
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol. , 03 August 2018
Sec. Pituitary Endocrinology
Volume 9 - 2018 | https://doi.org/10.3389/fendo.2018.00434
This article is part of the Research Topic Diabetes Secondary to Pituitary Diseases View all 7 articles
 Chiara Graziadio*
Chiara Graziadio* Valeria Hasenmajer
Valeria Hasenmajer Mary A. Venneri
Mary A. Venneri Daniele Gianfrilli
Daniele Gianfrilli Andrea M. Isidori
Andrea M. Isidori Emilia Sbardella
Emilia SbardellaSecondary adrenal insufficiency (SAI) is a potentially life-threatening endocrine disorder due to an impairment of corticotropin (ACTH) secretion from any process affecting the hypothalamus or pituitary gland. ACTH deficit can be isolated or associated with other pituitary failures (hypopituitarism). An increased mortality due to cardiovascular, metabolic, and infectious diseases has been described in both primary and secondary adrenal insufficiency. However, few studies have provided compelling evidences on the underlying mechanism in SAI, because of the heterogeneity of the condition. Recently, some studies suggested that inappropriate glucocorticoid (GCs) replacement therapy, as for dose and/or timing of administration, may play a role. Hypertension, insulin resistance, weight gain, visceral obesity, increased body mass index, metabolic syndrome, impaired glucose tolerance, diabetes mellitus, dyslipidemia have all been associated with GC excess. These conditions are particularly significant when SAI coexists with other pituitary alterations, such as growth hormone deficiency, hypogonadism, and residual tumor. Novel regimen schemes and GC preparations have been introduced to improve compliance and better mimick endogenous cortisol rhythm. The controlled trials on the improved replacement therapies, albeit in the short-term, show some beneficial effects on cardiovascular risk, glucose metabolism, and quality of life. This review examines the current evidence from the available clinical trials investigating the association between different glucocorticoid replacement therapies (type, dose, frequency, and timing of treatment) and glycometabolic alterations in SAI.
Secondary adrenal insufficiency (SAI) is a rare potentially life-threatening condition due to any disturbance involving the pituitary gland (space-occupying lesions, trauma or genetic abnormalities) and interfering with corticotropin (ACTH) secretion (1).
The impairment of ACTH secretion can be isolated or can be associated with other defected pituitary secretions (hypopituitarism) (1). Studies on outcomes in hypopituitary patients have shown that the frequency of SAI is between 41 and 94% (2). SAI shows a prevalence of 150–280 per million with a higher prevalence in women (3).
Moreover iatrogenic suppression of the hypothalamic–pituitary–adrenal axis caused by long-term glucocorticoid exposure should be carefully considered and patients taking long-term oral, inhaled, intranasal, intra-articular, or topical glucocorticoids, should be assessed for possible hypocortisolism (4).
Several studies have demonstrated an increased mortality in patients with cortisol deficiency attributable to cardiovascular, metabolic, neoplastic and infective diseases (5). The incidence of adrenal crises is increasing, at an estimated 3–6 % per year among SAI, with a dramatic 1% mortality rate (5).
On the basis of the Endocrine Society Clinical Practice guidelines, the management of patients with adrenal insufficiency (AI) consists in treatment with hydrocortisone (HC) or cortisone acetate (CA), which are the most physiological choice for glucocorticoid replacement. The recommended daily hydrocortisone dose is 10–12 mg/m2 divided in two to three doses, administering half to two thirds of the total daily dose in the morning, generally suggesting lower daily dosage in SAI (1, 6). However, optimal glucocorticoid replacement therapy for adrenally insufficient patients is still controversial due to lack of evidence from randomized trials.
One of the main concerns in the management of AI is balancing the need to increase the usual glucocorticoid (GC) dose during stress conditions. The goal is the prevention of adrenal crises on one side, with the need to reduce over-exposure in the uneventful daily life. Even when administered according to current guidelines, multiple daily doses of glucocorticoids (md-GC) have been associated with bone loss and reduced quality of life (7, 8) probably from a higher time exposure in the evening (9).
Hypercortisolism is associated to hyperglycaemia and insulin resistance (IR), increasing glicogenolisis, and hepatic gluconeogenesis and playing a direct role on pancreatic insulin secretion (10). Phosphatidylinositol-3 kinase pathway is involved in insulin signaling reducing nitric oxide synthase activation and causing endothelial impairment, ultimately increasing cardiovascular risk (11). Hypertension, IR, weight gain, visceral obesity, increased body mass index (BMI), metabolic syndrome, impaired glucose tolerance, diabetes mellitus, dyslipidemia are associated with GC excess or unphysiological GC replacement in patients with SAI (12). All these complications are more relevant when SAI coexists with other pituitary disorders, such as neoplasms of the sellar region, growth hormone deficiency (GHD), hypogonadism, and all the subsequent comorbidities that commonly affect the hypopituitary patient (2, 13).
