
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 18 February 2020
Sec. Cancer Endocrinology
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00060
This article is part of the Research Topic Endocrinology in Cancer and Aging View all 16 articles
 Huan Tao1
Huan Tao1 Adrienne O'Neil2,3
Adrienne O'Neil2,3 Yunseon Choi4
Yunseon Choi4 Wei Wang5
Wei Wang5 Junfeng Wang6
Junfeng Wang6 Yafeng Wang7*
Yafeng Wang7* Yongqian Jia1*
Yongqian Jia1* Xiong Chen8*
Xiong Chen8*Objective: The relationship between diabetes and all- and cause-specific mortality in individuals with common cancers (breast, colorectal, and prostate) remains both under-researched and poorly understood.
Methods: Cancer survivors (N = 37,993) from the National Health Interview Survey with linked data retrieved from the National Death Index served as our study participants. Cox proportional-hazards models were used to assess associations between pre- and post-diabetes and all-cause and cause-specific mortality.
Results: Over a median follow-up period of 13 years, 2,350 all-cause, 698 cancer, and 506 CVD deaths occurred. Among all cancer survivors, patients with diabetes had greater risk of: all-cause mortality [hazard ratio (HR) 1.35, 95% CI = 1.27–1.43], cancer-specific mortality (HR: 1.14, 95% CI = 1.03–1.27), CVD mortality (HR: 1.36, 95% CI = 1.18–1.55), diabetes related mortality (HR: 17.18, 95% CI = 11.51–25.64), and kidney disease mortality (HR: 2.51, 95% CI = 1.65–3.82), compared with individuals without diabetes. The risk of all-cause mortality was also higher amongst those with diabetes and specific types of cancer: breast cancer (HR: 1.28, 95% CI = 1.12–1.48), prostate cancer (HR: 1.20, 95% CI = 1.03–1.39), and colorectal cancer (HR: 1.29, 95% CI = 1.10–1.50). Diabetes increased the risk of cancer-specific mortality among colorectal cancer survivors (HR: 1.36, 95% CI = 1.04–1.78) compared to those without diabetes. Diabetes was associated with higher risk of diabetes-related mortality when compared to non-diabetic breast (HR: 9.20, 95% CI = 3.60–23.53), prostate (HR: 18.36, 95% CI = 6.01–56.11), and colorectal cancer survivors (HR: 12.18, 95% CI = 4.17–35.58). Both pre- and post-diagnosis diabetes increased the risk of all-cause mortality among all cancer survivors. Cancer survivors with diabetes had similar risk of all-cause and CVD mortality during the second 5 years of diabetes and above 10 years of diabetes as compared to non-diabetic patients.
Conclusions: Diabetes increased the risk of all-cause mortality among breast, prostate, and colorectal cancer survivors, not for pre- or post-diagnosis diabetes. Greater attention on diabetes management is warranted in cancer survivors with diabetes.
Diabetes is a major public health burden. The prevalence of diabetes is projected to increase to 439 million adults by 2030. By this time, the disease burden in adults living with diabetes is estimated to increase by 20 and 69% in developing and developed countries, respectively (1, 2).
Diabetes mellitus (DM) and cancer are two common diseases affecting aging populations worldwide, which commonly co-occur. These associations are complex and may be cancer specific (3). On one hand, strong evidence links diabetes to an increased risk of breast and colorectal cancer onset (4, 5). On the other hand, there is some evidence it may be associated with decreased risk of prostate cancer onset (6). For those with a cancer diagnosis or history of the condition, there is evidence that diabetes may increase overall mortality risk. Previous studies have reported that diabetes increased the all-cause and cancer-specific mortality for cancer survivors compared to those without diabetes (7), including breast (8), colorectal (7–9), and prostate cancer survivors (10, 11). Interestingly, individuals with both diabetes and a history of cancer also have higher rates of cardiovascular mortality when compared to those without diabetes among the general population (12, 13). However, the relationship between diabetes and all- and cause-specific mortality in individuals with common cancers (breast, colorectal, and prostate) remains both under-researched and poorly understood.
This study aimed to estimate the associations of pre- and post-diagnosis diabetes, and duration of diabetes with all-cause, cancer-specific and CVD mortality among U.S. breast, prostate and colorectal cancer survivors in a large prospective cohort study.
The National Health Interview Survey (NHIS) is a stratified, multistage probability survey that samples an average of 57,000 adults per year to estimate the health of the U.S. population, the prevalence and incidence of disease, the extent of disability, and the use of health care services. One adult is randomly selected from each selected household for a detailed interview on health and other behaviors.
