
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Endocrinol. , 22 October 2019
Sec. Cancer Endocrinology
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00713
 Lin Lu1†
Lin Lu1† Qingqing Meng1,2†
Qingqing Meng1,2† Xiaoping Xing1*
Xiaoping Xing1* Tao Yuan1
Tao Yuan1 Huabing Zhang1
Huabing Zhang1 Naishi Li1
Naishi Li1 Yining Wang3
Yining Wang3 Yuejuan Cheng4
Yuejuan Cheng4 Chunmei Bai4*
Chunmei Bai4* Hao Wang5
Hao Wang5 Xin Cheng5
Xin Cheng5 Yu Xiao6
Yu Xiao6 Boju Pan6
Boju Pan6 Yuan Li6
Yuan Li6 Jian Sun6
Jian Sun6 Zhiyong Liang6
Zhiyong Liang6 Huijuan Zhu1
Huijuan Zhu1 Renzhi Wang7
Renzhi Wang7 Zhaolin Lu1
Zhaolin Lu1Ectopic adrenocorticotropic hormone (ACTH) syndrome (EAS) is a rare cause of Cushing syndrome. If routine imaging examinations cannot identify the source of ACTH production, long-term follow-up observation is necessary to determine the etiology. We present the case of a middle-aged male with gradual weight gain and a Cushingoid appearance over 4 years; he provided written informed consent. Laboratory and endocrine tests strongly suggested EAS, although the origin was not detected by multiple imaging methods. Bilateral adrenalectomy was performed to prevent severe complications in the patient. Two and a half years later, a cardiac mass 18 × 23 × 27 mm in size at the junction between the anterior wall of the left ventricle and the middle septum was found together with multiple bone metastases by 18F-FDG PET/CT, while the 68Ga-DOTATE PET/CT findings were negative. Biopsy of the lumbar vertebrae revealed a neuroendocrine tumor (NET) with positive ACTH staining. The patient underwent chemotherapy by CAPTEM, resulting in shrinkage of the cardiac mass and a significant decrease in the ACTH level. In the case of EAS with an unusual cause, long-term follow-up observation is necessary to determine the source of ACTH production. Cardiac NETs are quite rare in EAS, so treatment selection was also challenging. CAPTEM chemotherapy proved effective in controlling the progression of tumor growth and decreasing the ACTH level in this patient.
Cushing syndrome (CS) is a rare disease accompanied by hypercortisolemia due to various causes. It is necessary to diagnose CS as early as possible to prevent the occurrence of fatal complications. Pituitary adrenocorticotropic hormone (ACTH)-secreting adenoma and ectopic ACTH syndrome (EAS) account for the etiology of CS in 70% and 10–20% of cases, respectively (1). The most commonly used dexamethasone suppression test (DST) cannot completely distinguish between the two conditions, with a false-negative rate of approximately 20%. Bilateral inferior petrosal sinus sampling (BIPSS) has been considered the gold standard for detecting the origin of ACTH. Bronchial and mediastinal ACTH-producing carcinoid tumors are the most common causes of EAS, representing nearly 90% of all EAS cases. Here, we report a very rare case of EAS caused by a neuroendocrine tumor (NET) that possibly originated in the heart with bone metastasis.
The study protocol was approved by the Ethics Committees of the Peking Union Medical College Hospital, Beijing, and written informed consent was obtained from the patient for the publication of this case report.
