
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 15 April 2020
Sec. Thyroid Endocrinology
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00201
Background: Previous studies indicate the effects of thyroid dysfunction on adverse obstetric outcomes and fetal neurodevelopment, of which the results on gestational anemia are controversial. Here, we evaluated the influence of thyroid dysfunction on gestational anemia via published epidemiological articles and a new prospective study conducted by our team, respectively.
Methods: We searched studies on the PubMed, Embase, MEDLINE, and Cochrane databases as of November 2019, and conducted a prospective study in which participants underwent thyroid function and blood routine testing throughout pregnancy.
Results: The meta-analysis showed that pregnancies with overt hypothyroidism [OH; odds ratio (OR) = 3.74, 95% confidence interval (CI): 1.95–7.15] or that were thyroid peroxidase antibody (TPOAb)-positive (OR = 1.97, 95%CI: 1.19–3.26) had increased anemia risk, but similar results were not found in pregnancies with subclinical hypothyroidism (SCH) and hyperthyroidism. In the prospective study from our new data, the hypothyroid group had significant reductions in hemoglobin (Hb) (P = 0.048) and increased anemia risk (OR = 6.384, 95%CI: 2.498–16.311) during the second half of pregnancy. From the first to second half of pregnancy, the longitudinal reductions in Hb, erythrocyte (RBC), and hematocrit (Hct) levels were significantly increased in hypothyroid group.
Conclusions: Our meta-analysis indicates that untreated OH or TPOAb-positive pregnant women have increased risk of anemia. In addition, our new data showed that treated hypothyroidism is also a risk factor for anemia in the second half of pregnancy rather than in the first half. The results may guide strengthening of Hb monitoring in pregnancies with thyroid dysfunction.
Thyroid dysfunction is a common endocrine disease during pregnancy, and consists of overt hypothyroidism (OH), subclinical hypothyroidism (SCH), overt hyperthyroidism (OHyper), and thyroid peroxidase antibody (TPOAb)-positive status. In China, SCH prevalence in the first half of pregnancy is 5.96%, which is higher than that of OH (1). Thyroid dysfunction is associated with adverse pregnancy and neonatal outcomes, including placental abruption (2), preeclampsia (3), miscarriage (4, 5), gestational diabetes (6), neonatal death (7), intrauterine growth restriction (8) and neuropsychological development (9).
Anemia is a worldwide health problem affecting 33% of non-pregnant women and 38% of pregnant women (10). The prevalence of gestational anemia increases with the progress of pregnancy (11). The World Health Organization (WHO) divides anemia into normal cell, micro cell, and large cell anemia according to the form of the red blood cells (RBC). Iron deficiency anemia (IDA) accounts for 75% of anemia during pregnancy (12). Pregnant women are highly susceptible to IDA due to the increased demand for iron (13). Some studies have noted that anemia can weaken thyroid function by reducing TPO activity (14). Women with thyroid dysfunction were more likely to have anemia compared with euthyroid women (15). A cross-sectional study by Veltri et al. (16) showed that iron deficiency (ID) is related to high prevalence of thyroid autoimmune disease (TAI), higher serum thyroid-stimulating hormone (TSH), and lower free thyroxine (FT4) levels during the first trimester of pregnancy.
A clinical study that involved 15,000 pregnant women showed that SCH was not related to pregnancy anemia, whether treated or not (17). Sahu et al. (18) and Wang et al. (19) drew the same conclusion. However, Morchiladze et al. (20) and Hou et al. (21) drew the opposite conclusion. The impact of TPOAb on anemia is also controversial. In addition, the above studies are all cross-sectional studies. Therefore, we aimed to evaluate the relationship between thyroid dysfunction and anemia via meta-analysis and a longitudinal study.
We searched for studies published on the PubMed, Embase, MEDLINE, and Cochrane databases by November 2019. The search terms used were: thyroid function, thyroid dysfunction, thyroid disease, hypothyroid, hypothyroidism, subclinical hypothyroid, subclinical hypo thyroidism, hyperthyroidism, hyperthyroid, subclinical hyperthyroid, subclinical hyperthyroidism, thyroid peroxidase antibody, anti-TPO, TPOAb, anemia, iron deficiency, Hb, hemoglobin, pregnancy, gestation.
Studies were considered eligible if they met the following criteria: investigated the relationship between gestational thyroid dysfunction and anemia; data on maternal thyroid dysfunction and gestational anemia could be extracted to analyze the 95% confidence interval (CI). Comments, conference abstracts, books, reports, and articles that could not be analyzed further were excluded. We also excluded studies that analyzed various types of thyroid dysfunction as a whole or studies that only compared Hb levels in patients with thyroid dysfunction. Studies involving subjects who were formerly diagnosed with other hematological disease and studies in which patients were treated with iron supplements were excluded from the study.
Two authors carefully extracted the following information from each study: first author's name, publication year, pregnancy phase, country of population, diagnostic criteria for thyroid disease and anemia. In addition, we collected primary data on thyroid disease and anemia.
We used the Newcastle-Ottawa scale (NOS) to assess the quality of the selected studies from three aspects: selection, comparability, and results, with a maximum of four, two, and three stars, respectively. Studies assigned >6 stars are considered high-quality studies (22).
