- 1Ministry of Education Key Laboratory of Assisted Reproduction, Beijing Key Laboratory of Reproductive Endocrinology and Assisted Reproductive Technology, Center of Reproductive Medicine, Peking University Third Hospital, Beijing, China
- 2Department of Reproductive Medicine, The First People's Hospital of Yunnan Province, Kunming, China
- 3Robinson Research Institute and Fertility SA, University of Adelaide, Adelaide, SA, Australia
Growth hormone (GH) has been considered as an adjuvant treatment in human assisted reproductive technology (ART) for several years. Its action was largely attributed to an improvement of ovarian function and less emphasis was paid to its role in the uterus. However, there is increasing evidence that GH and its receptors are expressed and have actions in the endometrium and may play an important role in modifying endometrial receptivity. Thus, in this review, we firstly describe the existence of GH receptors in endometrium and then summarize the effects of GH on the endometrium in clinical situations and the underlying mechanisms of GH in the regulation of endometrial receptivity. Finally, we briefly review the potential risks of GH in ART and consider rationalized use of GH treatment in ART.
Introduction
Growth hormone (GH), also called as somatotropin, is a protein of 191 amino acids with a three-dimensional structure that interacts with its receptor (1). The synthesis and secretion of GH is dynamically regulated by growth-hormone releasing hormone and somatostatin, which are both produced by the hypothalamus (2). In recent decades, GH has been considered to regulate many physiological functions including growth, metabolism, and reproduction (3, 4). Its therapeutic use in reproduction has been growing but remains controversial for its efficacy (4, 5). It has been used in assisted reproductive technologies (ART) in humans with an emphasis on potentially improved oocyte quality and pregnancy rates (6, 7). Little attention has been paid to the endometrium, despite the documented presence of GH receptors in this tissue (8). Our review of the literature indicates that study and application of GH for modulating the endometrium has been overlooked in understanding normal endometrial function in ART and reproduction generally. The endometrium undergoes changes via integrated interactions between the different uterine cell types and various growth factors and hormones (9). Several methods can be used to evaluate receptivity of the endometrium, including ultrasound, histology and molecular biomarkers (9, 10). Recent evidence has indicated that GH supplements may modulate endometrial receptivity and improve pregnancy outcomes in ART (10–13). Among women undergoing in vitro fertilization (IVF) treated with GH, changes of endometrial thickness (EMT) and endometrial perfusion have been described by ultrasound evaluation (11–14). The alteration of several biological markers of endometrial receptivity has also been detected with adjuvant administration of GH in animal models and cell-line studies (15–18). While short-term use of GH would not be expected to have problems currently, it could potentially have side-effects on other diseases, especially active cancer and metabolic diseases (19).
We describe the data on evidence of GH action and its receptor in the endometrium. We then mainly focus on the current evidence for the influence of GH on endometrial receptivity. Finally, we look at the potential risks of GH in co-treatment in ART.
Methods
A comprehensive search of the literature available in the PubMed, Web of Science, Embase, and CNKI was conducted using the following keywords, MeSH terms and phrases in combination with one another; “growth hormone,” “somatotropin,” “endometrium/uterine receptivity,” “endometrial thickness,” “endometrium perfusion,” “endometrium cancer,” “disease,” “metabolism,” “side effect/adverse event” “uterus/endometrium,” “growth hormone knockout,” “infertility,” “reproduction” through July 2019. Both human studies and animal data were used.
GH and Its Related Receptor in Endometrium
GH mediates its functions by binding to the GH receptor (GHR) (2). GHRs are most abundant in the liver (20), but also have been found in the reproductive system. GHRs have been reported in human granulosa cells and GH co-treatment in women receiving ART could regulate the expression of GHRs to improve pregnancy outcomes (7). The uterus also appears to be a site of both GH and GHR expression (2). GH has been detected in the cytoplasm of proliferating uterine epithelium cells in dogs (21) and also in human endometrial glandular cells during the mid and late luteal phases and in decidual tissue cells throughout pregnancy (22). GHRs can be found in uterine cells from various species including the mouse where localization of GHR mRNA in the endometrium, glands, stroma and myometrium have been described (23). GHR mRNA was also detected in the uterine epithelium, glands, vessels and placenta from bovine species (24) with biomolecular expressions including GHR and insulin-like growth factor-I (IGF-I) demonstrated in the uterus of dairy cows (25). In the pig, mRNA analyses demonstrated a high level of expression for endometrial somatotropin receptors (STR) (26). In women, GHR mRNA has been detected in the nuclei and cytoplasm of both human myometrial and leiomyoma cells (8). All these findings indicate a potential role for GH on the endometrium.
