- 1Department of Geriatric Cardiology, Chinese People's Liberation Army General Hospital, Beijing, China
- 2Department of Cardiology and Hainan Branch, Chinese People's Liberation Army General Hospital, Beijing, China
- 3Institute of Geriatrics, Chinese People's Liberation Army General Hospital, Beijing, China
- 4Beijing Key Laboratory of Geriatrics, Chinese People's Liberation Army General Hospital, Beijing, China
- 5Central Laboratory, Hainan Branch of Chinese People's Liberation Army General Hospital, Sanya, China
As the first time worldwide, this study aimed to investigate the relationships of hyperhomocysteinemia and hyperuricemia with metabolic syndrome (MetS) and renal function in Chinese centenarians. The China Hainan Centenarian Cohort Study was performed in 18 cities and counties of the Hainan Province. Home interview, physical examination, and blood analysis were performed on 808 centenarians following standard procedures. All centenarians had a median age of 102 (100–115) years. Prevalence of hyperhomocysteinemia and hyperuricemia was 91.6% (740 centenarians) and 28.5% (230 centenarians), respectively. The MetS was present in 117 centenarians (14.5%). In simple correlation analyses, hyperhomocysteinemia and hyperuricemia were significantly correlated with MetS and glomerular filtration rate (GFR) < 60 ml/min/1.73 m2 (P < 0.05 for all). Serum homocysteine levels were significantly correlated with GFR, waist circumference (WC), and triglyceride levels, while serum uric acid levels were significantly correlated with these variables plus high-density lipoprotein cholesterol (HDL-C) levels (P < 0.05 for all). In logistic regression analyses, hyperhomocysteinemia and hyperuricemia were significantly associated with MetS and GFR < 60 ml/min/1.73 m2 (P < 0.05 for all). In linear regression analyses, serum homocysteine levels were significantly associated with GFR, WC, and triglyceride, while serum uric acid levels were significantly associated with these variables plus HDL-C (P < 0.05 for all). Both hyperhomocysteinemia and hyperuricemia had important relationships with MetS and renal function in Chinese centenarians. Hyperuricemia and hyperhomocysteinemia that could help identify, while also affecting, the development of MetS and renal function may unfold complex relationships between MetS, renal function, and cardiovascular risk and provide effective prevention strategies for these conditions.
Introduction
As a complex pathophysiological entity, metabolic syndrome (MetS) comprises a number of metabolic abnormalities and is characterized by insulin resistance (1). The MetS has been estimated to occur in between 20 and 25% of the adult population and suggested as a leading cause of cardiovascular diseases and mortality (2–4). Homocysteine, a thiol-containing amino acid produced during the conversion of methionine to cysteine, has been found to correlate with insulin resistance and MetS in previous studies (5–8). However, it is being debated whether these correlations exist or do not persist, thus, making it a contentious issue in the literature (9–11). Meanwhile, there is growing interest in the relationship between hyperuricemia and MetS, which has been investigated previously in different populations, but with conflicting results (12, 13). In addition to MetS, renal function decline is also an important worldwide public health problem and has gained much attention in recent years (14). Epidemiological studies have realized a relationship between hyperuricemia and renal function (15–17). However, these studies have shown disputable results and it is, therefore, difficult to draw definite conclusions (18). Meanwhile, studies have also reported that hyperhomocysteinemia is an important indicator for renal function (19, 20). There is also, however, controversial and limited evidence about hyperhomocysteinemia and renal function in different populations (21).
The centenarians have been suggested to have delayed or escaped onset and interaction of age-related illnesses, such as hyperuricemia, hyperhomocysteinemia, MetS, and renal function decline (22). Some centenarians may experience delayed onset of age-related illnesses (delayers), while others may not succumb to any age-related illnesses (escapers) (23). Thus, the centenarians may represent a prototype of successful aging (24). However, this trend is still under scientific debate (25). More importantly, what is this model of successful aging? Studies analyzing this model in the centenarians could provide valuable information for early promotion of successful aging and prevention of age-related diseases. As a possible part of this model, whether the relationships of hyperuricemia and hyperhomocysteinemia with MetS and renal function decline exist in the aging process of centenarians is still unclear and needs further studies. Most of the current understandings about these relationships are derived from studies on general adults and the elderly population from Caucasians of European origin (26). It is well recognized that age and ethnicity may have a remarkable effect on these relationships (27). Specifically, the senile population and Asian adults are recognized to be more susceptible to adverse effects of MetS (28). To our knowledge, hardly any studies have confirmed these relationships in centenarians, especially in China. It is interesting to analyze these relationships in Chinese centenarians. Hainan is an area where longevity is high, with the highest population density of centenarians in China. The China Hainan Centenarian Cohort Study (CHCCS), with a considerable sample size, provides a significant population-based sample of Chinese centenarians.