To assess the strength of the relationship between thyroid disease and anemia risk, we used Stata (version 15.1) to analyze the 95%CI to calculate the composite odds ratio (OR). The significance of the combined OR calculated using the Mantel–Haenszel statistical method was determined by the Z-test. P < 0.05 was considered significant. Heterogeneity was studied using the Cochrane Q test (P < 0.05 indicated statistical significance) and I2 statistic. I2 values of 25, 50, and 75% were used as evidence of low, moderate, or high heterogeneity, respectively. Random-effects model was used to pool the results when high heterogeneity was considered existed; fixed-effects model was used to pool the results if heterogeneity is low.
To assess the stability of the results, we performed a sensitivity analysis by omitting one report from each rotation and re-computing the pooled estimates of the remaining studies employing the metaninf command. Begg's test was conducted to assess publication bias.
The participants had been diagnosed with thyroid dysfunction from February 2016 to October 2019 at the First Affiliated Hospital of China Medical University endocrinology clinic before pregnancy and had been treated at our department until delivery. Women with history of other metabolic diseases, thyroid surgery, radioiodine treatment, or who had been treated with iron supplements before the first antenatal visit during pregnancy were excluded from the study. The control pregnant women were from the Subclinical Hypothyroidism Early Pregnancy (SHEP) project (23). The experimental procedure here has been approved by the China Medical University Ethics Committee and is consistent with the Helsinki Declaration. All participants signed written informed consent forms.
Up to October 2019, we enrolled a total of 198 pregnant women aged 23–45 years. During the first and second half of pregnancy, the thyroid function results and treatment in pregnant women with thyroid dysfunction were monitored. All participants completed questionnaires on history of thyroid disease, birth history, smoking, drinking, chronic diseases, and medication, and to monitor their Hb, RBC, and hematocrit (Hct) during the first and second half of pregnancy.
Serum TSH, FT4, free triiodothyronine (FT3), and TPOAb were measured using an electrochemiluminescence immunoassay on a Cobas Elecsys 601 unit (Roche Diagnostics, Basel, Switzerland).
For pregnancy, the TSH and FT4 reference intervals were 0.14–4.87 mIU/L and 12.35–20.71 pmol/L, respectively. For non-pregnant women, the TSH and FT4 reference intervals were 0.69–5.64 mIU/L and 12.27–19.10 pmol/L, respectively (24). TPOAb ≥ 50 IU/mL was considered positive (1). The diagnostic criteria for hyperthyroidism were decreased TSH levels combined with elevated FT4 levels. Hypothyroidism included OH with elevated TSH levels combined with decreased FT4 levels and SCH with elevated TSH levels with normal FT4 levels. According to the WHO guidelines, the lower threshold value for Hb in pregnancy is 110 g/L (25).
Statistical analysis was performed using SPSS 25.0 (SPSS Inc., Chicago, IL, USA). Normally distributed data are expressed as the mean ± SD; non-normally distributed data are expressed as the median (5 and 95% interquartile range). We summarized demographic and laboratory characteristics as medians and interquartile ranges for continuous variables, or numbers and percentages for categorical variables. Normally and non-normally distributed count data were compared between groups using independent sample t-tests and the Mann-Whitney U-test, respectively. The chi-square test was used to test for categorical variables. The risk factors for anemia during pregnancy were assessed using logistic regression after adjusted for TPOAb, maternal age, body mass index (BMI), TSH, smoking and drinking, and P < 0.05 was considered statistically significant.
The keyword search retrieved a total of 1,393 articles from the online databases. We excluded 998 articles by reading their titles and abstracts, and evaluated the remaining studies in their entirety, and 10 of them were included [(16–21, 23, 26–28); Figure 1]. According to the NOS, the included articles were high-quality articles. Table 1 shows the characteristics of all included articles.
Meta-analysis of the five studies that reported relevant data on the relationship between anemia and OH showed that the combined OR of anemia for OH pregnant women was 3.74 (95%CI: 1.95–7.15, P = 0, I2 = 59.7%), indicating that OH is associated with gestational anemia. Among the five articles, two did not mention antibodies and two identified type of anemia as IDA (18, 21, 23, 26). To explore the source of heterogeneity, we divided the five articles into two subgroups based on whether patients were treated. Subgroup meta-analysis indicated that untreated OH in pregnancy increased anemia risk (OR = 6.03, 95%CI: 3.85–9.43, P = 0, I2 = 0%), while treated OH in pregnancy was not associated with anemia (OR = 1.76, 95%CI: 0.59–5.21, P = 0.308, I2 = 49.5%). Heterogeneity was low following subgroup meta-analysis, indicating that the previous heterogeneity may have been due to different treatments (Figure 2). Begg's test (P = 0.462) did not indicate publication bias. Sensitivity analysis showed that the combined OR values of the remaining studies after one study had been removed remained stable.
