- 1Department of Urology, Miller School of Medicine, University of Miami, Miami, FL, United States
- 2Miller School of Medicine, The Interdisciplinary Stem Cell Institute, University of Miami, Miami, FL, United States
- 3Division of Endocrinology, Diabetes and Hypertension, Brigham and Woman's Hospital, Harvard Medical School, Boston, MA, United States
Objective: The cause of age-related changes in testosterone remains unclear. We hypothesized that increased nitroso-redox imbalance with aging could affect testosterone production.
Materials and Methods: We measured several markers of nitroso-redox imbalance (4-HNE, 3-NT, and NT) in serum of S-nitrosoglutathione reductase knock out (GSNOR KO) mice that have increased nitroso-redox imbalance and compared these to wild-type (WT) mice. We evaluated the impact of age-induced nitroso-redox imbalance on serum luteinizing hormone (LH) and testosterone (T) in WT young (<2 months), middle-aged (2–6 months), and aged (>12 months) mice. Finally, to elucidate the susceptibility of testes to nitroso-redox imbalance, we measured 4-HNE protein levels in the testes of WT and KO mice.
Results: We identified 4-HNE as a reliable marker of nitroso-redox imbalance, as evidenced by increased protein levels in serum of GSNOR KO mice compared with WT mice. We demonstrated that 4-HNE serum protein levels increase in WT mice with age but do not accumulate in the testes. We also found that T levels were similar in all age groups. Interestingly, we found that serum LH levels in aged and middle-aged mice were increased when compared to young mice (n = 5) consistent with the phenotype of subclinical hypogonadism.
Conclusions: Increased serum 4-HNE and LH levels without changes in T with age suggest that nitroso-redox imbalance is associated with subclinical hypogonadism in aged mice. Recognizing the relationship and etiology of a currently poorly understood classification of hypogonadism could be a paradigm shift in how age-related testosterone change is diagnosed and treated.
Introduction
Age-related reproductive and sexual decline is a ubiquitous process. Recently, interest in identifying sources of age-related decline in sexual function in males has grown due to an increasing proportion of older people in the population (1). The hypothalamic-pituitary-gonadal (HPG) axis, which regulates sexual function in males, is responsible for the control of production of luteinizing hormone (LH) and testosterone (T). Serum levels of T change with age (2, 3), and these changes can be due to a combination of testicular (primary hypogonadism, high LH, and low testosterone) and pituitary or hypothalamic (secondary or tertiary hypogonadism, low, or normal LH with low testosterone) failure. Although it has been suggested that hypogonadism effects as much as 23.3% of men aged 40–79 years, the pathophysiology of age-related hypogonadism and its specific classifications have not been well-defined. In one study, in addition to those with low T, an additional 9.5% of men in the study had compensated or subclinical hypogonadism (increased LH and normal T) (4). The mechanism of this type of hypogonadism remains unknown.
Currently, the free radical theory of aging is the most accepted hypothesis to explain factors that contribute to functional decline associated with aging. (5, 6) This theory of aging proposes that reactive oxygen species (ROS), such as superoxide (O2−) and hydrogen peroxide (H2O2) and reactive nitrogen species (RNS) including nitrogen dioxide (NO2) and peroxynitrite (ONOO−) play key roles by inhibiting cellular function, growth, and apoptosis. (7–13) When ROS and RNS are produced in excess, a situation of oxidative stress, or nitrosative stress ensues, respectively. However, instead of implicating one or the other in biological systems, many have reasoned that the balance between both types of reactive species is responsible for biological effects (14). A disarray in this balance is better known as nitroso-redox imbalance; it has been associated with several degenerative diseases and negative cellular impacts and have further been shown to increase as a function of age (14, 15). We have previously demonstrated that young adult S-nitrosoglutathione reductase (GSNOR) knock-out (KO) mice have decreased circulating T and LH levels compared to wild-type (WT) controls (16). In the absence of GSNOR, these mice develop excess RNS (17), and their lower levels of T and LH suggest that the nitroso-redox imbalance may disrupt hormone production.