Clinical Evidence of GH on Endometrial Receptivity
Endometrial thickness (EMT) and uterine perfusion are important clinical indicators of endometrial receptivity in ultrasound studies (10). It has been suggested that ultrasonographic parameters including EMT and uterine perfusion can predict implantation potential in infertile patients undergoing embryo transfer (27). Although this is controversial (28), recent studies suggest a positive relationship between EMT and pregnancy outcome (29–32). Patients with positive pregnancy outcomes following IVF treatment had thicker endometrium readings on the day of hCG administration compared with those where a pregnancy did not result (29). The thicker the endometrium evaluated on the day of human chorionic gonadotropin administration, the higher the pregnancy rates reported following IVF (30, 31). EMT can also be measured on the day of oocyte retrieval and have been alleged to predict the endometrial receptivity during fresh IVF cycles (32). In general, EMT should exceed 8 mm as the threshold of endometrial receptivity in fresh embryo transfer cycles (33), although other studies suggest 10 mm of EMT may be better for a more stable implantation of embryos and minimization of pregnancy losses (34). Hence, increasing endometrial thickness and uterine perfusion might be beneficial goals for improving endometrial receptivity.
Two reports of women with panhypopituitarism causing either primary or secondary infertility who were treated with GH and gonadotropins are illustrative of the potential role for GH in fertility promotion (35, 36). After GH treatment, an improvement in their response to gonadotrophin stimulation was demonstrated with an acceptable endometrial growth and successful pregnancies ensued (35, 36). Standard infertile patients also show different endometrial changes and different pregnancy outcomes after adjuvant GH treatment (Table 1). For infertile women classified as poor responders, GH treatment has been promoted for improving the chances of pregnancy and live birth outcomes. Although no significant increases in implantation or clinical pregnancy rates are consistently demonstrated, there appears to be an increase of retrieved oocyte numbers and EMT (39, 42). A large scale retrospective clinical trial of infertile women classified as normal responders also had an increase in endometrial thickness in the older group (age ≥ 35 years) utilizing GH treatment and an improvement of implantation rate (IR) and clinical pregnancy rate (CPR) was claimed in the GH treatment group across all ages (45). An effect on weight-related infertility has also been seen with a significant improvement of EMT, IR and CPR in a group of infertile women who were overweight and obese (BMI ≥ 24 kg/m2) (43, 45).
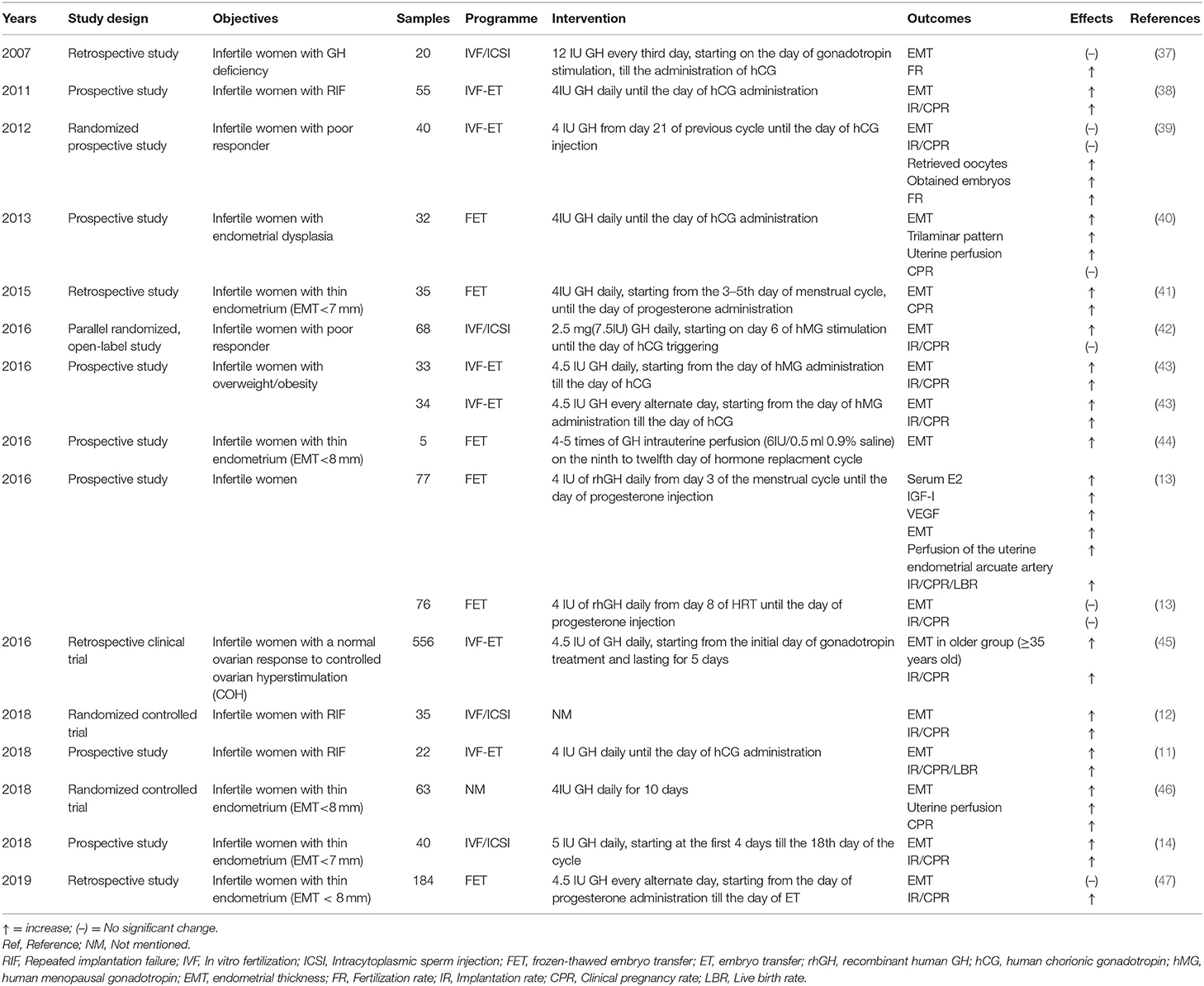
Table 1. The clinical evidence of GH on endometrial receptivity to improve pregnancy outcomes in ART.
