.2001.81.2.629
47. Feldman R, Vengrober A, Ebstein RP. Affiliation buffers stress: cumulative genetic risk in oxytocin–vasopressin genes combines with early caregiving to predict PTSD in war-exposed young children. Transl Psychiatry. (2014) 4:e370. doi: 10.1038/tp.2014.6
48. Schneiderman I, Kanat-Maymon Y, Ebstein RP, Feldman R. Cumulative risk on the oxytocin receptor gene (OXTR) underpins empathic communication difficulties at the first stages of romantic love. Soc Cogn Affect Neurosci. (2014) 9:1524–9. doi: 10.1093/scan/nst142
49. Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene-environment interactions predict cortisol responses after acute stress: implications for the etiology of depression. Psychoneuroendocrinology. (2009) 34:1294–303. doi: 10.1016/j.psyneuen.2009.03.017
50. Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. (2012) 75:747–61. doi: 10.1016/j.neuron.2012.08.016
51. Perkonigg A, Pfister H, Stein MB, Höfler M, Lieb R, Maercker A, et al. Longitudinal course of posttraumatic stress disorder and posttraumatic stress disorder symptoms in a community sample of adolescents and young adults. Am J Psychiatry. (2005) 162:1320–7. doi: 10.1176/appi.ajp.162.7.1320
52. Zero to Three. DC:0-3R: Diagnostic Classification of Mental Health and Developmental Disorders of Infancy and Early Childhood. Revised ed. Washington, DC: Zero To Three Press (2005).
53. Feldman R, Vengrober A. Posttraumatic stress disorder in infants and young children exposed to war-related trauma. J Am Acad Child Adolesc Psychiatry. (2011) 50:645–58. doi: 10.1016/j.jaac.2011.03.001
54. Degnan KA, Almas AN, Henderson HA, Hane AA, Walker OL, Fox NA. Longitudinal trajectories of social reticence with unfamiliar peers across early childhood. Dev Psychol. (2014) 50:2311–23. doi: 10.1037/a0037751
55. Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. (2006) 26:6399–405. doi: 10.1523/JNEUROSCI.0666-06.2006
56. Jarcho JM, Davis MM, Shechner T, Degnan KA, Henderson HA, Stoddard J, et al. Early-childhood social reticence predicts brain function in preadolescent youths during distinct forms of peer evaluation. Psychol Sci. (2016) 27:821–35. doi: 10.1177/0956797616638319
57. Michalska KJ, Feldman JS, Ivie EJ, Shechner T, Sequeira S, Averbeck B, et al. Early-childhood social reticence predicts SCR-BOLD coupling during fear extinction recall in preadolescent youth. Dev Cogn Neurosci. (2019) 36:100605. doi: 10.1016/j.dcn.2018.12.003
58. Halevi G, Djalovski A, Vengrober A, Feldman R. Risk and resilience trajectories in war-exposed children across the first decade of life. J Child Psychol Psychiatry. (2016) 57:1183–93. doi: 10.1111/jcpp.12622
59. Feldman R, Vengrober A, Eidelman-Rothman M, Zagoory-Sharon O. Stress reactivity in war-exposed young children with and without posttraumatic stress disorder: relations to maternal stress hormones, parenting, and child emotionality and regulation. Dev Psychopathol. (2013) 25:943–55. doi: 10.1017/S0954579413000291
60. Apter-Levy Y, Feldman M, Vakart A, Ebstein RP, Feldman R. Impact of maternal depression across the first 6 years of life on the child's mental health, social engagement, and empathy: the moderating role of oxytocin. Am J Psychiatry. (2013) 170:1161–8. doi: 10.1176/appi.ajp.2013.12121597
61. Avinun R, Ebstein RP, Knafo A. Human maternal behaviour is associated with arginine vasopressin receptor 1A gene. Biol Lett. (2012) 8:894–6. doi: 10.1098/rsbl.2012.0492
62. Bakermans-Kranenburg MJ, van IJzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci. (2008) 3:128–34. doi: 10.1093/scan/nsn004
63. Feldman R, Gordon I, Influs M, Gutbir T, Ebstein RP. Parental oxytocin and early caregiving jointly shape children's oxytocin response and social reciprocity. Neuropsychopharmacology. (2013) 38:1154–62. doi: 10.1038/npp.2013.22
64. Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, et al. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry. (2012) 72:175–81. doi: 10.1016/j.biopsych.2011.12.025
65. Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland adaptive behavior scales and cognition. Mol Psychiatry. (2008) 13:980–8. doi: 10.1038/sj.mp.4002087
