
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 08 August 2019
Sec. Thyroid Endocrinology
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00506
This article is part of the Research Topic The Role of Thyroid Hormones in Vertebrate Development View all 16 articles
Thyroid hormones (THs) are ancient hormones that not only influence the growth, development and metabolism of vertebrates but also affect the metabolism of (at least some) bacteria. Synthesized in the thyroid gland (or follicular cells in fish not having a discrete thyroid gland), THs can act on target cells by genomic or non-genomic mechanisms. Either way, THs need to get from their site of synthesis to their target cells throughout the body. Despite being amphipathic in structure, THs are lipophilic and hence do not freely diffuse in the aqueous environments of blood or cerebrospinal fluid (in contrast to hydrophilic hormones). TH Distributor Proteins (THDPs) have evolved to enable the efficient distribution of THs in the blood and cerebrospinal fluid. In humans, the THDPs are albumin, transthyretin (TTR), and thyroxine-binding globulin (TBG). These three proteins have distinct patterns of regulation in both ontogeny and phylogeny. During development, an additional THDP with higher affinity than those in the adult, is present during the stage of peak TH concentrations in blood. Although TTR is the only THDP synthesized in the central nervous system (CNS), all THDPs from blood are present in the CSF (for each species). However, the ratio of albumin to TTR differs in the CSF compared to the blood. Humans lacking albumin or TBG have been reported and can be asymptomatic, however a human lacking TTR has not been documented. Conversely, there are many diseases either caused by TTR or that have altered levels of TTR in the blood or CSF associated with them. The first world-wide RNAi therapy has just been approved for TTR amyloidosis.
Thyroid hormones (THs) are fundamentally involved in the regulation of growth, development, and overall metabolism, particularly of the CNS. Despite being amphipathic in structure, THs are lipophilic compounds and readily partition between the lipid phase and the aqueous phase with a ratio of about 20,000:1 (1). Therefore, THs are not freely diffusible in the aqueous environments of the blood and cerebrospinal fluid (CSF).
THs are considered evolutionarily “old” hormones, as THs and their derivatives impact the metabolism of not only vertebrates but also bacteria (2), ascidians, tunicates, and other invertebrate species [for review see Holzer et al. (3)]. Furthermore, the endostyle of tunicates can incorporate iodine into tyrosine residues which are then incorporated into proteins, rendering the endostyle the functional precursor (from a TH perspective) to the thyroid gland (4). In amphibians, reptiles, birds, and mammals, THs are synthesized in the thyroid gland, which is a discrete gland located at the base of the neck. In fish, however, the shape and location of the thyroid gland varies considerably between species e.g. diffuse follicles around the ventral aorta (cyclostomes), or near the branchial arteries of the gills (some teleosts) or a compact gland near the branchial arch (elasmobranchs) (5).
Following synthesis in the thyroid gland (or follicles, as for fish), the THs are secreted via TH transmembrane transporters into the blood. In vertebrate species studied to date, most TH secreted by the thyroid is in the form of 3,3′,5,5′-tetraiodo-L-thyronine (thyroxine; T4) and less is in the form of 3,3′5-triiodo-L-thyronine (T3) (6) (see Figure 1). Due to the lipophilicity of THs (as mentioned above), they preferentially partition into the lipid environment of membranes (11). However, this can be counteracted with the presence of plasma proteins that bind THs and enable distribution of the THs from their site of synthesis to their target cells throughout the body. Thus, these proteins are termed “TH Distributor Proteins” (THDPs) (12). In humans, the THDPs are albumin, transthyretin (TTR), and thyroxine-binding globulin (TBG) (see Figure 1). Greater than 99.7% of THs in the blood of mammals is bound to THDPs, rendering a very small fraction in the free form. Because only the free (non-protein bound) THs can enter cells, it is very important that the THDPs regulate the amount of free THs in the blood (and CSF).
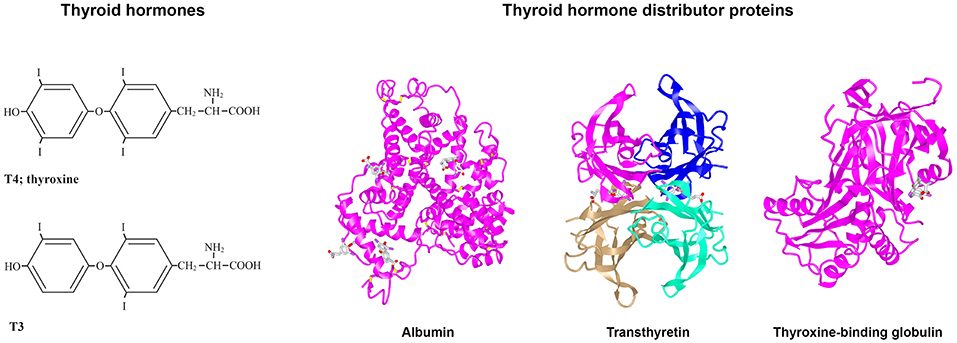
Figure 1. Structures of T4, T3, albumin, TTR, and TBG. (Left) Structures of T3 and T4. (Right) 3D protein structures and T4 binding sites of the three THDPs in humans: albumin (7), transthyretin (8), and thyroxine-binding globulin (7–10).
