- 1Research Center for Reproductive Medicine, Gynecological Endocrinology and Menopause, University of Pavia, Pavia, Italy
- 2Department of Clinical, Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Pavia, Italy
- 3Obstetrics and Gynecology Unit, IRCCS San Matteo Foundation, University of Pavia, Pavia, Italy
Vaginal health is an essential component of active and healthy aging in women at midlife and beyond. As a consequence of hormonal deprivation and senescence, the anatomy and function of urogenital tissues are significantly affected and vulvovaginal atrophy (VVA) may occur. In a high proportion of postmenopausal women, progressive and chronic VVA symptoms have a strong impact on sexual function and quality of life. The new definition of genitourinary syndrome of menopause (GSM) comprises genital symptoms (dryness, burning, itching, irritation, bleeding), sexual symptoms (dyspareunia and other sexual dysfunctions) and urinary symptoms (dysuria, frequency, urgency, recurrent urinary infections). Many variables (age, sexual activity and partnership status) influence the clinical impact VVA/GSM symptoms and attitudes of elderly women to consult for receiving effective treatments. Psychosocial factors play a critical role in sexual functioning, but the integrity of the urogenital system is as well important affecting many domains of postmenopausal women's health, including sexual function. Several international surveys have extensively documented the need to improve VVA/GSM management because of the strong impact on women's daily life and on couple's intimacy. Health care providers (HCPs) need to be proactive in the early recognition of VVA/GSM in order to preserve urogenital and sexual longevity, by using hormonal and non-hormonal strategies. The clinical diagnosis is based on genital examination to identify objective signs and on the use of subjective scales to rate most bothersome symptoms (MBS), especially vaginal dryness. Recent studies point to the importance of addressing VVA/GSM as a potential early marker of poor general health in analogy with vasomotor symptoms. Therefore, a standard of VVA/GSM care in elderly women is desirable to enhance physical, emotional and mental well-being.
Introduction
Women live longer than men all around the world (1) and in developed countries they expect to survive more than 30 years following natural menopause, which usually occurs between 48 and 52 of age (2). That being so, the impact of reproductive aging on healthy longevity becomes increasingly important because of the potential conditions associated with menopause-related hormonal deficiency (3). Estrogen deprivation is the hallmark of ovarian exhaustion leading to the manifestation of several signs and symptoms with a significant impact on quality of life (QoL) and on physical, mental and sexual health (4). Even androgen insufficiency, an endocrine feature more evident in women with premature ovarian failure (natural, surgical, iatrogenic), may contribute to the clinical events related to menopause (5). Separating the effect of menopause from the variety of changes associated with senescence is quite difficult, but recent observations bring about the idea that menopause accelerates biological aging, especially when reproductive failure occurs prematurely (6).
The present narrative review points to the importance of addressing the chronic condition of vulvovaginal atrophy (VVA)/genitourinary syndrome of menopause (GSM) in the context of promoting urogenital and sexual longevity in women at midlife and beyond. It merely reflects the expert opinion of the authors by analyzing the amount of available evidence (1990–2019) in this complex field of research. Therapeutic strategies to effectively manage sexual symptoms associated with VVA/GSM have been reviewed extensively elsewhere (7–12) and, in here, they will be discussed briefly to serve the scope of preventing severe VVA/GSM in elderly women.
Menopause and Urogenital Aging
Among the multitude of menopausal complaints, vasomotor symptoms (hot flushes and cold or night sweats) and vaginal dryness have clearly shown a strong relationship with low estrogens during and after the menopausal transition (13). Up to 80% of women experience vasomotor symptoms during menopause with an average duration of 10 years and a variable degree of severity (14). Untreated vasomotor symptoms may represent a biomarker of chronic postmenopausal conditions such as cardiovascular disorders and osteoporosis (15). However, they do not usually progress over time (16) and remain problematic for a lower number of postmenopausal women aged 60–65 years (17). Unlike vasomotor symptoms, vaginal dryness is highly present also in older women because it is the cardinal symptom of vulvovaginal atrophy (VVA) (18), a chronic condition starting around menopause, mainly as a consequence of estrogen deficiency (19), and progressing with chronological aging and medical morbidity (20). The majority of postmenopausal women have signs of VVA upon physical examination, especially if they consult for vaginal dryness (21), but less than half of the postmenopausal population report VVA symptoms as bothersome in international surveys (22–25). There is a lack of understanding surrounding vagina health (26) and elderly women do not discuss VVA symptoms so easily because sexual health is a sensitive topic (27). In addition, the condition is believed to be transient and part of the natural aging phenomena (28, 29). In the Vaginal Health: Insights, Views & Attitudes (VIVA) survey, 55% of women with vaginal discomfort reported experiencing symptoms for 3 years or longer and only a minority (4%) attributed their symptoms to vaginal atrophy (25). Age, attitudes toward menopause, sexual activity, chronic disorders, previous and/or current use of menopausal hormone therapy and other biopsychosocial determinants influence the level of distress associated with VVA symptoms and the rate of reporting female sexual dysfunction (FSD) (30, 31). General and sexual health of the partner, as well as the quality and duration of the relationship, are also very important and addressing age-related changes in both members of a couple may contribute to a better management of VVA and sexual dysfunctions (32).
Urogenital aging is an old problem, newly recognized, which can be highly prevented upon early recognition of signs and symptoms (33). Vaginal dryness, followed by dyspareunia, is the most common symptoms reported by postmenopausal women both in surveys (22) and in clinical studies (21, 34). In the REVIVE surveys conducted both in United States (US) (23) and in Europe (EU) (24) the onset of VVA symptoms has already been reported in the majority of women within the perimenopause/early postmenopause. Interestingly, in the AGATA study, which included a sample of Italian women asking for a routine gynecological examination, a clinical diagnosis of VVA displayed a prevalence ranging from 64.7 to 84.2%, starting from 1 to 6 years after menopause (35). It is essential that health care providers (HCPs) are proactive to uncover the topic of vaginal health because women who discuss VVA with HCPs are twice as likely to be current specific-treatment users (59.7% as compared to 22.7% who did not discuss VVA) (28). It is frequent to encounter a disconnection in education, communication, and information between HCPs and their menopausal patients (36). The WISDOM survey outlined that the comfort level of HCPs when prescribing VVA treatment is still suboptimal, in particular in case they are not gynecologists (37). Education of women, adequate training of HCPs and provision of communication tools in order to facilitate the “uncomfortable” dialogue are potential solutions to address the barriers currently impeding patient–clinician interactions around sexual health (38).
Basic counseling is the first step in the management of postmenopausal sexual dysfunctions (39) and a standard process of care developed by the International Society for the Study of Women's Sexual Health (ISSWSH) may provide guidance to HCPs to effectively recognize sexual concerns and problems in women (40).
Vulvovaginal Atrophy (VVA) or Genitourinary Syndrome of Menopause (GSM): What is in These two Names?