In patients with repeated implantation failure (RIF), a thicker endometrium on the day of hCG and an increase of IR, CPR and live birth rate (LBR) was found in a GH treated group, consistent with the previous results reported by others (11, 38). In another recent randomized clinical trial (RCT), the patients with RIF in an oocyte donation program also showed an increase of EMT, CPR, and LBR with GH supplements. Since the oocytes were donated by fertile women, further effects of GH on endometrium could be claimed (12).
In infertile women with poor endometrial development (EMT <7 mm), additional GH treatment is alleged to improve the EMT through uterine perfusion as well as the classical endometrial trilaminar pattern, although there was no significant alteration of pregnancy outcomes in this study (40). A meta-analysis including four RCTs demonstrated an enhancement effect of GH on EMT in infertile women with poor endometrial development (EMT <6 mm or non-trilaminar type endometrium) [OR = 10.62, 95% CI (2.97, 38.00)] (48). Other studies also demonstrated that EMT with GH treatment was significantly increased on day 3 (the 18th day of cycle) with subsequent increased IR and CPR (14) and an increased EMT was also detected in five patients with thin endometrium (EMT < 8 mm) after intrauterine perfusion of GH or parenteral injection of GH (41, 44, 46). These observations are not confirmed by others however (13, 37, 39, 47). Different patient selection, doses, starting time as well as the different measurements and interpretations may be the reasons resulting in the varied outcomes of GH treatment (Table 1). This is illustrated in a recent prospective study where the same dose of recombinant human GH (rhGH) was applied to infertile women but with different starting times resulting in quite different clinical outcomes (13). Those women who started with rhGH treatment earlier in the cycle had a significant increase of EMT, perfusion of the uterine artery index, IR, CPR, and LBR as well as estradiol, IGF-I and vascular endothelial growth factor (VEGF) on the day of embryo transfer (13).
Potential Mechanisms of GH on Endometrial Receptivity
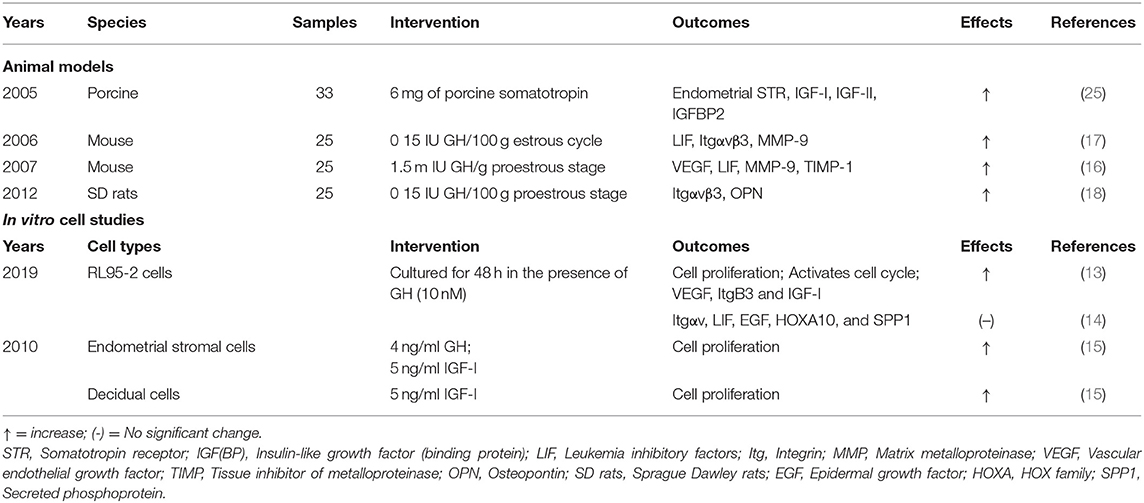
The mechanisms of GH effects on the endometrium to improve the EMT and uterine perfusion and IVF outcomes are still unclear. Currently, several molecules, including IGF, leukemia inhibitory factors (LIF), integrins (Itg), homeobox-containing transcription factors-HOX family genes, etc. contribute to the molecular basis of regulating endometrial receptivity while some molecules closely related to the implantation process have been demonstrated to be involved in the potential mechanisms of GH effects on endometrium (9, 49) (Table 2).
