
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 04 July 2019
Sec. Thyroid Endocrinology
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00440
 Stanley U. Ogbonna
Stanley U. Ogbonna Ignatius U. Ezeani*
Ignatius U. Ezeani*Background: Thyroid dysfunction has been widely reported among persons with diabetes (DM) in other parts of the World. In Nigeria, few studies have been reported. This study focused on risk factors for thyroid dysfunction in type 2 diabetes mellitus (T2DM) patients and will therefore add to the Nigerian literature, more so, as it is the first in South-East Nigeria.
Objective: To determine the risk factors of thyroid dysfunction in patients with Type 2 DM.
Methodology: Three hundred and fifty-four T2DM patients and 118 non-diabetic persons (controls) were recruited for the study. A pretested questionnaire was filled for each subject after due explanations. The subjects were subsequently examined and the findings, including anthropometric values and clinical parameters were documented. Their blood samples were tested for HbA1c, fT3, fT4, and TSH. Information retrieved from patients medical records included: age at diagnosis of DM, duration of DM, complications of DM. The Student's t-test, chi square test and regression analysis were used in the analysis of the data obtained. P < 0.05 was taken to be statistically significant.
Results: About 56.5% of the T2DM patients who participated in this study were females and 62.7% of the controls were females. The T2DM patients had significantly higher BMI than controls (27.6 ± 5.0 kg/m2 vs. 26.2 ± 3.8 kg/m2, p = 0.002). Mean HbA1c was significantly higher in T2DM patients than in the controls (7.8 ± 2.0% vs. 5.8 ± 1.2%, p = 0.001). Female gender (OR = 3.8, p = 0.002), central obesity (OR = 2.5, 95%CI = 1.5–5.2, p = 0.001), DM nephropathy (OR = 4.8, p = 0.001), HbA1c ≥7% (OR = 4.3, p = 0.025) and duration of DM >5years (OR = 3.3, p = 0.012) were significantly associated with thyroid dysfunction in T2DM patients in this study.
Conclusion: Female gender, central obesity, DM nephropathy, above normal HbA1c, and duration of DM were risk factors of thyroid dysfunction in type 2 DM patients in this study.
Thyroid dysfunction is a spectrum of disorders of the thyroid gland which manifests either as hyperthyroidism or hypothyroidism and is reflected in the circulating levels of thyroid stimulating hormone (TSH) (1, 2). Thyroid dysfunction may present in one of the following ways- thyroid enlargement (diffuse or nodular); symptoms of thyroid hormone deficiency (hypothyroidism); symptoms of thyroid hormone excess (thyrotoxicosis); some have no symptoms (i.e., the subclinical state) (3). Imbalance in the production of thyroid hormones arises from dysfunction of the thyroid gland itself, the pituitary gland, which produces TSH, or the hypothalamus, which regulates the pituitary gland via Thyrotropin Releasing Hormone (TRH) (4).
Thyroid dysfunctions are common in the general population and it is second only to diabetes as the most common condition to affect the endocrine system. As a result it is common for an individual to be affected by both thyroid diseases and diabetes (5, 6).
Patients with DM are at increased risk of thyroid disease, especially those with poor glycaemic control. The following mechanisms are thought to be responsible. In patients with DM, the nocturnal TSH peak is blunted or abolished; and the TSH response to TRH, from the hypothalamus, is impaired thus leading to hypothyroidism (7). Low T3 levels have been observed in uncontrolled DM. This has been ascribed to the impairment in peripheral conversion of T4 to T3 which normalizes with improvement in glycaemic control (5, 8). This is as a result of the hyperglycaemia-induced reversible reduction of the activities and hepatic concentration of thyroxine 5‘deiodinase (8). Higher levels of circulating insulin associated with insulin resistance has been shown to have a proliferative effect on thyroid tissue resulting in larger thyroid size with increased formation of nodules (5, 9, 10). This may lead to thyroid dysfunction (hyperthyroidism) in patients with type 2 DM.
Metformin decreases thyrotropin levels in patients with hypothyroidism. Prospective and retrospective studies (11) showed that patients with prediabetes and type 2 diabetes mellitus (TDM) had a significantly increased thyroid volume and a higher prevalence of incident goiter and nodules. Furthermore, diabetic patients treated with metformin had a smaller thyroid volume and a lower risk for the formation of thyroid nodules when compared with controls (12, 13). These results suggested that metformin exerts an anti-proliferative activity, providing a rationale for an innovative therapy of thyroid proliferative diseases with metformin.
Metformin is hypothesized to change the affinity and/or quantity of thyroid hormone receptors, increase the central dopaminergic tone or induce activation of the TSH receptor, thus enhancing the effects of thyroid hormones in the pituitary (14).
