- 1Chronic Disease Epidemiology Laboratory, Pennington Biomedical Research Center, Baton Rouge, LA, United States
- 2Shanghai Business School, Shanghai, China
- 3Department of Endocrinology and Metabolism, Fengxian Hospital of Southern Medical University, Shanghai, China
- 4Tianjin Women's and Children's Health Center, Tianjin, China
- 5Department of Public Health, University of Helsinki, Helsinki, Finland
- 6Population Cancer Research Program, Dalhousie University, Halifax, NS, Canada
- 7Department of Epidemiology, School of Public Health, Tianjin Medical University, Tianjin, China
- 8Department of Environmental Health Sciences, Columbia University Mailman School of Public Health, New York, NY, United States
- 9Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
Objectives: Either maternal gestational diabetes mellitus (GDM) or hypertensive disorder of pregnancy (HDP) is associated with an increased risk of obesity in the offspring. However, their joint associations with obesity in offspring remain unclear. We investigated the joint associations of maternal GDM and HDP with childhood overweight in offspring.
Methods: We performed a large study in 1967 mother-child pairs. Maternal GDM was diagnosed according to the 1999 World Health Organization (WHO) criteria. HDP was defined as self-reported doctor-diagnosed hypertension or treatment of hypertension (including gestational hypertension, preeclampsia, sever preeclampsia or eclampsia) after 20 weeks of gestation on the questionnaire. Body mass index (BMI) for age Z-score and childhood overweight were evaluated according to WHO growth reference. We used the general linear models to compare children's Z score for BMI and logistic regression models to estimate odds ratios of childhood overweight according to maternal different status of GDM and HDP.
Results: Offspring of mothers with both GDM and HDP had a higher BMI for age Z-score (0.63 vs. 0.03, P < 0.001) than children born to normotensive and normoglycemic pregnancy. After adjustment for maternal and children's major confounding factors, joint GDM and HDP were associated with increased odds ratios of offspring's overweight compared with normotensive and normoglycemic pregnancy (2.97, 95% confidence intervals [CIs] 1.65–5.34) and GDM alone (2.06, 95% CIs 1.20–3.54), respectively. After additional adjustment for maternal pre-pregnancy BMI and gestational weight gain, joint maternal GDM, and HDP was still associated with an increased risk of offspring's overweight compared with the maternal normotensive, and normoglycemic group but became to have a borderline increased risk compared with the maternal GDM alone group.
Conclusions: Maternal GDM alone or joint GDM and HDP were associated with increased ratios of offspring's overweight.
Introduction
Children with overweight and obesity are more likely to be affected by obesity into adulthood and develop non-communicable diseases including metabolic syndrome, cardiovascular diseases, and type 2 diabetes (1–3). The prevalence of obesity among children has been increasing globally in the past 25 years (4). In China, the prevalence of overweight and obesity among preschoolers reached 10.1% in 2010, and obesity was much worse in children than in adolescents (5, 6). China now has the largest number of children with obesity in the world (4).
Changes in children's behaviors, such as unhealthy eating habit, lacking moderate-to-vigorous physical activity, and increasing sedentary time, mainly account for the increasing prevalence of childhood obesity (7–9). Other factors, such as genetic susceptibility, socioeconomic inequalities, maternal education, children's sleep disturbance, neonatal macrosomia, may also play an important role in obesity formation (8–11). Recent studies have indicated that maternal metabolic disorders during pregnancy, such as gestational diabetes mellitus (GDM), and hypertensive disorder of pregnancy (HDP), were associated with an increased risk of obesity in children over 5 years old. Several but not all studies have found that maternal GDM was positively associated with general overweight/obesity, central obesity, and subcutaneous adiposity (skinfold thickness) in the offspring after 5 years old (12–17). Meanwhile, researchers pointed out that maternal HDP was associated with a greater risk of childhood overweight or obesity (18–20). A previous study from our team also demonstrated that maternal HDP was a risk factor for childhood overweight or obesity in the offspring of mothers with GDM (21). Nevertheless, it is unclear whether GDM concomitant with HDP represents an extra higher risk factor for obesity in offspring. We aimed to examine the joint association of maternal GDM and HDP with children's overweight among 1,967 mother-child pairs, including 1,263 mothers with GDM, and 704 without GDM.