What is unknown is whether age-related changes in T are associated with increasing RNS and ROS (nitroso-redox imbalance). We hypothesized that increased nitroso-redox imbalance can occur with aging and be associated with changes in T production. To investigate this relationship, we confirmed that 4-hydroxynonenal (4-HNE), a sensitive marker for oxidative damage (18) can also reliably be used to measure nitroso-redox imbalance. We then evaluated 4-HNE levels in the serum of young and aged WT mice and compared them to young and aged KO mice. We compared the levels of 4-HNE to changes in LH and T levels that could occur with aging in WT mice. Finally, in an effort to identify the mechanism of the effects of nitroso-redox imbalance we measured 4-HNE levels in the testes of WT mice.
Materials and Methods
Mice
Male WT mice (C57/BL6) (Jackson Laboratories, Bar Harbor, ME, USA) and mice lacking GSNOR (KO) (The University of Miami Miller School of Medicine, Miami, FL, USA) were used. Pups were genotyped and sacrificed at the indicated ages. Mice were kept in a barrier-protected animal facility with 12-h light-dark cycles. All experiments were carried out before 10 am to reduce the impact of diurnal variation of LH and T levels. Blood was obtained from WT mice by cardiac puncture followed by euthanasia for the young (< 2 months), middle aged (6 month), and aged (12–15 months) time points. We obtained blood via saphenous vein draw for the 2, 3, and 4 month time points. Saphenous vein blood collections were done on the same mice over time as they aged. All saphenous vein and cardiac puncture blood collections were done under isoflurane anesthesia. Euthanasia was performed by cervical dislocation under isoflurane anesthesia. All methods were performed in accordance with the approved institutional animal care and use committee protocol at our institution. All methods were performed with the approval of the University of Miami Institutional Animal Care and Use Committee (IACUC) protocol at our institution (Protocol # 15167).
Western Blotting
Serum proteins were isolated from total blood and processed with RIPA lysis buffer. Lysates were mixed with 5 × sample buffer, heated to 100°C for 5 min, and separated by 10% odium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Resolved proteins were transferred onto a 0.45-μm polyvinylidene fluoride (PVDF) membrane. Proteins were studied by exposing the membranes to antibodies against 4-HNE (Alpha Diagnostic International Intl. Inc., HNE11-S), Transferrin (Abcam, ab84036), and Albumin (Abcam, ab19195), 3-Nitrotyrosine (Abcam, ab61392), and Nitrotyrosine (Abcam, ab42789). Immunoreactive bands were visualized using the Thermo Scientific Chemiluminescent Pico Kit.
Testes Protein Isolation
Testes from mice were dissected, and the tunica albuginea was removed and collected in chilled RIPA buffer (150 mM NaCl. 0.1% SDS, 1% NP-40, 50 mM Tris pH 7.5, 0.5% Sodium-Deoxycholate) for preparation of whole testis protein lysates. Tissues were disrupted mechanically and incubated on ice for 30 min and lysates were cleared from residual cell debris by centrifugation for 10 min at 10,000 × g. The supernatant was collected, and protein concentrations were determined by Bradford colorimetric assay.
Testosterone and LH Assay
Serum total testosterone and LH levels were measured using the Ligand Assay & Analysis Core of the Center for Research in Reproduction at the University of Virginia (Charlottesville, VA, USA). Techniques for measurements of each hormone are described in detail at https://med.virginia.edu/research-in-reproduction/ligand-assay-analysis-core/assay-methods/. For serum testosterone, the mean intra-assay variation was 12.8% and the intraassay variation was 9.3%. Serum LH was measured using an ultrasensitive enzyme-linked immunosorbent assay. In brief, 6 μL of whole blood was collected in 54 μL of assay buffer for analysis. Intraassay coefficients of variation were 7.3% (low quality control [QC]; 0.13 ng/mL), 5.0% (medium QC; 0.8 ng/mL), and 6.5% (high QC; 2.3 ng/mL). All experiments were performed in the morning to minimize the impact of diurnal variation of hormones.
Epididymal Sperm Concentration
After the mice were euthanized, epididymides were collected and used for semen analysis. Fresh epididymis was cut into small pieces and dispersed in F12 medium 200 μL (Invitrogen, Waltham, MA, USA) containing 0.1% bovine serum albumin (Invitrogen) pre-warmed to 37°C and incubated for 15 min to facilitate the transmigration of sperm from the epididymis. Each epididymal sperm suspension was subjected to sperm counting and sperm motility analyses by a computer-aided semen analysis (CASA) system (Microptic SL, Barcelona, Spain).