Other authors suggested that TSH-lowering effect of metformin may be explained by a metformin-induced activation of the adenosine monophosphate-activated protein kinase (AMPK), which is involved in a variety of cellular functions and regulates cellular energy metabolism (15). Indeed, it may be plausible that any central effects of metformin on the TRH/TSH regulation involve the AMPK system. Metformin is proved to have an inhibitory effect on AMPK activity in the hypothalamus where it opposes T 3 (16).
Thyroid hormones affect glucose metabolism through several other mechanisms (17–26). Several studies have reported the prevalence of thyroid dysfunction in diabetic patients but only few studies have assessed the risk factors of thyroid dysfunction in diabetic patients, hence the need for this study. The objective of this study is to determine the risk factors of thyroid dysfunction in patients with Type 2 DM.
The study was conducted at the University of Nigeria Teaching Hospital (UNTH), Enugu, a Federal government tertiary hospital located in Ituku-Ozalla, Enugu state in South-East geographical region of Nigeria. This is a descriptive cross-sectional study involving patients with T2DM attending the Diabetes Clinic, or receiving treatment in the Medical Wards of the UNTH Enugu. Subjects were recruited using systematic sampling. A total number of 360 subjects were recruited from consenting persons with T2DM in the Diabetes Clinic and Medical Wards of the UNTH Enugu. For every three study subjects selected, one consenting person who did not have DM was recruited from the out-patients clinics and other parts of the hospital to serve as the control. Screening for DM was done in controls using the random blood glucose (RBG). Those with RBG of <11.1 mmol/l, had no classic symptoms of hyperglycaemia and were not on any hypoglycemic medications were accepted as controls (27). The inclusion criteria included
a) All patients with T2DM irrespective of blood pressure status.
b) Patients who have attained 40 years of age at the time of diagnosis of DM (28).
c) Those who had no thyroid surgery nor trauma to the neck.
d) Subjects with no history of previous exposure to radiation to the neck.
e) Those consenting to the study.
Exclusion criteria included:
1. Patients <40 years of age at diagnosis of DM
2. Those with history of neck trauma or surgery
3. Pregnant women
4. Subjects with history of previous exposure of radiation in the neck
5. Non-consenting patients
6. Patients on drugs like amiodarone, lithium, interferon alpha, iodides, beta blockers, carbimazole, propylthiouracil, potassium iodide, lugol's iodine
7. Patients with thyroid disorders
8. Patients previously diagnosed to have T1DM.
Ethical approval was obtained from the University of Nigeria Health Research Ethics Committee of the UNTH Enugu before commencing the study. Written informed consent was obtained from all subjects participating in the study.
All subjects were interviewed, using a pre-tested structured questionnaire. Demographic information and other relevant data were obtained. The neck was examined for presence of enlarged thyroid gland. Routine examinations for the complications of DM were also carried out. Fundoscopy was done with the assistance of an Ophthalmologist. The participants were examined for peripheral neuropathy using tuning fork (vibration sense) and tendon hammer (deep tendon reflex). Anthropometric measurements such as weight and height were taken.
Anthropometric measurements such as weight and height were taken using a standard scale and stadiometer from Lincoln Mark Medical England; waist and hip circumference were measured using a measuring tape. Body Mass Index (BMI) was calculated from their weights measured in kilograms (kg) and heights in meters (m) using the standard formula (29, 30). Subjects with BMI of between 18 and 25 kg/m2 were classified as having normal weight, while those with BMI of 30 kg/m2 and above were classified as being obese (31). Waist circumference (WC) was measured in centimeters (cm) along the mid-point between the costal margin and the iliac crest along the mid axillary line (32). The International Diabetes Federation (IDF)'s reference values for male and female were used. In males, WC of ≤94 cm was regarded as normal, while WC of >94 cm was regarded as abnormal. In females, WC of ≤80 cm was regarded as normal while WC of >80 cm was taken to be abnormal (33).
Three consecutive pulse rates were obtained and the mean recorded. Blood pressure (BP) was taken on the right arm with a mercury sphygmomanometer (34). The systolic and diastolic pressures were obtained. The disappearance of the korotkoff's sound (phase V) was the criterion for the diastolic blood pressure, and the average of three consecutive BP readings was recorded. BP was measured in a sitting position after 5 min rest. Normal BP was defined as a systolic BP of <130 mmHg, and or diastolic BP of <80 mmhHg. Those who did not satisfy these criteria were considered to have a high BP, in accordance with the BP target set by the American Diabetes Association (ADA) (35).
All the participants had their blood glucose estimated using the Accu-chek Active glucometer. The patients with type 2 DM had their urine samples tested for presence of urinary protein (albumin) using Combi-3 urinary strips from Medi-Test, Germany. The presence of one + and above of urine albumin was taken to be positive for albuminuria.
