- 1Department of Maternal and Child Health, School of Public Health, Sun Yat-sen University, Guangzhou, China
- 2Health Promotion Centre for Primary and Secondary Schools of Guangzhou Municipality, Guangzhou, China
- 3Department of Sports Science and Physical Education, The Chinese University of Hong Kong, Hong Kong, China
- 4Department of Health and Physical Education, The Education University of Hong Kong, Hong Kong, China
Whether irisin concentrations are associated with physical activity (PA) and sedentary time (ST) remains unknown. The role of irisin on cardiometabolic health among children has been contradictory and scarce. This study aimed to examine associations of PA and ST with irisin concentrations and relationships between irisin concentrations and cardiometabolic parameters among children. Additionally, we assessed the interaction between PA or ST and irisin concentrations on cardiometabolic parameters. Basing on a cross-sectional survey of 3,651 general children aged 7–12 years, 575 with different self-reported PA (moderate-vigorous intensity PA ≥ 60 min/day or <150 min/week) and ST (gender-, age-specific ST ≥ 75% or <25% percentile) levels were selected. PA and ST were assessed by the validated international physical activity questionnaires. Fasting blood glucose and lipid profile levels were measured with standard methods by biochemistry analyzer. Plasma irisin concentrations were measured by ELISA. The associations of PA and ST with circulating irisin concentrations were examined by linear regression. Linear regression analysis was also used to estimate associations of circulating irisin concentrations with cardiometabolic variables. Interactions between PA or ST and irisin concentrations on cardiometabolic parameters were calculated using multiple linear regression models with dichotomized factors (low PA and high PA; low ST and high ST). No significant association was observed between circulating irisin concentrations and habitual PA or ST. Irisin concentrations were negatively associated with body mass index (BMI) (β = −0.220), BMI z-score (β = −0.098), waist circumference (β = −0.621), diastolic blood pressure (DBP) (β = −0.561), and triglyceride (β = −0.019) in low PA subgroup, and negatively related to fasting blood glucose (β = −0.040) among high PA subgroup. Moreover, irisin concentrations were negatively associated with BMI (β = −0.157) and fasting blood glucose (β = −0.026) only in high ST subgroup (all P < 0.05). We also observed a significant interaction between PA and irisin concentrations on BMI (Pinteraction = 0.0350), BMI z-score (Pinteraction = 0.0173), and DBP (Pinteraction = 0.0068). In summary, irisin concentrations were not associated with habitual PA or ST in children. The negative associations of irisin concentrations with BMI, BMI z-score, and DBP were found only among children being inactive, implying that irisin may contribute to an improvement in health, especially among children with unhealthy lifestyles.
Introduction
There has been a remarkable worldwide increase in the prevalence of cardiometabolic risk factors among children, including obesity, hypertension, hyperglycemia, and dyslipidemia (1–3). Evidence suggests that cardiometabolic risk factors in childhood potentially play a crucial role in influencing later susceptibility to cardiometabolic disease and portend higher mortality (4, 5).
Irisin, a newly identified myokine, has been suggested to regulate energy metabolism (6). It is speculated that irisin induces the browning of white adipose tissue and has a beneficial impact on energy homeostasis (6). Therefore, irisin administration has been suggested to potentially serve as a therapeutic target for cardiometabolic disorders in the future. Some studies have put emphasis on associations of circulating irisin concentrations with cardiometabolic outcomes but conflicting conclusions have been observed (7–10). The controversial findings across studies may be linked with the discrepancy of various characteristics among study populations, such as age, sex, body mass index (BMI) status, diet, and physical activity (PA) levels (7, 11, 12). Research from Spain indicated that irisin concentrations were positively associated with metabolic risk factors among inactive adults, but not among active subjects (12). It is of interest whether associations between circulating irisin concentrations and cardiometabolic parameters are differentially regulated by PA levels. Although evidence has raised concerns about the role of irisin on cardiometabolic improvement, irisin has not been widely studied among children. Variations in different growth development stages may affect the role of irisin. Generalizing findings from adults to children may not be appropriate.
In addition, it has been suggested that increased PA and decreased sedentary time (ST) are important contributors in the protection against cardiometabolic risk factors via myokine released from skeletal muscle (13, 14), and both variables may be independently associated with cardiometabolic risk factors (15). However, the effects of PA on circulating irisin concentrations in humans remain controversial. Several studies have indicated that circulating irisin concentrations were increased in response to acute exercise or habitual PA levels (16, 17), whereas others failed to confirm a significant association between PA and irisin concentrations (18–20). Furthermore, despite the epidemic of ST, to our knowledge no data exist on the relationship between ST and irisin concentrations.
Given this partially unclear situation, the study aimed to (1) examine associations of PA and ST with circulating irisin concentrations and (2) investigate the relationship between irisin concentrations and cardiometabolic parameters among children. (3) In addition, we assessed the interaction between PA or ST and irisin concentrations on cardiometabolic parameters.