In recent years, VVA has a new name, genitourinary syndrome of menopause (GSM), to underline the multitude of genital, sexual and urinary symptoms associated with the anatomical and functional changes of vulvo-vaginal tissues occurring with menopause and aging (41). A terminology consensus conference cosponsored by the North American Menopause Society (NAMS) and by ISSWSH was held in May 2013 to review the most relevant scientific literature in the field of postmenopausal urogenital and sexual health. Following a 2-day discussion, acknowledged experts agreed on the need of having a new term to describe more accurately the condition previously known as VVA. The choice of GSM was the result of many considerations, including the need of a term more acceptable in the medical and public arena to improve and increase communication, research, education and management of urogenital and sexual symptoms in postmenopausal women. The definition of syndrome is used to describe a collection of clinical signs and symptoms (genitourinary) correlated with each other, that do not have to be all present and related to a single identifiable pathogenesis, but occur in a particular circumstance (menopause). That being so, GSM is defined as “a collection of symptoms and signs associated with a decrease in estrogen and other sex steroids involving changes to the labia majora/minora, clitoris, vestibule/introitus, vagina, urethra, and bladder. The syndrome may include but is not limited to genital symptoms of dryness, burning, and irritation; sexual symptoms of lack of lubrication, discomfort or pain, and impaired function; and urinary symptoms of urgency, dysuria, and recurrent urinary tract infections (Table 1). Women may present with some or all of the signs and symptoms, which must be bothersome and should not be better accounted for by another diagnosis” (41).
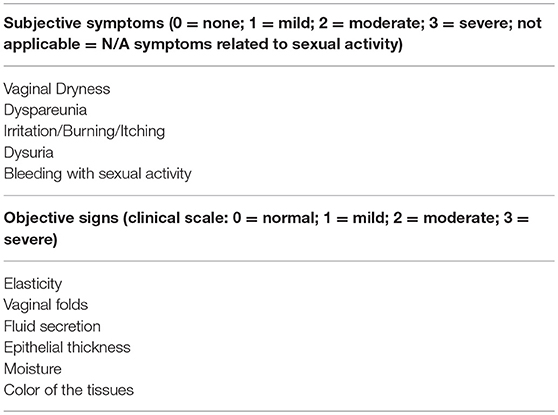
Table 1. Most common subjective and objective symptoms to diagnose vulvovaginal atrophy (VVA)/genitourinary syndrome of menopause (GSM) in daily practice.
VVA is strictly related to estrogen deficiency and is an integral part of GSM (10). However, the new definition GSM includes signs and symptoms that cannot be all reversed by estrogen replacement and may require different strategies according to their true etiology (42). As examples, vulvar dermatological conditions (43), vulvodynia (44), and pelvic floor dysfunction (45) have an increased prevalence in postmenopausal women, may co-occur with VVA, but have their own specific treatment protocols. At present, the majority of data were published with available questionnaires and scales validated to identify VVA-associated signs and symptoms and further studies are need to fully understand the multitude of disturbances included in the GSM definition. Recently, a novel patient-reported outcome measure exploring experiences of women with GSM was designed for use in both clinical care and research (46). The hope is to gain new insight into the biopsychosocial determinants of GSM in order to tailor evidence-based treatments for the individual woman across different stages of post reproductive lifespan.
Physio-Pathological Aspects of VVA/GSM
Hormonal fluctuations driving the female reproductive life cycle highly modulate the functional anatomy of the uro-genital and pelvic tract. Early data showed that untreated postmenopausal women displaying <50 pg/ml of circulating estradiol suffer more from symptoms associated with VVA (47). A historical study (the only citation prior 1990) demonstrated that even endogenous androgens may play a role because objective signs of VVA were less evident in postmenopausal women with significantly higher mean levels of androgens (androstenedione and testosterone) and gonadotropins (particularly LH). These women were more sexually active (intercourse frequency, three or more times monthly) as opposed to the sexually inactive women (intercourse frequency, <10 times yearly) (48). Whether stronger sexual desire and responsiveness driven by androgens protected against VVA or, alternatively, androgens had a direct action on peripheral tissues was not established by the “the use it or lose it” theory. However, these data are in line with the evidence that both circulating estradiol and its androgen precursors (dehydroepiandrosterone/dehydroepiandrosteronesulphate [DHEA/DHEAS], androstenedione, testosterone), as well as their local metabolites, are vital to maintain normal structure and function of the vagina and surrounding uro-genital tissues (49). Indeed, the science of intracrinology supports the idea that the age-related decline of circulating DHEA translates into a local intracellular deficiency of both estrogens and androgens, significantly contributing to poor vaginal health (50). During reproductive life, the vagina, vulva, pelvic floor muscles, endopelvic fascia, urethra, and bladder trigone display a significant amount of estrogen receptors (ERs, both α and β), which decline with menopause and may be restored by the use of systemic and local estrogen treatment. ERs are mainly expressed in the epithelium and in stromal and muscle cells of the human vagina. Even androgen receptors (ARs) are largely expressed at multiple levels (mucosa, submucosa, stroma, smooth muscles, and vascular endothelium) and cross-talk with ERs, influencing neurovascular and neuromuscular function under different endocrine conditions (51). Estradiol controls a plethora of cellular pathways regulating growth and proliferation, barrier function and pathogen defense (52). The main consequence of lacking estrogen stimulation is the loss of tissue elasticity by inducing fusion and hyalinization of collagen fibers and fragmentation of elastin fibers. The mucosa of the vagina, introitus, and labia minora becomes thin and pale and appears less hydrated. The vaginal canal becomes shorter and narrow because the vaginal rugae, the epithelial folds that allow for distensibility, progressively disappear. In addition, there is significant reduction of vascular support leading to a decrease of the volume of vaginal transudate and of other glandular secretions (53). Both estrogens and androgens contribute to pelvic nerve-stimulated genital blood flow, tissue response to neurotransmitters and sensory threshold to stimuli (51). Over time, intercellular acid mucopolysaccharide and hyaluronic acid are significantly reduced in the dermal layer. Moreover, there is a progressive dominance of parabasal cells with fewer intermediate and superficial cells. This means the vaginal squamous epithelium is quite completely estrogen deprived. Therefore, it becomes friable with petechiae, ulcerations, and eventually bleeding after minimal trauma (54–61). A thinner vaginal epithelium is also associated with a significant reduction of glycogen which translates into a lower amount of lactobacilli causing an increase in vaginal pH (between 5.0 and 7.5). The subsequent decrease of vaginal hydrogen peroxide allows the growth of other pathogenic bacteria (staphylococci, group B streptococci, and coliforms) causing atrophic vaginitis, vaginal discharge and odor. Indeed, lactobacilli diversity and abundance significantly decreased following menopause (62) and the vaginal microbiota of women with mild or moderate atrophy had a distinct bacterial community state, which may predispose to develop vaginitis and other uro-genital infections (63).
The neurovascular and neuromuscular substrates of the pelvic area are also impaired because the vulva, as well as the pelvic floor and the urinary tract, manifest similar anatomical and functional changes (64–66). In particular, entry dyspareunia, irritation, burning and itching of external genitals may be the result of the stenosis of the vulvar introitus. Indeed, hymeneal carunculae and the vestibule display less elasticity and the urethral meatus appears prominent and more vulnerable to trauma. Several changes of the urinary system (reduced urethral closure pressure, reduced sensory threshold in the bladder, and, in some cases, increased risk of rUTIs) may be observed as a consequence of the thinning of the urinary epithelium and weakening of the surrounding tissue (53).
