
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Endocrinol., 11 October 2019
Sec. Reproduction
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00685
This article is part of the Research TopicNew Aspects in HypogonadismView all 10 articles
Nearly two decades have passed since the release of Women's Health Initiative (WHI) postmenopausal hormone therapy trial findings, yet the medical community, and general public remain unsettled by ongoing debate over the benefits and safety of sex hormone replacement therapy (HRT). Among the contentious issues is the elevated risk of venous thromboembolism (VTE) and stroke observed in HRT users (1). While major guidelines rightly recommend the use of transdermal estradiol in women with risk factors, little attention has been given to the potential impact of the type of estrogen molecule. This review aims to highlight the importance of selecting appropriate estrogen therapy to enhance safety.
Hypogonadism in women of pre-menopausal age group is more frequent than is commonly anticipated; spontaneous or autoimmune primary ovarian insufficiency affects ~1% of the female population, and an estimated 5% experience early menopause prior to age 45 (2). Other important causes of premature estrogen deficiency include congenital conditions such as Turner syndrome and Kallmann syndrome, as well as surgical oophorectomy. For these patients, HRT is a well-established endocrine treatment aimed to replace estrogen physiologically until at least the average age of menopause. Untreated individuals are at substantial risk of sexual dysfunction, genitourinary symptoms, accelerated bone loss, vasomotor symptoms, and coronary heart disease (CHD) (3).
Similarly, in the management of postmenopausal women suffering from climacteric syndrome, estrogen is unequivocally more efficacious compared to non-estrogen-based pharmacological treatments, and plays a crucial role in holistic menopause management particularly in those with impaired quality of life from persistent vasomotor symptoms.
Unfortunately, many healthcare providers and patients became resistant to the use of HRT in the aftermath of WHI. Not only are menopausal women in their 50s and those with vasomotor symptoms unnecessarily deprived of HRT (4), there are also worrying signs of under-treatment among young hypogonadal women; a recent Swedish report on women with central hypogonadism found that at least half of the cohort failed to receive adequate replacement during their estrogen-deficient premenopausal years, placing them at heightened risk of complications in the ensuing years (5).
Prior to the landmark WHI trial, several large-scale observational studies were actually in favor of HRT's protective effects, as treated women were found to be at lower risk of CHD and mortality (6). To substantiate these observations, WHI postmenopausal hormone trials set out to investigate HRT in women aged 50–79 years; participants randomized to intervention arms received either conjugated equine estrogen (CEE) 0.625 mg alone (absent uterus) or with cyclical medroxyprogesterone acetate (MPA) 2.5 mg (intact uterus). Not only did WHI unexpectedly fail to demonstrate cardiovascular benefits, a disconcerting increase in incidence of breast cancer, stroke and VTE in treatment arms led to the premature closure of study after a median follow-up of 5.6 years (7).
Around the same time, the Heart and Estrogen/Progestin Replacement Study (HERS) trial also reported neutral effect of HRT (CEE+MPA vs. placebo) on CHD risk along with increased VTE events (8). Since then, the “timing hypothesis” has been widely proposed to explain the discordance in observational and trial findings because, unlike in typical clinical settings where most patients considered for HRT are early post-menopausal, the average age at which WHI subjects were initiated on HRT was 63.3 years (9). Indeed, post-hoc analyses showed better outcomes including reduction in CHD risk in subgroups of age <60 or <10 years from the time of menopause (10), with corroborative CT evidence of lower coronary calcified-plaque burden compared to placebo arm (11).
Aside from age factor, the type of estrogen therapy should also be carefully considered in HRT decision-making. It is imperative that estrogen products with the greatest safety margins be selected. However, this is an aspect that has often been overlooked in HRT guidance, with results of WHI/HERS often being inappropriately applied to all estrogen formulations. As will be elaborated further, non-physiological estrogenic compounds—by virtue of having greater propensity in inducing prothrombogenic state across ages—should be avoided in patients prescribed HRT.
