
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 07 February 2020
Sec. Pediatric Endocrinology
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00027
This article is part of the Research Topic Metabolically Healthy and Unhealthy Obese Children and Adolescents View all 9 articles
 Simonetta Genovesi1,2*
Simonetta Genovesi1,2* Laura Antolini3
Laura Antolini3 Antonina Orlando1
Antonina Orlando1 Luisa Gilardini2
Luisa Gilardini2 Simona Bertoli4,5
Simona Bertoli4,5 Marco Giussani6
Marco Giussani6 Cecilia Invitti2
Cecilia Invitti2 Elisa Nava7
Elisa Nava7 Maria Grazia Battaglino1
Maria Grazia Battaglino1 Alessandro Leone4
Alessandro Leone4 Maria Grazia Valsecchi3
Maria Grazia Valsecchi3 Gianfranco Parati2
Gianfranco Parati2Background: In pediatric age the prevalence of obesity is high. Obese children who do not have other risk factors than excess weight have been defined as “metabolically healthy obese” (MHO).
Aim: The aim of this study is to evaluate, in a population of obese children, the prevalence of the MHO and “metabolically unhealthy obese” (MUO) phenotype. Furthermore, we evaluated the distribution of Uric Acid, HOMA index and Waist-Height ratio (W-Hr) in the MHO and MUO sub-groups and the impact of these non-traditional risk factors on the probability to be MUO.
Methods: In 1201 obese children and adolescents [54% males, age (±SD) 11.9 (±3.0) years] weight, height, waist circumference, systolic (SBP) and diastolic (DBP) blood pressure, pubertal status, glucose, insulin, HDL cholesterol, triglycerides and Uric Acid serum values were assessed. MUO phenotype was defined as the presence of at least one of the following risk factors: SBP or DBP ≥ 90th percentile, glycaemia ≥ 100 mg/dl, HDL cholesterol <40 mg/dl, triglycerides ≥100 mg/dl (children <10 years) or ≥130 mg/dl (children ≥10 years). A multivariate logistic regression analysis was used to estimate the association between MUO phenotype and non-traditional cardiovascular risk factors.
Results: The prevalence of the MUO status was high (61%). MUO subjects were more often male, older and pubertal (p < 0.001). The levels of the three non-traditional risk factors were significantly higher in MUO children compared to MHO children (p < 0.001) and all of them were independent predictors of the fact of being MUO [OR 1.41 (95% CI 1.24–1.69); 1.15 (95% CI 1.06–1.23) and 1.03 (95% CI1.01–1.05) for Uric Acid, HOMA index and W-Hr, respectively]. About 15% of MHO subjects had serum Uric Acid, HOMA index and W-Hr values within the highest quartile of the study population.
Conclusion: The prevalence of MUO subjects in a large pediatric population is high and serum Uric Acid, HOMA index and W-Hr values are independent predictors of the probability of being MUO. A non-negligible percentage of subjects MHO has high values of all three non-traditional risk factors.
The increasing prevalence of obesity in children and adolescents is becoming a global concern (1). Obesity is a complex, chronic condition characterized by multiple causes and adverse health consequences (2, 3) and it is associated with increase all-cause mortality in adults (4). Excess weight in childhood is a major risk factor for adulthood obesity (5) and increases the risk of morbidity and mortality later in life (6). Not all obese individuals show an equal health risk. Metabolically healthy obesity (MHO) is defined as a condition in which, despite the significant excess weight, traditional risk factors as insulin resistance (IR), dyslipidemia, and hypertension are not present (7–10), contrary to what occurs in the metabolically unhealthy obesity (MUO) condition.
The MHO phenotype has been described since the 1980s (11). For adults MHO is generally defined as a condition in which metabolic syndrome (MetS) factors are absent (2, 12).
For children and adolescents, there is still no universally accepted definition of MetS (13, 14). For this reason, the American Academy of Pediatrics recommended to focus on the concept of cardiometabolic risk factor clustering, rather than on the presence of MetS to define MHO in pediatric age (15). Recently, a consensus of international experts proposed a definition of MHO including cutoff values for high density lipoprotein-cholesterol [>40 mg/dl, triglycerides ≤ 150 mg/dl), systolic and diastolic blood pressure (≤ 90th percentile), and a measure of glycaemia (16).
