- 1Department of Otorhinolaryngology, Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 2Department of Otorhinolaryngology, Head and Neck Surgery, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
Background: Considerable modifications have been introduced in the new edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) TNM staging system. Based on the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database, this study aimed to compare the 7th and 8th editions of the AJCC/UICC TNM staging system for patients with papillary thyroid microcarcinoma (PTMC) and follicular variant papillary thyroid microcarcinoma (FVPTMC).
Methods: A Data from 2004 to 2014 of 39,032 patients registered in the SEER database were included. The 7th and 8th editions of the AJCC/UICC staging system were compared in terms of TNM staging, age cutoff, and clinical staging. Patient survival was evaluated using Kaplan-Meier and multivariable Cox proportional hazards models. The American Thyroid Association (ATA) risk stratification system was integrated with the AJCC/UICC staging system for further investigation. Receiver operating characteristic (ROC) curves, Harrell's C-index, Akaike information criterion (AIC), and the Bayesian information criterion (BIC) were used to assess the models' performances.
Results: Revised TNM categories, age cutoff, and clinical staging in the 8th edition resulted in reclassification of the overall stage. Applying the 8th edition, 1,278 stage III and 425 stage IV patients were reclassified as stage I; 950 stage III and 459 stage IV patients were reclassified as stage II; 77 stage IV patients were reclassified as stage III; and only 88 patients remained in stage IV. All patients in stage I, according to the 7th edition, remained in this stage when using the 8th edition. Patients classified into higher stages (III and IV) in the 8th edition showed a worse prognosis than those classified into same stages in the 7th edition. The 8th edition proved to be a better model with higher prognostic efficacy survival (higher AUC and C-index, lower AIC and BIC) than the 7th edition. When integrated with the ATA risk stratification system, the 8th edition still showed better discriminative power for patients in the higher risk group.
Conclusion: Based on the SEER database, the 8th edition of the AJCC/UICC staging system has better prognostic efficacy than the 7th edition for patients with PTMC and FVPTMC.
Introduction
Thyroid cancer is one the most common endocrine malignancies and its incidence is rapidly increasing (1, 2). Most patients are diagnosed with papillary thyroid carcinoma (PTC) and approximately half of cases are identified as papillary thyroid microcarcinoma (PTMC), most of whom are classified as typical PTMC or follicular variant papillary thyroid microcarcinoma (FVPTMC) (2, 3). According to the World Health Organization (WHO) classification, PTMCs are small thyroid tumors with a maximum diameter of 1 cm (4). Papillary thyroid microcarcinoma usually has favorable prognosis and is often regarded as an indolent malignancy; however, lymph node metastasis (LNM) or local and/or distant recurrence may occur in the minority of patients, even after surgery or radioactive iodine treatment, leading to worse prognosis (5, 6).
The American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) updated its tumor node metastasis (TNM) staging system and published the 8th edition in 2018, in which conspicuous changes were introduced, reflecting a better understanding of the clinicopathological factors combined with cancer-specific survival regarding thyroid cancer (7). The main changes include an increased age cutoff, the new definition of the T3 and N categories and the updated clinical staging definition (Supplementary Table 1). As a result, a large proportion of patients with advanced stages (stages III and IV), according to the 7th edition of the AJCC/UICC TNM staging system, are now classified into earlier stages (stages I and II) (7, 8).
To further investigate the impact of the new TNM staging system on PTMC and FVPTMC, and compare it with the previous 7th edition, we conducted a retrospective analysis using data from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program.
Materials and Methods
Data Source and Study Subjects
We conducted a retrospective cohort analysis using the SEER database from 2004 to 2014. Patients with confirmed histological diagnosis of papillary thyroid cancer (International Classification of Diseases for Oncology (ICD-O) code C73.9) were selected and the definition of papillary thyroid microcarcinoma relied on the tumor size code 001-010 (millimeters (mm) and 991 [described as “less than 1 centimeter (cm)”]. The histologic subtypes were selected as follows: 8050/3 (papillary carcinoma, NOS). 8260/3 (papillary adenocarcinoma, NOS), 8340/3 (papillary carcinoma, follicular variant), 8341/3 (papillary microcarcinoma), 8343/3 (papillary carcinoma, encapsulated).
Demographic data included sex, age at diagnosis, year of diagnosis and ethnicity. The cancer characteristics included tumor extension, lymph node metastasis, distant metastasis, and TNM/clinical stage in the 7th AJCC/UICC staging system. The TNM stage in the 8th AJCC/UICC staging system was defined according to the SEER data of tumor extension, lymph node metastasis, and distant metastasis (Supplementary Table 2). The clinical stage of patients was then defined according to the TNM stage in the 8th AJCC/UICC staging system. All variables were defined using the SEER specific codes. Stage T2 (in both editions) and T3a (in the 8th edition) were excluded as they do not meet the definition criteria of thyroid microcarcinoma (tumor size with <1 cm). Patients with unknown variables (code 999) whose stage could not be classified were excluded.
Although the American Thyroid Association (ATA) risk stratification system was designed to provide estimation of the risk of disease recurrence, it still includes several variables that may affect cancer-specific survival (9). Thus, we also integrated the ATA risk stratification system with the two editions of the AJCC/UICC system. We used the 2009 ATA risk stratification system, which partially differs from the most recent updated version, due to limitations in the available clinicopathological data in the SEER database (10).