A rare but interesting cause of SAI is the homozygous or composed heterozygous loss-of-function mutations of POMC gene, leading to early onset adrenal insufficiency, obesity, and altered pigmentation (14). Given the pivotal role of POMC in regulating appetite and metabolism, whether the lack of POMC secretion in all-causes SAI can play a role in metabolic complications has never been investigated and should prompt further studies.
Recently, modified-release preparations have been developed to mimic the physiological cortisol rhythm and improve patients' compliance (15, 16). The once-daily modified-release hydrocortisone tablet (od-MRHC) has been introduced to prevent the afternoon peaks given by conventional therapies administered in multiple daily doses. Treatment with the od-MRHC seems to improve cardiovascular risk factors, glucose metabolism, and quality of life in controlled trials (17–19).
The objective of this review is to evaluate the association between inadequate glucocorticoid replacement therapy (type, dose, frequency, and timing of treatment) and glycometabolic alterations in SAI, on the basis of currently available data.
Glucocorticoids (GCs) are so named based on the results of Houssay's studies published in 1954, where it was shown that they exert an important action on glucose regulation (20) through intracellular glucocorticoid receptor protein.
Polymorphisms of glucocorticoid receptor may influence the peripheral activity of cortisol (21). In target tissues, the activation and inactivation of GCs are regulated by the enzyme 11β-hydroxysteroid dehydrogenase (22).
The GCs role on glucose homeostasis is due to the impairment of various mechanisms including β cell alterations (glucose sensitivity and insulin release) and IR, leading to a reduction in glucose disposal and to an increased production of endogenous glucose (10). Their ability to counterbalance the insulin action and to increase glucose levels are a major concern in patients with Cushing's syndrome/disease or under GCs treatment for immunomodulatory purposes (23). Physiologically, the alterations in peripheral insulin sensitivity is counterbalanced by increased β-cell function (24–27). Insulin demand is increased by GCs, and when the compensative insulin secretion is inadequate or compromised, fasting and/or postprandial hyperglycaemia may occur (10).
GCs interfere with the insulin receptor signaling cascade affecting these processes, and glucose homeostasis is usually maintained by β-cell compensations (10). Individuals with any susceptibility to glucose homeostasis alterations, such as reduced sensitivity to insulin (28), an impaired insulin response to glucose (29), first degree relatives with type 2 diabetes (30), obesity (31) and older age (32), are more prone to develop GCs induced alterations. A possible mechanism is the inadequate β-cells response. Hence, considering the negative effects of GCs on glucose homeostasis, their clinical use should be limited, especially in patients with risk factors for diabetes.
An interesting finding is that in the majority of in vivo studies, GCs treatment also induces an increase in glucagon and amylin plasma levels. Increased plasma amylin levels have been shown to exert a diabetogenic effect due to the higher rates of toxic amylin aggregation mediated by the islet amyloid polypeptide (IAPP) (33). Additionally, hyperglucagonaemia could oppose insulin actions and increase hepatic glucose output, a mechanism described in diabetes (34).
Replacement therapy in SAI does not require the mineralocorticoid administration because the renin-angiotensin-aldosterone axis is preserved (35, 36), instead of in primary adrenal insufficiency (PAI). Nevertheless, GCs replacement therapy shows wide variability in the type, dose, frequency, and timing of treatment (37).
However, there is general consent that replacement treatment should aim to obtain the physiological daily dose and to simulate the circadian cortisol rhythm.
Patients with SAI due to iatrogenic suppression of the hypothalamic–pituitary–adrenal axis requires conventional treatment and the same patient education as patients with central hypocortisolism due to disturbance involving the pituitary gland (4).
Hydrocortisone (HC), cortisone acetate (CA) and prednisone are the most frequently used drugs (37) for replacement therapy. HC and CA are usually administered with a higher dose in the morning in the fasting state and one or two fractioned doses later in the day (before 06:00 pm) (38). Compared to hydrocortisone, prednisone and dexamethasone have greater glucocorticoid activity (respectively 5 and 30- to 50-fold) and a longer half-life, exposing the patients to a greater time-exposure, and the risk of over-treatment (at the currently recommended doses), thus explaining the cardio-metabolic complications observed in these subjects (12, 39).
In patients with partial cortisol insufficiency, signs and symptoms should be evaluated to decide if the starting dose can be lower than conventional replacement doses (5–10 mg/day) or to limit exogenous glucocorticoids intake to rescue treatment when needed.
Considering a progressive worsening of the metabolic status in hypopituitary patients receiving doses greater than 20 and greater than 30 mg/day, the mean daily dose of hydrocortisone prescribed should be lower than 30 mg (40).
This is especially relevant in SAI, where many factors can influence the daily requirement of glucocorticoids, such as residual ACTH secretion, changes in binding proteins, other concomitant hormone replacements and related comorbidities.
