
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol. , 28 January 2020
Sec. Reproduction
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00008
This article is part of the Research Topic Reproduction and the Inflammatory Response View all 11 articles
The inflammasome is a key regulator of innate immunity involved in the inflammatory response to infections as well as disease through the activation of caspase-1 and the processing of the inflammatory cytokines interleukin (IL)-1β and IL-18. Even though the inflammasome was first described in the context of infections, most research in recent years has focused on targeting the inflammasome as a therapeutic option in sterile inflammatory events. Recent evidence indicates a clear involvement of the inflammasome in Reproductive Biology such as infertility and preeclampsia. In this mini-review, I summarize the current findings on the inflammasome that have been described in the field of Reproductive Biology and highlight the potential that the inflammasome has as a novel therapeutic option in this field. The topics covered in this review as it pertains to the inflammasome field cover the literature published on male and female infertility, endometriosis, preeclampsia, placental inflammation, and reproductive senescence.
The inflammasome is a multiprotein complex with a dual role, one on inflammation and the other one on cell death. The most studied role of the inflammasome involves the activation of the cysteine aspartase caspase-1, resulting in the processing of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 (1). The most recently identified role of the inflammasome is the cell death mechanism of pyroptosis, which involves the cleavage of gasdermin-D and the release, but not activation, of IL-1β (2). The inflammasome is comprised of three basic components: a nucleotide oligomerization domain (NOD)-like receptor (NLR) such as NLRP1, NLRP2, or NLRP3 as well as the adaptor protein known as apoptosis-associated speck-like protein containing a caspase activating recruitment domain (ASC) and the inflammatory cysteine protease caspase-1 (Figure 1).
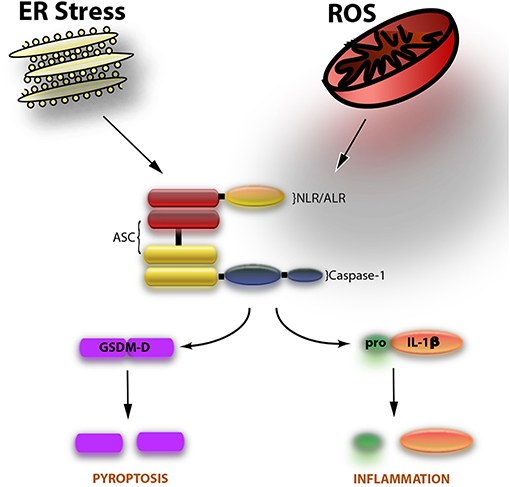
Figure 1. The inflammasome is comprised of caspase-1, ASC, and an NLR such as NLRP1 or NLRP3. Two events involved in the activation of the inflammasome are endoplasmic reticulum (ER) stress and the formation of reactive oxygen species (ROS). Upon activation of the inflammasome, caspase-1 is cleaved. Once cleaved (activated), caspase-1 goes on to cleave the pro-inflammatory cytokine IL-1β to induce inflammation. In addition, the substrate of pyroptosis (inflammasome-mediated cell death) gasdermin-D (GSDM-D) is cleaved. GSDM-D cleavage results in the formation of pores through which IL-1β is then released as well as cell death.
The inflammasome was initially discovered by the late Tschopp and colleagues in 2002 as a multiprotein complex involved in the activation of caspase-1, which is responsible for activating IL-1β and IL-18 (1). Most of the initial studies on the inflammasome started focusing on bacterial infections (3). Then these studies were further extended to the role of inflammasomes in viral (4) and fungal infections (5, 6) as well as autoimmune diseases (7). In the mid 2000s, the first studies on the inflammasome in a sterile event were carried on vitiligo (8) and central nervous system injury (9). Since then, the inflammasome field has started to expand into other indications such as atherosclerosis (10), diabetes (11), nephropathies (12), liver diseases (13), aging (14, 15) as well as in the field of reproductive biology (16, 17), which extent even to the effects of obesity and the inflammatory contribution of the inflammasome to male subfertility (18).
In the context of reproductive biology, the inflammasome has been studied in areas as diverse as female (19) and male infertility (16, 17), fetal growth (20), endometriosis (21), preeclampsia (22), gestational diabetes (23), perinatal depression (24), placental inflammation (25), preterm births (26), and reproductive senescence (27) (Table 1).