A total of 493,365 adults from the 13 cross-sectional waves (i.e., from 1997 to 2013) and their linked mortality data ending in December 31, 2015 was included in this analysis. After excluding missing data on diabetes, we identified 37,993 adults cancer survivors, including 6,330 breast cancer patients, 3,916 prostate cancer patients, and 2,656 colorectal cancer patients. A total of 5,174 cancer survivors diagnosed as diabetes, including 1,020 in breast cancer patients, 743 in prostate cancer patients and 509 in colorectal cancer patients. Totally, we identified 4,724 patients with diabetes prior to cancer diagnosis and 450 patients with diabetes after cancer diagnosis (Figure 1).
Self-reported diabetes was based on responses to questions “Have you ever been told by a doctor or other health professional that you had…” Women reporting diabetes occurring only during pregnancy, or adults who responded they had borderline diabetes were considered to not have diabetes. Diabetes was dichotomized into the presence or absence of diabetes (14, 15). Duration of diabetes was calculated by the present age minus the age at diagnosis of diabetes, and was categorized as <5, 5–10, and >10 years. After excluding individuals without diabetes and those with possible type 1 diabetes adults defined by using insulin and age of onset <30 years old which has been validated as accurate in 97% of cases (16).
We included demographic variables (i.e., race, gender, education, and marital status), lifestyle variables (i.e., body mass index, physical activity, and alcohol consumption) and comorbid conditions [i.e., hypertension, coronary heart disease (CHD), and stroke]. Body mass index (BMI) was calculated as weight in kilograms divided by height squared (<25, 25–30, and >30 kg/m2). A participant's physical activity was categorized into three groups according to metabolic equivalent task (MET) hours per week (low, 600 MET-h/wk; moderate, 600–3,000 MET-h/wk; high, >3,000 MET-h/wk). Alcohol consumption was categorized into three groups: lifetime abstainer; former drinker; current drinker. Cancer was defined as the presence of the same National Cancer Registry with a specific code (C code) more than three times within a year or an in patient hospitalization with a C code (17). Cancer diagnosed age was defined as the age at diagnosis of cancer and was categorized into <45, 45–65, and >65 years. Duration of cancer was calculated by the present age minus the cancer diagnosed age.
Ascertainment of mortality was established using the International Classification of Diseases-10th Revision codes, and study outcomes were defined using as follows: (1) all-cause mortality; (2) cancer-specific mortality (i.e., codes C00–C97); (3) CVD-mortality (i.e., codes I00–I09, I11, I13, and I20–I51, I60–I69); (4) diabetes mellitus (E10–E14); (5) chronic lower respiratory diseases (J40–J47); (6) influenza and pneumonia (J09–J18); (7) Alzheimer disease (G30) and (8) nephritis, Nephrotic syndrome and nephrosis (N00–N07, N17–N19, N25–N27).
We compared participants with and without diabetes for basic characteristics using chi-square test to examine categorical differences in the weighted percentages. According to the baseline hazard to capture the increase in hazard due to aging, age was used as the underlying timescale. To evaluate the association of diabetes with all-cause, cancer and CVD mortality, we computed multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CI) using cause-specific Cox proportional hazards models. All analyses were adjusted for age (using it as a timescale), sex, race, education level, income level, marital status, body mass index, smoking status, physical activity, alcohol intake, cancer diagnosed age, duration of cancer, history of hypertension, CHD, stroke, and missing data. We also analyzed the association between duration of diabetes and the risk of disease-specific and all-cause mortality. Besides, the relationship between pre- and post-diagnosis diabetes and mortality was examined among the 5,174 men and women cancer survivors. Two sensitivity analyses were also performed: (1) exclusion of individuals who died within the first 2 years; (2) exclusion of participants with CHD or stroke at interview; (3) excluding individuals with possible type 1 diabetes (defined by being on insulin and having an age of onset <30 years).
All analyses incorporated the complex survey design and were performed by using STATA version 15.0 (Stata Corp, College Station, TX, USA). Two-sided p-values <0.05 were considered significant for statistical inferences.
Descriptive statistics were reported in Table 1. The mean age of cancer survivors was 63 years at baseline. Compared to cancer survivors without diabetes, cancer survivors with diabetes were more likely to be obesity (i.e., BMI > 30 kg/m2) and be less physically active (i.e., low physical activity). Cancer survivors with diabetes tend to have a degree less than high school (24.1% in patients with diabetes, 15.09% in patients without diabetes). Patients with diabetes tend to have a history of hypertension (75.51% in patients with diabetes, 43.49% in patients without diabetes), CHD (24.17% in patients with diabetes, 9.52% in patients without diabetes), and stroke (12.44% in patients with diabetes, 5.38% in patients without diabetes).