Materials and Methods
GDM Screening Process
Tianjin is the fourth largest city in China, only 30-min distance by train from Beijing. There are six central districts in Tianjin with about 4.3 million residents. In 1999, the Tianjin Women's and Children's Health Center launched an urban universal screening of GDM using the 1999 World Health Organization (WHO)'s criteria in all six central districts. The screening rate was reported to be >91% between 1999 and 2008 (22). We first invited all pregnant women (at their 26–30 gestational weeks) to participate in the 1-h 50-g glucose screening test in their community health centers. Then, those with glucose reading ≥7.8 mmol/L were referred to the Tianjin Women's and Children's Health Center to undergo a 2-h oral glucose tolerance test (OGTT) with 75-g glucose load. If the pregnant women met the 1999 WHO's criteria of diabetes (fasting glucose ≥7 mmol/L or 2-h glucose ≥11.1 mmol/L), or impaired glucose tolerance (IGT) (2-h glucose ≥7.8 mmol/L and <11.1 mmol/L), they would be diagnosed as GDM (23).
Study Population
Totally 76,325 women were screened from 2005 to 2009, among whom 4,644 women were diagnosed as GDM, and 71,681 were free of GDM. We invited all 4,644 women with GDM to participate in the Tianjin Gestational Diabetes Mellitus Prevention Program (TGDMPP). From August 2009 to July 2011, a total of 1,263 women with GDM and their children finished the baseline survey. There were no differences at 26–30 gestational weeks OGTT test in age (28.9 vs. 28.7 years), fasting glucose (5.34 vs. 5.34 mmol/l), 2-h glucose (9.23 vs. 9.16 mmol/l), and the prevalence of IGT (90.9 vs. 91.8%) and diabetes (9.1 vs. 8.2%) between the returned and unreturned GDM women. We randomly chose and recruited 704 non-GDM mother-child pairs, with age and sex frequency-matched to children of GDM mothers at 1–2 years after the baseline survey of GDM mother-child pairs.
This study was carried out in accordance with the recommendations of the Human Subjects Committee of Tianjin Women's and Children's Health Center with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Human Subjects Committee of Tianjin Women's and Children's Health Center.
Questionnaires and Measurements
A self-administered questionnaire was implemented to collect mothers' information, including socio-demographic characteristics, such as age, education (<13 years, 13–16 years, and ≥16 years), history of GDM and its treatment during pregnancy; pregnancy outcomes (pre-pregnancy weight, weight gain during pregnancy, gestational age, etc.); and lifestyle in the past year, such as smoking status. HDP was defined as self-reported doctor-diagnosed hypertension or treatment of hypertension (including gestational hypertension, preeclampsia, sever preeclampsia or eclampsia) after 20 weeks of gestation on the questionnaire (8). Children's information was collected by another questionnaire completed by their mothers, including children's general information, such as sex, birth date, age, birth weight, birth length, lactation (exclusive formula, mixed, or exclusive breast) and lactation duration; history of diseases, and medication; dietary habits [using a validated food frequency questionnaire [FFQ]] (24); and routine activities (indoor and outdoor activities, screening watching time, and sleep duration) (25). Macrosomia was defined when birth weight ≥4,000 g.
All mother-child pairs underwent a physical examination. Using the standardized protocol, all participants' height and weight were measured in light indoor clothing and without shoes by trained research doctors. Body mass index (BMI) was obtained by dividing weight in kilograms by the square of height in meters. All mothers' pre-pregnancy BMI used their self-reported pre-pregnancy weight and their measured height. Children's BMI calculation used their body weight and height examined in the study visit. Children's Z scores for BMI-for-age were calculated based on the WHO growth reference (26, 27). Children's BMI was classified as normal weight, BMI <85th percentiles; overweight, 85th percentile ≤ BMI <95th percentile; and obesity, BMI ≥95th percentile, according to the WHO age-, and sex-specific growth reference (26, 27).
