- 1Laboratory of Endocrinology and Metabolism, Department of Endocrinology and Metabolism and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Orthopedics, West China Hospital, Sichuan University, Chengdu, China
Bone marrow adipose tissue (MAT) is distinct from white adipose tissue (WAT) or brown adipose tissue (BAT) for its location, feature and function. As a largely ignored adipose depot, it is situated in bone marrow space and resided with bone tissue side-by-side. MAT is considered not only as a regulator of bone metabolism through paracrine, but also as a functionally particular adipose tissue that may contribute to global metabolism. Adipokines, inflammatory factors and other molecules derived from bone marrow adipocytes may exert systematic effects. In this review, we summary the evidence from several aspects including development, distribution, histological features and phenotype to elaborate the basic characteristics of MAT. We discuss the association between bone metabolism and MAT, and highlight our current understanding of this special adipose tissue. We further demonstrate the probable relationship between MAT and energy metabolism, as well as glucose metabolism. On the basis of preliminary results from animal model and clinical studies, we propose that MAT has its unique secretory and metabolic function, although there is no in-depth study at present.
Introduction
Adipose tissue, distributed in distinct depots in the whole body, may affect overall health through endocrine function. White adipose tissue (WAT), as an energy-storing reservoir, principally locates in the subcutaneous and visceral depots; while brown adipose tissue (BAT), specializing in utilizing energy to produce heat, is primarily present above the clavicle and in the subscapular region of the back (1, 2). In addition to brown adipocytes, brite/beige adipocytes, also expressing uncoupling protein (Ucp) 1, principally store lipids and can be stimulated to transdifferentiate into a “brown-like” state with well characterized-thermogenic function (2–4). The brite/beige fat has been discovered in WAT in response to activators like cold exposure, indicating the involvement of sympathetic signaling (5, 6). The adipocytes also exist in the bone marrow (BM), and such marrow adipose tissue (MAT) accounts for over 10% of total adipose tissue mass in humans (7, 8). MAT has long been considered as a relatively inert and underappreciated component in the BM microenvironment but it has been recognized recently to have potentially significant and diverse functions.
The tenet widely accepted is that the amount of MAT is increased with age, obesity, and some metabolic disorders (9, 10). As the adipose tissue is one of the main components within the BM niche, there is a need to characterize the properties and functions of MAT. Previous review literatures mainly focused on the relationship between MAT and bone metabolism (11)/hematopoiesis (12). Although it has been summarized the ability of MAT to secrete traditional adipokines (7, 13) (including leptin and adiponectin), this review, combining the latest reports, has discussed the regulation of other small molecules derived from MAT, including inflammatory factors and cytokines. In addition, we have summarized the current evidence regarding the fundamental features and regulatory factors of MAT and discussed the inner relationships between MAT and some metabolic disorders.
The Basic Characteristics of MAT
Development of MAT
BM, primarily consisting of adipocytes and hematopoietic red blood cells (red marrow), is located in the cavities of trabecular bone. At birth, BM cavities mainly contain active hematopoietic red marrow. Then, MAT accumulates centripetally in a time-dependent way: the process starts from the terminal phalanges, continues to the appendicular skeleton and finally appears in the axial skeleton (14, 15). Significantly, this depot makes up ~50–70% of the marrow volume by the age of 25 and accounting for over 10% of total adipose mass in lean, healthy adults (8, 16–18). Afterwards, the BM transforms slowly into MAT throughout the rest of lifetime.
Distribution of MAT
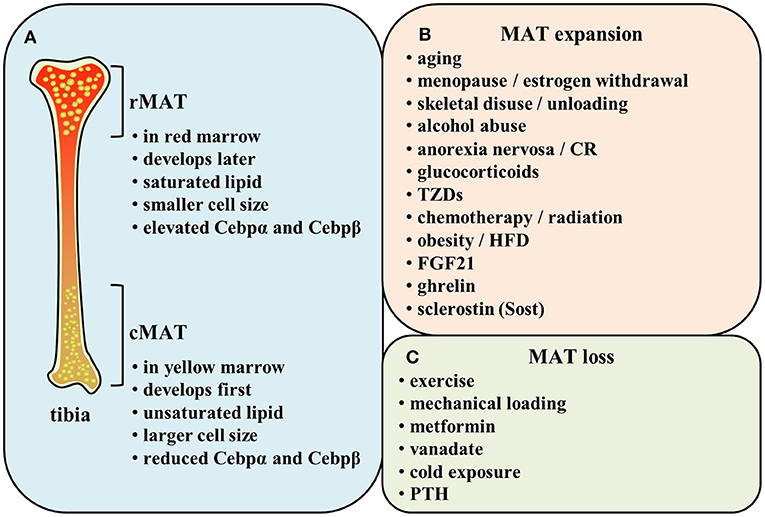
Deep analysis of the BM requires researchers to deal with the anatomy of bone. This explains why most of the initial work on BM in the late 1800s and early 1900s was relied on the relatively larger animals like rabbits or cats (19–22), so as to appropriately observe and analyze anatomical structure of BM. The existence of MAT, traditionally considered to be yellow marrow, has been neglected for many decades although it was mentioned in the early literature as a long-standing knowledge (23). However, one such cell population known as MAT or yellow marrow has attracted increasing attention by the scientific community recent years (24–26). In 1976, Tavassoli began to characterize the marrow adipocytes and delineate their morphologic features (24). Two histochemically distinct populations of fat cells, one is presented within the red marrow staining positively with performic acid-Schiff (PFAS) and the other is located in the yellow marrow non-staining with PFAS (24). This staining reaction is considered to rely on the oxidation of ethylene to acetaldehyde in the unsaturated fat and treatment with Schiff's reagent to produce a red/purple color. PFAS-positive adipocytes in red marrow disappeared in response to experimentally induced hemolysis, while non-stained adipocytes remain unaffected (25). This implies that red marrow with PFAS-positive cells is mainly composed of unsaturated lipids, while yellow marrow with PFAS-negative cells primarily consists of saturated lipids.
Indeed, 40 years later, Scheller and collaborators demonstrated a different distribution of lipid saturation in the BM by proton MRS (1H-MRS) from the findings of Tavassoli. MAT arisen first and early in life in the distal skeleton (e.g., distal tibia and caudal vertebrae) is identified as constitutive marrow adipose tissue (cMAT) within the yellow marrow. The other subtype of MAT exists in the lumbar/thoracic vertebrae, proximal limb skeleton, hip, and ribs, which is formed late and in a more scattered way. This type of MAT is known as regulated marrow adipose tissue (rMAT) within the red marrow. Researchers have found that distal marrow adipocytes (cMAT) contain more unsaturated lipids than those in proximal/central skeletal regions (rMAT) (26) (Figure 1A). This implies that the populations of rMAT fail to stain with PFAS while their constitutive counterparts readily display the characteristic pattern of PFAS staining (24, 27). Of note, this study yielded a conclusion of the increase in cMAT unsaturation which is opposite to the theory based on the proposed mechanism of PFAS staining. Nevertheless, there is possibility that both regulated and constitutive MAT adipocytes exist in the same position (26). It is possible that the rMAT can be matured into the stable cMAT in some conditions.
Histological Features of MAT
Similarly to WAT, MAT pre-adipocytes accumulate lipid that merges into a unilocular droplet, replacing nucleus and cytoplasm gradually (14, 28). The average MAT adipocyte diameter is measured and calculated to be 30–40 mm through osmium-based MAT analysis (26). BAT and brite/beige adipocytes are smaller and occupied by high mitochondrial content, as well as multilocular lipid droplet (29), while neither of which is observed in BM adipocytes (8, 30). Analysis of C3H mice and Sprague-Dawley rats demonstrated that cMAT adipocytes are significantly larger than rMAT adipocytes (26). MAT in diet-induced obesity mice show greater adipocyte size and number, while both measures are reduced with exercise (31–33).
Phenotype of MAT
It has been hypothesized that the metabolic profile of MAT resembles both BAT and WAT. On the one hand, MAT is assumed to have a similar feature to BAT, as the volume of MAT is adjusted by temperature (34). Compared with BAT, the entire tibia bones express higher BAT-specific gene markers, including modulators of thermogenesis such as deiodinase (Dio) 2 and peroxisome proliferator-activated receptor-gamma coactivator (Pgc) 1α, as well as transcription factor positive regulatory domain-containing (Prdm) 16 in C57BL6/J mice (35). However, the expression of Ucp1 in tibia was not suggested to be higher. Sulston et al. demonstrated that antidiabetic thiazolidinedione (TZD) rosiglitazone upregulated Ucp1 in BAT, but not in MAT of tibia (30), revealing that MAT of tibia may not share the thermogenic properties. Moreover, Ambrosi et al. indicated that the gene expression patterns of MAT in tibia are similar to inguinal WAT (iWAT) rather than BAT. MAT expresses similar levels of peroxisome proliferator-activated receptor (Ppar) γ and CCAAT/enhancer-binding protein (Cebp) α and low level of Ucp1 (36). However, high expression of Ucp1 was found in vertebral BM in humans and ICR mice (37). Taken together, the seemingly contradictory results tend to suggest that the potential presence of active brite/beige adipocytes in MAT of vertebrae in mammals. That means, marrow adipocytic lineage may possess the BAT-like thermogenic properties in vertebrae, but often exhibits a WAT-related phenotype in tibia.