Considering that a residual ACTH secretion and HPA-axis function may be present, lower glucocorticoid doses, especially in the afternoon, might be sufficient to replace patients with SAI when compared to PAI. Despite this, in clinical practice there is a tendency to use the same doses of GCs in both SAI and PAI, given at the same timing. It might be that in patients with SAI, even those with a non-reversible deficit, the residual ACTH secretion is sufficient to maintain the low levels that are physiologically required and produced to cover the afternoon and evening.
The modified release preparations, and the continuous infusions, has been developed to avoid the afternoon administrations, causing multiples peaks at a time when glucocorticoid sensitivity is higher, and thus replicate the shape of a gradual cortisol decline during the day of adrenal sufficient subject (15, 38). Although initially thought to treat PAI, this advantage of the modified release preparation could well apply to SAI.
Compared to the past, the current guidelines recommend lower doses of HC to align with cortisol production rates of healthy volunteers (41). In non-AI subjects, there is a correlation between hypercortisolism and metabolic impairment, but in AI has yet to be proven (42).
An open interventional study was conducted in 17 SAI patients to evaluate the effect of increased dose of HC (or equivalent) to 30 mg/d on cardiovascular system and the role of insulin in the impairment of cardiovascular function. No significant differences in fasting and post-glucose load pulse wave velocities were observed on the higher glucocorticoid regimen. This study showed that fasting augmentation index and reactive hyperemia index were lower on the higher glucocorticoid dose and post-glucose load changes were not significantly different. The insulin sensitivity or secretion didn't show alterations on the higher glucocorticoid regimen (43).
Several studies have evaluated the effects on metabolism and glucose homeostasis of reducing the daily dose of hydrocortisone (Table 1).
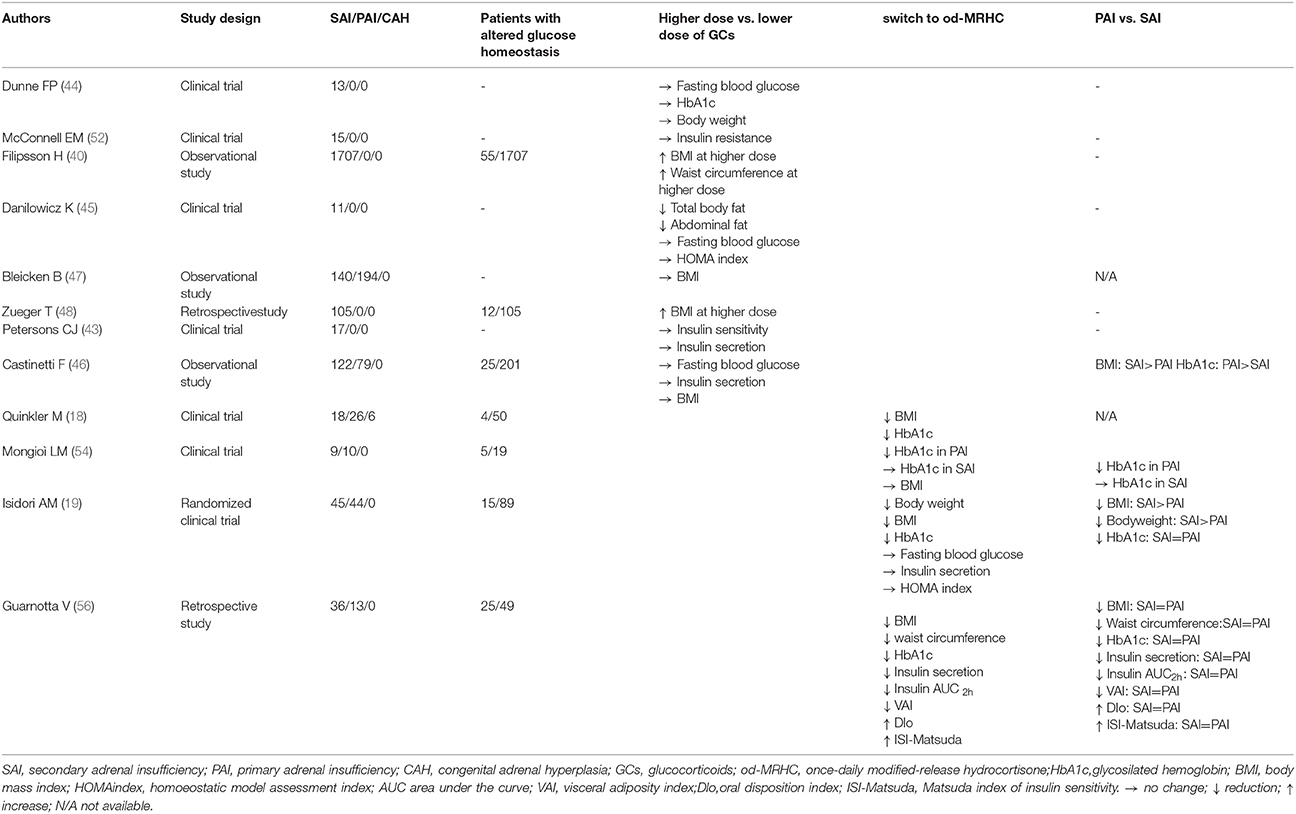
Table 1. Studies evaluating glycometabolic effects of glucocorticoid therapeutic regimens in Secondary Adrenal Insufficiency (SAI).