Key-Elements of VVA/GSM Diagnosis
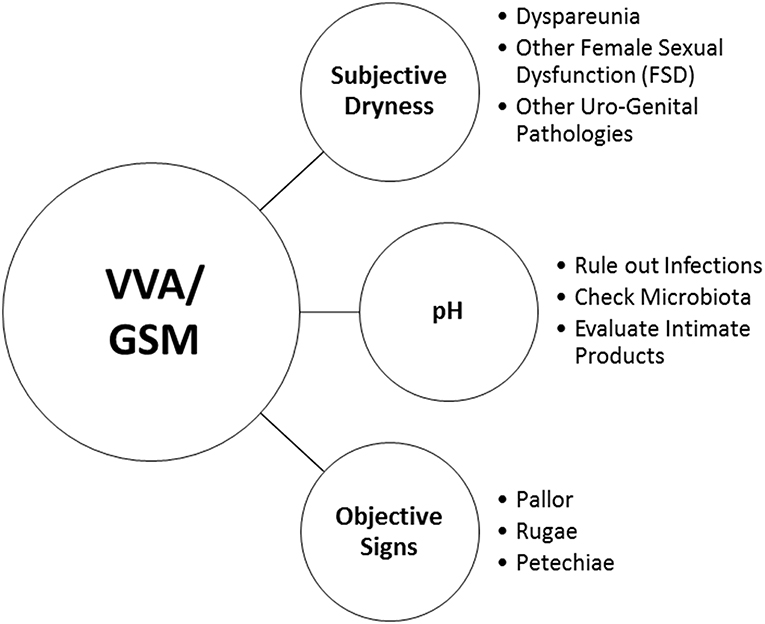
Clinical interviews and rating scales to score the most bothersome symptoms (MBS) (Table 1) are useful instruments to measure subjective symptoms and to identify risk factors for VVA/GSM. Objective diagnosis is confirmed by an accurate pelvic examination, including gentle inspection of the vulva, vestibule, vagina, and urethra in order to recognize the signs of VVA/GSM (Table 1) which can be rated on validated scales (67). The Vaginal Health Index Score is a clinical tool that, by evaluating 5 parameters (vaginal elasticity, vaginal secretions, pH, epithelial mucous membrane, vaginal hydration), allows to obtain a final score defining the degree of atrophy in the genitourinary tract by assigning a single score to each parameter. Total score ranges from 5 to 25, with lower scores corresponding to greater urogenital atrophy (68). Vulva Health Index evaluates labia, urethra, clitoris, introitus as well as elasticity and pain during intercourse; total score ranges from 0 to 24, with higher scores corresponding with greater vulvar atrophy. If the Vulva Health Index is over 8 or there is score of 3 (severe) in any category, vulvar atrophy is suggested (69). In the most severe cases, tissues may be easily traumatized and irritated by touching or inserting the speculum (70). Organ prolapse or hypertonicity of the pelvic floor with secondary vaginism may be also present, as well as vulvovaginal signs which require a differential diagnosis by performing colposcopy or carrying out bacteriological analyses (11). In general, VVA/GSM is typically a clinical diagnosis and few laboratory tests may be used to support the evidence. Among them, the evaluation of vaginal pH and the vaginal maturation index (VMI) are the most used (41). With the VMI it is possible to identify the relative proportion of parabasal, intermediate, and superficial vaginal epithelial cells. Hypoestrogenism and atrophy are suggested when there is a dominance of parabasal cells, calculated on specimens obtained directly from the lateral upper vaginal walls. Thus, the shift to a higher number of superficial cells is a primary end-point of any treatments prescribed to relieve symptoms of VVA (71). Even, vaginal pH alone is a simple outpatient procedure, influenced by infections and intimate products, which reflects the hormonal milieu and its effects on the vaginal epithelium. Indeed, it consistently correlated with parabasal and superficial cells and the visual vaginal epithelial changes and symptoms of dryness and dyspareunia (72).
In both clinical and research settings, subjective assessment (the MBS approach) and objective assessments of VVA (measurement of vaginal maturation index and vaginal pH) should be combined according to a recent systematic literature search (73). Even though a high rate of subjective symptoms is associated with a clinical diagnosis of VVA/GSM in over 90% of the cases (21), objective signs and subjective symptoms have a different prevalence distribution in the years after menopause and are not strictly associated (35). However, self-reported and visible vaginal dryness do correlate and together with ph> 5, mucosal pallor, and rugae thinning seem to be the most important objective signs to make a diagnosis (35). On the other hand, the presence of other vulvar and urinary signs are relevant to the severity of VVS/GSM and its impact on women's daily living (74).
Notwithstanding these findings, HCPs may pose very simple questions to facilitate an open conversation on urogenital health and to record the variety of vaginal, vulvar and urinary symptoms. Visual vaginal, vulvar and pelvic assessment by HCPs is a useful measure for diagnosing VVA/GSM and assessing response to treatment. Moreover, it may help HCPs to identify women at risk of vaginal dryness and dyspareunia, and allow them to proactively engage in conversations about sexual health (75). Figure 1 reports a very simple check-list to diagnose VVA/GSM in routine clinical practice.
Women with breast cancer and other gynecological malignancies are at very high risk of VVA and associated symptoms. Indeed, endocrine chemotherapy, surgery and/or radiation may induce profound changes at urogenital levels which have to be timely recognized in the oncologic care (76, 77). Moreover, we lack data on VVA/GSM in women with spontaneous premature ovarian insufficiency, even though it is likely that the condition is more distressing due to the younger age of these patients (78). Older women and those who abstain from sexual activity may suffer even more of VVA/GSM with vaginal and introital stenosis, fusion of the labia minora to the labia majora, and other urogenital conditions (79). Preventive gynecology is significantly challenged by the presence of severe VVA/GSM. Indeed, it may be difficult to adequately assess both cytologic and colposcopic findings to prevent cervical cancer. On the other hand, an episode of postmenopausal bleeding, very common in women with VVA/GSM, may cause an urgent referral to exclude endometrial cancer and other malignancies. Finally, even if less common, an early diagnosis of cancer may be delayed by vaginal synechiae and hematocolpos due to vaginal occlusion (80–82).
The Burden of VVA/GSM on Women's Sexual Function and Quality of Life (QoL)
In the last decade, many international surveys attempted to clarify the impact of VVA/GSM on sexual function and QoL (Table 2) indicating that a proactive approach to conversations about vulvovaginal discomfort would improve diagnosis and treatment (22). Even though the proportion of women who are sexually active decreases with advancing age, the value of discussions about sexual health is still high in elderly women who are in partnership (83). In the survey of Midlife Development in the United States (MIDUS II) women who were married or cohabitating had approximately 8 times higher odds of being sexually active, with more than 30% of women over 65 years reporting sexual activity at least once a week (84). Sexual satisfaction is highly dependent on many psychosocial aspects related to well-being (85). In addition, dimensions of sexual response are part of the domino effect of menopausal symptoms, including weight gain, depression, anxiety and poor physical health (86). VVA/GSM is a clear medical condition that can be associated with impairment of sexual activity and intimacy within couples at menopause (19). VVA symptoms have an approximately linear relationship with sexual functioning (87) and VVA correlates with sexual inactivity in the Hormone Therapy (HT) Trials of the Women's Health Initiative (WHI) (88). These findings are in contrast with an early study showing in a little sample of pre- and postmenopausal women that current sexual activity was not associated with differences in vaginal length or introital caliber (89). On the other hand, the international CLOSER survey investigated the impact of VVA on postmenopausal women and on male partners demonstrating that intimacy avoidance was attributed to painful sex by a significant proportion of women (55%) and men (61%) (90). That being so, the assessment of sexual well-being at menopause should rule out not only the clinical signs of VVA/GSM but also the multitude of aspects associated with it, especially hypoactive sexual desire disorder (HSDD) which is a strong determinant of maintaining sexual activity and emotional intimacy within the relationship (91).

Table 2. Most common dimensions affected by vulvovaginal atrophy (VVA)/genitourinary syndrome of menopause (GSM) in international surveys.
