
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 07 August 2019
Sec. Cancer Endocrinology
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00512
This article is part of the Research Topic Testicular Cancer: New Insights on the Origin, Genetics, Treatment, Fertility, General Health, Quality of Life and Sexual Function View all 18 articles
 Francesco Pierconti1*
Francesco Pierconti1* Maurizio Martini1
Maurizio Martini1 Giuseppe Grande2
Giuseppe Grande2 Luigi M. Larocca1
Luigi M. Larocca1 Emilio Sacco3
Emilio Sacco3 Dario Pugliese3
Dario Pugliese3 Gaetano Gulino3
Gaetano Gulino3 Pier F. Bassi3
Pier F. Bassi3 Domenico Milardi2
Domenico Milardi2 Alfredo Pontecorvi2
Alfredo Pontecorvi2Purpose: The testis-sparing surgery (TSS) is surgical technique accepted for small testicular masses (STMs). Frozen section examination (FSE) is an essential assessment at the time of TSS. The aim of this study is to measure the maximum distance of the foci of ITGCN from STMs.
Methods: In our hospital between June 2010 and October 2017 a total of 68 patients with STM underwent a TSS. All the testis specimens were totally embedded and processed via the whole-mount method and a diagnosis of germ cell tumor with GCNIS were made. The distance between STMs and GCNIS were calculated by two pathologists directly on the slides considering for the third dimension the number of the paraffin blocks in which the foci of GCNIS were found.
Results: The STMs were classic seminoma in 62 out 68 cases, embryonal carcinoma in 4 cases, while in 2 case a diagnose of mixed germ cell tumor were made. The size of the STMs was between 0.5 and 2 cm and the foci of GCNIS were observed in seminiferous tubules very closed to SMTs or as skip lesions in the surrounding testicular parenchyma, dispersed in normal testis. In 48 out of 68 cases (70.5%) foci of GCNIS were at the distance from SMTS of 1.5 cm or below and in 60 out of 68 cases (88%) at the distance of 2 cm or below The distance of GCNIS from the STMs was not related to the histological subtype of the germ cell tumor, while there is a linear correlation between size of the STMs and the distance of foci of GCNIS (p = 0.0105; r = 0.9167).
Conclusion: Our data showed that foci of ITGCN were not observed beyond 2.5 cm from the STM. In particular we demonstrated that exist a linear correlation between size of STMs and distance of the foci of GCNIS from STMs (p = 0.0105; r = 0.9167). In conclusion mapping the tissue around the tumor not randomly but in targeted areas could reduce the false negative biopsies of the testis with GCNIS, increasing the radicality of the TSS procedure.
Malignant germ cell tumors appear clinically as palpable masses and radical orchiectomy is considered the standard surgical treatment for these lesions (1). The recent use of high-frequency ultrasonography has led to an increase in detection of incidental small testicular masses (STMs) defined as non-palpable, <25 mm in diameter, intrascrotal masses (2). The testis-sparing surgery (TSS) is an accepted surgical technique for STMs. Since most STMs are benign, unnecessary orchiectomy is to be considered too “costly” from a hormonal and reproductive point of view (3–5). Organ-sparing surgery has numerous advantages from an endocrine point of view in avoiding hypogonadism (6). Orchiectomy in fact causes a reduction in testosterone levels, which in turns represents a risk factor for cardiovascular diseases (7), osteoporosis (8), and lower urinary tract syndrome (LUTS) (9). As a consequence, patients who underwent orchiectomy often require life-long androgen replacement therapy and have a higher risk of low bone mineral density (10) and LUTS (11). Furthermore, preservation of fertility is another issue in young patients affected by testicular tumors. Moreover, it has been reported that unilateral orchiectomy has disruptive effects on overall spermatogenesis (4). Liu et al. reported no significant changes in terms of sperm concentrations and motility in 11 patients with TSS history for benign testicular tumors (12).
The indications for TSS are still controversial, especially for patients with normal contralateral testis. According to the German Cancer Study Group, TSS can be considered only for selected patients with malignant tumors in solitary testis or with a bilateral tumor with a lesion diameter <2 cm and no invasion of rete testis, with normal preoperative serum (LH) levels (13).
Moreover in 2011, the update of the EAU Guidelines considered TSS as an alternative surgical treatment only for patients with synchronous bilateral testicular tumors, metachronous contralateral tumors or for lesions in patients with solitary testis, and normal preoperative testosterone levels if the volume of the tumors represents <30% of the testicular volume.