Materials and Methods
Study Design and Participants
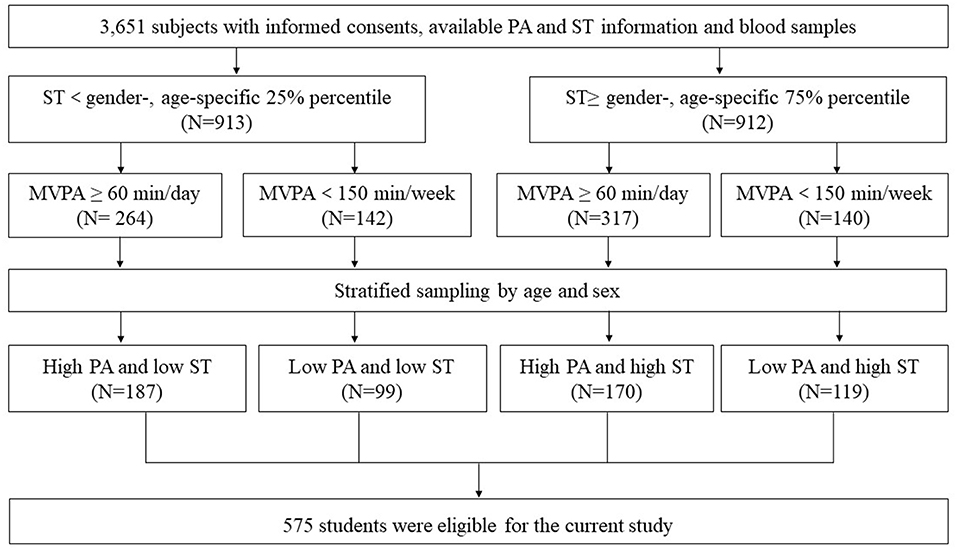
This study was a cross-sectional survey based on a National Natural Science Foundation project of China (Registration number: NCT 03582709), which was conducted between 2017 and 2018. The project had been approved by the Ethical Committee of Sun Yat-sen University and all the participants and their parents signed the informed consents voluntarily prior to participation in the study. In brief, one primary school was randomly chosen from each of the selected 5 different districts in Guangzhou. All the students from these schools were invited to participate in our study. Subjects with significant congenital anomalies or severe handicaps, or prescription medication that might affect endocrine function were excluded. Eventually, 3,651 subjects aged 7–12 years with available PA and ST information as well as blood samples were involved for further analysis.
According to the information on PA and ST obtained by the validated international physical activity questionnaire (IPAQ) (21), subjects with ST equal to or above the gender-, age-specific 75% percentile were selected as the high ST subgroup, while those with ST below the gender-, age-specific 25% percentile were selected as the low ST subgroup. Among each ST subgroup, subjects with moderate-vigorous physical activity (MVPA) ≥ 60 min/day were assigned to the high PA subgroup and those with MVPA <150 min/week were assigned to the low PA subgroup, respectively (22). Subsequently, 863 subjects who met the PA and ST criterion mentioned above were assigned into 4 groups with different ST and PA levels. After stratified sampling by age and sex, 575 children were eligible for the current study (Figure 1).
Measurements
Physical Activity and Sedentary Time Measurements
Information on PA, ST, food intake, and demography was collected by a validated questionnaire, which was filled out by the participants together with their parents. To determine the habitual PA and ST levels, IPAQ (21) was used to assess the frequency and duration of vigorous activity (VPA), moderate activity (MPA), walking, and ST in the last 7 days. VPA referred to activities that take hard physical effort and make you breathe much harder than normal. MPA referred to activities that take moderate physical effort and make you breathe somewhat harder than normal. VPA (min/day) and MPA (min/day) were continuous variables which could be calculated directly from the questionnaire. MVPA was the sum of MPA and VPA. ST covered time which spent in homework, television viewing, and computer using, but not in class or motor vehicle. Only sustained activities of more than 10 min would be noted. To determine participants' food intakes, the frequency and amount of fruit, vegetable, grain, fish, meat, fried food, milk, and sugar-sweetened beverage intake in the last 7 days was collected. Average daily intakes of these foods were calculated as the following formula: average daily intake = [days × (amount in each of those days)]/7.
Anthropometric Measurements
Anthropometric variables were measured by professional doctors, following a standard protocol. Participants were asked to wear light clothing and no shoes when measuring height, weight, and waist circumference (WC). Height was measured to the nearest millimeter by a portable stadiometer. Weight was measured to the nearest 0.1 kg by a lever type weight scale. WC was measured to the nearest millimeter at the level of 1 cm above umbilicus with a steel tape. BMI was calculated as body weight in kilograms divided by height in meters squared (kg/m2). BMI z-score were calculated according to the age-, gender-specific standards from WHO (5–19 years) (23). Waist height ratio (WHtR) was calculated as WC in centimeters divided by height in centimeters.
Blood pressure was measured on the right arm by a validated electronic sphygmomanometer. Participants were required to stay calm for at least 5 min prior to measuring. The cuff was placed 2 cm above the crease of elbow and the size was depended on the mid upper arm circumference. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded. Blood pressure was measured 3 times with 1-min intervals. The average of these three blood pressure recordings was used in the statistical analysis.
Biochemical Analysis
Blood samples were collected via venipuncture from the median cubital vein by qualified nurses at school in the morning, after a 12-h overnight fast. Participants were asked to rest and remain quiet for at least 5 min before the blood draw. Blood samples were immediately centrifuged at 3,000 rpm for 15 min. Plasma samples were separated as soon as possible and stored at −80°C for further assay. Fasting blood glucose levels were measured by the glucose oxidase method (HITACHI 7180 automated biochemistry analyzer, Tokyo, Japan). Concentrations of total cholesterol, triglycerides, high-density lipoprotein (HDL-C) and low-density lipoprotein (LDL-C) were determined by enzymatic assays (HITACHI 7180 automated biochemistry analyzer, Tokyo, Japan). Plasma irisin concentrations were measured with a specific commercial enzyme-linked immunosorbent assay (ELISA) kit (catalog no. EK-067-29; Phoenix Pharmaceuticals, CA, USA) with intra- and inter-assay variations of <10 and <15%, respectively.