Effective fertility requires a fine balance between pro- and anti-inflammatory mediators. Thus, an imbalance in the inflammatory response during fertilization and early embryogenesis dooms the process toward pregnancy failure (31). Witkin and colleagues showed that a polymorphism in the gene encoding for NLRP3 (CIAS1) is associated with female infertility. Interestingly, this polymorphism increased the likelihood of mycoplasma infection-associated female infertility (19). Moreover, another role for NLRP2 was also described for infertility. The NLRP2 inflammasome was first described to be formed in the nervous system (32). In the context of fertility, NLRP2 regulates oocyte quality, which is involved in age-associated fertility loss (33). In addition, a role for NLRP3 in the immune response in the testes has also been described (34). In addition, in the sperm of patients with spinal cord injury, inflammasome proteins are elevated (16), and this increase in inflammasome protein expression is consistent with decrease sperm motility that is improved by inhibition of ASC (16). In a rodent model of spinal cord injury, similar findings have been recently reported (35).
An abnormal imbalance between pro- and anti-inflammatory proteins in the endometrium results in recurrent miscarriages. Inflammatory proteins like tumor necrosis factor, IL-6, IL-10, and interferon-γ are dysregulated in women with recurrent pregnancy loss (36). Thus, highlighting the importance of an adequate pro- to anti-inflammatory milieu. Similarly, significant research has started to be published in the rea of the inflammasome and the endometrium (37). Accordingly, NLRP3, caspase-1, ASC, IL-1β, and IL-18 are increased in the endometrium of women with recurrent pregnancy loss (28). Thus, future therapeutic alternatives that aim to rebalance the pro- to anti-inflammatory milieu in the endometrium should also consider the inflammasome as part of the equation.
A disorder associated with hypertension and proteinuria starting on the 20th week of pregnancy (38), preeclampsia has a significantly heightened inflammatory response in which the inflammasome plays a contributing role (39). In regards to inflammasome regulation in preeclampsia, Weel and colleagues showed that the NLRP3 inflammasome is upregulated, and that it contributes to the damaging effects of inflammation present in preeclampsia (29), a finding that was then corroborated by Stodle et al. who showed that cholesterol and uric acid crystals activated the NLRP3 inflammasome in preeclampsia (30). A similar role for NLRP3 was suggested in a model of nanosilica-induced placental inflammation in rodents, but not for ASC (25). However, ASC is significantly increased in the amniotic fluid of women who undergo spontaneous labor at term (40). More recently, extracellular vesicles (EV) have been shown to activate the inflammasome in trophoblasts, thus promoting preeclampsia (41). Moreover, in women with anti-phospholipid syndrome, NLRP3 and ASC are responsible for placental dysfunction that increases adverse pregnancy outcomes (42). For instance, ASC specks have been detected in choriodecidual leukocytes isolated from women who underwent spontaneous labor at term (43).
In addition, exacerbated inflammation in the placenta is associated with fetal growth restriction (44), and protein levels of caspase-1 and IL-1β were elevated in cytotrophoblasts exposed to uric acid crystals, suggesting that inflammasome activation may contribute to placental inflammation by exposure to uric acid crystals, which are known to be associated with fetal growth restriction, preeclampsia and inflammasome activation. Taken together, these findings indicate a clear role for the inflammasome in preeclampsia and placental inflammation.
Reproductive senescence in females is characterized by heightened inflammation, which makes females more prone to the development of certain diseases. Inflammasome proteins have been shown to be present in EV (45). Interestingly, in reproductive senescent females, EV containing a cargo of inflammasome proteins originate in the female reproductive organs such as the ovaries; EV are then transported through the bloodstream to the nervous system by crossing the blood brain barrier, resulting in inflammasome activation in the brain (27). This heightened inflammasome activation in the brain makes females more susceptible to the damaging effects of central nervous system events such as stroke.
As a result of inflammasome involvement in several indications affecting several organ systems, the inflammasome is well-poised for the development of therapeutic interventions that can improve outcomes in a variety of diseases. Recently, as a result of this tremendous therapeutic potential, Big Pharma and the Biotechnology Industry have garnered special interest in licensing and developing therapeutic interventions that are meant to inhibit the inflammasome in a variety of diseases such as neurodegenerative diseases, liver diseases or gout, among others. The therapeutic potential of the inflammasome is so vast that it has been proven difficult to decide what indication to choose for clinical trials targeting the inflammasome.