66. Wu N, Li Z, Su Y. The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. J Affect Disord. (2012) 138:468–72. doi: 10.1016/j.jad.2012.01.009
67. Furman DJ, Chen MC, Gotlib IH. Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology. (2011) 36:891–7. doi: 10.1016/j.psyneuen.2010.12.004
68. Inoue H, Yamasue H, Tochigi M, Abe O, Liu X, Kawamura Y, et al. Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biol Psychiatry. (2010) 68:1066–72. doi: 10.1016/j.biopsych.2010.07.019
69. Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. (2005) 58:74–7. doi: 10.1016/j.biopsych.2005.03.013
70. Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci USA. (2009) 106:21437–41. doi: 10.1073/pnas.0909579106
71. Lerer E, Levi S, Israel S, Yaari M, Nemanov L, Mankuta D, et al. Low CD38 expression in lymphoblastoid cells and haplotypes are both associated with autism in a family-based study. Autism Res. (2010) 3:293–302. doi: 10.1002/aur.156
72. Munesue T, Yokoyama S, Nakamura K, Anitha A, Yamada K, Hayashi K, et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci Res. (2010) 67:181–91. doi: 10.1016/j.neures.2010.03.004
73. Avinun R, Israel S, Shalev I, Gritsenko I, Bornstein G, Ebstein RP, et al. AVPR1A variant associated with preschoolers' lower altruistic behavior. PLoS ONE. (2011) 6:e25274. doi: 10.1371/journal.pone.0025274
74. Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, et al. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry. (2002) 7:503–7. doi: 10.1038/sj.mp.4001125
76. Feldman R, Eidelman AI, Sirota L, Weller A. Comparison of skin-to-skin (kangaroo) and traditional care: parenting outcomes and preterm infant development. Pediatrics. (2002) 110:16–26. doi: 10.1542/peds.110.1.16
77. Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry. (2009) 48:919–27. doi: 10.1097/CHI.0b013e3181b21651
78. Feldman R, Keren M, Gross-Rozval O, Tyano S. Mother-child touch patterns in infant feeding disorders: relation to maternal, child, and environmental factors. J Am Acad Child Adolesc Psychiatry. (2004) 43:1089–97. doi: 10.1097/01.chi.0000132810.98922.83
79. Feldman R, Masalha S. Parent-child and triadic antecedents of children's social competence: cultural specificity, shared process. Dev Psychol. (2010) 46:455–67. doi: 10.1037/a0017415
80. Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The development and well-being assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry Allied Discipl. (2000) 41:645–55. doi: 10.1111/j.1469-7610.2000.tb02345.x
81. Mansbach-Kleinfeld I, Apter A, Farbstein I, Levine SZ, Ponizovsky AM. A population-based psychometric validation study of the strengths and difficulties questionnaire–Hebrew version. Front Psychiatry. (2010) 1:151. doi: 10.3389/fpsyt.2010.00151
82. Levy J, Yirmiya K, Goldstein A, Feldman R. Chronic trauma impairs the neural basis of empathy in mothers: relations to parenting and children's empathic abilities. Dev Cogn Neurosci. (2019) 38:100658. doi: 10.1016/j.dcn.2019.100658
83. Tal I, Abeles M. Cleaning MEG artifacts using external cues. J Neurosci Methods. (2013) 217:31–8. doi: 10.1016/j.jneumeth.2013.04.002
84. Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. (2011) 2011:156869. doi: 10.1155/2011/156869
85. Mak LE, Minuzzi L, MacQueen G, Hall G, Kennedy SH, Milev R. The default mode network in healthy individuals: a systematic review and meta-analysis. Brain Connect. (2017) 7:25–33. doi: 10.1089/brain.2016.0438
86. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. (2012) 8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049
87. Van Leeuwen JMC, Vink M, Fernández G, Hermans EJ, Joëls M, Kahn RS, et al. At-risk individuals display altered brain activity following stress. Neuropsychopharmacology. (2018) 43:1954–60. doi: 10.1038/s41386-018-0026-8
88. Sripada RK, Swain JE, Evans GW, Welsh RC, Liberzon I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology. (2014) 39:2244–51. doi: 10.1038/npp.2014.75
89. Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. (2009) 34:187–94.