After a median follow-up period of 13 years, 2,346 all-cause, 698 cancer-specific, and 506 CVD deaths occurred. Figure 2 showed the risk of all-cause, cancer and CVD mortality among diabetic cancer survivors compared to non-diabetic cancer survivors. Compared to non-diabetes cancer survivors, cancer survivors with diabetes had a 35% higher risk of all-cause mortality (HR: 1.35, 95% CI = 1.27–1.43), 14% higher risk of cancer mortality (HR: 1.14, 95% CI = 1.03–1.27), and 36% higher risk of CVD mortality (HR: 1.36, 95% CI = 1.18–1.55). These relationships were stronger for diabetes related mortality (HR: 17.18, 95% CI = 11.51–25.64) and kidney disease mortality 2.51 for (95% CI = 1.65–3.82) who were diabetic survivors when compared to non-diabetic survivors, but no associations were observed between diabetes and risk of chronic lower respiratory disease, influenza and pneumonia, and Alzheimer disease mortality.
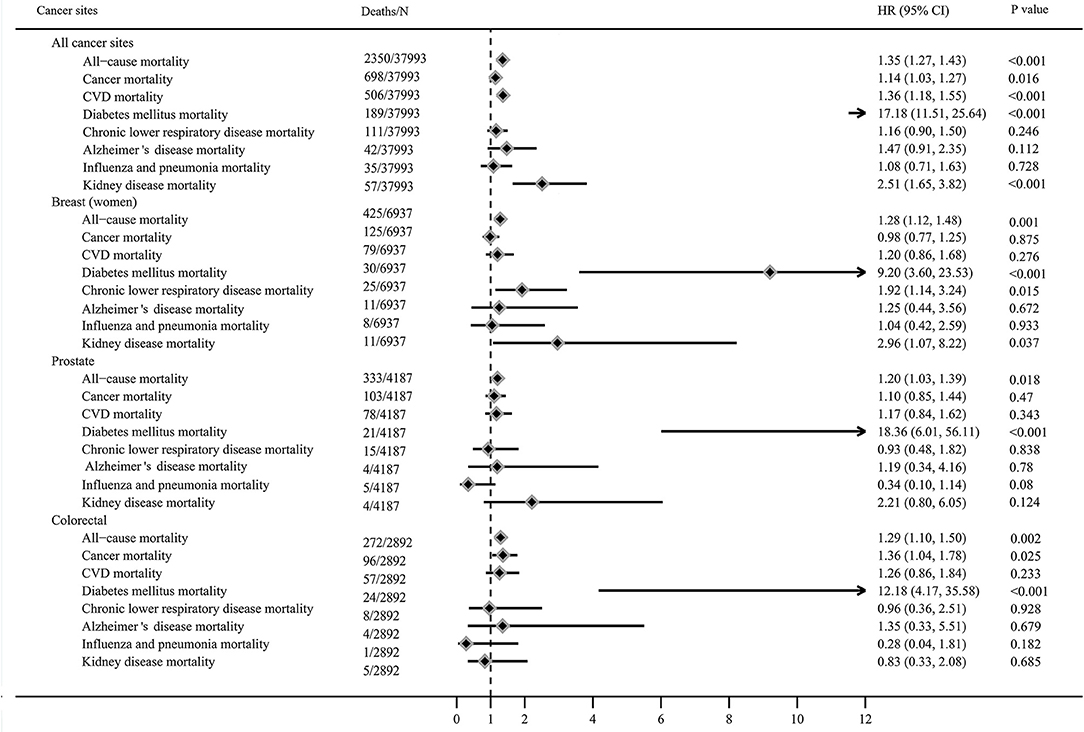
Figure 2. Mortality of cancer survivors with diabetes compared to non-diabetic cancer survivors (adjusted for age, sex, race, education level, income level, marital status, body mass index, smoking status, alcohol intake, physical activity, cancer diagnosed age, duration of cancer, history of hypertension, coronary heart disease and stroke, and missing data).
The HRs for all-cause mortality varied from 1.20 to 1.30 among survivors with diabetes diagnosed with breast cancer (HR: 1.28, 95% CI = 1.12–1.48), prostate cancer (HR: 1.20, 95% CI = 1.03–1.39), and colorectal cancer (HR = 1.29, 95% CI = 1.10–1.50). Diabetes increased the risk of cancer-specific mortality among colorectal cancer survivors (HR: 1.36, 95% CI = 1.04–1.78) compared to non-diabetic survivors, but not in breast and prostate cancer survivors. Diabetes was associated with higher risk of diabetes related mortality in breast (HR: 9.20, 95% CI = 3.60–23.53), prostate (HR: 18.36, 95% CI = 6.01–56.11), and colorectal cancer survivors (HR: 12.18, 95% CI = 4.17–35.58), compared to non-diabetic cancer survivors. Diabetes increased the risk of chronic lower respiratory disease mortality (HR: 1.92, 95% CI = 1.14–3.24) and kidney disease mortality (HR: 2.96, 95% CI = 1.07–8.22) among breast survivors compared to non-diabetic survivors, but not in colorectal and prostate cancer survivors.