There are three main types of estrogen formulations available for therapeutic purposes, namely 17β-estradiol (E2), ethinylestradiol (EE) and CEE. The former (available in oral and transdermal formulations) is the predominant endogenous human estrogen. To overcome its poor oral bioavailability (<10%), E2 is typically esterified or micronized; pro-drug esters, such as estradiol valerate and estradiol acetate, undergo hydrolysis rapidly following absorption to release E2 into the systemic circulation, while the microcrystalline structure of micronised estradiol (principally as estradiol hemihydrate) facilitates accelerated absorption by its larger compound surface area and thus minimizing first-pass metabolism (12). Conversely, transdermal application of E2, which has moderate skin permeability, avoids first-pass effect, and hence generates an E2:E1 (estrone, a metabolite of E2) profile similar to normal physiology, whereas E1 concentrations are higher after oral E2 administration (13). However, the weak potency of E1 does not have significant impact on the overall estrogenic bioactivity (14).
In contrast, CEE and EE are non-physiological because of their different molecular structure and properties. EE—a near-universal component of combined oral contraceptives (COCs)—is a potent synthetic E2 analog with a 17α-ethinyl substitution that binds to estrogen receptors α and β with high affinity, prevents the oxidation of the 17β-hydroxy group, as well as irreversibly inhibits CYP enzymes involved in the metabolism of steroids, resulting in a very reactive intermediate (12). CEEs are urine derivatives from pregnant horses and is a complex mix of numerous estrogenic compounds with varying receptor affinity, pharmacokinetics and biologic potency, as well as other non-estrogenic steroids with unknown effects (12). Additionally, both EE and CEE have considerably greater hepatic stimulatory effect, altering the synthesis of various proteins including angiotensinogen, SHBG and coagulation factors.
Given these fundamental pharmacological differences, biological effects are expected to be dissimilar, and hence the adverse effects observed in older trials employing non-physiological estrogen would not be generalisable to all estrogen formulations. Indeed, emerging data are demonstrating comparatively greater safety and efficacy associated with E2 use.
In a population-based, case-control study of ~400 postmenopausal women aged 30–79 years using oral hormone therapy, CEE use was significantly associated with increased venous thrombosis risk (odds ratio 2.08) and a trend toward increased myocardial infarction (MI) risk when compared with E2 (15). Further investigations demonstrated a higher endogenous thrombin potential-based normalized activated protein C (APC) sensitivity ratio as one of the mechanisms for the elevated clotting propensity observed in CEE users. This is in line with a recent large UK observational study of general female population aged 40–79 which found that among oral HRT, CEE(+MPA) had the greatest risk while E2(+dydrogesterone) had the lowest risk (16). Likewise, Danish Osteoporosis Prevention Study showed no evidence of increased thrombotic or stroke risk in women with recent menopause onset who received E2(± norethisterone) replacement and followed for up to 16 years (17). Moreover, a significant reduction in combined end-point of mortality and hospitalisations for congestive heart failure or MI was demonstrated.
Similarly, recent HRT intervention trials in younger women with premature ovarian failure have reported encouraging data with E2 therapy (Table 1). Improvement in BMD, particularly at lumbar spine, was consistently observed across studies, with E2 demonstrating superiority to COCs (19, 21). Furthermore, E2 has beneficial effects on several cardiovascular and uterine parameters, which could have far-reaching impact on long-term cardiovascular health and possibly fertility treatment outcomes, respectively (20, 22). These studies also provided evidence for dose titration to achieve physiological serum E2 levels (21, 23). More importantly, both oral and transdermal E2 therapy were safe and well-tolerated in these trials.