As it is the first in the world, the current study aims to investigate the relationships of hyperhomocysteinemia and hyperuricemia with MetS and renal function in Chinese centenarians.
Methods
Study Population
As a population-based study, the CHCCS was carried out in 18 cities and counties of the Hainan Province, China. Its cohort profile has been described previously (29). It started in July 2014 and ended in December 2016. Based on the National Civil Registry, there were 1,002 centenarians (at least 100 years of age) identified by the Hainan Civil Affairs Bureau and ssurveyed in the current study. Age was ascertained from national identification cards. The following inclusion criteria were used to recruit study participants: (1) were 100 years or older; (2) had volunteered to participate in the study and provided written informed consent; and (3) were conscious and could cooperate to complete the home interview, physical examination, and blood analysis. The following were participant exclusion criteria: (1) personal identity information was not complete or identification cards showed an age < 100 years; and (2) refused to comply with the requirements of the study, including the collection of physical information or blood samples. There were 808 centenarians included in the final analysis. All centenarians had no certain malignancies and received no agent that obviously changed serum homocysteine and uric acid levels. The Ethics Committee of Hainan branch of Chinese People's Liberation Army General Hospital (Sanya, Hainan) has approved of the current study (Number: 301hn11201601), and all centenarians gave written informed consent upon their entry.
Standard Procedures
Home interview, physical examination, and blood analysis were performed following standard procedures (30). The research team included internists, geriatricians, cardiologists, endocrinologists, nephrologists, and nurses. Waist circumference (WC) was measured with a soft tape in the middle point of the lowest rib and iliac crest. After resting in supine position for 5 min, systolic and diastolic blood pressures (SBP and DBP) were measured twice on the right arm of the centenarians with a 1-min interval. The average of these measurements was used for the analysis. Samples of venous blood were drawn from the centenarians and transported in chilled bio-transport containers (4°C) to our Central Laboratory within 4 h. Serum concentrations of triglyceride, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), and creatinine were tested using the enzymatic assays (Roche Products Ltd, Basel, Switzerland) on a fully automatic biochemical autoanalyzer (Cobas c702; Roche Products Ltd., Basel, Switzerland). All assays were performed by qualified technicians without knowledge of clinical data.
Variable Definitions
According to the worldwide consensus of the International Diabetes Federation (31), the centenarians with WC ≥85 cm in males and ≥80 cm in females along with any two or more of the following abnormalities were considered to have MetS: (1) SBP ≥130 mmHg or DBP ≥85 mmHg (or use of blood pressure-lowering agents); (2) FBG ≥5.6 mmol/L (or use of glucose-lowering agents); (3) triglyceride ≥1.7 mmol/L (or use of lipid-regulating agents); and (4) HDL-C < 1.0 mmol/L in males and < 1.3 mmol/L in females. Estimated glomerular filtration rate (GFR) was calculated using a modified version of the Modification of Diet in Renal Disease (MDRD) equation based on the data from Chinese population as follows: 175 × serum creatinine (mg/dL)−1.234 × age (year)−0.179 × 0.79 (if female) (32). Hyperhomocysteinemia was diagnosed as serum homocysteine levels above 15 umol/L. Hyperuricemia was diagnosed as serum uric acid levels above 420 umol/L in males or 350 umol/L in females.
Statistical Analyses
To describe the variables of Chinese centenarians, mean (standard deviation), median (interquartile range) and number (percentage) were applied for normally distributed continuous variables, non-normally distributed continuous variables, and categorical variables, respectively. Pearson's (normally distributed continuous variables) and Spearman's (non-normally distributed continuous variables and categorical variables) correlations were applied to evaluate their simple correlations with serum homocysteine (hyperhomocysteinemia) and uric acid (hyperuricemia) levels. Logistic regression analyses were applied to evaluate the associations of hyperhomocysteinemia and hyperuricemia with GFR < 60 ml/min/1.73 m2 after adjusting for age, sex, WC, SBP, DBP, triglyceride, HDL-C, LDL-C, and FBG, and with MetS after adjusting for age and sex. With the same adjustment, linear regression analyses were applied to evaluate the associations of serum homocysteine and uric acid levels with GFR and all features of MetS. Two-tailed tests were adopted throughout, considering significant P < 0.05. The Statistical Package for Social Science (SPSS) version 17 was applied to carry out all statistical analyses (SPSS Inc., Chicago, IL, United States).