Additionally, both in human and rodent bones, cMAT is reported to express elevated levels of the adipogenic transcription factors (e.g., Cebpα and Cebpβ) and be principally composed of unsaturated lipids. Conversely, rMAT is described to express reduced Cebpα and Cebpβ and be mainly constituted of saturated lipids (26, 28) (Figure 1A). These evidences imply that constitutive and regulated MATs not only differ in their position but also exhibit distinct features in their function. Since the time for identification of MAT classification is not long, there still remains much unclear about cMAT and rMAT. It is unknown whether they originate from a common progenitor or not and how the microenvironment affects their development. In addition, we also need to explore the effect of each subtype on metabolism not only in bone turnover but also in the whole body during some specific physiopathological conditions.
Regulatory Factors of MAT
MAT Expansion
MAT alters its volume in order to adapt to various physiologic and pathologic conditions. Indeed, MAT is a potentially critical participant in bone homeostasis since osteoblasts and adipocytes are derived from the same common progenitor cells-bone marrow mesenchymal stem cells (MSCs) (38–41). Marrow adiposity is correlated with low bone mass, indicating that the decision for MSCs to differentiate into osteoblasts or adipocytes may create a tug-of-war between MAT and bone tissue (42, 43). Many osteoporotic states in humans, including aging (36, 44–46), menopause (47, 48), skeletal disuse or unloading (49, 50), alcohol abuse (51), and anorexia nervosa (52, 53), are associated with increased bone marrow adiposity, suggesting the balance between MAT and bone mineral density (BMD) has been broken. Researchers have also shown that glucocorticoids (18), TZDs (30, 31, 41, 54–59), caloric restriction (8, 18, 60), chemotherapy and/or radiation (8), obesity (36, 45, 61), high-fat diet (HFD) (33, 61–64), and hormonal factors such as estrogen withdrawal (46, 48), fibroblast growth factor (FGF) 21 administration (65) are related to a significant increase in MAT. However, increased marrow fat led by HFD or obesity is coincided with preserved or increased bone density (33, 61–64). Thompson et al. also demonstrated that des-octanoyl ghrelin, a major circulating form of ghrelin, promotes BM adipogenesis in vivo by a direct peripheral action (66). Besides, a recent work demonstrates a direct role for sclerostin (Sost), secreted from osteocytes, to induce BM adipogenesis through inhibiting Wnt signaling (67) (Figure 1B). It has been reported that inhibition of Wnt signaling increased expression of adipogenic transcription factors Pparγ and Cebpα and stimulated adipogenesis (68–70). Levels of mRNA expression adipogenesis markers Pparγ2, lipoprotein lipase (LPL), adipocyte-specific fatty acid binding protein (aP2), and adiponectin were lower when incubated with adipocytes induction medium containing wnt3a than without wnt3a (71). Osteocyte-derived Sost induced adipogenesis in mouse primary bone marrow MSCs, increased the expression of Pparγ and Cebpα, and simultaneously decreased the expression of β-catenin responsive genes Axin2 and Smad6 (67). The above results demonstrate Wnt signaling inhibits adipogenic differentiation of mouse MSCs and human MSCs, and Sost derived from osteocytes could inhibit Wnt signaling, thus promoting adipogenesis in BM.
MAT Loss
The exercise or mechanical loading have been reported to lower MAT volume (31, 33, 57, 63, 64, 72–75). The exercise can reduce MAT adipocytes in both lean and obese mice (33). Moreover, metformin, the most widely prescribed medicine for type 2 diabetes (T2D) worldwide, ameliorates elevated MAT induced by HFD in tibia (61). Besides, vanadate impedes adipogenesis significantly in MSCs within BM (76). A recent study revealed that proximal rMAT adipocytes are decreased in size and number in response to cold exposure (26). Some endocrine signals like parathyroid hormone (PTH) also strongly influence the extent of MAT. Fan et al. found MAT increased through conditional deletion of the PTH/PTHrP receptor (PTH1R) in MSCs using Prx1-Cre recombinase (77). Moreover, intermittent PTH administration can effectively reduce the increased marrow fat in mice and osteoporotic patients (77, 78) (Figure 1C). Therefore, many regulatory factors lead to the changes of MAT. This reflects the strong plasticity of MAT and implies its vital functions.
Secretory Property of MAT
Extracellular Vesicles
The adipogenic/osteogenic differentiation of MSCs has always been considered to affect bone metabolism. In fact, MSC differentiation and even bone metabolism could be directly regulated by mature BM fat cells. Human MSC-derived osteoblasts demonstrated an elevated adipogenic profile and reduced osteogenic markers such as osteocalcin (OC) upon co-culturing with human MSC-derived adipocytes in the early study (79). In recent years, the same research group has explored the mechanism underlying this modulation. Adipocytes have been described as liberating extracellular vesicles (EVs) (80) (Figure 2). However, the definition of EVs is still lacking. It is conventionally believed that EVs are heterogeneous in size, encompassing the so-called microparticles/microvesicles (>100 nm) and exosomes (< 100 nm) in diameter (81, 82). The EVs from the human MSC-derived adipocytes were observed ~30–100 nm in size under transmission electron microscopy, but their protein profile remains to be characterized to classify (80). The EVs contain adipocyte specific transcripts e.g., Pparγ, leptin, Cebpα, Cebpδ, and anti-osteoblastic miRNAs including miR-138, miR-30c, miR-125a, miR-125b, and miR-31 (80). These EVs are probably involved in the down-regulation of osteogenesis in the co-culture system. Early studies have demonstrated that adipocytes have the ability to secrete exosomes (83, 84). Thus, the EVs in this study should be more accurately called exosomes. The evidence suggests that BM fat cells impact the phenotype of osteoblasts through paracrine of adipogenic transcripts and anti-osteoblastic miRNAs.
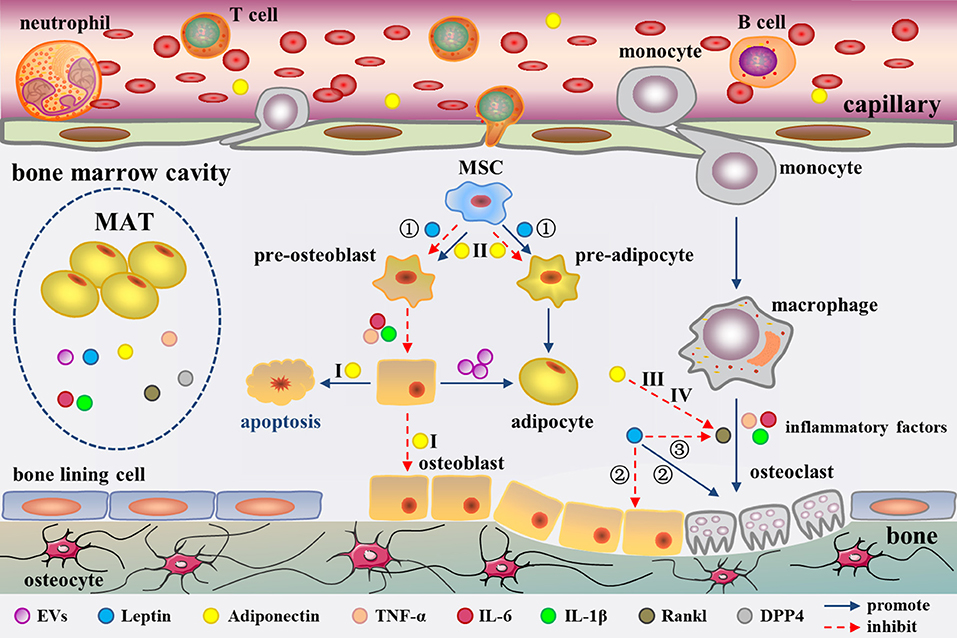
Figure 2. Secretion of MAT as well as adipocyte-derived molecules in the regulation of bone metabolism in the bone marrow cavity. MAT could secrete EVs, leptin, adiponectin, inflammatory factors, RANKL, and DPP4. These factors regulate bone metabolism from different aspects. Among them, adiponectin has been confirmed to enter into the circulation. EVs cause a phenotypic transformation of osteoblast to adipocyte. Leptin regulates bone metabolism in three ways (①-③). Moreover, Adiponectin regulates bone metabolism through four pathways (I-IV). Inflammatory cytokines promote osteoclast formation and adipocyte differentiation. RANKL promote osteoclast formation. The role of DPP4 in bone marrow cavity remains unclear.
Leptin
Leptin and adiponectin are known for their role in the regulation of global energy metabolism from 1990s (8, 85–88). Leptin is closely correlated with obesity, which forms a sense of satiety in the brain and reserves energy in peripheral tissues via leptin receptor (LepR) (89). Expression of leptin in primary culture system of human BM adpocytes was first confirmed in 1998 (88). Later studies proved that human MSC-derived mature adipocytes and human primary BM adipocytes can express and secrete leptin (90–94). Moreover, Liu et al. compared the expression of leptin in MAT and epididymal WAT (eWAT) in male C57BL/6J mice by microarray analysis. Their results showed that leptin is expressed at lower level in MAT than in eWAT (95). However, MAT expresses the similar level of leptin as iWAT and perirenal WAT (pWAT) in rabbits, while MAT secretes distinct concentration of leptin from WAT in humans (8). Nevertheless, the different secretion volume of leptin between MAT and WAT has not been studied in depth. It remains to be answered whether MAT could release more metabolic leptin that the traditional WAT.