Statistical Methods
Numerical data were expressed as mean ± standard deviation, and categorical data were expressed as percentages. The chi-square and Fisher's exact tests were used to evaluate the relationship between clinical characteristics. Survival was estimated using the Kaplan-Meier method and comparisons between groups were made using a log-rank test. The effects of potential predictors of overall survival (OS) and cancer-specific survival (CSS) were assessed using Cox proportional hazards regression and reported as hazard ratios (HRs) with 95% confident intervals (CIs). Receiver operating characteristic (ROC) curves and the Harrell's C concordance index (C-index), a method used for assessing the probability of concordance between expected and observed outcomes, was used to evaluate the prognostic efficacy of CSS of the 7th and 8th AJCC/UICC staging systems using stage-determinant variables (age, T, N, and M categories) (11). In order to measure the relative quality of the two editions of the AJCC/UICC staging system, we used the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). AIC and BIC served as standards to measure the quality of model fitness by providing asymptotically unbiased estimators between the true model and the fitted approximating model (12, 13). In summary, the model with higher C-index and lower AIC and BIC is considered to have better prognostic efficacy. A p-value lower than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and R statistical software v3.5.1 (The R Project for Statistical Computing, Vienna, Austria) with package Survival (14). ECharts 4.0 (Baidu Corp., Beijing, China), an open-source data visualization software, was used to draw the alluvial flow diagram.
Ethical Statement
This retrospective study used data from the SEER database, which is designed and maintained by the National Cancer Institute. The research was limited to the secondary use of previously collected information and data were anonymized before statistical analyses. The study was approved by the ethical review board of the Beijing Tongren Hospital, Capital Medical University, and complied with the ethical standards of the Declaration of Helsinki, as well as with relevant national and international guidelines.
Results
Demographic and Follow-Up Data
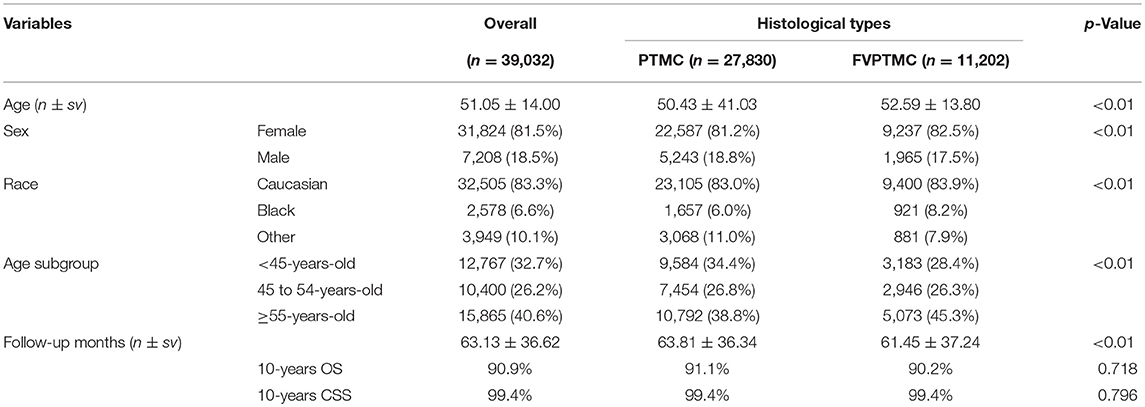
Our study involved 39,032 patients, out of whom 27,830 had PTMC and 11,202 had FVPTMC. All patients were staged according to the 7th AJCC/UICC staging system, which was compared to the recently published 8th edition. Detailed demographic data and follow-up information of patients are shown in Table 1. The 10-year OS of all patients was 90.9%, and the 10-year CSS was 99.4%. Patients with PTMC showed longer but not statistically different 10-year OS than those with FVPTMC (90.9 vs. 91.1%, p = 0.718), and showed same 10-year CSS with FVPTMC patients (both 99.4%, p = 0.796).
Changing of Patients' TNM Stages
The definition of TNM stages were modified in the 8th edition. Revision of the T category in the 8th edition resulted in reclassification of almost half of T3 categories (1,078/2,063, 52.3%) to T1a, due to the removal of minimal extrathyroidal extension to the perithyroidal tissue as a standard for the T3 category, in relation to the 7th edition. Thus, the proportion of T3 patients decreased from 5.3 to 2.5%, while the proportion of T1a patients increased from 94.1 to 97.1% in the 7th and 8th editions, respectively.
Based on the 7th edition, the change of the T categories resulted in increased hazard ratios (HRs) of OS and CSS, either in univariate or multivariate analyses. Exceptions were observed when comparing the HRs of T1a and T3, as no significant statistical differences were found in multivariate analyses of both OS and CSS, and univariate analysis of OS (but were found in univariate analysis of CSS). As for the 8th edition, similar results were observed, as increased T categories led to increased HRs (the same exceptions were found when comparing the HRs of OS and CSS between T1a and T3) (Table 2).