Results
As shown in Table 1, the current study included 808 centenarians with a median age of 102 (100-115) years. It consisted of 155 men (19.2%) and 653 women (80.8%). Prevalence of hyperhomocysteinemia and hyperuricemia was 91.6% (740 centenarians) and 28.5% (230 centenarians), respectively. The MetS was presented in 117 centenarians (14.5%). In simple correlation analyses, hyperhomocysteinemia and hyperuricemia were significantly correlated with MetS and GFR < 60 ml/min/1.73 m2 (P < 0.05 for all).. Serum homocysteine levels were significantly correlated with GFR, WC, and triglyceride levels, while serum uric acid levels were significantly correlated with these variables plus HDL-C levels (P < 0.05 for all). In logistic regression analyses (Table 2), hyperhomocysteinemia and hyperuricemia were significantly associated with MetS and GFR < 60 ml/min/1.73 m2 (P < 0.05 for all). In linear regression analyses (Table 3), serum homocysteine levels were significantly associated with GFR, WC, and triglyceride, while serum uric acid levels were significantly associated with these variables plus HDL-C (P < 0.05 for all).
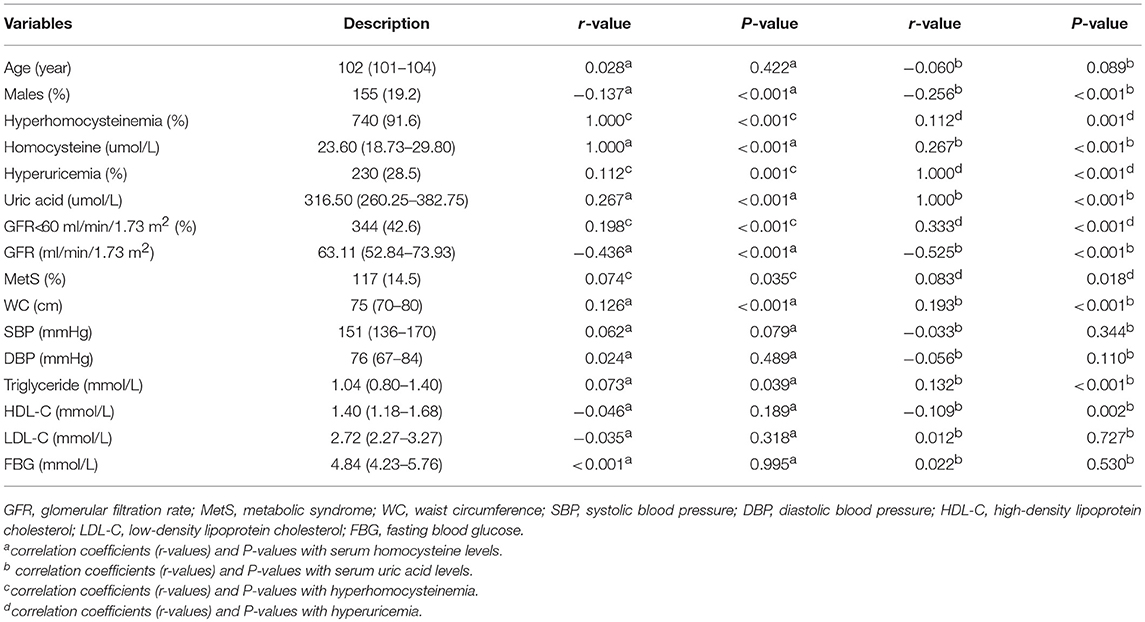
Table 1. Variables of Chinese centenarians and their correlations with serum homocysteine (hyperhomocysteinemia) and uric acid (hyperuricemia) levels.
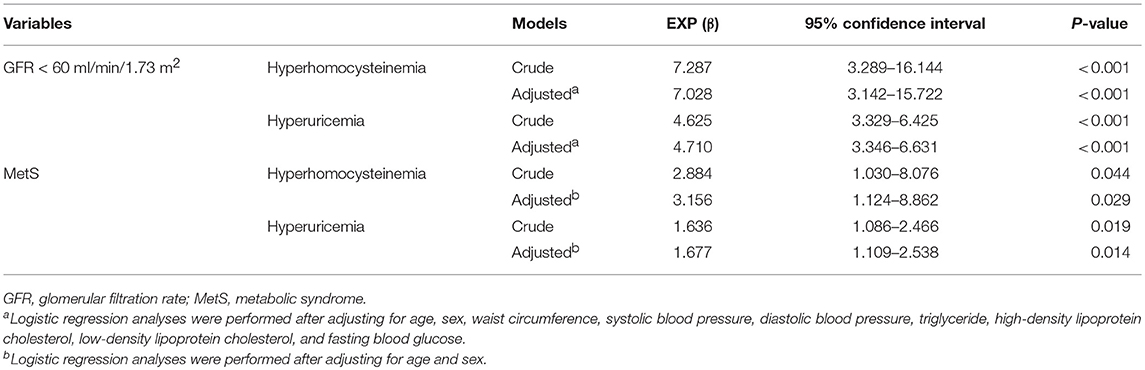
Table 2. Associations of hyperhomocysteinemia and hyperuricemia with GFR < 60 ml/min/1.73 m2 and MetS in Chinese centenarians.