In humans, the main THDPs are albumin, TTR, and TBG. Albumin is a single polypeptide chain protein with a molecular mass of about 67 kDa that is rich in alpha helical structure (see Figure 1). At physiological pH, albumin has a heart shape globular structure but under acidic pH adopts an elongated “cigar conformation” (these conformational changes are reversible). Albumin comprises about half the total protein in blood (~40 g/l) and can bind many compounds weakly: THs, fatty acid, drugs etc. (13). TTR is a 55 kDa homotetrameric protein rich in beta sheet structure and can be considered a dimer of dimers which come together with a central channel that has two TH binding sites [Blake et al. (14); see Figure 1], although under physiological conditions only one site is filled due to negative co-operativity (9). TTR is present in healthy adult blood at about 0.25 mg/l and can also bind up to two molecules of retinol-binding protein, which in turn bind retinol (15). Thus, TTR distributes two ligands for nuclear hormone receptors. TBG is a 54 kDa monomeric protein that has only a single site for TH binding and is highly glycosylated (see Figure 1). Whilst due to its structure TBG can be considered a serpin (serine protease inhibitor), it does not actually function as a serpin. Albumin, TTR, and TBG each have differing affinities and on/off rates for T4 and for T3 [for greater details and discussion, see (16)]. In general, albumin binds quite weakly, TTR has intermediate affinity and TBG has the highest affinity for THs. In mammals, each of these three THDPs binds T4 with higher affinity than T3. This provides a buffering like system, for maintaining the free level of THs [see (12)]. The range is from the concentration of free T4 in blood up to the maximum solubility of T4 at pH 7.4. Some text books claim that the reason for hydrophobic signal molecules being bound to protein in the blood is due to poor solubility of the hydrophobic signal compound in the blood, but this is not true. The maximum solubility of T4 at pH 7.4 is 2.3 μM (17) i.e., 100,000 times the concentration of free T4 in human blood (24 pM). The function of THDPs in enabling TH distribution was shown by a set of elegant experiments by Mendel et al. (11): rat livers perfused with T4 in the absence of THDPs resulted in T4 partitioning into the first cells they came in contact with; whereas when livers were perfused with T4 together with THDPs, this resulted in a uniform distribution of T4 throughout the liver and T4 also in the perfusate.
Albumin and TBG have higher affinity for T4 than for T3 in all species studied. However, this is not the case for TTR: in all studied species of birds, reptiles, amphibians, and fish [with the notable exception of sea bream, where TTR had similar affinity for T3 and T4 (18)],TTR has higher affinity for T3 than for T4 (19–24). Only in mammals, does TTR have higher affinity for T4 than for T3 [for a detailed discussion on how and why this may have occurred during evolution, see (25)].
Not all vertebrates have all three THDPs in their blood. There are clear patterns based on various groups of vertebrates, during both ontogeny and phylogeny. The traditional way of identifying THDPs in blood of various species was analyzing serum or plasma directly for radioactively-labeled T3 or T4 binding to proteins. The discussion in this paragraph relates to data collected in that way. Of the ~150 species of adult vertebrates studied, all had albumin as a THDP in their blood (26–30). For some groups of animals, albumin was the only THDP. In general: fish, amphibians, and reptiles and monotremes (echidna and platypus) and some polyprotodont marsupials. For another set of animals, both albumin and TTR were present in blood. In general: birds, diprotodont marsupials, and some eutherians (“placental mammals”). The final set had all three THDPs. This group only comprised some eutherian mammals but we could not discern a clear pattern within eutherians for presence/absence of TBG [previous studies had suggested TBG was present in “larger mammals” but this no longer holds true e.g., (28)]. In general, there has been an increase in TH distribution capacity during vertebrate evolution, both in the number of THDPs and in light of each “new” THDP having higher affinity for THs than the previous i.e., albumin (original THDP with weak affinity for THs) then TTR (second THDP appearing during evolution, with higher affinity for THs than albumin) then TBG [third THDP appearing, with higher affinity for THs than TTR; see (31)].
More recently, with the ever-expanding number of genomes and transcriptomes that are accessible via publicly available databases, it has been possible to identify genes and mRNA or expressed sequence tags (ESTs) corresponding to proteins of interest. This approach can be valuable for identification of low abundance proteins. Whilst this can be an alternative approach to identification of proteins being synthesized in a given tissue of a particular species at a defined stage of life, this can also generate many “false positives” if only considered superficially. It is very time-consuming to check each transcript for complete integrity (full length, lack of internal stop codons etc.). Never the less, this approach has been used successfully to identify TTR in many species of fish (32). Given the huge diversity of fish (>25,000 species), the data presented in Table 1 that were collected the “traditional” way, are probably overly simplistic and not truly representative of the actual situation in nature. “Omics” approaches are required to further investigate the distribution of TTR synthesis in each group of fish and the corresponding ontogenic analyses. Furthermore, the variation in piscine TTRs to date has already revealed that some fish TTRs bind T3 with higher affinity than T4 whereas others bind both ligands with similar affinity (see above).
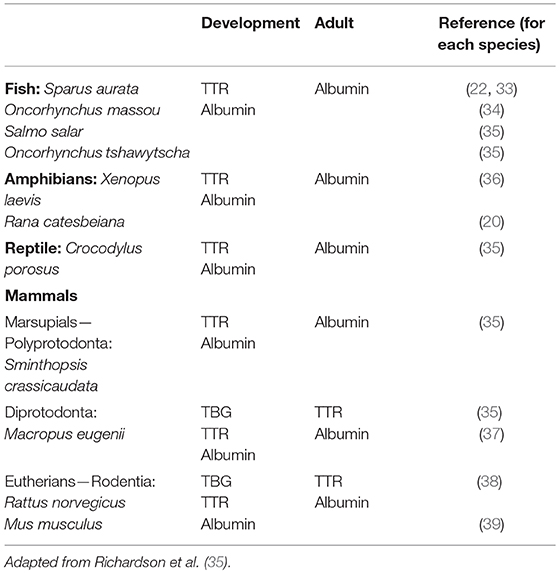
Table 1. Vertebrate species with additional THDP with higher affinity for TH in blood during development.
