
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 26 September 2019
Sec. Thyroid Endocrinology
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00664
This article is part of the Research Topic Combination Therapy for Hypothyroidism View all 17 articles
Background: For significant numbers of patients dissatisfied on standard levothyroxine (LT4) treatment for hypothyroidism, patient-specific responses to T4 could play a significant role.
Aim: To assess response heterogeneity to LT4 treatment, identifying confounders and hidden clusters within a patient panel, we performed a secondary analysis using data from a prospective cross-sectional and retrospective longitudinal study.
Methods: Multivariate and multivariable linear models adjusted for covariates (gender, age, and BMI) were stratified by disease-specific treatment indication. During follow-up, pooled observations were compared from the same patient presenting either with or without self-reported symptoms. Statistical analysis was extended to multilevel models to derive intra-class correlation coefficients and reliability measures during follow-up.
Results: Equilibria between TSH, FT4, and FT3 serum concentrations in 342 patients were examined by treatment indication (benign goiter, autoimmune thyroiditis, thyroid carcinoma), consequently displaying complex interactive response patterns. Seventy-seven patients treated with LT4 and monitored for thyroid carcinoma presented, in association with changes in LT4 dose, either with hypothyroid symptoms or symptom-free. Significant biochemical differences appeared between the different presentations. Leveled trajectories by subject to relief from hypothyroid symptoms differed significantly, indicating distinct responses, and denying a single shared outcome. These were formally defined by a high coefficient of the intraclass correlation (ICC1, exceeding 0.60 in all thyroid parameters) during follow-up on multiple visits at the same LT4 dose, when lacking symptoms. The intra-personal clusters were clearly differentiated from random variability by random group resampling. Symptomatic change in these patients was strongly associated with serum FT3, but not with FT4 or TSH concentrations. In 25 patients transitioning from asymptomatic to symptomatically hyperthyroid, FT3 concentrations remained within the reference limits, whilst at the same time marked biochemical differences were apparent between the presentations.
Conclusions: Considerable intra-individual clustering occurred in the biochemical and symptomatic responses to LT4 treatment, implying statistically multileveled response groups. Unmasking individual differences in the averaged treatment response hereby highlights clinically distinguishable subgroups within an indiscriminate patient panel. This, through well-designed larger clinical trials will better target the different therapeutic needs of individual patients.
Despite lacking the minor component of triiodothyronine (T3) physiologically co-secreted with thyroxine (T4) by the healthy human thyroid gland, in thyroid failure monotherapy by levothyroxine (LT4) replacement remains the standard treatment for patients with primary hypothyroidism (1, 2). LT4 is one of the most frequently prescribed drugs with a long history of successful use and favorable safety record (3–5). Administered in variable doses, dose adequacy for a hypothyroid patient is determined by biochemically defined treatment targets based mainly on TSH measurements (2). This marks a historical shift from earlier regimens primarily aiming at symptom relief (6). However, despite achieving appropriate biochemical treatment targets with LT4, as defined by current guidelines (2), a substantial fraction of patients continues to report persisting symptomatology expressing their dissatisfaction with the standard treatment (7). The magnitude of the problem has recently been re-emphasized by a large online survey conducted by the American Thyroid Association where satisfaction with LT4 treatment reported by the 12,146 respondents was only at median 5 on a scale of 1–10 (8). A prospective study by Winther et al. using a validated thyroid specific QoL questionnaire and following hypothyroid patients with autoimmune thyroiditis, concluded that QoL outcome measures improved but a full recovery was not achieved after 6 months of treatment with LT4 (9). While patients and doctors reported some success with the addition of T3 and guidelines by the European Thyroid Association acknowledge a potential benefit of T3/T4 combinations to some patients this subject overall remains contentious (7, 10–12).
Variable patient experiences with LT4 treatment and the possible existence of differently responding subgroups of patients have long been suspected (13), but formal analysis of this problem with robust statistical methods is seriously lacking. A biochemical dissociation in the equilibria or so called setpoint between TSH, FT4, and FT3 has been increasingly recognized, both in untreated subjects and in patients treated with LT4 (5, 13). Patients on LT4 display considerable variation in their biochemical and symptomatic treatment response, along with the manifestation of a pronounced disjoint between FT3 and TSH concentrations, compared to the relationship in thyroid health (14–16). This may also pertain to such intrinsic differences in patient response as to encourage an exploration of risk stratification. In this respect, the Rotterdam study documented an increased risk of both atrial fibrillation and sudden cardiac death in an untreated euthyroid population with higher LT4 serum concentrations within its reference range, yet uncorrelated with TSH concentrations (17, 18).
In the present study, we question to what extent the response to LT4 treatment, as expressed in the respective equilibria between the thyroid parameters TSH, FT4, and FT3, may differ between athyreotic patients with thyroid carcinoma and benign entities such as autoimmune thyroiditis or goiter post-surgery. In a panel of athyreotic patients with thyroid carcinoma followed long term on LT4 replacement, we assessed the biochemical alterations in individual subjects with symptoms before and after symptom relief. We were particularly interested in possible implications of ergodicity arising during long-term follow-up from a narrow intra-individual variation of thyroid hormones.
Data for this secondary analysis were collected as part of two previously reported trials, a cross-sectional prospective trial and a retrospective longitudinal study (19–21). The prospective trial was registered (www.ClinicalTrials.gov, NCT 01969552), ethically approved, and all participants gave written informed consent, and the retrospective study was approved by the local authorities in data protection. Both studies were conducted in an outpatient setting, prospectively from 2013 to 2014 in 1912 patients with various thyroid diseases and retrospectively from 2008 to 2016 in 319 patients with thyroid carcinoma routinely monitored at 2,309 visits (19, 21). Only LT4-treated out-patients without known comorbidity were included in the present study, and we also included only visits after hypothyroidism was biochemically controlled as defined by both a TSH < 4 mIU/l and FT4 > 10 pmol/l, while FT3 concentrations were within the reference limits. All measurements were obtained on unchanged stable medication in equilibrium. Indication for LT4 treatment resulted from three different indications, namely benign goiter, primary hypothyroidism due to thyroid autoimmune disease as evidenced by the presence of peroxidase antibodies (TPO Ab), and total thyroidectomy due to thyroid carcinoma. Patients with thyroid carcinoma were regularly monitored at 6 month intervals for the first 5 years after thyroidectomy and 12 month intervals thereafter in tumor-free patients, and followed long-term. Patient characteristics are tabulated as relevant for this study (Tables 1, 2).
Details were collected on patient history and medication, demographic factors (gender, age, BMI), physical examination, ultrasound, and laboratory tests (FT3, FT4, TSH, TPO Ab, and TSH-receptor antibodies (TSH R Ab) in TPO Ab positive cases only). In the longitudinal study only, any patient complaints, specific and non-specific, were freely communicated during visits in an open format, avoiding suggestive or standardized questions, and documented as such. The documented complaints were later independently categorized by a specialist into thyroid-unrelated symptoms (e.g., back pain), hypothyroid symptoms (e.g., tiredness, fatigue, lack of energy, cold intolerance, weight gain) and hyperthyroid symptoms (e.g., nervousness, irritability, restlessness, anxiety, rapid pulse, palpitations, trembling, heat intolerance, unwanted weight loss). The terms complaint and symptom are used synonymously here. During follow-up adjustments were made to the LT4 dose, either in response to individual patient complaints or according to the general treatment strategy including the individual risk profile and changes in guideline recommendations over the years (21).