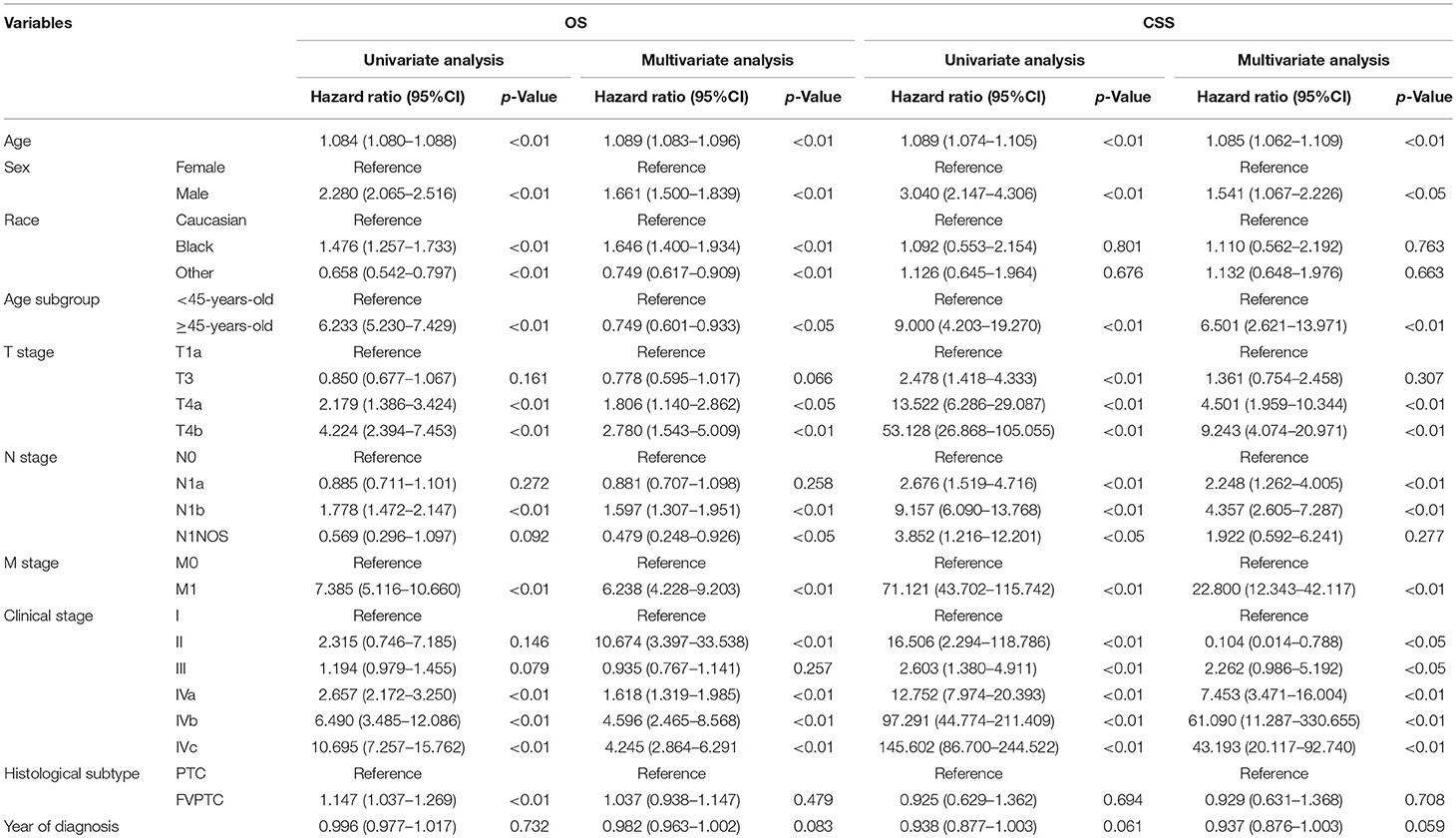
Table 2A. Univariate and multivariate analysis for patient survival according to the 7th AJCC/UICC staging system.
Out of 1532 patients classified as N1b according to the 7th edition, 154 (10.1%) patients were reclassified into the N1a stage according to the 8th edition, as level VII lymph node metastasis without lateral cervical lymph node metastasis is now classified as N1a. Therefore, the proportion of N1b patients decreased from 3.9 to 3.5%, while the proportion of N1a patients increased from 6.1 to 6.5%.
As shown in Table 2, according to both editions, significant differences in the HRs of OS were only observed between N0 and N1b patients, whereas no statistically significant difference was observed between N0 and N1a patients. Regarding CSS, increased N categories yielded dramatically increased HRs, both between N0 and N1a and between N0 and N1b patients.
Survival analysis revealed that older patients had worse prognosis than younger patients, either regarding OS or CSS. With an age cutoff of 45 years old in the 7th edition, older patients had a worse HR than younger patients (Table 2A). As the age cutoff changed to 55 years old in the 8th edition, up to 10,400 patients were moved to the younger group (<55 years old), nearly 15% of whom were in advanced clinical stages: 8,971 (86.3%) in stage I, 988 (9.5%) in stage III and 441 (4.2%) in stage IV. This age cutoff was still associated with OS and CSS, as significant differences in the HRs of OS and CSS were observed between the two age subgroups (<55 years old and ≥55 years old) (Table 2B).