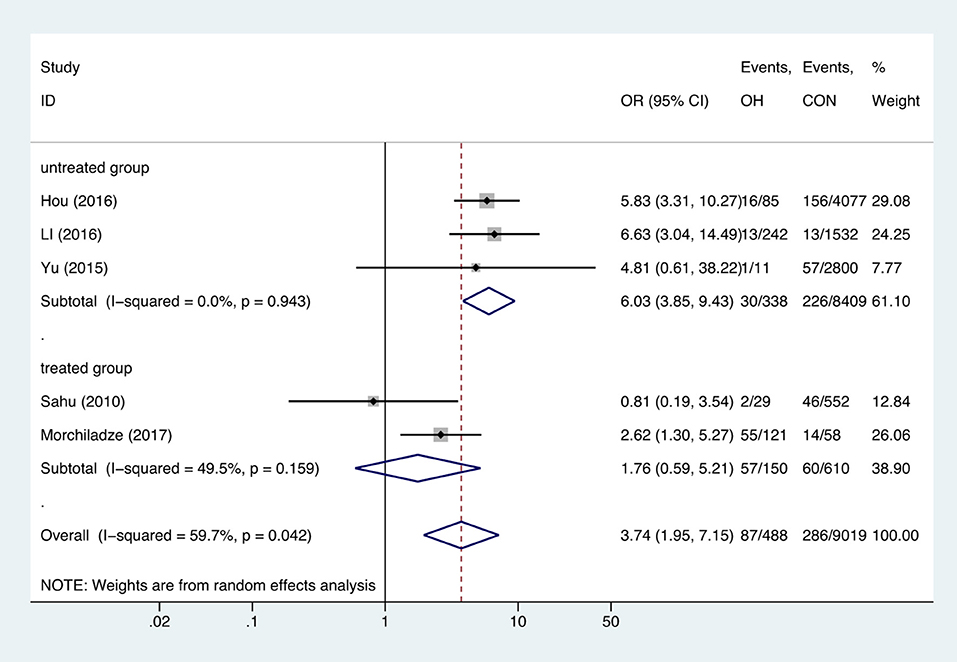
Figure 2. Forest plots of odds ratio and 95% confidence interval of pooled studies comparing pregnant women with overt hypothyroidism (OH) to euthyroid pregnant women (CON) for risk of gestational anemia.
Meta-analysis of the seven studies that reported relevant data on the association between anemia and SCH showed that SCH was not associated with anemia (OR = 1.55, 95%CI: 0.99–2.44, P = 0.056, I2 = 83.4%; Figure 3). Of these articles, there were five in which the patients were untreated. The combined results of the five studies found no significant association between untreated SCH with anemia (OR = 1.59, 95%CI: 0.94–2.67, P = 0.082, I2 = 88.6%; Figure 4). Among the seven included articles, three adopted the 2011 American Thyroid Association (ATA) guidelines as TSH > 2.5 mIU/L; the remaining four studies used pregnancy-specific reference ranges for diagnosing SCH. Subgroup analysis based on diagnostic criteria found that SCH remained unrelated to gestational anemia (Supplementary Figure 1). High heterogeneity persisted following the subgroup analysis, probably because the included articles had different diagnostic time of hypothyroidism and anemia. Begg's test (P = 0.548) did not indicate publication bias. Sensitivity analysis showed that the combined OR values of the remaining studies after one study had been removed remained stable.
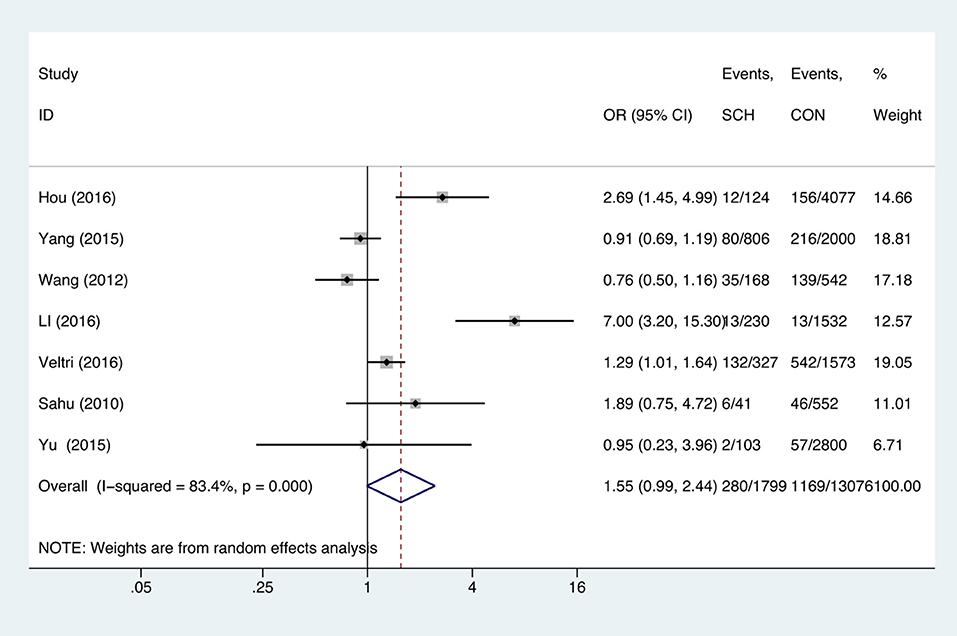
Figure 3. Forest plots of odds ratio and 95% confidence interval of pooled studies comparing pregnant women with subclinical hypothyroidism (SCH) to euthyroid pregnant women (CON) for risk of gestational anemia.