Glycated hemoglobin (HbA1c) was estimated using the In-2-it HbA1c device from BIO-RAD Laboratories Flintshire UK. It involves the use of boronate affinity chromatography to separate the glycated fraction from the non-glycated fraction (36). It measures the HbA1c level which reflects the average blood glucose level over the previous 2 or 3 months.
Glycaemic control was assessed with the values of the HbA1c. HbA1c value was used to categorize the DM patients into two groups: good glycaemic control (HbA1c<7%), and poor glycaemic control (HbA1c≥7%) (37).
Participants with raised TSH, and low fT3 and fT4 were regarded to have primary hypothyroidism; those that had elevated TSH, but with normal fT3 and fT4 were taken to have subclinical hypothyroidism (38–40). In the same vein, those who had low TSH, and high fT3 and fT4 were regarded to have primary hyperthyroidism; those that had low TSH but with normal fT3, and fT4 were taken to have subclinical hyperthyroidism (39–41). While the subjects with low or normal TSH, but had low fT3 and fT4 were taken to have secondary hypothyroidism (40, 42).
Frozen sera from the T2DM subjects and controls were thawed and allowed to attain room temperature. The samples were assayed for free T3, free T4, and TSH, respectively in batches, in three runs, each on a different day. Control samples provided in the reagents kits were analyzed with each run of the analytes following the manufacturer's instructions.
The data generated from the study was analyzed using the Statistical Package for the Social Sciences (SPSS) IBM version 17. A statistical comparison was made with the student t-test for quantitative variables like weight, height, blood pressure, serum TSH, serum T3; while Chi-square test was used for comparison of proportions. A p < 0.05 was taken as being statistically significant.
A total of 480 subjects were studied. Complete data for analysis was available for 472 subjects. All the subjects (100%) were of African descent. Three hundred and fifty-four (354) of them were patients with type 2 DM, while 118 subjects who did not have type 2 DM served as the controls.
Females formed the majority of the study population accounting for 56.5% of the type 2 DM patients and 62.7% of the controls. Most of the participants had either tertiary education (38.3%) or secondary level of education (22.7%). Majority of the participants (65.3%) were married. Retirees (28.8%) and Civil servants (26.3%) formed the majority of the participants as shown in Table 1.
The mean age of the T2DM patients in this study was 57.5 (±9.3) years, while the controls had a similar mean age of 57.7 ± 8.9 (p = 0.17). The mean age at diagnosis of DM was 54 ± 7.6 years. The mean duration of DM for all the T2DM patients was 6.5 ± 2.8 years. Table 2 below shows that T2DM patients had higher mean BMI than the controls (27.6 ± 5.0 vs. 26.2 ± 3.8). This difference was statistically significant (t = 2.7; p = 0.002). The T2DM patients also had higher mean waist circumference (90.2 ± 13.9 vs. 87.5 ± 11.0), and this difference was also statistically significant (t = −2.2, p = 0.03).
The type 2 DM patients in this study had the following chronic complications of DM: DM retinopathy (49.1%), peripheral neuropathy (48.1%), DM nephropathy (13.0%), and DM foot ulcer (19.8%)
Thyroid dysfunction had a statistically significant relationship with DM nephropathy in this study as shown in the Table 3. The other chronic complications of DM did not have significant relationship with thyroid dysfunction in this study.
Using a combination of TSH, fT3, and fT4 values, 12.4% of the T2DM patients were observed to have thyroid dysfunction (hypothyroidism-11.6%; or hyperthyroidism- 0.8%) as shown in the Table 4.
There was a significant association between the presence of thyroid dysfunction and female gender (χ2 = 20.3; p = 0.001), duration of DM >5 years (χ2 = 7.7, p = 0.005), central obesity (χ2 = 24.8; p = 0.001), nephropathy (χ2 = 29.2, p = 0.001), and HbA1c ≥7% (χ2 = 5.0, p = 0.04) as shown in Table 4.
Binary logistic regression showed that female gender (OR = 3.8, p = 0.002), central obesity (OR = 2.5, p = 0.001), DM duration >5 years (OR = 3.3, p = 0.012), HbA1c ≥7 (OR = 4.3, p = 0.025), and DM nephropathy (OR = 4.8, p = 0.001) were risk factors for thyroid dysfunction.
Females formed 58.1% of the participants. Females also constituted 56.5% of the type 2 DM patients. This female preponderance is similar to the 60.5% reported by the Diabcare Nigeria study group in 2012 in a multi-center study that assessed the profile of Nigerians with DM (43). It is also close to 57.9% reported by Okafor et al. in Enugu, Nigeria in 2012 in a study that evaluated the cardio-metabolic risk factors in Nigerians living with type 2 DM (44).