LepR is highly expressed on MSCs (96). Previous research has suggested that leptin directly inhibits adipogenic differentiation and enhances osteogenesis in MSCs (97). Central or peripheral administration of leptin notably decreases the size and number of BM adipocytes in the leptin-deficient ob/ob mice (98–100). Besides, leptin treatment also blunts marrow fat in type 1 diabetic mice and calorie-restricted mice (101, 102). Compared to earlier studies, researchers have gotten different results recent years. Yue et al. found that limb bones exhibited a lower adipogenesis with conditional deletion of LepR from limb MSCs using Prx1-Cre, but not from the axial skeleton or hypothalamic neurons (103). Further studies suggested leptin increased adipogenesis by activating Janus kinase (Jak) 2/signal transducer and activator of transcription (Stat) 3 signaling pathway in MSCs (103) (Figure 2). The conflicting results may be resulted from the different animal models. A global gene knockout mouse model was used in the previous studies. However, the animal model with conditional deletion of leptin/LepR signaling in MSCs of limb bones was used in recent years, which is more intuitive and rigorous for exploring the role of leptin in MAT.
Adiponectin
Since its discovery in 1995, adiponectin has gradually been considered as a biomarker for increased risk of insulin resistance (IR), cardiovascular diseases, bone loss, and certain cancers (104). Although adiponectin is derived from adipocytes, the plasma concentration of adiponectin is paradoxically decreased during obesity (105). As early as 2003, Delporte et al. have found that the plasma level of adiponectin is elevated in women with anorexia nervosa (106). Adiponectin is expressed in adipocytes derived from human and mouse MSCs (107, 108) and human MSC-derived adipocytes can secrete adiponectin (91, 109). However, the results from different groups showed diverse amount of adiponectin produced by BM adipocyte or MAT. Compared to peripheral adipocytes, mature human MSC-derived adipocytes, and human primary BM adipocytes express lower level adiponectin (110), which has also been confirmed in primary BM adipocytes from mice (95). In addition, the amount of adiponectin secreted by MSC-derived adipocytes is reported to be very low (94), even after stimulated with glucocorticoids (111, 112). However, Cawthorn et al. demonstrated that adiponectin secretion is greater from MAT than from WAT in conditions such as anorexia nervosa and cancer therapy in humans (8, 13). This discrepancy could be related to the fact that some groups analyzed the isolated BM adipocytes while Cawthorn et al. studied the intact MAT, which has been discussed before (7). It is undeniable that MAT has the ability to express and secrete adiponectin which can be released into the circulation (8, 30) to modulate systemic metabolism through endocrine effects (Figure 2). In addition, osteoblasts and osteoclasts are both in close contact with adipocytes in the BM, which creates a favorable topographic distribution for the crosstalk among them.
Inflammatory Factors
The theme of MAT related pro-inflammatory factor has been of great significance. Compared with the epididymal white adipocytes, an independent transcriptomic study has revealed a unique phenotype for BM adipocytes characterized by higher levels of inflammatory response genes, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β (95) (Figure 2). The expression of TNF-α and IL1-β in BM adipocytes was also higher than that of visceral adipocytes under normal diet in C57BL/6J mice. Of note, mRNA level of pro-inflammatory cytokines was increased in the visceral adipocytes, while it was decreased in the BM adipocytes in high fat-induced obese mice (113). This suggests that MAT does not show the similar inflammatory response as WAT when stimulated by HFD. However, another research reported that human BM adipocytes in primary culture secrete only little amounts of IL1-β and TNF-α, but significant levels of IL-6 (114). Whatever, the BM adipocytes have a great potential to secrete pro-inflammatory factors, which may regulate bone metabolism (115, 116) and haematopoiesis (117) through paracrine, as well as affect the whole body metabolism by entering into the circulation.
RANKL and DPP4
Fan and collaborators revealed BM adipocytes secrete receptor activator of nuclear factor kappa B ligand (RANKL) (77). In this study, they generated a mouse model with PTH1R deleted in the bone MSCs. They found that Pref-1 (pre-adipocyte marker) and RANKL tag synchronously were up-regulated in B220 (B cell lineage marker) negative cells in the knockout mice by flow cytometry. In addition, mRNA expression of RANKL is remarkable higher in the isolated marrow adipocytes than the whole BM. The above evidence suggests that MAT secretes RANKL (77) (Figure 2). Moreover, primary human BM adipocytes can also express RANKL to promote osteoclast differentiation by co-cultures of pre-osteoclasts with BM adipocytes (118).
As we know, dipeptidyl peptidase (DPP) 4 is a protease and its inhibitors have been widely used for the treatment of T2D (119). CD26, the membrane-bound form of DPP4, is enriched on the surface of adipogenic cell populations, but not osteochondrogenic progenitor cells. The shed CD26, also called DDP4, enters into the BM sera after adipogenic differentiation (36). The amounts of DPP4 are increased in distal tibiae with aging (36). These findings imply MAT may be involved in glucose metabolism through secreting DDP4. Overall, more potential of MAT for secreting factors remains to be clarified.
MAT and Metabolism
MAT and Bone Metabolism
As early as 2001, Justesen et al. found an age-related increase in MAT and decline in trabecular bone volume by iliac crest bone biopsies (120). Compared with age-matched controls, patients with osteoporosis exhibited an increased MAT (120), which suggests a strong correlation between osteoporosis and MAT. Furthermore, in diabetic mice, the trabecular bone loss is significantly correlated with the increased BM adiposity (121). In the young people, MAT was found to be negatively correlated with the amount of bone in the axial and appendicular skeleton (122, 123). Similar performance also occurs in the pelvis of the elderly (124) and children (125). In African-American and Caucasian men and women, a negative relationship existed between MRI-measured MAT and hip and lumbar BMD (126, 127). However, opposite result has revealed a positive relationship between MAT and bone mineral content (BMC) in white and non-Hispanic black girls aged at 4–10 years (128). Subjects with metabolic disorders such as morbid obesity and T2D have higher MAT but also higher femoral neck BMD (45). In short, most studies have shown a negative relationship between MAT and BMD in humans. But some studies suggest that BMC or BMD is positively associated with MAT, which reveals the relationship between marrow adiposity and bone mass is not a simple inverse correlation.
The communication between MAT and bone is complex. Factors including EVs, adipokines (leptin and adiponectin, etc.), inflammatory factors (IL-6 and TNF-α, etc.), and RANKL derived from MAT can regulate bone metabolism. EVs, containing adipocyte specific transcripts, down-regulate osteogenesis (79, 80). Leptin regulates bone metabolism at least in three ways. First, leptin decreases bone formation by activating Jak2/Stat3 signaling in MSCs (103). Second, leptin increases sympathetic activity through an indirect way depending on inhibition of 5-HT synthesis in raphe nuclei of brainstem (129, 130). The sympathetic signaling strengthens bone resorption through an ATF4-mediated process, and reduces bone formation through a CREB-mediated process (131, 132). Third, leptin also directly stimulates LepR in hypothalamic arcuate nuclei neurons and elevates Cart (cocaine amphetamine regulated transcript) expression, which decreases RANKL expression via an unknown mechanism, and thereby decreases bone resorption (133) (Figure 2).
The effect of adiponectin on bone metabolism presents a confusing situation. Serum adiponectin is negatively correlated with BMD in young healthy men (134), male hemodialysis patients (135), and men with spinal cord injury (136). In addition, adiponectin is also associated with decrease of bone mass in childhood (137). However, studies have shown that circulating level of adiponectin is not related with BMD in postmenopausal women (138, 139). Significant decrease of BMD was observed in adiponectin-knockout mice (140), suggesting a positive effect of adiponectin on bone geometry and density. Adiponectin regulates bone metabolism through four pathways. First, adiponectin signals could activate p38/mitogen-activated protein kinase (MAPK) pathway to increase RANKL expression in osteoblast through adiponectin receptor (AdipoR) 1 (141). Moreover, another signaling phosphoinositide 3-kinase (PI3K)/AKT pathway in osteoblasts is also induced simultaneously, resulting in inhibition of forkhead box protein O (FoxO) 1 and decrease of osteoblasts proliferation, as well as increase of osteoblasts apoptosis (142). The synergistic effect is to reduce osteogenesis and increase bone resorption, leading to decreased bone mass. Second, in MSCs, adiponectin serially activates AdipoR1, p38/MAPK, and the c-Jun signaling pathway to induce cyclooxygenase (COX) 2 expression (143). In this way, the adipocyte differentiation of MSCs is inhibited (144) and osteogenic differentiation is promoted. Third, in pre-osteoclast, adiponectin treatment significantly induces Appl1-mediated down-regulation of AKT1 activity and removes glycogen synthase kinase (GSK)-3β-mediated phosphorylation of nuclear factor of activated T cells (NFAT)2, giving rise to expressions of NFAT2-targeted genes decreased (145). The activation of this pathway leads to the inhibition of RANKL-induced osteoclastogenesis and increase of bone mass. Forth, adiponectin signals decrease the sympathetic tone also through FoxO1 in neurons of the locus coeruleus, further lower expression of RANKL, thereby inhibiting bone resorption (142). In the four signaling pathways above, the first one reduces bone mass while the other three increase bone mass. Previous study has suggested that adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1 (142) (Figure 2). It is unclear that the net effect of adiponectin on bone mass. Under different pathophysiological conditions, there must be a dominant pathway for adiponectin signal transduction.