Statistical Analysis
Differences in the general characteristics (continuous and categorical variables) of both mothers and children according to maternal different status of GDM and HDP were tested using the univariate analysis of variance or chi-square test. We used the general linear models.
to compare children's Z scores for BMI and used logistic regression models to estimate odds ratios of childhood overweight according to maternal different status of GDM and HDP. All analyses were adjusted for maternal age, gestational age, education, smoking status, history of treatment of GDM, and HDP, children's sex, age, birth weight, and feeding status (Model 1), and then children's lifestyles including outdoor physical activity time, screen watching time, sleep time, daily energy intake, energy from fat and dietary fiber intake (Model 2), and further for maternal pre-pregnancy BMI and gestational weight gain (Model 3). All the statistical analyses were performed with SPSS 25.0 for windows software package (IBM SPSS statistics 25) and SAS Proprietary Software 9.4 (SAS Institute Inc., Cary, NC, USA). Two-sided P < 0.05 was considered statistically significant.
Results
As shown in Table 1, there were significant differences in maternal age at delivery, pre-pregnancy BMI, gestational weight gain, gestational age at delivery, and education among 4 different groups (all P < 0.05). There were also differences in children's age, birth weight, outdoor activity time, screen watching time, Z score for BMI-for-age, prevalence of overweight/obesity, and daily energy intake from fat according to various maternal GDM and HDP status (P < 0.001).
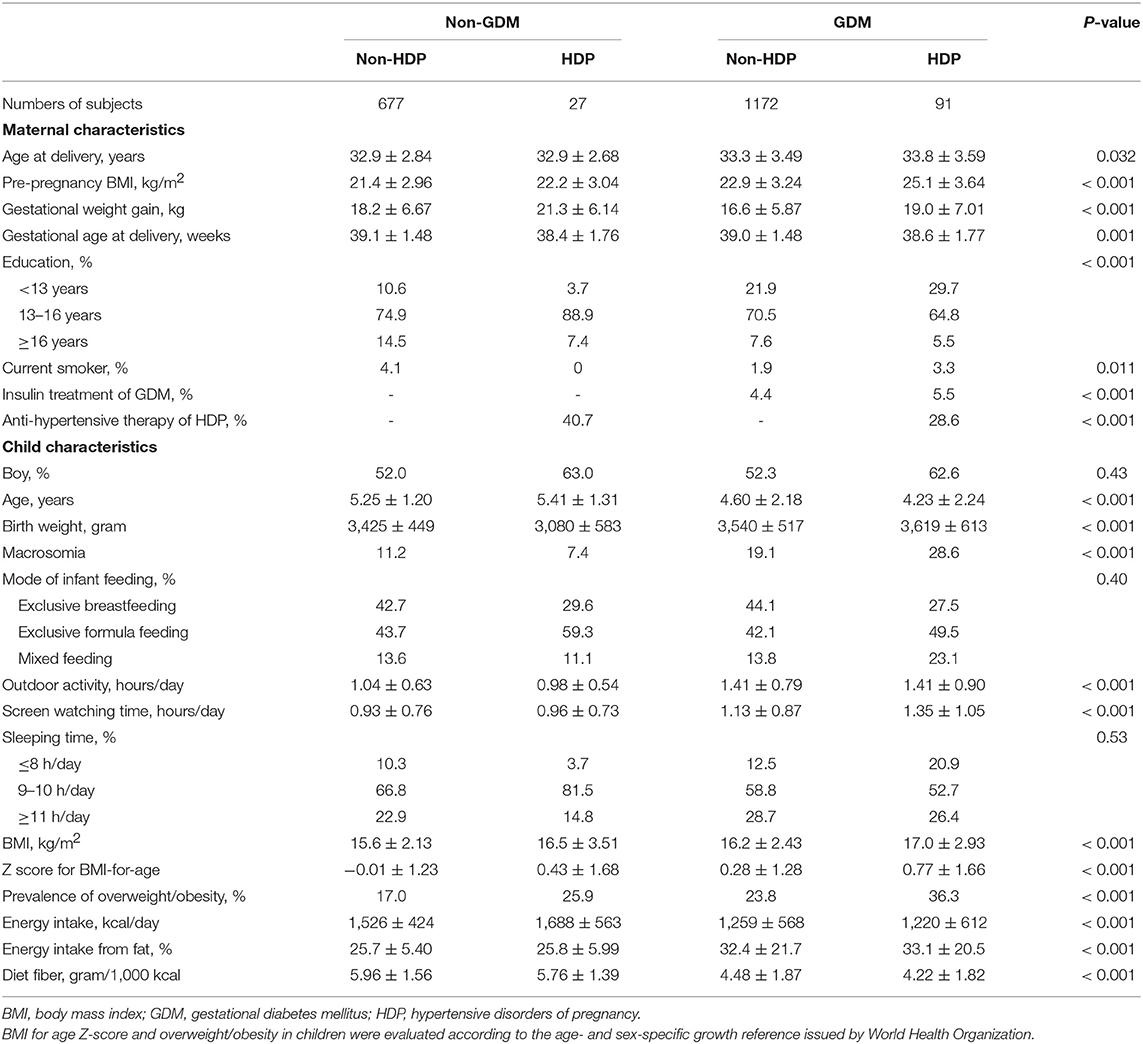
Table 1. Maternal and child characteristics according to maternal gestational diabetes and hypertensive disorders of pregnancy.