The mean age of type 2 DM patients in this study was 57.5 years. This may be due to the fact that the prevalence of type 2 DM increases with age (37, 45, 46). This reflects the pattern observed by Chinenye et al. (57.1) in a multi-center study involving DM patients (43). Ofoegbu et al. reported a mean age of 59.2 years in Enugu in a study that evaluated the body composition of Nigerians with DM (47). Okafor et al. in Enugu reported 55.7 years as the mean age of type 2 DM patients they evaluated for cardio-metabolic risk factors (44). This is lower than the figures reported from developed countries like New Zealand, and the USA (48, 49). This can be attributed to the lower life expectancy of Africans, and Nigerians in particular, compared to those of patients in the developed world. The mean age at diagnosis of type 2 DM in this study was 54 years which is similar to that observed in the UKPDS (54 years) (50), but is a bit higher than 48.3 years reported by the Diabcare Nigeria study group (43). The mean duration of DM of 6.5 years is similar to 6.7 years observed by Okafor et al. in Enugu and reflects the pattern reported in other Nigerian studies (43, 44), but is low when compared to that observed in the developed world (49). This might mean that most diabetics in Enugu and other parts of Nigeria do not survive long enough, which might be a pointer to the disease burden and quality of care available to the patients.
Diabetic retinopathy was observed in 49.1% of the type 2 DM patients in this study. This can be attributed to the increasing prevalence of DM retinopathy. This is close to 42.1% reported by Ashaye et al. (51) in a study that evaluated retinopathy among type 2 DM patients in Ibadan, Nigeria (51), but is high when compared to the 34.6% global prevalence reported by Yau et al. (52). The prevalence of peripheral neuropathy in this study was 48.1%. This is lower than 75% prevalence reported by Ugoya et al. in Jos, Nigeria (53), but is higher than 31.2% observed by Ibrahim et al. in Kano, Nigeria (54).
Diabetic nephropathy was observed in 13% of the patients with type 2 DM in this study. This is a bit lower than 16.6% observed by Ulasi et al. in Enugu, Nigeria (55) and is much lower than 72.6% reported by Onovughakpo-Sakpa et al. in Benin, Nigeria (56).
Diabetic nephropathy was the only microvascular complication of DM that has a significant relationship with the presence of thyroid dysfunction (χ2 = 29.2, p = 0.001); and had a strong association with thyroid dysfunction on logistic regression (OR = 4.8, 95%CI = 2.2–10.5). This shows that type 2 DM patients with nephropathy are 4.8 times as likely to also have thyroid dysfunction as their counterparts who do not have nephropathy. This agrees with the report of Yau et al., that hypothyroidism is a risk factor for nephropathy (57).
This study observed female gender, central obesity, duration of DM >5 years, and HbA1c ≥7% as independent predictors of thyroid dysfunction in type 2 DM (see Table 5).
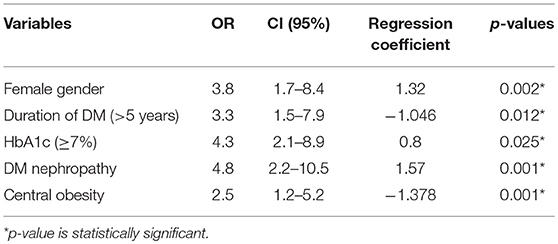
Table 5. Multivariate Analysis (logistic regression) of predictors/risk factors of thyroid dysfunction in type 2 DM patients.
Females who had type 2 DM were 3.8 times more likely to develop thyroid dysfunction than their male counterparts. This reflects the findings of a study from India which reported that the prevalence of thyroid disorders were more in females as compared to males (69 vs. 31%) (58). These findings are also consistent with several other studies (59–62). Thus, the prevalence of thyroid disorders in diabetic patients is influenced by female gender.
Central obesity (abnormal waist circumference) was significantly associated with thyroid dysfunction (OR = 2.5, 95%CI = 1.5–5.2, p = 0.001). This is similar to the report of Udenze et al. in Lagos, Nigeria who reported that waist circumference had a significant association with thyroid dysfunction (63). Biondi et al. also found associations between thyroid dysfunction and obesity in the metabolic syndrome (64). This may be as a result of the link between obesity and leptin. Leptin is known to be an important neuro-endocrine regulator of the hypothalamo-pituitary-thyroid axis by regulating TRH gene expression in the paraventricular nucleus (65). Iodine deficiency, autoimmune thyroiditis and mutations in the TSH receptor genes are some of the other hypothesis put forward to explain the association between increasing TSH, obesity and subclinical hypothyrodism in some populations (63, 66).