A 35 year-old Chinese man was admitted to Peking Union Medical College Hospital in April 2012 with complaints of weight gain and a round and red face for 4 years. The patient denied any underlying medical condition, such as hypertension, diabetes and heart disease. There was no significant personal or family history. There were no inherited diseases in his family. His blood pressure was 170/120 mmHg on admission. The laboratory workup showed hypokalemia (serum potassium, 2.5 mmol/l) and elevated morning plasma total cortisol (37.04 μg/dl; normal range, 4–22 μg/dl), morning plasma ACTH (235.0 pg/ml; normal range, 0–46 pg/ml) and 24 h urinary free cortisol (1,136.39 μg; normal range, 12–103 μg) levels. High-dose DST was not suppressible. There were no detectable tumors on dynamic enhanced MRI of the sella or CT of the chest, abdomen or pelvis. BIPSS with the desmopressin stimulation test did not show an ACTH concentration gradient. Therefore, EAS was highly suspected. Further imaging evaluations, including somatostatin receptor scanning, 18F-FDG PET/CT and 68Ga-DOTATE PET/CT, showed negative findings. Meanwhile, pulmonary infection and hypoxemia aggravated rapidly. Considering the rapid progression of illness and the unclear origin of the ectopic ACTH, bilateral adrenalectomy was performed in August 2013 immediately to improve the life-threatening condition. Subsequently, the patient's general condition significantly improved.
The patient participated in regular follow-up examinations annually. His Cushingoid symptoms recovered gradually. The patient's blood pressure and hypokalemia returned to normal. The patient's weight decreased obviously, with disappearance of the roundness and redness of the face. However, his skin color incrementally darkened. Annual chest, abdominal and pelvic CT and somatostatin receptor scanning did not identify any tumors during 2014 and 2015. By the end of 2015, his skin color had darkened to a great extent (Figures 1A–C). His morning ACTH level had increased to over 1,250 pg/ml (Figure 2). In January 2016, although the results of somatostatin receptor scanning were still negative, 18F-FDG PET/CT revealed abnormal, high uptake in the anterior wall of the left ventricle near the pericardium, as well as in the sacral region, with osteolytic change. Ultrasonic cardiography and enhanced cardiac CT showed thickening of the aortic root and the left ventricular wall. Furthermore, bone scans revealed abnormal lesions in several regions, including the left occipital bone, right sacroiliac joint, spine and right shoulder joint, indicating multiple bone metastases. Cardiac MRI revealed a mass 18 × 23 × 27 mm in size at the junction between the anterior wall of the left ventricle and the middle septum, involving the adjacent myocardium and pericardium (Figure 3A). This suspicious cardiac lesion was considered a very likely source of ACTH production. However, the patient and family refused cardiac biopsy considering the high risk of the procedure. In November 2016, cardiac MRI revealed a slightly enlarged, irregular cardiac lesion (20 × 24 × 28 mm) at the same location (Figure 3B). Biopsy of the lumbar vertebrae was performed, and the pathological results showed neuroendocrine tumor (NET) infiltration in the bone and bone marrow. The tumor cells were well-differentiated, and mitosis and necrosis were frequent (Figure 3D). The tumor cells showed positive staining for ACTH, chromogranin A and synaptophysin (Figures 3E–G), as well as cytokeratin. The Ki-67 index was 20% (Figure 3H). Since the patient had no evidence of detectable lesions apart from the cardiac mass and multiple bone metastases after long-term follow-up observation, it was strongly suspected that his cardiac lesion was a primary ACTH-secreting, high-grade NET.

Figure 1. Hyperpigmentation of the patient's skin (A), knuckles and nails (B), and palms (C) was photographed in January 2016.
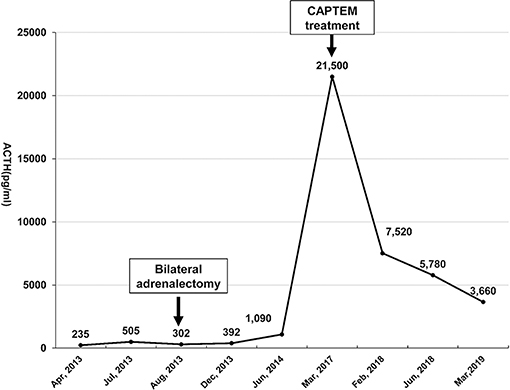
Figure 2. The morning serum ACTH level was monitored from April 2013 to March 2019. Bilateral laparoscopic adrenalectomy was performed in August 2013. Note that his ACTH level increased to over 1,250 pg/mL after January 2015, with the highest level reaching 21,500 pg/ml. However, the ACTH level dropped significantly after the patient underwent CAPTEM treatment. The last follow-up test indicated that the ACTH level dropped to 3,660 pg/ml in March 2019.