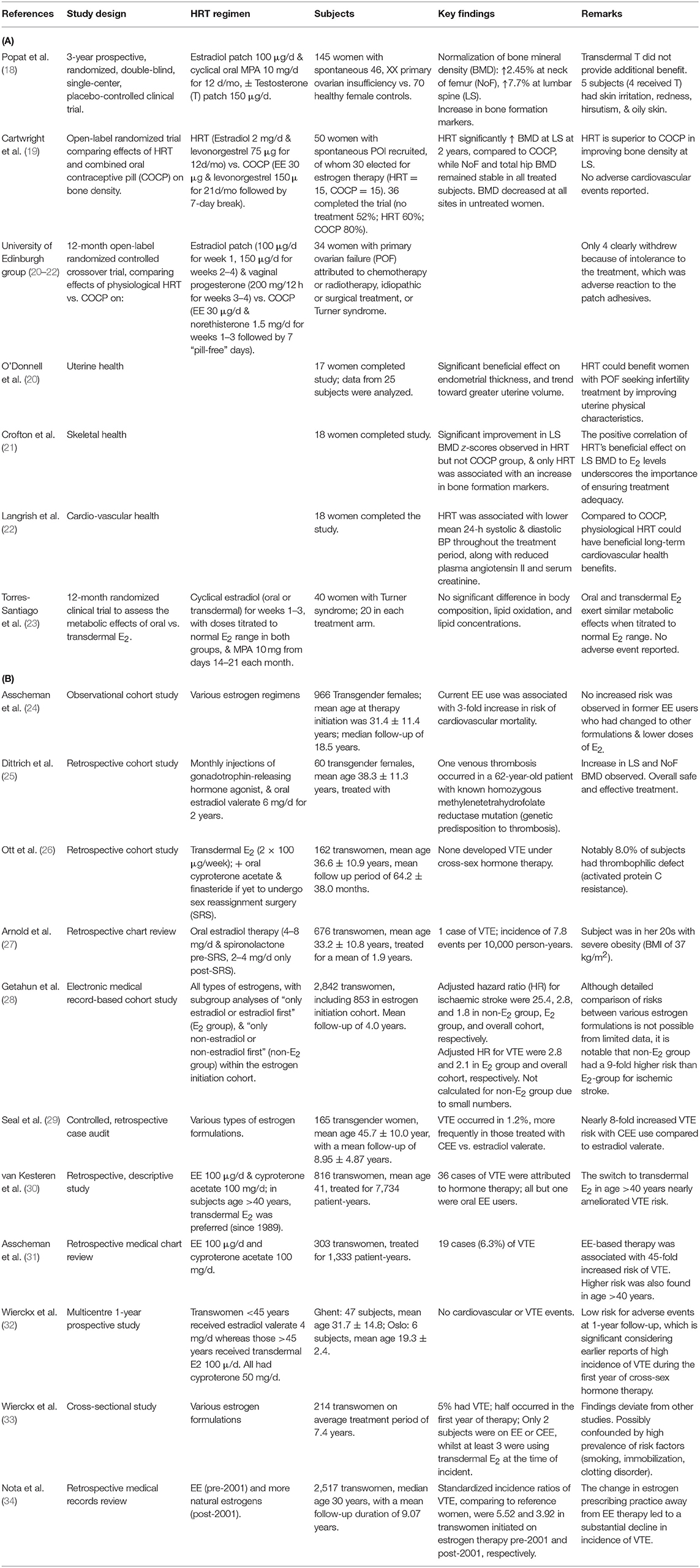
Table 1. Summary of hormone therapy studies in (A) young women with premature ovarian failure (recent HRT trials), and (B) transgender females (retrospective & cross-sectional studies).
Despite that, the current evidence base remains disproportionately influenced by older randomized controlled trials which employed non-physiological estrogens, with little regards for their differential effects. In a recent Cochrane review examining the risk of cardiovascular events in HRT trials, WHI and HERS—both of which employed CEE in intervention arms—accounted for 79%(425/540) of stroke, 88%(312/353) of VTE, and 90%(149/166) of pulmonary embolism events (35). That would inevitably serve to confuse clinicians with a skewed picture of HRT-associated risks being presented. More clarity is certainly needed.
Furthermore, most guidelines on the management of female hypogonadism (e.g., NICE 2017) continue to list COCs as reasonable replacement therapy—with the exception of those for Congenital Hypogonadotropic Hypogonadism, which only recommend E2-based HRT (36). In contrast, WHO guidance for the treatment of male hypogonadism has long emphasized that only native testosterone should be prescribed, rather than synthetic androgens. Similarly, HRT prescribing practices in transgender medicine have also evolved over the past 2–3 decades following accumulating data of the significantly lower risk with E2 therapy compared to EE/CEE. Another commonality between guidance for androgen replacement in males and E2 replacement in trans-females is that it emphasizes the importance of monitoring serum sex hormone with the aim of achieving physiological levels. By contrast, guidance for both young hypogonadal women and older post-menopausal women do not recommend biochemical monitoring (37).