Pre-diagnosis diabetes increased the risk of all-cause mortality (HR: 1.35, 95% CI = 1.27–1.44), cancer mortality (HR: 1.14, 95% CI = 1.02–1.28), CVD mortality (HR: 1.38, 95% CI = 1.20–1.59), diabetes mellitus mortality (HR: 17.82, 95% CI = 11.86–26.79), Alzheimer's disease mortality (HR: 1.66, 95% CI = 1.01–2.72), and kidney disease mortality (HR: 2.45, 95% CI = 1.60–3.75), respectively among all cancer survivors. Post-diagnosis diabetes increased the risk of all-cause mortality (HR: 1.35, 95% CI = 1.12–1.63) and diabetes mellitus mortality (HR: 8.62, 95% CI = 3.83–19.39), but not in other mortality. For specific cancer sites, pre-diagnosis diabetes increased the risk of all-cause mortality in prostate (HR: 1.21, 95% CI = 1.01–1.43) and breast cancer survivors (HR: 1.31, 95% CI = 1.10–1.57), not in colorectal cancer survivors. Post-diagnosis diabetes did not confer obvious increased risk of all-cause mortality for prostate and colorectal cancer sites except breast cancer (HR: 1.25, 95% CI = 1.02–1.52). Additional information were detailed in Table 2.
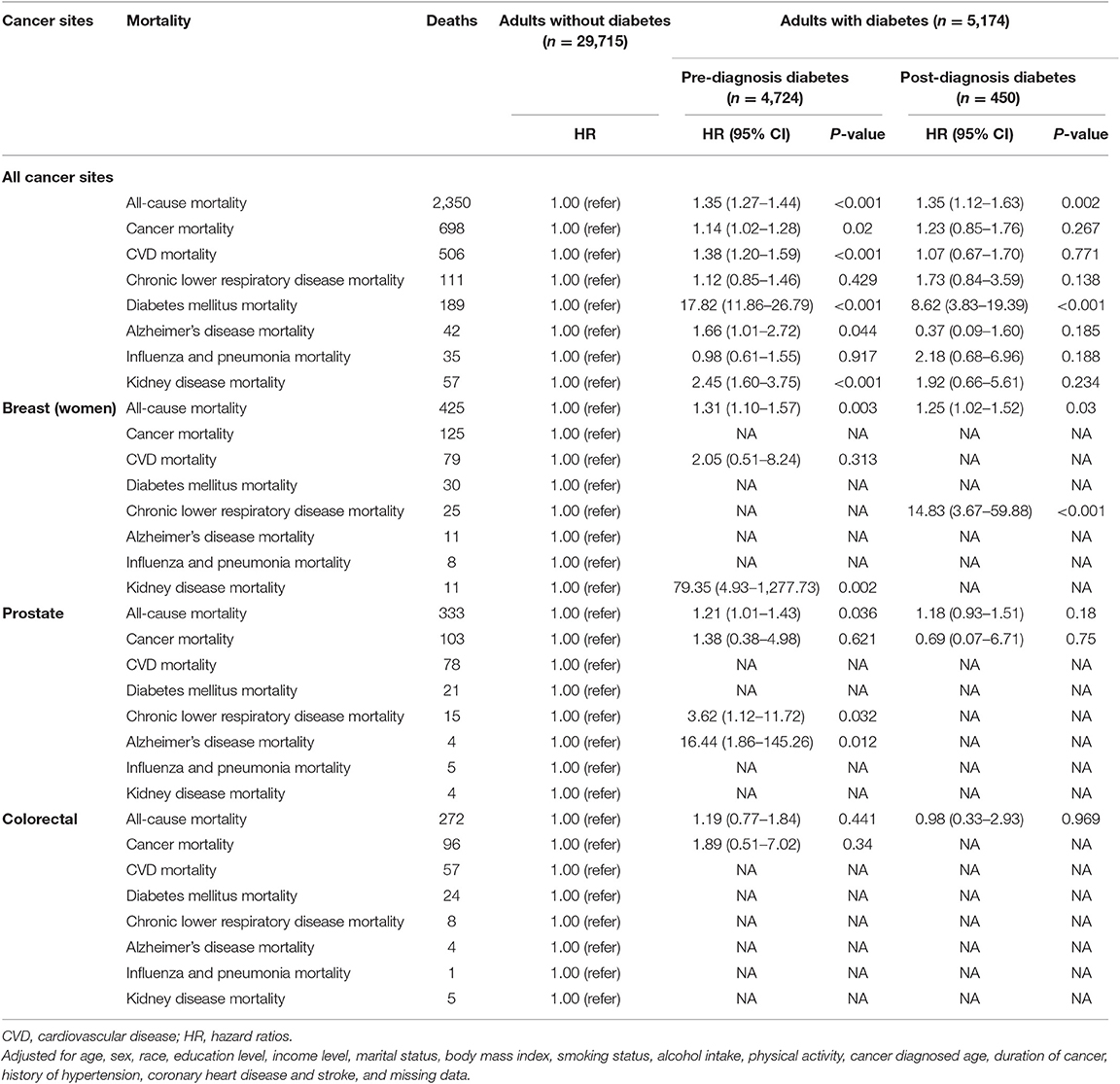
Table 2. Mortality of cancer survivors with pre-diagnosis diabetes group compared to non-diabetic group.