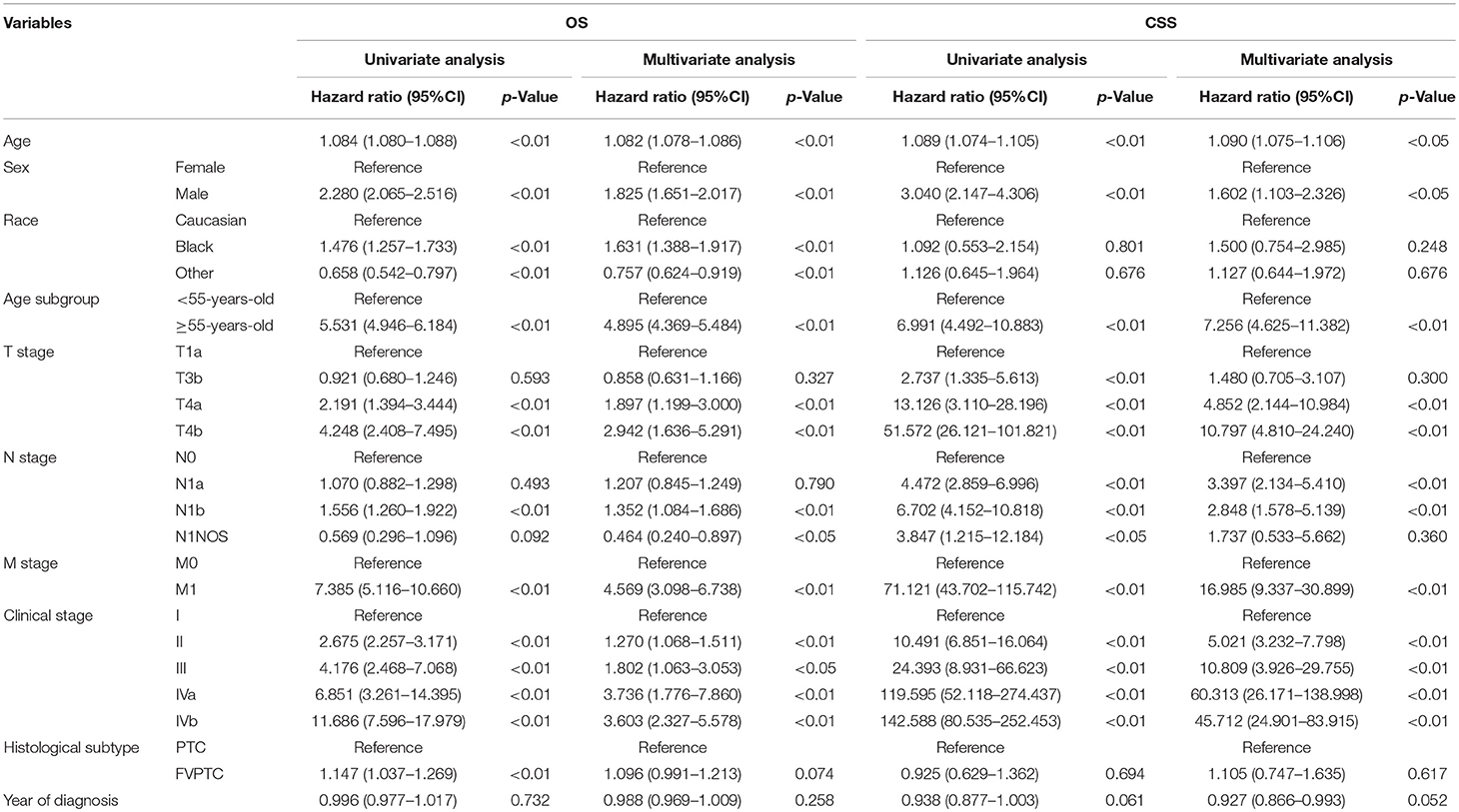
Table 2B. Univariate and multivariate analysis for patients' survival according to the 8th AJCC/UICC staging system.
Changing of Clinical Stages
Modification of the T and N categories and age cutoff led to conspicuous changes in the patients' clinical stage (Figure 1). The Kaplan-Meier survival curves showed a better separation of the stage curves in the 8th edition, when compared to the 7th edition (Figure 2). A better 10-year OS of patients with stage I in the 8th edition was observed, when compared to the same stage in the 7th edition, although patients with stages II, III, and IV in the 8th edition had worse 10-year OS. A similar pattern was seen for 10-year CSS in patients with stage III and IV in the 8th edition. However, patients with stage I and II in the 8th edition had better 10-year CSS (Table 3).
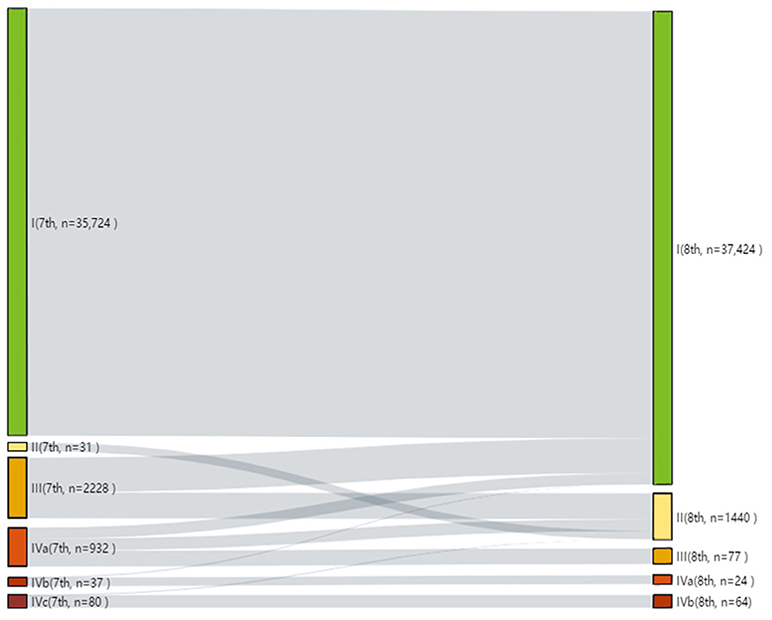
Figure 1. Alluvial flow diagram representing the re-staging of patient cohorts from the 7th to the 8th edition of the AJCC/UICC TNM staging system.