To avoid secondary surgery, the urologist needs to know the quality of the resected small tumor and the surrounding tissue. The diameter of the mass is an important parameter for TSS; several studies demonstrated that in the case of non-palpable, symptomatic masses with a diameter of <2 cm, TSS represents the best surgical management because the prevalence of benign histology is ~80% (14–19).
Multiple biopsies of the surrounding tissue, in order to evaluate the presence of microscopic areas of tumor infiltration, is performed after the enucleation of the mass. The presence of foci of GCNIS in the multiple biopsies surrounding the small testicular mass (STM), at frozen section examination (FSE), could help the pathologist discern benign from malignant neoplasms, when a non-conclusive diagnosis of STMs is made. In fact, the presence of GCNIS near the mass seems sufficient for the diagnosis of malignant testicular germ cell tumors (TGCTs) (20–25).
In case of the diagnosis of TGCTs, adjuvant approaches such as radiotherapy, chemotherapy (one cycle of carbolatin AUC7 in case of seminoma) or surveillance must be considered.
The goal of our study is to define the maximum distance of the expected presence of foci of GCNIS from malignant STMs, in order to provide the surgeon with significant information during the intraoperative procedures of TSS.
Sixty-eight patients with a STM <2 cm and with a volume <30% of the testicular volume, underwent TSS in our hospital between June 2010 and October 2017. All patients had a testicular mass <2 cm at inspection and a scrotal high frequency ultrasound (US); no physical or radiological signs of malignancy or metastases were observed.
During surgical procedures the FSE of the STMS and multiple biopsies of the surrounding testicular tissue were performed. An operative ultrasound was applied to permit the surgeon to measure and perform the biopsies at the right distance.
As a result of the diagnosis, a traditional radical orchiectomy was performed in case of diagnosis of GCT and/or GCNIS identification in FSE, in order to ensure the best oncological result.
All the diagnosis made on frozen section biopsies were confirmed at histological examination after immune-histochemical staining with PLAP and CD117.
All testis specimens were totally embedded and processed via the whole-mount method and a final pathology reports of germ cell tumors with GCNIS were made (26, 27). For the diagnosis of the GCNIS we isolated the areas with histological features of GCNIS in the whole mount section. A confirmative immune-histochemical staining with PLAP and CD117 was performed.
The distance between STMs and GCNIS was calculated by two pathologists (LML and FP) on the whole mount section, directly on the slides, considering the third dimension for the number of paraffin blocks in which the foci of GCNIS were found. Each paraffin block was 5 mm in thickness.
For the immune-histochemical studies, the avidin-biotin-peroxidase complex method was performed on the paraffin sections, applying a technical procedure previously reported, using a commercially available kit (Dako LSAB2, Dakopatts, Glostrup, Denmark) and the following commercially available monoclonal antibodies: PLAP, CD117 (28, 29).
Statistical analysis was performed using GraphPad Prism (version 5, La Jolla, CA) or MedCalc (version 10.2, Ostend, Belgium) software. Continuous variables normally distributed were reported as the mean and standard deviation (SD). A comparison of continuous variables normally distributed was performed using the Student's t-test (U-paired or Mann-Whitney t-test). A comparison of categorical variables between the two groups was performed by the Chi-square statistic or using the Fisher exact test when appropriate. All p-values are considered statistically significant when p < 0.05.
The main clinical features of the patients, including the main risk factors for TGCTs, are reported in Table 1. Figure 1 shows an ultrasound image explicative of a STM.
Classic seminoma was found in 62 out of 68 cases with STMs, embryonal carcinoma was found in four, while a mixed germ cell tumor was found in two (seminoma, embryonal carcinoma, and yolk sac tumor). No histological signs of neoplastic invasion of rete testis or necrosis were observed.
The size of the STMs was between 0.5 and 2 cm and the foci of GCNIS were observed in seminiferous tubules very close to SMTs or as skip lesions in the surrounding testicular parenchyma. In 24 cases, the foci of GCNIS were at a distance of 1.5 cm or below from SMTS and in 31 cases at a distance of 2 cm or below. GCNIS was not detected in 13 FSE biopsies.
The distance of GCNIS from the STMs was not related to the histological subtype of the germ cell tumor, while there was a linear correlation between the size of the STMs and the distance of foci of ITGCN (p = 0.0105; r = 0.9167; Figure 2).