For individuals receiving cross-sex hormone treatment, the major goal is to suppress endogenous sex hormone levels and thus reduce biological secondary sexual characteristics, and to replace sex hormone levels consistent with those of the affirmed sex. Importantly, there are no fundamental sex differences in response to sex steroids, and the principles of treatment are very similar to that of HRT in hypogonadal patients. Hence such data are wholly applicable to cis-gender patients.
In 1989, a key publication from a major center in Netherlands on the estrogen treatment outcomes in transwomen reported an alarming 45-fold increase in risk of VTE with EE compared with cisgender controls (31). This finding triggered a change in the treatment protocol to switch patients of age >40 years to transdermal E2 in order to lower VTE risk. Although that led to an overall reduction in adverse events, the VTE risk remained high at 20-fold, largely because EE was still being used by a significant proportion of entire cohort, albeit at lower doses (30). It was also concerning that a 3-fold increased risk of cardiovascular mortality was found to be independently associated with long-term users of EE, consistent with the deleterious effects on haemostatic cascade induced by EE (24). Similarly, CEE proved to be greatly unsafe, with an 8-fold increased risk of VTE compared with E2 in transwomen seen in a large transgender service in UK (29).
On the other hand, E2 demonstrates an excellent safety profile in several transgender studies (Table 1). In a large US cohort of ~700 subjects on E2 4–8 mg/day for a mean duration of 1.9 years, only a single case of VTE occurred (27). Similarly, in a German cohort of 60 subjects on a relatively high oral E2 dose of 6 mg/day, including three with underlying thrombotic tendency, only one VTE event was observed (25). Furthermore, in an Austrian study of 162 subjects on transdermal E2, no VTE was observed over a median follow-up of 64.2 ± 38.0 months despite a high prevalence of smokers (~60%) and the presence of confirmed thrombophilic disorder (APC resistance) in nearly 10% (26).
EE has been shown to induce APC resistance similar to that of factor V Leiden mutation, as well as increase in plasma protein C and a decrease in plasma protein S, in a dose-dependent manner. Additionally, non-physiological estrogens lead to elevated inflammatory markers such as C-reactive protein and interleukin-6, which could contribute to the prothrombotic milleu (38). Besides first-pass liver effect driving haemostatic dysregulation, higher prothrombotic tendencies are present with other modes of administration (transdermal and transvaginal) as well, providing evidence for a direct pathway induced by the molecular structure (39, 40).
Another major advantage of E2 over EE/CEE is the feasibility for dose adjustment according to serum E2 concentration. This is important as bioequivalence between different administrative forms (oral tablet, gel, and patch) is not well-established and subject to wide interindividual variation (13). Moreover, titrating to robust physiological E2 levels has been correlated with positive outcomes on metabolic parameters and carotid intima media (23, 41). Normative E2 values derived from healthy normally menstruating females would serve as a good guide to dosing (14).
The choice of estrogen formulation is vital to ensure optimisation of safety and treatment efficacy. Compelling data from recent literature supports the use of E2 over EE/CEE to avoid the excessive vascular risk that the latter formulations are associated with. Further studies should seek to build on available evidence and provide greater clarity on estrogen replacement to empower clinicians and patients to make better therapeutic decisions.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Canonico M1, Plu-Bureau G, O'Sullivan MJ, Stefanick ML, Cochrane B, Scarabin PY, et al. Age at menopause, reproductive history, and venous thromboembolism risk among postmenopausal women: the Women's Health Initiative hormone therapy clinical trials. Menopause. (2014) 21:214–20. doi: 10.1097/GME.0b013e31829752e0
2. Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. (1986) 67:604–6.
3. Sullivan SD, Sarrel PM, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. (2016) 106:1588–99. doi: 10.1016/j.fertnstert.2016.09.046
4. Crawford SL, Crandall CJ, Derby CA, El Khoudary SR, Waetjen LE, Fischer M, et al. Menopausal hormone therapy trends before versus after 2002: impact of the Women's Health Initiative Study Results. Menopause. (2018) 26:588–97. doi: 10.1097/GME.0000000000001282
5. Olivius C, Landin-Wilhelmsen K, Olsson DS, Johannsson G, Tivesten Å. Prevalence and treatment of central hypogonadism and hypoandrogenism in women with hypopituitarism. Pituitary. (2018) 21:445–453. doi: 10.1007/s11102-018-0895-1
6. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. (1998) 19:55–72. doi: 10.1146/annurev.publhealth.19.1.55
7. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. (2002) 288:321–33.
8. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. (1998) 280:605–13. doi: 10.1001/jama.280.7.605
9. Lobo RA. Where are we 10 years after the Women's Health Initiative? J Clin Endocrinol Metab. (2013) 98:1771–80. doi: 10.1210/jc.2012–4070
10. Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. (2007) 297:1465–77. doi: 10.1001/jama.297.13.1465
11. Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. (2007) 356:2591–602. doi: 10.1056/NEJMoa071513
12. Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. (2005) 8(Suppl 1):3–63. doi: 10.1080/13697130500148875
13. Järvinen A1, Nykänen S, Paasiniemi L. Absorption and bioavailability of oestradiol from a gel, a patch and a tablet. Maturitas. (1999) 32:103–13. doi: 10.1016/S0378-5122(99)00021-3
14. Taboada M, Santen R, Lima J, Hossain J, Singh R, Klein KO, et al. Pharmacokinetics and pharmacodynamics of oral and transdermal 17β estradiol in girls with Turner syndrome. J Clin Endocrinol Metab. (2011) 96:3502–10. doi: 10.1210/jc.2011-1449
15. Smith NL, Blondon M, Wiggins KL, Harrington LB, van Hylckama Vlieg A, Floyd JS, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. (2014) 174:25–31. doi: 10.1001/jamainternmed.2013.11074
16. Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. (2019) 364:k4810. doi: 10.1136/bmj.k4810
17. Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. (2012) 345:e6409 doi: 10.1136/bmj.e6409
18. Popat VB, Calis KA, Kalantaridou SN, Vanderhoof VH, Koziol D, Troendle JF, et al. Bone mineral density in young women with primary ovarian insufficiency: results of a three-year randomized controlled trial of physiological transdermal estradiol and testosterone replacement. J Clin Endocrinol Metab. (2014) 99:3418–26. doi: 10.1210/jc.2013-4145
19. Cartwright B, Robinson J, Seed PT, Fogelman I, Rymer J. Hormone replacement therapy versus the combined oral contraceptive pill in premature ovarian failure: a randomized controlled trial of the effects on bone mineral density. J Clin Endocrinol Metab. (2016) 101:3497–505. doi: 10.1210/jc.2015-4063
20. O'Donnell RL, Warner P, Lee RJ, Walker J, Bath LE, Kelnar CJ, et al. Physiological sex steroid replacement in premature ovarian failure: randomized crossover trial of effect on uterine volume, endometrial thickness and blood flow, compared with a standard regimen. Hum Reprod. (2012) 27:1130–8. doi: 10.1093/humrep/des004
21. Crofton PM, Evans N, Bath LE, Warner P, Whitehead TJ, Critchley HO, et al. Physiological versus standard sex steroid replacement in young women with premature ovarian failure: effects on bone mass acquisition and turnover. Clin Endocrinol. (2010) 73:707–14. doi: 10.1111/j.1365-2265.2010.03868.x
22. Langrish JP, Mills NL, Bath LE, Warner P, Webb DJ, Kelnar CJ, et al. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension. (2009) 53:805–11. doi: 10.1161/HYPERTENSIONAHA.108.126516
23. Torres-Santiago L, Mericq V, Taboada M, Unanue N, Klein KO, Singh R, et al. Metabolic effects of oral versus transdermal 17β-estradiol (E 2): a randomized clinical trial in girls with turner syndrome. J Clin Endocrinol Metab. (2013) 98:2716–24. doi: 10.1210/jc.2012-4243
24. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. (2011) 164:635–42. doi: 10.1530/EJE-10-1038
25. Dittrich R, Binder H, Cupisti S, Hoffmann I, Beckmann MW, Mueller A. Endocrine treatment of male-to-female transsexuals using gonadotropin-releasing hormone agonist. Exp Clin Endocrinol Diabetes. (2005) 113:586–92. doi: 10.1055/s-2005-865900
26. Ott J, Kaufmann U, Bentz EK, Huber JC, Tempfer CB. Incidence of thrombophilia and venous thrombosis in transsexuals under cross-sex hormone therapy. Fertil Steril. (2010) 93:1267–72. doi: 10.1016/j.fertnstert.2008.12.017