Cultural aspects are strongly related to the interpretation of results from surveys on VVA/GSM and explain differences in reporting bothersome symptoms and consequences associated with them. For example, women reporting VVA in Southern Europe stopped having sex in 18 % of the cases (92), in Northern Europe in 22% (92), in UK in 27% (93) and in North America (US and Canada) in 29% (94). In addition, both US and EU REVIVE surveys underlined the strong impact of VVA on sexual satisfaction and sexual spontaneity, as well as on intimacy and relationship with the partner (23, 24). Of interest, EU participants acknowledged a significantly higher impact of VVA symptoms on sexual intercourse and partner interaction than US participants, and both cohorts were observed to have differences between their respective VVA symptom profiles (95). Apart cultural attitudes in the health care system or in the importance to maintain sexual activity over time, other elements of difference may be found between US and EU samples at baseline, including age, marital status, education, and working activity (95). Indeed, other studies indicate that the true prevalence of each symptom and the rate of distress associated with it are significantly influenced by many factors, namely age and sexual activity (96, 97). Dyspareunia is generally less reported later in life mainly because older women are less likely to still have a spousal or other intimate relationship (83). Behavioral profiles of postmenopausal women play also a role in disclosing VVA symptomatology and actively seeking treatment (98). Data collected in the CLOSER survey indicated that the VVA condition related to many dimensions of womanhood, in particular perception of aging and poor health (90, 99). In the “women's voices in the menopause” survey (27), 52% of respondents with vaginal discomfort reported an impact on their QoL. Both VIVA and CLOSER international surveys further explored the dimension affected by self-reported VVA symptoms demonstrating an influence on working, social activity and other aspects of personal well-being (24, 25). In addition, other data indicated that VVA is associated with a clinically significant impact on QoL that may be comparable to that seen in serious conditions such as arthritis, chronic obstructive pulmonary disease, asthma and irritable bowel syndrome (100, 101).
The EVES study collected very accurate information in a clinical population of EU (Italy and Spain) postmenopausal women aged 45–75 years reporting at least one subjective VVA symptom and objectively diagnosed with VVA during gynecological examination. Women scored 19 potentially VVA-related complaints on a 4-point severity scale (absent, mild, moderate and severe) and filled in both the EuroQol questionnaire (EQ-5D-3L) (102) and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire to measure the impact of VVA on several dimensions of QoL (103). Sexual function and distress were also evaluated by validated questionnaires (104, 105). During gynecological clinical assessment, signs of VVA were rated in order to calculate the Vaginal Health Index (68) and the Vulva Health Index (69). The main outcomes of EVES showed that of a total of 2,160 evaluable women, 66.3, 30.5, and 11.2% suffered from severe vaginal, vulvar, and urinary symptoms, respectively. VVA was confirmed in more than 90% of the participants. Both generic and vaginal aging-related QoL scores showed a significant relationship with the different types of severe VVA symptoms. QoL questionnaires displayed worse scores in women where the diagnosis of VVA was confirmed by gynecologic examination. The severity of urinary symptoms showed a more strong impact on all DIVA components (daily activities, emotional well-being, sexual functioning and self-concept/body image) compared to vaginal and vulvar symptoms (74). This data confirmed recently reported observations on predictors of impact of vaginal symptoms, in which women with urinary incontinence reported a higher impact of VVA symptoms on three of the four DIVA dimensions (not sexual functioning) (106). In the Italian subset of 1,226 postmenopausal women, those with objective confirmation of VVA had worsened sexual function and distress when compared with the patients having only subjective VVA symptoms (107). Interestingly enough, postmenopausal women with VVA receiving treatment complained of more severe symptoms than those untreated. Moreover, time since menopause was significantly higher in women treated for VVA. Collectively, EVES data indicate that VVA treatments should ideally be initiated at a younger age when symptoms commence and cause distress, before the condition becomes very severe and difficult to be reverted (108).
General Principles for VVA/GSM Treatment
The chronic nature of VVA/GSM indicates that effective treatments should preferably be prescribed at the onset of the symptoms and signs of atrophic changes of the vagina, early before severe pictures of the condition occur, and should be continued over time in order to maintain their benefits (109). The therapeutic approach needs to be personalized and women's preferences have to be taken into account because the level of comfort with a given therapy is strongly influenced by a multitude of individual and socio-environmental factors (110). Apart the embarrassment to discuss an intimate condition, fears of hormones are a major barrier (24), in spite of the very reassuring safety data obtained with local estrogen therapy (LET) (111), the first-line hormonal treatment for VVA/GSM according to guidelines of menopausal scientific societies (53, 112, 113). Various local estrogen treatments are equally effecting in reversing VVA/GSM symptoms, including dyspareunia and other associated sexual dysfunction, alone or even combined with systemic HT. With low-dose LET, systemic estrogen absorption is minimal, and serum estradiol levels remain in the postmenopausal range permitting the use in women with or at high risk for breast cancer, after a discussion of risks and benefits and review with oncologists (76). Local androgens, such as DHEA pessaries and testosterone cream, are new therapeutic options that await for further confirmation (49). Another option approved by Medical Authorities is ospemifene, a third-generation selective estrogen receptor modulator, which is an oral medication for the treatment of VVA associated symptoms (114). It is currently indicated for women, who are not candidates for LET or whenever other treatments, including LET, were not effective to relieve vaginal dryness and dyspareunia (15).
Non-hormonal strategies may be used in women of any age in which hormonal treatments are contraindicated or co-treating women prescribed with systemic/vaginal hormone therapy. The prescription of vaginal moisturizers and lubricants and the maintenance of sexual activity may be helpful in improving vaginal dryness-related symptoms. However, a few clinical trials have been performed to assess the efficacy of such products. Lubricants are short-acting substances (water-, silicone-, or oil-based) which are useful to reduce friction during sexual activity, whereas moisturizers are longer acting than lubricants and may exert a trophic effect (115). Pelvic floor muscle training (PFMT) program in postmenopausal women with urinary incontinence is feasible and improves VVA/GSM symptoms and signs, as well as displays a positive impact on activities of daily living, QoL and sexual function (116). Microablative fractional CO2 laser, the non-ablative vaginal Erbium YAG laser (VEL) and energy-based devices are increasingly used to alleviate VVA/GSM symptoms with promising results and a good safety profile (117).
VVA/GSM as a Negative Marker of Women's Aging: Is There Enough Evidence?
Urogenital and sexual longevity is an integral part of healthy aging in postmenopausal women and their partners. The severity of VVA/GSM and the type of prevailing symptoms are mostly influenced by the multitude of clinical phenotypes of postmenopausal women depending on a wide range of biopsychosocial variables which are difficult to estimate in large scale trials. It is known that women loose less years of sexually active life because of poor health than men (118). This data confirm the multidimensional nature of women's sexuality with psychosocial factors (relationship satisfaction, communication with romantic partner, and importance of sex) mattering more than biological aging to sexual satisfaction among midlife and older women (84). That being so, the presence of severe VVA/GSM cannot be considered a negative marker of general health as it had been demonstrated for erectile dysfunction in aging males (119). However, coital sexual activity is associated with an excellent or very good general health also in women, as it is in men (83), and it is certainly influenced by a healthy genital response. Even if it has been difficult to establish a clear link between cardiovascular and metabolic health and women's sexual dysfunctions (120), there is no doubt that several chronic conditions may be associated with poor sexual functioning (121). It is fascinating to speculate on the evidence that vaginal dryness is the only other symptom very sensitive to estrogen deprivation apart hot-flushes (13). Given the clear association of vasomotor symptoms with negative long-term health consequences across aging (15), we cannot exclude that even severe VVA/GSM may represent an early marker of poor general health, a hypothesis that needs further exploration by investigating objective parameters of such chronic condition in relationship with other aspects of women's well-being. Interestingly, baseline characteristics and medical history were tabulated for a VVA cohort identified from two US administrative claims databases (9,080 women aged 40–79 years) and matched controls without VVA. The mean age at baseline was 60.2 years for both but the Deyo-Charlson comorbidity index was significantly higher, with a significantly higher proportion of women in the VVA cohort with a diagnosis of angina, osteoporosis, migraines, insomnia, or anxiety. As expected VVA patients had a significantly higher incidence of each of six genitourinary conditions (“urinary tract infections,” “other/unspecified genitourinary symptoms,” “other inflammatory diseases of female pelvic organs,” “menopausal disorders,” “female genital pain and other symptoms,” and “other/unspecified female genital disorders”) compared to controls (122).