Statistical Analysis
Data were analyzed for significance using analysis of variance with the Student t-test, as indicated. All analyses were performed using GraphPad Prism 4.03 (GraphPad, South San Francisco, CA, USA), and a P < 0.05 was considered significant. All data are presented as mean ± standard error.
Results
Serum 4-HNE Levels Are a Reliable Marker of Nitroso-Redox Imbalance
We evaluated markers of nitroso-redox imbalance such as 4-HNE, 3-Nitrotyrosine (3-NT), and Nitrotyrosine (NT) in the serum of WT and GSNOR KO mice. We identified 4-HNE as a reliable marker of nitroso stress as evidenced by higher serum protein levels in GSNOR KO mice compared to WT mice (Figure 1). The other markers, 3-NT and NT, did not demonstrate significant differences in serum protein levels between GSNOR KO and WT mice (data not shown).
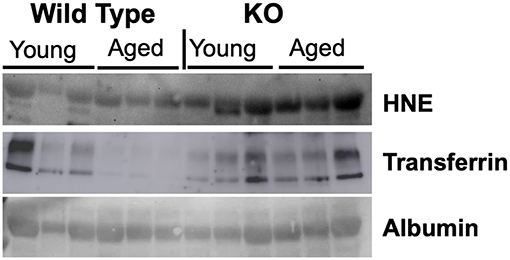
Figure 1. 4-HNE is a reliable marker of nitroso-redox imbalance. To identify a reliable marker of nitroso-redox imbalance in serum, we evaluated several markers such as 4-HNE, 3-NT, and NT. Western blot indicated that 4-HNE protein levels are a reliable marker of nitroso-redox imbalance, as evidenced by increased expression in GSNOR KO mice compared to WT mice.
Age Is Associated With Increased Nitroso-Redox Imbalance
Nitroso-redox imbalance is associated with increasing age. We investigated whether 4-HNE protein levels increase with age. We evaluated the levels of 4-HNE in serum samples from young (<2 months) and aged (>12 months) mice using Western blot. We found that 4-HNE levels were higher in older mice when compared to young mice (Figure 2). This result suggests that nitroso-redox imbalance increases with age.
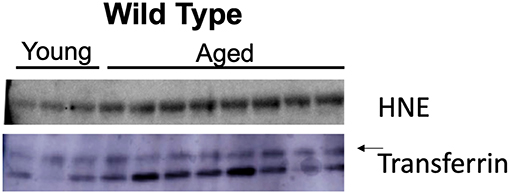
Figure 2. Age is associated with increased nitroso-redox imbalance. To validate that nitroso-redox imbalance is increased with age, we compared 4-HNE serum protein levels in young (<2 months) and aged mice (>12 months). Western blot demonstrated 4-HNE serum protein levels are increased with age.
Aging Affects Circulating LH, Testosterone, and Sperm Concentration
We have previously demonstrated that nitroso-redox imbalance in GSNOR KO mice decreased serum T and LH levels in addition to impairing sperm parameters, compared to WT controls (16). We examined if age-associated nitroso-redox imbalance impacts LH, T, and epididymal sperm concentration. For these experiments, we measured T, LH, and epididymal sperm concentration in young (<2 months), middle-aged (2–6 months), and aged mice (>12 months) over time. We found that LH levels in aged (n = 8) and middle-aged (n = 5) were increased when compared to young mice (n = 5) (Figure 3A). T levels were not significantly different between any of the age groups (n = 5) (Figure 3B). We also did not identify any changes in epididymal sperm concentration (Figure 3C). Collectively, LH concentration increased with age, while T and sperm concentration were not significantly impacted (Supplementary Figure 1). Taken together, these results suggest that age-induced increase in nitroso-redox imbalance is associated with subclinical hypogonadism.