In adulthood, individuals with MUO show increased mortality rates and cardiovascular risk compared to obese individuals with normal metabolic profiles (17). It is therefore possible that children and adolescents with the MUO phenotype may have higher cardiovascular and mortality risk compared to MHO subjects when they become adults.
The aims of the present study are (i) to determine the prevalence of the MUO phenotype in a population sample of obese Italian children and adolescents referred to secondary level Clinical Centers, (ii) to evaluate the distribution of Waist-Height ratio (W-Hr), Homeostatic Model Assessment (HOMA) index of insulin resistance and serum Uric Acid values in MHO and MUO children (iii) to investigate whether these non-traditional cardiovascular risk factors are independent predictors of the MUO phenotype, if added to the risk factors already present in the definition.
We studied a cohort of 1201 obese children and adolescents (4–18 years) referred from January 2002 to August 2019 by their primary care pediatricians to the IRCCS Istituto Auxologico Italiano and to the International Center for the Assessment of Nutritional Status, ICANS. Cardiovascular risk factors were evaluated at Istituto Auxologico Italiano, IRCCS, Cardiologic Unit. Inclusion criteria for the study were: (1) age ≤18 years (2) informed consent obtained from parents or legal representatives to participate in the study. Exclusion criteria were: (1) syndromic obesity; (2) dysthyroidism; (3) diabetes; (4) congenital cardiovascular diseases (5) any form of secondary hypertension (6) presence of chronic kidney disease and (7) treatment with antihypertensive drugs, lipid-lowering drugs, and glucose altering medications such as metformin.
The study protocol was approved by the local institutional ethics committee and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Anthropometric measurements were taken following international guidelines (18). Weight was measured with 700 SECA gram scale and height with 417 SECA stadiometer (®SECA Medical Measuring Systems and scales, Birmingham UK), approximated to the nearest 100 g and 0.5 cm, respectively. BMI was calculated as weight (kg)/height (m)2. BMI z-scores were calculated using the Centers for Disease and Control prevention charts available at http://www.cdc.gov/nchs/. Weight class (Obesity) was defined according to the International Obesity Task Force classification (19). Waist circumference was measured in standing position at the midpoint between the last rib and the iliac crest with a non-stretch tape to the nearest 0.5 cm. Waist–height ratio was calculated dividing WC by height obtaining a pure number. Pubertal stage was assessed by a medical examination and children were classified into two categories: pre-pubertal and pubertal according to Tanner (20). Blood pressure (BP) was measured using an aneroid sphygmomanometer with the appropriate cuff for the child's upper arm size (Heine GAMMA® G7, Germany). The sphygmomanometer was calibrated before starting the study and once a month thereafter with a mercury sphygmomanometer. Systolic BP (SBP) was defined by the first Korotkoff sound (appearance of sounds) and diastolic BP (DBP) was identified by the fifth Korotkoff sound (disappearance of sounds). Measurements were performed after at least 5 min of rest. BP measures were taken 3 times (at 3–5 min intervals). The average of the two last SBP and diastolic BP (DBP measurements was calculated. Systolic BP and DBP 90th percentiles were calculated according to the nomograms of the National High Blood Pressure Education Program (NHBPEP) Working Group on High Blood Pressure in Children and Adolescents (21).
Fasting blood samples were drawn after a 12-h fasting period in order and analyzed in the same morning. Glucose, triglycerides, HDL- cholesterol, alanine transaminase (ALT), gamma- glutamyl-transferase (GGT) and uric acid were measured by means of an enzymatic method (Cobas Integra 400 Plus, Roche Diagnostics, Rotkreuz, Switzerland), with intra-and inter-assay CVs < 2%. Circulating insulin was measured in duplicate by an autoanalyzer (Cobas e411 Hitachi, Roche Diagnostics). The homeostatic model assessment-insulin resistance was calculated as [fasting glucose (mg/dL) × fasting insulin (mU/L)/405] (22).
The following definition to identify MHO subjects was applied: SBP and DBP <90th percentile by gender, age and height percentile, glycaemia <100 mg/dl, HDL cholesterol >40 mg/dl, triglycerides <100 mg/dl (children <10 years) or <130 mg/dl (children ≥10 years) (16).
MUO phenotype was defined as the presence of at least one of the following risk factors: SBP or DBP≥ 90th percentile, glycaemia >100 mg/dl, HDL cholesterol ≤40 mg/dl, triglycerides ≥100 mg/dl (children <10 years) or ≥130 mg/dl (children ≥10 years).