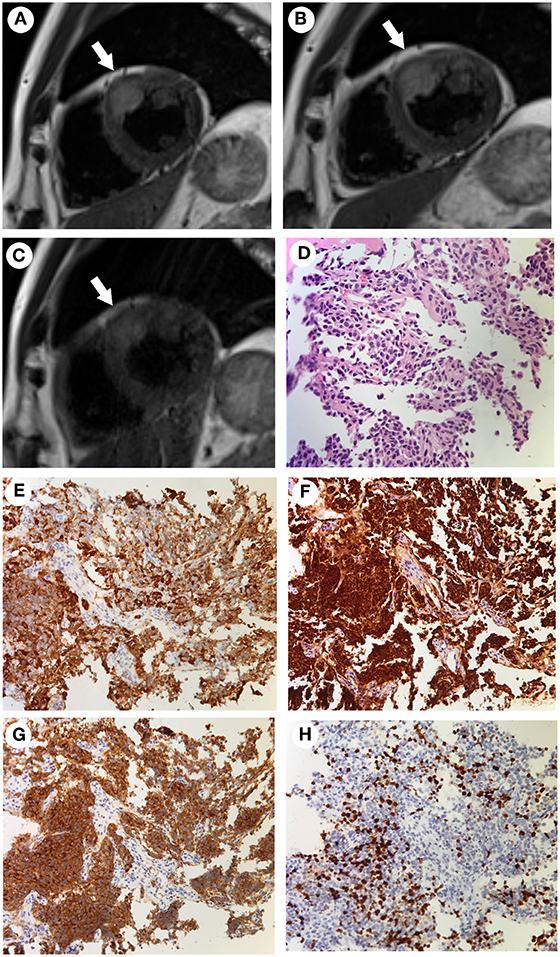
Figure 3. (A–C) Cardiac MRI findings acquired at different time points. The tumor was located at the junction between the anterior wall of the left ventricle and the middle septum (arrows). The tumor was 18 × 23 × 27 mm in size when initially found in January 2016 (A). It had increased in size to 20 × 24 × 28 mm in November 2016 (B). After combined chemotherapy with capecitabine/temozolomide for 12 months, the tumor size was 16 × 22 × 22 mm in January 2018 and remained stable to March 2019 (C). (D–H) The pathological findings of the bone biopsy of the metastatic vertebrae. Hematoxylin and eosin (HandE) staining showed an invasive tumor cell growth pattern, with abundant eosinophilic cytoplasm and finely granular nuclear chromatin. Mitosis and necrosis were frequent (D). Positive immunohistochemistry staining for ACTH (E), chromogranin A (F), and synaptophysin (G). The Ki-67 index was approximately 20% (H) (Magnification, ×200).
The challenging location of the cardiac lesion made surgical resection very risky. After consultation with a multidisciplinary team, it was decided to treat the patient with combined oral chemotherapy with a CAPTEM (capecitabine/temozolomide) regimen (orally taking capecitabine 2.5 g/day on the 1st to 14th day and temozolomide 300 mg/d on the 10th to 15th day of each month, followed by discontinuation until the next cycle). Beginning in March 2017 to March 2019, he underwent 24 cycles of chemotherapy without any evidence of further progression of the cardiac lesion (Figure 3C). During the periods medication was administered, he developed the side effects of mild bloating, nausea, and vomiting, which could be tolerated without medical treatment. The above symptoms improved during the periods medication was not administered. There were no other side effects, such as skin rash, bone marrow inhibition and abnormal liver function and renal function. Cardiac MRI indicated shrinkage of the cardiac lesion to 16 × 22 × 22 mm after 8 cycles of chemotherapy, and the bone scan indicated stable conditions. In addition, the ACTH level dropped from 21,500 pg/ml (before CAPTEM treatment) to 3,660 pg/ml. Both the image and hormone test results indicated a partial response to CAPTEM chemotherapy.