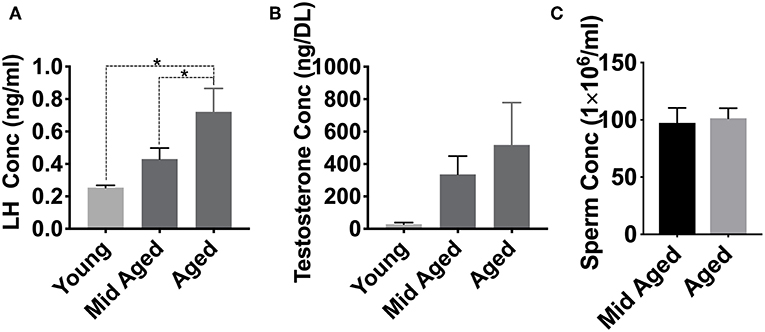
Figure 3. Aging effects on LH and testosterone levels. We examined if age-associated nitroso-redox imbalance impacts serum LH, Testosterone (T), and epididymal sperm concentration. (A) LH levels are higher in aged (>12 months) mice than in young (<2 months) or middle-aged (2–6 months) mice (*p < 0.05) (B) T concentrations show no significant differences with age (C) Epididymal sperm concentrations are not different between middle-aged and aged mice.
4-HNE Protein Levels Are Not Accumulated in the Testes With Aging
Previous studies have demonstrated that testicular tissue is particularly susceptible to nitroso-redox imbalance (19). To examine this, we measured 4-HNE protein levels in the testes of young WT, middle-aged WT, and young KO mice. Our results demonstrated that testicular 4-HNE protein levels were similar in all groups (Figure 4). This result suggests that the testis does not accumulate nitroso-redox imbalance with age, or that and that testicular 4-HNE protein levels may not be a reliable indicator of nitroso-redox imbalance.
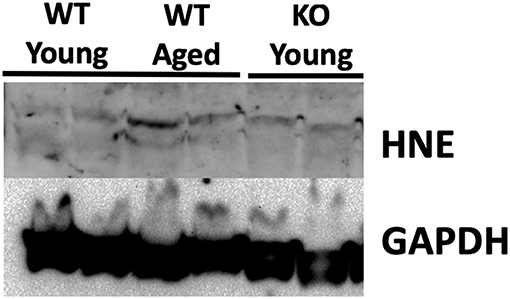
Figure 4. 4-HNE protein expression does not accumulate in the testis with age. We measured protein levels of 4-HNE in the testes of young WT, middle-aged WT, and young KO. Protein levels were similar in all of the groups suggesting that 4-HNE protein levels in the testes may not be a reliable indicator of nitroso-redox imbalance.
Discussion
The mechanism of age-associated changes in testosterone production remains unclear. Accumulating free radicals with nitrosative and oxidative stress can lead to cellular aging. We investigated whether increasing nitroso-redox imbalance with aging can explain changes in T. We identified that age is not only associated with increasing nitroso-redox imbalance as reflected by serum 4-HNE protein levels but is also associated with increased LH with unchanged T levels, suggesting a profile of compensated hypogonadism. As this imbalance increases with age, T production can decrease, resulting in a reduction of the negative feedback inhibiting LH production. The result is an increase in LH that is enough to stimulate steroidogenesis to produce T levels in the normal range; increased LH “compensates” for the decreased T production. As a result, T levels remain within normal reference ranges concurrently with elevated LH levels. While the mechanism remains uncertain, our results indicate that Leydig cells capacity for T production is not diminished since T levels remain in normal range. However, the mechanism may lie in the HPG axis in the Leydig cells ability to respond to LH and stimulate T production. Thus, we suggest that nitroso-redox imbalance may lead to compensated hypogonadism through effects of the HPG axis. Our results suggest an association between nitroso-redox imbalance and subclinical hypogonadism, but the cause must still be elucidated.
Compensated hypogonadism, also referred to as subclinical hypogonadism, has been suggested to be its own clinical condition (20, 21). The overall clinical and physiological significance of compensated hypogonadism is poorly understood, but it has been found to be associated with both aging and increased physical symptoms of T deficiency (22). This association, combined with its surprising prevalence in men [9.5% of all men aged 40–79 (4)], raises the question of whether this classification of hypogonadism should be diagnosed or potentially even treated. Men, and especially older men, may benefit from having LH included in the initial screening for hypogonadism in order to diagnose subclinical hypogonadism. It also should be investigated whether treating subclinical hypogonadism, much like treating subclinical hypothyroidism, can be of benefit. In this study, we show that increased levels of nitroso-redox imbalance occur with age suggesting a possible etiology for compensated hypogonadism in older men. This discovery provides an aim for future studies to evaluate if measuring nitroso-redox imbalance in older men may be of diagnostic benefit by identifying a potential therapeutic target in the aging process. Novel therapeutic strategies such as anti-oxidant therapy, may have a significant impact on treating subclinical hypogonadism. Since subclinical hypogonadism represents a form of testicular failure future studies should continue to investigate the mechanism of nitroso-redox imbalance at the level of Leydig cell steroidogenesis and whether treating the nitroso-redox imbalance could reverse these HPG axis changes and potentially prevent a progression of testicular failure resulting in primary hypogonadism.