In the proposed definition of MHO (16) it is not specified which glycemia cut-off should be used. However, most of the studies reviewed by the expert consensus used fasting glucose <100 mg/dL. For this reason, we used the same value in our study. The triglyceride cut-offs were based on the indications of the National Cholesterol Education Program (NCEP) Expert Panel on Cholesterol Levels in Children (23, 24). Since the proposed definition of MHO suggests 150 mg as a single cut-off for triglycerides, we also performed a further analysis (sensitivity analysis) by entering this triglyceride cut-off.
Data analysis was conducted separately for male and female subjects. Categorical variables were described by percentages and continuous variables were reported as means and standard deviations. Comparison across groups was performed by Chi-square and T-test considering 0.05 as significance level to judge p-values. Logistic regression was used to relate the binary status (MHO or MUO) to explanatory variables through univariate and multivariate models. The discrimination potential of explanatory variables was investigated via receiver operating characteristic curves. Data analysis and graphics was conducted by the STATA 16 software (StataCorp LLC 4905 Lakeway Drive College Station, TX 77845 USA).
Table 1 shows the characteristics of the study population by gender. About 46.0% of the subjects were male and 56% were pubertal. Serum Uric Acid and W-Hr values were significantly higher in males [5.1 (±1.5) vs. 4.7 (±1.0) mg/dl, p < 0.001 and 60.6 (±8.4) vs. 59.4 (±7.4)%, p = 0.016, respectively].
As expected, the pre-pubertal subjects were younger, more sensitive to insulin, and had lower Uric Acid and W-Hr values than pubertal, [age, 9.2 (±1.9) vs. 13.7 (±2.3) years, p < 0.001; HOMA index 2.8 (±1.9) vs. 3.7 (±2.4), p < 0.001; Uric Acid 4.4 (±0.9) vs. 5.2 (±1.3) mg/dl, p < 0.001 and W-Hr58.0 (±7.0) vs. 61.0 (±8.0)%, p < 0.001].
Seven hundred and thirty-two subjects (61.0%) were MUO. Table 2 shows the characteristics of the study population by metabolic status. MUO subjects were more frequently male [OR 1.26 (95% CI 0.99–1.59), p = 0.053], and more often pubertal [OR 2.0 (95% CI 1.56–2.55), p < 0.001] compared to MHO children. The risk of being MUO increased with increasing age [OR 1.14 (95% CI 1.09–1.18), per 1-year increment, p < 0.001]. Both SBP and DBP z-scores were higher in MUO compared to MHO subjects (p < 0.001). Moreover, plasma values of glycaemia and triglycerides were higher and those of HDL cholesterol were lower in MUO children (p < 0.001). Serum Uric Acid values were significantly higher in the MUO group than in the MHO group [5.2 (±1.3) vs. 4.5 (±1.1) mg/dl, p < 0.001], as well as those of HOMA index [3.7 (±2.4) vs. 2.8 (±1.8), p < 0.001] and W-Hr [61.2 (±8.1) vs. 58.0 (±7.2)%, p < 0.001]. It is interesting to note that MUO subjects were not different from MHO subjects as far as the BMI z-score values were concerned (p = 0.117).
Among MUO children, 389 (53.1%) presented only one risk factor, 266 (36.3%) 2 risk factors, 57 (7.8%) 3 risk factors and only 20 (2.7%) had all four risk factors of the definition of the MUO phenotype. Table 3 shows the risk factor distribution (SBP ≥ 90th percentile, DBP ≥ 90th percentile, low HDL cholesterol, high triglycerides and high glycaemia) among MUO children according to gender. Hypertriglyceridemia and high SBP were the most represented risk factor (42.6%), subjects with DBP≥ 90th percentile and those with low HDL were also frequent (33.2%). Instead, a low prevalence of elevated glycaemia was observed (8.5%).
After adjustment for gender, age and pubertal status, Uric Acid values [OR 1.53 (95% CI 1.35–1.74), p < 0.001], those of HOMA index [OR 1.28 (95% CI 1.12–1.30), p < 0.001] and those of W-Hr [OR 102.23 (95% CI 15.80–661.51), p < 0.001] were all independent predictors of the probability of being MUO.
When Uric Acid, HOMA index and W-Hr were put together in the logistic regression model, all three variables maintained their predictive power on the probability of being MUO and the result did not change after adjustment for gender, age and pubertal status. The probability of being classified as MUO increased by 41% for each 1 mg/dl increase in serum Uric Acid, by 15% for each increment of 1 unit of HOMA index and by 3% for each percentage unit of W-Hr (Table 4).