Statistical Analysis
Statistical analyses were conducted using the SPSS software version 22.0. Continuous variables were expressed as mean ± standard deviation (SD). Values were presented as percentages for categorical variables. Differences in characteristics between groups were tested by analysis of variance (ANOVA) or chi-square test. Variables with non-normal distributions were log-transformed before analysis. The associations of PA and ST with circulating irisin concentrations were examined by linear regression. Model 1 was adjusted for age and sex. Model 2 was further adjusted for parental educational levels, family income, and food intake. Linear regression analysis was also used to estimate associations of circulating irisin concentrations with anthropometric and metabolic variables, adjusting for age, sex, parental educational levels, family income, PA (min/day), ST (min/day), and food intake. Interactions between PA or ST and circulating irisin concentrations on cardiometabolic parameters were calculated using multiple linear regression models with dichotomized factors (low PA and high PA; low ST and high ST), adjusting for age, sex, parental educational levels, family income, PA (min/day), ST (min/day), and food intake. A two-sided of P-value below 0.05 was considered as statistically significant.
Results
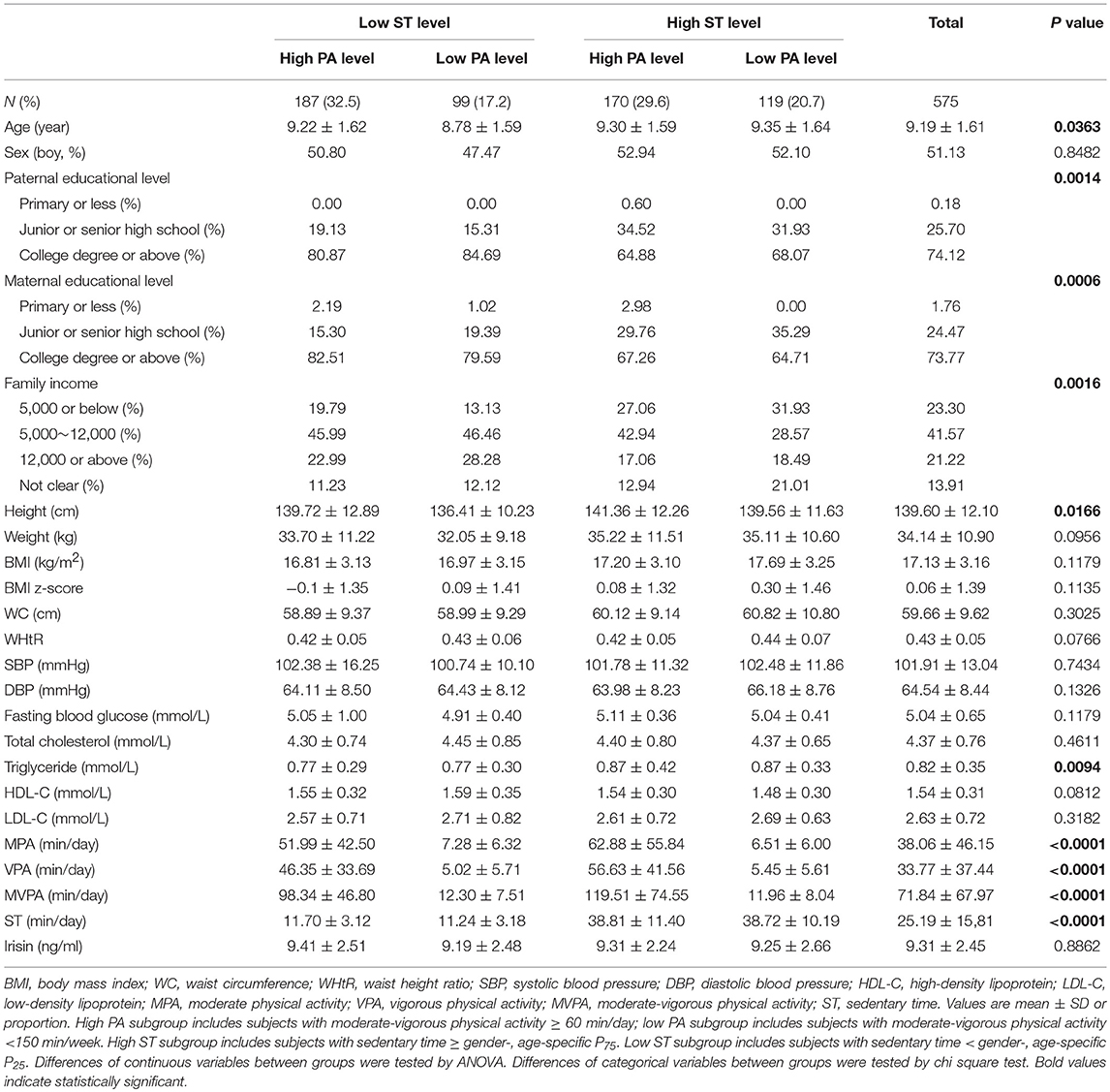
The general characteristics of subjects are shown in Table 1. A total of 575 students were included in the study, of whom 32.5% with low ST and high PA level, 17.2% with low ST and low PA level, 29.6% with high ST and high PA level, and 20.7% with high ST and low PA level. Subjects had a mean age of 9.19 ± 1.61 years. Individuals with high ST and high PA level had higher body height and triglyceride levels compared with their counterparts. Circulating irisin concentrations and other cardiometabolic parameters were not significantly different among groups.