Animal Models
Increased concentrations of cytosolic estrogen receptor but not the concentration of progesterone receptor was found in the rabbit uterus after GH treatment, indicating a potential estrogen mediated function (50). Consistent with this, an increase in the concentration of estrogen receptor in the guinea-pig uterus after treating with GH has also been demonstrated (51). Research in the ovine uterus indicates that GH could regulate endometrial gland proliferation via interferon tau (52) and could alter the endometrial gene expression related to maintenance of pregnancy. GH may increase the expression of oxytocin receptor, progesterone receptor mRNA, and the mRNA of estrogen receptor α in non-lactating cows (53) with an increased pregnancy rate in lactating cows after injection of GH at the initiation of timed artificial insemination following a synchronized ovulation protocol (54). Knockout of the GHR in mice leads to a negative impact on reproduction with fewer uterine implantation sites during early pregnancy (55).
GH-mediated increase in uterine IGF-I levels may be the mechanism underlying the increase in endometrial thickness (56). Exogenous porcine GH elevates the expression of endometrial STR, IGF-I mRNA, IGF-II mRNA, and IGFBP2 mRNA in the uterus, supporting the role of the so-called GH/IGF axis in the uterus (25, 56). Both IGF-I and IGF-II can be detected in endometrial stroma, but have different roles (2, 57). IGF-II is more closely related to endometrial differentiation (57), while IGF-I is a potential mediator of the mitogenic effects of estrogen on the uterus, so-called oestromedin (56). Other studies in the mouse also indicate an alteration of other molecular biomarkers of endometrial receptivity after treating GH (16, 17). GH-treated mice show a significant increase of leukemia inhibitory factors (LIF), integrin alpha v beta 3(Itgαvβ3) and matrix metalloproteinase (MMP)-9 in the endometrium, molecules which have been implicated in implantation (16, 17). Exogenous GH supplementation in Sprague Dawley (SD) rats may also increase osteopontin (OPN) and Itgαvβ3 expression with an associated improvement in endometrial receptivity (18).
In vitro Studies
Addition of hGH to the cultured endometrial and decidual cells increases the proliferation of endometrial and decidual cells, when these cells were harvested and separated from the human endometrial pieces and decidual tissue, respectively (15). When transfected to the human endometrial cell line RL95-2, there is an enhancement of cell proliferation, survival and invasion (58) and increased cell proliferation and expression of VEGF, Itgβ3, and IGF-I (14). Janus kinase (JAK) 2 inhibitor AG490 addition with GH suppresses VEGF, Itgβ3, and IGF-I expression in RL95-2 cells, indicating that GH might regulate the expression of these factors via the JAK2 pathway. However, no change in expression of LIF, Itgαv, Hox family gene (HOXA10) and SPP1 could be found in RL95-2 cells after treatment with GH (14). Therefore, the effects of GH on endometrial receptivity-related molecules in vitro remains to be elucidated.
The Potential Risks of GH Treatment in Art
GH may play a pathological role in body systems in view of the fact that it may have a potential of being an oncogene (59). Transplantation of GH transfected-RL95-2 cells suspension to BALBc nu/nu mice led to a larger tumor size and a more aggressive progression of endometrial carcinoma (58). When RL95-2 cells cultured with the GH receptor antagonist (pegvisomant) were inoculated into immunodeficient NIH-III mice and continuously treated the mice with antagonist for 16 weeks, a delayed tumor growth rate and decreased IGF serum level could also be found (60). A systematic review concluded that long-term GH treatment actually had a positive effect on reducing cardiovascular disease, stroke, and fractures, without a simultaneous increase in malignancy risk (61). While there are few indications of problems, the long-term safety of GH for the cancer risk, metabolic disorder and other unforeseen adverse events should be under constant surveillance (62).
Although metabolic sequelae improve with GH in GH deficient patients (63), some advocate that addition of GH in patients with diabetes mellitus should be cautioned due to its potential negative effects on insulin resistance and glucose tolerance (19, 64). GH can also result in significant metabolic changes by elevating cholesterol and disturbing the renin-angiotensin mechanism (65). Therefore, in consideration of the potential risks, personalized comprehensive assessment, and professional guidance in usage and dosage is required before deciding to use GH in ART.
Conclusion
Clinical evidence for efficacy of GH in improving reproductive function remains controversial. Generally, the majority of studies show positive effects of GH on endometrial receptivity, but there is no general agreement about the dosage and usage of GH in ART and there are few useful RCTs (Table 1). Hence, more evidence is still required to determine the purported value of GH treatment in ART and provide more specific guidance in the clinical setting.