Conclusions
The management of VVA/GSM is increasingly important in light of the feminilization of aging. Postmenopausal women are becoming aware that preserving urogenital and sexual longevity is a major step in gender equality and healthy living. HCPs should address the issue in daily clinical practice with the aim to prevent the long-term health consequences associated with estrogen deprivation (123). Early recognition of signs and symptoms of VVA/GSM, individual counseling and personalized treatment strategies are key-steps in helping women to maintain QoL.
Author Contributions
RN: conception and design. EM, LC, LT, AI, EB, SM, and DB: acquisition, analysis, and interpretation of data. CC and BG: drafting the article. RN and BG: revising for intellectual content. RN, EM, LC, SM, LT, AI, DB, CC, and BG: final approval.
Conflict of Interest Statement
During the past 2 years, RN had a financial relationship (lecturer, member of advisory boards and/or consultant) with Bayer HealthCare, Endoceutics, Exceltis, Gedeon Richter, MSD, Novo Nordisk, Palatin, Pfizer, Shionogi, Teva, and Theramex. These companies have no involvement with the study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. GBD 2017 Mortality Collaborators. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1684–735. doi: 10.1016/S0140-6736(18)31891-9
2. Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric. (2010) 13:419–28. doi: 10.3109/13697137.2010.507886
3. Monteleone P, Mascagni G, Giannini A, Genazzani AR, Simoncini T. Symptoms of menopause—global prevalence, physiology and implications. Nat Rev Endocrinol. (2018) 14:199–215. doi: 10.1038/nrendo.2017.180
4. Davis SR, Lambrinoudaki I, Lumsden M, Mishra GD, Pal L, Rees M, et al. Menopause. Nat Rev Dis Primers. (2015) 1:15004. doi: 10.1038/nrdp.2015.54
5. Davis SR, Wahlin-Jacobsen S. Testosterone in women–the clinical significance. Lancet Diabetes Endocrinol. (2015) 3:980–92. doi: 10.1016/S2213-8587(15)00284-3
6. Levine ME, Lu AT, Chen BH, Hernandez DG, Singleton AB, Ferrucci L, et al. Menopause accelerates biological aging. Proc Natl Acad Sci USA. (2016) 113:9327–32. doi: 10.1073/pnas.1604558113
7. Palacios S, Castelo-Branco C, Currie H, Mijatovic V, Nappi RE, Simon J, et al. Update on management of genitourinary syndrome of menopause: a practical guide. Maturitas. (2015) 82:308–13. doi: 10.1016/j.maturitas.2015.07.020
8. Nappi RE, Cucinella L. Advances in pharmacotherapy for treating female sexual dysfunction. Expert Opin Pharmacother. (2015) 16:875–87. doi: 10.1517/14656566.2015.1020791
9. Faubion SS, Sood R, Kapoor E. Genitourinary syndrome of menopause: management strategies for the clinician. Mayo Clin Proc. (2017) 92:1842–9. doi: 10.1016/j.mayocp.2017.08.019
10. Shifren JL. Genito-urinary syndrome of menopause. Clin Obstet Gynecol. (2018) 61:508–16. doi: 10.1097/GRF.0000000000000380
11. Naumova I, Castelo-Branco C. Current treatment options for postmenopausal vaginal atrophy. Int J Womens Health. (2018) 10:387–95. doi: 10.2147/IJWH.S158913
12. Donders GGG, Ruban K, Bellen G, Grinceviciene S. Pharmacotherapy for the treatment of vaginal atrophy. Expert Opin Pharmacother. (2019) 20:821–35. doi: 10.1080/14656566.2019.1574752
13. Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. (2000) 96:351–8. doi: 10.1097/00006250-200009000-00007
14. Thurston RC. Vasomotor symptoms: natural history, physiology, and links with cardiovascular health. Climacteric. (2018) 21:96–100. doi: 10.1080/13697137.2018.1430131
15. Nappi RE, Murina F, Perrone G, Villa P, Biglia N. Clinical profile of women with vulvar and vaginal atrophy who are not candidates for local vaginal estrogen therapy. Minerva Ginecol. (2017) 69:370–80. doi: 10.23736/S0026-4784.17.04064-3
16. Avis NE, Crawford SL, Green R. Vasomotor symptoms across the menopause transition: differences among women. Obstet Gynecol Clin North Am. (2018) 45:629–40. doi: 10.1016/j.ogc.2018.07.005
17. Gartoulla P, Worsley R, Bell RJ, Davis SR. Moderate to severe vasomotor and sexual symptoms remain problematic for women aged 60 to 65 years. Menopause. (2018) 25:1331–8. doi: 10.1097/GME.0000000000001237
18. Nappi RE, Lachowsky M. Menopause and sexuality: prevalence of symptoms and impact on quality of life. Maturitas. (2009) 63:138–41. doi: 10.1016/j.maturitas.2009.03.021
19. Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. (2014) 17:3–9. doi: 10.3109/13697137.2013.871696
20. Mitchell CM, Waetjen LE. Genitourinary changes with aging. Obstet Gynecol Clin North Am. (2018) 45:737–50. doi: 10.1016/j.ogc.2018.07.010
21. Palacios S, Nappi RE, Bruyniks N, Particco M, Panay N, EVES Study Investigators. The European Vulvovaginal Epidemiological Survey (EVES): prevalence, symptoms and impact of vulvovaginal atrophy of menopause. Climacteric. (2018) 21:286–91. doi: 10.1080/13697137.2018.1446930
22. Parish SJ, Nappi RE, Krychman ML, Kellogg-Spadt S, Simon JA, Goldstein JA, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. (2013) 5:437–47. doi: 10.2147/IJWH.S44579
23. Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women's VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. (2013) 10:1790–9. doi: 10.1111/jsm.12190
24. Nappi RE, Palacios S, Panay N, Particco M, Krychman ML. Vulvar and vaginal atrophy in four European countries: evidence from the European REVIVE Survey. Climacteric. (2016) 19:188–97. doi: 10.3109/13697137.2015.1107039
25. Nappi RE, Kokot-Kierepa M. Vaginal Health: Insights, Views & Attitudes (VIVA)—results from an international survey. Climacteric. (2012) 15:36–44. doi: 10.3109/13697137.2011.647840
26. Nappi RE, Liekens G, Brandenburg U. Attitudes, perceptions and knowledge about the vagina: the International Vagina Dialogue Survey. Contraception. (2006) 73:493–500. doi: 10.1016/j.contraception.2005.12.007
27. Nappi RE, Kokot-Kierepa M. Women's voices in the menopause: results from an international survey on vaginal atrophy. Maturitas. (2010) 67:233–8. doi: 10.1016/j.maturitas.2010.08.001
28. Nappi RE, Palacios S, Particco M, Panay N. The REVIVE (REal Women's VIews of Treatment Options for Menopausal Vaginal ChangEs) survey in Europe: country-specific comparisons of postmenopausal women's perceptions, experiences and needs. Maturitas. (2016) 91:81–90. doi: 10.1016/j.maturitas.2016.06.010
29. Krychman M, Graham S, Bernick B, Mirkin S, Kingsberg SA. The women's EMPOWER survey: women's knowledge and awareness of treatment options for vulvar and vaginal atrophy remains inadequate. J Sex Med. (2017) 14:425–33. doi: 10.1016/j.jsxm.2017.01.011
30. Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. (2009) 6:2133–42. doi: 10.1111/j.1743-6109.2009.01335.x
31. Nappi RE, Cucinella L, Martella S, Rossi M, Tiranini L, Martini E. Female sexual dysfunction (FSD): prevalence and impact on quality of life (QoL). Maturitas. (2016) 94:87–91. doi: 10.1016/j.maturitas.2016.09.013