27. Arnold JD, Sarkodie EP, Coleman ME, Goldstein DA. Incidence of venous thromboembolism in transgender women receiving oral estradiol. J Sex Med. (2016) 13:1773–7. doi: 10.1016/j.jsxm.2016.09.001
28. Getahun D, Nash R, Flanders WD, Baird TC, Becerra-Culqui TA, Cromwell L, et al. Cross-sex hormones and acute cardiovascular events in transgender persons: a cohort study. Ann Intern Med. (2018) 169:205–213. doi: 10.7326/M17-2785
29. Seal LJ, Franklin S, Richards C, Shishkareva A, Sinclaire C, Barrett J. Predictive markers for mammoplasty and a comparison of side effect profiles in transwomen taking various hormonal regimens. J Clin Endocrinol Metab. (2012) 97:4422–8. doi: 10.1210/jc.2012-2030
30. van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol. (1997) 47:337–42. doi: 10.1046/j.1365-2265.1997.2601068.x
31. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender hormone treatment. Metabolism. (1989) 38:869–73. doi: 10.1016/0026-0495(89)90233-3
32. Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher AD, Toye K, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med. (2014) 11:1999–2011. doi: 10.1111/jsm.12571
33. Wierckx K, Elaut E, Declercq E, Heylens G, De Cuypere G, Taes Y, et al. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol. (2013) 169:471–8. doi: 10.1530/EJE-13-0493
34. Nota NM, Wiepjes CM, de Blok CJM, Gooren LJG, Kreukels BPC, den Heijer M. Occurrence of acute cardiovascular events in transgender individuals receiving hormone therapy. Circulation. (2019) 139:1461–1462. doi: 10.1161/CIRCULATIONAHA.118.038584
35. Boardman HM, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. (2015) 10:CD002229. doi: 10.1002/14651858.CD002229.pub4
36. Boehm U, Bouloux PM, Dattani MT, de Roux N, Dodé C, Dunkel L, et al. Expert consensus document: european consensus statement on congenital hypogonadotropic hypogonadism-pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. (2015) 11:547–64. doi: 10.1038/nrendo.2015.112
37. Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, et al. Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2016) 101:3888–921. doi: 10.1210/jc.2016-2118
38. Wakatsuki A, Ikenoue N, Shinohara K, Watanabe K, Fukaya T. Effect of lower dosage of oral conjugated equine estrogen on inflammatory markers and endothelial function in healthy postmenopausal women. Arterioscler Thromb Vasc Biol. (2004) 24:571–6. doi: 10.1161/01.ATV.0000115383.49802.0c
39. Toorians AW, Thomassen MC, Zweegman S, Magdeleyns EJ, Tans G, Gooren LJ, et al. Venous thrombosis and changes of hemostatic variables during cross-sex hormone treatment in transsexual people. J Clin Endocrinol Metab. (2003) 88:5723–9. doi: 10.1210/jc.2003-030520
40. Sitruk-Ware R, Plu-Bureau G, Menard J, Conard J, Kumar S, Thalabard JC, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. (2007) 92:2074–9. doi: 10.1210/jc.2007–0026
Keywords: hormonal replacement therapy, estrogen (17b-estradiol), conjugated estrogen, ethinyl estradiol (EE), female hypogonadism, premature ovarian failure, transgender, cross-sex hormonal treatment
Citation: Swee DS, Javaid U and Quinton R (2019) Estrogen Replacement in Young Hypogonadal Women—Transferrable Lessons From the Literature Related to the Care of Young Women With Premature Ovarian Failure and Transgender Women. Front. Endocrinol. 10:685. doi: 10.3389/fendo.2019.00685
Received: 23 June 2019; Accepted: 20 September 2019;
Published: 11 October 2019.
Edited by:
Evangelos G. Papanikolaou, Aristotle University of Thessaloniki, GreeceReviewed by:
Ljiljana Marina, Clinical Center of Serbia, SerbiaCopyright © 2019 Swee, Javaid and Quinton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Du Soon Swee, c3dlZS5kdS5zb29uQHNpbmdoZWFsdGguY29tLnNn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.