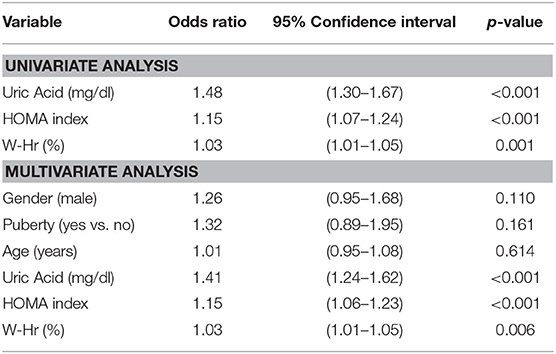
Table 4. Effect of Uric Acid, HOMA index and W-Hr on the risk to be MUO by a multiple logistic regression.
Figure 1 shows the three ROC curves related to the sensitivity and specificity of Uric Acid (Panel 1), HOMA index (Panel 2) and W-Hr (Panel 3) in the prediction of being a MUO subject. The areas under ROC curves were 0.66, 0.64, and 0.61 for Uric Acid, HOMA index and W-Hr, respectively.
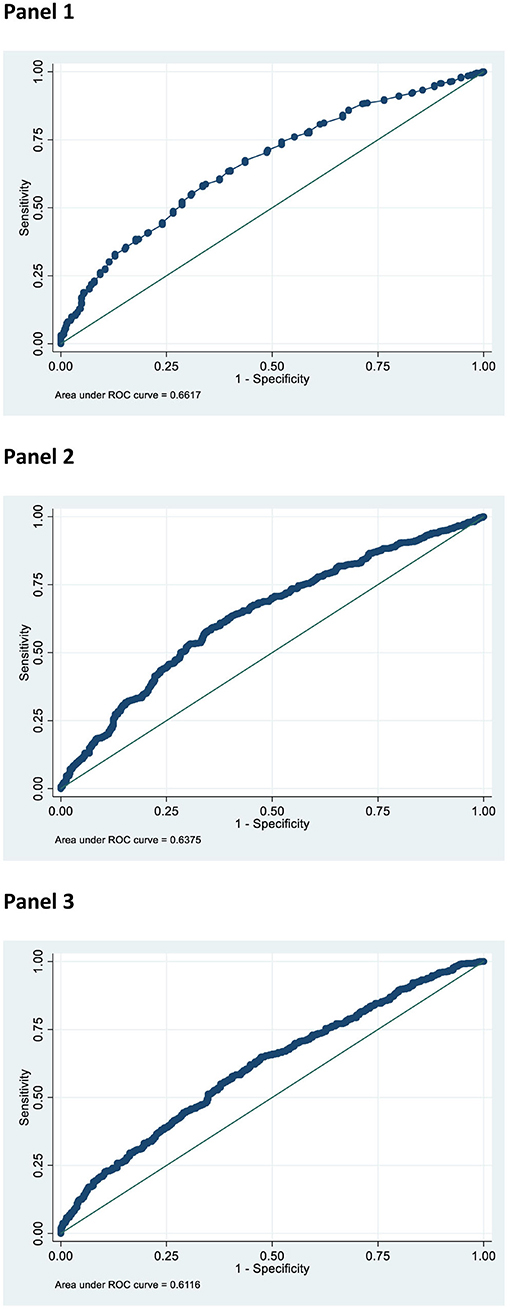
Figure 1. Receiver Operating Characteristic (ROC) curve of Uric Acid (mg/dl) (Panel 1), HOMA index (Panel 2), and W-Hr (Panel 3) to detect the condition of metabolic unhealthy obesity.
A high percentage of MUO children showed Uric Acid, HOMA index and W-Hr values within the highest quartile. In the MUO group the percentage of children who had Uric Acid, HOMA index and W-Hr values within the fourth quartile was about twice the percentage observed in the MHO group (p < 0.001) (Table 5).
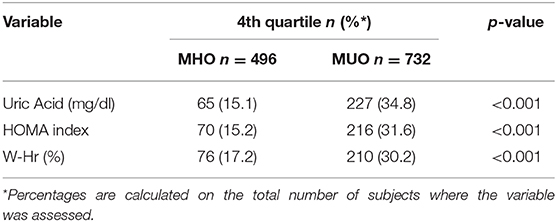
Table 5. Children with Uric Acid, HOMA index and W-Hrvalues within the fourth quartile in MHO/MUO groups.