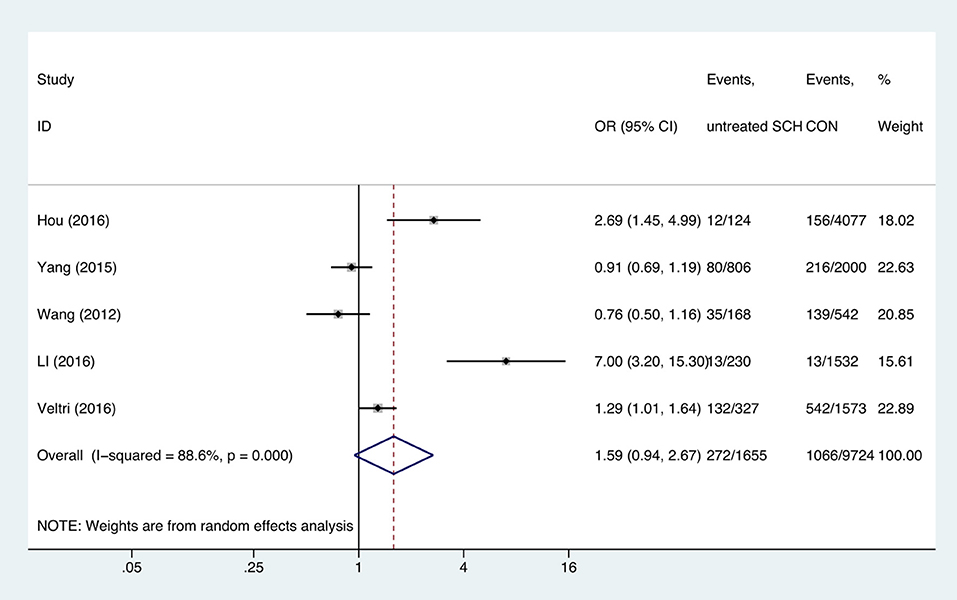
Figure 4. Forest plots of odds ratio and 95% confidence interval of pooled studies comparing untreated subclinical hypothyroid pregnant women (SCH) to euthyroid pregnant women (CON) for risk of gestational anemia.
Two studies analyzed the effect of hyperthyroidism on gestational anemia. The combined OR of anemia for hyperthyroid pregnant women was 1.27 (95%CI: 0.43–3.73, P = 0.664, I2 = 0%), indicating that hyperthyroidism had no influence on gestational anemia (Figure 5).
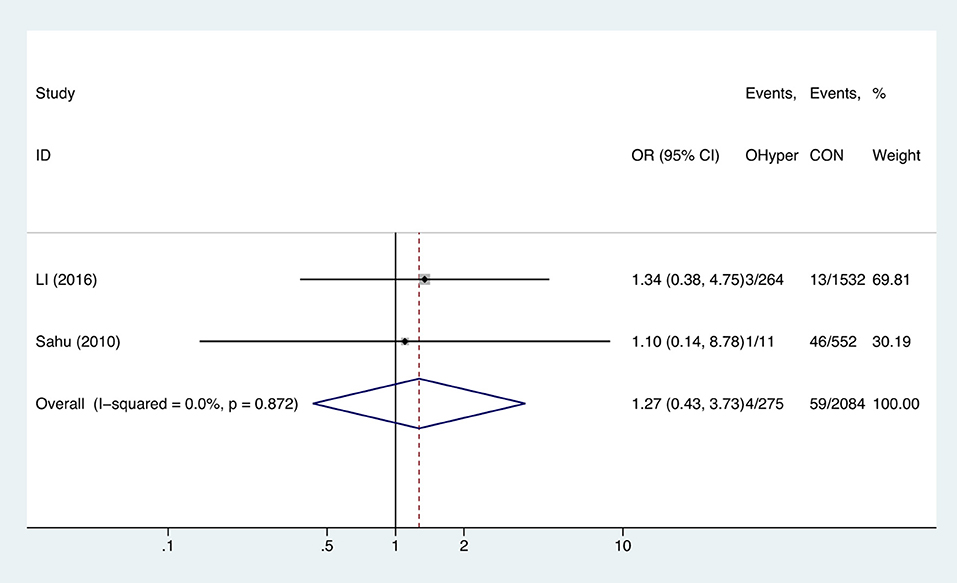
Figure 5. Forest plots of odds ratio and 95% confidence interval of pooled studies comparing pregnant women with hyperthyroidism (OHyper) to euthyroid pregnant women (CON) for risk of gestational anemia.
Compared with TPOAb-negative pregnant women, TPOAb-positive pregnant women had higher risk of anemia (OR = 1.97, 95%CI: 1.19–3.26, P = 0.009, I2 = 79.4%; Figure 6). Begg's test (P = 0.462) did not indicate publication bias, and sensitivity analysis showed that the combined OR values of the remaining studies after one study had been removed remained stable.
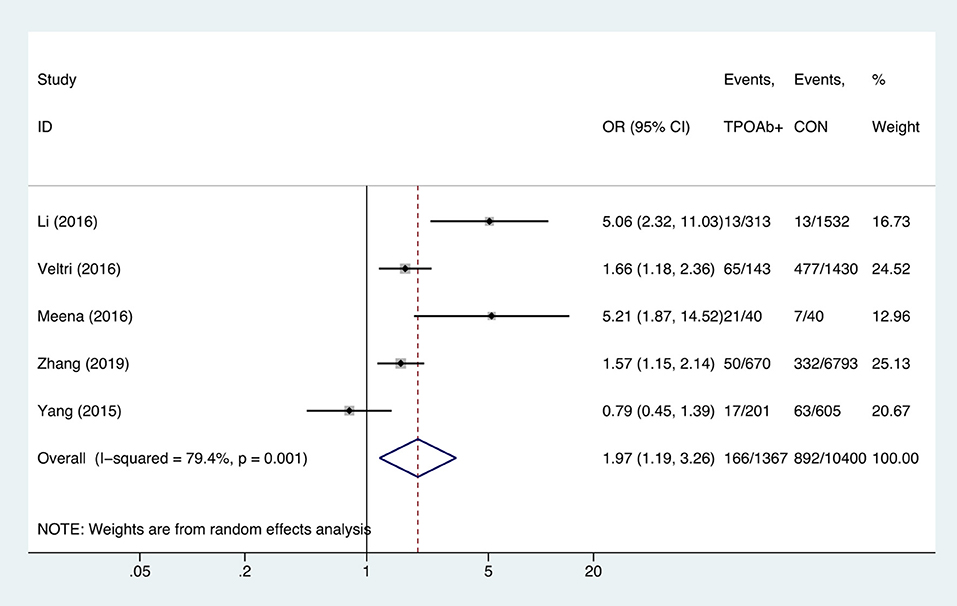
Figure 6. Forest plots of odds ratio and 95% confidence interval of pooled studies comparing thyroid peroxidase antibody-positive pregnant women (TPOAb+) to euthyroid pregnant women (CON) for risk of gestational anemia.