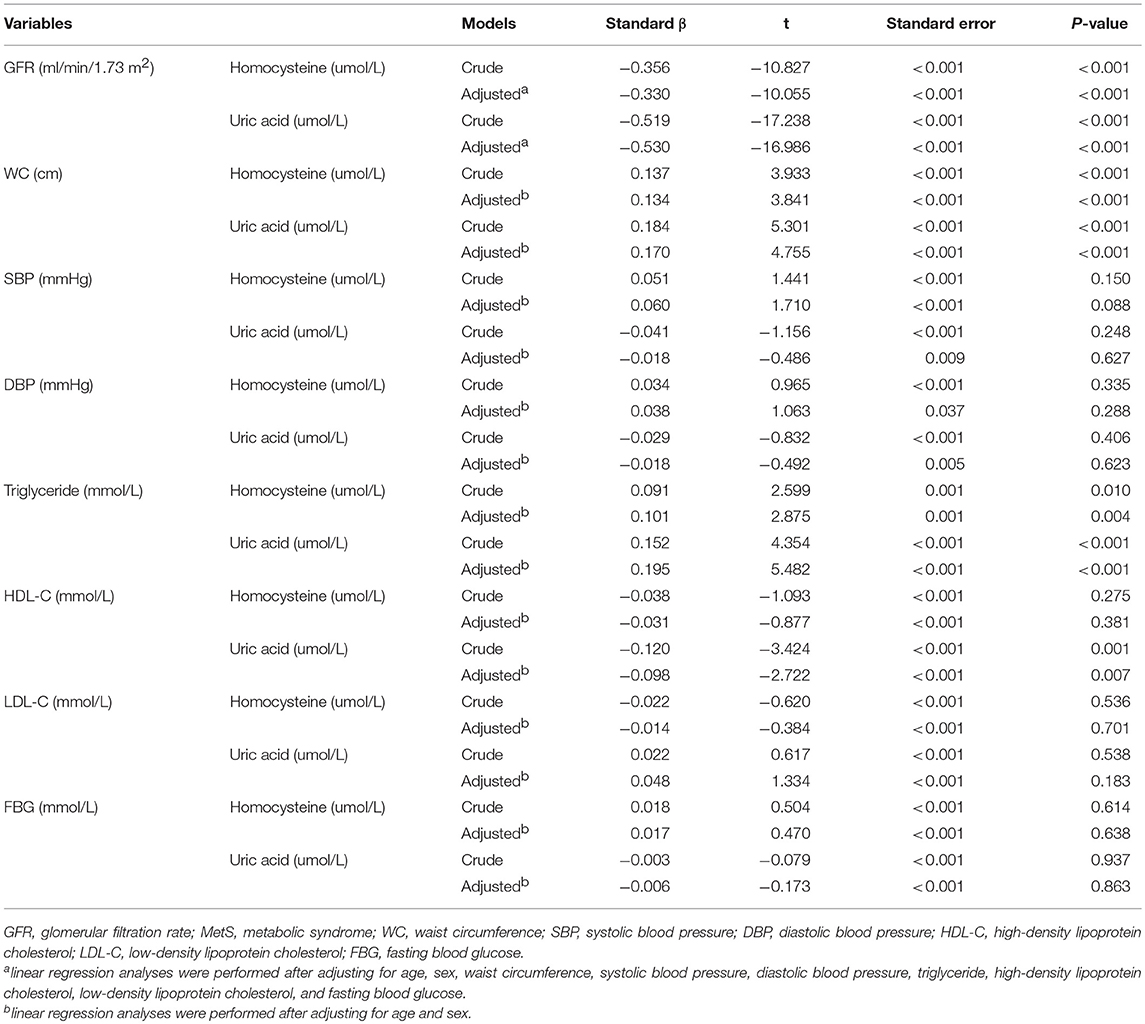
Table 3. Associations of serum homocysteine and uric acid levels with GFR and all features of MetS in Chinese centenarians.