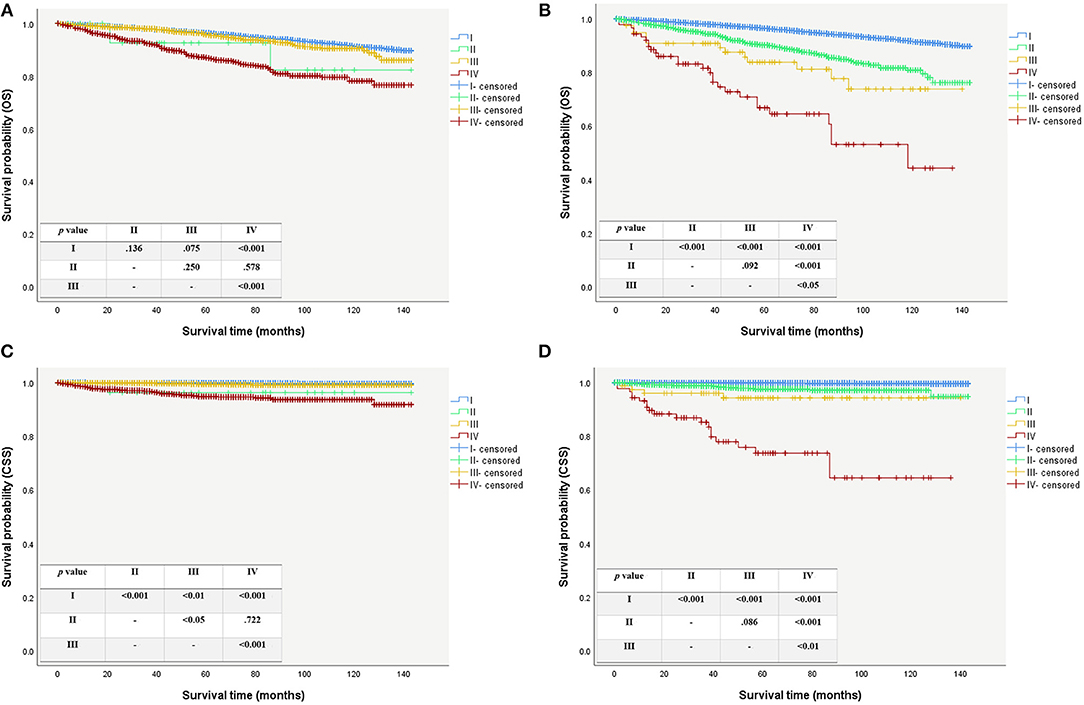
Figure 2. Survival curves for patient using the AJCC/UICC staging system. (A) Overall survival curves using the 7th edition. (B) Overall survival curves using the 8th edition. (C) Caner specific survival curves using the 7th edition. (D) Caner specific survival curves using the 8th edition.
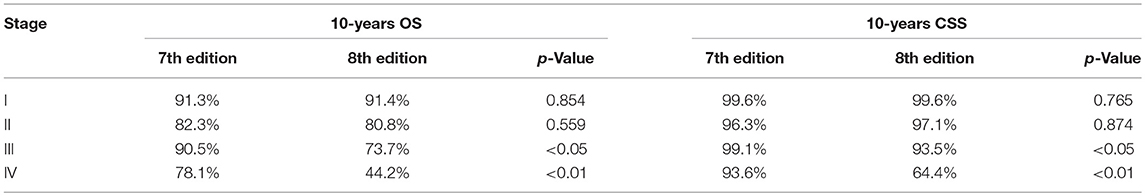
Table 3. Comparison of patient 10-years OS and CSS among clinical stages between the 7th and 8th edition.
When evaluating the prognostic efficacy of the two editions, the 8th edition showed higher AUC and C-index, as well as lower AIC and BIC, thus indicating a better model performance than the 7th edition (Figure 3).
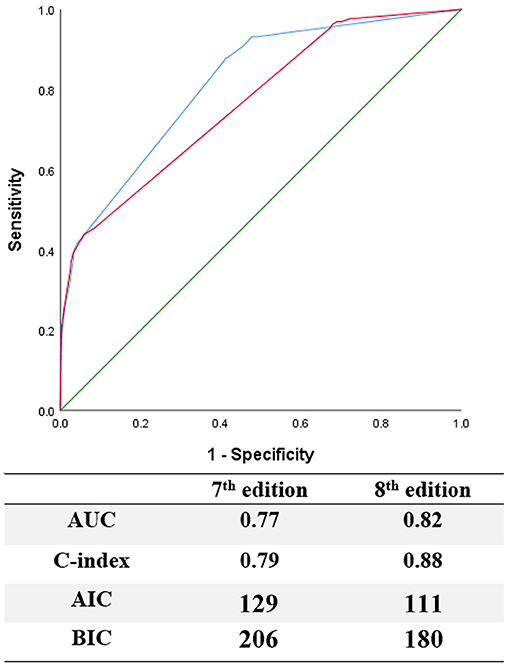
Figure 3. Evaluation of predictive performance between the 7th and 8th edition by using ROC curves, C-index, AIC, and BIC.
Integrating ATA Risk Stratification With 7th and 8th Edition
According to the ATA risk stratification system, 33,428 (85.6%, all of whom were stage I in both editions) were classified as low-risk, 4,301 (11.0%) as intermediate-risk, and 1,303 (3.3%) as high-risk. When the ATA system was integrated with the AJCC/UICC staging system, the risk stratification changed, as follows: the intermediate-risk group shifted in stage I from 1,916 (44.5%) to 3,303 (76.8%), in stage II from 0 (0%) to 998 (23.2%), in stage III and stage IV both from 1649 (38.3%) and 736 (17.1%) to 0 (0%), respectively; the high-risk group shifted in stage I from 380 (29.2%) to 696 (53.4%), in stage II from 31 (2.4%) to 442 (33.9%), in stage III from 579 (44.4%) to 77 (5.9%) and in stage IV from 313 (24.0%) to 88 (6.8%).
Even with the updated AJCC/UICC staging system, the composition of the low-risk group remained unchanged, and patients in this group had a 10-years CSS of 99.6%. The 10-years CSS decreased as patients' stage increased in the intermediate-risk group, in line with the changing trend of the high-risk group. The Kaplan-Meier survival curves still showed a better separation of the stage curves in the 8th edition, when compared to the 7th edition (Figure 4).