For those animals with three THDPs, TTR is responsible for most of the delivery (bioavailability) of T4 (40). This is in contrast to the previously held belief that because TBG binds about 75% of T4 in blood (albumin binds about 10% and TTR binds about 15%), that TBG is responsible for the delivery of T4. We think of the situation as analogous to Goldilocks and the Three Bears (41): albumin binds so weakly that it is not very efficient in distributing T4; TBG binds so tightly that it is not efficient in releasing T4 (more like a storage reservoir in the blood); but due to the combination of off rates and capillary transit times, TTR is responsible for most of the delivery of T4 to tissues (42).
The pattern of THDPs in blood during development differs from that in adults (see below). In general, it was revealed that there was an additional THDP present during specific stages of development, compared to adulthood. For example, in two species of salmon, where albumin is the only THDP in adults, TTR was also present at smoulting (35) and in juvenile fish (22, 33, 34). Whereas, adult amphibian had only albumin as a THDP in blood, around the time of metamorphosis TTR was also present (20, 23, 36). Juvenile saltwater crocodiles were found to have TTR in addition to albumin, and the polyprotodont marsupial (fat-tailed dunnart) had TTR in addition to albumin during development (35). The diprotodont marsupial tammar wallaby had a TBG-like protein during development in addition to the albumin and TTR present in adults (37). All vertebrates have a transient surge in TH levels at a specific stage in development (6). The additional THDPs appear to coincide with the elevated TH levels in blood (35). This provides an augmented TH distribution capacity at the time when TH levels in blood are elevated during development (see Table 1). In this table, we consider only plasma proteins. However, whilst plasma proteins are responsible for the majority of TH distribution in homeotherms, some fish are known to have the bulk of their THs distributed in the blood by lipoproteins (43) and a small proportion of THs in human blood (44). Oviparous animals (including some fish, amphibians, reptiles, birds, monotremes, and some invertebrates) also synthesize another lipoprotein: vitellogenin, an egg yolk precursor protein synthesized in the liver. Vitellogenin is a TH binding protein. In females, vitellogenin levels cycle according to estrogen levels. However, TH can regulate the levels of vitellogenin by inducing estrogen receptor alpha (45).
THs must cross the blood-brain barrier or the blood-CSF barrier in order to enter the CNS. These two barriers have been studied most extensively in mammals but some information is known about other vertebrates also. However, to the best of our knowledge, the quantitative contribution of each pathway (crossing the blood-brain barrier or crossing the blood-CSF barrier, relative to the other) for TH entering the brain is not yet known.
The blood-CSF barrier is formed by the tight junctions between the epithelial cells of the choroid plexus. The choroid plexus is a villous structure located in the lateral, third and fourth ventricles of the brain and is responsible for secreting about 70% of the CSF (46). In adults, the major protein synthesized and secreted by the choroid plexus from studied species of mammals (eutherians, marsupials, and monotremes), birds, and reptiles is TTR (24, 29, 47–50). This TTR is secreted toward the CSF and not into the blood (51) and has been implicated in moving T4 (but not T3) from the blood across the choroid plexus into the CSF (1, 52, 53). The major protein synthesized and secreted by the choroid plexus of amphibians is a lipocalin, specifically: prostaglandin D synthetase (54, 55) also known as Cpl1 (55) and β-trace (56). This protein has a calyx structure which could be used for binding small hydrophobic molecules and thus could have been a functional precursor to TTR, although possibly not transporting THs.
In those species studied, the choroid plexus has the highest concentration of TTR mRNA per tissue weight compared to other tissues in the body e.g., 11- to 22-fold higher than in the liver [see (57)]. However, the timing for the maximal TTR mRNA levels in the choroid plexus differs between animals. Animals who are fairly independent soon after birth/hatching (e.g., chickens and sheep) are described as precocial and their brains are further developed at birth compared to altricial animals, whose brains are less developed at birth and are dependent on their mothers (e.g., rats, mice, marsupials). Precocial animals were found to have the peak of TTR mRNA in their choroid plexus before birth, whereas altricial animals had the peak of TTR mRNA in the choroid plexus after birth (58). For both groups of animals, the peak in TTR mRNA is just prior to the maximal growth rate in the brain. Given that the blood-brain barrier starts to develop when the first blood vessel grows into the brain (59) and that the choroid plexus develops faster than other parts of the brain, producing most of the CSF (and thus regulating its composition), and the peak of TTR mRNA just prior to the maximal growth rate of the brain, it follows that the choroid plexus-derived TTR could have a significant role in moving T4 from the blood into the CSF (57).
Whilst TTR is the only THDP known to be synthesized in the CNS (to the best of our knowledge, albumin and TBG are exclusively synthesized in the liver), this does not mean that albumin and TBG are absent from the CSF. In adult mammals, the protein concentration of the CSF is about 0.43 g/l compared to that in blood of about 70 g/l (60). Plasma proteins are present in the CSF at a concentration inversely proportional to their Stokes radius (61). Thus, whilst albumin is not synthesized in the CSF, it is present in the CSF; similarly for TBG in species where TBG is synthesized by the liver. It follows that, for example, although the choroid plexus of fish and amphibians do not synthesize TTR, their CSF would contain some albumin. Similarly, the CSF in reptiles and birds contains albumin and TTR; and the CSF of humans contains albumin, TTR, and TBG. However, the ratio of TTR to albumin in the CSF is very different to that in the blood: whereas in the blood albumin comprises ~50% total protein and TTR comprises ~0.4% total protein, in the CSF albumin comprises ~40% total protein and TTR comprises about 4% total protein (45). Thus, the TTR to albumin ratios and TH distribution kinetics would differ significantly. TTR is the main carrier of TH in the CSF (62, 63). Presumably, during the peak in TH concentration in blood during development, when the liver is synthesizing an “additional” THDP, some of that protein will enter the CSF. To date, it is unknown if TH levels in the CSF peak when (or soon after) the TH levels in the blood peak. Indeed, it might not be a reasonable question to consider, as the concentration of proteins and other molecules is not consistent throughout the CSF (46). In contrast to the blood, which mixes within minutes and is fairly homogeneous, the CSF flows in a directional “pipeline-like” manner and measurements of concentrations of its components differ depending on the sampling site [see (12)].