Elevated HbA1c (poor glycaemic control) has been shown to be strongly associated with chronic complications of DM (50, 67). This study observed that type 2 DM patients with elevated HbA1c were 4.3 times more likely to develop thyroid dysfunction than their counterparts with good glycaemic control (HbA1c<7%). This may be due the adverse effects of chronic hyperglycaemia on the hypothalamo-pituitary axis where it blunts or abolishes the nocturnal TSH peak (7). Several previous studies have categorized clinical and subclinical hypothyroidism as insulin resistant states. Bazrafshan et al. in his study (68) found a significant correlation between HbA1c levels and TSH levels which is similar to our findings. In a study by Ardekani et al. (69), HbA1c were significantly higher in patients with diabetes having thyroid disorders which is in keeping with findings from our study.
Hyperglycaemia also inhibits the peripheral deiodination of T4 to T3 by reducing the activities of thyroxine deiodinase (8). Schlienger et al. in their study (70) on the “Effect of diabetic control on the level of circulating thyroid hormones” reported that a poor diabetic control (glycosylated hemoglobin ≥12%) is associated with a low T3 syndrome as a result of impairment of T4 to T3 conversion.
This study reported that DM duration >5 years (OR = 3.3, p = 0.012) was a risk factor for thyroid dysfunction. Furthermore, there was a significant difference (p = 0.001) in the mean duration of DM between those that have thyroid dysfunction (9.5 years) and those that are euthyroid (6.0 years). This might be an indication that increasing duration of DM may be a risk factor in the prevalence of thyroid dysfunction as chronic hyperglycaemia impairs the peripheral deiodination of T4 to T3 leading to thyroid dysfunction. Which is also in keeping with findings by Telwani et al. (58) who reported that the prevalence of thyroid disorders was significantly more in diabetics with duration of diabetes = 5 years as compared to duration < 5 years (75.9 vs. 24.1%). However, study by Diez et al., found no significant relationship between presence of thyroid dysfunction and duration of diabetes (71).
In conclusion, female gender, central obesity, HbA1c (≥7%), duration of DM (>5 years), and DM nephropathy were risk factors of thyroid dysfunction in type 2 DM patients in this study.
Assaying for thyroid hormones using the more sensitive chemilumniscent immunoassay method would have sought out more patients with thyroid dysfunction. Another limitation is the inability of the authors to do fasting blood glucose in the control group and in T2DM patients in this study. Thirdly, limitations deriving from the possible influence of metformin on thyroid function and from the use of a qualitative method to measure proteinuria deserve mention.
Ethical approval was obtained from the University of Nigeria Health Research Ethics Committee of the UNTH Enugu before commencing the study. Written informed consent was obtained from all subjects participating in the study.
SO and IE conceived the study, participated in its design and coordination, helped to draft the manuscript, participated in the design of the study, and performed the statistical analysis. IE participated in the sequence alignment. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank staff of the Department of Medicine, UNTH, Enugu, Nigeria who contributed toward the article by making substantial contributions to conception and revision of manuscript for important intellectual content.
BMI, Body Mass Index; CLIA, chemilumniscent immunoassay; DM, diabetes mellitus; fT3, free 3,5,3-triiodothyronine; fT4, free 3,5,3′,5′-tetraiodothyronine; HbA1c, glycosylated hemoglobin; IBM, International Business Machine; NHANES III, National Health and Nutritional Education Survey III; RBG, random blood glucose; SPSS, Statistical Package for the Social Sciences; T1DM, type 2 diabetes mellitus; T2DM, type 2 diabetes mellitus; TSH, thyroid stimulating hormone; UKPDS, United Kingdom Prospective Diabetes Study; UNTH, University of Nigeria Teaching Hospital.
1. Tunbridge WMG, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol. (1977) 7:481–93. doi: 10.1111/j.1365-2265.1977.tb01340.x
2. Ghazali SM, Abbiyesuku FM. Thyroid dysfunction in type 2 diabetics seen at the University College Hospital, Ibadan, Nigeria. Niger J Physiol Sci. (2010) 25:173–9.
3. Masharani U, German MS. Pancreatic hormones and diabetes mellitus. In: Gardner DG, Shoback D, editors. Greenspan's Basic and Clinical Endocrinology. New York, NY: McGraw-Hill Medical (2007). p. 661–747.
4. Peters RP, Wouters PJ, Kaptein E. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab. (2003) 88:3202–11. doi: 10.1210/jc.2002-022013
5. Singh G, Gupta V, Sharma AK, Gupta N. Evaluation of thyroid dysfunction among type 2 diabetic Punjabi population. Adv Biores. (2011) 2:3–9.
7. Gursoy NT, Tuncel E. The relationship between the glycaemic control and hypothalamus-pituitary-thyroid axis in diabetic patients. Turkish J Endocrinol Metab. (1999) 12:163–8.