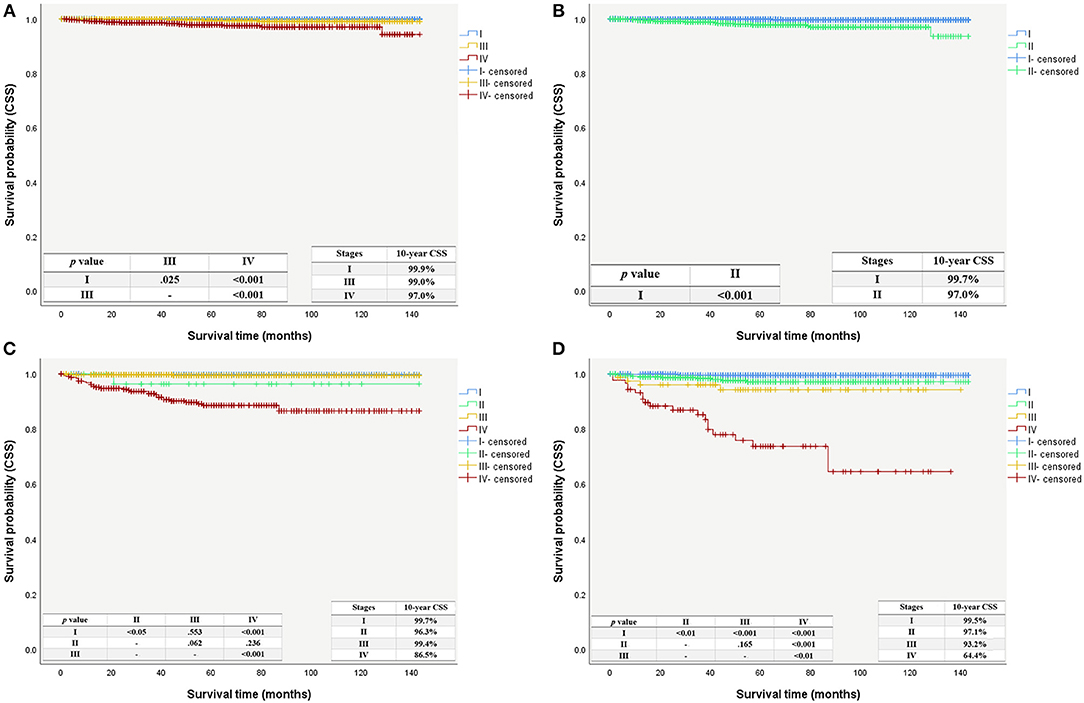
Figure 4. Cancer specific survival curves with patient using the AJCC/UICC staging system stratified by the ATA risk stratification system. (A) Cancer specific survival curves using the 7th edition stratified as intermediate-risk group. (B) Cancer specific survival curves using the 8th edition stratified as intermediate-risk group. (C) Cancer specific survival curves using the 7th edition stratified as high-risk group. (D) Cancer specific survival curves using the 8th edition stratified as high-risk group.
Discussion
The purpose of this study was to compare the survival values and the prognostic efficacy between the 7th and 8th editions of the AJCC/UICC staging system for papillary thyroid microcarcinoma using the SEER database.
Although many patients with papillary thyroid microcarcinoma have favorable prognosis, part of them have worse prognosis with tumor recurrence or distant metastasis. Kazaure et al. reported that some PTMC variants may contribute to worse prognosis (15). Typical PTMC, as well as FVPTMC were reported to have excellent prognosis (16–18). In this study, we assessed data of patients with PTMC and FVPTMC from the SEER database. Since the HRs in Cox regression analyses, 10-years OS and CSS were all found without significant differences between PTMC and FVPTMC, we combined the data of PTMC and FVPTMC together rather than severally in our subsequent analyses.
In the 7th edition, tumors with extrathyroidal extension (ETE) were classified as T3, regardless of the range of the extension into the perithyroidal tissue. Advanced T categories have been consistently reported as risk factors for OS and CSS (19–21), while the lack of association between T3 categories with minimal ETE with worse survival was controversial (21–23). Consequently, microscopic/minimal perithyroidal tissue extension is no longer considered as a criterion for defining T3 in the 8th edition. Considering the new edition, about half of patients with advanced T categories were reclassified as early T stages in the current study. Dispense with considering tumor size, all patients who were reclassified as T3b, according to the 8th edition, in this study had extrathyroidal extension to the strap muscle, and all patients with minimal ETE were reclassified as T1a rather than T3. On the other hand, no significant differences in HRs were found between T1a and T3b patients. Patients with tumor extension code 450 (i.e., minimal extra thyroid extension, including strap muscles) were classified as T1a, and those with tumor extension code 480 (i.e., extension to the pericapsular soft tissue/connective tissue) were classified as T3b in this study. Due to the uncertain definitions of tumor extension codes in the SEER database, these reclassifications might cause downstaging of patients with gross strap muscle extension into T1a or upstaging of patients with minimal pericapsular extension into T3b. As strap muscles can be resected en bloc with the thyroid gland during surgery, it is still lack of further investigation as to why especially strap muscle extension is considered to be a greater risk otherwise than microscopic perithyroidal tissue extension.
In our study, according to the 8th edition, the N1a category did not lead to worse OS when compared to the N0 category, while N1b did it. As for CSS, our results were in accordance with a previous study assessing 10,000 PTC patients using the SEER database, which found that both N1a and N1b led to an increased risk of cancer-related death (24). Regarding the N categories, the new edition of the AJCC/UICC staging system de-emphasized the risk of superior mediastinal lymph node (level VII) metastasis, when compared to the 7th edition, as there were no obvious anatomical boundaries between the superior mediastinal (level VII) and central cervical (level VI) lymph nodes. As reported by a number of studies, cervical lymph node metastases can impair CSS, especially in older patients; however, their impact seems to be weaker when compared to advanced T categories (T4a/b) or distant metastases (M1). Furthermore, although lateral cervical lymph node metastasis contributes to worse prognosis, it is also reported that other characteristics, such as the metastatic lymph node size, number, and extra-nodal extension, also influence prognosis and still remain to be deeply investigated (25, 26).