32. Jannini EA, Nappi RE. Couplepause: a new paradigm in treating sexual dysfunction during menopause and andropause. Sex Med Rev. (2018) 6:384–95. doi: 10.1016/j.sxmr.2017.11.002
33. Bachmann G. Urogenital ageing: an old problem newly recognized. Maturitas. (1995) 22(Suppl. 1):S1–5. doi: 10.1016/0378-5122(95)00956-6
34. Palma F, Xholli A, Cagnacci A, as the Writing Group of the AGATA Study. The most bothersome symptom of vaginal atrophy: evidence from the observational AGATA study. Maturitas. (2018) 108:18–23. doi: 10.1016/j.maturitas.2017.11.007
35. Palma F, Volpe A, Villa P, Cagnacci A, Writing Group of GATA Study. Vaginal Atrophy of Women in Postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. (2016) 83:40–4. doi: 10.1016/j.maturitas.2015.09.001
36. Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. (2017) 14:413–24. doi: 10.1016/j.jsxm.2017.01.010
37. Kingsberg SA, Larkin L, Krychman M, Parish SJ, Bernick B, Mirkin S. WISDOM survey: attitudes and behaviors of physicians toward vulvar and vaginal atrophy (VVA) treatment in women including those with breast cancer history. Menopause. (2019) 26:124–31. doi: 10.1097/GME.0000000000001194
38. Kingsberg SA, Schaffir J, Faught BM, Pinkerton JV, Parish SJ, Iglesia CB, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health. (2019) 28:432–43. doi: 10.1089/jwh.2018.7352
39. Al-Azzawi F, Bitzer J, Brandenburg U, Castelo-Branco C, Graziottin A, Kenemans P, et al. Therapeutic options for postmenopausal female sexual dysfunction. Climacteric. (2010) 13:103–20. doi: 10.3109/13697130903437615
40. Parish SJ, Hahn SR, Goldstein SW, Giraldi A, Kingsberg SA, Larkin L, et al. The International Society for the Study of Women's Sexual Health Process of Care for the Identification of Sexual Concerns and Problems in Women. Mayo Clin Proc. (2019) 94:842–56. doi: 10.1016/j.mayocp.2019.01.009
41. Portman DJ, Gass ML. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause. (2014) 21:1063–8. doi: 10.1097/GME.0000000000000329
42. Gandhi J, Chen A, Dagur G, Suh Y, Smith N, Cali B, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. (2016) 215:704–11. doi: 10.1016/j.ajog.2016.07.045
43. Lewis FM. Vulval symptoms after the menopause—Not all atrophy!. Post Reprod Health. (2015) 21:146–50. doi: 10.1177/2053369115608019
44. Vieira-Baptista P, Donders G, Margesson L, Edwards L, Haefner HK, Pérez-López FR. Diagnosis and management of vulvodynia in postmenopausal women. Maturitas. (2018) 108:84–94. doi: 10.1016/j.maturitas.2017.11.003
45. Johnston SL. Pelvic floor dysfunction in midlife women. Climacteric. (2019) 11:1–7. doi: 10.1080/13697137.2019.1568402
46. Shifren JL, Zincavage R, Cho EL, Magnavita A, Portman DJ, Krychman ML, et al. Women's experience of vulvovaginal symptoms associated with menopause. Menopause. (2019) 26:341–9. doi: 10.1097/GME.0000000000001275
47. Sarrel PM. Sexuality and menopause. Obstet Gynecol. (1990) 75:26S−35S. doi: 10.1097/00006250-199004001-00006
48. Leiblum S, Bachmann G, Kemmann E, Colburn D, Swartzman L. Vaginal atrophy in the postmenopausal woman. The importance of sexual activity and hormones JAMA. (1983) 249:2195–8. doi: 10.1001/jama.1983.03330400041022
49. Simon JA, Goldstein I, Kim NN, Davis SR, Kellogg-Spadt S, Lowenstein L, et al. The role of androgens in the treatment of genitourinary syndrome of menopause (GSM): International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Menopause. (2018) 25:837–47. doi: 10.1097/GME.0000000000001138
50. Labrie F. Intracrinology and menopause: the science describing the cell-specific intracellular formation of estrogens and androgens from DHEA and their strictly local action and inactivation in peripheral tissues. Menopause. (2019) 26:220–4. doi: 10.1097/GME.0000000000001177
51. Traish AM, Vignozzi L, Simon JA, Goldstein I, Kim NN. Role of androgens in female genitourinary tissue structure and function: implications in the genitourinary syndrome of menopause. Sex Med Rev. (2018) 6:558–71. doi: 10.1016/j.sxmr.2018.03.005
52. Cotreau MM, Chennathukuzhi VM, Harris HA, Han L, Dorner AJ, Apseloff G, et al. A study of 17beta-estradiol-regulated genes in the vagina of postmenopausal women with vaginal atrophy. Maturitas. (2007) 58:366–76. doi: 10.1016/j.maturitas.2007.09.009
53. Sturdee DW, Panay N, International Menopause Society Writing Group. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric. (2010) 13:509–22. doi: 10.3109/13697137.2010.522875
54. Forsberg JG. A morphologist's approach to the vagina – age-related changes and estrogen sensitivity. Maturitas. (1995) 22:S7–S15. doi: 10.1016/0378-5122(95)00957-4
55. Caillouette JC, Sharp CF Jr, Zimmerman GJ, Roy S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol. (1997) 176:1270–5. doi: 10.1016/S0002-9378(97)70345-4
56. Robinson D, Cardozo LD. The role of estrogens in female lower urinary tract dysfunction. Urology. (2003) 62:45–51. doi: 10.1016/S0090-4295(03)00676-9
57. Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Juliá MD. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas. (2005) 52 (Suppl. 1):S46–52. doi: 10.1016/j.maturitas.2005.06.014
58. Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. (2008) 51:549–55. doi: 10.1097/GRF.0b013e3181809a26
59. Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. (2009) 12:279–85. doi: 10.1080/13697130902814751
60. Mac Bride MB, Rhodes DJ, Shuster LT. Vulvo-vaginal atrophy. Mayo Clin Proc. (2010) 85:87–94. doi: 10.4065/mcp.2009.0413
61. Stika CS. Atrophic vaginitis. Dermatol Ther. (2010) 23:514–22. doi: 10.1111/j.1529-8019.2010.01354.x
62. Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. (2016) 91:42–50. doi: 10.1016/j.maturitas.2016.05.015
63. Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver MI, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. (2018) 25:1321–30. doi: 10.1097/GME.0000000000001236
64. Basaran M, Kosif R, Bayar U, Civelek B. Characteristics of external genitalia in pre- and postmenopausal women. Climacteric. (2008) 11:416–21. doi: 10.1080/13697130802366670
65. Mannella P, Palla G, Bellini M, Simoncini T. The female pelvic floor through midlife and aging. Maturitas. (2013) 76:230–4. doi: 10.1016/j.maturitas.2013.08.008
66. Calleja-Agius J, Brincat M.P. The urogenital system and the menopause. Climacteric. (2015) 18(Suppl. 1):18–22. doi: 10.3109/13697137.2015.1078206
67. Nappi RE. New attitudes to sexuality in the menopause: clinical evaluation and diagnosis. Climacteric. (2007) 10(Suppl. 2):105–8. doi: 10.1080/13697130701599876
68. Bachmann GA, Notelovitz M, Kelly SJ, Thompson C, Owens A. Long-term non-hormonal treatment of vaginal dryness. Clin Pract Sexuality. (1992) 8:3–8.