Our study has strengths and limitations. A strength of this study is that it is the first study to show that 4-HNE can be a reliable marker for nitroso-redox imbalance. This study also demonstrates that nitroso-redox imbalance increases with age. Limitations of the study include the small sample size (limited by the breeding capabilities of GSNOR KO mice) and variability in serum LH and T levels in mice. Mice are also known to have variable T levels due to lack of circulating sex hormone binding globulin (23). We accounted for this variability by consistently drawing the blood in the morning (before 10 a.m.).
Conclusion
Aging is associated with increased nitroso-redox imbalance as reflected by serum 4-HNE protein levels and subclinical hypogonadism. Recognizing the relationship and etiology of a currently poorly understood classification of hypogonadism could be a paradigm shift in how we diagnose and treat age-related hypogonadism. Future studies can focus on identifying markers of nitroso-redox imbalance in testis and the mechanisms of how they impact steroidogenesis.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
JL, MK, HA, SK, TM, JH, UK, and RR designed the research. JL, MK, HA, and EG performed the research. JL, MK, HA, SK, and RR analyzed the data. JL, MK, and RR wrote the paper.
Conflict of Interest Statement
JH discloses a relationship with Vestion, Inc., that includes equity, board membership, and consulting; is the chief scientific officer, a compensated consultant and advisory board member for Longeveron, and holds equity in Longeveron; and is the co-inventor of intellectual property licensed to Longeveron.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00190/full#supplementary-material
Figure S1. LH and testosterone concentrations further broken down by age show the linear increase in LH concentration as the mice aged.
Abbreviations
WT, wild-type; GSNOR, S-nitrosoglutathione reductase; LH, luteinizing hormone; HPG, hypothalamic-pituitary-gonadal; ROS, reactive oxygen species; RNS, reactive nitrogen species; KO, knock-out; 4-HNE, 4-hydroxynonenal; 3-NT, 3-Nitrotyrosine; NT, Nitrotyrosine.
References
1. Corona G, Rastrelli G, Maseroli E, Forti G, Maggi M. Sexual function of the ageing male. Best Pract Res Clin Endocrinol Metab. (2013) 27:581–601. doi: 10.1016/j.beem.2013.05.007
2. Hammar M. Impaired in vitro testicular endocrine function in elderly men. Andrologia. (1985) 17:444–9.
3. Arora H, Zuttion MSSR, Nahar B, Lamb D, Hare JM, Ramasamy R. Subcutaneous leydig stem cell autograft: a promising strategy to increase serum testosterone. Stem Cells Transl Med. (2019) 8:58–65. doi: 10.1002/sctm.18-0069
4. Tajar A, Forti G, O'Neill TW, Lee DM, Silman AJ, Finn JD, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. (2010). 95:1810–8. doi: 10.1210/jc.2009-1796
5. Ljubuncic GE, Reznik AZ. Nitrosative Stress in aging- Its Importance and Biological Implications in NF-kB Signaling. In: Bondy S, Maiese K, editors. Aging and Age Related Disorders. New York, NY: Springer (2010). p. 27–54.
6. Kuchakulla M, Masterson T, Arora H, Kulandavelu S, Ramasamy R. Effect of nitroso-redox imbalance on male reproduction. Transl Androl Urol. (2018) 7:968–77. doi: 10.21037/tau.2018.08.14
7. Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. (1998) 356:1–11.
8. Stamler JS, Hausladen A. Oxidative modifications in nitrosative stress. Nat Struct Biol. (1998) 5:247–9. doi: 10.1038/nsb0498-247
9. Eu JP, Liu L, Zeng M, Stamler JS. An apoptotic model for nitrosative stress. Biochemistry. (2000) 39:1040–7. doi: 10.1021/bi992046e
10. Simon DI, Mullins ME, Jia L, Gaston B, Singel DJ, Stamler JS. Polynitrosylated proteins: characterization, bioactivity, and functional consequences. Proc Natl Acad Sci USA. (1996) 93:4736–41.