Even if clinical evidence currently encourages the view that GH might be helpful for endometrial receptivity, the mechanisms are still not known. The studies of molecular biomarkers included in this review are few (Table 2), but also may provide some foundations for future exploration of the mechanisms of GH in the endometrium. As GH is administered systemically during ART, it is difficult to separate the effect on GHR in the ovary from that in the endometrium and if GH alters receptor action in the ovary, it could potentially have an effect on the endometrium receptors too.
The risk of GH used in ART should be noticed but not overstated. There is a relationship between autocrine GH and endometrial cancer, but further studies of the mechanisms under this phenomenon and the confirmation of increased diseases risk of exogenous GH are still needed. In general, further explorations of the mechanisms underlying the effects of GH on endometrium should spread some light on our basic knowledge and clinical actions.
Author Contributions
F-TL and RL were responsible for writing the first draft. RL, ZW, JY, and RN performed the critical revisions. All authors listed did contributed to the writing and review of the manuscript.
Funding
This study was supported by National Key Research and Development Program of China (Grant No. 2016YFC1000201), Program for Innovative Research Team of Yunnan, China (Grant No. 2017HC009), and National Natural Science Foundation of China (Grant Nos. 81771650 and 81660266). RN acknowledges funding from the Australian National Health and Medical Research Council.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our gratitude to Prof. Rebecca L. Robker, NHMRC Senior Research Fellow, Robinson Research Institute, The University of Adelaide. She provided us with important suggestions on our manuscript.
References
1. Ranke MB, Wit JM. Growth hormone — past, present and future. Nat Rev Endocrinol. (2018) 14:285–300. doi: 10.1038/nrendo.2018.22
2. Varela-Nieto I, Chowen JA. Basic physiology of the growth hormone/insulin-like growth factor axis. In: Varela-Nieto I, editor. The Growth Hormone/Insulin-Like Growth Factor Axis During Development. Boston, MA: Springer (2005). p. 1–25. doi: 10.1007/b106814
3. Bergan-Roller HE, Sheridan MA. The growth hormone signaling system: Insights into coordinating the anabolic and catabolic actions of growth hormone. Gen Comp Endocrinol. (2018) 258:119–33. doi: 10.1016/j.ygcen.2017.07.028
4. Xu YM, Hao GM, Gao BL. Application of growth hormone in in vitro fertilization. Front endocrinol. (2019) 10:502. doi: 10.3389/fendo.2019.00502
5. Norman RJ, Alvino H, Hull LM, Mol BW, Hart RJ, Kelly TL, et al. Human growth hormone for poor responders: a randomized placebo-controlled trial provides no evidence for improved live birth rate. Reprod Biomed Online. (2019) 38:908–15. doi: 10.1016/j.rbmo.2019.02.003
6. Hou HY, Wang X, Yu Qi, Li HY, Li SJ, Tang RY, et al. Evidence that growth hormone can improve mitochondrial function in oocytes from aged mice. Reprod. (2018) 157:345–58. doi: 10.1530/REP-18-0529
7. Regan SLP, Knight PG, Yovich JL, Arfuso F, Dharmarajan A. Growth hormone during in vitro fertilization in older women modulates the density of receptors in granulosa cells, with improved pregnancy outcomes. Fert Steril. (2018) 110:1298–310. doi: 10.1016/j.fertnstert.2018.08.018
8. Sharara FI, Nieman LK. Growth hormone receptor messenger ribonucleic acid expression in leiomyoma and surrounding myometrium. Am J Obstet Gynecol. (1995) 173:814–19. doi: 10.1016/0002-9378(95)90346-1
9. Tu Z, Ran H, Zhang S, Xia G, Wang B, Wang H. Molecular determinants of uterine receptivity. Int J Dev Biol. (2014) 58:147–54. doi: 10.1387/ijdb.130345wh
10. Bonilla-Musoles F, Raga F, Osborne NG, Castillo JC, Bonilla F Jr. Endometrial receptivity evaluation with ultrasound. Ultrasound Q. (2013) 29:3–20. doi: 10.1097/RUQ.0b013e318281b60a
11. Chen Y, Liu FH, Nong YQ, Ruan JX, Guo QQ, Luo M, et al. Clinical efficacy and mechanism of growth hormone action in patients experiencing repeat implantation failure. Can J Physiol Pharmacol. (2018) 96:929–32. doi: 10.1139/cjpp-2017-0786
12. Altmäe S, Mendoza-Tesarik R, Mendoza C, Mendoza N, Cucinelli F, Tesarik J. Effect of growth hormone on uterine receptivity in women with repeated implantation failure in an oocyte donation program: a randomized controlled trial. J Endocr Soc. (2018) 2:96–105. doi: 10.1210/js.2017-00359
13. Xue-Mei W, Hong J, Wen-Xiang Z, Yang L. The effects of growth hormone on clinical outcomes after frozen-thawed embryo transfer. Int J Gynaecol Obstet. (2016) 133:347–50. doi: 10.1016/j.ijgo.2015.10.020
14. Cui N, Li AM, Luo ZY, Zhao ZM, Xu YM, Zhang J, et al. Effects of growth hormone on pregnancy rates of patients with thin endometrium. J Endocrinol Invest. (2019) 42:27–35. doi: 10.1007/s40618-018-0877-1
15. Strowitzki T, Wiedemann R, Hepp H. Influence of Growth Factors EGF, IGF-1, and Human growth hormone on human endometrial stromal cells in vitro. Ann N Y Acad Sci. (2010) 626:308–11. doi: 10.1111/j.1749-6632.1991.tb37925.x
16. Xiang YG, Tan L, Dong FL. Effect of GH on the Expressions of VEGF, LIF, MMP-9 and TIMP-1 in Endometrium of Mouse. Reprod Contracept. (2007) 27:639–42.