Discussion
The MetS is clinically important as it causes a three- to four-fold increased incidence of cardiovascular diseases and worsens survival prognosis by increasing cardiovascular events and mortality (1–4). About one-fifth of the adult population in the United States had high cardiovascular risk, with the prevalence of MetS being estimated at 22.9% (2). Homocysteine is a thiol-containing amino acid that is formed during the conversion of methionine to cysteine (5, 6). As studies have suggested, serum homocysteine levels have been related to MetS in general adults and the elderly population (7, 8). Nevertheless, the relationship between hyperhomocysteinemia and MetS has been a matter of debate (9–11). Moreover, only a limited number of studies have discussed this relationship in China, not to say in Chinese centenarians. The current study indicated a tight connection between hyperhomocysteinemia and MetS, and identified WC and triglyceride as the factors that linked MetS to serum homocysteine levels in Chinese centenarians. Hyperhomocysteinemia may thus, turn out to be a good marker in the development of MetS. Several mechanisms may explain their relationship, and hyperhomocysteinemia may be the cause and/or the consequence of insulin resistance (10). Insulin resistance has been proposed as the main regulator of serum homocysteine levels (33). In previous animal experiments, hyperhomocysteinemia has been found to be a result of hyperinsulinemia (34). Cystathionine-b-synthase, the key enzyme of transsulfuration pathway in homocysteine metabolism, is downregulated in an insulin-resistant state (35). At the same time, elevated levels of serum homocysteine may result in insulin resistance through an inhibition of insulin-receptor kinase activity in vitro (36). Hyperhomocysteinemia has been reported before the onset of MetS, and may be the initial signature before the development of MetS (37).
The relationship between hyperuricemia and MetS has been studied before; however, it is yet to be studied in the centenarians, especially in China. Moreover, this relationship remains a debatable topic to be studied in general populations (12, 13). In the current study, hyperuricemia was associated with MetS through WC, triglyceride, and HDL-C. The underlying mechanism is still not well understood, though insulin resistance is suspected to be the mechanism interlinking hyperuricemia with the development of MetS (13). A previous study has proposed that uric acid-mediated upregulation of the adipose renin–angiotensin system may cause insulin resistance (38). Uric acid may also directly contribute to the development of insulin resistance in adipose tissue, possibly through redox modulation or adiponectin (39). Hyperuricemia may indicate MetS, which itself is associated with cardiovascular risk (40). Furthermore, lowering serum levels of uric acid may be a useful strategy for reducing MetS and cardiovascular burden (13).
As a subject that has been previously studied, the relationship between hyperuricemia and renal function has not been confirmed in the centenarians, especially in China (15). Even in general population, previous studies have pointed out disputable results about this relationship (16–18). The current study supported that hyperuricemia significantly interacted with renal function, mediated by WC, triglyceride, and HDL-C. Uric acid, which is a final product of purine metabolism, is mainly eliminated in the urine. Elevated levels of serum uric acid have been considered to be a marker for renal function (41). Furthermore, hyperuricemia may affect renal function through an induction of endothelial dysfunction, inflammatory reaction, and oxidative stress (42, 43). This effect occurs mainly because uric acid stimulates the renin–angiotensin system and inhibits vascular nitric oxide synthesis (44). Experimental studies have also shown that hyperuricemia induces the development of glomerular arteriolopathy that impairs renal autoregulation and causes glomerular hypertension, leading eventually to glomerulosclerosis and interstitial fibrosis (45). It will probably be necessary to control uric acid levels so as to improve renal function.
In previous studies, hyperhomocysteinemia has been found to have a positive correlation with renal function (19). However, the number of studies analyzing this correlation is limited and their results are contradicting, especially in Chinese centenarians (20). The current study illustrated that hyperhomocysteinemia was significantly associated with renal function through WC and triglyceride. Renal function decline may not only reduce renal extraction of homocysteine due to decreasing plasma flow, but also affect homocysteine metabolism through homocysteine remethylation, one of the main metabolitic pathways in homocysteine degradation (46, 47). Meanwhile, hyperhomocysteinemia has been suggested as an important pathogenic factor leading to glomerular injury, dysfunction, and sclerosis (48). Increased oxidative stress and decreased antioxidant defense function, caused by hyperhomocysteinemia, have been proved to be associated with renal function (21).