Dunne FP et al. investigated the impact of the reduction of daily HC from 30 mg to 15 mg on cardiovascular function and body weight in 13 patients with SAI. After 3 months there was no change in body weight (44). Danilowicz et al. successively demonstrated that the reduction of HC on average by 50% in 11 patients with SAI decreased the body weight by 7 kg while insulin levels and fasting glucose unchanged (45).
In hypopituitary patients with severe untreated GHD, GC daily doses are positively correlated with BMI and serum cholesterol levels. Patients receiving HC equivalent (HCeq) doses of less than 20 mg/day did not diverge in metabolic parameters from adrenal sufficient patients. After increasing HCeq dose, higher BMI, serum lipids were observed. Baseline serum insulin growth factor I (IGF-I) varied on the basis of the type and dose of GC therapy, whereas the 1-yr response to GH treatment in metabolic risk factors was not influenced by GC regimen (40).
In a large French cohort (79 PAI, 122 SAI), Castinetti et al. found only a small difference in HC doses between patients with or with-out dyslipidemia, but not in other metabolic variables (46). In a German study (194 PAI, 140 SAI), BMI was not different in groups supplemented by high (>30 mg/day) or lower doses of HC (47). In other real-life surveys, from Poland and France, no correlation was found between the daily dose of HC and fasting glycaemia or insulinemia (46). Slightly different is the case of SAI, where an apparent relationship between weight-adjusted HC dose and high blood pressure has been described, but again not with diabetes mellitus (46, 48). Recently, a double blind, randomized, crossover trial compared the effects of 3 dosing regimens in 18 patients with SAI (49). Subjective improvement was observed with only a twice daily regimen, namely HC 10 mg at 07:00 am and 5 mg at 03:00 pm. Conversely, another Swedish study with 15 patients with PAI found that a 4-dose regimen was preferred, with higher QOL scores (50). However, this regimen resulted in a much higher cortisol exposure (24 h-cortisol area under the curve), which in the long-term might be deleterious from a metabolic point of view (50).
Recently, increased awareness about the detrimental effects of mild glucocorticoid over treatment in patients with AI has led to a lower GC daily dose. However, none of these studies found an inverse association between glucocorticoid exposure and the incidence of adrenal crises (<12.5 mg/day, 12.5–30 mg/day or >30 mg/day) (51).
McConnell et al. (52) in a randomized crossover study with 15 patients with SAI, did not find any difference in hepatic and peripheral insulin sensitivity comparing conventional oral HC replacement with HC infusions (52).
Several studies have investigated the effects of the new formulations of GCs focusing on metabolic effects of these treatments (Table 1). In particular, the od-MRHC treatment has been demonstrated to have a positive effect on body weight and glycosilated hemoglobin (HbA1c) in patients with PAI (17, 53). The od-MRHC regimen has been shown to slightly reduce BMI andHbA1c in a previous parallel open, non-randomized, prospective trial on a mixed AI population (PAI, SAI and congenital adrenal hyperplasia) (18).
An open clinical trial evaluated the effects of od-MRHC on the glycometabolic profile and health-related quality of life of 10 PAI and 9 SAI patients. After 12 months therapy with the od-MRHC, no changes in fasting glucose, insulin resistance and BMI were observed in either population while HbA1c levels were significantly reduced in patients with PAI (54).
In a single-blind randomized active comparator, non-intervention parallel-group controlled trial, Isidori et al. (19) demonstrated that a restoration of physiological circadian glucocorticoid rhythm by replacement of conventional GC therapy with od-MRHC regimen reduced the body weight of 44 PAI and 45 SAI patients. In particular, at 24 weeks, body weight reduction was superior in patients in the od-MRHC group compared with those in the conventional treatment group (−2.1 kg [95%CI,– 4.0 to −0.3] vs.1.9 kg [– 0.1 to 3.9]; treatment difference – 4.0 kg, 95% CI −6.9 to −1.1; p = 0.008). Subgroup analysis for type of AI showed greater reductions in BMI (p = 0.081) and body weight (p = 0.074) in SAI than PAI patients. Conversely, any change in fasting blood glucose, insulin and the homoeostatic model assessment (HOMA) index wasn't found between the two intervention groups (conventional treatment/od-MRHC treatment) (19). This suggest that obesity in SAI can be targeted by improving GC administration.