8. Hage M, Zantout MS, Azar ST. Thyroid disorders and diabetes mellitus. J Thyr Res. (2011) 31:39–45. doi: 10.4061/2011/439463
9. Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomnisczce H. Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid. (2008) 18:461–4. doi: 10.1089/thy.2007.0223
10. Ayturk S, Gursoy A, Kut A, Aml C, Nar A, Tutuncu NB. Metabolic syndrome and its components are associated with increased thyroid volume and nodule prevalence in a mild-to-moderate iodine deficient area. Eur J Endocrinol. (2009) 161:599–605. doi: 10.1530/EJE-09-0410
11. Anil C, Akkurt A, Ayturk S, Kut A, Gursoy A. Impaired glucose metabolism is a risk factor for increased thyroid volume and nodule prevalence in a mild-to-moderate iodine deficient area. Metabolism. (2013) 62:970–5. doi: 10.1016/j.metabol.2013.01.009
12. Blanc E, Ponce C, Brodschi D, Nepote A, Barreto A, Schnitman M, et al. Association between worse metabolic control and increased thyroid volume and nodular disease in elderly adults with metabolic syndrome. Metab Syndr Relat Dis. (2015) 13:221–6. doi: 10.1089/met.2014.0158
13. Ittermann T, Markus MR, Schipf S, Derwahl M, Meisinger C, Volzke H. Metformin inhibits goitrogenous effects of type 2 diabetes. Eur J Endocrinol. (2013) 169:9–15. doi: 10.1530/EJE-13-0101
14. Vigersky RA, Filmore-Nassar A, Glass AR. Thyrotropin suppression by metformin. J Clin Endocrinol Metab. (2006) 91:225–7. doi: 10.1210/jc.2005-1210
15. Duntas LH, Orgiazzi J, Brabant G. The interface between thyroid and diabetes mellitus. Clin Endocrinol. (2011) 75:1–9. doi: 10.1111/j.1365-2265.2011.04029.x
16. Alevizaki M. Metformin and the thyroid: some questions still remain. Clin Endocrinol. (2013) 78:503–4. doi: 10.1111/cen.12005
17. O'Meara NM, Blackman JD, Sturis J, Polonsky KS. Alterations in the kinetics of c-peptide and insulin secretion in hyperthyroidism. J Clin Endocrinol Metab. (1993) 76:79–84. doi: 10.1210/jcem.76.1.8421108
18. Dimitriadis G, Baker B, Marsh H. Effect of thyroid hormone excess on action, secretion, and metabolism of insulin in humans. Am J Physiol. (1985) 248:593–601. doi: 10.1152/ajpendo.1985.248.5.E593
19. Beer SF, Pair JH, Temple RC, Hales CN. The effect of thyroid disease on pro-insulin and c-peptide levels. Clin Endocrinol. (1989) 30:379–83. doi: 10.1111/j.1365-2265.1989.tb00435.x
20. Levine RJ, Smyth DH. The effect of the thyroid gland on intestinal absorption of hexoses. J Physiol. (1963) 169:755–69. doi: 10.1113/jphysiol.1963.sp007294
21. Matty AJ, Seshadri B. Effect of thyroxine on the isolated rat intestine. Gut. (1965) 6:200–2. doi: 10.1136/gut.6.2.200
22. Kemp HF, Hundal HS, Taylor PM. Glucose transport correlates with GLUT 2 abundance in rat liver during altered thyroid status. Molecular Cell Endocrinol. (1997) 128:97–102. doi: 10.1016/S0303-7207(97)04026-4
23. Mokuno T, Uchimura K, Hayashi R. Glucose transporter 2 concentrations in hyper and hypothyroid rat livers. J Endocrinol. (1999) 160:285–9. doi: 10.1677/joe.0.1600285
24. Vaughan M. An in vitro effect of triiodothyronine on rat adipose tissue. J Clin Invest. (1967) 46:1482–91. doi: 10.1172/JCI105640
25. Sola E, Morillas C, Garzon S, Gomez-Balaguer M, Hernandez-Mijares A. Association between diabetic ketoacidosis and thyrotoxicosis. Actadiabetologica. (2002) 39:235–7. doi: 10.1007/s005920200040
26. Bhattacharya A, Wiles PG. Diabetic ketoacidosis precipitated by thyrotoxicosis. Postgrad Med J. (1999) 75:291–2. doi: 10.1136/pgmj.75.883.291
27. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2012) 35:S11–9. doi: 10.2337/dc12-s064
28. Gale EAM, Anderson JV. Diabetes mellitus and other disorders of metabolism. In: Kumar P, Clark M, editors. Kumar and Clark's Clinical Medicine. London; Elsevier Ltd. (2012). p. 1001–46.