69. Panay N. Genitourinary syndrome of the menopause–dawn of a new era? Climacteric. (2015) 18(Suppl. 1):13–7. doi: 10.3109/13697137.2015.1070564
70. Goldstein I. Recognizing and treating urogenital atrophy in postmenopausal women. J Womens Health. (2010) 19:425–32. doi: 10.1089/jwh.2009.1384
71. Nilsson K, Risberg B, Heimer G. The vaginal epithelium in the post menopause—cytology, histology and pH as methods of assessment. Maturitas. (1995) 21:51–6. doi: 10.1016/0378-5122(94)00863-3
72. Tucker KM, Godha K, Mirkin S, Archer DF. Vaginal pH: a simple assessment highly correlated with vaginal morphology and symptoms in postmenopausal women. Menopause. (2008) 25:762–6. doi: 10.1097/GME.0000000000001081
73. Weber MA, Limpens J, Roovers JP. Assessment of vaginal atrophy: a review. Int Urogynecol J. (2015) 26:15–28. doi: 10.1007/s00192-014-2464-0
74. Nappi RE, Palacios S, Bruyniks N, Particco M, Panay N, EVES Study Investigators. The burden of vulvovaginal atrophy on women's daily living: implications on quality of life from a face-to-face real-life survey. Menopause. (2019) 26:485–91. doi: 10.1097/GME.0000000000001260
75. Simon JA, Archer DF, Kagan R, Bernick B, Graham S, Constantine GD, et al. Visual improvements in vaginal mucosa correlate with symptoms of VVA: data from a double-blind, placebo-controlled trial. Menopause. (2017) 24:1003–10. doi: 10.1097/GME.0000000000000880
76. Faubion SS, Larkin LC, Stuenkel CA, Bachmann GA, Chism LA, Kagan R, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. (2018) 25:596–608. doi: 10.1097/GME.0000000000001121
77. Sadovsky R, Basson R, Krychman M, Morales AM, Schover L, Wang R, et al. Cancer and sexual problems. J Sex Med. (2010) 7:349–73. doi: 10.1111/j.1743-6109.2009.01620.x
78. Nappi RE, Cucinella L, Martini E, Rossi M, Tiranini L, Martella S, et al. Sexuality in premature ovarian insufficiency. Climacteric. (2019) 22:289–95. doi: 10.1080/13697137.2019.1575356
79. Doumouchtsis SK, Chrysanthopoulou EL. Urogenital consequences in ageing women. Best Pract Res Clin Obstet Gynaecol. (2013) 27:699–714. doi: 10.1016/j.bpobgyn.2013.03.007
80. Bolton PJ, Selo-Ojeme DO. Endometrial adenocarcinoma: an unusual presentation with acute urinary retention secondary to haematocolpos. J Obstet Gynaecol. (1999) 19:553–4. doi: 10.1080/01443619964508
81. Segal S, Harvie HS, Siegelman E, Arya LA. Severe atrophic vaginitis causing vaginal synechiae and hematocolpos at menopause. Menopause. (2011) 18:333–5. doi: 10.1097/gme.0b013e3181f3285a
82. Stiles M, Redmer J, Paddock E, Schrager S. Gynecologic issues in geriatric women. J WomensHealth. (2012) 21:4–9. doi: 10.1089/jwh.2011.2803
83. Lindau ST, Schumm LP, Laumann EO, Levinson W, O'Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. (2007) 357:762–74. doi: 10.1056/NEJMoa067423
84. Thomas HN, Hess R, Thurston RC. Correlates of sexual activity and satisfaction in midlife and older women. Ann Fam Med. (2015) 13:336–42. doi: 10.1370/afm.1820
85. Nappi RE, Albani F, Santamaria V, Tonani S, Magri F, Martini E, et al. Hormonal and psycho-relational aspects of sexual function during menopausal transition and at early menopause. Maturitas. (2010) 67:78–83. doi: 10.1016/j.maturitas.2010.05.008
86. Nappi RE, Verde JB, Polatti F, Genazzani AR, Zara C. Self-reported sexual symptoms in women attending menopause clinics. Gynecol Obstet Invest. (2002) 53:181–7. doi: 10.1159/000058371
87. Pinkerton JV, Bushmakin AG, Komm BS, Abraham L. Relationship between change in vulvar-vaginal atrophy and changes in sexual functioning. Maturitas. (2017) 100:57–63. doi: 10.1016/j.maturitas.2017.03.315
88. Gass ML, Cochrane BB, Larson JC, Manson JE, Barnabei VM, Brzyski RG, et al. Patterns and predictors of sexual activity among women in the Hormone Therapy trials of the Women's Health Initiative. Menopause. (2011) 18:1160–71. doi: 10.1097/gme.0b013e3182227ebd
89. Weber AM, Walters MD, Schover LR, Mitchinson A. Vaginal anatomy and sexual function. Obstet Gynecol. (1995) 86:946–9. doi: 10.1016/0029-7844(95)00291-X
90. Nappi RE, Kingsberg S, Maamari R, Simon J. The CLOSER (CLarifying Vaginal Atrophy's Impact On SEx and Relationships) survey: implications of vaginal discomfort in postmenopausal women and in male partners. J Sex Med. (2013) 10:2232–41. doi: 10.1111/jsm.12235
91. Simon JA, Davis SR, Althof SE, Chedraui P, Clayton AH, Kingsberg SA, et al. Sexual well-being after menopause: an International Menopause Society White Paper. Climacteric. (2018) 21:415–27. doi: 10.1080/13697137.2018.1482647
92. Nappi RE, Mattsson LÅ, Lachowsky M, Maamari R, Giraldi A. The CLOSER survey: impact of postmenopausal vaginal discomfort on relationships between women andtheir partners in Northern and Southern Europe. Maturitas. (2013) 75:373–9. doi: 10.1016/j.maturitas.2013.05.003
93. Domoney C, Currie H, Panay N, Maamari R, Nappi RE. The CLOSER survey: impact of postmenopausal vaginal discomfort on women and male partners in the UK. Menopause Int. (2013) 19:69–76. doi: 10.1177/1754045313484139
94. Simon JA, Kokot-Kierepa M, Goldstein J, Nappi RE. Vaginal health in the United States: results from the vaginal health: insights, views & attitudes survey. Menopause. (2013) 20:1043–8. doi: 10.1097/GME.0b013e318287342d
95. Nappi RE, Krychman ML. The American-European difference in vulvar and vaginal atrophy views: a lesson from the REVIVE Survey. Climacteric. (2016) 19:252–5. doi: 10.3109/13697137.2016.1173026
96. Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women's Health Initiative. Maturitas. (2004) 49:292–303. doi: 10.1016/j.maturitas.2004.06.019
97. Gass M, Larson J, Cochrane B, Manson JE, Lane D, Barnabei V, et al. Sexual activity and vaginal symptoms in the postintervention phase of the Women's Health Initiative Hormone Therapy Trials. Menopause. (2018) 25:252–64. doi: 10.1097/GME.0000000000000994
98. Castelo-Branco C, Biglia N, Nappi RE, Schwenkhagen A, Palacios S. Characteristics of post-menopausal women with genitourinary syndrome of menopause: implications for vulvovaginal atrophy diagnosis and treatment selection. Maturitas. (2015) 81:462–9. doi: 10.1016/j.maturitas.2015.05.007