The current study had several limitations. Firstly, several parameters, such as a specific diet, vitamin B12, and mental status, were difficult to be obtained in this epidemiological study. These parameters needed to be evaluated with great complications by nutritionists and psychiatrists. Secondly, there was no evidence for a causal relationship provided and no correlation with mortality tested in the current study. Thirdly, only data from centenarians were analyzed in the current study, and there was no comparison with the elderly aged < 100 years. Future studies should be performed to not only compare these centenarians with a cohort of the elderly aged < 100 years from the same region, but also provide evidence for a causal relationship and test the correlation with mortality in the following years.
Conclusion
As the first in the world, the current study demonstrated that both hyperhomocysteinemia and hyperuricemia had important relationships with MetS and renal function in Chinese centenarians. Hyperuricemia and hyperhomocysteinemia that could help identify, while also affecting, the development of MetS and renal dysfunction may unfold complex relationships between MetS, renal function, and cardiovascular risk, and provide effective prevention strategies for these conditions.
Author Contributions
SF, YY, YZ, and FL designed the study, collected and analyzed the data, and wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the Key Research and Development Program of Hainan (ZDYF2016135 and ZDYF2017095), Sanya Medical and Health Science and Technology Innovation Project (2016YW21), and Clinical Scientific Research Supporting Fund of Chinese People's Liberation Army General Hospital (2017FC-CXYY-3009). The sponsors had no role in the design, conduct, interpretation, review, approval, or control of this article.
References
1. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA (2015) 313:1973. doi: 10.1001/jama.2015.4260
2. Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of Metabolic Syndrome in the adult US population, 1999–2010. J Am Coll Cardiol. (2013) 62:697–703. doi: 10.1016/j.jacc.2013.05.064
3. Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation (2004) 110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E
4. Levantesi G, Macchia A, Marfisi R, Franzosi MG, Maggioni AP, Nicolosi GL, et al. Metabolic syndrome and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. (2005) 46:277–83. doi: 10.1016/j.jacc.2005.03.062
5. Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res. (2007) 4:143–50. doi: 10.3132/dvdr.2007.033
6. Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. (2006) 355:2631–9. doi: 10.1056/NEJMoa055373
7. de Carvalho SC, Muniz MT, Siqueira MD, Siqueira ER, Gomes AV, Silva KA, et al. Plasmatic higher levels of homocysteine in non-alcoholic fatty liver disease (NAFLD). Nutr J. (2013) 12:37. doi: 10.1186/1475-2891-12-37
8. Meigs JB, Jacques PF, Selhub J, Singer DE, Nathan DM, Rifai N, et al. Fasting plasma homocysteine levels in the insulin resistance syndrome: the Framingham offspring study. Diabetes Care (2001) 24:1403–10. doi: 10.2337/diacare.24.8.1403
9. Vaya A, Carmona P, Badia N, Perez R, Hernandez Mijares A, Corella D. Homocysteine levels and the metabolic syndrome in a Mediterranean population: a case-control study. Clin Hemorheol Microcirc. (2011) 47:59–66. doi: 10.3233/CH-2010-1366
10. Hajer GR, van der Graaf Y, Olijhoek JK, Verhaar MC, Visseren FL. Levels of homocysteine are increased in metabolic syndrome patients but are not associated with an increased cardiovascular risk, in contrast to patients without the metabolic syndrome. Heart (2007) 93:216–20. doi: 10.1136/hrt.2006.093971
11. Rhee EJ, Hwang ST, Lee WY, Yoon JH, Kim BJ, Kim BS, et al. Relationship between metabolic syndrome categorized by newly recommended by International Diabetes Federation criteria with plasma homocysteine concentration. Endocr J. (2007) 54:995–1002. doi: 10.1507/endocrj.K07E-018
12. shizaka N, Ishizaka Y, Toda E, Nagai R, Yamakado M. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arterioscler Thromb Vasc Biol. (2005) 25:1038–44. doi: 10.1161/01.ATV.0000161274.87407.26