THs can exert both genomic and non-genomic actions. Regardless of which type of action a given TH will have, it is still required to get from its site of synthesis to its target tissue/cell and this is mediated via the THDPs: TTR, albumin, and TBG (depending on the species and stage of development).
Once THs have arrived at their target cell and have dissociated from the THDP, they are able to enter cells via TH transmembrane transporter proteins. These TH transmembrane transporters belong to the family of solute carriers and those known to move THs into and out of cells are the monocarboxylate transporters MCT8 and MCT10; L-amino acid transporters LAT1 and (depending on the species) LAT2; and organic anion transporter peptide OATP1C1 [for review see (64)]. Of these, only MCT8 and MCT10 are exclusive for the transmembrane transport of THs.
As mentioned above, the majority of TH secreted from the thyroid gland is in the form of T4 and around 80% of T3 is generated by local deiodination in target cells e.g., various regions of the brain produce differing proportions of T3 via local deiodination (65). Deiodination is carried out by a family of deiodinase enzymes, each of which can remove a specific iodine atom from a TH. Deiodinases can be classified by their broad reactions as either Outer Ring Deiodinases or Inner Ring Deiodinases, according to the position of the iodine atom being removed. Deiodinases can also be classified via their structures (amino acid sequences), locations and substrate preferences: Dio1, Dio2, and Dio3 [for review see (66)]. Deiodinases can either activate or inactivate THs within a cell. Whereas, genomic pathways are regulated mainly by T3, non-genomic pathways may be regulated by a greater number of TH derivatives (67).
Albumin, TTR, and TBG are negative acute phase plasma proteins (68) i.e., following stress, illness, surgery, or injury, their rates of synthesis in the liver decrease. This is thought to result in a transient increase in free TH in blood, which can then enter cells and direct anabolic reactions to restore health and homeostasis. Humans lacking either albumin (www.albumin.org) or TBG [see (69)] have been reported and were essentially without overt symptoms. Until now, no human lacking TTR has been reported. Could lack of TTR be incompatible with human life? Could this be due to TTR being the main protein synthesized and secreted by the choroid plexus or due to TTR being the main source of delivery of THs to tissues?
On the other hand, albumin and TBG have very few diseases associated with them: analbuminaemia [(70), www.albumin.org] and a variant of TBG in Australian Aborigines which has low affinity for THs (71), whereas TTR has major diseases associated with it: the various forms of TTR amyloidosis. Familial Amyloidotic Polyneuropathy (FAP) is a late onset autosomal dominant form of amyloidosis. More than 100 point mutations have been associated with causing TTR FAP (72). This is a significant number, as the polypeptide chain has only 127 amino acids in total (TTR is a homo-tetramer). In addition, wild type TTR can also form amyloid: Senile Systemic Amyloidosis. This occurs most frequently in the hearts of elderly men (73). The cause for wild type TTR to spontaneously form amyloid in the heart is currently unknown.
The first approved anti-TTR amyloid drug to come onto the market was Tafamidis (trade name Vyndaqel), a compound designed by Jeff Kelly and colleagues (74). Very recently, Patisiran (trade name ONPATTRO) has been approved by the European Commission and the United States Food and Drug Administration for the treatment of TTR amyloidosis. This is the first world-wide approved RNAi therapeutic! Apparently, patients receiving this RNAi therapy did not show deficiencies in thyroid or vitamin A metabolism. This could be due to only a small proportion of TTR circulating in blood having a TH or RBP-retinol bound.
An increasing body of knowledge is building around associations of altered TTR concentrations in the blood and/or CSF and a variety of diseases such as Alzheimer's Disease (75–77), rheumatoid arthritis (78, 79), schizophrenia (80, 81), preeclampsia (82–84), Guillain-Barre syndrome (85–87), and depression (88), and references in Alshehri et al. (41).
Whether TH actions are via genomic or non-genomic pathways, THs need to get from their site of synthesis to their sites of action via THDPs in the blood and CSF. The network of THDPs is augmented during the developmental surge in THs in blood, providing increased distribution capacity. The THDPs have distinct profiles during both ontogeny and phylogeny, such that very fine regulation of free TH available to enter cells is highly controlled. In adult humans, lack of albumin or TBG are tolerated, yet a single point mutation in TTR can lead to disease. Humans lacking TTR have not yet been identified. The implication is that lack of TTR in humans is incompatible with life. This could be due to TTR being responsible for most delivery of TH to tissues or due to its role in moving TH from the blood into the CSF via the choroid plexus. Abnormal levels of TTR in humans are increasingly being associated with a variety of diseases. It is unknown if the altered TTR levels are a cause or a consequence of these diseases.
Overall structure was suggested by SJR. SAR and IG wrote most drafts with guidance from MP and NO. SJR edited the final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Dickson PW, Aldred AR, Menting JG, Marley PD, Sawyer WH, Schreiber G. Thyroxine transport in choroid plexus. J Biol Chem. (1987) 262:13907–15.