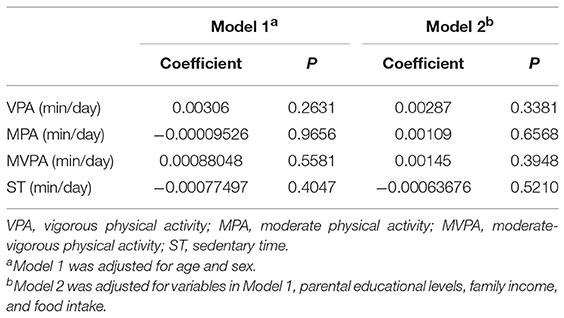
The associations of PA and ST with circulating irisin concentrations are presented in Table 2. After adjustments for covariates, there was no significant association between circulating irisin concentrations and PA. In addition, ST was not significantly related to irisin concentrations as well. Stratified analysis by gender showed similar results in both boys and girls (Table S1).
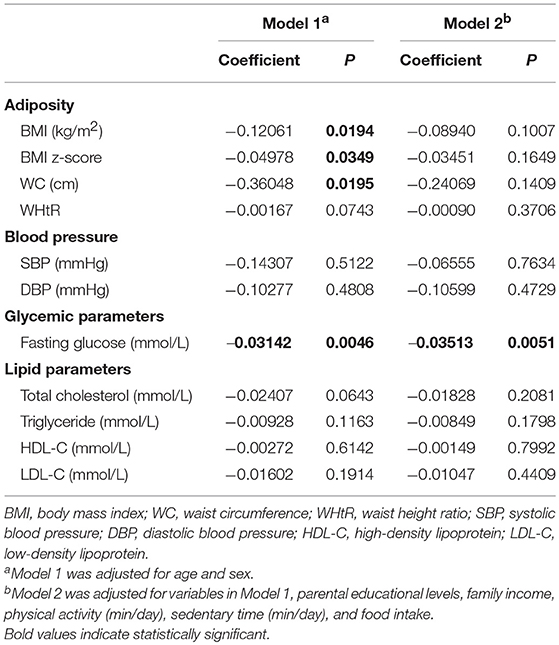
Table 3 presents the associations of subjects' circulating irisin concentrations and cardiometabolic parameters in the overall group. Circulating irisin concentrations were negatively associated with BMI (β = −0.12061, P = 0.0194), BMI z-score (β = −0.04978, P = 0.0349), WC (β = −0.36048, P = 0.0195), and fasting blood glucose levels (β = −0.03142, P = 0.0046), when adjusting for age and sex. The negative correlation between circulating irisin concentrations and fasting glucose levels remained significant in Model 2 (β = −0.03513, P = 0.0051). However, the associations of circulating irisin concentrations with BMI, BMI z-score, and WC became non-significant after further adjustment for other potential confounders. No significant association was found between circulating irisin concentrations and blood pressure as well as lipid profiles in the overall subjects. When stratified by gender, a negative association between irisin concentration and fasting glucose was observed only in boys (Table S2).
To explore the interactions of PA or ST and circulating irisin concentrations on cardiometabolic parameters, we divided subjects into 2 subgroups by PA or ST levels (Table 4). When stratified by PA status, we found that higher circulating irisin concentrations were associated with lower BMI (β = −0.220, P = 0.0164), BMI z-score (β = −0.098, P = 0.0177), WC (β = −0.621, P = 0.0362), DBP (β = −0.561, P = 0.0222), and triglyceride levels (β = −0.019, P = 0.0428) only in the low PA subgroup. In addition, a negative association was observed between circulating irisin concentrations and fasting blood glucose among individuals with high PA status (β = −0.040, P = 0.0411). Significant interactions were observed between circulating irisin concentrations and PA levels on BMI (P = 0.0350 for interaction), BMI z-score (P = 0.0173 for interaction), and DBP (P = 0.0068 for interaction). Moreover, the interaction between circulating irisin concentrations and PA levels on WC was at the edge of significance (P = 0.0533 for interaction). Analysis stratified by ST levels was also conducted. Circulating irisin concentrations were inversely related to BMI (β = −0.157, P = 0.0346) and fasting blood glucose levels (β = −0.026, P = 0.0082) only in high ST subgroup. No significant interaction was observed between circulating irisin concentrations and ST levels.