29. Papazafiropoulou A, Sotiropoulos A, Kokolaki A, Lardara M, Stamataki P, Pappas S. Prevalence of thyroid dysfunction among Greek type 2 diabetic patients. J Clin Med Res. (2010) 2:75–8. doi: 10.4021/jocmr2010.03.281w
30. Akbar DH, Ahmed MM, Al-Mughales J. Thyroid dysfunction and thyroid autoimmunity in Saudi type 2 diabetics. Actadiabetologica. (2006) 43:14–8. doi: 10.1007/s00592-006-0204-8
31. World Health Organisation (WHO). Obesity: Preventing and Managing the Global Epidemic. Report of WHO consultation. WHO technical report series 894. Geneva: World Health Organisation (2000).
32. Gezawa ID, Puepet FH, Mubi MB, Haliru I, Bakki B, Tella MA. Anthropometric correlates of insulin resistance: a study of healthy Nigerian adults. Kamen J Med Sci. (2010) 4:14–8. doi: 10.4103/1118-8561.149495
33. Sicree R, Shaw J, Zimmet P. Diabetes and Impaired glucose tolerance. In: Gan D, editor. Diabetes Atlas. 3rd ed. Belgium: International Diabetes Federation (2006). p. 15–103.
34. Nuttal FQ. Comparison of percent total GHb with percent HbA1C in people with and without known diabetes. Diabetes Care. (1998) 21:1475–80. doi: 10.2337/diacare.21.9.1475
35. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2012) 35:S11–9.
36. O'Gara PT, Loscalzo J. Physical examination of the cardiovascular system In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. Vol. 19. New York, NY: McGraw- Hill Medical (2014). p. 1442–56.
37. Turner HE, Wass TAH. Diabetes. In: Turner HE, Wass TAH, editors. Oxford Handbook of Endocrinology and Diabetes. New York, NY: Oxford University Press (2010). p. 724–822. doi: 10.1093/med/9780198567394.003.0111
38. Cooper DS, Greenspan FS, Ladenson PN. The thyroid gland. In: Gardner DG, Shoback D, editors. Greenspan's Basic and Clinical Endocrinology. New York, NY: McGraw-Hill Medical (2007). p. 209–80.
39. Jameson JL, Mandel SJ, Weetman AP. Disorders of the thyroid gland. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Localzo J, editors. Harrison's Principles of Internal Medicine. New York, NY: McGraw-Hill Medical (2014). p. 2251–334.
40. Turner HE, Wass TAH. Thyroid. In: Turner HE, Wass TAH, editors. Oxford Handbook of Endocrinology and Diabetes. New York, NY: Oxford University press (2010). p. 2–70. doi: 10.1093/med/9780198567394.003.0009
41. Jameson JL, Weetman AP. Disorders of thyroid gland. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Localzo J, editors. Harrison's Principles of Internal Medicine. New York, NY: McGraw-Hill Medical (2012). p. 2911–39.
42. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. (2014) 37:S14–80. doi: 10.2337/dc14-S014
43. Chinenye S, Uloko AE, Ogbera AO, Ofoegbu EN, Fasanmade OA, Fasanmade AA, et al. Profile of Nigerians with diabetes mellitus—Diabcare Nigeria study group (2008): results of a multi-centre study. Indian J Endocrinol Metab. (2012) 16:558–64. doi: 10.4103/2230-8210.98011
44. Okafor CI, Ofoegbu EN. Control to goal of cardiometabolic risk factors among Nigerians living with type 2 diabetes mellitus. Niger J Clin Pract. (2012) 15:15–8. doi: 10.4103/1119-3077.94089
45. Powers AC. Diabetes mellitus: diagnosis, classification, management and complications. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Localzo J, editors. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill Medical (2014). p. 2399–430.
46. Gale EAM, Anderson JV. Diabetes mellitus and other disorders of metabolism. In: Kumar P, Clark M, editors. Kumar and Clark's Clinical Medicine. London: Elsevier Ltd. (2012). p. 1001–46.
47. Ofoegbu EN, Oli JM, Igwe JC. Body composition of Nigerian diabetics using Bioimpedance analysis (BIA). Niger Health Biomed Sci. (2004) 3:37–9. doi: 10.4314/njhbs.v3i1.11505
48. Chuang LM, Tsai ST, Huang BY, Tai TY, on behalf of Diabcare Asia 1998 Study Group. The status of diabetes control in Asia- a cross-sectional survey of 24,317 patients with diabetes mellitus in 1998. Diabet Med. (2002) 19:978–85. doi: 10.1046/j.1464-5491.2002.00833.x
49. McMicheal AJ, Beaglehole R. The changing global concept of public health. Lancet. (2000) 356:495–9. doi: 10.1016/S0140-6736(00)02564-2
50. United Kingdom Prospective Study Group 1998. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes mellitus. Lancet. (1998) 352:837–53. doi: 10.1016/S0140-6736(98)07019-6
51. Ashaye A, Ayodeji A, Kuti M, Olusanya B, Ayeni E, Fasanmade A, et al. Retinopathy among type 2 DM patients seen at a tertiary hospital in Nigeria. Clin Ophthalmol. (2008) 2:103–6. doi: 10.2147/OPTH.S1532
52. Yau WJ, Rogers SL, Kawasaki R, Lamoureux EL, Bek T, Chen SJ, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. (2012) 35:556–64. doi: 10.2337/dc11-1909
53. Ugoya S, Echejoh GO, Ugoya T, Agaba E, Puepet HF, Oguniyi A. Clinically diagnosed diabetic neuropathy: frequency, types, and severity. J Nat Med Assoc. (2006) 98:1763–6.