2. Distefano JJ, Deluze A, Nguyen TT. Binding and degradation of 3,5,3'-triiodothyronine and thyroxine by rat intestinal bacteria. Am J Physiol. (1993) 264:E966–72. doi: 10.1152/ajpendo.1993.264.6.E966
3. Holzer G, Roux N, Laudet V. Evolution of ligands, receptors and metabolizing enzymes of thyroid signaling. Mol Cell Endocrinol. (2017) 459:5–13. doi: 10.1016/j.mce.2017.03.021
4. Roche J, Salvatore G, Rametta G. Sur la presence et la biosynthese d'hormones thyroidiennes chez un tunicier Ciona intestinalis. Biochim Biophys Acta. (1962) 63:154–65. doi: 10.1016/0006-3002(62)90348-7
5. Chanet B, Meunier FJ. The anatomy of the thyroid gland among “fishes”: phylogenetic implications for the vertebrata. Cybium. (2014) 38:89–116. doi: 10.26028/cybium/2014-382-002
6. Hulbert AJ. Thyroid hormones and their effects: a new perspective. Biol Rev Camb Philos Soc. (2000) 75:519–631. doi: 10.1017/S146479310000556X
7. Petitpas I, Petersen CE, Ha CE, Bhattacharya AA, Zunszain PA, Ghuman J, et al. Structural basis of albumin-thyroxine interactions and familial dysalbuminemic hyperthyroxinemia. Proc Natl Acad Sci USA. (2003) 100:6440–5. doi: 10.1073/pnas.1137188100
8. Zhou A, Wei Z, Read RJ, Carrell RW. Structural mechanism for the carriage and release of thyroxine in the blood. Proc Natl Acad Sci USA. (2006) 103:13321–6. doi: 10.1073/pnas.0604080103
9. Neumann P, Cody V, Wojtczak A. Ligand binding at the transthyretin dimer-dimer interface: structure of the transthyretin-T4Ac complex at 2.2 Angstrom resolution. Acta Crystallogr D Biol Crystallogr. (2005) 61(Pt 10):1313–9. doi: 10.1107/S0907444905022523
10. Madej T, Lanczycki CJ, Zhang D, Thiessen PA, Geer RC, Marchler-Bauer A, et al. MMDB and VAST+: tracking structural similarities between macromolecular complexes. Nucleic Acids Res. (2014) 42(Database issue):D297–303. doi: 10.1093/nar/gkt1208
11. Mendel CM, Weisiger RA, Jones AL, Cavalieri RR. Thyroid hormone binding proteins in plasma facilitate uniform distribution of thyroxine within tissues - a perfused rat liver study. Endocrinology. (1987) 120:1742–9. doi: 10.1210/endo-120-5-1742
12. Schreiber G, Richardson SJ. The evolution of gene expression, structure and function of transthyretin. Compar Biochem Physiol Part B Biochem Mol Biol. (1997) 116:137–60. doi: 10.1016/S0305-0491(96)00212-X
13. Peters T. Ligand binding by albumin. In: All About Albumin. 1st ed. Biochemistry, Genetics and Medical Applications. San Diego, CA: Academic Press (1996). p. 76–132. doi: 10.1016/B978-012552110-9/50005-2
14. Blake CC, Geisow MJ, Oatley SJ, Rerat B, Rerat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J Mol Biol. (1978) 121:339–56. doi: 10.1016/0022-2836(78)90368-6
15. Monaco HL, Rizzi M, Coda A. Structure of a complex of two plasma proteins: transthyretin and retinol-binding protein. Science. (1995) 268:1039–41. doi: 10.1126/science.7754382
16. Richardson SJ. Cell and molecular biology of transthyretin and thyroid hormones. Int Rev Cytol. (2007) 258:137–93. doi: 10.1016/S0074-7696(07)58003-4
17. Rotzsch W, Kohler H, Martin H. Zur loslichkeit von thyroxin. Hoppe-Seyler's Z Physiol Chem. (1967) 348:939–40.
18. Morgado I, Santos CR, Jacinto R, Power DM. Regulation of transthyretin by thyroid hormones in fish. Gen Comp Endocrinol. (2007) 152:189–97. doi: 10.1016/j.ygcen.2006.12.017
19. Kasai K, Nishiyama N, Yamauchi K. Molecular and thyroid hormone binding properties of lamprey transthyretins: The role of an N-terminal histidine-rich segment in hormone binding with high affinity. Mol Cell Endocrinol. (2018) 474:74–88. doi: 10.1016/j.mce.2018.02.012
20. Yamauchi K, Kasahara T, Hayashi H, Horiuchi R. Purification and characterization of a 3,5,3'-L-triiodothyronine-specific binding protein from bullfrog tadpole plasma: a homolog of mammalian transthyretin. Endocrinology. (1993) 132:2254–61. doi: 10.1210/endo.132.5.8477670
21. Chang L, Munro SLA, Richardson SJ, Schreiber G. Evolution of thyroid hormone binding by transthyretins in birds and mammals. Eur J Biochem. (1999) 259:534–42. doi: 10.1046/j.1432-1327.1999.00076.x
22. Santos CRA, Power DM. Identification of transthyretin in fish (Sparus aurata): cDNA cloning and characterisation. Endocrinology. (1999) 140:2430–3. doi: 10.1210/en.140.5.2430
23. Prapunpoj P, Yamauchi K, Nishiyama N, Richardson SJ, Schreiber G. Evolution of structure, ontogeny of gene expression, and function of Xenopus laevis transthyretin. Am J Physiol. (2000) 279:R2026–41. doi: 10.1152/ajpregu.2000.279.6.R2026
24. Prapunpoj P, Richardson SJ, Schreiber G. Crocodile transthyretin: structure, function, and evolution. Am J Physiol. (2002) 283:R885–96. doi: 10.1152/ajpregu.00042.2002
25. Richardson SJ. Tweaking the structure to radically change the function: the evolution of transthyretin from 5-hydroxyisourate hydrolase to triiodothyronine distributor to thyroxine distributor. Front Endocrinol. 5:245. doi: 10.3389/fendo.2014.00245