In all subjects, thyroid volume, echo-density, and nodularity were examined by ultrasound (10 MHz transducer). Thyroid volume was determined by the ellipsoid formula (longitudinal diameter × width × depth × 0.5 cm3) and summation of lobe volumes. Reference values are <18 ml for female and <25 ml for male subjects.
TSH was measured with an automated direct chemiluminescence method (TSH3-Ultra ADVIA Centaur XP, Siemens Healthcare Diagnostics, Erlangen, Germany). The standard curve was calibrated with the 3rd WHO Standard for hTSH (IRP 81/565). Functional sensitivity was 0.008 mIU/l, intra-assay variation 1.4–2.4%, and inter-assay imprecision 0.9–2.9%. FT3 and FT4 were measured on the same platform, showing intra-assay CVs from 2.4 to 3.1% or 2.2 to 3.3% and inter-assay CVs from 2.3 to 3.9% or 2.5 to 4.0%, respectively. Assay performance characteristics have been reported (22). Laboratory-evaluated reference intervals were as follows, 0.4–4 mIU/l for TSH, 3.1–6.8 pmol/l for FT3, 10–23 pmol/l for FT4.
TPO Abs were measured with a competitive chemiluminescence method (ADVIA Centaur XP, Siemens Healthcare Diagnostics, Erlangen, Germany) and TSH-R Abs with an ELISA (EUROIMMUN AG, Lübeck, Germany). Reference ranges were for TPO Ab <60 IU/ml and for TSH-R Ab <2 IU/l.
Descriptive data are shown as mean (standard deviation, SD) or median (interquartile range, IQR). Non-normally distributed TSH values were natural logarithmically transformed. Between-two-group comparisons for continuous variables were based on Welch's t-test or, if normality could not be assumed, Wilcoxon's rank-sum test. More than three independent groups were compared using ANOVA or a Kruskal-Wallis test. Chi-squared test with Yates' correction for continuity was used for categorical variables. Pooled observations at either symptomatic or asymptomatic presentations derived from the same patient were compared using a paired t-test or the signed rank Wilcoxon test. Multivariable and multivariate linear models, adjusted for disease entity, gender, age, and BMI were used to assess associations across subjects between thyroid parameters including–when significant–more complex multiplicative interactions between them. MANOVA tests for the multivariate models relied on Pillai's test statistic. Residual plots were inspected to verify model assumptions. Changes during follow-up in the binary outcomes for the presence or absence of symptoms and continuous thyroid parameters were assessed using generalized linear mixed models with a restricted maximum likelihood estimator (REML) and a binomial or Gaussian link function, respectively, appropriately accounting for within-variation and intra-subject correlations for repeated measurements per subject in the longitudinal design (23). Effect plots predict the binary outcome as a probability response on a linearized logit scale or the natural response of a continuous outcome. Relative risks (RR) are reported in Results. Model performance was compared by both F-test and Akkaike's information criteria (AIC). These models were formulated as unconditional means models to derive estimates on intra-class correlation coefficients (ICC1) and reliability (ICC2) for thyroid hormones obtained at multiple occasions under stable conditions during follow-up. Random group resampling was performed to differentiate personal group-level properties from random group variability (24, 25). Power simulations were done according to the methods described and implemented by Bliese (24, 25). All tests were two-sided with p < 0.05 denoting statistical significance. Variables were considered explanatory without adjusting for multiple comparisons. We used the R statistical software environment (version 3.5.2 for Mac) (26) with the added packages lme4 1.1-19 (23), effects 4.1-0 (27), heplots 1.3.-5 (28), sjstats 0.17-3 (29), and multilevel 2.6 (24, 25).
Patient characteristics of LT4-treated patients are summarized in Table 1 for the cross-sectional study and in Table 2 for the longitudinal study.
In 342 patients treated with LT4, we examined their equilibria and biochemical response heterogeneity by disease entity, benign goiter (n = 111), autoimmune thyroiditis (n = 95), thyroid carcinoma after thyroidectomy (n = 136). Using FT3 levels achieved as dependent outcome in a multivariable linear model, disease category (significantly steeper relationship in the carcinoma group, p = 0.02), FT4 (0.03 pmol/l per pmol/l, 95% CI [0.014, 0.052], p < 0.001), and TSH (lnTSH −0.17 pmol/l per mIU/l, 95% CI [−0.21, −0.13], p < 0.001) concentrations were all significantly independent predictors. The influence of the other adjusted covariates present in the model was as follows, gender (0.42 pmol/l higher for men, 95% CI [0.29, 0.56], p < 0.001), age (−0.012 pmol/l per year 95% CI [−0.015, −0.008], p < 0.001), BMI (−0.002 pmol/l per kg/m2, 95% CI [−0.011, 0.006], p = 0.60). Figure 1A shows the TSH-dependent and FT4-adjusted FT3 response by treatment category. Conversely, in a multivariate model, FT3 concentrations interacted with the treatment category in predicting the combined outcomes for FT4 and TSH, used as surrogates for setpoints. This interaction was highly significant (Pillai test, p < 0.001), and remained so after adjusting for gender, age and BMI (Pillai test, p < 0.001). Figure 1B shows the derived trajectories for the relationships and estimates the resulting FT3-dependent equilibria (setpoints) between FT4 and TSH serum concentrations by treatment category and FT3 levels.
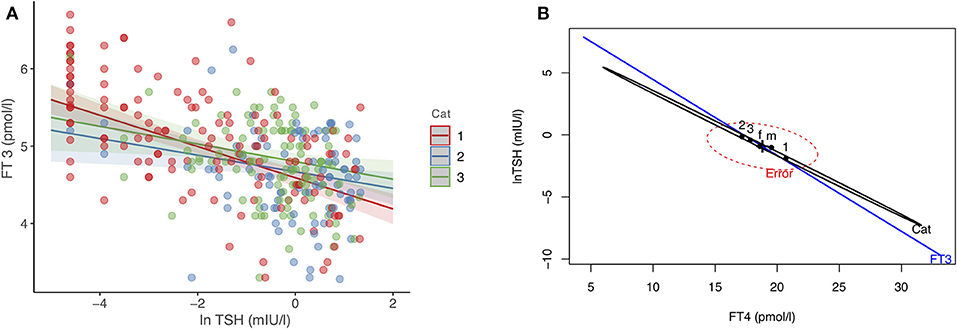
Figure 1. (A,B) Effect plots of the complex interdependency between FT3, FT4, and TSH. (A) shows the relationship between FT3 and TSH, examined by treatment category, after adjusting for FT4 concentrations, gender, age, and BMI. (B) The resulting equilibria between FT4 and TSH (as surrogate markers for setpoints), associated with treatment category and FT3 concentrations in a significant interaction (Pillai-test p < 0.001, see Results for details). The three treatment categories refer to patients with thyroid carcinoma (1), autoimmune thyroiditis (2), and benign goiter (3). f indicates female, and m male gender. Cat, disease category. Regression lines are surrounded by the 95% confidence limit.