90. Charquero-Ballester M, Kleim B, Vidaurre D, Ruff C, Stark E, Tuulari JJ, et al. Effective psychological treatment for PTSD changes the dynamics of specific large—scale brain networks. BioRxiv. (2020). doi: 10.1101/2020.01.07.891986
91. Andreescu C, Wu M, Butters MA, Figurski J, Reynolds CF, Aizenstein HJ. The default mode network in late-life anxious depression. Am J Geriatr Psychiatry. (2011) 19:980–3. doi: 10.1097/JGP.0b013e318227f4f9
92. Zhao XH, Wang PJ, Li CB, Hu ZH, Xi Q, Wu WY, et al. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol. (2007) 63:373–8. doi: 10.1016/j.ejrad.2007.02.006
93. Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, et al. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. (2013) 38:1883–94. doi: 10.1016/j.psyneuen.2013.06.019
94. Ulmer-Yaniv A, Djalovski A, Yirmiya K, Halevi G, Zagoory-Sharon O, Feldman R. Maternal immune and affiliative biomarkers and sensitive parenting mediate the effects of chronic early trauma on child anxiety. Psychol Med. (2018) 48:1020–33. doi: 10.1017/S0033291717002550
95. Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Dev Sci. (2011) 14:752–61. doi: 10.1111/j.1467-7687.2010.01021.x
96. Vittner D, McGrath J, Robinson J, Lawhon G, Cusson R, Eisenfeld L, et al. Increase in oxytocin from skin-to-skin contact enhances development of parent-infant relationship. Biol Res Nurs. (2018) 20:54–62. doi: 10.1177/1099800417735633
97. Behar E, DiMarco ID, Hekler EB, Mohlman J, Staples AM. Current theoretical models of generalized anxiety disorder (GAD): conceptual review and treatment implications. J Anxiety Disord. (2009) 23:1011–23. doi: 10.1016/j.janxdis.2009.07.006
98. Andrews-Hanna JR. The brain's default network and its adaptive role in internal mentation. Neuroscientist. (2012) 18:251–70. doi: 10.1177/1073858411403316
99. Li W, Mai X, Liu C. The default mode network and social understanding of others: what do brain connectivity studies tell us. Front Hum Neurosci. (2014) 8:74. doi: 10.3389/fnhum.2014.00074
Keywords: OXTR, magnetoencephalography, trauma exposure, anxiety disorders, longitudinal studies, genetics
Citation: Zeev-Wolf M, Levy J, Ebstein RP and Feldman R (2020) Cumulative Risk on Oxytocin-Pathway Genes Impairs Default Mode Network Connectivity in Trauma-Exposed Youth. Front. Endocrinol. 11:335. doi: 10.3389/fendo.2020.00335
Received: 03 February 2020; Accepted: 29 April 2020;
Published: 22 May 2020.
Edited by:
Adam Smith, University of Kansas, United StatesReviewed by:
Hilary Marusak, Wayne State University, United StatesCopyright © 2020 Zeev-Wolf, Levy, Ebstein and Feldman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruth Feldman, ZmVsZG1hbi5ydXRoQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.