99. Simon JA, Nappi RE, Kingsberg SA, Maamari R, Brown V. Clarifying Vaginal Atrophy's Impact on Sex and Relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. (2014) 21:137–42. doi: 10.1097/GME.0b013e318295236f
100. DiBonaventura M, Luo X, Moffatt M, Bushmakin AG, Kumar M, Bobula J. The Association Between Vulvovaginal Atrophy Symptoms and Quality of Life Among Postmenopausal Women in the United States and Western Europe. J Womens Health. (2015) 24:713–22. doi: 10.1089/jwh.2014.5177
101. Lang K, Alexander IM, Simon J, Sussman M, Lin I, Menzin J, et al. The impact of multimorbidity on quality of life among midlife women: findings from a U.S. nationally representative survey. J Womens Health. (2015) 24:374–83. doi: 10.1089/jwh.2014.4907
102. EuroQoL Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. (1990) 16:199–208. doi: 10.1016/0168-8510(90)90421-9
103. Huang AJ, Gregorich SE, Kuppermann M, Nakagawa S, Van Den Eeden SK, Brown JS, et al. Day-to-Day Impact of Vaginal Aging questionnaire: a multidimensional measure of the impact of vaginal symptoms on functioning and well-being in postmenopausal women. Menopause. (2015) 22:144–54. doi: 10.1097/GME.0000000000000281
104. Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. (2000) 26:26191–208. doi: 10.1037/t28568-000
105. Derogatis L, Clayton A, Lewis-D'Agostino D, Wunderlich G, Fu Y. Validation of the female sexual distress scale-revised for assessing distress in women with hypoactive sexual desire disorder. J Sex Med. (2008) 5:357–64. doi: 10.1111/j.1743-6109.2007.00672.x
106. Hunter MM, Nakagawa S, Van Den Eeden SK, Kuppermann M, Huang AJ. Predictors of impact of vaginal symptoms in postmenopausal women. Menopause. (2016) 23:40–6. doi: 10.1097/GME.0000000000000482
107. Nappi RE, Seracchioli R, Salvatore S, Cagnacci A, Di Paolantonio T, Busacca M, et al. Impact of vulvovaginal atrophy of menopause: prevalence and symptoms in Italian women according to the EVES study. Gynecol Endocrinol. (2019) 35:453–9. doi: 10.1080/09513590.2018.1563883
108. Panay N, Palacios S, Bruyniks N, Particco M, Nappi RE, EVES Study Investigators. Symptom severity and quality of life in the management of vulvovaginal atrophy in postmenopausal women. Maturitas. (2019) 124:55–61. doi: 10.1016/j.maturitas.2019.03.013
109. Nappi RE, Biglia N, Cagnacci A, Di Carlo C, Luisi S, Paoletti AM. Diagnosis and management of symptoms associated with vulvovaginal atrophy: expert opinion on behalf of the Italian VVA study group. Gynecol Endocrinol. (2016) 32:602–6. doi: 10.1080/09513590.2016.1183627
110. Kingsberg SA, Krychman ML. Resistance and barriers to local estrogen therapy in women with atrophic vaginitis. J Sex Med. (2013) 10:1567–74. doi: 10.1111/jsm.12120
111. Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. (2016) 31:CD001500. doi: 10.1002/14651858.CD001500.pub3
112. Rees M, Pérez-López FR, Ceasu I, Depypere H, Erel T, Lambrinoudaki I, et al. EMAS clinical guide: low-dose vaginal estrogens for postmenopausal vaginal atrophy. Maturitas. (2012) 73:171–4. doi: 10.1016/j.maturitas.2012.06.009
113. NAMS. (2013). Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 20, 888–902; quiz 903-4. doi: 10.1097/GME.0b013e3182a122c2
114. Bruyniks N, Nappi RE, Castelo-Branco C, de Villiers TJ, Simon J. Effect of ospemifene on moderate or severe symptoms of vulvar and vaginal atrophy. Climacteric. (2016) 19:60–5. doi: 10.3109/13697137.2015.1113517
115. Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. (2016) 19:151–61. doi: 10.3109/13697137.2015.1124259
116. Mercier J, Morin M, Zaki D, Reichetzer B, Lemieux MC, Khalifé S, et al. Pelvic floor muscle training as a treatment for genitourinary syndrome of menopause: a single-arm feasibility study. Maturitas. (2019) 125:57–62. doi: 10.1016/j.maturitas.2019.03.002
117. Tadir Y, Gaspar A, Lev-Sagie A, Alexiades M, Alinsod R, Bader A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. (2017) 49:137–59. doi: 10.1002/lsm.22637
118. Lindau ST, Gavrilova N. Sex, health, and years of sexually active life gained due to good health: evidence from two US population based cross sectional surveys of ageing. BMJ. (2010) 340:c810. doi: 10.1136/bmj.c810
119. Corona G, Rastrelli G, Maseroli E, Forti G, Maggi M. Sexual function of the ageing male. Best Pract Res Clin Endocrinol Metab. (2013) 27:581–601. doi: 10.1016/j.beem.2013.05.007
120. Miner M, Esposito K, Guay A, Montorsi P, Goldstein I. Cardiometabolic risk and female sexual health: the Princeton III summary. J Sex Med. (2012) 9:641–51; quiz 652. doi: 10.1111/j.1743-6109.2012.02649.x
121. Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. (2007) 369:409–24. doi: 10.1016/S0140-6736(07)60197-4
122. Constantine GD, Bruyniks N, Princic N, Huse D, Palmer L, Lenhart G, et al. Incidence of genitourinary conditions in women with a diagnosis of vulvar/vaginal atrophy. Curr Med Res Opin. (2014) 30:143–8. doi: 10.1185/03007995.2013.850068
Keywords: vulvovaginal atrophy (VVA), genito-urinary syndrome of menopause (GSM), aging, longevity, vaginal dryness, dyspareunia, female sexual dysfunction (FSD), quality of life (QoL)
Citation: Nappi RE, Martini E, Cucinella L, Martella S, Tiranini L, Inzoli A, Brambilla E, Bosoni D, Cassani C and Gardella B (2019) Addressing Vulvovaginal Atrophy (VVA)/Genitourinary Syndrome of Menopause (GSM) for Healthy Aging in Women. Front. Endocrinol. 10:561. doi: 10.3389/fendo.2019.00561
Received: 22 May 2019; Accepted: 31 July 2019;
Published: 21 August 2019.
Edited by:
Sandro La Vignera, University of Catania, ItalyReviewed by:
Erika Limoncin, University of Rome Tor Vergata, ItalyRoberta Venturella, Università degli Studi Magna Græcia di Catanzaro, Italy
Copyright © 2019 Nappi, Martini, Cucinella, Martella, Tiranini, Inzoli, Brambilla, Bosoni, Cassani and Gardella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rossella E. Nappi, cmVuYXBwaUB0aW4uaXQ=
 Rossella E. Nappi
Rossella E. Nappi Ellis Martini1
Ellis Martini1 Alessandra Inzoli
Alessandra Inzoli Chiara Cassani
Chiara Cassani Barbara Gardella
Barbara Gardella