In a longitudinal series of 319 patients with thyroid carcinoma followed at 2,309 visits for 63 months (median, IQR 46, 81), we assessed the individual treatment responses. Of particular interest were 77 patients with changing hypothyroid symptomatology following LT4 dose adjustment during follow-up. The statistical comparison between pooled paired observations, averaged over multiple visits (median 9, IQR 6, 12) at either symptomatically hypothyroid or asymptomatic presentations, was as follows, weight-adjusted LT4 dose (−0.081 μg/kg BW, 95% CI [−0.14, −0.024], paired t-test: p = 0.006), TSH concentrations (0.14 mIU/l [−0.03, 0.31], paired signed Wilcoxon test: p = 0.47), FT4 concentrations (−1.05 pmol/l, 95% CI [−1.83, −0.28], paired t-test: p = 0.009), and FT3 levels (−0.22 pmol/l, 95% CI [−0.36, −0.08], paired t-test: p = 0.002), all except TSH being significantly lower at presentations with hypothyroid symptoms. A difference plot of FT3 measurements between the symptomatic and asymptomatic pairs revealed considerable diversity among individual patients in their start and end levels and respective distances between the two levels (Figure 2A). After mean-standardizing the FT3 concentrations, we plotted the z-scores for FT3 of either symptomatic or asymptomatic presentations against the average scores over all visits per patient (Figure 2B). This shows that the corrective effect size required for relief of hypothyroid symptoms increased with the distance from the center. Looking at FT3 change relative to TSH, symptomatic and asymptomatic observations did not move along a shared trajectory but were significantly shifted (0.23 pmol/l 95% CI [0.36, 0.11], p < 0.001) (Figure 2C). On average, the rate of hypothyroid symptoms increased with lower FT3 concentrations (Figure 2D). FT3 serum concentrations (RR 0.70 per pmol/l, 95% CI [0.49, 0.96], p = 0.03), but not FT4 concentrations (RR 0.95 per pmol/l, 95% CI [0.91, 1.00], p = 0.053), and TSH concentrations (lnTSH RR 0.98 per mIU/l, 95% CI [0.87, 1.09], p = 0.73) were significantly predictive of the presence of hypothyroid symptoms in these patients. In this respect, a combination of all three covariates FT3, FT4, and TSH was not more informative, compared to FT3 measurements alone (F-test p = 0.14, AIC difference 0.11). Confounders in the cross-sectional study, namely gender (p = 0.30), age (p = 0.57), and BMI (p = 0.93), were non-influential but adjusting for these covariates slightly reduced the variation of the significant FT3 influence (adjusted RR 0.55, 95% CI [0.31, 0.87], p = 0.008).
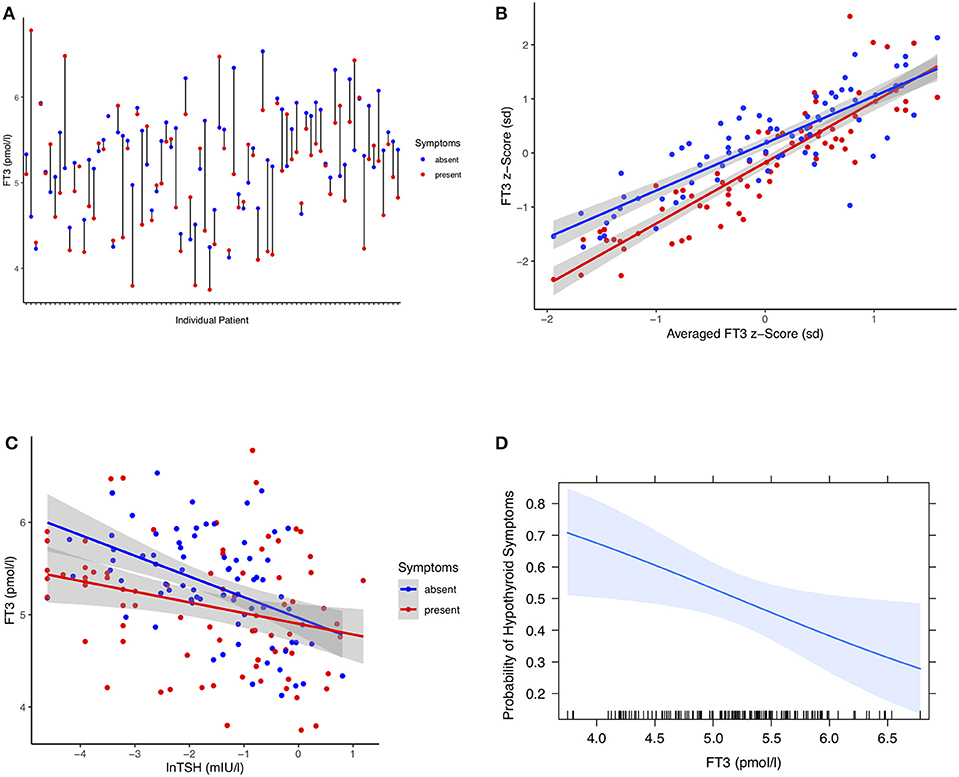
Figure 2. (A) Difference plot of serum FT3 concentrations in 77 individual patients at either visits with hypothyroid symptoms or asymptomatic presentations. Each point refers to pooled measurements obtained from a single patient during follow-up and averaged over multiple either symptomatic or asymptomatic visits after adjusting LT4 dose (see Results). (B) FT3 z-scores of either symptomatic or asymptomatic presentations plotted against the averaged scores over all visits per patient. FT3 concentrations are mean-centered and units are expressed in standard deviations. This shows that the corrective FT3 difference associated with relief of self-reported hypothyroid symptoms increased progressively with increasing distance from the center. Shaded areas indicate the 95% confidence limit for the fitted regression line. (C) FT3 change relative to TSH stratified by symptomatic and asymptomatic presentations of the same patients. The intersecting points did not move along a shared trajectory between the two conditions but were significantly shifted, progressively so toward lower TSH concentrations (see Results). (D) Probability of hypothyroid symptoms as a function of circulating FT3 concentrations. The probability of the presence of hypothyroid symptoms at a given FT3 level for these patients was derived by a multilevel model accounting for intra-class and between-subject variation (see Methods and Results). The shaded area indicates the 95% confidence limit of the probability curve. The vertical ticks on the x axis indicate the observed individual values.