13. Al-Daghri NM, Al-Attas OS, Wani K, Sabico S, Alokail MS. Serum uric acid to creatinine ratio and risk of metabolic syndrome in saudi type 2 diabetic patients. (2017)Sci Rep. 7:12104. doi: 10.1038/s41598-017-12085-0
14. Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al. Chronic kidney disease as a global public health problem: approaches and initiatives-a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. (2007) 72:247–59. doi: 10.1038/sj.ki.5002343
15. Xue C, Ye XD, Li W, Peng Q, Ding HY, Zhang YH, et al. Prevalence of chronic kidney disease in Jing adults in China: a village-based study. Clin Nephrol. (2013) 79:50–6. doi: 10.5414/CN107511
16. Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: a systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. (2014) 15:122. doi: 10.1186/1471-2369-15-122
17. Lin F, Zhang H, Huang F, Chen H, Lin C, Zhu P. Influence of changes in serum uric acid levels on renal function in elderly patients with hypertension: a retrospective cohort study with (2016) 3.5-year follow-up. BMC Geriatr. 16:35. doi: 10.1186/s12877-016-0209-2
18. Macías N, Goicoechea M, de Vinuesa MS, Verdalles U, Luño J. Urate reduction and renal preservation: what is the evidence? Curr Rheumatol Rep. (2013) 15:386. doi: 10.1007/s11926-013-0386-3
19. Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. (2009) Risk factors for end-stage renal disease: 25-year follow up. Arch Intern Med. 169:342–50. doi: 10.1001/archinternmed.2008.605
20. Ye Z, Zhang Q, Li Y, Wang C, Zhang J, Ma X, et al. High prevalence of hyperhomocysteinemia and its association with target organ damage in chinese patients with chronic kidney disease. Nutrients (2016) 8:E645. doi: 10.3390/nu8100645
21. Ostrakhovitch EA, Tabibzadeh S. Homocysteine in chronic kidney disease. Adv Clin Chem. (2015) 72:77–106. doi: 10.1016/bs.acc.2015.07.002
22. Evert J, Lawler E, Bogan H, Perls T. Morbidity profles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci. (2003) 58:232–7. doi: 10.1093/gerona/58.3.M232
23. Ismail K, Nussbaum L, Sebastiani P, Andersen S, Perls T, Barzilai N, et al. Compression of morbidity is observed across cohorts with exceptional longevity. J Am Geriatr Soc. (2016) 64:1583–91. doi: 10.1111/jgs.14222
24. Motta M, Bennati E, Ferlito L, Malaguarnera M, Motta L, Italian Multicenter Study onCentenarians (IMUSCE). Successful aging in centenarians: myths and reality. Arch Gerontol Geriatr. (2005) 40:241–51. doi: 10.1016/j.archger.2004.09.002
25. Jopp DS, Park MK, Lehrfeld J, Paggi ME. Physical, cognitive, social and mental health in near-centenarians and centenarians living in New York City: findings from the Fordham Centenarian Study. BMC Geriatr. (2016) 16:1. doi: 10.1186/s12877-015-0167-0
26. Qin X, Huo Y, Xie D, Hou F, Xu X, Wang X. Homocysteine-lowering therapy with folic acid is effective in cardiovascular disease prevention in patients with kidney disease: a meta-analysis of randomized controlled trials. Clin Nutr. (2013) 32:722–7. doi: 10.1016/j.clnu.2012.12.009
27. Forouhi NG, Sattar N. CVD risk factors and ethnicity-a homogeneous relationship. Atheroscler Suppl. (2006) 7:11–9. doi: 10.1016/j.atherosclerosissup.2006.01.003
28. Esteghamati A, Hafezi-Nejad N, Zandieh A, Sheikhbahaei S, Ebadi M, Nakhjavani M. Homocysteine and metabolic syndrome: from clustering to additional utility in prediction of coronary heart disease. J Cardiol. (2014) 64:290–6. doi: 10.1016/j.jjcc.2014.02.001
29. He Y, Zhao Y, Yao Y, Yang S, Li J, Liu M, et al. Cohort profile: the China Hainan Centenarian Cohort Study (CHCCS). Int J Epidemiol. (2018) 47, 694-695h. doi: 10.1093/ije/dyy017
30. Yang SH, Dou KF, Song WJ. Prevalence of diabetes among men and women in China. N Engl J Med. (2010) 362:2425–6. doi: 10.1056/NEJMoa0908292
31. Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group. The metabolic syndrome: a new worldwide definition. Lancet (2005) 366:1059–62. doi :10.1016/S0140-6736(05)67402-8
32. Ma YC, Zuo L, Chen JH. Modified glomerular filtration rate estimating equation for chinese patients with chronic kidney disease. J Am Soc Nephrol. (2006) 17:2937–44. doi: 10.1681/ASN.2006040368