This is consistent with the demonstration that HC administration in the evening, compared to a morning administration, produces a much more pronounced delayed hyperglycaemic effect with deleterious consequence on metabolism (55). In 1999, authors hypothesized this effect could be due to a greater sensitivity to GCs in the evening (55).
An Italian 36-months retrospective study evaluated the effects of od-MRHC in 13 PAI e 36 SAI patients with normal glucose tolerance (NGT) and pre-diabetes including impaired fasting glucose (IFG), and/or impaired glucose tolerance (IGT), on insulin secretion, and sensitivity, anthropometric parameters and cardiometabolic risk, indirectly expressed by the visceral adiposity index (VAI). In patients with AI-NGT and AI-pre-diabetes a significant decrease in BMI (p = 0.017 and p < 0.001), waist circumference (p = 0.008 and p < 0.001), HbA1c (p = 0.034 and p = 0.001) and a significant increase in high-density lipoprotein cholesterol (p = 0.036 and p = 0.043) were respectively observed. In addition, in pre-diabetic patients only a significant decrease in insulinemia (p = 0.014), AUC2hinsulinemia (p = 0.038) and VAI (p = 0.001), in concomitance with a significant increase in the oral disposition index (DIo; p = 0.041) and Matsuda index of insulin sensitivity (ISI-Matsuda; p = 0.038) were observed. No differences in metabolic parameters, insulin secretion and sensitivity index were found comparing PAI and SAI patients (56).
Secondary adrenal insufficiency has been reportedly associated with altered glycometabolic profile and increased cardiovascular risk (57), possibly due to over-replacement. However, a reduction in glucocorticoid daily dose has not been universally associated with improved glucose homeostasis, cardiovascular risk and overall morbidity. Randomized clinical trial addressing this point are very few.
In fact, specific data on glucose homeostasis alterations and improvement with different GC regimens in SAI are scarce and influenced by many confounding factors such as comorbidities, age, multiple pituitary hormone deficiencies and other replacement therapies.
In SAI patients with a residual ACTH secretion, the daily requirement of glucocorticoid might be lower than in PAI. Nevertheless, in the clinical practice, tailoring of GC replacement is often suboptimal, and there is a tendency to over-treat patients with SAI. This could be even more problematic than in PAI because of their increased metabolic risk.
Recently, the introduction of a new modified release formulation with a more physiological cortisol circadian profile has been associated with significant metabolic improvement in body weight, HbA1c and insulin sensitivity in SAI.
This prompted the hypothesis that a disruption of the physiological circadian rhythm could have a role in the observed glicometabolic alterations. Recent finding that patients with alterations of clock-related genes, such as patients with sleep disturbances or psychiatric disorders, and as recently demonstrated patients with AI (58) are exposed to increased cardiovascular risk and metabolic syndrome support this concept (59, 60).
A greater attention toward the dose and timing of glucocorticoid administration should be placed in patients with AI, tailoring the overall exposure to the residual HPA function and circadian rhythm of affected patients in order to limit their adverse metabolic profile.
CG, VH, MV, DG, AI, and ES have equally contributed to the paper, the literature review, analysis, interpretation, drafting, and editing for the final submission.
CG and AI have received honoraria from Shire and Novartis for speaking or serving on advisory boards and grants. ES has received honoraria from Shire for speaking or serving on advisory boards and grants. AI and DG have received honoraria for speaking and serving on advisory boards from Novartis, IPSEN, Otsuka, and menarini.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet (2014) 383:2152–67. doi: 10.1016/S0140-6736(13)61684-0
2. Sherlock M, Ayuk J, Tomlinson JW, Toogood AA, Aragon-Alonso A, Sheppard MC, et al. Mortality in patients with pituitary disease. Endocr Rev. (2010) 31:301–42. doi: 10.1210/er.2009-0033