Similar to the hypothyroid complaints, the pooled observations from 25 individual patients were compared when they were either symptom-free or presented with hyperthyroid symptoms (Figures 3A–C). Differences were highly significant for weight-adjusted LT4 dose (0.23 μg/kg BW, 95% CI [0.12, 0.35], paired t-test: p < 0.001), TSH concentrations (−0.44 mIU/l [−0.60, −0.29], paired signed Wilcoxon test: p < 0.001), FT4 concentrations (4.20 pmol/l, 95% CI [2.83, 5.58], paired t-test: p < 0.001), and FT3 levels (0.53 pmol/l, 95% CI [0.26, 0.80], paired t-test: p < 0.001). For individuals, FT3 and symptomatic change are shown in Figure 3A. Standardized effect sizes are depicted in Figure 3B. The probability of hyperthyroid symptoms increased with higher serum concentrations of FT3, as shown in Figure 3C. Relative risk estimates with increasing concentrations were as follows, FT3 1.54 per pmol, 95% CI [1.13, 1.79], p < 0.001), FT4 1.17 per pmol, 95% CI [1.06, 1.28], p = 0.003), and lnTSH 0.42 per mIU/l, 95% CI [0.24, 0.70], p < 0.001).
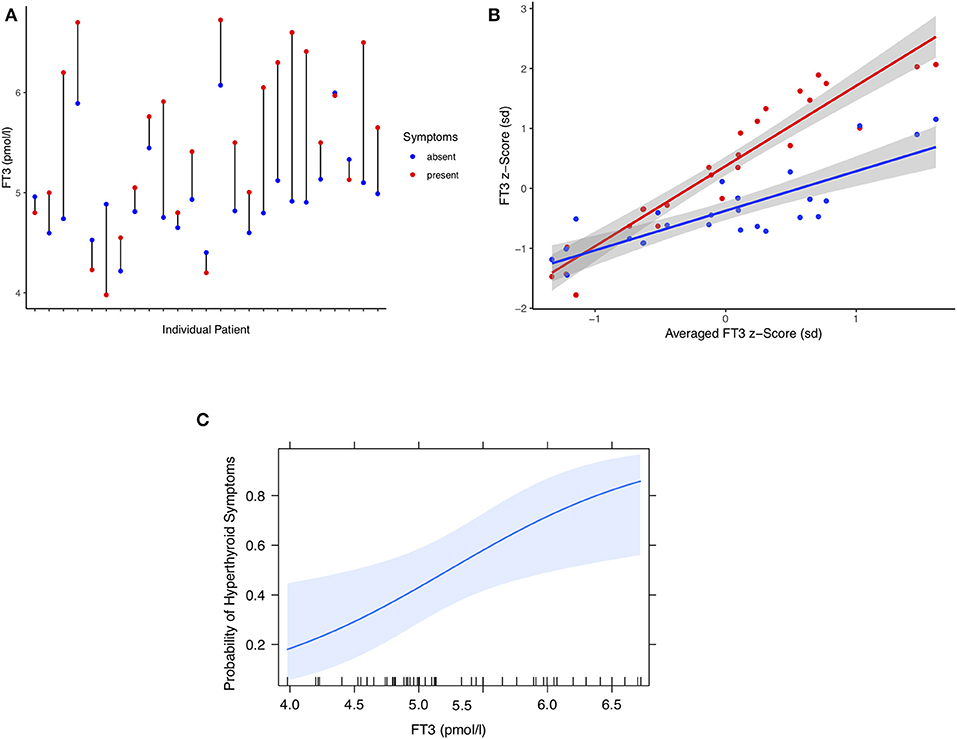
Figure 3. (A) Difference plot of serum FT3 concentrations in 25 individual patients at either hyperthyroid or symptom-free presentations. Each point refers to the pooled measurements over multiple either symptomatic or asymptomatic visits (see Results). (B) FT3 z-scores of either symptomatic or asymptomatic presentations plotted against the averaged scores over all visits per patient. Shown are mean-centered standardized FT3 concentrations and a fitted regression line surrounded by its 95% confidence limit (shaded area). (C) Probability of hyperthyroid symptoms in these patients as a function of circulating FT3 concentrations. A multilevel model was used to estimate the probability (see Methods and Results). The shaded area indicates the 95% confidence limit of the fitted curve. The vertical ticks on the x axis indicate the observed individual values.
We estimated the intra-class correlation coefficient (ICC1) and reliability (ICC2) over the follow-up period in 141 patients at 435 visits on a stable unchanged LT4 dose of 125 μg/day and in the absence of symptoms. The individual patients displayed a high intraclass correlation for all thyroid parameters, FT3 0.61, FT4 0.67, and TSH 0.67. FT3-dependent multilevel trajectories to relief of hypothyroid symptoms also proved highly individually variable, ICC1 0.64.
All parameters showed excellent group-mean reliability FT3 0.83, FT4 0.86, TSH 0.86, indicating that the individuals do not form random groups and can be reliably differentiated. To estimate how much appropriately accounting for level properties may reduce variance or, conversely, if ignored, inflate variance we compared the variance of randomly resampled pseudo-groups with the real variance in the actual groups where every patient formed their own group during follow-up. This demonstrates a highly significant (p < 0.001) and pronounced influence of personal grouping, as opposed to random grouping, the mean within-group variance for the random FT3 sample being 0.46, compared to 0.26 for the real data for FT3.
A bias may arise, for instance in an RCT, if the individual levels are disregarded and the data is treated as though it was independent. Simulating a hypothetical design with two groups, 40 subjects per group, moderate between-variable correlation (r = 0.47) and intra-class correlation of 0.61 for the outcome variable, the t-test-based power estimate of such a trial would be reduced from 92 to 48% if the analysis fails to account for the observed multilevel structure in the data. A sufficiently powered (93%) larger trial with a group size of 200 subjects and a lower correlation of 0.25 under otherwise identical conditions would become underpowered by ignoring the level properties in the sample (power estimate 63%). Increasing group size to 500 subjects in the case of three groups and two weakly correlated variables (r = 0.10) would not remedy the lack of power caused by averaging (54%), compared to leveling (91%) the outcome.
Although in health a narrow intra-individual variation of thyroid hormones has long been recognized (30), its application to the treatment of patients with LT4 has not been rigorously examined. This study has uncovered considerable inter-individual variability and intra-class correlations in the biochemical and symptomatic responses to LT4 treatment within a patient panel. For instance, failure to account for a multilevel structure in the data, if present in a randomized controlled clinical trial (RCT), may mask potential treatment effects. This will result in the reduction of statistical power when predicting treatment-associated outcomes in hypothyroid patients on LT4.
In probability theory, the definition of an ergodic dynamical system is that it displays the same behavior when averaged over time as averaged over the space of all the system's states in its phase space (31). The mathematical definition emphasizes that for group membership all group members must share the same moments, namely means, variances and covariances (32). Ergodicity is a requirement when generalizing from the population to the individual level (32–35). Because the implicitly assumed ergodicity of thyroid parameters does not hold true, thyroid reference ranges are fundamentally inappropriate and should be replaced by personal setpoints (5). Ergodicity is most particularly challenged in situations where both trait-like differences exist among individuals and structural change occurs over time. In thyroid patients, the concept relates to trait-like personal setpoints (equilibria between TSH and FT4, FT3) that undergo structural change during follow-up. The determination of intra-class correlations (ICC) provides a quantitative measure on influences associated with either a subject or a particular situation.