The sensitivity analysis performed by entering 150 mg/dl as single triglyceride cut-off confirmed the independent predictive value of Uric Acid, HOMA index and W-Hr on the risk of being MUO. With the additional analysis, BMI z-score was greater in MHO compared to MUO subjects, however the difference disappeared at multivariate analysis. Furthermore, male gender was an independent predictor of MUO phenotype (Supplementary Tables 2–5). The areas under ROC curves did not change with the sensitivity analysis.
The main results of our study are: (i) the prevalence of MUO subjects in a large pediatric population is high (about 60%) (ii) three risk factors not included in the definition of MHO (serum Uric Acid, HOMA index and W-Hr) are significantly higher in MUO children compared to MHO children and all three are independent predictors of the possibility of being MUO iii) considering the study population, a non-negligible percentage of MHO subjects has values of serum Uric Acid, HOMA index and W-Hr within the highest quartile.
The prevalence of the MHO phenotype in children described in the literature varies from 20 to 68%, but it should be emphasized that the MHO definitions used in the different studies are not always the same. Moreover, in most cases these studies are performed in populations composed of a relatively small number of children (2). The prevalence of MHO subjects we found in a large sample is higher than that described by other authors (25). However, it must be considered that this sample does not originate from a population screening. These young patients, in fact, are referred to us by their family pediatricians, when they have some difficulty in managing them. Our clinics, in fact, constitute a second-level clinical care center. For this reason, we cannot consider the study population as representative of the Italian pediatric population. The prevalence of individual risk factors in our sample of MUO children is similar to that reported in a previous study performed in a large pediatric population (26).
Previous studies demonstrated that all three non-traditional cardiovascular risk factors considered have a significant and positive association with BP z-scores and the presence of arterial hypertension in pediatric populations (27–31). The well-documented association of hyperuricemia with metabolic syndrome in adults (32) agrees with the observation made in a cohort of children with severe obesity (33), and further supports the role for Uric Acid as an independent risk factor for cardiovascular diseases. Several studies show that MUO subjects have a higher degree of obesity (i.e., a BMI z-score) than MHO subjects (34, 35). In our population, on the contrary, there is no difference in BMI z-score values between MHO children and MUO children. Despite this, W-Hr is significantly higher in MUO subjects, suggesting the presence of a different distribution of abdominal adipose tissue in this sub-group. In children, a high W-Hr value is associated with alterations of a series of cytokines that can lead to negative consequences on the cardiovascular system (36–38). In the present study population, although there are no significant differences in BMI z-score between the two phenotypes, the values of HOMA index are significantly higher in MUO children. This finding suggests a close relationship between visceral fat and insulin resistance, a known cardiovascular risk factor (29). The INTERHEART study, a case-control study that looked at 29,972 patients in 52 countries, showed that the relationship between waist circumference and waist-to-hip ratio and myocardial infarction is stronger than the relationship between myocardial infarction and BMI (39).
The abovementioned evidence helps us to interpret our results, suggesting a series of reasons why, individually, the non-traditional cardiovascular risk factors considered are associated with the MUO phenotype. However, our study provides an additional observation concerning these data. As a matter of fact, in our population serum Uric Acid, HOMA index and W-Hr may be helpful, independently of each other, to identify among obese children those showing the MUO phenotype. It is important to underline that 15% of children with the MHO phenotype show high values of one or more of the three non-traditional cardiovascular risk factors. It has been shown that obesity is a powerful predictor of cardiac hypertrophy in children, regardless of the presence of hypertension (40). Di Bonito et al. described a prevalence of hepatic steatosis of 36.5% and a proportion of 56.0% of left ventricular hypertrophy in a pediatric MHO population (41). Moreover, Dangardt et al. showed, in a large population of children and adolescents, that persistence of high total fat mass during adolescence, assessed using dual-energy x-ray absorptiometry, was positively associated with increased arterial stiffness, assessed by carotid-femoral pulse wave velocity. This adverse association (with arterial stiffness) was absent with BMI z-score (42). It is therefore conceivable that even MHO subjects cannot be considered truly “healthy” and that they may present initial organ damage of some kind or that they may, over time, become hypertensive.