26. Farer LS, Robbins J, Blumberg BS, Rall JE. Thyroxine-serum protein complexes in various animals. Endocrinology. (1962) 70:686–96. doi: 10.1210/endo-70-5-686
27. Tanabe Y, Ishii T, Tamaki Y. Comparison of thyroxine-binding plasma proteins of various vertebrates and their evolutionary aspects. Gen Comp Endocrinol. (1969) 13:14–21. doi: 10.1016/0016-6480(69)90216-0
28. Larsson M, Pettersson T, Carlstrom A. Thyroid hormone binding in serum of 15 vertebrate species: isolation of thyroxine-binding globulin and prealbumin analogs. Gen Comp Endocrinol. (1985) 58:360–75. doi: 10.1016/0016-6480(85)90108-X
29. Richardson SJ, Bradley AJ, Duan W, Wettenhall RE, Harms PJ, Babon JJ, et al. Evolution of marsupial and other vertebrate thyroxine-binding plasma proteins. Am J Physiol. (1994) 266(4 Pt 2):R1359–70. doi: 10.1152/ajpregu.1994.266.4.R1359
30. Richardson SJ, Wettenhall RE, Schreiber G. Evolution of transthyretin gene expression in the liver of Didelphis virginiana and other American marsupials. Endocrinology. (1996) 137:3507–12. doi: 10.1210/endo.137.8.8754780
31. McLean TR, Rank MM, Smooker PM, Richardson SJ. Evolution of thyroid hormone distributor proteins. Mol Cell Endocrinol. (2017) 459:43–52. doi: 10.1016/j.mce.2017.02.038
32. Power DM, Morgado I, Cardoso I. Evolutionary insights from fish transthyretin. In: Richardson SJ, Cody V, editors. Recent Advances in Transthyretin Evolution, Structure and Biological Functions. Berlin: Springer (2009). p. 59–75. doi: 10.1007/978-3-642-00646-3_4
33. Funkenstein B, Perrot V, Brown CL. Cloning of putative piscine (Sparus aurata) transthyretin: developmental expression and tissue distribution. Mol Cell Endocrinol. (1999) 157:67–73. doi: 10.1016/S0303-7207(99)00160-4
34. Yamauchi K, Nakajima J, Hayashi H, Hara A. Purification and characterization of thyroid-hormone-binding protein from masu salmon serum - A homolog of higher-vertebrate transthyretin. Eur J Biochem. (1999) 265:944–9. doi: 10.1046/j.1432-1327.1999.00825.x
35. Richardson SJ, Monk JA, Shepherdley CA, Ebbesson LO, Sin F, Power DM, et al. Developmentally regulated thyroid hormone distributor proteins in marsupials, a reptile, and fish. Am J Physiol Regul Integr Comp Physiol. (2005) 288:R1264–72. doi: 10.1152/ajpregu.00793.2004
36. Yamauchi K, Takeuchi H, Overall M, Dziadek M, Munro SL, Schreiber G. Structural characteristics of bullfrog (Rana catesbeiana) transthyretin and its cDNA - comparison of its pattern of expression during metamorphosis with that of lipocalin. Eur J Biochem. (1998) 256:287–96. doi: 10.1046/j.1432-1327.1998.2560287.x
37. Richardson SJ, Aldred AR, Leng SL, Renfree MB, Hulbert AJ, Schreiber G. Developmental profile of thyroid hormone distributor proteins in a marsupial, the tammar wallaby Macropus eugenii. Gen Compar Endocrinol. (2002) 125:92–103. doi: 10.1006/gcen.2001.7729
38. Vranckx R, Rouaze M, Savu L, Nunez EA, Beaumont C, Flink IL. The hepatic biosynthesis of rat thyroxine binding globulin (TBG): demonstration, ontogenesis, and up-regulation in experimental hypothyroidism. Biochem Biophys Res Commun. (1990) 167:317–22. doi: 10.1016/0006-291X(90)91767-M
39. Vranckx R, Savu L, Maya M, Nunez EA. Characterization of a major development-regulated serum thyroxine-binding globulin in the euthyroid mouse. Biochem J. (1990) 271:373–9. doi: 10.1042/bj2710373
40. Robbins J. Transthyretin from discovery to now. Clin Chem Lab Med. (2002) 40:1183–90. doi: 10.1515/CCLM.2002.208
41. Alshehri B, D'Souza DG, Lee JY, Petratos S, Richardson SJ. The diversity of mechanisms influenced by transthyretin in neurobiology: development, disease and endocrine disruption. J Neuroendocrinol. (2015) 27:303–23. doi: 10.1111/jne.12271
42. Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. (1989) 10:232–74. doi: 10.1210/edrv-10-3-232
43. Babin PJ. Binding of thyroxine and 3,5,3'-triiodothyronine to trout plasma lipoproteins. Am J Physiol. (1992) 262(5 Pt 1):E712–20. doi: 10.1152/ajpendo.1992.262.5.E712
44. Benvenga S. A thyroid hormone binding motif is evolutionarily conserved in apolipoproteins. Thyroid. (1997) 7:605–11. doi: 10.1089/thy.1997.7.605
45. Nelson ER, Habibi HR. Thyroid hormone regulates vitellogenin by inducing estrogen receptor alpha in the goldfish liver. Mol Cell Endocrinol. (2016) 436:259–67. doi: 10.1016/j.mce.2016.08.045
46. Cserr HF. Physiology of the choroid plexus. Physiol Rev. (1971) 51:273–311. doi: 10.1152/physrev.1971.51.2.273
47. Duan W, Achen MG, Richardson SJ, Lawrence MC, Wettenhall RE, Jaworowski A, et al. Isolation, characterization, cDNA cloning and gene expression of an avian transthyretin. Implications for the evolution of structure and function of transthyretin in vertebrates. Eur J Biochem. (1991) 200:679–87. doi: 10.1111/j.1432-1033.1991.tb16232.x