3. Paragliola RM, Corsello SM. Secondary adrenal insufficiency: from the physiopathology to the possible role of modified-release hydrocortisone treatment. Minerva Endocrinol. (2017) 43:183–97. doi: 10.23736/S0391-1977.17.02701-8
4. Swords FM. Uncertainties in endocrine substitution therapy for central hypocortisolism. Handb Clin Neurol. (2014) 124:387–96. doi: 10.1016/B978-0-444-59602-4.00026-5
5. Hahner S, Spinnler C, Fassnacht M, Burger-Stritt S, Lang K, Milovanovic D, et al. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab. (2015) 100:407–16. doi: 10.1210/jc.2014-3191
6. Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2016) 101:364–89. doi: 10.1210/jc.2015-1710
7. Lovas K, Gjesdal CG, Christensen M, Wolff AB, Almas B, Svartberg J, et al. Glucocorticoid replacement therapy and pharmacogenetics in Addison's disease: effects on bone. Eur J Endocrinol. (2009) 160:993–1002. doi: 10.1530/EJE-08-0880
8. Bleicken B, Hahner S, Loeffler M, Ventz M, Allolio B, Quinkler M. Impaired subjective health status in chronic adrenal insufficiency: impact of different glucocorticoid replacement regimens. Eur J Endocrinol. (2008) 159:811–7. doi: 10.1530/EJE-08-0578
9. Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev. (2017) 38:3–45. doi: 10.1210/er.2015-1080
10. Rafacho A, Ortsater H, Nadal A, Quesada I. Glucocorticoid treatment and endocrine pancreas function: implications for glucose homeostasis, insulin resistance and diabetes. J Endocrinol. (2014) 223:R49–62. doi: 10.1530/JOE-14-0373
11. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia (2010) 53:1270–87. doi: 10.1007/s00125-010-1684-1
12. Mazziotti G, Formenti AM, Frara S, Roca E, Mortini P, Berruti A, Giustina A. management of endocrine disease: risk of overtreatment in patients with adrenal insufficiency: current and emerging aspects. Eur J Endocrinol. (2017) 177:R231–48. doi: 10.1530/EJE-17-0154
13. Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet (2007) 369:1461–70. doi: 10.1016/S0140-6736(07)60673-4
14. Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. (1998) 19:155–7. doi: 10.1038/509
15. Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, Campbell MJ, Newell-Price J, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. (2009) 94:1548–54. doi: 10.1210/jc.2008-2380
16. Johannsson G, Bergthorsdottir R, Nilsson AG, Lennernas H, Hedner T, Skrtic S. Improving glucocorticoid replacement therapy using a novel modified-release hydrocortisone tablet: a pharmacokinetic study. Eur J Endocrinol. (2009) 161:119–30. doi: 10.1530/EJE-09-0170
17. Johannsson G, Nilsson AG, Bergthorsdottir R, Burman P, Dahlqvist P, Ekman B, et al. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. J Clin Endocrinol Metab. (2012) 97:473–81. doi: 10.1210/jc.2011-1926
18. Quinkler M, Miodini Nilsen R, Zopf K, Ventz M, Oksnes M. Modified-release hydrocortisone decreases BMI and HbA1c in patients with primary and secondary adrenal insufficiency. Eur J Endocrinol. (2015) 172:619–26. doi: 10.1530/EJE-14-1114
19. Isidori AM, Venneri MA, Graziadio C, Simeoli C, Fiore D, Hasenmajer V, et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): a single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. (2017) 6:173–85. doi: 10.1016/S2213-8587(17)30398-4
20. Yuen KC, Chong LE, Riddle MC. Influence of glucocorticoids and growth hormone on insulin sensitivity in humans. Diabet Med. (2013) 30:651–63. doi: 10.1111/dme.12184
21. Giordano R, Marzotti S, Berardelli R, Karamouzis I, Brozzetti A, D'Angelo V, et al. BClI polymorphism of the glucocorticoid receptor gene is associated with increased obesity, impaired glucose metabolism and dyslipidaemia in patients with Addison's disease. Clin Endocrinol. (2012) 77:863–70. doi: 10.1111/j.1365-2265.2012.04439.x
22. Gathercole LL, Lavery GG, Morgan SA, Cooper MS, Sinclair AJ, Tomlinson JW, et al. 11beta-Hydroxysteroid dehydrogenase 1: translational and therapeutic aspects. Endocr Rev. (2013) 34:525–55. doi: 10.1210/er.2012-1050
23. Raul Ariza-Andraca C, Barile-Fabris LA, Frati-Munari AC, Baltazar-Montufar P. Risk factors for steroid diabetes in rheumatic patients. Arch Med Res. (1998) 29:259–62.
24. Beard JC, Halter JB, Best JD, Pfeifer MA, Porte D Jr. Dexamethasone-induced insulin resistance enhances B cell responsiveness to glucose level in normal men. Am J Physiol. (1984) 247:E592–596.