Testing therapeutic interventions aimed at inhibiting inflammasome activation is of utmost importance since the ultimate role should be to gain a better mechanistic understanding so that efficient and more specific therapies can be eventually tested in patients. In the field of Reproductive Biology, miR-520c-3p has been shown to inhibit the NLRP3 inflammasome in preeclampsia (46). In addition, the NLRP3 inhibitor MCC950 has been shown to reduce preterm birth by 35.7% and neonatal mortality by 26.7% (47). Similarly, the NLRP3 inflammasome inhibitor glibenclamide also decreases inflammasome activation in human trophoblasts, thus highlighting the therapeutic potential of the NLRP3 inflammasome for the treatment of placental disorders (22).
Moreover, other inflammasomes such as the NLRP1 and AIM2 inflammasomes are also promising targets in this field. For instance, omega-3 fatty acids inhibit NLRP1 and AIM2 inflammasome activation and trophoblast cathepsin S release into the cytosol from lysosomes, thus reducing preterm birth associated with infection and inflammation (48).
Taken together, these findings in the area of Reproductive Biology highlight the important role of the inflammasome, and indicate that therapeutic targeting of the inflammasome is a viable option to treat reproduction-related problems. Current evidence points at NLRP1, NRLP2, NLRP3, AIM2, caspase-1, ASC, and IL-1β as potential targets for therapeutic intervention in this field.
Inflammasome research in the field of Reproductive Biology needs to focus on more mechanistic insights beyond understanding the expression of inflammasome signaling proteins like caspase-1, ASC, and IL-1β (Figure 1). Future research should take a deeper look into the potential mechanisms of inflammasome activation such as extracellular potassium levels (22); the role of oxidative stress on inflammasome activation (49, 50); or whether the inflammasome-mediated process of pyroptosis, or the non-canonical inflammasome pathways, involving caspase-11 in rodents (caspase-4/5 in humans), or caspase-8 are involved in conditions associated with reproduction. To this extent, a recent article has been published showing that hypoxia and endoplasmic reticulum stress activate the NLRP3 inflammasome in primary human trophoblasts, resulting in increased expression of Thioredoxin-interacting protein (TXNIP), a key regulator of inflammasome activation (51). Moreover, these findings were consistent with increased cleavage of caspase-1 and GSDM-D, thus indicating that placental pyroptosis contributes to the systemic release of factors involved in preeclampsia (52).
In conclusion, whether it involves female or male reproductive biology, the data published so far indicate that it is critical to maintain an adequate ratio of pro-inflammatory to anti-inflammatory proteins to increase the possibility of successful reproduction. Thus, targeting the inflammasome to decrease the pro-inflammatory environment is a promising approach, but further research in the area of biomarkers will be useful in gaining a better understanding as to what are the right protein concentrations for relevant pro-inflammatory and anti-inflammatory markers that can be used to help patients with reproductive problems. For instance, one of such studies has been carried looking at increased ASC levels in amniotic fluid obtained from women with clinical chorioamnionitis at term (53). Therefore, further research should focus on mechanistic insights with the goal of developing better therapies and on biomarkers with the goal of diagnosis and monitoring patients once those treatments are tested in clinical trials or delivered to patients in the clinical setting.
JR contributed fully to the writing of this article.
This work was supported by the 2019 Stanley J. Glaser Foundation Research Award to JR and The Miami Project to Cure Paralysis.
JR is a co-founder and managing member of InflamaCORE, LLC and has licensed patents on inflammasome proteins as biomarkers of injury and disease as well as on targeting inflammasome proteins for therapeutic purposes. JR is a Scientific Advisory Board Member for ZyVersa Therapeutics.
1. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. (2002) 10:417–26. doi: 10.1016/S1097-2765(02)00599-3
2. He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. (2015) 25:1285–98. doi: 10.1038/cr.2015.139
3. Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. (2004) 14:1929–34. doi: 10.1016/j.cub.2004.10.027
4. Johnston JB, Barrett JW, Nazarian SH, Goodwin M, Ricciuto D, Wang G, et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. (2005) 23:587–98. doi: 10.1016/j.immuni.2005.10.003
5. Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. (2009) 5:487–97. doi: 10.1016/j.chom.2009.05.002
6. Lev-Sagie A, Prus D, Linhares IM, Lavy Y, Ledger WJ, Witkin SS. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. (2009) 200:303.e301–6. doi: 10.1016/j.ajog.2008.10.039
7. Agostini L, Martinon F, Burns K, Mcdermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. (2004) 20:319–25. doi: 10.1016/S1074-7613(04)00046-9
8. Taieb A. NALP1 and the inflammasomes: challenging our perception of vitiligo and vitiligo-related autoimmune disorders. Pigment Cell Res. (2007) 20:260–2. doi: 10.1111/j.1600-0749.2007.00393.x
9. De Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. (2008) 28:3404–14. doi: 10.1523/JNEUROSCI.0157-08.2008
10. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. (2010) 464:1357–61. doi: 10.1038/nature08938
11. Pontillo A, Brandao L, Guimaraes R, Segat L, Araujo J, Crovella S. Two SNPs in NLRP3 gene are involved in the predisposition to type-1 diabetes and celiac disease in a pediatric population from northeast Brazil. Autoimmunity. (2010) 43:583–9. doi: 10.3109/08916930903540432
12. Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. (2010) 21:1732–44. doi: 10.1681/ASN.2010020143
13. Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. (2009) 119:305–14. doi: 10.1172/JCI35958
14. Mawhinney LJ, De Rivero Vaccari JP, Dale GA, Keane RW, Bramlett HM. Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci. (2011) 12:123. doi: 10.1186/1471-2202-12-123
15. Mejias NH, Martinez CC, Stephens ME, De Rivero Vaccari JP. Contribution of the inflammasome to inflammaging. J Inflamm. (2018) 15:23. doi: 10.1186/s12950-018-0198-3
16. Ibrahim E, Castle SM, Aballa TC, Keane RW, De Rivero Vaccari JP, Lynne CM, et al. Neutralization of ASC improves sperm motility in men with spinal cord injury. Hum Reprod. (2014) 29:2368–73. doi: 10.1093/humrep/deu230
17. Ibrahim E, Lynne CM, Brackett NL. Male fertility following spinal cord injury: an update. Andrology. (2016) 4:13–26. doi: 10.1111/andr.12119
18. Fan W, Xu Y, Liu Y, Zhang Z, Lu L, Ding Z. Obesity or overweight, a chronic inflammatory status in male reproductive system, leads to mice and human subfertility. Front Physiol. (2017) 8:1117. doi: 10.3389/fphys.2017.01117
19. Witkin SS, Bierhals K, Linhares I, Normand N, Dieterle S, Neuer A. Genetic polymorphism in an inflammasome component, cervical mycoplasma detection and female infertility in women undergoing in vitro fertilization. J Reprod Immunol. (2010) 84:171–5. doi: 10.1016/j.jri.2009.11.005
20. Brien ME, Duval C, Palacios J, Boufaied I, Hudon-Thibeault AA, Nadeau-Vallee M, et al. Uric acid crystals induce placental inflammation and alter trophoblast function via an IL-1-dependent pathway: implications for fetal growth restriction. J Immunol. (2017) 198:443–51. doi: 10.4049/jimmunol.1601179
21. Bullon P, Navarro JM. Inflammasome as a key pathogenic mechanism in endometriosis. Curr Drug Targets. (2017) 18:997–1002. doi: 10.2174/1389450117666160709013850
22. Tamura K, Ishikawa G, Yoshie M, Ohneda W, Nakai A, Takeshita T, et al. Glibenclamide inhibits NLRP3 inflammasome-mediated IL-1beta secretion in human trophoblasts. J Pharmacol Sci. (2017) 135:89–95. doi: 10.1016/j.jphs.2017.09.032