Table 2 shows the general characteristics and thyroid function of the included pregnant women. The age, body mass index (BMI), parity, and smoking and drinking status in the hypothyroid and hyperthyroid pregnant women were similar to that of euthyroid pregnant women. Hypothyroid pregnant women had higher FT4 levels after treatment (13.79 vs. 13.02 pmol/L, P < 0.05). In conservative observation or medication-treated hyperthyroid pregnant women, even though FT4, FT3, and TSH were in the normal range, the levels were nevertheless higher than that in euthyroid pregnant women (15.28 vs. 13.02 pmol/L, P < 0.001; 5.37 vs. 4.34 pmol/L, P < 0.001; 0.01 vs. 1.2 mIU/L, P < 0.001, respectively). The rates of TPOAb positive in both the hyperthyroid group and the hypothyroid group were higher than those in the euthyroid group.
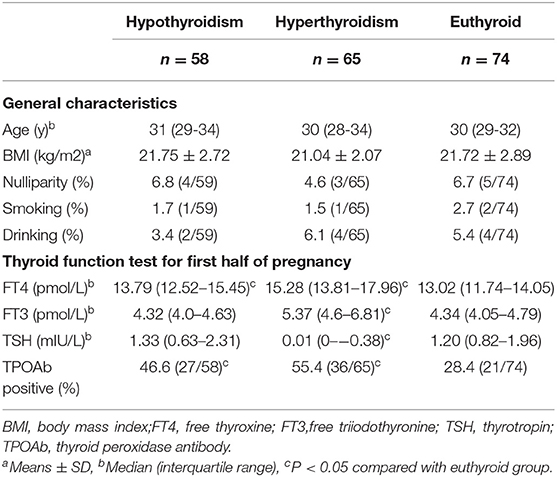
Table 2. Demographic data and thyroid function test for thyroid dysfunction and euthyroid pregnant women.
Compared with euthyroid pregnant women, there were no significant differences in Hb, RBC, and Hct levels in hypothyroid pregnant women during the first half of pregnancy. In the second half of pregnancy, Hb levels were significantly reduced (115 vs. 120 g/L, P = 0.048) and the prevalence of anemia was increased (OR = 6.384, 95%CI: 2.498–16.311) in the hypothyroid pregnant women after adjusted for TPOAb, maternal age, body mass index (BMI),nulliparity, smoking and drinking (Tables 3, 4). RBC levels were significantly higher in hyperthyroid pregnant women throughout pregnancy, while other hematological parameters showed no differences (Table 3).
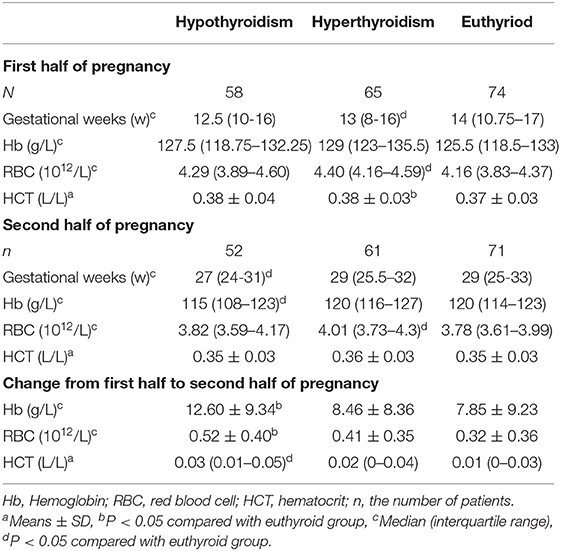
Table 3. Comparison of hematological parameters in thyroid dysfunction and euthyroid pregnant women.
Compared with euthyroid pregnant women longitudinal reductions in the Hb were significantly in increased (12.60 ± 9.34 g/L vs. 7.85 ± 9.23, P = 0.006) in hypothyroid pregnant women from the first to second half of pregnancy. In addition, longitudinal reductions in the RBC and Hct levels were also increased (0.52 ± 0.40 vs. 0.32±0.36 1012/L, P = 0.01; 0.03 vs. 0.01 L/L, P = 0.015) in pregnant women with hypothyroidism. (Table 3).
The present article assessed the relationship between maternal thyroid dysfunction and gestational anemia. The meta-analysis showed that OH and TPOAb-positive status were risk factors of gestational anemia, while SCH and hyperthyroidism were not. In new data from our team, we found that Hb levels of pregnant women with hypothyroidism were lower during the second half of pregnancy and longitudinal reductions in the Hb, RBC, and Hct levels were higher from the first to second half of pregnancy. Moreover, RBC levels were significantly increased in the hyperthyroid pregnant women throughout pregnancy.