33. Fonseca V, Keebler M, Dicker-Brown A, Desouza C, Poirier LA, Murthy SN, et al. The effect of troglitazone on plasma homocysteine, hepatic and red blood cell S-adenosyl methionine, and S-adenosyl homocysteine and enzymes in homocysteine metabolism in Zucker rats. Metabolism (2002) 51:783–6. doi: 10.1053/meta.2002.32731
34. Oron-Herman M, Rosenthal T, Sela BA. Hyperhomocysteinemia as a component of syndrome X. Metabolism (2003) 52:1491–5. doi: 10.1016/S0026-0495(03)00262-2
35. Fonseca V, Dicker-Brown A, Ranganathan S, Song W, Barnard RJ, Fink L, et al. Effects of a high-fat-sucrose diet on enzymes in homocysteine metabolism in the rat. Metabolism (2000) 49:736–41. doi: 10.1053/meta.2000.6256
36. Najib S, Sanchez-Margalet V. (2005) Homocysteine thiolactone inhibits insulinstimulated DNA and protein synthesis: possible role of mitogen-activated protein kinase (MAPK), glycogen synthase kinase- 3 (GSK-3) and p70 S6K phosphorylation. J Mol Endocrinol. 34:119–26. doi: 10.1677/jme.1.01581
37. Sakamuri A, Pitla S, Putcha UK, Jayapal S, Pothana S, Vadakattu SS, et al. Transient Decrease in circulatory testosterone and homocysteine precedes the development of metabolic syndrome features in fructose-fed sprague dawley rats. J Nutr Metab. (2016) 2016:7510840. doi: 10.1155/2016/7510840
38. Zhang JX, Zhang YP, Wu QN, Chen B. (2015)Uric acid induces oxidative stress via an activation of the renin-angiotensin system in 3T3-L1 adipocytes. Endocrine 48:135–42. doi: 10.1007/s12020-014-0239-5
39. Norvik JV, Storhaug HM, Ytrehus K, Jenssen TG, Zykova SN, Eriksen BO, et al. Overweight modifies the longitudinal association between uric acid and some components of the metabolic syndrome: the Tromsø Study. BMC Cardiovasc Disord. (2016) 16:85. doi: 10.1186/s12872-016-0265-8
40. Baker JF, Krishnan E, Chen L, Schumacher HR. Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? Am J Med. (2005) 118:816–26. doi: 10.1016/j.amjmed.2005.03.043
41. Feig DI. Uric acid: a novel mediator and marker of risk in chronic kidney disease? Curr Opin Nephrol Hypertens (2009) 18:526–30. doi: 10.1097/MNH.0b013e328330d9d0
42. Kocyigit I, Yilmaz MI, Orscelik O, Sipahioglu MH, Unal A, Eroglu E, et al. Serum uric acid levels and endothelial dysfunction in patients with autosomal dominant polycystic kidney disease. Nephron Clin Pract. (2013) 123:157–64. doi: 10.1159/000353730
43. Menon V, Rudym D, Chandra P, Miskulin D, Perrone R, Sarnak M. Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin J Am Soc Nephrol. (2011) 6:7–13. doi: 10.2215/CJN.04140510
44. Yu MA, Sánchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin–angiotensin system inhuman vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. (2010) 28:1234–42. doi: 10.1097/HJH.0b013e328337da1d
45. Kohagura K, Kochi M, Miyagi T, Kinjyo T, Maehara Y, Nagahama K, et al. An association between uric acid levels and renal arteriolopathy in chronic kidney disease: a biopsy-based study. Hypertens Res. (2013) 36:43–9. doi: 10.1038/hr.2012.135
46. Guttormsen AB, Ueland PM, Svarstad E, Refsum H. Kinetic basis of hyperhomocysteinemia in patients with chronic renal failure. Kidney Int. (1997) 52:495–502. doi: 10.1038/ki.1997.359
47. van Guldener C, Kulik W, Berger R, Dijkstra DA, Jakobs C, Reijngoud DJ, et al. Homocysteine and methionine metabolism in ESRD: a stable isotope study. Kidney Int. (1999) 56:1064–71. doi: 10.1046/j.1523-1755.1999.00624.x
Keywords: Chinese centenarians, hyperhomocysteinemia, hyperuricemia, metabolic syndrome, renal function
Citation: Fu S, Yao Y, Zhao Y and Luan F (2018) Relationships of Hyperhomocysteinemia and Hyperuricemia With Metabolic Syndrome and Renal Function in Chinese Centenarians. Front. Endocrinol. 9:502. doi: 10.3389/fendo.2018.00502
Received: 31 May 2018; Accepted: 09 August 2018;
Published: 11 September 2018.
Edited by:
Michal Masternak, University of Central Florida, United StatesReviewed by:
Marcello Pinti, Università degli Studi di Modena e Reggio Emilia, ItalyJames Harper, Sam Houston State University, United States
Copyright © 2018 Fu, Yao, Zhao and Luan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yali Zhao, emhhb3lsMzAxQDE2My5jb20=
Fuxin Luan, YmFpc3VpMzAxQDE2My5jb20=
†Joint first authors
 Shihui Fu
Shihui Fu Yao Yao
Yao Yao Yali Zhao5*
Yali Zhao5*