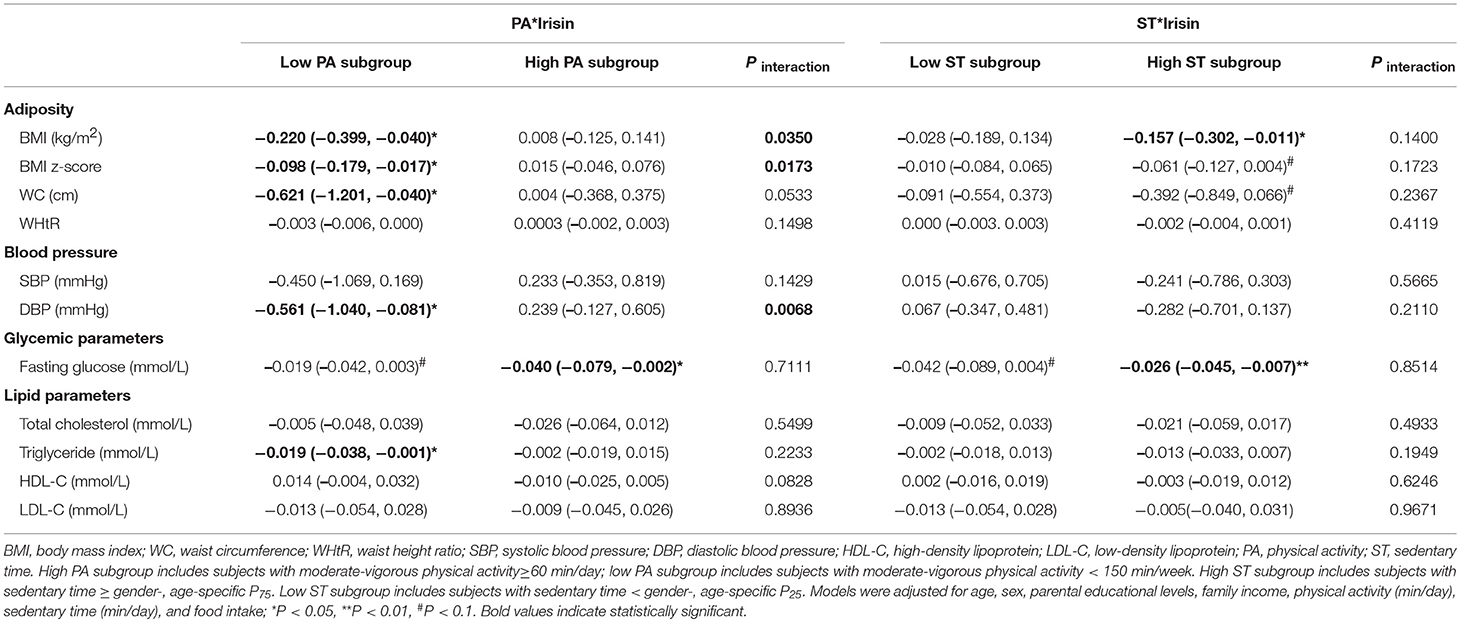
Table 4. Associations of irisin concentrations with cardiometabolic parameters and interactions between irisin concentrations and PA/ST status.
Discussion
In this study, we found that circulating irisin concentrations were not associated with habitual self-reported PA or ST among children. Moreover, irisin concentrations were negatively associated with BMI, BMI z-score, WC, DBP, and triglyceride levels among children with low PA levels, and negatively related to fasting blood glucose among children with high PA levels. As for ST stratifications, irisin concentrations were negatively associated with BMI and fasting blood glucose only among children with high ST status. Additionally, our study provided new evidence that there was a significant interaction between PA and irisin concentrations on BMI, BMI z-score, and DBP.
Some studies had questioned the regulatory role of PA on circulating irisin concentrations in humans. In the present study, we aimed to provide evidence of associations between habitual PA as well as ST and circulating irisin concentrations. Our results were in agreement with the findings which reported a non-significant association of PA with circulating irisin concentrations (18, 24–26). Various researches found an increase in irisin concentrations after intensive exercise (17, 24, 26). However, the acute exercise-induced increase in irisin concentrations seemed to be a transient phenomenon. Irisin concentrations were up-regulated by prolonged aerobic exercise at a moderate intensity, but were no longer elevated by 90 min and significantly lowered by 20 min of recovery (17). Moreover, another study aimed to explore detailed time-course changes in the irisin response to acute exercise, and suggested that irisin concentrations declined to the baseline at 6 h after exercise (16). In addition, a 6-month randomized trail suggested that irisin was stable within-person with high long-term reliability (27). Accordingly, possible explanations for the uncorrelation between habitual PA and irisin concentrations are as follows. Firstly, exercise could induce a transient irisin elevation but irisin concentrations tend to decline to the stable baseline soon after exercise. In this study, we did not measure participants' irisin concentrations immediately after PA. Secondly, only a short bout of PA is necessary to mobilize irisin from its precursor and the further increase will only occur when new FNDC5 is recruited to the membrane (26). With regard to ST, previous studies largely defined subjects who did not meet the PA guideline as sedentary individuals but seldom took account into the exact ST levels. However, sedentary behavior is not necessarily the same as a lack of PA. The relationship between ST and irisin concentrations has been under-investigated. Taking different types of ST into consideration, our findings do not support a significant association between ST and circulating irisin concentrations in children. However, further evidence in this area is needed before definite conclusions can be drawn.
As for associations of circulating irisin concentrations with cardiometabolic risk factors, there is no general agreement among different researches. In the current study, irisin concentrations were not associated with any cardiometabolic outcomes, except fasting blood glucose in the overall group, but negatively associated with BMI, BMI z-score, and WC among children with low PA levels. Meanwhile, irisin concentrations were negatively associated with fasting blood glucose levels among children with high PA levels. Previous studies had also reported negative associations of irisin concentrations with markers of adiposity and blood glucose (8, 28). According to the research by Boström et al. (6), irisin had an efficacious effect on the browning of white adipose tissues both in culture and in vivo to simulate uncoupling protein 1 (UCP1) expression and a broad program of brown fat-like development. Subsequently, it caused a significant increase in whole-body energy expenditure, and modified susceptibility to weight gain and improved glucose homeostasis. In contrast, some studies showed that circulating irisin concentrations were positively correlated with adiposity indices and fasting blood glucose, putting forward the presumption that irisin concentrations were increased in compensation for the “irisin resistance” among obese or diabetic populations (7, 29, 30). Moreover, some researches failed to detect a relationship between irisin concentrations and adiposity indices as well as blood glucose levels (31, 32). The discrepant findings across several studies in humans may be linked with the differences in study population. In our study, the general population of children were analyzed, which exhibited normal glucose homeostasis and without “irisin resistance.” Additionally, it is interesting that irisin concentrations were negatively associated with BMI, BMI z-score, and WC when adjusting for age and sex. However, the relationship became non-significant after further adjustment for other potential confounders. Further analysis indicated that food intake might have an effect on the magnitude of these associations (data not shown). According to previous research, irisin concentrations might not be affected by food ingestion (11, 25). Still, evidence for the impact of food intake on the magnitude of the relationship between irisin and adiposity indices is insufficient. Further work is needed before definitive conclusions can be made.