25. Nicod N, Giusti V, Besse C, Tappy L. Metabolic adaptations to dexamethasone-induced insulin resistance in healthy volunteers. Obes Res. (2003) 11:625–31. doi: 10.1038/oby.2003.90
26. Ahren B. Evidence that autonomic mechanisms contribute to the adaptive increase in insulin secretion during dexamethasone-induced insulin resistance in humans. Diabetologia (2008) 51:1018–24. doi: 10.1007/s00125-008-0995-y
27. Rafacho A, Giozzet VA, Boschero AC, Bosqueiro JR. Functional alterations in endocrine pancreas of rats with different degrees of dexamethasone-induced insulin resistance. Pancreas (2008) 36:284–93. doi: 10.1097/MPA.0b013e31815ba826
28. Larsson H, Ahren B. Insulin resistant subjects lack islet adaptation to short-term dexamethasone-induced reduction in insulin sensitivity. Diabetologia (1999) 42:936–43. doi: 10.1007/s001250051251
29. Wajngot A, Giacca A, Grill V, Vranic M, Efendic S. The diabetogenic effects of glucocorticoids are more pronounced in low- than in high-insulin responders. Proc Natl Acad Sci USA. (1992) 89:6035–9. doi: 10.1073/pnas.89.13.6035
30. Jensen DH, Aaboe K, Henriksen JE, Volund A, Holst JJ, Madsbad S, Krarup T. Steroid-induced insulin resistance and impaired glucose tolerance are both associated with a progressive decline of incretin effect in first-degree relatives of patients with type 2 diabetes. Diabetologia (2012) 55:1406–16. doi: 10.1007/s00125-012-2459-7
31. Besse C, Nicod N, Tappy L. Changes in insulin secretion and glucose metabolism induced by dexamethasone in lean and obese females. Obes Res. (2005) 13:306–11. doi: 10.1038/oby.2005.41
32. Novelli M, De Tata V, Bombara M, Lorenzini A, Masini M, Pollera M, et al. Insufficient adaptive capability of pancreatic endocrine function in dexamethasone-treated ageing rats. J Endocrinol. (1999) 162:425–32. doi: 10.1677/joe.0.1620425
33. Couce M, Kane LA, O'Brien TD, Charlesworth J, Soeller W, McNeish J, et al. Treatment with growth hormone and dexamethasone in mice transgenic for human islet amyloid polypeptide causes islet amyloidosis and beta-cell dysfunction. Diabetes (1996) 45:1094–101. doi: 10.2337/diab.45.8.1094
34. Quesada I, Tuduri E, Ripoll C, Nadal A. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol. (2008) 199:5–19. doi: 10.1677/JOE-08-0290
35. Grossman AB. Clinical Review#: the diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. (2010) 95:4855–63. doi: 10.1210/jc.2010-0982
36. Crowley RK, Argese N, Tomlinson JW, Stewart PM. Central hypoadrenalism. J Clin Endocrinol Metab. (2014) 99:4027–36. doi: 10.1210/jc.2014-2476
37. Murray RD, Ekman B, Uddin S, Marelli C, Quinkler M, Zelissen PM, et al. Management of glucocorticoid replacement in adrenal insufficiency shows notable heterogeneity - data from the EU-AIR. Clin Endocrinol. (2017) 86:340–6. doi: 10.1111/cen.13267
38. Higham CE, Johannsson G, Shalet SM. Hypopituitarism. Lancet (2016) 388:2403–15. doi: 10.1016/S0140-6736(16)30053-8
39. Quinkler M, Ekman B, Marelli C, Uddin S, Zelissen P, Murray RD, et al. Prednisolone is associated with a worse lipid profile than hydrocortisone in patients with adrenal insufficiency. Endocr Connect. (2017) 6:1–8. doi: 10.1530/EC-16-0081
40. Filipsson H, Monson JP, Koltowska-Haggstrom M, Mattsson A, Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab. (2006) 91:3954–61. doi: 10.1210/jc.2006-0524
41. Esteban NV, Loughlin T, Yergey AL, Zawadzki JK, Booth JD, Winterer JC, et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab. (1991) 72:39–45. doi: 10.1210/jcem-72-1-39
42. Falorni A, Minarelli V, Morelli S. Therapy of adrenal insufficiency: an update. Endocrine (2013) 43:514–28. doi: 10.1007/s12020-012-9835-4
43. Petersons CJ, Mangelsdorf BL, Thompson CH, Burt MG. Acute effect of increasing glucocorticoid replacement dose on cardiovascular risk and insulin sensitivity in patients with adrenocorticotrophin deficiency. J Clin Endocrinol Metab. (2014) 99:2269–76. doi: 10.1210/jc.2013-4305
44. Dunne FP, Elliot P, Gammage MD, Stallard T, Ryan T, Sheppard MC, et al. Cardiovascular function and glucocorticoid replacement in patients with hypopituitarism. Clin Endocrinol. (1995) 43:623–9. doi: 10.1111/j.1365-2265.1995.tb02928.x
45. Danilowicz K, Bruno OD, Manavela M, Gomez RM, Barkan A. Correction of cortisol overreplacement ameliorates morbidities in patients with hypopituitarism: a pilot study. Pituitary (2008) 11:279–85. doi: 10.1007/s11102-008-0126-2