In our longitudinal series, we documented substantial intra-class correlation for all thyroid parameters. This characterizes the thyroid status largely as a personal trait, which varies under stable medication more between subjects, and less so within a person on different occasions. Random variability was ruled out by a permutation test, confirming both the multi-level properties and individual heterogeneity among the patients within the panel. Whenever the intra-class correlation coefficient is found to be large, we cannot confidently use aggregated statistical methods on these data that assume independence, because estimates of variance, and therefore p-values, become insufficiently robust. As pointed out by Fisher et al., best-practice guidelines derived from RCTs in such conditions tend to overestimate the accuracy of aggregated statistical estimates (35). Ecological fallacy, collider stratification bias and Simpson's paradox are variations of the problem with serious implications, as exemplified by the market retraction of an approved drug (34–38).
Setpoint theory may explain the high degree of individuality physiologically observed in thyroid parameters (39, 40). In the thyroid healthy state, the so-called setpoint delivers a homeostatically defined multivariate expression of the stable equilibrium between interlocked pairs of TSH and FT4 (41). The resulting distribution of clustered setpoints is fundamentally different from the process of using univariate reference ranges for TSH or FT4, rather requiring multivariate and multileveled approaches (5, 41). In the event of a disease or under the influence of LT4 treatment, personal set points may be conditionally redistributed (13, 14). Consequently, the TSH level previously appropriate for thyroid health cannot equally serve as a treatment target in the same person (42).
From an evolutionary point of view, moderate diversity in their personal setpoints and response heterogeneity among individuals within a human or animal population makes sense, attenuating excessive reactions, and abrupt transitions to changing environmental conditions in a community (43–45).
The reasons for persisting patient complaints are not well-understood and thyroid-related symptoms may overlap with a plethora of non-specific complaints (8, 46–60). Also, drugs containing LT4 or LT3 may display mild antidepressant pharmacological properties (61). In an ergodic framework, we may question whether complaints relate to the dynamic structure of the underlying thyroid process or are independent “traits” of the individual and derive quantitative estimates for the two components. Concerns about inexplicable variation (62, 63) should be advanced from the descriptive level to analytical study. A focus on idiographic patterns following individual patients receiving LT4 long term on multiple occasions, as in this retrospective study, limits the impact of inter-personal variation. It reduces thereby the importance of chief non-thyroidal confounders of cross-sectional studies such as gender, age and BMI as well as treatment-related variation in the biochemical equilibria across different disease entities, such as thyroid carcinoma, autoimmune thyroiditis, and goiter. This may uncover subtle differences that may otherwise remain hidden within a noisy background and go undetected by statistically inappropriate averaging.
The present study is one of the first of its kind in conducting an intra-class correlation analysis in the treatment response to LT4 over multiple presentations in a large sample of patients with thyroid carcinoma followed for several years. It has however several limitations. This is a secondary analysis; the primary clinical study outcomes of both the prospective cross-sectional trial and retrospective longitudinal study have previously been reported (19, 21). Although patients with known comorbidities, interfering comedication, and clinical conditions in which elevated TSH levels persisted (e.g., non-adherence, LT4 malabsorption) were excluded from this analysis remaining subclinical pathologies are part of the biological variation (64). The present analysis focusses on the framework of ergodicity and multileveled patterns of the responses to LT4 treatment within a patient panel. Although the study design was uncontrolled, the findings of the ICC analysis are pertinent to prospective studies and RCTs and may aid in improving future trials. As hypothyroid symptoms inherently overlap with non-specific or hyperthyroid complaints, a few misclassifications are inevitable but are of little apparent influence on the main tendencies for each symptom category (Figures 2, 3). More importantly, patient expectancy remains a bias that has not been robustly addressed in any thyroid trial including RCTs (65). The American Food and Drug Administration (FDA) therefore demands drugs to be evaluated under “actual conditions of use”–a requirement met by none of the many RCTs on QoL outcomes for LT4 and T4 T3 combinations (10, 11, 65).
Subjective symptoms as experienced by the patient were freely communicated during routine visits in an open format, being retrospectively and independently categorized into hypothyroid, hyperthyroid, or thyroid-unrelated complaints. While unstandardized, this process avoids any suggestive interrogation. The presence of leading symptoms may successfully substitute for the use of more complex QoL questionnaires, and instantaneous assessment offers higher precision and sensitivity to change, compared to retrospective ratings (66, 67). Due to their non-ergodic behavior TSH and thyroid hormone levels associated with the presence or absence of hypothyroid symptoms considerably overlap among individual patients. Individual trajectories to symptom relief start from different levels and end at different targets. Intra-class clustering and shifts in the treatment response reduce the discriminatory power of averaged between-subject comparisons in trials, including RCTs, as demonstrated in Results.
T4 mainly acts as a circulating pro-hormone, requiring both prior transmembrane transport and enzymatic activation to exert a multitude of genomic actions through nuclear thyroid hormone receptor binding (68–71). Both T3 conversion rates and thyroidal T3 secretion are subject to central control by TSH (72–76). The loss of functioning thyroid tissue and/or the TSH-lowering effect of LT4 treatment may impair this compensatory mechanism (16, 19, 77). Due to the expression of distributional individuality and subsequent disruption of the TSH-FT4 correlation, subclinical hypothyroidism is an indeterminate and unreliable disease classifier (5, 13). This dissolves the existing relationships in an individual prior to thyroidectomy, re-adjusting the setpoint and re-setting the equilibria between TSH, FT4, and FT3 in thyroid disease, compared to the healthy state (14, 15, 42, 78). While regarded as essential in maintaining narrow individual serum concentrations of the respective hormones (30), the non-ergodic behavior has yet to be transferred to the treatment situation where population range-based recommendations are paramount in guidelines (2).
Dissimilar clusters or individuals may have conditional requirements for optimum treatment success different from the averaged population and risk profiles may also be shifted. This is in accord with a recent prospective study defining the optimum TSH target slightly below the lower reference limit for patients with thyroid carcinoma treated with LT4, based on the examination of surrogate markers (79). TSH-independent risk profiles have also been demonstrated by the Rotterdam study in euthyroid subjects, although this study did not include a sufficient number of LT4-treated subjects (17, 18). We note that in our study patients with uncontrolled or refractory hypothyroidism (TSH > 4 mIU/l) were excluded and TSH suppression was primarily motivated by tumor control–which is now managed differently–not symptom control (80–83). We do not infer that patients should have a suppressed TSH, rather that the personal levels expressed in the treated condition have different meanings, compared to the untreated situation. Neither does a TSH measurement within its reference limits guarantee that a patient will be symptom-free, nor that a presumably healthy person by this definition may not suffer serious adverse consequences such as atrial fibrillation (17). We and others proposed a more personal definition of “euthyroid,” based on individual traits (setpoints) and dynamic changes between the relationships of all three thyroid parameters TSH, FT4, and FT3 (5). This extends to both genetically determined fingerprints and treatment-related alterations in the expression of personal setpoints and includes other allostatic expressions of individuality (e.g., in their gut microbiome) affecting iodothyronine homeostasis (84–90).