In comparison with children born to normotensive and normoglycemic pregnancy, offspring of mothers with HDP alone, GDM alone, and concomitant GDM and HDP had higher levels of Z score for BMI-for-age after adjustment for maternal age at delivery, gestational age at delivery, education, current smoking, history of treatment of GDM and HDP, and children's age, sex, birth weight, and feeding status (Model 1, Table 2). After further adjustment for children's lifestyle factors, maternal pre-pregnancy BMI, and gestational weight gain, the difference of Z score for BMI was still significant among children who born to mothers with GDM alone and concomitant GDM and HDP, but no longer significant among children born to HDP pregnancy alone, compared with those born to normotensive and normoglycemic mothers during pregnancy.
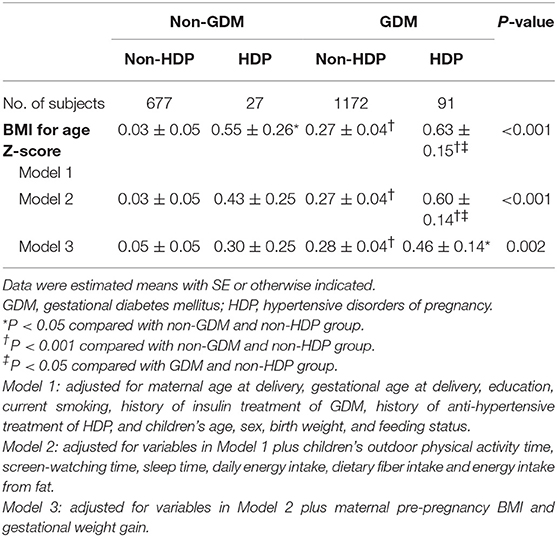
Table 2. Comparison of BMI for age Z-score according to maternal gestational diabetes and hypertensive disorders of pregnancy.
Compared with children born to normotensive and normoglycemic pregnancy, multi-variable (maternal age, gestational age, education and smoking status, history of treatment of GDM, and HDP, children's sex, age, birth weight, and feeding status) adjusted odds ratios (95% confidence intervals [95% CI]) of overweight among children who born to mothers with HDP alone, with GDM only, and with both GDM and HDP were 2.61 (95% CI 0.98–6.93), 1.51 (95% CI 1.17–1.95), and 3.11 (95% CI 1.78–5.43), respectively (Model 1, Table 3). After additional adjustment for children's lifestyle factors, maternal pre-pregnancy BMI, and gestational weight gain, children who born to mothers with GDM only or with both GDM and HDP still had significantly increased odds ratios of overweight compared with children who born to mothers with normotension and normoglycaemia during pregnancy (Model 2-3, Table 3). There was no difference in odds ratios of childhood overweight between children who born to mothers with HDP only and children who born to mothers with GDM only. Children who were born to mothers with joint GDM and HDP had ~1-fold higher multivariable-adjusted risk of overweight compared with children who were born to mothers with GDM only, but this association changed to borderline significance after additional adjustment for maternal pre-pregnancy BMI and gestational weight gain.