The definition of an MHO phenotype was created in the adult population, in which it is possible to evaluate the incidence of cardiovascular events and mortality, and to compare this between MHO and MUO patients (17). Of course, this cannot be done in the pediatric population. Moreover, in adults, obesity is frequently a stable condition, not of recent onset, and may therefore exert its negative effects over time. In children, instead, the presence of obesity can be transient and represents a condition with a more recent clinical history than in adults. However, Reinehret al. demonstrated that, in their large pediatric population, after 1 year of follow-up, the majority of the MHO children remained MHO (68.0%) (26). Our data show that older and post-pubertal subjects, in whom it can be assumed that obesity has been present for longer, are more frequently MUO. This would suggest that it takes some time for metabolic alterations to occur. Furthermore, for what regards non-traditional risk factors, it should be noted that also insulin resistance and Uric Acid serum values increase with age and puberty. Our multivariate model however, shows an association between MHO phenotype and these non-traditional risk factors independent of the age of the child, suggesting the importance of a very early intervention. It is reasonable to hypothesize that a part of the children who present the MHO phenotype could become MUO adults; this could be influenced by their genetic predisposition, as well as by eating habits and lifestyle (43). Only prospective studies will allow us to understand what percentage of children who are obese in childhood remain obese in adulthood and how many of the subjects showing the MHO phenotype in childhood, continue to be MHO even when they become adults.
A strength of the study is the size of the study-population, but a limitation is the cross-sectional design. For this reason, we can only describe associations between cardiovascular risk factors and the presence of MU phenotype, but we cannot say whether or not there is a cause/effect relationship. In addition, our sample is made up of subjects referred to a Center for the prevention and treatment of cardiovascular risk in pediatric age and may not represent the totality of obese children.
In conclusion, on the one hand distinguishing between the MHO and MUO phenotype in children can be potentially useful, as it allows the pediatrician to more closely follow-up the subjects with highest cardiovascular risk (8). The definition proposed by Damanhoury et al. (16) however, can lead to an underestimation of the number of obese children actually at risk, as obesity per se can lead to early organ damage even in the absence of metabolic alterations (40, 41). Furthermore, the definition does not cover all potential cardiovascular risk factors, such as insulin resistance, high levels of Uric Acid and visceral adiposity (44). In our opinion, the definition proposed by Damanhoury et al. (16) is a bit limiting and pediatricians would need a more detailed definition to identify obese MUO subjects.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics committee of Istituto Auxologico Italiano, RICARPE Study, code 2015_10_20_02. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
SG and MG conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. AO, EN, MB, MG, and AL collected data. LA and MV performed data analysis. LG, SB, CI, and GP reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with the authors LG and CI.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00027/full#supplementary-material
1. Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. (2005) 111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10
2. Blüher S, Schwarz P. Metabolically healthy obesity from childhood to adulthood – does weight status alone matter? Metabolism. (2014) 63:1084–92. doi: 10.1016/j.metabol.2014.06.009
3. Vukovic R, Mitrovic K, Milenkovic T, Todorovic S, Soldatovic I, Sipetic-Grujicic S, et al. Insulin-sensitive obese children display a favorable metabolic profile. Eur J Pediatr. (2013) 172:201–6. doi: 10.1007/s00431-012-1867-5
4. Flegal K, Kit B, Orpana H, Graubard B. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. (2013) 309:71–82. doi: 10.1001/jama.2012.113905
5. Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. (2013) 128:1682–171. doi: 10.1161/CIR.0b013e3182a5cfb3
6. Juonala M, Magnussen C, Berenson G, Venn A, Burns T, Sabin M, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. (2011) 17:1876–85. doi: 10.1056/NEJMoa1010112
7. Blüher M. Mechanisms in endocrinology: are metabolically healthy obese individuals really healthy? Eur J Endocrinol. (2014) 171:209–19. doi: 10.1530/EJE-14-0540
8. Prince RL, Kuk JL, Ambler K, Dhaliwal J, Ball GDC. Predictors of metabolically healthy obesity in children. Diabetes Care. (2014) 37:1462–8. doi: 10.2337/dc13-1697
9. vanVliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, Foco L, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. (2014) 14:9. doi: 10.1186/1472-6823-14-9
10. Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. (2013) 1:152–62. doi: 10.1016/S2213-8587(13)70062-7
11. Sims E. Characterization of the syndromes of obesity. In: Brodoff BN, Bleicher SJ, editors. DiabetesMellitus and Obesity. Baltimore, MD: Williams & Wilkins (1982). p. 219–26.