Dissimilarities in the treatment responses between individual patients are particularly apparent when patients on LT4 display substantial variation in their T4–T3 conversion efficiency and pronounced disjoints between their serum TSH and FT3 concentrations (16, 77). Importantly, FT3 levels relative to TSH in symptomatic vs. asymptomatic presentations of the same patients did not move along a shared trajectory but were shifted upward when the patients transitioned from the symptomatically hypothyroid to the asymptomatic condition. Depending on the patient presentation, the addition of LT3 is increasingly considered by thyroid experts worldwide (12). Differential treatment of identifiable dissimilar subpopulations, e.g., with persistently low FT3 concentrations despite normalized TSH (77), appears feasible, but was not tested in this study and awaits further proof.
Complex patterns emerge between TSH, FT4, and FT3 in patients treated with LT4 during follow-up in response to the treatment and changes in LT4 dose, displaying a high degree of intra-class correlation and multileveled structure. This invokes the danger of inappropriate statistical averaging in clinical trials, mandating a stronger focus on within-subject analyses according to ergodic principles. It emphasizes a need to better define personal treatment outcomes and individual risk profiles in patients receiving LT4 alone or, similarly, a combination of T3 and T4.
The prospective trial was registered (www.ClinicalTrials.gov, NCT 01969552) and the protocol was ethically approved by the Ethical Committee of the University of Münster, Germany. All participants gave written informed consent. The retrospective analysis was specifically approved by the local authorities in data protection. The study was carried out in accordance with the Declaration of Helsinki.
All authors have significantly contributed to the findings reported here, and all authors have jointly conceptualized the study and agreed to the final submitted manuscript.
JD is co-owner of the intellectual property rights for the patent “System and Method for Deriving Parameters for Homeostatic Feedback Control of an Individual” (Singapore Institute for Clinical Sciences, Biomedical Sciences Institutes, Application Number 201208940-5, WIPO number WO/2014/088516).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Biondi B, Wartofsky L. Treatment with thyroid hormone. Endocr Rev. (2014) 35:433–512. doi: 10.1210/er.2013-1083
2. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Replacement ATATFOTH. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. (2014) 24:1670–751. doi: 10.1089/thy.2014.0028
3. McAninch EA, Bianco AC. The history and future of treatment of hypothyroidism. Ann Intern Med. (2016) 164:50–6. doi: 10.7326/M15-1799
4. Razvi S, Korevaar TIM, Taylor P. Trends, determinants, and associations of treated hypothyroidism in the United Kingdom, 2005-2014. Thyroid. (2019) 29:174–82. doi: 10.1089/thy.2018.0251
5. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Individualised requirements for optimum treatment of hypothyroidism: complex needs, limited options. Drugs Cont. (2019) 8:1–18. doi: 10.7573/dic.212597
6. Slater S. The discovery of thyroid replacement therapy. Part 1: in the beginning. J R Soc Med. (2011) 104:15–8. doi: 10.1258/jrsm.2010.10k050
7. Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J. (2012) 1:55–71. doi: 10.1159/000339444
8. Peterson SJ, Cappola AR, Castro MR, Dayan CM, Farwell AP, Hennessey JV, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid. (2018) 28:707–21. doi: 10.1089/thy.2017.0681
9. Winther KH, Cramon P, Watt T, Bjorner JB, Ekholm O, Feldt-Rasmussen U, et al. Disease-specific as well as generic quality of life is widely impacted in autoimmune hypothyroidism and improves during the first six months of levothyroxine therapy. PLoS ONE. (2016) 11:e0156925. doi: 10.1371/journal.pone.0156925
10. Hennessey JV, Espaillat R. Current evidence for the treatment of hypothyroidism with levothyroxine/levotriiodothyronine combination therapy versus levothyroxine monotherapy. Int J Clin Pract. (2018) 72:e13062–14. doi: 10.1111/ijcp.13062
11. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Lessons from randomised clinical trials for triiodothyronine treatment of hypothyroidism: have they achieved their objectives. J Thyroid Res. (2018) 2018:3239197. doi: 10.1155/2018/3239197
12. Jonklaas J, Tefera E, Shara N. Prescribing therapy for hypothyroidism: influence of physician characteristics. Thyroid. (2019) 29:44–52. doi: 10.1089/thy.2018.0369
13. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Recent advances in thyroid hormone regulation: toward a new paradigm for optimal diagnosis and treatment. Front Endocrinol. (2017) 8:364. doi: 10.3389/fendo.2017.00364
14. Ito M, Miyauchi A, Morita S, Kudo T, Nishihara E, Kihara M, et al. TSH-suppressive doses of levothyroxine are required to achieve preoperative native serum triiodothyronine levels in patients who have undergone total thyroidectomy. Eur J Endocrinol. (2012) 167:373–8. doi: 10.1530/EJE-11-1029
15. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Is pituitary TSH an adequate measure of thyroid hormone-controlled homoeostasis during thyroxine treatment. Eur J Endocrinol. (2013) 168:271–80. doi: 10.1530/EJE-12-0819
16. Midgley JEM, Larisch R, Dietrich JW, Hoermann R. Variation in the biochemical response to L-thyroxine therapy and relationship with peripheral thyroid hormone conversion efficiency. Endocr Connect. (2015) 4:196–205. doi: 10.1530/EC-15-0056
17. Chaker L, Van Den Berg ME, Niemeijer MN, Franco OH, Dehghan A, Hofman A, et al. Thyroid function and sudden cardiac death: a prospective population-based cohort study. Circulation. (2016) 134:713–22. doi: 10.1161/CIRCULATIONAHA.115.020789
18. Baumgartner C, Da Costa BR, Collet TH, Feller M, Floriani C, Bauer DC, et al. Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. (2017) 136:2100–16. doi: 10.1161/CIRCULATIONAHA.117.028753
19. Hoermann R, Midgley JEM, Giacobino A, Eckl WA, Wahl HG, Dietrich JW, et al. Homeostatic equilibria between free thyroid hormones and pituitary thyrotropin are modulated by various influences including age, body mass index and treatment. Clin Endocrinol. (2014) 81:907–15. doi: 10.1111/cen.12527
20. Hoermann R, Midgley JEM, Dietrich JW, Larisch R. Dual control of pituitary thyroid stimulating hormone secretion by thyroxine and triiodothyronine in athyreotic patients. Ther Adv Endocrinol Metab. (2017) 8:83–95. doi: 10.1177/2042018817716401
21. Larisch R, Midgley JEM, Dietrich JW, Hoermann R. Symptomatic relief is related to serum free triiodothyronine concentrations during follow-up in levothyroxine-treated patients with differentiated thyroid cancer. Exp Clin Endocrinol Diabetes. (2018) 126:546–52. doi: 10.1055/s-0043-125064
22. Larisch R, Giacobino A, Eckl WA, Wahl HG, Midgley JEM, Hoermann R. Reference range for thyrotropin. Post hoc assessment. Nuklearmedizin. (2015) 54:112–7. doi: 10.3413/Nukmed-0671-14-06
23. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. (2015) 67:1–48. doi: 10.18637/jss.v067.i01
24. Bliese P. Within-group agreement, non-independence, and reliability: implications for data aggregation and analysis. In: Klein KJ, Kozlowski SW, editors. Multilevel Theory, Research, and Methods in Organizations. San Francisco, CA: Jossey-Bass, Inc. (2000). p. 349–81.