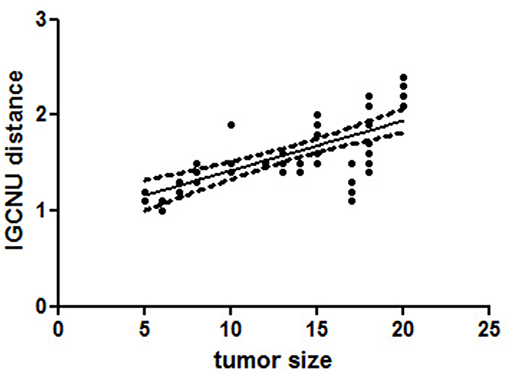
Figure 2. A linear regression analysis performed to evaluate the significant association between different variables [tumor size and distance of foci germ cell neoplasia in situ (GCNIS) showed a linear correlation between size of the small testicular masses (STMs) and the distance of foci of GCNIS (p = 0.0105; r = 0.9167)]. All p-values are considered statistically significant when p < 0.05.
In detail, foci of GCNIS have been found within 2 cm from the STMs with a diameter ≤ 1.5 cm. GCNIS have been found up to 2.5 cm from the neoplastic lesion, when tumor size ranged from 1.5 cm up to 2 cm, We did not observe foci of GCNIS beyond 2.5 cm from the STMs (Figures 3A–C).
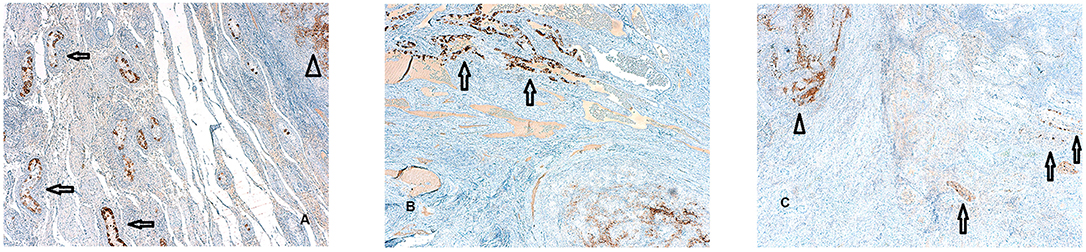
Figure 3. (A–C) Foci of germ cell neoplasia in situ (GCNIS) identified by PLAP immunostaining surrounding the small testicular mass (STM) diagnosed as seminoma. A focal rather than random distribution of the GCNIS in testicular tissue adjacent to TGCTS was evident (PLAP immunostaining magnification 5X). This foci of GCNIS (arrows) are separated form the STM (arrows head) by tubular testis without the sign of intratubular germ cell neplasia and in all the cases examined,foci of GCNIS were not observed beyond 2,5 cm from the STM.
The use of an ultrasound in the primary evaluation of patients with infertility and with local scrotal symptoms has led to high incidence in the early detection of small, mostly benign, testicular masses (3). According to the literature, testis sparing surgery is a safe and feasible procedure for patients presenting a benign small testis mass. It has been demonstrated that more than two thirds of asymptomatic testicular masses <2 cm are pathologically benign (13, 16, 17, 19). Although TSS is a controversial approach (30), it is justified in highly selected clinical scenarios.
In our study we performed TSS and FSE in a population of selected patients with STMs. When a diagnosis of malignant tumors was made after TSS, the orchiectomy was performed in our study. However, several papers demonstrated that the surveillance of STMs is an option along with testis sparing surgery (30).
In our study, FSE of the primary lesion and the surrounding tissue was performed to determine the nature of the testicular mass and to evaluate the radicality of the surgical procedure (3, 31). FSE has gained increasing accuracy, after initial skepticism about its diagnostic power. FSE represents a useful tool, especially with the aim to discriminate between benign or malignant testicular neoplasms when a histological diagnosis of STM is not conclusive (16, 20–22, 25). Sometimes in fact, the FSE of the STM is unable to discriminate between benign and malignant tumors, especially when the STM is very small, when the material for the FSE is poor or when the tissue resected is affected by artifacts, caused by technical frozen procedures (18, 32, 33). In these cases, identifying foci of GCNIS near the STM may help pathologists to classify STM as a germ cell tumor. In fact, previous data have been reported demonstrating that foci of GCNIS were found in up to 82% of tissues surrounding a germ cell tumor and that the risk of progression to invasive malignancy is 50 and 70% at 5- and 7-years follow-ups, respectively (13, 34).