As summarized in Table 3, cancer survivors with diabetes had similar risk of all-cause and CVD mortality during the second 5 years of diabetes [(HR: 1.44, 95% CI = 1.29–1.60); (HR: 1.43, 95% CI = 1.13–1.82)] and above 10 years [(HR = 1.41, 95% CI = 1.30–1.52); (HR: 1.45, 95% CI = 1.22–1.71)] as compared to non-diabetic participants. However, cancer survivors with duration of diabetes 5–10 years (HR: 1.40, 95% CI = 1.15–1.70) achieved the highest risk of cancer mortality compared to those with duration of diabetes <5 years or more than 10 years. Compared to non-diabetic survivors, cancer survivors with diabetes with duration for 0–5 years (HR: 10.97, 95% CI = 6.28–19.16), 5–10 years (HR: 11.04, 95% CI = 6.20–19.64), and more than 10 years (HR: 24.11, 95% CI = 15.55–37.38) were at increased risk of diabetes related mortality.
We conducted a sensitivity analysis excluding individuals who were followed up for less than the first 2 years, those with CHD, stroke. Exclusion of individuals with possible type 1 diabetes did not largely affect the HRs indicating the robustness of results (data not shown).
This large prospective study showed that compared to cancer survivors without diabetes, cancer survivors with diabetes had higher risk of all-cause, cancer-specific, and CVD mortality. Further they had an increased risk of all-cause, but not cancer specific mortality as the duration of diabetes increased. These findings add to a largely equivocal evidence base on this relationship.
Diabetes elevates all-cause and cancer specific mortality is consistent with a recent study which showed similar findings in individuals with established prostate cancer (11) as well as other studies in patients with breast and colorectal cancer (8). Some types of cancer are treated less aggressively in cancer survivors with diabetes than those without diabetes (18). In our study, pre-diagnosis diabetes increased the risk of all-cause mortality after adjusting for covariables in breast and prostate cancer sites, which is not consistent with previous findings (19, 20). But additional evidence indicated that pre-existing diabetes increased the risk of all-cause mortality among women with breast cancer after adjusting for covariables related to delayed diagnosis and therapy (21). In addition, pre-diagnosis diabetes increased the risk of all-cause mortality in colorectal cancer survivors in our study, which is consistent with findings in previous study (20).
Our second finding, that all-cause (but not cancer specific) mortality risk increased with diabetes duration has been observed previously in those with colorectal and breast cancer, respectively. With respect to the former group, diabetes duration of more than 10 years was associated with a 49% higher risk of all-cause mortality (22), which is slightly greater than the magnitude of association we observed (33%). With respect to the latter group, we found the risk of all-cause mortality increased in breast cancer patients who have had diabetes for more than 10 years, while others have shown increased risk from 7 years (23).
Among non-diabetic patients, hyperinsulinemia associated with reduced insulin sensitivity may play a role in the pathogenesis of prostate carcinoma (24). Thus, our study might underestimate the effect between insulin secretion or increase of insulin resistance and mortality among cancer survivors. Cancer survivor with diabetes have the same CVD risk of mortality during the second 5 years of diabetes and above 10 years as compared to non-diabetic patients. But this is not aspected since type 2 diabetes abnormalities (such as reduced insulin secretion, increase of insulin resistance) start several years before that the diabetes clinical manifestations appears.
Previous studies indicated the possible relationship between diabetic status and less aggressive oncological treatment and worse overall survival (18). In our study, all-cause mortality (but not cancer-specific) increase in long-standing diabetes diagnosis (>10 years) is likely to be related with the increase of potentially fatal events related to diabetic complications partially explaining the reduced cancer-mortality observed in this group in comparison with the 5–10 years group. A slight increase cancer-related mortality in recently diagnosed diabetic patient (0–5 years group) is likely to be related with short-time exposure to the risk of development of diabetes complications able to influence the oncological treatment strategies.