23. Lappas M. Activation of inflammasomes in adipose tissue of women with gestational diabetes. Mol Cell Endocrinol. (2014) 382:74–83. doi: 10.1016/j.mce.2013.09.011
24. Leff-Gelman P, Mancilla-Herrera I, Flores-Ramos M, Cruz-Fuentes C, Reyes-Grajeda JP, Garcia-Cuetara Mdel P, et al. The immune system and the role of inflammation in perinatal depression. Neurosci Bull. (2016) 32:398–420. doi: 10.1007/s12264-016-0048-3
25. Shirasuna K, Usui F, Karasawa T, Kimura H, Kawashima A, Mizukami H, et al. Nanosilica-induced placental inflammation and pregnancy complications: different roles of the inflammasome components NLRP3 and ASC. Nanotoxicology. (2015) 9:554–67. doi: 10.3109/17435390.2014.956156
26. Winship A, Dimitriadis E. Interleukin-11 induces preterm birth and modulates decidual inflammasome gene expression in mice. Placenta. (2017) 50:99–101. doi: 10.1016/j.placenta.2017.01.006
27. Raval AP, Martinez CC, Mejias NH, De Rivero Vaccari JP. Sexual dimorphism in inflammasome-containing extracellular vesicles and the regulation of innate immunity in the brain of reproductive senescent females. Neurochem Int. (2019) 127:29–37. doi: 10.1016/j.neuint.2018.11.018
28. D'ippolito S, Tersigni C, Marana R, Di Nicuolo F, Gaglione R, Rossi ED, et al. Inflammosome in the human endometrium: further step in the evaluation of the “maternal side”. Fertil Steril. (2016) 105:111–8.e111-4. doi: 10.1016/j.fertnstert.2015.09.027
29. Weel IC, Romao-Veiga M, Matias ML, Fioratti EG, Peracoli JC, Borges VT, et al. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J Reprod Immunol. (2017) 123:40–7. doi: 10.1016/j.jri.2017.09.002
30. Stodle GS, Silva GB, Tangeras LH, Gierman LM, Nervik I, Dahlberg UE, et al. Placental inflammation in pre-eclampsia by Nod-like receptor protein (NLRP)3 inflammasome activation in trophoblasts. Clin Exp Immunol. (2018) 193:84–94. doi: 10.1111/cei.13130
31. Dahm-Kahler P, Ghahremani M, Lind AK, Sundfeldt K, Brannstrom M. Monocyte chemotactic protein-1 (MCP-1), its receptor, and macrophages in the perifollicular stroma during the human ovulatory process. Fertil Steril. (2009) 91:231–9. doi: 10.1016/j.fertnstert.2007.07.1330
32. Minkiewicz J, De Rivero Vaccari JP, Keane RW. Human astrocytes express a novel NLRP2 inflammasome. Glia. (2013) 61:1113–21. doi: 10.1002/glia.22499
33. Kuchmiy AA, D'hont J, Hochepied T, Lamkanfi M. NLRP2 controls age-associated maternal fertility. J Exp Med. (2016) 213:2851–60. doi: 10.1084/jem.20160900
34. Walenta L, Schmid N, Schwarzer JU, Kohn FM, Urbanski HF, Behr R, et al. NLRP3 in somatic non-immune cells of rodent and primate testes. Reproduction. (2018) 156:231–8. doi: 10.1530/REP-18-0111
35. Nikmehr B, Bazrafkan M, Hassanzadeh G, Shahverdi A, Sadighi Gilani MA, Kiani S, et al. The correlation of gene expression of inflammasome indicators and impaired fertility in rat model of spinal cord injury: a time course study. Urol J. (2017) 14:5057–63. doi: 10.22037/uj.v14i6.4085
36. Banerjee P, Jana SK, Pasricha P, Ghosh S, Chakravarty B, Chaudhury K. Proinflammatory cytokines induced altered expression of cyclooxygenase-2 gene results in unreceptive endometrium in women with idiopathic recurrent spontaneous miscarriage. Fertil Steril. (2013) 99:179–87. doi: 10.1016/j.fertnstert.2012.08.034
37. Di Nicuolo F, Specchia M, Trentavizi L, Pontecorvi A, Scambia G, Di Simone N. An emerging role of endometrial inflammasome in reproduction: new therapeutic approaches. Protein Pept Lett. (2018) 25:455–62. doi: 10.2174/0929866525666180412160045
38. De Oliveira LG, Karumanchi A, Sass N. Preeclampsia: oxidative stress, inflammation and endothelial dysfunction. Rev Bras Ginecol Obstet. (2010) 32:609–16. doi: 10.1590/S0100-72032010001200008