FSE in surrounding tissue is a very accurate procedure. It has been reported in fact that the false-negative biopsies for the diagnosis of GCNIS has been found in only 0.5% of the biopsies (35). The majority of false negative biopsies are caused by sampling errors when a biopsy is not taken from a representative area.
Our data showed that foci of GCNIS have not been observed beyond 2.5 cm from the STM. To provide detail, we demonstrated the linear correlation between the size of STMs and the distance of the GCNIS foci from STMs. For small masses with a diameter up to 1.5 cm, GCNIS was observed up to 2 cm from the tumor. When the tumor diameter was more than 1.5 cm, GCNIS foci was detected up to 2.5 cm from the tumor. These results are in accordance with GCNIS-mapping studies that reported a focal rather than random distribution of the GCNIS in testicular tissue adjacent to TGCTs (36, 37).
Despite the limitations of this study, since it is a retrospective study analyzing TSS with targeted biopsies, it is the first study providing data on the correct distance to perform FSE in TSS from. Further studies are needed to provide confirmation in total orchiectomies and namely to analyze the linear correlation between the size of GCT and the distance from GNIS in orchiectomy samples. These studies will provide clearer evidence to support partial orchiectomy.
Moreover, targeted testicular biopsies performed at the right distance from STMs and measured by intraoperative US, might reduce the false negative rates in the diagnosis of GCNIS, thus supporting pathologists in detecting clinical situations at risk for malignant TGCTs.
The knowledge of the distance, from STM beyond which we did not find GCNIS, could help surgeons during intraoperative procedures, suggesting the best site at which to perform the testicular biopsies for the FSE.
In conclusion, the histological characterization by FES of the tissue around tumors in targeted areas could reduce false negative biopsies of testis with GCNIS. This approach may improve the efficacy of radical excision through the TSS procedure, reducing the risk of leaving residual cancer during TSS and subsequently of the disease recurrence.
All the procedures described in this paper are included in the consensus statement of our hospital that the patients signed when a surgical approach is recommended. The FSE of the STMS, the multiple biopsies of the surrounding testicular tissue and the processing of the neoplastic nodule via the whole-mount method are routinary processes in our department of pathology as well as the immunohistochemical staining with PLAP and CD117 performed in order to make a correct histopathological diagnosis. For this reasons, we believe that an ethical review process was not required for this study.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, et al. European Association of Urology EAU guidelines on testicular cancer: 2011 update. Eur Urol. (2012) 60:304–19. doi: 10.1016/j.eururo.2011.05.038
2. Steiner H, Höltl L, Maneschg C, Berger AP, Rogatsch H, Bartsch G, et al. Frozen section analysis-guided organ-sparing approach in testicular tumors: technique, feasibility, and long-term results. Urology. (2003) 62:508–13. doi: 10.1016/S0090-4295(03)00465-5
3. Carmignani L, Gadda F, Gazzano G, Nerva F, Mancini M, Ferruti M, et al. High incidence of benign testicular neoplasms diagnosed by ultrasound. J Urol. (2003) 170:1783–6. doi: 10.1097/01.ju.0000092066.01699.90
4. Ferreira U, Netto Júnior NR, Esteves SC, Rivero MA, Schirren C. Comparative study of the fertility potential of men with only one testis. Scand J Urol Nephrol. (1991) 25:255–9. doi: 10.3109/00365599109024555
5. Arai Y, Kawakita M, Okada Y, Yoshida O. Sexuality and fertility in long-term survivors of testicular cancer. J Clin Oncol. (1997) 15:1444–8. doi: 10.1200/JCO.1997.15.4.1444