11. Marshall HE, Merchant K, Stamler JS. Nitrosation anad oxidation in the regulation of gene expression. FASEB J. (2000) 14:1889–900. doi: 10.1096/fj.00.011rev
12. Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. (2002) 416:337–9. doi: 10.1038/416337a
13. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. (1956) 11:298–300.
14. Raju SV, Barouch LA, Hare JM. Nitric oxide and oxidative stress in cardiovascular aging. Sci Aging Knowl Environ. (2005) 2005:re4. doi: 10.1126/sageke.2005.21.re4
15. Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. (2001) 928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x
16. Masterson TA, Arora H, Kulandavelu S, Carroll RS, Kaiser UB, Gultekin SH, et al. S-Nitrosoglutathione Reductase (GSNOR) Deficiency Results in Secondary Hypogonadism. J Sex Med. (2018) 15:654–61. doi: 10.1016/j.jsxm.2018.03.002
17. Hatzistergos KE, Paulino EC, Dulce RA, Takeuchi LM, Bellio MA, Kulandavelu S, et al. S-Nitrosoglutathione reductase deficiency enhances the proliferative expansion of adult heart progenitors and myocytes post myocardial infarction. J Am Heart Assoc. (2015) 4:e001974. doi: 10.1161/JAHA.115.001974
18. Argüelles S, Gómez A, Machado A, Ayala A. A preliminary analysis of within-subject variation in human serum oxidative stress parameters as a function of time. Rejuvenation Res. (2007) 10:621–36. doi: 10.1089/rej.2006.0528
19. Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. J Clin Diagn Res. (2017) 11:IE01–5. doi: 10.7860/JCDR/2017/23927.9886
20. Giannetta E, Gianfrilli D, Barbagallo F, Isidori AM, Lenzi A. Subclinical male hypogonadism. Best Pract Res Clin Endocrinol Metab. (2012) 26:539–50. doi: 10.1016/j.beem.2011.12.005
21. Eendebak RJAH, Ahern T, Swiecicka A, Pye SR, O'Neill TW, Bartfai G, et al. Elevated luteinizing hormone despite normal testosterone levels in older men-natural history, risk factors and clinical features. Clin Endocrinol. (2018) 88:479–90. doi: 10.1111/cen.13524
22. van den Beld A, Huhtaniemi IT, Pettersson KS, Pols HA, Grobbee DE, de Jong FH, et al. Luteinizing hormone and different genetic variants, as indicators of frailty in healthy elderly men. J Clin Endocrinol Metab. (1999) 84:1334–9.
Keywords: subclinical hypogonadism, compensated hypogonadism, aging, nitroso-redox imbalance, nitrosative stress, 4-hydroxynonenal, S-nitrosoglutathione reductase
Citation: Lee JA, Kuchakulla M, Arora H, Kulandavelu S, Gonzalez E, Masterson TA, Hare JM, Kaiser UB and Ramasamy R (2019) Age Induced Nitroso-Redox Imbalance Leads to Subclinical Hypogonadism in Male Mice. Front. Endocrinol. 10:190. doi: 10.3389/fendo.2019.00190
Received: 10 December 2018; Accepted: 07 March 2019;
Published: 28 March 2019.
Edited by:
Richard Quinton, Newcastle University, United KingdomReviewed by:
Ashok Agarwal, Cleveland Clinic, United StatesNannan Thirumavalavan, Baylor College of Medicine, United States
Copyright © 2019 Lee, Kuchakulla, Arora, Kulandavelu, Gonzalez, Masterson, Hare, Kaiser and Ramasamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manish Kuchakulla, bS5rdWNoYWt1bGxhQHVtaWFtaS5lZHU=
Ranjith Ramasamy, cmFtYXNhbXlAbWlhbWkuZWR1
 John Alden Lee
John Alden Lee Manish Kuchakulla
Manish Kuchakulla Himanshu Arora
Himanshu Arora Shathiyah Kulandavelu2
Shathiyah Kulandavelu2 Thomas A. Masterson
Thomas A. Masterson Joshua M. Hare
Joshua M. Hare Ranjith Ramasamy
Ranjith Ramasamy