Age at diagnosis is an important factor for survival in virtually all thyroid cancer staging systems. This non-anatomic factor, as a dichotomous variable, has been combined with other anatomic factors for staging thyroid cancer. Older patients were distributed among very advanced stages and had poorer prognosis. The age cutoff of 45 years old was used as a categorical variable in the 7th edition of AJCC/UICC staging system; however, this cutoff has been challenged. Mazurat et al. suggested the cutoff of 55 years as a better indicator of cancer-specific death risk (27). Moreover, after assessing 9,484 patients in a multicenter study, Nixon et al. reported that the age cutoff of 55 years old improved both the outcome prediction according to different stages and prognostic information (28). Our results support these findings, as patients changed from advanced to earlier cancer stages after the age cutoff was changed, and no markedly decreased OS or CSS were observed. Of note, several studies have reported higher risk with age as a continuous variable, and suggested using nomograms or multiple age classifications, instead of a single age cutoff, to predict the patient's survival risk (29, 30).
In our study, no statistically significant differences in OS and CSS were seen between patients with stages I and II, irrespective of the AJCC/UICC edition. However, patients with stage III and IV in the 8th edition had worse OS and CSS than those with the same stages in the 7th edition. The discriminative power of both editions to reveal survival was compared and our data suggests that the 8th edition is a better model than the 7th edition, as also evidenced by recently published reports (31, 32).
When integrated with the ATA risk stratification system, major changes were observed in the intermediate-risk and high-risk groups. The 8th edition showed a more precise risk stratification, especially for the high-risk group, as the survival curves showed a better separation of stages. A recent study showed a higher risk of persistence or recurrence in patients with PTC in advanced stages in the 8th edition (33). A study focusing on younger (under 55 years old) patients also found a higher mortality in patients from the high-risk group with early stages (stages I and II) (34) after integrating the 8th AJCC/UICC staging system and the ATA risk stratification system. These findings add to the improvements of the 8th edition to discriminate patients better in higher risk group and may remind physicians that the decrease of stages does not reflect less disease aggressiveness. Not only staging but also risk stratification should be assessed when caring patients with PTMC. However, other risk factors for recurrence, such as 131I-avid metastatic foci in the neck on the first post-treatment whole-body radionuclide scan (intermediate risk), incomplete tumor resection (high risk) and detection of elevated postoperative serum thyroglobulin suggestive of distant metastases (high risk), were not analyzed in our study due to the lack of available data in the SEER database (10).
This study has some notable limitations. First, the study probably had a selection bias, as its study focused on patients with PTMC and FVPTMC, and most cases were early-stage tumors. As we did not use the updated SEER database (which include follow-up data from 2015), the follow-up period for part of the patients assessed here may not have been sufficient to observe recurrence or cancer-specific death. Continuing surveillance of these patients is still necessary. Potential coding errors may not be ruled out, although the SEER database is standardized and appropriately audited. Finally, the current study did not include treatment information, as well as disease recurrence and novel outcome predictors, such as molecular markers, that were not included in the SEER database. Despite of these limitations, this study assessed a large cohort with a relatively long follow-up period, which valorize its contributions to the evaluation and comparison of the prognostic efficacies of the 7th and 8th editions of the AJCC/UICC staging system.
Conclusion
In this study, we compared the prognostic values of the 7th and 8th versions of the AJCC/UICC staging system for patients with PTMC and FVPTMC and integrated them with the ATA risk stratification system. The 8th edition model provided a meaningful risk stratification and had a higher accuracy than the 7th edition, thus appearing to be superior to the 7th edition for evaluating patient survival.
Author Contributions
JF and ML contributed to the conception and design of the work. FY and QZ participated to data analysis and text editing. FY and ML participated to data collection. JF and ZH contributed to text revision.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was financially supported by Capital's Funds for Health Improvement and Research (CFH, 2018-2-2054, 2018-1-2052) and the Thyroid Research Program of Young and Middle-aged Physicians (2017-N-14) funded by China International Medical Foundation (CIMF).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00010/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. (2016) 375:1054–67. doi: 10.1056/NEJMra1501993
3. Liu Z, Zhao Q, Liu C, Zeng W, Ming J, Chen C, et al. Is biopsy enough for papillary thyroid microcarcinoma?: ananalysis of the SEER database 2004 to 2013 with propensity score matching. Medicine (2018) 97:e11791. doi: 10.1097/MD.0000000000011791
5. Leboulleux S, Tuttle RM, Pacini F, Schlumberger M. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance? Lancet Diabetes Endocrinol. (2016) 4:933–42. doi: 10.1016/S2213-8587(16)30180-2
6. Liu Z, Huang T. Papillary thyroid microcarcinoma and active surveillance. Lancet Diabetes Endocrinol. (2016) 4:974–5. doi: 10.1016/S2213-8587(16)30269-8
7. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer (2017).
8. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer (2010).
9. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26:1–133. doi: 10.1089/thy.2015.0020
10. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid (2010) 20:1341–9. doi: 10.1089/thy.2010.0178
11. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. (1996) 15:361–87.
12. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control (1974) 19:716–23.
14. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer (2000).