6. Keske M, Canda AE, Atmaca AF, Cakici OU, Arslan ME, Kamaci D, et al. Testis-sparing surgery: experience in 13 patients with oncological and functional outcomes. Can Urol Assoc J. (2019) 13:E83–E88. doi: 10.5489/cuaj.5379
7. Milardi D, Grande G, Giampietro A, Vendittelli F, Palumbo S, Tartaglione L, et al. Circulating endothelial cells as marker of endothelial damage in male hypogonadism. J Androl. (2012) 33:1291–7. doi: 10.2164/jandrol.112.016600
8. Ferlin A, Selice R, Carraro U, Foresta C. Testicular function and bone metabolism–beyond testosterone. Nat Rev Endocrinol. (2013) 9:548–54. doi: 10.1038/nrendo.2013.135
9. La Vignera S, Condorelli RA, Russo GI, Morgia G, Calogero AE. Endocrine control of benign prostatic hyperplasia. Andrology. (2016) 4:404–11. doi: 10.1111/andr.12186
10. Ondrusova M, Spanikova B, Sevcikova K, Ondrus D. Testosterone deficiency and bone metabolism damage in testicular cancer survivors. Am J Mens Health. (2018) 12:628–33. doi: 10.1177/1557988316661986
11. Cheng CL, de Groat WC. Effect of orchiectomy and testosterone replacement on lower urinary tract function in anesthetized rats. Am J Physiol Renal Physiol. (2016) 311:F864–F870. doi: 10.1152/ajprenal.00016.2016
12. Liu B, Su H, Cheng G, Li P, Hua L, Song N, et al. Experiences and outcomes of organ-sparing surgery for testicular tumors with benign tendency. Can Urol Assoc J. (2015) 9:E785–8. doi: 10.5489/cuaj.2972
13. Heidenreich A, Weissbach L, Höltl W, Albers P, Kliesch S, Köhrmann KU, et al. Organ sparing surgery for malignant germ cell tumor of the testis. J Urol. (2001) 166:2161–5. doi: 10.1016/S0022-5347(05)65526-7
14. Gentile G, Brunocilla E, Franceschelli A, Schiavina R, Pultrone C, Borghesi M, et al. Can testis-sparing surgery for small testicular masses be considered a valid alternative to radical orchiectomy? A prospective single-center study. Clin Genitourin Cancer. (2013) 11:522–6. doi: 10.1016/j.clgc.2013.04.033
15. Rolle L, Tamagnone A, Destefanis P, Bosio A, Timpano M, Fiori C, et al. Microsurgical “testis-sparing” surgery for nonpalpable hypoechoic testicular lesions. Urology. (2006) 68:381–5. doi: 10.1016/j.urology.2006.02.028
16. Shilo Y, Zisman A, Raz O, Lang E, Strauss S, Sandbank J, et al. Testicular sparing surgery for small masses. Urol Oncol. (2012) 30:188–91. doi: 10.1016/j.urolonc.2009.12.021
17. De Stefani S, Isgrò G, Varca V, Pecchi A, Bianchi G, Carmignani G, et al Microsurgical testis-sparing surgery in small testicular masses: seven years retrospective management and results. Urology. (2012) 79:858–62. doi: 10.1016/j.urology.2011.12.039
18. Hopps CV, Goldstein M. Ultrasound guided needle localization and microsurgical exploration for incidental nonpalpable testicular tumors. J Urol. (2002) 168:1084–7. doi: 10.1016/S0022-5347(05)64580-6
19. Giannarini G, Dieckmann KP, Albers P, Heidenreich A, Pizzocaro G. Organ-sparing surgery for adult testicular tumors: a systematic review of the literature. Eur Urol. (2010) 57:780–90. doi: 10.1016/j.eururo.2010.01.014
20. Tokuc R, Sakr W, Pontes JE, Haas GP. Accuracy of frozen section examination of testicular tumors. Urology. (1992) 40:512–6. doi: 10.1016/0090-4295(92)90405-L
21. Winstanley AM, Mikuz G, Debruyne F, Schulman CC, Parkinson MC, Eurpean Association of Pathologists Uropathology Division in Florence. Handling and reporting of biopsy and surgical specimens of testicular cancer. Eur Urol. (2004) 45:564–73. doi: 10.1016/j.eururo.2003.10.015
22. Elert A, Olbert P, Hegele A, Barth P, Hofmann R, Heidenreich A. Accuracy of frozen section examination of testicular tumors of uncertain origin. Eur Urol. (2002) 41:290–3. doi: 10.1016/S0302-2838(02)00004-0
23. Leroy X, Rigot JM, Aubert S, Ballereau C, Gosselin B. Value of frozen section examination for the management of nonpalpable incidental testicular tumors. Eur Urol. (2003) 44:458–60. doi: 10.1016/S0302-2838(03)00316-6