48. Harms PJ, Tu GF, Richardson SJ, Aldred AR, Jaworowski A, Schreiber G. Transthyretin (prealbumin) gene expression in choroid plexus is strongly conserved during evolution of vertebrates. Compar Biochem Physiol. (1991) 99:239–49. doi: 10.1016/0305-0491(91)90035-C
49. Achen MG, Duan W, Pettersson TM, Harms PJ, Richardson SJ, Lawrence MC, et al. Transthyretin gene expression in choroid plexus first evolved in reptiles. Am J Physiol. (1993) 265(5 Pt 2):R982–9. doi: 10.1152/ajpregu.1993.265.5.R982
50. Duan W, Richardson SJ, Babon JJ, Heyes RJ, Southwell BR, Harms PJ, et al. Evolution of transthyretin in marsupials. Eur J Biochem. (1995) 227:396–406. doi: 10.1111/j.1432-1033.1995.tb20402.x
51. Schreiber G, Aldred AR, Jaworowski A, Nilsson C, Achen MG, Segal MB. Thyroxine transport from blood to brain via transthyretin synthesis in choroid plexus. Am J Physiol. (1990) 258(2 Pt 2):R338–45. doi: 10.1152/ajpregu.1990.258.2.R338
52. Chanoine JP, Alex S, Fang SL, Stone S, Leonard JL, Korhle J, et al. Role of transthyretin in the transport of thyroxine from the blood to the choroid plexus, the cerebrospinal fluid, and the brain. Endocrinology. (1992) 130:933–8. doi: 10.1210/endo.130.2.1733735
53. Southwell BR, Duan W, Alcorn D, Brack C, Richardson SJ, Kohrle J, et al. Thyroxine transport to the brain: role of protein synthesis by the choroid plexus. Endocrinology. (1993) 133:2116–26. doi: 10.1210/endo.133.5.8404661
54. Achen MG, Harms PJ, Thomas T, Richardson SJ, Wettenhall RE, Schreiber G. Protein synthesis at the blood-brain barrier. The major protein secreted by amphibian choroid plexus is a lipocalin. J Biol Chem. (1992) 267:23170–4.
55. Lepperdinger G. Amphibian choroid plexus lipocalin, Cpl1. Biochim Biophys Acta. (2000) 1482:119–26. doi: 10.1016/S0167-4838(00)00143-6
56. Beuckmann CT, Lazarus M, Gerashchenko D, Mizoguchi A, Nomura S, Mohri I, et al. Cellular localization of lipocalin-type prostaglandin D synthase (beta-trace) in the central nervous system of the adult rat. J Compar Neurol. (2000) 428:62–78. doi: 10.1002/1096-9861(20001204)428:1<62::AID-CNE6>;3.0.CO;2-E
57. Richardson SJ. Expression of transthyretin in the choroid plexus: Relationship to brain homeostasis of thyroid hormones. In: Zheng W, Chodobski A, editors. The Blood-Cerebrospinal Fluid Barrier. Boca Raton, FL: CRC Press (2005). p. 275–304. doi: 10.1201/9781420023404.ch11
58. Schreiber G, Aldred A. Extrahepatic synthesis of acute phase proteins. In: Mackiewicz A, Kushner I, Baumann H, editors. Acute Phase Proteins: Molecular Biology, Biochemistry and Clinical Applications. Boca Raton, FL: CRC Press (1993). p. 39–76.
59. Saunders NR, Habgood MD, Dziegielewska KM. Barrier mechanisms in the brain, II. Immature brain. Clin Exp Pharmacol Physiol. (1999) 26:85–91. doi: 10.1046/j.1440-1681.1999.02987.x
60. Bock E. Quantification of plasma proteins in cerebrospinal fluid. In: Axelson NH, Kroll J, Weeke B, editors. A Manual of Quantitative Immunoelectrophoresis. Oslo: Universitetsforlaget. (1976). p. 111–7. doi: 10.1111/j.1365-3083.1973.tb03789.x
61. Felgenhauer K. Protein size and cerebrospinal fluid composition. Klin Wochensch. (1974) 52:1158–64. doi: 10.1007/BF01466734
62. Hagen GA, Elliott WJ. Transport of thyroid hormones in serum and cerebrospinal fluid. J Clin Endocrinol Metab. (1973) 37:415–22. doi: 10.1210/jcem-37-3-415
63. Hagen GA, Solberg LAJr. Brain and cerebrospinal fluid permeability to intravenous thyroid hormones. Endocrinology. (1974) 95:1398–410. doi: 10.1210/endo-95-5-1398
64. Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. (2011) 25:1–14. doi: 10.1210/me.2010-0095
65. van Doorn J, Roelfsema F, van der Heide D. Concentrations of thyroxine and 3,5,3'-triiodothyronine at 34 different sites in euthyroid rats as determined by an isotopic equilibrium technique. Endocrinology. (1985) 117:1201–8. doi: 10.1210/endo-117-3-1201
66. Darras VM, Houbrechts AM, Van Herck SL. Intracellular thyroid hormone metabolism as a local regulator of nuclear thyroid hormone receptor-mediated impact on vertebrate development. Biochim Biophys Acta. (2015) 1849:130–41. doi: 10.1016/j.bbagrm.2014.05.004
67. Koehrle J. Thyroid hormones and derivatives: endogenous thyroid hormones and their targets. In: Plateroti J, Samarut J, editors. Thyroid Hormone Nuclear Receptor. Springer (2018). p. 85–104. doi: 10.1007/978-1-4939-7902-8_9
68. Schreiber G. Synthesis, processing, and secretion of plasma proteins by the liver and other organs and their regulation. In: Putnam FW, editor. The Plasma Proteins. New York, NY: Academic Press (1987). p. 293–363. doi: 10.1016/B978-0-12-568405-7.50011-4
69. Refetoff S. Inherited thyroxine-binding globulin abnormalities in man. Endocr Rev. (1989) 10:275–93. doi: 10.1210/edrv-10-3-275