Besides, it is noteworthy that we found that the association between irisin concentration and fasting blood glucose was more evident among boys in parallel with girls. The gender-specific influence of irisin in glucose status may be explained by differences in hormones and adipose tissue distribution between genders. Compared with girls, boys might have greater volumes of brown adipose tissue (33).
Here, we also presented an inverse association between irisin concentrations and DBP among the low PA subgroup. Our findings were consistent with an observational study from China (34). Nevertheless, Yan et al. (10) demonstrated a non-significant correlation between circulating irisin concentrations and blood pressure in obese Chinese adults. In view of existing evidence, our knowledge about the association of irisin concentrations with blood pressure in humans is still incomplete. Several experiments were performed to detect the modulating effect of irisin on blood pressure in rats. Zhang et al. (35) suggested that peripheral irisin reduced blood pressure in rats by acting on blood vessels, and that both smooth muscle and endothelial cells were affected by irisin. Another study concluded that administration of irisin decreased blood pressure in spontaneously hypertensive rats by an improvement of endothelial dysfunction of the mesenteric artery through the AMPK-Akt-eNOS-NO signaling pathway (36). However, the biological mechanisms by which irisin regulates blood pressure are not totally unraveled, especially in humans.
A growing body of epidemiological evidence indicates that irisin may have an impact on lipid metabolism. In this study, we found that circulating irisin concentrations were negatively related to triglyceride levels among children with low PA status. This negative association was also presented in other previous research (28, 37). According to the experimental evidence, irisin administration might increase the secretion of glycerol and decrease lipid accumulation by regulating the expression of adipose triglyceride lipase, hormone-sensitive lipase and fatty acid-binding protein 4 (38). Conversely, numerous studies demonstrated a positive relationship between irisin concentrations and lipid metabolism markers (9, 39, 40). Diversities of metabolic and endocrine status among study populations might lead to the inconsistent results (38). Based on the current evidence, little is known about the physiological association between irisin and lipid metabolism in humans. Further research is also needed to elucidate the underlying mechanisms.
Interestingly, we also found significant interactions between circulating irisin concentrations and PA levels on BMI, BMI z-score and DBP. Significantly negative associations of irisin concentrations with BMI, BMI z-score, and DBP were found only among children being inactive. These findings highlighted the possibility that the associations mentioned above were differentially regulated by PA status. Meanwhile, we displayed evidence to support the hypothesis that associations of irisin concentrations with cardiometabolic risk factors among individuals being inactive might raise a compensatory role to counterbalance the increased risk of unhealthy life styles (12). On the other hand, it had been reported that various myokines were secreted and released into circulation induced by exercise (41). Exercise might orchestrate the interplay of various myokines and contribute to benefits for health. We speculate that irisin's interplay with other myokines might counteract its association with cardiometabolic outcomes among subjects being active.
Notably, the present study is the first to evaluate the association of ST levels with circulating irisin concentrations, as well as to provide a further analysis of the interactions between PA and irisin concentrations on cardiometabolic outcomes. However, our study also has some limitations. Firstly, our findings were based on a cross-sectional design, and the causality could not be determined. Secondly, PA and ST levels were subjectively measured using a self-report questionnaire. As compared to accelerometer, questionnaires might underestimate the absolute values of MVPA and ST (42). Nevertheless, the questionnaire was on the basis of the widely used IPAQ and had a moderate reliability and validity according to the pre-investigation. A comparison of IPAQ-SF with accelerometer also indicated that MVPA estimated from IPAQ-SF was moderately correlated with accelerometers (42). Thirdly, the low PA subgroup (MVPA <150 min/week) in our study may only represent those children being typically physically inactive. Fourthly, the acute increases in irisin during exercise might be an important mechanism for exercise-induced improvement in cardiometabolic risk factors. However, we did not able to demonstrate whether irisin is simply a brief intermediary pathway in the relationship between PA and cardiometabolic risk factors, for the reason that we did not conduct blood drawing immediately after exercise. Further research is needed to expound the potential pathways. Moreover, circulating irisin concentrations varied a lot in different studies, which may in part be attributed to the fact that different assay kits were used. In the present study, we used reliable and widely used kits (catalog no. EK-067-29; Phoenix Pharmaceuticals, CA, USA), which were specifically designed to measure circulating irisin concentrations. The irisin concentrations in the current study are comparable with those reported by Thomas et al. (9), who used the same kit in their study. Lastly, we measured irisin concentrations in circulation, but were unable to clarify the detailed source of irisin secretion, which led to difficulties in unraveling the precise associations.
In summary, our study found that there was no association between circulating irisin concentrations and habitual PA or ST levels among children. Furthermore, associations of circulating irisin concentrations with BMI, BMI z-score, and DBP were differentially regulated by PA status. The negative associations of irisin concentrations with BMI, BMI z-score, and DBP were found only among inactive children. These findings imply that irisin may contribute to improvement in cardiovascular system and metabolism, especially among children with unhealthy lifestyles. Future studies are needed to provide a better understanding on the function of irisin and the underlying biological mechanism in humans.
Data Availability
The datasets for this manuscript are not publicly available. Requests to access the datasets should be directed to YC (Y2hlbnlqNjhAbWFpbC5zeXN1LmVkdS5jbg==).
Ethics Statement
This project had been approved by the Ethical Committee of Sun Yat-sen University and all the participants and their parents signed the informed consents voluntarily prior to participation in the study.