25. Bliese P. Multilevel: Multilevel Functions. R package version 2.6. 2016. Available online at: https://cran.r-project.org/package=multilevel (accessed January 14, 2019).
26. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing (2019). Available online at: https://www.R-project.org/ (accessed January 14, 2019).
27. Fox J, Weisberg S. An R Companion to Applied Regression. 3rd ed. Thousand Oaks, CA: SAGE Publications (2019). Available online at: http://tinyurl.com/carbook (accessed January 14, 2019).
28. Fox J, Friendly M, Monette G. Visualizing Tests in Multivariate Linear Models. R package version 1.3–5. (2018). Available online at: https://cran.r-project.org/package=heplots (accessed January 14, 2019).
29. Luedecke D. Statistical Functions for Regression Models. (2019). Available online at: https://cran.r-project.org/package=sjstats (accessed January 14, 2019).
30. Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. (2002) 87:1068–72. doi: 10.1210/jcem.87.3.8165
31. Feller W. An Introduction to Probability Theory and Its Applications. 2nd ed. Vol. 2. New Delhi: Wiley India Pvt. Limited. (2008). p. 1–800.
32. Molenaar PCM, Campbell CG. The new person-specific paradigm in psychology. Curr Dir Psychol Sci. (2009) 18:112–7. doi: 10.1111/j.1467-8721.2009.01619.x
33. Boker SM, Molenaar PC, Nesselroade JR. Issues in intraindividual variability: individual differences in equilibria and dynamics over multiple time scales. Psychol Aging. (2009) 24:858–62. doi: 10.1037/a0017912
34. Hamaker EL. Why researchers should think “within-person”: a paradigmatic rationale. In: Mehl MR, Conner TS, editors. Handbook of Research Methods for Studying Daily Life. New York: Guilford Press. (2012). p. 43–61.
35. Fisher AJ, Medaglia JD, Jeronimus BF. Lack of group-to-individual generalizability is a threat to human subjects research. Proc Natl Acad Sci USA. (2018) 115:E6106–15. doi: 10.1073/pnas.1711978115
36. Simpson EH. The interpretation of interaction in contingency tables. J Roy Stat Soc. (1951) 13:238–41.
37. Rücker G, Schumacher M. Simpson's paradox visualized: the example of the rosiglitazone meta-analysis. BMC Med Res Methodol. (2008) 8:34. doi: 10.1186/1471-2288-8-34
38. Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol. (2011) 62:583–619. doi: 10.1146/annurev.psych.093008.100356
39. Andersen S, Bruun NH, Pedersen KM, Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid. (2003) 13:1069–78. doi: 10.1089/105072503770867237
40. Dietrich JW, Landgrafe G, Fotiadou EH. TSH and thyrotropic agonists: key actors in thyroid homeostasis. J Thyroid Res. (2012) 2012:351864. doi: 10.1155/2012/351864
41. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Advances in applied homeostatic modelling of the relationship between thyrotropin and free thyroxine. PLoS ONE. (2017) 12:e0187232. doi: 10.1371/journal.pone.0187232
42. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Relational stability in the expression of normality, variation, and control of thyroid function. Front Endocrinol. (2016) 7:142. doi: 10.3389/fendo.2016.00142
43. Seely AJE, Macklem PT. Complex systems and the technology of variability analysis. Crit Care. (2004) 8:R367–84. doi: 10.1186/cc2948
44. Jones JC. Honey bee nest thermoregulation: diversity promotes stability. Science. (2004) 305:402–4. doi: 10.1126/science.1096340
45. Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. (2015) 160:816–27. doi: 10.1016/j.cell.2015.02.010
46. Canaris GJ, Steiner JF, Ridgway EC. Do traditional symptoms of hypothyroidism correlate with biochemical disease? J Gen Int Med. (1997) 12:544–50. doi: 10.1046/j.1525-1497.1997.07109.x
47. Ott J, Promberger R, Kober F, Neuhold N, Tea M, Huber JC, et al. Hashimoto's thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid. (2011) 21:161–7. doi: 10.1089/thy.2010.0191
48. Quinque EM, Villringer A, Kratzsch J, Karger S. Patient-reported outcomes in adequately treated hypothyroidism - insights from the German versions of thydqol, thysrq and thytsq. Health Qual Life Outcomes. (2013) 11:68. doi: 10.1186/1477-7525-11-68
49. Kelderman-Bolk N, Visser TJ, Tijssen JP, Berghout A. Quality of life in patients with primary hypothyroidism related to BMI. Eur J Endocrinol. (2015) 173:507–15. doi: 10.1530/EJE-15-0395
50. Applewhite MK, James BC, Kaplan SP, Angelos P, Kaplan EL, Grogan RH, et al. Quality of life in thyroid cancer is similar to that of other cancers with worse survival. World J Surg. (2016) 40:551–61. doi: 10.1007/s00268-015-3300-5
51. Massolt ET, Van Der Windt M, Korevaar TIM, Kam BLR, Burger JW, Franssen GJH, et al. Thyroid hormone and its metabolites in relation to quality of life in patients treated for differentiated thyroid cancer. Clin Endocrinol. (2016) 85:781–8. doi: 10.1111/cen.13101
52. Blum MR, Wijsman LW, Virgini VS, Bauer DC, Den Elzen WP, Jukema JW, et al. Subclinical thyroid dysfunction and depressive symptoms among the elderly: a prospective cohort study. Neuroendocrinology. (2016) 103:291–9. doi: 10.1159/000437387
53. Carlé A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Andersen S, et al. Hypothyroid symptoms fail to predict thyroid insufficiency in old people: a population-based case-control study. Am J Med. (2016) 129:1082–92. doi: 10.1016/j.amjmed.2016.06.013
54. Pollock MA, Sturrock A, Marshall K, Davidson KM, Kelly CJ, Mcmahon AD, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: randomised double blind placebo controlled crossover trial. BMJ. (2001) 323:891–5. doi: 10.1136/bmj.323.7318.891
55. Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. (2006) 91:145–53. doi: 10.1210/jc.2005-1775
56. Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RGJ, Mooijaart SP, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. (2017) 376:2534–44. doi: 10.1056/NEJMoa1603825
57. Samuels MH, Kolobova I, Niederhausen M, Janowsky JS, Schuff KG. Effects of altering levothyroxine (L-T4) doses on quality of life, mood, and cognition in L-T4 treated subjects. J Clin Endocrinol Metab. (2018) 103:1997–2008. doi: 10.1210/jc.2017-02668
58. Michaelsson LF, La Cour JL, Medici BB, Watt T, Faber J, Nygaard B. Levothyroxine/liothyronine combination therapy and quality of life: is it all about weight loss. Eur Thyroid J. (2018) 7:243–50. doi: 10.1159/000490383
59. Hedman C, Djärv T, Strang P, Lundgren CI. Fear of recurrence and view of life affect health-related quality of life in patients with differentiated thyroid carcinoma: a prospective Swedish population-based study. Thyroid. (2018) 28:1609–17. doi: 10.1089/thy.2018.0388