17. Xiang YG, Tan L, Zhang J. Effect of growth hormone on endometrial receptivity in implantation endometrium of mouse. J Med Forum. (2006) 27:35–7. doi: 10.3969/j.issn.1672-3422.2006.24.015
18. Chen Y, Liu FH, Li L, Long XL, Shi Y, Du HZ. Impact of growth hormone on endometrial receptivity in SD rats. Chinese J Biomed Eng. (2012) 18:173–6. doi: 10.3760/cma.j.issn.1674-1927.2012.03.002
19. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine Society. Evaluation and treatment of adult growth hormone deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:1587–609. doi: 10.1210/jc.2011-0179
20. Reindl KM, Sheridan MA. Peripheral regulation of the growth hormone-insulin-like growth factor system in fish and other vertebrates. Comp Biochem Physiol A Mol Integr Physiol. (2012) 163:231–45. doi: 10.1016/j.cbpa.2012.08.003
21. Bhatti SF, Rao NA, Okkens AC, Mol JA, Duchateau L, Ducatelle R, et al. Role progestin-induced mammary-derived growth hormone in the pathogenesis of cystic endometrial hyperplasia in the bitch. Domest Anim Endocrinol. (2007) 33:294–312. doi: 10.1016/j.domaniend.2006.06.005
22. Sbracia M, Scarpellini F, Poverini R, Alo PL, Rossi G, Di Tondo U. Immunohistochemical localization of the growth hormone in human endometrium and decidua. Am J Reprod Immunol. (2004) 51:112–6. doi: 10.1046/j.8755-8920.2003.00127.x
23. Sharara FI, Bhartiya D, Nieman LK. Growth hormone receptor gene expression in the mouse uterus: modulation by gonadal steroids. J Soc Gynecol Invest. (1994) 1:285–9. doi: 10.1177/107155769400100407
24. Kölle S, Sinowatz F, Boie G, Lincoln D, Waters MJ. Differential expression of the growth hormone receptor and its transcript in bovine uterus and placenta. Mol Cell Endocrinol. (1997) 131:127–36. doi: 10.1016/S0303-7207(97)00097-X
25. Rhoads ML, Meyer JP, Kolath SJ, Lamberson WR, Lucy MC. Growth hormone receptor, insulin-like growth factor (IGF)-1, and IGF-binding protein-2 expression in the reproductive tissues of early postpartum dairy cows. J Dairy Sci. (2008) 91:1802–13. doi: 10.3168/jds.2007-0664
26. Freese LG, Rehfeldt C, Fuerbass R, Kuhn G, Okamura CS, Ender K, et al. Exogenous somatotropin alters IGF axis in porcine endometrium and placenta. Domest Anim Endocrinol. (2005) 29:457–75. doi: 10.1016/j.domaniend.2005.02.012
27. Khan MS, Shaikh A, Ratnani R. Ultrasonography and doppler study to predict uterine receptivity in infertile patients undergoing embryo transfer. J Obstet Gynaecol India. (2016) 66:377–82. doi: 10.1007/s13224-015-0742-5
28. Arce H, Velilla E, Lopez-Teijon M. Association between endometrial thickness in oocyte donation cycles and pregnancy success rates. Reprod Fertil Dev. (2015) 28:1288–94. doi: 10.1071/RD14459
29. Al-Ghamdi A, Coskun S, Al-Hassan S, Al-Rejjal R, Awartani K. The correlation between endometrial thickness and outcome of in vitro fertilization and embryo transfer (IVF-ET) outcome. Reprod Biol Endocrinol. (2008) 6:37. doi: 10.1186/1477-7827-6-37
30. Wu Y, Gao X, Lu X, Xi J, Jiang S, Sun Y, et al. Endometrial thickness affects the outcome of in vitro fertilization and embryo transfer in normal responders after GnRH antagonist administration. Reprod Biol Endocrinol. (2014) 12:96. doi: 10.1186/1477-7827-12-96
31. Zhao J, Zhang Q, Wang Y, Li Y. Endometrial pattern, thickness and growth in predicting pregnancy outcome following 3319 IVF cycle. Reprod BioMed Online. (2014) 29:291–8. doi: 10.1016/j.rbmo.2014.05.011
32. Zhang T, Li Z, Ren X, Huang B, Zhu G, Yang W, et al. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: a retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Medicine. (2018) 97:e9689. doi: 10.1097/MD.0000000000009689
33. Bu Z, Wang K, Dai W, Sun Y. Endometrial thickness significantly affects clinical pregnancy and live birth rates in frozen-thawed embryo transfer cycles. Gynecol Endocrinol. (2016) 32:524–8. doi: 10.3109/09513590.2015.1136616