EAS is the most challenging etiology in the management of CS. Among tumors in EAS, the prevalent types are bronchial carcinoid tumors (5–40%), thymic carcinoid tumors (5–42%), small cell lung carcinoma (3.3–50%), pancreatic carcinoid tumors (7.5–25%) and pheochromocytoma (2.5–25%) (1, 2). Based on previous publications, no cases of EAS due to a cardiac NET, either primary or metastatic, have been reported thus far.
Cardiac NETs are rarely documented, and most of them are metastatic. According to the current literature, few cases of primary cardiac NETs have been reported (3), and nearly all reported metastatic cases have origins in the gastrointestinal tract, lungs or mediastinum (4). It is noteworthy that cardiac paragangliomas (PGLs), which could arise from paraganglia located at the base of the heart and atria, are another common type of cardiac endocrine tumor (5, 6). However, cardiac NETs and PGLs can be differentiated under the microscope by the main components of PGLs, including clusters of chief cells and characteristic sustentacular cells (7). In addition, the positive staining for cytokeratin in this case ruled out the diagnosis of PGL. In the present case, metastatic high grade NETs with positive ACTH staining were confirmed by pathology. Furthermore, there was no evidence of the primary origin of the metastatic bone NETs other than the cardiac lesion after 6 years of follow-up observation. Therefore, a primary cardiac ACTH-secreting NET and a metastatic cardiac NET of unknown origin were considered highly as possibilities.
During imaging examinations to detect the EAS source, the cardiac lesion was not detected until 2.5 years after adrenalectomy by 18F-FDG PET/CT. Therefore, the lack of sensitive imaging methods for the early detection of cardiac NETs may have resulted in a delayed diagnosis. It is not uncommon that some ectopic ACTH-producing NETs are such latent tumors that are quite difficult to detect during the onset of the disease. It is speculated that the deficiency of negative feedback to ACTH production by ectopic NETs after bilateral adrenalectomy may promote tumor proliferation. On the other hand, the previously occult lesion gradually progressed and could be detected by imaging examinations as the disease duration increased. Considering the special location of the lesion, surgery was quite risky for this patient. In recent years, the CAPTEM regimen has been shown to be highly effective and well-tolerated for the management of both well-differentiated and metastatic NETs (8–10). Finally, the CAPTEM regimen was administered to the patient. After this treatment, some shrinkage of the cardiac mass was observed, and the ACTH level dropped 83.0%. Thus, CAPTEM treatment had a beneficial effect on the patient.
In conclusion, cardiac ACTH-producing NETs, either primary or metastatic, are extremely rare. In the beginning, with combined imaging methods, including CT, MRI, somatostatin receptor scanning and PET/CT, the exact location of the cardiac NET remained undetected, leading to a delay in the diagnosis until multiple bone metastases had occurred. After nearly 24 cycles of chemotherapy with the CAPTEM regimen, slight shrinkage of the cardiac mass, no progression of the metastatic bone lesions and significant improvement in the ACTH level were observed. Additionally, the patient tolerated the treatment well. Thus, considering the possibility of disease progression and other distant metastases, lifelong follow-up observation is needed to monitor the disease in this case.
The study protocol was approved by the Ethics Committees of the Peking Union Medical College Hospital, Beijing, and the participant provided written informed consent.