12. Pajunen P1, Kotronen A, Korpi-Hyövälti E, Keinänen-Kiukaanniemi S, Oksa H, Niskanen L, et al. Metabolically healthy and unhealthy obesity phenotypes in the general population: the FIN-D2D Survey. BMC Public Health. (2011) 11:754. doi: 10.1186/1471-2458-11-754
13. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents - findings from the third national health and nutrition examination survey, 1988-1994. Arch Pediatr Adolesc Med. (2003) 157:821–7. doi: 10.1001/archpedi.157.8.821
14. Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolicsyndromeinchildren and adolescents. Lancet. (2007) 369:2059–61. doi: 10.1016/S0140-6736(07)60958-1
15. Magge SN, Goodman E, Armstrong SC, Committee on nutrition, section on endocrinology, section on obesity. The metabolic syndrome in children and adolescents; shifting the focus to cardiometabolic risk factor clustering. Pediatrics. (2017) 140:e20171603. doi: 10.1542/peds.2017-1603
16. Damanhoury S, Newton AS, Rashid M, Hartling L, Byrne JLS, Ball GDC. Defining metabolically healthy obesity in children: a scoping review. Obes Rev. (2018) 19:1476–91. doi: 10.1111/obr.12721
17. Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions? a systematic review and meta-analy-sis. Ann Intern Med. (2013) 159:758–69. doi: 10.7326/0003-4819-159-11-201312030-00008
18. Lohman GT, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human kinetics book (1991).
19. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. (2000) 320:1240–3. doi: 10.1136/bmj.320.7244.1240
20. Tanner JM. Growth and maturation during adolescence. Nutr Rev. (1981) 39:43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x
21. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatrics. (2004) 114:555–76. doi: 10.1542/peds.114.2.S2.555
22. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/BF00280883
23. National Cholesterol Education Program (NCEP). Expert panel on cholesterol levels in children (national cholesterol education program 1992 report of the expert panel on blood cholesterol levels in children and adolescents. Pediatrics. (1992) 89(Suppl):525–84.
24. Kwiterovich PO Jr. Recognition and management of dyslipidemia in children and adolescents. J Clin Endocrinol Metab. (2008) 93:4200–9. doi: 10.1210/jc.2008-1270
25. Sénéchal M, Wicklow B, Wittmeier K, Hay J, MacIntosh A, Eskicioglu P, et al. Cardiorespiratory fitness and adiposity in metabolically healthy overweight and obese youth. Pediatrics. (2013) 132:e85–92. doi: 10.1542/peds.2013-0296
26. Reinehr T1, Wolters B, Knop C, Lass N, Holl RWJ. Strong effect of pubertal status on metabolic health in obese children: a longitudinal study. Clin Endocrinol Metab. (2015) 100:301–8. doi: 10.1210/jc.2014-2674
27. Genovesi S, Antolini L, Giussani M, Pieruzzi F, Galbiati S, Valsecchi MG, et al. Usefulness of waist circumference for the identification of childhood hypertension. J Hypertens. (2008) 26:1563–70. doi: 10.1097/HJH.0b013e328302842b
28. Genovesi S, Antolini L, Giussani M, Brambilla P, Barbieri V, Galbiati S, et al. Hypertension, prehypertension, and transient elevated blood pressure in children: association with weight excess and waist circumference. Am J Hypertens. (2010) 23:756–61. doi: 10.1038/ajh.2010.50
29. Genovesi S, Brambilla P, Giussani M, Galbiati S, Mastriani S, Pieruzzi F, et al. Insulin resistance, prehypertension, hypertension and blood pressure values in paediatric age. J Hypertens. (2012) 30:327–35. doi: 10.1097/HJH.0b013e32834e4aaa
30. Viazzi F, Antolini L, Giussani M, Brambilla P, Galbiati S, Mastriani S, et al. Serum uric acid and blood pressure in children at cardiovascular risk. Pediatrics. (2013) 132:e93–9. doi: 10.1542/peds.2013-0047
31. Viazzi F, Rebora P, Giussani M, Orlando A, Stella A, Antolini L, et al. Increased serum uric acid levels blunt the antihypertensive efficacy of lifestyle modifications in children at cardiovascular risk. Hypertension. (2016) 67:934–40. doi: 10.1161/HYPERTENSIONAHA.115.06852
32. Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. (2007) 120:442–7. doi: 10.1016/j.amjmed.2006.06.040
33. Invitti C, Maffeis C, Gilardini L, Pontiggia B, Mazzilli G, Morabito F, et al. Metabolicsyndromein obese Caucasianchildren: prevalenceusing WHO-derivedcriteria and association with non traditionalcardiovascularriskfactors. Int J Obesity. (2006) 30:627–33. doi: 10.1038/sj.ijo.0803151
34. Cadenas-Sanchez C, Ruiz JR, Labayen I, Huybrechts I, Manios Y, González-Gross M, et al. Prevalence of metabolically healthy but overweight/obese phenotype and its association with sedentary time, physical activity, and fitness. J Adolesc Health. (2017) 61:107–14. doi: 10.1016/j.jadohealth.2017.01.018
35. Li L, Yin J, Cheng H, Wang Y, Gao S, Li M, et al. Identification of genetic and environmental factors predicting metabolically healthy obesity in children: data from the BCAMS study. J Clin Endocrinol Metab. (2016) 101:1816–25. doi: 10.1210/jc.2015-3760
36. Orlando A, Viazzi F, Giussani M, Nava E, Cazzaniga E, Bonino B, et al. Endothelin-1/nitricoxide balance and HOMA index in children with excessweight and hypertension: a pathophysiological model of hypertension. Hypertens Res. (2019) 42:1192–9. doi: 10.1038/s41440-019-0253-3
37. Luordi C, Maddaloni E, Bizzarri C, Pedicelli S, Zampetti S, D'Onofrio L, et al. Wrist circumference is a biomarker of adipose tissue dysfunction and cardiovascular risk in children with obesity. J Endocrinol Invest. (2019) 43:101–7. doi: 10.1007/s40618-019-01086-7
38. Brambilla P, Antolini L, Street ME, Giussani M, Galbiati S, Valsecchi MG, et al. Adiponectin and hypertension in normal-weight and obese children. Am J Hypertens. (2013) 26:257–64. doi: 10.1093/ajh/hps033
39. Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, INTERHEART Study Investigators, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. (2005) 366:1640–9. doi: 10.1016/S0140-6736(05)67663-5
40. Pieruzzi F, Antolini L, Salerno FR, Giussani M, Brambilla P, Galbiati S, et al. The role of blood pressure, body weight and fat distribution on left ventricular mass, diastolic function and cardiac geometry in children. J Hypertens. (2015) 33:1182–92. doi: 10.1097/HJH.0000000000000552
41. Di Bonito P, Miraglia Del Giudice E, Chiesa C, Licenziati MR, Manco M, Franco F, et al. Preclinical signs of liver and cardiac damage in youth with metabolically healthy obese phenotype. Metab Cardiovasc Dis. (2018) 28:1230–6. doi: 10.1016/j.numecd.2018.08.007
42. Dangardt F, Chen Y, Berggren K, Osika W, Friberg P. Increased rate of arterial stiffening with obesity in adolescents: a five-year follow-up study. PLoS ONE. (2013) 8:e57454. doi: 10.1371/journal.pone.0057454
43. Huang LO, Loos RJF, Kilpeläinen TO. Evidence of genetic predisposition for metabolically healthy obesity and metabolically obese normal weight. Physiol Genomics. (2018) 50:169–78. doi: 10.1152/physiolgenomics.00044.2017
Keywords: children, metabolically healthy obesity, Uric Acid, HOMA index, waist-height ratio
Citation: Genovesi S, Antolini L, Orlando A, Gilardini L, Bertoli S, Giussani M, Invitti C, Nava E, Battaglino MG, Leone A, Valsecchi MG and Parati G (2020) Cardiovascular Risk Factors Associated With the Metabolically Healthy Obese (MHO) Phenotype Compared to the Metabolically Unhealthy Obese (MUO) Phenotype in Children. Front. Endocrinol. 11:27. doi: 10.3389/fendo.2020.00027
Received: 25 November 2019; Accepted: 14 January 2020;
Published: 07 February 2020.
Edited by:
Claudio Chiesa, Italian National Research Council, ItalyReviewed by:
Rade Vukovic, The Institute for Health Protection of Mother and Child Serbia, SerbiaCopyright © 2020 Genovesi, Antolini, Orlando, Gilardini, Bertoli, Giussani, Invitti, Nava, Battaglino, Leone, Valsecchi and Parati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simonetta Genovesi, c2ltb25ldHRhLmdlbm92ZXNpQHVuaW1pYi5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.