60. Feller M, Snel M, Moutzouri E, Bauer DC, De Montmollin M, Aujesky D, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. (2018) 320:1349–59. doi: 10.1001/jama.2018.13770
61. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. (2018) 229:410–4. doi: 10.1016/j.jad.2017.12.071
62. Samuels MH, Kolobova I, Antosik M, Niederhausen M, Purnell JQ, Schuff KG. Thyroid function variation in the normal range, energy expenditure, and body composition in L-T4-treated subjects. J Clin Endocrinol Metab. (2017) 102:2533–42. doi: 10.1210/jc.2017-00224
63. Ylli D, Wartofsky L. Can we link thyroid status, energy expenditure, and body composition to management of subclinical thyroid dysfunction. J Clin Endocrinol Metab. (2019) 104:209–12. doi: 10.1210/jc.2018-01997
64. Virili C, Antonelli A, Santaguida MG, Benvenga S, Centanni M. Gastrointestinal malabsorption of thyroxine. Endocr Rev. (2019) 40:118–36. doi: 10.1210/er.2018-00168
65. George BJ, Li P, Lieberman HR, Pavela G, Brown AW, Fontaine KR, et al. Randomization to randomization probability: estimating treatment effects under actual conditions of use. Psychol Methods. (2018) 23:337–50. doi: 10.1037/met0000138
66. Hedman C, Djärv T, Strang P, Lundgren CI. Effect of thyroid-related symptoms on long-term quality of life in patients with differentiated thyroid carcinoma: a population-based study in Sweden. Thyroid. (2017) 27:1034–42. doi: 10.1089/thy.2016.0604
67. Boesen VB, Feldt-Rasmussen U, Bjorner JB, Cramon P, Groenvold M, Nygaard B, et al. How should thyroid-related quality of life be assessed? Recalled patient-reported outcomes compared to here-and-now measures. Thyroid. (2018) 28:1561–70. doi: 10.1089/thy.2018.0210
68. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. (2002) 23:38–89. doi: 10.1210/edrv.23.1.0455
69. Cheng S-Y, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. (2010) 31:139–70. doi: 10.1210/er.2009-0007
70. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. (2014) 94:355–82. doi: 10.1152/physrev.00030.2013
71. Schweizer U, Johannes J, Bayer D, Braun D. Structure and function of thyroid hormone plasma membrane transporters. Eur Thyroid J. (2014) 3:143–53. doi: 10.1159/000367858
72. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. (2008) 29:898–938. doi: 10.1210/er.2008-0019
73. Citterio CE, Veluswamy B, Morgan SJ, Galton VA, Banga JP, Atkins S, et al. De novo triiodothyronine formation from thyrocytes activated by thyroid-stimulating hormone. J Biol Chem. (2017) 292:15434–44. doi: 10.1074/jbc.M117.784447
74. Ishii H, Inada M, Tanaka K, Mashio Y, Naito K, Nishikawa M, et al. Induction of outer and inner ring monodeiodinases in human thyroid gland by thyrotropin. J Clin Endocrinol Metab. (1983) 57:500–5. doi: 10.1210/jcem-57-3-500
75. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Relational stability of thyroid hormones in euthyroid subjects and patients with autoimmune thyroid disease. Eur Thyroid J. (2016) 5:171–9. doi: 10.1159/000447967
76. Berberich J, Dietrich JW, Hoermann R, Müller MA. Mathematical modeling of the pituitary-thyroid feedback loop: role of a TSH-T3-shunt and sensitivity analysis. Front Endocrinol. (2018) 9:91. doi: 10.3389/fendo.2018.00091
77. Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS ONE. (2011) 6:e22552. doi: 10.1371/journal.pone.0022552
78. Peterson SJ, McAninch EA, Bianco AC. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy. J Clin Endocrinol Metab. (2016) 101:4964–73. doi: 10.1210/jc.2016-2660
79. Ito M, Miyauchi A, Hisakado M, Yoshioka W, Ide A, Kudo T, et al. Biochemical markers reflecting thyroid function in athyreotic patients on levothyroxine monotherapy. Thyroid. (2017) 27:484–90. doi: 10.1089/thy.2016.0426
80. Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest. (2017) 40:1289–301. doi: 10.1007/s40618-017-0706-y
81. Nieto H, Boelaert K. Women in cancer thematic review: thyroid-stimulating hormone in thyroid cancer: does it matter? Endocr Relat Cancer. (2016) 23:T109–21. doi: 10.1530/ERC-16-0328
82. Carhill AA, Litofsky DR, Ross DS, Jonklaas J, Cooper DS, Brierley JD, et al. Long-term outcomes following therapy in differentiated thyroid carcinoma: NTCTCS registry analysis 1987-2012. J Clin Endocrinol Metab. (2015) 100:3270–9. doi: 10.1210/JC.2015-1346
83. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
84. Thvilum M, Brandt F, Almind D, Christensen K, Hegedüs L, Brix TH. Excess mortality in patients diagnosed with hypothyroidism: a nationwide cohort study of singletons and twins. J Clin Endocrinol Metab. (2013) 98:1069–75. doi: 10.1210/jc.2012-3375
85. Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. (2013) 9:e1003266. doi: 10.1371/journal.pgen.1003266.s011
86. McAninch EA, Jo S, Preite NZ, Farkas E, Mohácsik P, Fekete C, et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J Clin Endocrinol Metab. (2015) 100:920–33. doi: 10.1210/jc.2014-4092
87. Chng CL, Lim AY, Tan HC, Kovalik JP, Tham KW, Bee YM, et al. Physiological and metabolic changes during the transition from hyperthyroidism to euthyroidism in Graves' disease. Thyroid. (2016) 26:1422–30. doi: 10.1089/thy.2015.0602
88. Massolt ET, Chaker L, Visser TJ, Gillis AJM, Dorssers LCJ, Beukhof CM, et al. Serum microRNA profiles in athyroid patients on and off levothyroxine therapy. PLoS ONE. (2018) 13:e0194259. doi: 10.1371/journal.pone.0194259
89. Carlé A, Faber J, Steffensen R, Laurberg P, Nygaard B. Hypothyroid patients encoding combined MCT10 and Dio2 gene polymorphisms may prefer L-T3 + L-T4 combination treatment - data using a blind, randomized, clinical study. Eur Thyroid J. (2017) 6:143–51. doi: 10.1159/000469709
Keywords: intra-class correlation, response heterogeneity, LT4 treatment, thyroid carcinoma, thyroid homeostasis, setpoint, ergodicity
Citation: Hoermann R, Midgley JEM, Larisch R and Dietrich JW (2019) Functional and Symptomatic Individuality in the Response to Levothyroxine Treatment. Front. Endocrinol. 10:664. doi: 10.3389/fendo.2019.00664
Received: 18 January 2019; Accepted: 13 September 2019;
Published: 26 September 2019.
Edited by:
Terry Francis Davies, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Salvatore Benvenga, University of Messina, ItalyCopyright © 2019 Hoermann, Midgley, Larisch and Dietrich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rudolf Hoermann, cnVkb2xmLmhvZXJtYW5uQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.