70. Bennhold H, Peters H, Roth E. Uber einen Fall von kompletter Analbuminaemie ohne wesentliche klinische Krankenheitszichen. Verh Dtsch Ges Inn Med. (1954) 60:630–4. doi: 10.1007/978-3-642-53819-3_139
71. Murata Y, Refetoff S, Sarne DH, Dick M, Watson F. Variant thyroxine-binding globulin in serum of Australian Aborigines: its physical, chemical and biological properties. J Endo Invest. ()1985 8:2250232. doi: 10.1007/BF03348482
72. Benson MD. Genetics: clinical implications of TTR amyloidosis. In: Richardson SJ, Cody V, editors. Recent Advances in Transthyretin Evolution, Structure and Biological Functions. Berlin; Heidelberg: Springer (2009). p. 173–89. doi: 10.1007/978-3-642-00646-3_11
73. Westermark P, Sletten K, Johansson B, Cornwell GG III. Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc Natl Acad Sci USA. (1990) 87:2843–5. doi: 10.1073/pnas.87.7.2843
74. Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci USA. (2012) 109:9629–34. doi: 10.1073/pnas.1121005109
75. Riisoen H. Reduced prealbumin (transthyretin) in CSF of severely demented patients with Alzheimer's disease. Acta Neurol Scand. (1988) 78:455–9. doi: 10.1111/j.1600-0404.1988.tb03687.x
76. Sousa JC, Cardoso I, Marques F, Saraiva MJ, Palha JA. Transthyretin and Alzheimer's disease: where in the brain? Neurobiol Aging. (2007) 28:713–8. doi: 10.1016/j.neurobiolaging.2006.03.015
77. Velayudhan L, Killick R, Hye A, Kinsey A, Guntert A, Lynham S, et al. Plasma transthyretin as a candidate marker for Alzheimer's disease. J Alzheimers Dis. (2012) 28:369–75. doi: 10.3233/JAD-2011-110611
78. Ni M, Wei W, Feng Q, Sun XG, Wang YC, Gu YJ, et al. Transthyretin as a potential serological marker for the diagnosis of patients with early rheumatoid arthritis. Clin Exp Rheumatol. (2013) 31:394–9. Available online at: https://www.clinexprheumatol.org/article.asp?a=6535
79. Lee J, Mun S, Kim D, Lee YR, Sheen DH, Ihm C, et al. Proteomics analysis for verification of rheumatoid arthritis biomarker candidates using multiple reaction monitoring. Proteomics Clin Appl. (2018) 13:e1800011. doi: 10.1002/prca.201800011
80. Wan C, Yang Y, Li H, La Y, Zhu H, Jiang L, et al. Dysregulation of retinoid transporters expression in body fluids of schizophrenia patients. J Proteome Res. (2006) 5:3213–6. doi: 10.1021/pr060176l
81. Martins-De-Souza D, Wobrock T, Zerr I, Schmitt A, Gawinecka J, Schneider-Axmann T, et al. Different apolipoprotein E, apolipoprotein A1 and prostaglandin-H2 D-isomerase levels in cerebrospinal fluid of schizophrenia patients and healthy controls. World J Biol Psychiatry. (2010) 11:719–28. doi: 10.3109/15622971003758748
82. Kalkunte SS, Neubeck S, Norris WE, Cheng SB, Kostadinov S, Vu Hoang D, et al. Transthyretin is dysregulated in preeclampsia, and its native form prevents the onset of disease in a preclinical mouse model. Am J Pathol. (2013) 183:1425–36. doi: 10.1016/j.ajpath.2013.07.022
83. Cheng SB, Nakashima A, Sharma S. Understanding pre-eclampsia using Alzheimer's etiology: an intriguing viewpoint. Am J Reprod Immunol. (2016) 75:372–81. doi: 10.1111/aji.12446
84. Tong M, Cheng SB, Chen Q, DeSousa J, Stone PR, James JL, et al. Aggregated transthyretin is specifically packaged into placental nano-vesicles in preeclampsia. Sci Rep. (2017) 7:6694. doi: 10.1038/s41598-017-07017-x
85. Jin T, Hu LS, Chang M, Wu J, Winblad B, Zhu J. Proteomic identification of potential protein markers in cerebrospinal fluid of GBS patients. Eur J Neurol. (2007) 14:563–8. doi: 10.1111/j.1468-1331.2007.01761.x
86. Yang YR, Liu SL, Qin ZY, Liu FJ, Qin YJ, Bai SM, et al. Comparative proteomics analysis of cerebrospinal fluid of patients with Guillain-Barre syndrome. Cell Mol Neurobiol. (2008) 28:737–44. doi: 10.1007/s10571-007-9257-7
87. Chiang HL, Lyu RK, Tseng MY, Chang KH, Chang HS, Hsu WC, et al. Analyses of transthyretin concentration in the cerebrospinal fluid of patients with Guillain-Barre syndrome and other neurological disorders. Clin Chim Acta. (2009) 405:143–7. doi: 10.1016/j.cca.2009.04.022
Keywords: albumin, development, evolution, phylogeny, thyroid hormones, thyroxine-binding globulin, transthyretin, vertebrates
Citation: Rabah SA, Gowan IL, Pagnin M, Osman N and Richardson SJ (2019) Thyroid Hormone Distributor Proteins During Development in Vertebrates. Front. Endocrinol. 10:506. doi: 10.3389/fendo.2019.00506
Received: 14 February 2019; Accepted: 11 July 2019;
Published: 08 August 2019.
Edited by:
Marco António Campinho, University of Algarve, PortugalReviewed by:
Kiyoshi Yamauchi, Shizuoka University, JapanCopyright © 2019 Rabah, Gowan, Pagnin, Osman and Richardson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samantha J. Richardson, c2FtYW50aGEucmljaGFyZHNvbkBybWl0LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.