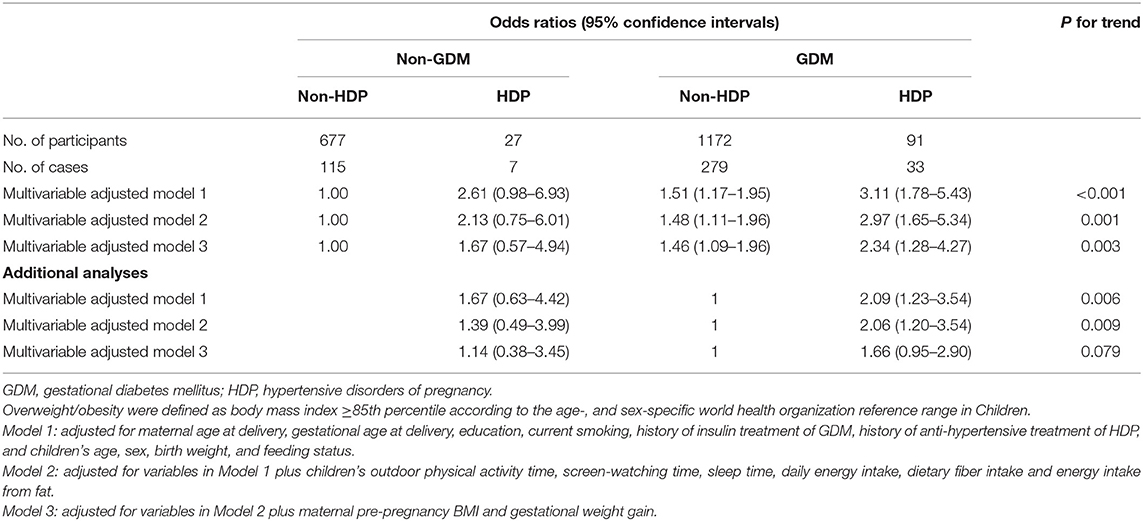
Table 3. Odds ratios for childhood overweight according to maternal gestational diabetes and hypertensive disorders of pregnancy.
Discussion
The present study indicated that maternal GDM alone or joint GDM and HDP was associated with an increased Z score for BMI-for-age and a higher risk of childhood overweight in offspring. Concomitant of maternal GDM and HDP showed an additionally higher risk for offspring's overweight than maternal GDM alone in offspring, but this association was not fully independent of maternal pre-pregnancy BMI and gestational weight gain.
Maternal GDM and HDP are two common complications during pregnancy, and are associated with adverse pregnant outcomes and exert long-term effect on their children (12, 28). Most but not all studies have indicated that children born to mothers with GDM are at higher risk of overweight or obesity compared with those born to mothers with normal glucose during pregnancy (15, 29–31). Studies on maternal HDP with children's obesity were controversial. Most studies demonstrated that maternal HDP was associated with elevated BMI or an increased risk for children's overweight and obesity (18–21). However, a US study showed that offspring of mothers with HDP had a reduced Z score for BMI compared with children of non-HDP mothers (32). This study also showed that children born to mothers with GDM alone had a 1.5-fold increased risk for overweight/obesity compared with children born to mothers with normotension and normoglycaemia during pregnancy, and there was no difference in odd ratio of overweight/obesity between children born to mothers with GDM alone and those born to mothers with HDP alone. Furthermore, although birth weight was a risk factor for childhood obesity (8), children born to HDP pregnancy had a lower or similar birth weight when compared with the controls (18, 19, 32), implying the association between maternal HDP and children's obesity was complicated and long lasting. Until now, very few studies have assessed the joint association of maternal GDM and HDP with the childhood obesity, and results remained unknown. Kvehaugen and colleagues reported a non-significant higher proportion of overweight or obesity in children of mothers with GDM or preeclampsia than children of mothers without GDM and HDP (33). The present study showed that children born to mothers with joint GDM and HDP were associated with higher odds ratios for overweight/obesity than those born to mothers with normotension and normoglycaemia during pregnancy, and with GDM alone.
The mechanism for the association of maternal GDM and HDP with children's overweight and obesity has not been fully understood. Shared familial genes and lifestyle may be the first consideration as the reason of this association (20). However, some researchers ascribed the mechanism to intrauterine programming of epigenome. Data indicated that siblings who were born to uncomplicated pregnancy had significantly lower prevalence of obesity and vascular abnormalities than those born to HDP- or GDM- complicated pregnancy (34, 35). Some studies also demonstrated that placental gene-specific hypomethylation occurred more often in early-onset than late-onset pre-eclampsia (36, 37). Further, amelioration of maternal GDM can help reduce the odds ratio for children's obesity (12). All these clues supported intrauterine programming of epigenome in the fetus, and implied the importance of early prevention, management and treatment of metabolic disorder for next generation's health.