Author Contributions
YC and LC designed the study. LC, MT, WT, XZ, NW, and JY contributed to the data collection. LC and MT analyzed the data and drafted the manuscript. YC, SW, JO'R, and FS critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study has been funded by National Natural Science Foundation Projects of China (Grant Nos. 81673193 and 81602862). Peiqi Ye and Xiaoyu Xu also received support from an undergraduate teaching project of Sun Yat-sen University (51000-18832609).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the support from all the participated students and their parents, local education, health staffs, our team members, and volunteers (Peiqi Ye and Xiaoyu Xu).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00549/full#supplementary-material
Abbreviations
BMI, Body mass index; DBP, Diastolic blood pressure; ELISA, Enzyme-linked immunosorbent assay; FBG, Fasting blood glucose; FNDC5, Fibronectin type III domain containing protein 5; HDL-C, High density lipoprotein-cholesterol; IPAQ, International physical activity questionnaire.; LDL-C, Low density lipoprotein-cholesterol; MPA, Moderate intensity physical activity; MVPA, Vigorous to moderate Intensity physical activity; PA, Physical activity; PGC-1α, PPAR γ coactivator 1 alpha; SBP, Systolic blood pressure; ST, Sedentary time; TC, Total cholesterol; TG, Triglyceride; UCP1, Uncoupling protein1; VPA, Vigorous intensity physical activity; WC, Waist circumference; WHO, World Health Organization; WHtR, Waist height ratio.
References
1. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2014) 384:766–81. doi: 10.1016/S0140-6736(14)60460-8
2. de Moraes ACF, Lacerda MB, Moreno LA, Horta BL, Carvalho HB. Prevalence of high blood pressure in 122,053 adolescents. Medicine. (2014) 93:e232. doi: 10.1097/MD.0000000000000232
3. Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. (2012) 35:2515–20. doi: 10.2337/dc12-0669
4. Eckel J. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. (2011) 128 (Suppl 5):S213–56. doi: 10.1542/peds.2009-2107C
5. Adair LS, Gordon-Larsen P, Du SF, Zhang B, Popkin BM. The emergence of cardiometabolic disease risk in Chinese children and adults: consequences of changes in diet, physical activity and obesity. Obes Rev. (2014) 15:49–59. doi: 10.1111/obr.12123
6. Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. (2012) 481:463–8. doi: 10.1038/nature10777
7. Jang HB, Kim H, Kang JH, Park SI, Park KH, Lee H. Association of circulating irisin levels with metabolic and metabolite profiles of Korean adolescents. Metabolism. (2017) 73:100–8. doi: 10.1016/j.metabol.2017.05.007
8. Choi Y, Kim M, Bae KH, Seo H, Jeong J, Lee W. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract. (2013) 100:96–101. doi: 10.1016/j.diabres.2013.01.007
9. Reinehr T, Elfers C, Lass N, Roth CL. Irisin and its relation to insulin resistance and puberty in obese children: a longitudinal analysis. J Clin Endocrinol Metab. (2015) 100:2123–30. doi: 10.1210/jc.2015-1208
10. Yan B, Shi X, Zhang H, Pan L, Ma Z, Liu S. Association of serum irisin with metabolic syndrome in obese Chinese adults. PLoS ONE. (2014) 9:e94235. doi: 10.1371/journal.pone.0094235
11. Anastasilakis AD, Polyzos SA, Saridakis ZG, Kynigopoulos G, Skouvaklidou EC, Molyvas D. Circulating irisin in healthy, young individuals: day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J Clin Endocrinol Metab. (2014) 99:3247–55. doi: 10.1210/jc.2014-1367
12. Moreno M, Moreno-Navarrete JM, Serrano M, Ortega F, Delgado E, Sanchez-Ragnarsson C. Circulating irisin levels are positively associated with metabolic risk factors in sedentary subjects. PLoS ONE. (2015) 10:e0124100. doi: 10.1371/journal.pone.0124100
13. Pedersen BK. The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol. (2009) 587:5559–68. doi: 10.1113/jphysiol.2009.179515
14. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. (2012) 8:457–65. doi: 10.1038/nrendo.2012.49
15. Ekelund U. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA. (2012) 307:704. doi: 10.1001/jama.2012.156
16. Tsuchiya Y, Ando D, Takamatsu K, Goto K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism. (2015) 64:1042–50. doi: 10.1016/j.metabol.2015.05.010
17. Kraemer RR, Shockett P, Webb ND, Shah U, Castracane VD. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm Metab Res. (2014) 46:150–4. doi: 10.1249/01.mss.0000494385.02684.8b
18. Pekkala S, Wiklund PK, Hulmi JJ, Ahtiainen JP, Horttanainen M, Pollanen E. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol. (2013) 591:5393–400. doi: 10.1113/jphysiol.2013.263707
19. Hecksteden A, Wegmann M, Steffen A, Kraushaar J, Morsch A, Ruppenthal S. Irisin and exercise training in humans - results from a randomized controlled training trial. BMC Med. (2013) 11:235. doi: 10.1186/1741-7015-11-235
20. Palacios-Gonzalez B, Vadillo-Ortega F, Polo-Oteyza E, Sanchez T, Ancira-Moreno M, Romero-Hidalgo S. Irisin levels before and after physical activity among school-age children with different BMI: a direct relation with leptin. Obesity. (2015) 23:729–32. doi: 10.1002/oby.21029
21. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
23. Li YP, Hu XQ, Jing-Zhao, Yang XG, Ma GS. Application of the WHO growth reference (2007) to assess the nutritional status of children in China. Biomed Environ Sci. (2009) 22:130–5. doi: 10.1016/S0895-3988(09)60035-0
24. Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. (2014) 281:739–49. doi: 10.1111/febs.12619
25. Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. (2012) 61:1725–38. doi: 10.1016/j.metabol.2012.09.002
26. Löffler D, Müller U, Scheuermann K, Friebe D, Gesing J, Bielitz J. Serum irisin levels are regulated by acute strenuous exercise. J Clin Endocrinol Metab. (2015) 100:1289–99. doi: 10.1210/jc.2014-2932
27. Panagiotou G, Mu L, Na B, Mukamal KJ, Mantzoros CS. Circulating irisin, omentin-1, and lipoprotein subparticles in adults at higher cardiovascular risk. Metabolism. (2014) 63:1265–71. doi: 10.1016/j.metabol.2014.06.001
28. Shim YS, Kang MJ, Yang S, Hwang IT. Irisin is a biomarker for metabolic syndrome in prepubertal children. Endocr J. (2018) 65:23–31. doi: 10.1507/endocrj.EJ17-0260
29. Qiu S, Cai X, Yin H, Zugel M, Sun Z, Steinacker JM. Association between circulating irisin and insulin resistance in non-diabetic adults: a meta-analysis. Metabolism. (2016) 65:825–34. doi: 10.1016/j.metabol.2016.02.006
30. Fukushima Y, Kurose S, Shinno H, Cao TTH, Tamanoi A, Tsutsumi H. Relationships between serum irisin levels and metabolic parameters in Japanese patients with obesity. Obes Sci Pract. (2016) 2:203–9. doi: 10.1002/osp4.43
31. Sanchis-Gomar F, Alis R, Pareja-Galeano H, Sola E, Victor VM, Rocha M. Circulating irisin levels are not correlated with BMI, age, and other biological parameters in obese and diabetic patients. Endocrine. (2014) 46:674–7. doi: 10.1007/s12020-014-0170-9
32. Gouni-Berthold I, Berthold HK, Huh JY, Berman R, Spenrath N, Krone W. Effects of lipid-lowering drugs on irisin in human subjects in vivo and in human skeletal muscle cells ex vivo. PLoS ONE. (2013) 8:e72858. doi: 10.1371/journal.pone.0072858
33. Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, Hu HH. Changes in brown adipose tissue in boys and girls during childhood and puberty. J Pediatr. (2012) 160:604–609.e1. doi: 10.1016/j.jpeds.2011.09.035
34. Zhang L, Xie Q, Tang C, Zhang A. Expressions of irisin and urotensin II and their relationships with blood pressure in patients with preeclampsia. Clin Exp Hypertens. (2017) 5:460–7. doi: 10.1080/10641963.2016.1273945
35. Zhang W, Chang L, Zhang C, Zhang R, Li Z, Chai B. Central and peripheral irisin differentially regulate blood pressure. Cardiovasc Drugs Ther. (2015) 29:121–7. doi: 10.1007/s10557-015-6580-y
36. Fu J, Han Y, Wang J, Liu Y, Zheng S, Zhou L. Irisin lowers blood pressure by improvement of endothelial dysfunction via AMPK-Akt-eNOS-NO pathway in the spontaneously hypertensive rat. J Am Heart Assoc. (2016) 5:e003433. doi: 10.1161/JAHA.116.003433
37. Oelmann S, Nauck M, Völzke H, Bahls M, Friedrich N. Circulating irisin concentrations are associated with a favourable lipid profile in the general population. PLoS ONE. (2016) 11:e0154319. doi: 10.1371/journal.pone.0154319
38. Gao S, Li F, Li H, Huang Y, Liu Y, Chen Y. Effects and molecular mechanism of GST-irisin on lipolysis and autocrine function in 3T3-L1 adipocytes. PLoS ONE. (2016) 11:e0147480. doi: 10.1371/journal.pone.0147480
39. Liu J, Wong MDS, Toy WC, Tan CSH, Liu S, Ng XW. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complicat. (2013) 27:365–9. doi: 10.1016/j.jdiacomp.2013.03.002
40. Hee Park K, Zaichenko L, Brinkoetter M, Thakkar B, Sahin-Efe A, Joung KE. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab. (2013) 98:4899–907. doi: 10.1210/jc.2013-2373
41. Pedersen BK. A muscular twist on the fate of fat. N Engl J Med. (2012) 366:1544–5. doi: 10.1056/NEJMcibr1201024
Keywords: irisin, cardiometabolic risk factors, children, physical activity, sedentary time
Citation: Cai L, Tan M, Tan W, Zeng X, Wan N, Wong SH, O'Reilly J, Sun F, Yang J and Chen Y (2019) Associations of Circulating Irisin Concentrations With Cardiometabolic Risk Factors Among Children Vary by Physical Activity or Sedentary Time Levels. Front. Endocrinol. 10:549. doi: 10.3389/fendo.2019.00549
Received: 04 May 2019; Accepted: 23 July 2019;
Published: 14 August 2019.
Edited by:
Madhusmita Misra, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Nicolas M. Oreskovic, Massachusetts General Hospital and Harvard Medical School, United StatesGeorge Paltoglou, National and Kapodistrian University of Athens, Greece
Copyright © 2019 Cai, Tan, Tan, Zeng, Wan, Wong, O'Reilly, Sun, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yajun Chen, Y2hlbnlqNjhAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work
 Li Cai1†
Li Cai1† Stephen Heung-sang Wong
Stephen Heung-sang Wong Fenghua Sun
Fenghua Sun Yajun Chen
Yajun Chen