The deletion of inflammatory factors like IL-6 and TNF-α has been reported to be a protective effect on HFD-induced trabecular bone loss (115, 116), but the molecular mechanisms is unclear. In addition, other adipocyte-secreted molecules, including chemerin (146–149), resistin (150, 151), visfatin (152), and omentin-1 (153, 154) have also been shown to modulate bone metabolism. But it is not clear if they are also expressed in BM adipocytes. Of course, only certain factors play a dominant role in regulating bone metabolism under specific conditions. This partly explains why adipocytes and osteoblasts share common precursor (39), but they are not always in a trade-off relationship. There are few studies on the molecular mechanism of MAT affecting bone metabolism. Future studies should pay more attention to it in order to predict the positive or negative regulation of MAT on bone metabolism under different pathophysiological conditions.
MAT and Energy Metabolism
MAT as an Energy Depot in Bone
WAT reflects the high capacity of storing lipids. However, the transfer of lipids from WAT to other depots reflects the ability of retaining lipids is not a unique feature of WAT. Marrow adipocytes store significant quantities of fat and express insulin receptor (InsR) (34), they also respond to insulin-sensitizing anti-diabetic TZDs (54). This evidence tightly links MAT with the energy metabolism. Fatty acids and lipids can be used to generate adenosine triphosphate (ATP) for osteoblasts via the tricarboxylic acid cycle, although much less is known about their utilization (155). Studies have demonstrated that fatty acids can also be metabolized to generate ATP via Wnt activation in osteoblasts (155, 156). Although the relative concentrations and degree of saturation may be different (26, 157), fatty acids and lipids constantly circulate and are also present in BM sera, which favoring MAT as an energy depot in bone.
HFD in mice and obesity in humans promote expansion of bone MAT (45, 113), suggesting a stage of energy reserve. A recent research showed that HFD significantly increased expression of lipid storage marker fat-specific protein (FSP) 27, LepR and perilipin (PLIN) 5 in total bone tissue (33). This data suggests that there is an increase in lipid storage in MAT. In addition, running can improve bone quantity and quality while reduce the diameter and number of marrow adipocytes in obese mice (33). PLIN3 has been reported to play an important role in the β-oxidation of lipids as well as in promoting basal lipolysis (158, 159). Expression of PLIN3 is increased in MAT after running. In summary, these findings indicate that the marrow fat may be utilized as fuel to enhance bone formation.
MAT May Not Provide Energy During Acute Starvation
Nevertheless, other studies suggested that MAT is not a preferential position to provide energy. It seems that there is no connection between MAT and visceral adipose tissue (VAT) (160). During malnutrition, MAT does not decrease as a result of energy supply. Studies have shown that caloric restriction in animals and anorexia nervosa in humans lead to high marrow adiposity (53, 60). The incremental amount of MAT during nutritional deprivation may result from the differentiation of MSCs into adipocytes (8, 60). As early as 1979, Bathija et al. found that MAT is not catabolized during acute starvation (161). Moreover, the latest research has shown that MAT has the capacity to respond to β-adrenergic stimuli, however, its responses are muted in states of fasting and caloric restriction (162). This suggests that MAT may not participate in lipiolysis stimulated by β-adrenergic neuron to provide energy as peripheral WAT in the absence of energy.
MAT and Glucose Metabolism
MAT and TZDs
TZD compounds, Pparγ agonists, have been widely used for the treatment of T2D and impaired glucose tolerance (IGT) with IR or hyperinsulinemia by reducing circulating free fatty acids (FFAs) and strengthening insulin sensitivity (163). TZDs stimulate Pparγ activation in adipose tissue and upregulate expression levels of genes involved in lipid metabolism, such as scavenger receptor CD36, fatty acid-binding protein (FABP) 4 and LPL (164, 165). Elevated expression of these genes promotes FFAs to translocate into adipose tissue, thus decreases serum FFAs concentration and eventually ameliorates IR. TZD-induced Pparγ activation contributes to MSCs differentiating into adipocytic lineage and is associated with bone loss and marrow adiposity, especially in aging female mice (56). These lines of evidence highlight the clinical observations that TZDs increase fracture risk in postmenopausal women (166, 167). Correspondingly, Pparγ inhibitor inhibits BM adiposity in mice after radiation exposure and in streptozotocin (STZ)-induced diabetic mice (168, 169). This evidence indicates the close relationship between MAT and glucose metabolism.
MAT Responds Differently to Insulin Stimuli
MAT may respond differently to insulin stimuli compared to peripheral WAT. Studies have shown that marrow adipocytes express InsR (34). In obesity and T2D, peripheral WAT develops IR and exhibits impaired insulin signaling (170). However, HFD-induced obesity did not impair insulin sensitivity in adipocytic progenitors of BM, as BM shows normal levels of pAKT as well as insulin signaling genes after insulin stimulation (113). This indicates that MAT has different insulin sensitivity from WAT.
MAT and IR
The augment of MAT has been observed in some conditions such as aging, and glucocorticoid-induced osteoporosis (GIOP), both of which are accompanied with IR (45, 171). Other results clearly showing a positive connection between MAT and glycated hemoglobin (HbA1c) or serum glucose concentrations (45, 160, 171). The adipocyte markers and marrow adiposity are increased in tibias in STZ-induced diabetic mouse model (121, 172). Moreover, patients with diabetes show a higher MAT compared to non-diabetic persons (173). In short, IR and diabetes status are strongly associated with increased MAT. However, no studies have investigated the molecular mechanisms as well as the crosstalk between MAT and glucose metabolism. We speculate MAT may regulate insulin sensitivity by secreting certain molecules like DPP4 and TNF-α. A latest research showed that hepatocyte DPP4 promotes VAT inflammation and IR in obesity (174). TNF-α is also involved in obesity-linked IR (175). Moreover, studies have shown that MAT can release DPP4 and TNF-α (36, 95). Thus, MAT may increase IR through secreting DPP4 and TNF-α, but more evidence is required to verify this view.
MAT and Insulin Sensitivity
Nonetheless, evidence also demonstrated there are no differences in MAT by diabetic status (176, 177). It has been summarized in the past that leptin and adiponectin can regulate food intake, lipid metabolism, glucose metabolism, etc. (178, 179). To our knowledge, MAT may secrete adiponectin and leptin to regulate blood glucose levels or insulin sensitivity. Previous study has shown that serum levels of adiponectin are positively associated with insulin sensitivity (105, 180). Insulin-resistant states including obesity and T2D are correlated closely to decreased adiponectin (105, 180, 181). Although adiponectin is increased in patients with type 1 diabetes (T1D), its levels are still positively correlated to insulin sensitivity (182). Studies have revealed that adiponectin can activate fatty acid oxidation and glucose uptake by increasing AMP-activated protein kinase (AMPK) phosphorylation and Pparα activity (183). A recent study of 50 obese and non-obese premenopausal women revealed a negative relationship between BM adiposity and IR, possibly mediated by increased secretion of adiponectin (184). In addition, it has been reported that more MAT content and adiponectin exist in the non-obese group (8, 184). Furthermore, leptin is increased in obesity inversely. The leptin signal is transmitted by Jak/Stat pathway to regulate food intake and body weight. In addition, PI3K signaling stimulated by leptin appears to take part in the decrease of blood glucose level (183). Therefore, adiponectin and leptin can increase insulin sensitivity and reduce blood glucose level, which is contrary to the effect of DPP4 and TNF-α derived from MAT. In brief, some studies have shown that MAT is positively related to IR and blood glucose levels, while some other studies have shown no correlation. More in-depth studies are needed to clarify the exact relationship.
Concluding Perspectives
Substantial evidence have indicated that MAT secretes EVs, leptin, adiponectin, inflammatory cytokines such as IL-6 and TNF-α, RANKL, as well as DPP4. However, it has not been proved synchronously that these factors are both derived from BM fat cells and also regulate bone metabolism, energy metabolism and glucose metabolism. Since BM adipocytes and osteoblasts are derived from the same precursor cells, both of which exhibit a push-pull relationship in most conditions. Nevertheless, some studies have also shown a positive correlation between adipocytes and osteoblasts. The reason for these contradictory results has not been studied in-depth. The available evidence have manifested MAT can provide energy for osteoblasts during exercise, while the hypothesis of MAT acting as an energy supplier has been doubted as MAT is increased during caloric restriction and HFD. Animal and human studies have found that MAT and IR are inextricably linked. We infer that DPP4 and TNF-α secreted by MAT may increase IR, yet adiponectin and leptin increase insulin sensitivity. Because of the opposite effects of these hormones, different studies have yielded different results. As reviewed herein, MAT may affect global metabolism as a novel endocrine organ. Future studies will be critical to gain insight into the role of MAT and its relationship to bone and global metabolism.