Affected by factors such as human chorionic gonadotropin (hCG), iodine level, and serum thyroxine-binding globulin (TBG), thyroid function levels fluctuate during pregnancy (29). This means that the diagnostic criteria for thyroid dysfunction during pregnancy differ from those in non-pregnancy. At present, several studies have noted that abnormal thyroid function during pregnancy can lead to obstetric adverse outcomes and adverse fetal neurodevelopment, including miscarriage, gestational diabetes mellitus, preeclampsia, and intrauterine growth restriction (IUGR) (30, 31). Therefore, it is necessary to assess thyroid function during pregnancy based on specific reference values for people with abnormal thyroid function.
Due to the increased demand for nutrition during pregnancy and the plasma volume increasing more quickly than red cell mass, anemia is prone to occur in middle and late pregnancy, especially IDA. A few studies have reported that ID can affect thyroid function (32, 33). Iron deficiency without anemia (ID – A) and severe iron deficiency with anemia (ID + A) rat models showed similar results that both of them could cause serum TT4 decreased as well as TPO activities reduction during pregnancy (34). However, the mechanism between anemia and thyroid function remains unclear; a few studies have shown that the mechanism by which ID affects thyroid function is likely to impair the efficacy of iodized salt and TPO activity as well as the conversion of T4 to T3 (14, 35, 36). A meta-analysis reported that when no consideration age and gender restrictions, the risk of anemia was increased in patients with OH and SCH compared with euthyroid participants. While prevalence of anemia increases with age. When conducting a subgroup analysis of age, the study found that the pooled OR for the SCH younger than 50 years old was 1.15 (0.77–1.74) indicating no relationship between anemia and SCH younger than 50 years old (15). However, no meta-analysis had been conducted to investigate the effect of maternal thyroid dysfunction on anemia. A cross-sectional study in China indicated that pregnancies with IDA show lower FT4 levels compared with the control group during the first trimester of pregnancy (37). Another article showed that Hb correlated positively with FT4 and FT3 and negatively with TSH (5).
OH is characterized by elevated TSH concentrations with low FT4 and FT3 concentrations. As OH has a definite adverse effect on obstetric and child development outcomes during pregnancy, the 2017 ATA guidelines recommend that OH be treated as early as possible (38). In the present meta-analysis, we found that the OR for anemia in treated OH pregnant women was 1.76 (95%CI: 0.59–5.21, P = 0.308), indicating that treatment can eliminate the effects of OH on anemia. The results prompt the diagnosis and treating of gestational OH as soon as possible.
SCH is a condition with elevated TSH concentrations. The 2011 ATA guidelines recommend using fixed upper limits of 2.5 or 3.0 mIU/L for the first and second or third trimesters, respectively (39). The present meta-analysis indicates that SCH is not associated with gestational anemia. Of seven included studies, three adopted the diagnostic criteria of the 2011 guidelines, but the present analysis suggests that, with either criteria, SCH is not a risk factor for anemia during pregnancy.
Currently, there only two studies analyzed the effect of hyperthyroidism on gestational anemia. One of them is a prospective study conducted by Sahu et al. (18). The article showed that both overt and subclinical hyperthyroidism had no influence on gestational anemia. And another article came to similar results (26). Nevertheless, more large-sample epidemiological studies are needed to clarify this issue.
Euthyroid TAI is easily overlooked because of the lack of specific clinical symptoms. It had also been proposed that positive TPOAb can adversely affect pregnancy and neonatal outcomes (9, 40, 41). Here, we focused the effect of TPOAb on gestational anemia and found increased risk of gestational anemia in TPOAb-positive pregnant women. On one hand, the probable reason is that TPO is a heme-dependent protein, which interacts with iron levels (14). On the other hand, it may be due to the effect of inflammatory mediators on erythropoiesis, similar to other autoimmune diseases (42).
In order to better explain the relationship between thyroid dysfunction and gestational anemia, we conducted a prospective study and observed that Hb levels decreased significantly in hypothyroid pregnant women, and precisely show that hypothyroidism is an independent risk factor for anemia in the second half of pregnancy rather than in the entire pregnancy. From the first to second half of pregnancy, there was increased Hb, RBC, and Hct reduction in the hypothyroid pregnant women. Compared with the results of our meta-analysis, the longitudinal study showed that treated hypothyroidism is also a risk factor for gestational anemia in the second half of pregnancy. The possible reasons are: (1) Anemia was diagnosed at different gestational periods. Two articles included in the meta-analysis on treated hypothyroidism did not mention the exact timing of the anemia diagnosis. However, our prospective study highlights the impact of treated hypothyroidism on anemia risk in the second half of pregnancy; (2) The course of hypothyroidism is different. Most of the patients included in the meta-analysis were new-onset hypothyroidism in the first trimester, while the patients in the prospective study had a longer course of hypothyroidism.