54. Ibrahim A, Owolabi LF, Borodo MM, Oguniyi A. Clinical profile of diabetic sensorimotor polyneuropathy in a tertiary hospital in North Western Nigeria. Niger J Basic Clin Sci. (2015) 12:13–9. doi: 10.4103/0331-8540.156669
55. Ulasi II, Ijeoma CK. The prevalence of diabetic nephropathy in Nigerian patients with end-stage renal disease. J Cou Med. (1998) 3:40–2.
56. Onovughakpo-Sakpa OE, Onyeneke EC, Olumese EF. Incidence of diabetic nephropathy in Southern Nigeria. J Med Sci. (2009) 9:264–9.
57. Oputa RN, Chinenye S. Diabetes mellitus: a global epidemic with potential solutions. Afr J Diabet Med. (2012) 20:2033–5.
58. Telwani AA, Wani ZH, Ashraf Y, Shah AA. Prevalence of thyroid dysfunction in type 2 diabetes mellitus: a case control study. Int J Res Med Sci. (2017) 5:4527–31. doi: 10.18203/2320-6012.ijrms20174590
59. Babu K, Kakar A, Byotra SP. Prevalence of thyroid disorder in type II diabetes mellitus patients. J Assoc Phys Ind. (2001) 49:43.
60. Michalek AM, Mahoney MC, Calebaugh D. Hypothyroidism and diabetes mellitus in an American Indian population. J Family Pract. (2000) 49:53–5.
61. Celani MF, Bonati ME, Stucci N. Prevalence of abnormal thyrotropin concentrations measured by a sensitive assay in patients with Type 2 diabetes mellitus. Diabete Res. (1994) 27:15–25.
62. Vondra K, Vrbikova J, Dvorakova K. Thyroid gland diseases in adult patients with diabetes mellitus. Minerva Endocrinol. (2005) 30:217–36.
63. Udenze I, Nnaji I, Oshodi T. Thyroid function in adult Nigerians with metabolic syndrome. Pan Afr Med J. (2014) 18:352–4. doi: 10.11604/pamj.2014.18.352.4551
64. Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab. (2010) 95:3614–7. doi: 10.1210/jc.2010-1245
65. Lagradi G, Emerson CH, Ahima RS. Leptin prevents fasting–induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. (1997) 138:2569–76. doi: 10.1210/endo.138.6.5209
66. Bandurska-Stankiewicz E. Thyroid hormones - obesity and metabolic syndrome. In: Proceedings of the 4th Congress of the Polish Thyroid Association. Łódz (2013). p. 11–3.
67. Diabetes Control and Complications Trial Research Group. Effects of intensive treatment of diabetes on the development of microvascular complications of diabetes mellitus. N Engl J Med. (1993) 329:304–9.
68. Bazrafshan HR, Ramezani A, Salehi A, Shir AAA, Mohammadian S, Faraj EM, et al. Thyroid dysfunction and its relationship with diabetes mellitus (NIDDM). J Gorgan Univ Med Sci. (2000) 2:5–11.
69. Ardekani MA, Rashidi M, Shojaoddiny A. Effect of thyroid dysfunction on metabolic response in type 2 diabetic patients. Iranian J Diabetes Obesity. (2010) 2:20–6.
70. Schlienger JL, Anceau A, Chabrier G, North ML, Stephan F. Effect of diabetic control on the level of circulating thyroid hormones. Diabetologia. (1982) 22:486–8. doi: 10.1007/BF00282596
Keywords: thyroid dysfunction, type 2 diabetes mellitus, hypothyroidism, South East Nigeria, predictors, risk factors
Citation: Ogbonna SU and Ezeani IU (2019) Risk Factors of Thyroid Dysfunction in Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 10:440. doi: 10.3389/fendo.2019.00440
Received: 24 January 2019; Accepted: 18 June 2019;
Published: 04 July 2019.
Edited by:
Bernadette Biondi, University of Naples Federico II, ItalyReviewed by:
Anthony Martin Gerdes, New York Institute of Technology, United StatesCopyright © 2019 Ogbonna and Ezeani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ignatius U. Ezeani, aWduYXRpdXNlenNAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.