LL, QM, XX, CB, YC, and YW carried out the studies, participated in collecting the data, and drafted the manuscript. YX, JS, BP, YL, and ZLi participated in the pathological research. TY, HZha, NL, XC, and HW performed the statistical analysis and participated in its design. HZhu, RW, and ZLu helped to draft the manuscript. All authors have read and approved the final manuscript.
This study was supported by the Chinese Academy of Medical Science Innovation Fund for Medical Science (CAMS-2017-I2M-1-011), the Nonprofit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2017PT32020, No. 2018PT32001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to express our deep thanks to Dr. Richard Auchus (Department of MEND, University of Michigan, Michigan, USA), who provided us with important suggestions for the management of this patient.
1. Alexandraki KI, Grossman AB. The ectopic ACTH syndrome. Rev Endocr Metab Disord. (2010) 11:117–26. doi: 10.1007/s11154-010-9139-z
2. Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab. (2005) 90:4955–62. doi: 10.1210/jc.2004-2527
3. Guajardo-Salinas GE, Anaya-Ayala JE, Rice DC, Moran CA, Reardon MJ. Primary high-grade neuroendocrine carcinoma of the heart. Tex Heart Inst J. (2013) 40:71–4.
4. Jann H, Wertenbruch T, Pape U, Ozcelik C, Denecke T, Mehl S, et al. A matter of the heart: myocardial metastases in neuroendocrine tumors. Horm Metab Res. (2010) 42:967–76. doi: 10.1055/s-0030-1267204
5. Burke A, Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol. (2016) 11:441–52. doi: 10.1016/j.jtho.2015.11.009
6. Patel J, Sheppard MN. Pathological study of primary cardiac and pericardial tumours in a specialist UK Centre: surgical and autopsy series. Cardiovasc Pathol. (2010) 19:343–52. doi: 10.1016/j.carpath.2009.07.005
7. Laudenschlager M, Nykamp V, Allard B, Japs R, Simmons J. Cardiac neuroendocrine tumor with absence of sustentacular cells: immunohistochemical and ultrastructural findings. Ultrastruct Pathol. (2012) 36:130–3. doi: 10.3109/01913123.2011.626890
8. Kotteas EA, Syrigos KN, Saif MW. Profile of capecitabine/temozolomide combination in the treatment of well-differentiated neuroendocrine tumors. Onco Targets Ther. (2016) 9:699–704. doi: 10.2147/OTT.S72155
9. Ramirez RA, Beyer DT, Chauhan A, Boudreaux JP, Wang YZ, Woltering EA. The role of capecitabine/temozolomide in metastatic neuroendocrine tumors. Oncologist. (2016) 21:671–5. doi: 10.1634/theoncologist.2015-0470
Keywords: Cushing syndrome, ACTH syndrome, ectopic, cardiac neoplasms, neuroendocrine tumor, chemotherapy
Citation: Lu L, Meng Q, Xing X, Yuan T, Zhang H, Li N, Wang Y, Cheng Y, Bai C, Wang H, Cheng X, Xiao Y, Pan B, Li Y, Sun J, Liang Z, Zhu H, Wang R and Lu Z (2019) Long-Term Follow-Up of Significant Improvement After CAPTEM Treatment for Rare Adrenocorticotropin-Producing Cardiac Neuroendocrine Tumor. Front. Endocrinol. 10:713. doi: 10.3389/fendo.2019.00713
Received: 29 April 2019; Accepted: 02 October 2019;
Published: 22 October 2019.
Edited by:
Wen Zhou, Case Western Reserve University, United StatesReviewed by:
Dario Giuffrida, Mediterranean Institute of Oncology (IOM), ItalyCopyright © 2019 Lu, Meng, Xing, Yuan, Zhang, Li, Wang, Cheng, Bai, Wang, Cheng, Xiao, Pan, Li, Sun, Liang, Zhu, Wang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoping Xing, eGluZ3hwMjAwNkAxMjYuY29t; Chunmei Bai, YmFpY2h1bm1laTE5NjRAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.