To our knowledge, this is the first meta-analysis and longitudinal study focusing on the influence of abnormal thyroid function on anemia during pregnancy. There remain some limitations to this study. Although positive TPOAb status had an effect on anemia, we did not consider antibody factors for the patients with hypothyroidism included in our meta-analysis and prospective study. Due to the influence of the region and the publication year, the diagnostic criteria for SCH were not consistent. Although we had performed subgroup analyses according to diagnostic criteria, the effects cannot be completely eliminated. We included 10 related articles in the meta-analysis, most of which came from Asia. Due to the small number of articles on the effects of thyroid dysfunction on gestational anemia, there were only two articles included in our subgroup analysis of treated or untreated overt hypothyroidism. In addition, the number of patients included in our longitudinal study was small. It is necessary to expand the population for further exploration.
In summary, untreated OH and positive TPOAb status are associated with increased anemia risk. Treated hypothyroidism is a risk factor for anemia in the second half of pregnancy rather than in the first half. The results may guide strengthening of Hb monitoring in pregnant women with thyroid dysfunction and guide the timely treatment of pregnant women with anemia.
All datasets generated for this study are included in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by China Medical University Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
ZS conceived the study, interpreted the data, and critically revised the report. WT critically revised the report. YY searched, collected, analyzed, and interpreted the data, and drafted and critically revised the report. YH, HW, XG, XW, and JL searched and collected data.
This study was supported by the Chinese National Natural Science Foundation (81570709, 81170730); the National Science and Technology Support Program (2014BAI06B02); the Research Foundation of Key Laboratory of Endocrine Diseases, Department of Education in Liaoning Province, China (LZ2014035); the Key Platform Foundation of Science and Technology for the Universities in Liaoning Province (16010); and the Health and Medicine Research Foundation, Shenyang City (17-230-9-02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful for the invaluable contribution of the experts and the participating pregnant women.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00201/full#supplementary-material
Supplementary Figure 1. Forest plots of odds ratio and 95% confidence interval of pooled studies comparing pregnant women with subclinical hypothyroidism (SCH) to euthyroid pregnant women (CON) for risk of gestational anemia based on different diagnostic criteria.
1. Shan ZY, Chen YY, Teng WP, Yu XH, Li CY, Zhou WW, et al. A study for maternal thyroid hormone deficiency during the first half of pregnancy in China. Eur J Clin Invest. (2009) 39:37–42. doi: 10.1111/j.1365-2362.2008.02055.x
2. Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. (2005) 105:239–45. doi: 10.1097/01.AOG.0000152345.99421.22
3. Leung AS, Millar LK, Koonings PP, Montoro M, Mestman JH. Perinatal outcome in hypothyroid pregnancies. Int J Gynecol Obstet. (1993) 43:349–53. doi: 10.1016/0020-7292(93)90343-U
4. Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab. (2010) 95:E44–E8. doi: 10.1210/jc.2010-0340
5. Gur E, Karadeniz M, Yalcin M, Inceefe H, Tatar S, Turan G, et al. Thyroid antibodies in euthyroid and subclinical hypothyroidic pregnant women with autoimmune hypothyroidism: effects on hematological parameters and postpartum hemorrhage. Pol Gynaecol. (2015) 86:666–71. doi: 10.17772/gp/57810
6. Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol. (2012) 119:983–8. doi: 10.1097/AOG.0b013e318250aeeb
7. Maraka S, Ospina NM, O'Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. (2016) 26:580–90. doi: 10.1089/thy.2015.0418
8. Tong Z, Xiaowen Z, Baomin C, Aihua L, Yingying Z, Weiping T, et al. The effect of subclinical maternal thyroid dysfunction and autoimmunity on intrauterine growth restriction: a systematic review and meta-analysis. Medicine (Baltimore). (2016) 95:e3677. doi: 10.1097/MD.0000000000003677
9. Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol. (2010) 72:825–9. doi: 10.1111/j.1365-2265.2009.03743.x
10. Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health. (2013) 1:e16–e25. doi: 10.1016/S2214-109X(13)70001-9
11. Lin L, Wei Y, Zhu W, Wang C, Su R, Feng H, et al. Prevalence, risk factors and associated adverse pregnancy outcomes of anaemia in Chinese pregnant women: a multicentre retrospective study. BMC Pregnancy Childbirth. (2018) 18:111. doi: 10.1186/s12884-018-1739-8
12. Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. (2016) 387:907–16. doi: 10.1016/S0140-6736(15)60865-0
13. Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. (2000). 72(Suppl):257S. doi: 10.1093/ajcn/72.1.257S
14. Hess SY, Zimmermann MB, Arnold M, Langhans W, Hurrell RF. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J Nutr. (2002) 132:1951–5. doi: 10.1093/jn/132.7.1951
15. Wopereis DM, Du Puy RS, van Heemst D, Walsh JP, Bremner A, Bakker SJL, et al. The relation between thyroid function and anemia: a pooled analysis of individual participant data. J Clin Endocrinol Metab. (2018) 103:3658–67. doi: 10.1210/jc.2018-00481
16. Veltri F, Decaillet S, Kleynen P, Grabczan L, Belhomme J, Rozenberg S, et al. Prevalence of thyroid autoimmunity and dysfunction in women with iron deficiency during early pregnancy: is it altered? Eur J Endocrinol. (2016) 175:191–9. doi: 10.1530/EJE-16-0288
17. Yang J, Guo H, Ding S, Tao B, Zhang X. Effect of the treatment acceptance on the perinatal outcomes in women with subclinical hypothyroidism, positive thyroid gland peroxidase antibody in early pregnancy. Zhonghua Fu Chan Ke Za Zhi. (2015) 50:652–7. doi: 10.3760/cma.j.issn.0529-567x.2015.09.003
18. Sahu MT, Das V, Mittal S, Agarwal A, Sahu M. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch Gynecol Obstet. (2010) 281:215–20. doi: 10.1007/s00404-009-1105-1
19. Wang S, Teng WP, Li JX, Wang WW, Shan ZY. Effects of maternal subclinical hypothyroidism on obstetrical outcomes during early pregnancy. J Endocrinol Invest. (2012) 35:322–5. doi: 10.3275/777
20. Morchiladze N, Tkeshelashvili B, Gagua T, Gagua D. Prognostic risk of obsteric and perinatal complications in pregnant women with thyroid dysfunction. Georgian Med News. (2017) 264:21–5.