34. Gallos I, Khairy M, Chu J, Rajkhowa M, Tobias A, Campbell A, et al. Optimal endometrial thickness to maximize live births and minimize pregnancy losses: analysis of 25,767 fresh embryo transfers. Reprod BioMed Online. (2018) 37:542–8. doi: 10.1016/j.rbmo.2018.08.025
35. Salle A1, Klein M, Pascal-Vigneron V, Dousset B, Leclere J, Weryha G. Successful pregnancy and birth after sequential cotreatment with growth hormone and gonadotropins in a woman with panhypopituitarism: a new treatment protocol. Fertil Steril. (2000) 74:1248–50. doi: 10.1016/S0015-0282(00)01619-8
36. Drakopoulos P, Pluchino N, Bischof P, Cantero P, Meyer P, Chardonnens D. Effect of growth hormone on endometrial thickness and fertility outcome in the treatment of women with panhypopituitarism: a case report. J Reprod Med. (2016) 61:78–82.
37. Rajesh H, Yong YY, Zhu M, Chia D, Yu SL. Growth hormone deficiency and supplementation at in-vitro fertilisation. Singapore Med J. (2007) 48:514–8.
38. Liu FH, Chen Y, Li L, Shi Y, Du HZ, Wei L. Explore the effect of growth hormone on patients with recurrent implantation failures undergoing in vitro fertilization-embryo transfer. Chin J Prac Gynecol Obstet. (2011) 27:609–12.
39. Eftekhar M, Aflatoonian A, Mohammadian F, Eftekhar T. Adjuvant growth hormone therapy in antagonist protocol in poor responders undergoing assisted reproductive technology. Arch Gynecol Obstet. (2013) 287:1017–21. doi: 10.1007/s00404-012-2655-1
40. Wu HB, Li LM, Li MJ, Yuan H. The effect of growth hormone on the endometrium and endometrial blood flow in frozen-thawed embryo transfer. J Reproduct Med. (2013) 22:914–7.
41. Ling DD, Shi XB. The role of 17 beta-estradiol and growth hormone in the preparation of frozen embryo transfer in patients with thin endometrium. J Reproduct Med. (2016) 25:72–6.
42. Bassiouny YA, Dakhly DMR, Bayoumi YA, Hashish NM. Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil Steril. (2015) 105:697–702. doi: 10.1016/j.fertnstert.2015.11.026
43. Fan W, Li DY, Yang XL, Ma L, Meng YS. The study of the value of growth hormone applied in IVF-ET in overweight and obese infertile patients. J Prac Obstet Gynecol. (2016) 32:774–7.
44. Yu H, Gao SY, Tang HZ, Chen HL, Deng ZH, Yang L, et al. Growth hormone intrauterine perfusion combined with replacement cycle in the treatment of non-response thin endometrium: report of 5 cases. Int J Clin Exp Med. (2016) 9:11982–9. doi: 10.3760/cma.j.issn.1008-1372.2015.09.010
45. Du XF, Yang XH, Li J, Hao M, Guo YH. Growth hormone co-treatment within a GnRH agonist long protocol improves implantation and pregnancy rates in patients undergoing IVF-ET. Arch Gynecol Obstet. (2016) 294:877–83. doi: 10.1007/s00404-016-4163-1
46. Mei DH. Clinical evaluation of growth hormone combined with 17β-estradiol for treating thin endometrium in 63 cases. Chin Pharm. (2018) 27:63–5. doi: 10.3969/j.issn.1006-4931.2018.02.017
47. Yang JY, Li H, Lu N, Li L, Sun XX. Influence of growth hormone supplementation in patients with thin endometrium undergoing frozen embryo transfer. Reprod Dev Med. (2019) 3:49–53. doi: 10.4103/2096-2924.255983
48. Yang L, Wei ZC, Zhang XH. A meta-analysis of the efficacy of growth hormone in patients with endometrial dysplasia during in vitro fertilization and embryo transplantation. Prog Obstet Gynecol. (2014) 23:560–3. doi: 10.13283/j.cnki.xdfckjz.2014.07.013
49. Karizbodagh MP, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H. Implantation Window and Angiogenesis. J Cell Biochem (2017) 118: 4141-51. doi: 10.1002/jcb.26088
50. Chilton BS, Daniel JC. Differences in the rabbit uterine response to progesterone as influenced by growth hormone or prolactin. J Reproduct Fertil. (1987) 79:581–7. doi: 10.1530/jrf.0.0790581
51. Bezecný I1, Bártová J, Skarda J. Growth hormone treatment increases oestrogen receptor concentration in the guinea-pig uterus. J Endocrinol. (1992) 134:5–9. doi: 10.1677/joe.0.1340005