24. Connolly SS, D'Arcy FT, Bredin HC, Callaghan J, Corcoran MO. Value of frozen section analysis with suspected testicular malignancy. Urology. (2006) 67:162–5. doi: 10.1016/j.urology.2005.07.041
25. Subik MK, Gordetsky J, Yao JL, di Sant'Agnese PA, Miyamoto H. Frozen section assessment in testicular and paratesticular lesions suspicious for malignancy: its role in preventing unnecessary orchiectomy. Hum Pathol. (2012) 43:1514–9. doi: 10.1016/j.humpath.2011.11.013
26. Pierconti F, Straccia P, Emilio S, Bassi PF, De Pascalis I, Marques RC, et al. Cytological and histological changes in the urothelium produced by electromotive drug administration (EMDA) and by the combination of intravescical hyperthermia and chemotherapy (thermochemotherapy). Pathol Res Pract. (2017) 213:1078–81. doi: 10.1016/j.prp.2017.07.026
27. Valentini AL, Gui B, Cina A, Pinto F, Totaro A, Pierconti F, et al. T2-weighted hypointense lesions within prostate gland: differential diagnosis using wash-in rate parameter on the basis of dynamic contrast-enhanced magnetic resonance imaging–hystopatology correlations. Eur J Radiol. (2012) 81:3090–5. doi: 10.1016/j.ejrad.2012.05.019
28. Martini M, Hohaus S, Petrucci G, Cenci T, Pierconti F, Massini G, et al. Phosphorylated STAT5 represents a new possible prognostic marker in Hodgkin lymphoma. Am J Clin Pathol. (2008) 129:472–7. doi: 10.1309/63H1A83HRTBQ07DB
29. Calarco A, Pinto F, Pierconti F, Sacco E, Marrucci E, Totaro A, et al. Role of SOCS3 evaluated by immunohistochemical analysis in a cohort of patients affected by prostate cancer: preliminary results. Urologia. (2012) 79 (Suppl. 19):4–8. doi: 10.5301/RU.2012.9392
30. Pizzocaro G, Nicolai N, Salvioni R. Evolution and controversies in the management of low-stage nonseminomatous germ-cell tumors of the testis. World J Urol. (1994) 12:113–9. doi: 10.1007/BF00192265
31. Bozzini G, Picozzi S, Gadda F, Colombo R, Decobelli O, Palou J, et al. Long-term follow-up using testicle-sparing surgery for Leydig cell tumor. Clin Genitourin Cancer. (2013) 11:321–4. doi: 10.1016/j.clgc.2012.12.008
32. Powell TM, Tarter TH. Management of nonpalpable incidental testicular masses. J Urol. (2006) 176:96–8. doi: 10.1016/S0022-5347(06)00496-4
33. Klatte T, de Martino M, Arensmeier K, Reiher F, Allhoff EP, Klatte D. Management and outcome of bilateral testicular germ cell tumors: a 25-year single center experience. Int J Urol. (2008) 15:821–6. doi: 10.1111/j.1442-2042.2008.02107.x
34. von der Maase H, Rørth M, Walbom-Jørgensen S, Sørensen BL, Christophersen IS, Hald T, et al. Carcinoma in situ of contralateral testis in patients with testicular germ cell cancer: study of 27 cases in 500 patients. Br Med J. (1986) 293:1398–401. doi: 10.1136/bmj.293.6559.1398
35. Dieckmann KP, Loy V. False-negative biopsies for the diagnosis of testicular intraepithelial neoplasia (TIN)–an update. Eur Urol. (2003) 43:516–21. doi: 10.1016/S0302-2838(03)00101-5
36. Prym C, Lauke H. Carcinoma-in situ of the human testis: tumors cells are distributed focally in the seminiferous tubules. Andrologia. (1994) 26:231–4. doi: 10.1111/j.1439-0272.1994.tb00793.x
Keywords: testis, small testicular mass, seminoma, intratubular germ cell neoplasia, PLAP
Citation: Pierconti F, Martini M, Grande G, Larocca LM, Sacco E, Pugliese D, Gulino G, Bassi PF, Milardi D and Pontecorvi A (2019) Germ Cell Neoplasia in situ (GCNIS) in Testis-Sparing Surgery (TSS) for Small Testicular Masses (STMs). Front. Endocrinol. 10:512. doi: 10.3389/fendo.2019.00512
Received: 30 January 2019; Accepted: 12 July 2019;
Published: 07 August 2019.
Edited by:
Vincenzo Pezzi, University of Calabria, ItalyReviewed by:
Patrick Fénichel, Centre Hospitalier Universitaire de Nice, FranceCopyright © 2019 Pierconti, Martini, Grande, Larocca, Sacco, Pugliese, Gulino, Bassi, Milardi and Pontecorvi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Pierconti, ZnJhbmNlc2NvLnBpZXJjb250aUB1bmljYXR0Lml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.