39. Cheng SB, Sharma S. Preeclampsia and health risks later in life: an immunological link. Semin Immunopathol. (2016) 38:699–708. doi: 10.1007/s00281-016-0579-8
40. Panaitescu B, Romero R, Gomez-Lopez N, Xu Y, Leng Y, Maymon E, et al. In vivo evidence of inflammasome activation during spontaneous labor at term. J Matern Fetal Neonatal Med. (2019) 32:1978–91. doi: 10.1080/14767058.2017.1422714
41. Kohli S, Ranjan S, Hoffmann J, Kashif M, Daniel EA, Al-Dabet MM, et al. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood. (2016) 128:2153–64. doi: 10.1182/blood-2016-03-705434
42. Mulla MJ, Salmon JE, Chamley LW, Brosens JJ, Boeras CM, Kavathas PB, et al. A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1beta production by human first trimester trophoblast. PLoS ONE. (2013) 8:e65237. doi: 10.1371/journal.pone.0065237
43. Gomez-Lopez N, Romero R, Xu Y, Garcia-Flores V, Leng Y, Panaitescu B, et al. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. Am J Reprod Immunol. (2017) 77:e12648. doi: 10.1111/aji.12648
44. Hulthen Varli I, Petersson K, Kublickas M, Papadogiannakis N. Both acute and chronic placental inflammation are overrepresented in term stillbirths: a case-control study. Infect Dis Obstet Gynecol. (2012) 2012:293867. doi: 10.1155/2012/293867
45. De Rivero Vaccari JP, Brand F III, Adamczak S, Lee SW, Perez-Barcena J, Wang MY, et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. (2016) 136(Suppl. 1):39–48. doi: 10.1111/jnc.13036
46. Liu Z, Zhao X, Shan H, Gao H, Wang P. microRNA-520c-3p suppresses NLRP3 inflammasome activation and inflammatory cascade in preeclampsia by downregulating NLRP3. Inflamm Res. (2019) 68:643–54. doi: 10.1007/s00011-019-01246-8
47. Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, et al. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomesdagger. Biol Reprod. (2019) 100:1306–18. doi: 10.1093/biolre/ioy264
48. Chen CY, Chen CY, Liu CC, Chen CP. Omega-3 polyunsaturated fatty acids reduce preterm labor by inhibiting trophoblast cathepsin S and inflammasome activation. Clin Sci. (2018) 132:2221–39. doi: 10.1042/CS20180796
49. Shirasuna K, Takano H, Seno K, Ohtsu A, Karasawa T, Takahashi M, et al. Palmitic acid induces interleukin-1beta secretion via NLRP3 inflammasomes and inflammatory responses through ROS production in human placental cells. J Reprod Immunol. (2016) 116:104–12. doi: 10.1016/j.jri.2016.06.001
50. Nunes PR, Peracoli MTS, Romao-Veiga M, Matias ML, Ribeiro VR, Da Costa Fernandes CJ, et al. Hydrogen peroxide-mediated oxidative stress induces inflammasome activation in term human placental explants. Pregnancy Hypertens. (2018) 14:29–36. doi: 10.1016/j.preghy.2018.07.006
51. Yang Y, Li J, Han TL, Zhou X, Qi H, Baker PN, et al. Endoplasmic reticulum stress may activate NLRP3 inflammasomes via TXNIP in preeclampsia. Cell Tissue Res. (2019). doi: 10.1007/s00441-019-03104-9. [Epub ahead of print].
52. Cheng SB, Nakashima A, Huber WJ, Davis S, Banerjee S, Huang Z, et al. Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death Dis. (2019) 10:927. doi: 10.1038/s41419-019-2162-4
Keywords: inflammasome, fertility, inflammation, caspase-1, reproduction
Citation: de Rivero Vaccari JP (2020) The Inflammasome in Reproductive Biology: A Promising Target for Novel Therapies. Front. Endocrinol. 11:8. doi: 10.3389/fendo.2020.00008
Received: 09 September 2019; Accepted: 07 January 2020;
Published: 28 January 2020.
Edited by:
John Even Schjenken, University of Adelaide, AustraliaReviewed by:
Alessandro Conforti, University of Naples Federico II, ItalyCopyright © 2020 de Rivero Vaccari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Pablo de Rivero Vaccari, amRlcml2ZXJvQG1lZC5taWFtaS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.