Insulin resistance may mediate GDM and HDP with intrauterine programming of epigenome, because insulin resistance was putative as pathogenesis of HDP and GDM in pregnant women (38–41), and cord plasma insulin level was positively associated with birth weight and neonatal fat mass (42). We postulate that the combination of GDM and HDP may imply more severe insulin resistance, as women with both GDM and HDP had the higher level of pre-pregnancy BMI, a parameter reflecting insulin resistance. This point of view needs further investigation.
Our study enrolled a larger sample of GDM and non-GDM mother-child pairs than previous studies. Data on a variety of confounding variables, such as the parameters of mothers before and during pregnancy; and indices of the children, including birth weight, lifestyles factors, and anthropometric indexes were collected and used in the final analysis. There were some limitations in our study. First, maternal HDP, and children's diet intake, physical activity and sleeping time were based on the self-reported questionnaire, which may bring retrospective bias. However, good concordance between self-reported HDP and clinical records has been validated in the United States and England (43). Second, the sample size in only HDP group was relatively small. Third, other confounders of children's obesity, such as the genetics, insulin resistance, have not been investigated in the present study.
In conclusion, the present study indicated that maternal GDM alone or joint GDM and HDP was associated with an increased Z score for BMI-for-age and a higher risk of childhood overweight in offspring. Maternal GDM and HDP had similar odds ratio for children's overweight. Joint maternal GDM and HDP were associated with extra higher odds ratios of offspring's overweight than maternal GDM alone but this association was not fully independent of maternal pre-pregnancy BMI and gestational weight gain.
Data Availability Statement
All datasets generated for this study are included in the manuscript/Supplementary Files.
Ethics Statement
This study was carried out in accordance with the recommendations of the Human Subjects Committee of Tianjin Women's and Children's Health Center with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Human Subjects Committee of Tianjin Women's and Children's Health Center.
Author Contributions
YG, JLu, and GH conceptualized and designed the study, performed statistical analyses, interpreted the results, and drafted and revised the manuscript. WqL, HL, LW, JLe, WL, SZ, and SW collected the data and revised the manuscript. JT, ZY, XY, AB, and LH critically revised the manuscript for important intellectual contents. All authors critically reviewed the scientific content and approved the final manuscript. GH is the guarantor of this work, and has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was supported by the Tianjin Women's and Children's Health Center, Tianjin Public Health Bureau, European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly programme for Collaborative Research between China and Europe. GH was partly supported by the grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100790) and the National Institute of General Medical Sciences (U54GM104940) of the National Institutes of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewers and handling editor declared their shared affiliation.
References
2. Kelsey MM, Zaepfel A, Bjornstad P, Nadeau KJ. Age-related consequences of childhood obesity. Gerontology. (2014) 60:222–8. doi: 10.1159/000356023
3. Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. (1997) 337:869–73. doi: 10.1056/nejm199709253371301
4. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
5. Xiao Y, Qiao Y, Pan L, Liu J, Zhang T, Li N, et al. Trends in the prevalence of overweight and obesity among Chinese preschool children from 2006 to 2014. PLoS ONE. (2015) 10:e0134466. doi: 10.1371/journal.pone.0134466
6. Zhou Y, Zhang Q, Wang T, Zhang Y, Xu B. Prevalence of overweight and obesity in Chinese children and adolescents from 2015. Ann Hum Biol. (2017) 44:642–3. doi: 10.1080/03014460.2017.1371224
7. Bracale R, Milani Marin LE, Russo V, Zavarrone E, Ferrara E, Balzaretti C, et al. Family lifestyle and childhood obesity in an urban city of Northern Italy. Eat Weight Disord. (2015) 20:363–70. doi: 10.1007/s40519-015-0179-y