Diabetes could increase the risk of all-cause mortality among population with different diseases. In our study, both pre- and post-diagnosis diabetes increased the risk of all-cause mortality by 35% than non-diabetic cancer survivors, which was higher than that with 23% among tuberculosis patients (25). Previous studies indicated that patients with diabetes who underwent incident amputation have a 55% higher risk for all-cause mortality than non-diabetic patients (26), and diabetes increased the total mortality markedly with a higher 70% among patients hospitalized with a confirmed acute myocardial infarction than non-diabetic patients (27). In addition, individuals with vs. those without diabetes were at increased higher risk of all-cause mortality in the earlier (144%) and later time (95%) periods in the Framingham Heart Study (28).
There are several mechanistic pathways by which diabetes may confer elevated mortality risk in individuals with cancer history. Cancer patients with diabetes often have other diabetes-related comorbid conditions, which may influence physicians' clinical decision making (29). For example, cancer survivors with diabetes have been shown to be treated less aggressively with chemotherapy and radiotherapy (18, 30). This could partially explain the worse prognosis compared to those without diabetes. Secondly, individuals with co-morbid cancer and diabetes may have poor response to cancer treatment, including increased infection rates and intraoperative mortality (31). Thirdly, they may have a greater risk of chemotherapy-related toxicity (i.e., dilated cardiomyopathy in breast cancer) (30). Thus, conservative clinical decision making or side effects of conventional cancer treatments may impair survival time for individuals with this comorbidity.
The presence of diabetes may itself influence cancer progression via physiologic processes of hyperinsulinemia, hyperglycemia, immunodeficiency, and chronic inflammation. It is possible that most glucose uptake in cancer cells is constitutively high and independent of insulin binding to insulin-like growth factor receptor, which could stimulate cancer cell proliferation and metastasis (32, 33). Another potential pathway is that acute exposure to hyperglycemia may increase endothelial cell permeability for reasons of increased generation of reactive oxidative species and structural changes in the basement membrane, increasing the probability of metastasis (34). The third possible pathway is that hyperinsulinemia related to underlying insulin resistance might stimulate tumor growth (35). Multiple downstream signaling pathways are activated after insulin receptors interact with their ligands, and can stimulate multiple cancer phenotypes contribute to tumor initiation and progression (36). Decreased immunity induced by diabetes can also lead to cancer progression. In this study, the risk of diabetes related mortality increased obviously among cancer survivors with diabetes when compared to non-diabetic survivors. The increased mortality can be reversed when diabetes is managed well in clinical practice.
Our study has several strengths including the large, prospective nature of the cohort used. The representativeness of the sample further adds to the robustness of the findings. Nonetheless, our study had certain limitations. Firstly, we used an epidemiological definition of type 1 diabetes, which implies that misclassification of diabetes type is possible. However, a validation study has shown that those with possible type 1 diabetes adults defined by using insulin and age of onset <30 years old which has been validated as accurate in 97% of cases (16). This could be a potential bias for patients selection in our study. Secondly, we did not have extensive clinical parameters regarding disease status for diabetes and its comorbidities and cancer. For instance, insulin therapy has been associated with increased risk of newly developed colorectal cancer (37), however we could not account for this in the model. Thirdly, lacking of informations about pharmacological and/or surgical treatment received for either diabetes or cancer fail to identify subgroups of cancer survivors who may have been at risk of high mortality rate because of the cancer treatment they received. Fourthly, although diabetes assessments were from self-reports, it is always possible to include the participants who had never received the appropriate diagnostic tests of diabetes, and to misclassify the diabetes as non-diabetes. Self-report of cancer duration or diabetes implies high probability of incorrect data assumed as correct. Finally, our findings may not be generalizable to other cancer sites except for breast, colorectal, and prostate cancer.
Breast, prostate, and colorectal cancer survivors with diabetes had higher risk of all-cause mortality than did cancer survivors without diabetes. Pre-diagnosis diabetes increased risk of all-cause mortality after adjusting for covariables in breast and prostate cancer sites. Duration of diabetes is associated with increased risk of all-cause but not cancer specific mortality when compared to non-diabetic breast and prostate cancer survivors. Greater attention on diabetes control might be warranted in those with comorbid cancer and diabetes.
The datasets generated for this study are available on request to the corresponding author Yafeng Wang (d29ueWhmb25AMTYzLmNvbQ==).
The studies involving human participants were reviewed and approved by all procedures performed in studies involving adult participant provided a written informed consent and the NHIS was approved by the National Center for Health Statistics ethics review board. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YW, XC, and YJ: conception and design of the study. YW and HT: acquisition of data and analysis, and statistical analysis. HT, YW, AO'N, YC, WW, JW, XC, and YJ: writing and revision of the manuscript. All authors read and approved the final manuscript.