Author Contributions
XY designed this review. YL, YM, and XY wrote the manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 81770875, 81572639, 81370969 to XY, 81702156 to YM), Department of Science and Technology Department of Sichuan Province (2018SZ0142 to XY), Postdoctoral Science Foundation of China (No. 2017M61060 to YM), and Postdoctoral Research Foundation of Sichuan University (No. 2017SCU12038 to YM).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes. (2011) 2011:490650. doi: 10.1155/2011/490650
2. Sanchez-Gurmaches J, Hung CM, Guertin DA. Emerging complexities in adipocyte origins and identity. Trends Cell Biol. (2016) 26:313–26. doi: 10.1016/j.tcb.2016.01.004
3. Chu DT, Gawronska-Kozak B. Brown and brite adipocytes: same function, but different origin and response. Biochimie (2017) 138:102–5. doi: 10.1016/j.biochi.2017.04.017
4. Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes (2015) 64:2361–8. doi: 10.2337/db15-0227
5. Qian S, Huang H, Tang Q. Brown and beige fat: the metabolic function, induction, and therapeutic potential. Front Med. (2015) 9:162–72. doi: 10.1007/s11684-015-0382-2
6. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell (2012) 150:366–76. doi: 10.1016/j.cell.2012.05.016
7. Scheller EL, Burr AA, Macdougald OA, Cawthorn WP. Inside out: bone marrow adipose tissue as a source of circulating adiponectin. Adipocyte (2016) 5:251–69. doi: 10.1080/21623945.2016.1149269
8. Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. (2014) 20:368–75. doi: 10.1016/j.cmet.2014.06.003
9. Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. (2015) 3:141–7. doi: 10.1016/S2213-8587(14)70007-5
10. Paccou J, Hardouin P, Cotten A, Penel G, Cortet B. The role of bone marrow fat in skeletal health: usefulness and perspectives for clinicians. J Clin Endocrinol Metab. (2015) 100:3613–21. doi: 10.1210/jc.2015-2338
11. Cornish J, Wang T, Lin JM. Role of marrow adipocytes in regulation of energy metabolism and bone homeostasis. Curr Osteoporos Rep. (2018) 16:116–22. doi: 10.1007/s11914-018-0425-0
12. Wang H, Leng Y, Gong Y. Bone marrow fat and hematopoiesis. Front Endocrinol. (2018) 9:694. doi: 10.3389/fendo.2018.00694
13. Sulston RJ, Cawthorn WP. Bone marrow adipose tissue as an endocrine organ: close to the bone? Horm Mol Biol Clin Investig. (2016) 28:21–38. doi: 10.1515/hmbci-2016-0012
14. Scheller EL, Rosen CJ. What's the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. (2014) 1311:14–30. doi: 10.1111/nyas.12327
15. Hardouin P, Rharass T, Lucas S. Bone marrow adipose tissue: to be or not to be a typical adipose tissue? Front Endocrinol. (2016) 7:85. doi: 10.3389/fendo.2016.00085
16. Scheller EL, Troiano N, Vanhoutan JN, Bouxsein MA, Fretz JA, Xi Y, et al. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol. (2014) 537:123–39. doi: 10.1016/B978-0-12-411619-1.00007-0
17. Blebea JS, Houseni M, Torigian DA, Fan C, Mavi A, Zhuge Y, et al. Structural and functional imaging of normal bone marrow and evaluation of its age-related changes. Semin Nucl Med. (2007) 37:185–94. doi: 10.1053/j.semnuclmed.2007.01.002
18. Cawthorn WP, Scheller EL, Parlee SD, Pham HA, Learman BS, Redshaw CM, et al. Expansion of bone marrow adipose tissue during caloric restriction is associated with increased circulating glucocorticoids and not with hypoleptinemia. Endocrinology (2016) 157:508–21. doi: 10.1210/en.2015-1477
19. Drummond WB, Muir R. On the structure of the bone-marrow in relation to blood-formation. J Anat Physiol. (1893) 28:125–41.
20. Stockman R. The action of arsenic on the bone-marrow and blood. J Physiol. (1898) 23:376–82. doi: 10.1113/jphysiol.1898.sp000734
21. Bunting CH. The formation of true bone with cellular (red) marrow in a sclerotic aorta. J Exp Med. (1906) 8:365–76. doi: 10.1084/jem.8.3.365
23. Zakaria E, Shafrir E. Yellow bone marrow as adipose tissue. Proc Soc Exp Biol Med. (1967) 124:1265–8. doi: 10.3181/00379727-124-31983
24. Tavassoli M. Marrow adipose cells. Histochemical identification of labile and stable components. Arch Pathol Lab Med. (1976) 100:16–8.
25. Tavassoli M, Houchin DN, Jacobs P. Fatty acid composition of adipose cells in red and yellow marrow: a possible determinant of haematopoietic potential. Scand J Haematol. (1977) 18:47–53. doi: 10.1111/j.1600-0609.1977.tb01476.x
26. Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. (2015) 6:7808. doi: 10.1038/ncomms8808
27. Lanske B, Rosen C. Bone marrow adipose tissue: the first 40 years. J Bone Miner Res. (2017) 32:1153–6. doi: 10.1002/jbmr.3140
28. Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, Macdougald OA. Marrow adipose tissue: trimming the fat. Trends Endocrinol Metab. (2016) 27:392–403. doi: 10.1016/j.tem.2016.03.016
29. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. (2013) 19:1252–63. doi: 10.1038/nm.3361
30. Sulston RJ, Learman BS, Zhang B, Scheller EL, Parlee SD, Simon BR, et al. Increased circulating adiponectin in response to thiazolidinediones: investigating the role of bone marrow adipose tissue. Front Endocrinol. (2016) 7:128. doi: 10.3389/fendo.2016.00128
31. Pagnotti GM, Styner M. Exercise regulation of marrow adipose tissue. Front Endocrinol. (2016) 7:94. doi: 10.3389/fendo.2016.00094
32. Maredziak M, Smieszek A, Chrzastek K, Basinska K, Marycz K. Physical activity increases the total number of bone-marrow-derived mesenchymal stem cells, enhances their osteogenic potential, and inhibits their adipogenic properties. Stem Cells Int. (2015) 2015:379093. doi: 10.1155/2015/379093
33. Styner M, Pagnotti GM, Mcgrath C, Wu X, Sen B, Uzer G, et al. Exercise decreases marrow adipose tissue through ss-oxidation in obese running mice. J Bone Miner Res. (2017) 32:1692–702. doi: 10.1002/jbmr.3159
34. Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone (2012) 50:534–9. doi: 10.1016/j.bone.2011.06.032
35. Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone (2012) 50:546–52. doi: 10.1016/j.bone.2011.06.016
36. Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell (2017) 20:771–84. doi: 10.1016/j.stem.2017.02.009
37. Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, et al. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. (2012) 16:394–406. doi: 10.1016/j.cmet.2012.08.001
38. Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. (2006) 2:35–43. doi: 10.1038/ncprheum0070
39. Rodriguez JP, Astudillo P, Rios S, Pino AM. Involvement of adipogenic potential of human bone marrow mesenchymal stem cells (MSCs) in osteoporosis. Curr Stem Cell Res Ther. (2008) 3:208–18. doi: 10.2174/157488808785740325
40. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science (1997) 276:71–4. doi: 10.1126/science.276.5309.71
41. Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology (2005) 146:1226–35. doi: 10.1210/en.2004-0735
42. Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. (2004) 113:846–55. doi: 10.1172/JCI19900
43. Cho SW, Yang JY, Her SJ, Choi HJ, Jung JY, Sun HJ, et al. Osteoblast-targeted overexpression of PPARgamma inhibited bone mass gain in male mice and accelerated ovariectomy-induced bone loss in female mice. J Bone Miner Res. (2011) 26:1939–52. doi: 10.1002/jbmr.366
44. Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell (2004) 3:379–89. doi: 10.1111/j.1474-9728.2004.00127.x
45. Yu EW, Greenblatt L, Eajazi A, Torriani M, Bredella MA. Marrow adipose tissue composition in adults with morbid obesity. Bone (2017) 97:38–42. doi: 10.1016/j.bone.2016.12.018
46. Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. (2008) 19:1323–30. doi: 10.1007/s00198-008-0574-6
47. Cohen A, Dempster DW, Stein EM, Nickolas TL, Zhou H, Mcmahon DJ, et al. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. (2012) 97:2782–91. doi: 10.1210/jc.2012-1477
48. Limonard EJ, Veldhuis-Vlug AG, van Dussen L, Runge JH, Tanck MW, Endert E, et al. Short-term effect of estrogen on human bone marrow fat. J Bone Miner Res. (2015) 30:2058–66. doi: 10.1002/jbmr.2557
49. Trudel G, Payne M, Madler B, Ramachandran N, Lecompte M, Wade C, et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol. (1985\2009) 107:540–8. doi: 10.1152/japplphysiol.91530.2008
50. Ahdjoudj S, Lasmoles F, Holy X, Zerath E, Marie PJ. Transforming growth factor beta2 inhibits adipocyte differentiation induced by skeletal unloading in rat bone marrow stroma. J Bone Miner Res. (2002) 17:668–77. doi: 10.1359/jbmr.2002.17.4.668