52. Spencer TE. Effects of recombinant ovine interferon tau, placental lactogen, and growth hormone on the ovine uterus. Biol Reprod. (1999) 61:1409–18. doi: 10.1095/biolreprod61.6.1409
53. Guzeloglu A, Bilby TR, Meikle A, Kamimura S, Kowalski A, Michel F, et al. Pregnancy and bovine somatotropin in nonlactating dairy cows: II. endometrial gene expression related to maintenance of pregnancy. J Dairy Sci. (2004) 87:3268–79. doi: 10.3168/jds.S0022-0302(04)73463-3
54. Santos JE, Juchem SO, Cerri RL, Galvão KN, Chebel RC, Thatcher WW, et al. Effect of bST and reproductive management on reproductive performance of Holstein dairy cows. J. Dairy Sci. (2004) 87:868–81. doi: 10.3168/jds.S0022-0302(04)73231-2
55. Zaczek D, Hammond J, Suen L, Wandji S, Service D, Bartke A, et al. Impact of growth hormone resistance on female reproductive function: new insights from growth hormone receptor knockout mice. Biol Reproduct. (2002) 67:1115–24. doi: 10.1095/biolreprod67.4.1115
56. Bondy CA, Zhou J. Growth hormone, insulin-like growth factors and the female reproductive system. In: Varela-Nieto I, editor. The Growth Hormone/Insulin-Like Growth Factor Axis During Development. Boston, MA: Springer (2005). p. 91–115. doi: 10.1007/0-387-26274-1_4
57. Rutanen EM. Insulin-like growth factors in endometrial function. Gynecol Endocrinol. (1998) 12:399–406. doi: 10.3109/09513599809012842
58. Pandey V1, Perry JK, Mohankumar KM, Kong XJ, Liu SM, Wu ZS, et al. Autocrine human growth hormone stimulates oncogenicity of endometrial carcinoma cells. Endocrinol. (2008) 149:3909–19. doi: 10.1210/en.2008-0286
59. Perry JK, Emerald BS, Mertani HC, Lobie PE. The oncogenic potential of growth hormone. Growth Horm IGF Res. (2006) 16:277–89. doi: 10.1016/j.ghir.2006.09.006
60. Evans A, Jamieson SM, Liu DX, Wilson WR, Perry JK. Growth hormone receptor antagonism suppresses tumour regrowth after radiotherapy in an endometrial cancer xenograft model. Cancer Lett. (2016) 379:117–23. doi: 10.1016/j.canlet.2016.05.031
61. van Bunderen CC, van Varsseveld NC, Erfurth EM, Ket JC, Drent ML. Efficacy and safety of growth hormone treatment in adults with growth hormone deficiency: a systematic review of studies on morbidity. Clin Endocrinol. (2014) 81:1–14. doi: 10.1111/cen.12477
62. Society GH. Critical evaluation of the safety of recombinant human growth hormone administration: statement from the growth hormone research society. J Clin Endocrinol Metab. (2001) 86:1868–70. doi: 10.1210/jc.86.5.1868
63. Chihara K, Kato Y, Kohno H, et al. Safety and efficacy of growth hormone (GH) during extended treatment of adult Japanese patients with GH deficiency (GHD). Growth Horm IGF Res. (2008) 18:307–17. doi: 10.1016/j.ghir.2007.12.001
64. Jeffcoate W, Jeffcoate W. Growth hormone therapy and its relationship to insulin resistance, glucose intolerance and diabetes mellitus: a review of recent evidence. Drug Saf. (2002) 25:199–212. doi: 10.2165/00002018-200225030-00005
Keywords: growth hormone, endometrial receptivity, mechanisms, risk, ART
Citation: Liu F-T, Wu Z, Yan J, Norman RJ and Li R (2020) The Potential Role of Growth Hormone on the Endometrium in Assisted Reproductive Technology. Front. Endocrinol. 11:49. doi: 10.3389/fendo.2020.00049
Received: 06 September 2019; Accepted: 27 January 2020;
Published: 14 February 2020.
Edited by:
John Lui Yovich, Pivet Medical Center, AustraliaReviewed by:
Jan Tesarik, MAR Gen Clinic, SpainYves Menezo, London Fertility Associates, United Kingdom
André Hazout, Consultant, Paris, France
Sheena Regan, Curtin University, Australia
Copyright © 2020 Liu, Wu, Yan, Norman and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Li, cm9zZWxpMDAxQHNpbmEuY29t
 Fen-Ting Liu
Fen-Ting Liu Ze Wu2
Ze Wu2 Robert J. Norman
Robert J. Norman Rong Li
Rong Li