This research was funded by the Department of Science and Technology of Sichuan Province (0040205302006) and Research Incubation Project of the First Affiliated Hospital of Wenzhou Medical University (FHY2019015).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the NHIS for access to data and we greatly appreciate the support from other members of the study team who provided their generous contribution of time and efforts help during the research.
NHIS, National Health Interview Survey; DM, Diabetes mellitus; CVD, Cardiovascular disease; CHD, Coronary heart disease; BMI, Body mass index; MET, Metabolic equivalent task; HRs, Hazard ratios; CI, Confidence intervals.
1. Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, et al. The evolving diabetes burden in the United States. Ann Intern Med. (2004) 140:945–50. doi: 10.7326/0003-4819-140-11-200406010-00035
2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. (2010) 87:4–14. doi: 10.1016/j.diabres.2009.10.007
3. Renehan AG, Yeh HC, Johnson JA, Wild SH, Gale EA, Moller H. Diabetes and cancer (2): evaluating the impact of diabetes on mortality in patients with cancer. Diabetologia. (2012) 55:1619–32. doi: 10.1007/s00125-012-2526-0
4. Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. (2005) 97:1679–87. doi: 10.1093/jnci/dji375
5. Johnson JA, Carstensen B, Witte D, Bowker SL, Lipscombe L, Renehan AG. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. (2012) 55:1607–18. doi: 10.1007/s00125-012-2525-1
6. Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. (2006) 15:2056–62. doi: 10.1158/1055-9965.EPI-06-0410
7. Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. (2008) 300:2754–64. doi: 10.1001/jama.2008.824
8. De Bruijn KM, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CH. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. (2013) 100:1421–9. doi: 10.1002/bjs.9229
9. Luo W, Cao Y, Liao C, Gao F. Diabetes mellitus and the incidence and mortality of colorectal cancer: a meta-analysis of 24 cohort studies. Colorectal Dis. (2012) 14:1307–12. doi: 10.1111/j.1463-1318.2012.02875.x
10. Snyder CF, Stein KB, Barone BB, Peairs KS, Yeh HC, Derr RL, et al. Does pre-existing diabetes affect prostate cancer prognosis? A systematic review. Prostate Cancer Prostatic Dis. (2010) 13:58–64. doi: 10.1038/pcan.2009.39
11. Cai H, Xu Z, Xu T, Yu B, Zou Q. Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: a meta-analysis of 11 cohort studies. Diabetes Metab Res Rev. (2015) 31:336–43. doi: 10.1002/dmrr.2582
12. Rawshani A, Sattar N, Franzen S, Rawshani A, Hattersley AT, Svensson AM, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. (2018) 392:477–86. doi: 10.1016/S0140-6736(18)31506-X
13. Sattar N, Rawshani A, Franzen S, Rawshani A, Svensson AM, Rosengren A, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. (2019) 139:2228–37. doi: 10.1161/CIRCULATIONAHA.118.037885
14. Miller EA, Tarasenko YN, Parker JD, Schoendorf KC. Diabetes and colorectal cancer screening among men and women in the USA: National Health Interview Survey: 2008, 2010. Cancer Causes Control. (2014) 25:553–60. doi: 10.1007/s10552-014-0360-z
15. Lipton BJ, Decker SL. Association between diagnosed diabetes and trouble seeing, National Health Interview Survey, 2011–13. J Diabetes. (2015) 7:743–6. doi: 10.1111/1753-0407.12311
16. Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. (2018) 391:2430–40. doi: 10.1016/S0140-6736(18)30314-3
17. Hwangbo Y, Kang D, Kang M, Kim S, Lee EK, Kim YA, et al. Incidence of diabetes after cancer development: a Korean National Cohort Study. JAMA Oncol. (2018) 4:1099–105. doi: 10.1001/jamaoncol.2018.1684
18. van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. (2007) 120:1986–92. doi: 10.1002/ijc.22532
19. Kiderlen M, de Glas NA, Bastiaannet E, Engels CC, van de Water W, de Craen AJ, et al. Diabetes in relation to breast cancer relapse and all-cause mortality in elderly breast cancer patients: a FOCUS study analysis. Ann Oncol. (2013) 24:3011–6. doi: 10.1093/annonc/mdt367
20. Strele I, Pildava S, Repsa I, Kojalo U, Vilmanis J, Brigis G. Pre-existing diabetes mellitus and all-cause mortality in cancer patients: a register-based study in Latvia. Acta Oncol. (2018) 57:973–82. doi: 10.1080/0284186X.2017.1420909