51. Maddalozzo GF, Turner RT, Edwards CH, Howe KS, Widrick JJ, Rosen CJ, et al. Alcohol alters whole body composition, inhibits bone formation, and increases bone marrow adiposity in rats. Osteoporos Int. (2009) 20:1529–1538. doi: 10.1007/s00198-009-0836-y
52. Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK, et al. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. (2010) 25:298–304. doi: 10.1359/jbmr.090805
53. Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. (2009) 94:2129–36. doi: 10.1210/jc.2008-2532
54. Liu L, Aronson J, Lecka-Czernik B. Rosiglitazone disrupts endosteal bone formation during distraction osteogenesis by local adipocytic infiltration. Bone (2013) 52:247–58. doi: 10.1016/j.bone.2012.09.038
55. Tornvig L, Mosekilde LI, Justesen J, Falk E, Kassem M. Troglitazone treatment increases bone marrow adipose tissue volume but does not affect trabecular bone volume in mice. Calcif Tissue Int. (2001) 69:46–50. doi: 10.1007/s002230020018
56. Ackert-Bicknell CL, Shockley KR, Horton LG, Lecka-Czernik B, Churchill GA, Rosen CJ. Strain-specific effects of rosiglitazone on bone mass, body composition, and serum insulin-like growth factor-I. Endocrinology (2009) 150:1330–40. doi: 10.1210/en.2008-0936
57. Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, et al. Exercise regulation of marrow fat in the setting of PPARgamma agonist treatment in female C57BL/6 mice. Endocrinology (2015) 156:2753–61. doi: 10.1210/en.2015-1213
58. Liu L, Aronson J, Huang S, Lu Y, Czernik P, Rahman S, et al. Rosiglitazone inhibits bone regeneration and causes significant accumulation of fat at sites of new bone formation. Calcif Tissue Int. (2012) 91:139–48. doi: 10.1007/s00223-012-9623-4
59. Grey A, Beckley V, Doyle A, Fenwick S, Horne A, Gamble G, et al. Pioglitazone increases bone marrow fat in type 2 diabetes: results from a randomized controlled trial. Eur J Endocrinol. (2012) 166:1087–91. doi: 10.1530/EJE-11-1075
60. Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. (2010) 25:2078–88. doi: 10.1002/jbmr.82
61. Bornstein S, Moschetta M, Kawano Y, Sacco A, Huynh D, Brooks D, et al. Metformin affects cortical bone mass and marrow adiposity in diet-induced obesity in male mice. Endocrinology (2017) 158:3369–85. doi: 10.1210/en.2017-00299
62. Doucette CR, Horowitz MC, Berry R, Macdougald OA, Anunciado-Koza R, Koza RA, et al. A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J Cell Physiol. (2015) 230:2032–7. doi: 10.1002/jcp.24954
63. Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone (2014) 64:39–46. doi: 10.1016/j.bone.2014.03.044
64. Lecka-Czernik B, Stechschulte LA, Czernik PJ, Dowling AR. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol Cell Endocrinol. (2015) 410:35–41. doi: 10.1016/j.mce.2015.01.001
65. Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci USA. (2012) 109:3143–8. doi: 10.1073/pnas.1200797109
66. Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC, et al. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology (2004) 145:234–42. doi: 10.1210/en.2003-0899
67. Fairfield H, Falank C, Harris E, Demambro V, Mcdonald M, Pettitt JA, et al. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. (2018) 233:1156–67. doi: 10.1002/jcp.25976
68. Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem. (2002) 277:30998–1004. doi: 10.1074/jbc.M204527200
69. Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibanez G, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone (2012) 50:477–89. doi: 10.1016/j.bone.2011.08.010
70. Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, et al. Inhibition of adipogenesis by Wnt signaling. Science (2000) 289:950–3. doi: 10.1126/science.289.5481.950
71. Qiu W, Andersen TE, Bollerslev J, Mandrup S, Abdallah BM, Kassem M. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. (2007) 22:1720–31. doi: 10.1359/jbmr.070721
72. David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, et al. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology (2007) 148:2553–62. doi: 10.1210/en.2006-1704
73. Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology (2008) 149:6065–75. doi: 10.1210/en.2008-0687
74. Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem. (2009) 284:34607–17. doi: 10.1074/jbc.M109.039453
75. Sen B, Guilluy C, Xie Z, Case N, Styner M, Thomas J, et al. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells (2011) 29:1829–36. doi: 10.1002/stem.732
76. Jacobs FA, Sadie-Van GH, van de Vyver M, Ferris WF. Vanadate impedes adipogenesis in mesenchymal stem cells derived from different depots within bone. Front Endocrinol. (2016) 7:108. doi: 10.3389/fendo.2016.00108
77. Fan Y, Hanai JI, Le PT, Bi R, Maridas D, Demambro V, et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. (2017) 25:661–72. doi: 10.1016/j.cmet.2017.01.001
78. Yang Y, Luo X, Xie X, Yan F, Chen G, Zhao W, et al. Influences of teriparatide administration on marrow fat content in postmenopausal osteopenic women using MR spectroscopy. Climacteric (2016) 19:285–91. doi: 10.3109/13697137.2015.1126576
79. Clabaut A, Delplace S, Chauveau C, Hardouin P, Broux O. Human osteoblasts derived from mesenchymal stem cells express adipogenic markers upon coculture with bone marrow adipocytes. Differentiation (2010) 80:40–5. doi: 10.1016/j.diff.2010.04.004
80. Martin PJ, Haren N, Ghali O, Clabaut A, Chauveau C, Hardouin P, et al. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol. (2015) 16:10. doi: 10.1186/s12860-015-0057-5
81. Muller G, Schneider M, Biemer-Daub G, Wied S. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell Signal. (2011) 23:1207–23. doi: 10.1016/j.cellsig.2011.03.013
82. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. (2007) 9:654–9. doi: 10.1038/ncb1596
83. Lazar I, Clement E, Dauvillier S, Milhas D, Ducoux-Petit M, Legonidec S, et al. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: a novel mechanism linking obesity and cancer. Cancer Res. (2016) 76:4051–7. doi: 10.1158/0008-5472.CAN-16-0651
84. Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature (2017) 542:450–5. doi: 10.1038/nature21365
85. Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. (1996) 271:10697–703. doi: 10.1074/jbc.271.18.10697
86. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. (1995) 270:26746–9. doi: 10.1074/jbc.270.45.26746
87. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature (1998) 395:763–70. doi: 10.1038/27376
88. Laharrague P, Larrouy D, Fontanilles AM, Truel N, Campfield A, Tenenbaum R, et al. High expression of leptin by human bone marrow adipocytes in primary culture. FASEB J. (1998) 12:747–52. doi: 10.1096/fasebj.12.9.747
89. Fairfield H, Rosen CJ, Reagan MR. Connecting bone and fat: the potential role for sclerostin. Curr Mol Biol Rep. (2017) 3:114–21. doi: 10.1007/s40610-017-0057-7
90. Laharrague P, Truel N, Fontanilles AM, Corberand JX, Penicaud L, Casteilla L. Regulation by cytokines of leptin expression in human bone marrow adipocytes. Horm Metab Res. (2000) 32:381–5. doi: 10.1055/s-2007-978658
91. Ryden M, Dicker A, Gotherstrom C, Astrom G, Tammik C, Arner P, et al. Functional characterization of human mesenchymal stem cell-derived adipocytes. Biochem Biophys Res Commun. (2003) 311:391–7. doi: 10.1016/j.bbrc.2003.10.010
92. Corre J, Planat-Benard V, Corberand JX, Penicaud L, Casteilla L, Laharrague P. Human bone marrow adipocytes support complete myeloid and lymphoid differentiation from human CD34 cells. Br J Haematol. (2004) 127:344–7. doi: 10.1111/j.1365-2141.2004.05198.x
93. Belaid-Choucair Z, Lepelletier Y, Poncin G, Thiry A, Humblet C, Maachi M, et al. Human bone marrow adipocytes block granulopoiesis through neuropilin-1-induced granulocyte colony-stimulating factor inhibition. Stem Cells (2008) 26:1556–64. doi: 10.1634/stemcells.2008-0068
94. Uchihashi K, Aoki S, Shigematsu M, Kamochi N, Sonoda E, Soejima H, et al. Organotypic culture of human bone marrow adipose tissue. Pathol Int. (2010) 60:259–67. doi: 10.1111/j.1440-1827.2010.02511.x
95. Liu LF, Shen WJ, Ueno M, Patel S, Kraemer FB. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics (2011) 12:212. doi: 10.1186/1471-2164-12-212
96. Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell (2014) 15:154–68. doi: 10.1016/j.stem.2014.06.008
97. Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology (1999) 140:1630–8. doi: 10.1210/endo.140.4.6637
98. Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. (2005) 20:994–1001. doi: 10.1359/JBMR.050103
99. Lindenmaier LB, Philbrick KA, Branscum AJ, Kalra SP, Turner RT, Iwaniec UT. Hypothalamic leptin gene therapy reduces bone marrow adiposity in ob/ob mice fed regular and high-fat diets. Front Endocrinol. (2016) 7:110. doi: 10.3389/fendo.2016.00110
100. Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone (2004) 34:376–83. doi: 10.1016/j.bone.2003.11.020
101. Motyl KJ, Mccabe LR. Leptin treatment prevents type I diabetic marrow adiposity but not bone loss in mice. J Cell Physiol. (2009) 218:376–84. doi: 10.1002/jcp.21608
102. Devlin MJ, Brooks DJ, Conlon C, Vliet M, Louis L, Rosen CJ, et al. Daily leptin blunts marrow fat but does not impact bone mass in calorie-restricted mice. J Endocrinol. (2016) 229:295–306. doi: 10.1530/JOE-15-0473
103. Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell (2016) 18:782–96. doi: 10.1016/j.stem.2016.02.015
104. Scherer PE. Adiponectin: basic and clinical aspects. Preface Best Pract Res Clin Endocrinol Metab. (2014) 28:1–2. doi: 10.1016/j.beem.2013.11.004
105. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. (1999) 257:79–83. doi: 10.1006/bbrc.1999.0255
106. Delporte ML, Brichard SM, Hermans MP, Beguin C, Lambert M. Hyperadiponectinaemia in anorexia nervosa. Clin Endocrinol. (2003) 58:22–9. doi: 10.1046/j.1365-2265.2003.01702.x
107. Ugarte F, Ryser M, Thieme S, Fierro FA, Navratiel K, Bornhauser M, et al. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp Hematol. (2009) 37:867–75. doi: 10.1016/j.exphem.2009.03.007
108. Xiao L, Sobue T, Esliger A, Kronenberg MS, Coffin JD, Doetschman T, et al. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone (2010) 47:360–70. doi: 10.1016/j.bone.2010.05.021
109. Martella E, Bellotti C, Dozza B, Perrone S, Donati D, Lucarelli E. Secreted adiponectin as a marker to evaluate in vitro the adipogenic differentiation of human mesenchymal stromal cells. Cytotherapy (2014) 16:1476–85. doi: 10.1016/j.jcyt.2014.05.005
110. Poloni A, Maurizi G, Serrani F, Mancini S, Zingaretti MC, Frontini A, et al. Molecular and functional characterization of human bone marrow adipocytes. Exp Hematol. (2013) 41:558–66. doi: 10.1016/j.exphem.2013.02.005
111. Hozumi A, Osaki M, Sakamoto K, Goto H, Fukushima T, Baba H, et al. Dexamethasone-induced plasminogen activator inhibitor-1 expression in human primary bone marrow adipocytes. Biomed Res. (2010) 31:281–6. doi: 10.2220/biomedres.31.281
112. Sakamoto K, Osaki M, Hozumi A, Goto H, Fukushima T, Baba H, et al. Simvastatin suppresses dexamethasone-induced secretion of plasminogen activator inhibitor-1 in human bone marrow adipocytes. BMC Musculoskelet Disord. (2011) 12:82. doi: 10.1186/1471-2474-12-82
113. Tencerova M, Figeac F, Ditzel N, Taipaleenmaki H, Nielsen TK, Kassem M. High-fat diet-induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J Bone Miner Res. (2018) 33:1154–65. doi: 10.1002/jbmr.3408
114. Laharrague P, Fontanilles AM, Tkaczuk J, Corberand JX, Penicaud L, Casteilla L. Inflammatory/haematopoietic cytokine production by human bone marrow adipocytes. Eur Cytokine Netw. (2000) 11:634–9.
115. Zhang K, Wang C, Chen Y, Ji X, Chen X, Tian L, et al. Preservation of high-fat diet-induced femoral trabecular bone loss through genetic target of TNF-alpha. Endocrine (2015) 50:239–49. doi: 10.1007/s12020-015-0554-5
116. Wang C, Tian L, Zhang K, Chen Y, Chen X, Xie Y, et al. Interleukin-6 gene knockout antagonizes high-fat-induced trabecular bone loss. J Mol Endocrinol. (2016) 57:161–70. doi: 10.1530/JME-16-0076
117. Mirantes C, Passegue E, Pietras EM. Pro-inflammatory cytokines: emerging players regulating HSC function in normal and diseased hematopoiesis. Exp Cell Res. (2014) 329:248–54. doi: 10.1016/j.yexcr.2014.08.017
118. Goto H, Osaki M, Fukushima T, Sakamoto K, Hozumi A, Baba H, et al. Human bone marrow adipocytes support dexamethasone-induced osteoclast differentiation and function through RANKL expression. Biomed Res. (2011) 32:37–44. doi: 10.2220/biomedres.32.37
119. Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. (2014) 35:992–1019. doi: 10.1210/er.2014-1035
120. Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology (2001) 2:165–71. doi: 10.1023/A:1011513223894
121. Botolin S, Mccabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology (2007) 148:198–205. doi: 10.1210/en.2006-1006
122. Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab. (2008) 93:2281–6. doi: 10.1210/jc.2007-2691
123. Di Iorgi N, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. J Clin Endocrinol Metab. (2010) 95:2977–82. doi: 10.1210/jc.2009-2336
124. Shen W, Chen J, Gantz M, Punyanitya M, Heymsfield SB, Gallagher D, et al. MRI-measured pelvic bone marrow adipose tissue is inversely related to DXA-measured bone mineral in younger and older adults. Eur J Clin Nutr. (2012) 66:983–8. doi: 10.1038/ejcn.2012.35
125. Shen W, Velasquez G, Chen J, Jin Y, Heymsfield SB, Gallagher D, et al. Comparison of the relationship between bone marrow adipose tissue and volumetric bone mineral density in children and adults. J Clin Densitom. (2014) 17:163–9. doi: 10.1016/j.jocd.2013.02.009
126. Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. (2007) 18:641–7. doi: 10.1007/s00198-006-0285-9
127. Shen W, Scherzer R, Gantz M, Chen J, Punyanitya M, Lewis CE, et al. Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and Caucasian participants: the CARDIA study. J Clin Endocrinol Metab. (2012) 97:1337–46. doi: 10.1210/jc.2011-2605
128. L NA, J HL, Davis M, Casazza K. The relationships among total body fat, bone mineral content and bone marrow adipose tissue in early-pubertal girls. Bonekey Rep. (2013) 2:315. doi: 10.1038/bonekey.2013.49
129. Oury F, Yadav VK, Wang Y, Zhou B, Liu XS, Guo XE, et al. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes Dev. (2010) 24:2330–42. doi: 10.1101/gad.1977210
130. Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. (2010) 95:4795–801. doi: 10.1210/jc.2010-1030
131. Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell (2005) 122:803–15. doi: 10.1016/j.cell.2005.06.028
132. Fu L, Patel MS, Karsenty G. The circadian modulation of leptin-controlled bone formation. Prog Brain Res. (2006) 153:177–88. doi: 10.1016/S0079-6123(06)53010-9
133. Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature (1998) 393:72–6. doi: 10.1038/29993
134. Frost M, Abrahamsen B, Nielsen TL, Frystyk J, Flyvbjerg A, Hagen C, et al. Adiponectin and peak bone mass in men: a cross-sectional, population-based study. Calcif Tissue Int. (2010) 87:36–43. doi: 10.1007/s00223-010-9376-x
135. Okuno S, Ishimura E, Norimine K, Tsuboniwa N, Kagitani S, Yamakawa K, et al. Serum adiponectin and bone mineral density in male hemodialysis patients. Osteoporos Int. (2012) 23:2027–35. doi: 10.1007/s00198-011-1789-5
136. Tan CO, Battaglino RA, Doherty AL, Gupta R, Lazzari AA, Garshick E, et al. Adiponectin is associated with bone strength and fracture history in paralyzed men with spinal cord injury. Osteoporos Int. (2014) 25:2599–607. doi: 10.1007/s00198-014-2786-2
137. Sayers A, Timpson NJ, Sattar N, Deanfield J, Hingorani AD, Davey-Smith G, et al. Adiponectin and its association with bone mass accrual in childhood. J Bone Miner Res. (2010) 25:2212–20. doi: 10.1002/jbmr.116
138. Tenta R, Kontogianni MD, Yiannakouris N. Association between circulating levels of adiponectin and indices of bone mass and bone metabolism in middle-aged post-menopausal women. J Endocrinol Invest. (2012) 35:306–11. doi: 10.3275/7744
139. Tohidi M, Akbarzadeh S, Larijani B, Kalantarhormozi M, Ostovar A, Assadi M, et al. Omentin-1, visfatin and adiponectin levels in relation to bone mineral density in Iranian postmenopausal women. Bone (2012) 51:876–81. doi: 10.1016/j.bone.2012.08.117
140. Naot D, Watson M, Callon KE, Tuari D, Musson DS, Choi AJ, et al. Reduced bone density and cortical bone indices in female adiponectin-knockout mice. Endocrinology (2016) 157:3550–61. doi: 10.1210/en.2016-1059
141. Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, et al. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. (2006) 21:1648–56. doi: 10.1359/jbmr.060707
142. Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, et al. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab. (2013) 17:901–15. doi: 10.1016/j.cmet.2013.04.009
143. Lee HW, Kim SY, Kim AY, Lee EJ, Choi JY, Kim JB. Adiponectin stimulates osteoblast differentiation through induction of COX2 in mesenchymal progenitor cells. Stem Cells (2009) 27:2254–62. doi: 10.1002/stem.144
144. Yokota T, Meka CS, Medina KL, Igarashi H, Comp PC, Takahashi M, et al. Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins. J Clin Invest. (2002) 109:1303–10. doi: 10.1172/JCI14506
145. Tu Q, Zhang J, Dong LQ, Saunders E, Luo E, Tang J, et al. Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. J Biol Chem. (2011) 286:12542–53. doi: 10.1074/jbc.M110.152405
146. Muruganandan S, Dranse HJ, Rourke JL, Mcmullen NM, Sinal CJ. Chemerin neutralization blocks hematopoietic stem cell osteoclastogenesis. Stem Cells (2013) 31:2172–82. doi: 10.1002/stem.1450
147. Muruganandan S, Roman AA, Sinal CJ. Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J Bone Miner Res. (2010) 25:222–34. doi: 10.1359/jbmr.091106
148. Ramos-Junior ES, Leite GA, Carmo-Silva CC, Taira TM, Neves KB, Colon DF, et al. Adipokine chemerin bridges metabolic dyslipidemia and alveolar bone loss in mice. J Bone Miner Res. (2017) 32:974–84. doi: 10.1002/jbmr.3072
149. Muruganandan S, Govindarajan R, Sinal CJ. Bone marrow adipose tissue and skeletal health. Curr Osteoporos Rep. (2018) 16:434–42. doi: 10.1007/s11914-018-0451-y
150. Thommesen L, Stunes AK, Monjo M, Grosvik K, Tamburstuen MV, Kjobli E, et al. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J Cell Biochem. (2006) 99:824–34. doi: 10.1002/jcb.20915
151. Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, et al. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. (2014) 19:484–97. doi: 10.1016/j.cmet.2014.01.013
152. Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ, et al. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. (2007) 80:201–10. doi: 10.1007/s00223-006-0155-7
153. Wu SS, Liang QH, Liu Y, Cui RR, Yuan LQ, Liao EY. Omentin-1 stimulates human osteoblast proliferation through PI3K/Akt signal pathway. Int J Endocrinol. (2013) 2013:368970. doi: 10.1155/2013/368970
154. Rao SS, Hu Y, Xie PL, Cao J, Wang ZX, Liu JH, et al. Omentin-1 prevents inflammation-induced osteoporosis by downregulating the pro-inflammatory cytokines. Bone Res. (2018) 6:9. doi: 10.1038/s41413-018-0012-0
155. Lee WC, Guntur AR, Long F, Rosen CJ. Energy metabolism of the osteoblast: implications for osteoporosis. Endocr Rev. (2017) 38:255–66. doi: 10.1210/er.2017-00064
156. Frey JL, Li Z, Ellis JM, Zhang Q, Farber CR, Aja S, et al. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. (2015) 35:1979–91. doi: 10.1128/MCB.01343-14
157. Pino AM, Miranda M, Figueroa C, Rodriguez JP, Rosen CJ. Qualitative aspects of bone marrow adiposity in osteoporosis. Front Endocrinol. (2016) 7:139. doi: 10.3389/fendo.2016.00139
158. Covington JD, Noland RC, Hebert RC, Masinter BS, Smith SR, Rustan AC, et al. Perilipin 3 differentially regulates skeletal muscle lipid oxidation in active, sedentary, and type 2 diabetic males. J Clin Endocrinol Metab. (2015) 100:3683–92. doi: 10.1210/JC.2014-4125
159. Covington JD, Galgani JE, Moro C, Lagrange JM, Zhang Z, Rustan AC, et al. Skeletal muscle perilipin 3 and coatomer proteins are increased following exercise and are associated with fat oxidation. PLoS ONE (2014) 9:e91675. doi: 10.1371/journal.pone.0091675
160. de Araujo IM, Salmon CE, Nahas AK, Nogueira-Barbosa MH, Elias JJ, de Paula FJ. Marrow adipose tissue spectrum in obesity and type 2 diabetes mellitus. Eur J Endocrinol. (2017) 176:21–30. doi: 10.1530/EJE-16-0448
161. Bathija A, Davis S, Trubowitz S. Bone marrow adipose tissue: response to acute starvation. Am J Hematol. (1979) 6:191–8. doi: 10.1002/ajh.2830060303
162. Scheller EL, Khandaker S, Learman BS, Cawthorn WP, Anderson LM, Pham HA, et al. Bone marrow adipocytes resist lipolysis and remodeling in response to beta-adrenergic stimulation. Bone (2018) 118:32–41. doi: 10.1016/j.bone.2018.01.016
163. He J, Xu C, Kuang J, Liu Q, Jiang H, Mo L, et al. Thiazolidinediones attenuate lipolysis and ameliorate dexamethasone-induced insulin resistance. Metabolism (2015) 64:826–36. doi: 10.1016/j.metabol.2015.02.005
164. Kawai M, Rosen CJ. PPARgamma: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol. (2010) 6:629–36. doi: 10.1038/nrendo.2010.155
165. Kawai M, Sousa KM, Macdougald OA, Rosen CJ. The many facets of PPARgamma: novel insights for the skeleton. Am J Physiol Endocrinol Metab. (2010) 299:E3–9. doi: 10.1152/ajpendo.00157.2010
166. Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. (2006) 91:3349–54. doi: 10.1210/jc.2005-2226
167. Schwartz AV, Chen H, Ambrosius WT, Sood A, Josse RG, Bonds DE, et al. Effects of TZD use and discontinuation on fracture rates in ACCORD bone study. J Clin Endocrinol Metab. (2015) 100:4059–66. doi: 10.1210/jc.2015-1215
168. Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature (2009) 460:259–63. doi: 10.1038/nature08099
169. Botolin S, Mccabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol. (2006) 209:967–76. doi: 10.1002/jcp.20804
170. Czech MP, Tencerova M, Pedersen DJ, Aouadi M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia (2013) 56:949–64. doi: 10.1007/s00125-013-2869-1
171. de Paula FJ, de Araujo IM, Carvalho AL, Elias JJ, Salmon CE, Nogueira-Barbosa MH. The relationship of fat distribution and insulin resistance with lumbar spine bone mass in women. PLoS ONE (2015) 10:e129764. doi: 10.1371/journal.pone.0129764
172. Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, Mccabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology (2005) 146:3622–31. doi: 10.1210/en.2004-1677
173. Sheu Y, Amati F, Schwartz AV, Danielson ME, Li X, Boudreau R, et al. Vertebral bone marrow fat, bone mineral density and diabetes: the Osteoporotic Fractures in Men (MrOS) study. Bone (2017) 97:299–305. doi: 10.1016/j.bone.2017.02.001
174. Ghorpade DS, Ozcan L, Zheng Z, Nicoloro SM, Shen Y, Chen E, et al. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature (2018) 555:673–7. doi: 10.1038/nature26138
175. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (1993) 259:87–91. doi: 10.1126/science.7678183
176. Slade JM, Coe LM, Meyer RA, Mccabe LR. Human bone marrow adiposity is linked with serum lipid levels not T1-diabetes. J Diabetes Compl. (2012) 26:1–9. doi: 10.1016/j.jdiacomp.2011.11.001
177. Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. (2013) 28:1721–8. doi: 10.1002/jbmr.1950
178. Triantafyllou GA, Paschou SA, Mantzoros CS. Leptin and hormones: energy homeostasis. Endocrinol Metab Clin North Am. (2016) 45:633–45. doi: 10.1016/j.ecl.2016.04.012
179. Lee B, Shao J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord. (2014) 15:149–56. doi: 10.1007/s11154-013-9283-3
180. Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. (2000) 20:1595–99. doi: 10.1161/01.ATV.20.6.1595
181. Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. (2001) 86:1930–5. doi: 10.1210/jcem.86.5.7463
182. Pereira RI, Snell-Bergeon JK, Erickson C, Schauer IE, Bergman BC, Rewers M, et al. Adiponectin dysregulation and insulin resistance in type 1 diabetes. J Clin Endocrinol Metab. (2012) 97:E642–7. doi: 10.1210/jc.2011-2542
183. Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta (2013) 417:80–4. doi: 10.1016/j.cca.2012.12.007
Keywords: marrow adipose tissue, bone marrow, endocrine, bone mesenchymal stem cell, adipokine
Citation: Li Y, Meng Y and Yu X (2019) The Unique Metabolic Characteristics of Bone Marrow Adipose Tissue. Front. Endocrinol. 10:69. doi: 10.3389/fendo.2019.00069
Received: 15 June 2018; Accepted: 24 January 2019;
Published: 08 February 2019.
Edited by:
Basem M. Abdallah, University of Southern Denmark, DenmarkReviewed by:
Jan Josef Stepan, Charles University, CzechiaHan Qiao, Shanghai Jiao-Tong University School of Medicine, China
Copyright © 2019 Li, Meng and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xijie Yu, eGlqaWV5dUBob3RtYWlsLmNvbQ==;eGlqaWV5dUBzY3UuZWR1LmNu
 Yujue Li1
Yujue Li1 Xijie Yu
Xijie Yu