8. Qiao Y, Zhang T, Liu H, Katzmarzyk PT, Chaput JP, Fogelholm M, et al. Joint association of birth weight and physical activity/sedentary behavior with obesity in children ages 9-11 years from 12 countries. Obesity. (2017) 25:1091–7. doi: 10.1002/oby.21792
9. Hjorth MF, Chaput JP, Ritz C, Dalskov SM, Andersen R, Astrup A, et al. Fatness predicts decreased physical activity and increased sedentary time, but not vice versa: support from a longitudinal study in 8- to 11-year-old children. Int J Obes. (2014) 38:959–65. doi: 10.1038/ijo.2013.229
10. Munthali RJ, Sahibdeen V, Kagura J, Hendry LM, Norris SA, Ong KK, et al. Genetic risk score for adult body mass index associations with childhood and adolescent weight gain in an African population. Genes Nutr. (2018) 13:24. doi: 10.1186/s12263-018-0613-7
11. Ballon M, Botton J, Charles MA, Carles S, de Lauzon-Guillain B, Forhan A, et al. Socioeconomic inequalities in weight, height and body mass index from birth to 5 years. Int J Obes. (2018) 42:1671–9. doi: 10.1038/s41366-018-0180-4
12. Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. (2007) 30:2287–92. doi: 10.2337/dc06-2361
13. Tam WH, Ma RCW, Ozaki R, Li AM, Chan MHM, Yuen LY, et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care. (2017) 40:679–86. doi: 10.2337/dc16-2397
14. Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. (2009) 94:2464–70. doi: 10.1210/jc.2009-0305
15. Tam WH, Ma RC, Yang X, Li AM, Ko GT, Kong AP, et al. Glucose intolerance and cardiometabolic risk in adolescents exposed to maternal gestational diabetes: a 15-year follow-up study. Diabetes Care. (2010) 33:1382–4. doi: 10.2337/dc09-2343
16. Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. (2009) 22:215–20. doi: 10.1038/ajh.2008.326
17. Shearrer GE, Whaley SE, Miller SJ, House BT, Held T, Davis JN. Association of gestational diabetes and breastfeeding on obesity prevalence in predominately Hispanic low-income youth. Pediatr Obes. (2015) 10:165–71. doi: 10.1111/ijpo.247
18. Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, et al. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. (2015) 5:e008136. doi: 10.1136/bmjopen-2015-008136
19. Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the avon longitudinal study of parents and children. Circulation. (2010) 122:1192–9. doi: 10.1161/circulationaha.110.936674
20. Alsnes IV, Vatten LJ, Fraser A, Bjorngaard JH, Rich-Edwards J, Romundstad PR, et al. Hypertension in pregnancy and offspring cardiovascular risk in young adulthood: prospective and sibling studies in the HUNT study (nord-trondelag health study) in Norway. Hypertension. (2017) 69:591–8. doi: 10.1161/hypertensionaha.116.08414
21. Zhang S, Wang L, Leng J, Liu H, Li W, Zhang T, et al. Hypertensive disorders of pregnancy in women with gestational diabetes mellitus on overweight status of their children. J Hum Hypertens. (2017) 31:731–6. doi: 10.1038/jhh.2017.17
22. Zhang F, Dong L, Zhang C, Li B, Wen J, Gao W, et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabet Med. (2011): 652–7. doi: 10.1111/j.1464-5491.2010.03205.x
23. WHO. Consultation, Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organisation (1999).
24. Li YP, He YN, Zhai FY, Yang XG, Hu XQ, Zhao WH, et al. [Comparison of assessment of food intakes by using 3 dietary survey methods]. Zhonghua Yu Fang Yi Xue Za Zhi. (2006) 40:273–80.
25. Zhang S, Liu H, Zhang C, Wang L, Li N, Leng J, et al. Maternal glucose during pregnancy and after delivery in women with gestational diabetes mellitus on overweight status of their children. Biomed Res Int. (2015) 2015:543038. doi: 10.1155/2015/543038
26. Lu J, Hou X, Zhang L, Hu C, Zhou J, Pang C, et al. Associations between clinical characteristics and chronic complications in latent autoimmune diabetes in adults and type 2 diabetes. Diabetes Metab Res Rev. (2015) 31:411–20. doi: 10.1002/dmrr.2626
27. Zhou Z, Xiang Y, Ji L, Jia W, Ning G, Huang G, et al. Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes. (2013) 62:543–50. doi: 10.2337/db12-0207
28. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. (2008) 358:1991–2002. doi: 10.1056/NEJMoa0707943
29. Vaarasmaki M, Pouta A, Elliot P, Tapanainen P, Sovio U, Ruokonen A, et al. Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. Am J Epidemiol. (2009) 169:1209–15. doi: 10.1093/aje/kwp020
30. Vohr BR, McGarvey ST, Tucker R. Effects of maternal gestational diabetes on offspring adiposity at 4-7 years of age. Diabetes Care. (1999) 22:1284–91.