21. Luo J, Hendryx M, Virnig B, Wen S, Chlebowski R, Chen C, et al. Pre-existing diabetes and breast cancer prognosis among elderly women. Br J Cancer. (2015) 113:827–32. doi: 10.1038/bjc.2015.249
22. Amshoff Y, Maskarinec G, Shvetsov YB, Raquinio PH, Grandinetti A, Setiawan VW, et al. Type 2 diabetes and colorectal cancer survival: the multiethnic cohort. Int J Cancer. (2018) 143:263–8. doi: 10.1002/ijc.31311
23. Maskarinec G, Shvetsov YB, Conroy SM, Haiman CA, Setiawan VW, Le Marchand L. Type 2 diabetes as a predictor of survival among breast cancer patients: the multiethnic cohort. Breast Cancer Res Treat. (2019) 173:637–45. doi: 10.1007/s10549-018-5025-2
24. Nandeesha H, Koner BC, Dorairajan LN. Altered insulin sensitivity, insulin secretion and lipid profile in non-diabetic prostate carcinoma. Acta Physiol Hung. (2008) 95:97–105. doi: 10.1556/APhysiol.95.2008.1.7
25. Ko PY, Lin SD, Hsieh MC, Chen YC. Diabetes mellitus increased all-cause mortality rate among newly-diagnosed tuberculosis patients in an Asian population: a nationwide population-based study. Diabetes Res Clin Pract. (2017) 133:115–23. doi: 10.1016/j.diabres.2017.08.011
26. Schofield CJ, Libby G, Brennan GM, MacAlpine RR, Morris AD, Leese GP, et al. Mortality and hospitalization in patients after amputation: a comparison between patients with and without diabetes. Diabetes Care. (2006) 29:2252–6. doi: 10.2337/dc06-0926
27. Mukamal KJ, Nesto RW, Cohen MC, Muller JE, Maclure M, Sherwood JB, et al. Impact of diabetes on long-term survival after acute myocardial infarction: comparability of risk with prior myocardial infarction. Diabetes Care. (2001) 24:1422–7. doi: 10.2337/diacare.24.8.1422
28. Preis SR, Hwang SJ, Coady S, Pencina MJ, D'Agostino RB Sr, Savage PJ, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. (2009) 119:1728–35. doi: 10.1161/CIRCULATIONAHA.108.829176
29. Richardson LC, Pollack LA. Therapy insight: influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. (2005) 2:48–53. doi: 10.1038/ncponc0062
30. Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. (2009) 27:2170–6. doi: 10.1200/JCO.2008.17.5935
31. Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB III, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. (2003) 21:433–40. doi: 10.1200/JCO.2003.07.125
32. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. (2001) 131:3109S−20. doi: 10.1093/jn/131.11.3109S
33. Zhang H, Pelzer AM, Kiang DT, Yee D. Down-regulation of type I insulin-like growth factor receptor increases sensitivity of breast cancer cells to insulin. Cancer Res. (2007) 67:391–7. doi: 10.1158/0008-5472.CAN-06-1712
34. Morss AS, Edelman ER. Glucose modulates basement membrane fibroblast growth factor-2 via alterations in endothelial cell permeability. J Biol Chem. (2007) 282:14635–44. doi: 10.1074/jbc.M608565200
35. Pollak M. Insulin, insulin-like growth factors and neoplasia. Best Pract Res Clin Endocrinol Metab. (2008) 22:625–38. doi: 10.1016/j.beem.2008.08.004
36. Mardilovich K, Pankratz SL, Shaw LM. Expression and function of the insulin receptor substrate proteins in cancer. Cell Commun Signal. (2009) 7:14. doi: 10.1186/1478-811X-7-14
Keywords: diabetes, all-cause, cancer, cardiovascular disease, mortality, cohort study
Citation: Tao H, O'Neil A, Choi Y, Wang W, Wang J, Wang Y, Jia Y and Chen X (2020) Pre- and Post-diagnosis Diabetes as a Risk Factor for All-Cause and Cancer-Specific Mortality in Breast, Prostate, and Colorectal Cancer Survivors: a Prospective Cohort Study. Front. Endocrinol. 11:60. doi: 10.3389/fendo.2020.00060
Received: 18 November 2019; Accepted: 30 January 2020;
Published: 18 February 2020.
Edited by:
Ralf Jockers, Université Paris-Sorbonne, FranceReviewed by:
Rosario Le Moli, University of Catania, ItalyCopyright © 2020 Tao, O'Neil, Choi, Wang, Wang, Wang, Jia and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yafeng Wang, d29ueWhmb25Ad2h1LmVkdS5jbg==; Yongqian Jia, amlhX3lxQHNjdS5lZHUuY24=; Xiong Chen, Y2hhc2VjeEAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.