21. Hou MQ, Wang ZJ, Hou KZ. Influence of hypothyroidism on pregnancy outcome and fetus during pregnancy. Zhonghua Liu Xing Bing Xue Za Zhi. (2016) 37:722–4. doi: 10.3760/cma.j.issn.0254-6450.2016.05.028
22. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
23. Yu X, Shan Z, Li C, Mao J, Wang W, Xie X, et al. Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant and nonpregnant women of childbearing age in China. J Clin Endocrinol Metab. (2015) 100:1594–601. doi: 10.1210/jc.2014-3887
24. Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, et al. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J Clin Endocrinol Metab. (2014) 99:73–9. doi: 10.1210/jc.2013-1674
25. WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva: World Health Organization (2011).
26. Li S, Gao X, Wei Y, Zhu G, Yang C. The relationship between iron deficiency and thyroid function in chinese women during early pregnancy. J Nutr Sci Vitaminol. (2016) 62:397–401. doi: 10.3177/jnsv.62.397
27. Meena M. The effect of anti-thyroid peroxidase antibodies on pregnancy outcomes in euthyroid women. J Clin Diagn Res. (2016) 10:4–7. doi: 10.7860/JCDR/2016/19009.8403
28. Zhang H, Teng X, Shan Z, Wang Z, Teng W. Association between iron deficiency and prevalence of thyroid autoimmunity in pregnant and non-pregnant women of childbearing age: a cross-sectional study. Chin Med J (Engl). (2019) 132:2143–9. doi: 10.1097/CM9.0000000000000409
29. Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ. (2014) 349:g4929. doi: 10.1136/bmj.g4929
30. Van Den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, et al. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. (2011) 17:605–19. doi: 10.1093/humupd/dmr024
31. Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab. (2010) 95:4227–34. doi: 10.1210/jc.2010-0415
32. Zimmermann MB. The influence of iron status on iodine utilization and thyroid function. Annu Rev Nutr. (2006) 26:367–89. doi: 10.1146/annurev.nutr.26.061505.111236
33. Dillman E, Gale C, Green W, Johnson DG, Mackler B, Finch C. Hypothermia in iron deficiency due to altered triiodothyronine metabolism. Am J Physiol. (1980) 239:377–81. doi: 10.1152/ajpregu.1980.239.5.R377
34. Hu X, Teng X, Zheng H, Shan Z, Li J, Jin T, et al. Iron deficiency without anemia causes maternal hypothyroxinemia in pregnant rats. Nutr Res. (2014) 34:604–12. doi: 10.1016/j.nutres.2014.06.007
35. Hess SY, Zimmermann MB, Pierre A, Toni T, Hurrell RF. Treatment of iron deficiency in goitrous children improves the efficacy of iodized salt in Côte d'Ivoire. Am J Clin Nutr. (2002) 75:743–8. doi: 10.1093/ajcn/75.4.743
36. Hess SY, Zimmermann MB. The effect of micronutrient deficiencies on iodine nutrition and thyroid metabolism. Int J Vitam Nutr Res. (2004) 74:103–15. doi: 10.1024/0300-9831.74.2.103
37. Fu J, Yang A, Zhao J, Zhu Y, Gu Y, Xu Y, et al. The relationship between iron level and thyroid function during the first trimester of pregnancy: a cross-sectional study in Wuxi, China. J Trace Elem Med Biol. (2017) 43:148–52. doi: 10.1016/j.jtemb.2017.01.004
38. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. (2017). Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. (2017) 27:315–89. doi: 10.1089/thy.2016.0457
39. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. (2011) 21:1081–125. doi: 10.1089/thy.2011.0087
40. Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ. (2011) 342:d2616. doi: 10.1136/bmj.d2616
41. Groer MW, Vaughan JH. Positive thyroid peroxidase antibody titer is associated with dysphoric moods during pregnancy and postpartum. J Obstet Gynecol Neonatal Nurs. (2012) 42:E26–E32. doi: 10.1111/j.1552-6909.2012.01425.x
Keywords: hypothyroidism, hyperthyroidism, TPOAb-positive, anemia, pregnancy
Citation: Yang Y, Hou Y, Wang H, Gao X, Wang X, Li J, Teng W and Shan Z (2020) Maternal Thyroid Dysfunction and Gestational Anemia Risk: Meta-Analysis and New Data. Front. Endocrinol. 11:201. doi: 10.3389/fendo.2020.00201
Received: 01 February 2020; Accepted: 23 March 2020;
Published: 15 April 2020.
Edited by:
Noriyuki Koibuchi, Gunma University, JapanReviewed by:
Izuki Amano, Gunma University, JapanCopyright © 2020 Yang, Hou, Wang, Gao, Wang, Li, Teng and Shan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongyan Shan, c2hhbnpob25neWFuQG1lZG1haWwuY29tLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.