15. Kazaure HS, Roman SA, Sosa JA. Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Ann Surg Oncol. (2012) 19:1874–80. doi: 10.1245/s10434-011-2129-x
16. Kuo EJ, Goffredo P, Sosa JA, Roman SA. Aggressive variants of papillary thyroid microcarcinoma are associated with extrathyroidal spread and lymph-node metastases: a population-level analysis. Thyroid (2013) 23:1305–11. doi: 10.1089/thy.2012.0563
17. Jeon MJ, Kim WG, Choi YM, Kwon H, Lee YM, Sung TY, et al. Features predictive of distant metastasis in papillary thyroid microcarcinomas. Thyroid (2016) 26:161–8. doi: 10.1089/thy.2015.0375
18. Liu Z, Zeng W, Huang L, Wang Z, Wang M, Zhou L, et al. Prognosis of FTC compared to PTC and FVPTC: findings based on SEER database using propensity score matching analysis. Am J Cancer Res. (2018) 8:1440–8.
19. Fukushima M, Ito Y, Hirokawa M, Miya A, Shimizu K, Miyauchi A. Prognostic impact of extrathyroid extension and clinical lymph node metastasis in papillary thyroid carcinoma depend on carcinoma size. World J Surg. (2010) 34:3007–14. doi: 10.1007/s00268-010-0776-x
20. Radowsky JS, Howard RS, Burch HB, Stojadinovic A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid (2014) 24:241–4. doi: 10.1089/thy.2012.0567
21. Jin BJ, Kim MK, Ji YB, Song CM, Park JH, Tae K. Characteristics and significance of minimal and maximal extrathyroidal extension in papillary thyroid carcinoma. Oral Oncol. (2015) 51:759–63. doi: 10.1016/j.oraloncology.2015.05.010
22. Moon HJ, Kim EK, Chung WY, Yoon JH, Kwak JY. Minimal extrathyroidal extension in patients with papillary thyroid microcarcinoma: is it a real prognostic factor? Ann Surg Oncol. (2011) 18:1916–23. doi: 10.1245/s10434-011-1556-z
23. Woo CG, Sung CO, Choi YM, Kim WG, Kim TY, Shong YK, et al. Clinicopathological significance of minimal extrathyroid extension in solitary papillary thyroid carcinomas. Ann Surg Oncol. (2015) 22 (Suppl. 3):S728–33. doi: 10.1245/s10434-015-4659-0
24. Smith VA, Sessions RB, Lentsch EJ. Cervical lymph node metastasis and papillary thyroid carcinoma: does the compartment involved affect survival? Experience from the SEER database. J Surg Oncol. (2012) 106:357–62. doi: 10.1002/jso.23090
25. Nixon IJ, Wang LY, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery (2014) 156:137–46. doi: 10.1016/j.surg.2014.03.027
26. Wada N, Nakayama H, Suganuma N, Masudo Y, Rino Y, Masuda M, et al. Prognostic value of the sixth edition AJCC/UICC TNM classification for differentiated thyroid carcinoma with extrathyroid extension. J Clin Endocrinol Metab. (2007) 92:215–8. doi: 10.1210/jc.2006-1443
27. Mazurat A, Torroni A, Hendrickson-Rebizant J, Benning H, Nason RW, Pathak KA. The age factor in survival of a population cohort of well-differentiated thyroid cancer. Endocr Connect. (2013) 2:154–60. doi: 10.1530/EC-13-0056
28. Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, et al. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid (2016) 26:373–80. doi: 10.1089/thy.2015.0315
29. Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, et al. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid (2015) 25:1106–14. doi: 10.1089/thy.2015.0104
30. Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. Exploring the relationship between patient age and cancer specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol. (2016) 34:4415–20. doi: 10.1200/jco.2016.68.9372
31. van Velsen EFS, Stegenga MT, van Kemenade FJ, Kam BLR, van Ginhoven TM, Visser WE, et al. Comparing the prognostic value of the Eighth Edition of the American Joint Committee on Cancer/Tumor Node Metastasis Staging System between papillary and follicular thyroid cancer. Thyroid (2018) 28:976–81. doi: 10.1089/thy.2018.0066
32. Kim TH, Kim YN, Kim HI, Park SY, Choe JH, Kim JH, et al. Prognostic value of the eighth edition AJCC TNM classification for differentiated thyroid carcinoma. Oral Oncol. (2017) 71:81–6. doi: 10.1016/j.oraloncology.2017.06.004
33. Shteinshnaider M, Muallem Kalmovich L, Koren S, Or K, Cantrell D, Benbassat C. Reassessment of differentiated thyroid cancer patients using the eighth TNM/AJCC classification system: a comparative study. Thyroid (2018) 28:201–9. doi: 10.1089/thy.2017.0265
34. Ghaznavi SA, Ganly I, Shaha AR, English C, Wills J, Tuttle RM. Using the American Thyroid Association Risk-Stratification System to refine and individualize the American Joint Committee on Cancer Eighth Edition disease-specific survival estimates in differentiated thyroid cancer. Thyroid (2018) 28:1293–300. doi: 10.1089/thy.2018.0186
Keywords: papillary thyroid microcarcinoma, AJCC/UICC staging system, surveillance, Epidemiology and End Results, survival, prognostic efficacy
Citation: Yang F, Zhong Q, Huang Z, Lian M and Fang J (2019) Survival in Papillary Thyroid Microcarcinoma: A Comparative Analysis Between the 7th and 8th Versions of the AJCC/UICC Staging System Based on the SEER Database. Front. Endocrinol. 10:10. doi: 10.3389/fendo.2019.00010
Received: 17 November 2018; Accepted: 09 January 2019;
Published: 24 January 2019.
Edited by:
Wen Zhou, Case Western Reserve University, United StatesReviewed by:
Amit Tirosh, Sheba Medical Center, IsraelPasqualino Malandrino, University of Catania, Italy
Copyright © 2019 Yang, Zhong, Huang, Lian and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Lian, bGlhbm1lbmcxOTg2MTIyMkAxNjMuY29t
Jugao Fang, ZmFuZ2p1Z2FvQDE2My5jb20=
 Fan Yang
Fan Yang Qi Zhong1
Qi Zhong1