31. Wang J, Pan L, Liu E, Liu H, Liu J, Wang S, et al. Gestational diabetes and offspring's growth from birth to 6 years old. Int J Obes. (2018) 43:663–72. doi: 10.1038/s41366-018-0193-z
32. Tripathi RR, Rifas-Shiman SL, Hawley N, Hivert MF, Oken E. Hypertensive disorders of pregnancy and offspring cardiometabolic health at midchildhood: project viva findings. J Am Heart Assoc. (2018) 7:e007426. doi: 10.1161/jaha.117.007426
33. Kvehaugen AS, Andersen LF, Staff AC. Anthropometry and cardiovascular risk factors in women and offspring after pregnancies complicated by preeclampsia or diabetes mellitus. Acta Obstet Gynecol Scand. (2010) 89:1478–85. doi: 10.3109/00016349.2010.500368
34. Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. (2010) 122:488–94. doi: 10.1161/circulationaha.110.941203
35. Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes. (1988) 37:622–8.
36. Yuen RK, Penaherrera MS, von Dadelszen P, McFadden DE, Robinson WP. DNA methylation profiling of human placentas reveals promoter hypomethylation of multiple genes in early-onset preeclampsia. Eur J Hum Genet. (2010) 18:1006–12. doi: 10.1038/ejhg.2010.63
37. Anton L, Brown AG, Bartolomei MS, Elovitz MA. Differential methylation of genes associated with cell adhesion in preeclamptic placentas. PLoS ONE. (2014) 9:e100148. doi: 10.1371/journal.pone.0100148
38. Sowers JR, Saleh AA, Sokol RJ. Hyperinsulinemia and insulin resistance are associated with preeclampsia in African-Americans. Am J Hypertens. (1995) 8:1–4. doi: 10.1016/0895-7061(94)00166-9
39. Caruso A, Ferrazzani S, De Carolis S, Lucchese A, Lanzone A, De Santis L, et al. Gestational hypertension but not pre-eclampsia is associated with insulin resistance syndrome characteristics. Hum Reprod. (1999) 14:219–23.
40. Jeon EJ, Hong SY, Lee JH. Adipokines and insulin resistance according to characteristics of pregnant women with gestational diabetes mellitus. Diabetes Metab J. (2017) 41:457–65. doi: 10.4093/dmj.2017.41.6.457
41. Kalafat E, Sukur YE, Abdi A, Thilaganathan B, Khalil A. Metformin for the prevention of hypertensive disorders of pregnancy in women with gestational diabetes and obesity: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2018) 52:706–14. doi: 10.1002/uog.19084
42. Brunner S, Schmid D, Huttinger K, Much D, Heimberg E, Sedlmeier EM, et al. Maternal insulin resistance, triglycerides and cord blood insulin in relation to post-natal weight trajectories and body composition in the offspring up to 2 years. Diabet Med. (2013) 30:1500–7. doi: 10.1111/dme.12298
Keywords: gestational diabetes mellitus, hypertensive disorders of pregnancy, overweight, obesity, childhood
Citation: Gu Y, Lu J, Li W, Liu H, Wang L, Leng J, Li W, Zhang S, Wang S, Tuomilehto J, Yu Z, Yang X, Baccarelli AA, Hou L and Hu G (2019) Joint Associations of Maternal Gestational Diabetes and Hypertensive Disorders of Pregnancy With Overweight in Offspring. Front. Endocrinol. 10:645. doi: 10.3389/fendo.2019.00645
Received: 29 November 2018; Accepted: 05 September 2019;
Published: 20 September 2019.
Edited by:
Tim S. Nawrot, University of Hasselt, BelgiumReviewed by:
Nelly Saenen, University of Hasselt, BelgiumMichelle Plusquin, University of Hasselt, Belgium
Copyright © 2019 Gu, Lu, Li, Liu, Wang, Leng, Li, Zhang, Wang, Tuomilehto, Yu, Yang, Baccarelli, Hou and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Hu, Z2FuZy5odUBwYnJjLmVkdQ==
†These authors have contributed equally to this work
 Yuying Gu1,2†
Yuying Gu1,2† Jun Lu
Jun Lu Jaakko Tuomilehto
Jaakko Tuomilehto Xilin Yang
Xilin Yang Lifang Hou
Lifang Hou Gang Hu
Gang Hu