46. Castinetti F, Sahnoun M, Albarel F, Morange I, Philippon M, Conte-Devolx B, et al. An observational study on adrenal insufficiency in a French tertiary centre: Real life versus theory. Ann Endocrinol. (2015) 76:1–8. doi: 10.1016/j.ando.2014.11.004
47. Bleicken B, Hahner S, Loeffler M, Ventz M, Decker O, Allolio B, et al. Influence of hydrocortisone dosage scheme on health-related quality of life in patients with adrenal insufficiency. Clin Endocrinol. (2010) 72:297–304. doi: 10.1111/j.1365-2265.2009.03596.x
48. Zueger T, Kirchner P, Herren C, Fischli S, Zwahlen M, Christ E, et al. Glucocorticoid replacement and mortality in patients with nonfunctioning pituitary adenoma. J Clin Endocrinol Metab. (2012) 97:E1938–42. doi: 10.1210/jc.2012-2432
49. Benson S, Neumann P, Unger N, Schedlowski M, Mann K, Elsenbruch S, et al. Effects of standard glucocorticoid replacement therapies on subjective well-being: a randomized, double-blind, crossover study in patients with secondary adrenal insufficiency. Eur J Endocrinol. (2012) 167:679–85. doi: 10.1530/EJE-12-0351
50. Ekman B, Bachrach-Lindstrom M, Lindstrom T, Wahlberg J, Blomgren J, Arnqvist HJ. A randomized, double-blind, crossover study comparing two- and four-dose hydrocortisone regimen with regard to quality of life, cortisol and ACTH profiles in patients with primary adrenal insufficiency. Clin Endocrinol. (2012) 77:18–25. doi: 10.1111/j.1365-2265.2012.04352.x
51. Smans LC, Van der Valk ES, Hermus AR, Zelissen PM. Incidence of adrenal crisis in patients with adrenal insufficiency. Clin Endocrinol. (2016) 84:17–22. doi: 10.1111/cen.12865
52. McConnell EM, Bell PM, Ennis C, Hadden DR, McCance DR, Sheridan B, et al. Effects of low-dose oral hydrocortisone replacement versus short-term reproduction of physiological serum cortisol concentrations on insulin action in adult-onset hypopituitarism. Clin Endocrinol. (2002) 56:195–201. doi: 10.1046/j.0300-0664.2001.01447.x
53. Giordano R, Guaraldi F, Marinazzo E, Fumarola F, Rampino A, Berardelli R, et al. Improvement of anthropometric and metabolic parameters, and quality of life following treatment with dual-release hydrocortisone in patients with Addison's disease. Endocrine (2016) 51:360–8. doi: 10.1007/s12020-015-0681-z
54. Mongioi LM, Condorelli RA, La Vignera S, Calogero AE. Dual-release hydrocortisone treatment: glycometabolic profile and health-related quality of life. Endocr Connect. (2018) 7:211–9. doi: 10.1530/EC-17-0368
55. Plat L, Leproult R, L'Hermite-Baleriaux M, Fery F, Mockel J, Polonsky KS, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. (1999) 84:3082–92. doi: 10.1210/jc.84.9.3082
56. Guarnotta V, Ciresi A, Pillitteri G, Giordano C. Improved insulin sensitivity and secretion in prediabetic patients with adrenal insufficiency on dual-release hydrocortisone treatment: a 36-month retrospective analysis. Clin Endocrinol. (2018) 88:665–72. doi: 10.1111/cen.13554
57. Johannsson G, Ragnarsson O. Cardiovascular and metabolic impact of glucocorticoid replacement therapy. Front Horm Res. (2014) 43:33–44. doi: 10.1159/000360556
58. Venneri MA, Hasenmajer V, Fiore D, Sbardella E, Pofi R, Graziadio C, et al. Circadian rhythm of glucocorticoid administration entrains clock genes in immune cells: a DREAM trial ancillary study. J Clin Endocrinol Metab. (2018). doi: 10.1210/jc.2018-00346 [Epub ahead of print].
59. Sookoian S, Gemma C, Fernandez Gianotti T, Burgueno A, Alvarez A, Gonzalez CD, et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. (2007) 261:285–92. doi: 10.1111/j.1365-2796.2007.01766.x
Keywords: secondary adrenal insufficiency, insulin resistance, weight gain, body mass index, metabolic syndrome, impaired glucose tolerance, diabetes mellitus, glucocorticoids
Citation: Graziadio C, Hasenmajer V, Venneri MA, Gianfrilli D, Isidori AM and Sbardella E (2018) Glycometabolic Alterations in Secondary Adrenal Insufficiency: Does Replacement Therapy Play a Role? Front. Endocrinol. 9:434. doi: 10.3389/fendo.2018.00434
Received: 26 February 2018; Accepted: 13 July 2018;
Published: 03 August 2018.
Edited by:
Carla Giordano, Università degli Studi di Palermo, ItalyReviewed by:
Masaaki Yamamoto, Cedars-Sinai Medical Center, United StatesCopyright © 2018 Graziadio, Hasenmajer, Venneri, Gianfrilli, Isidori and Sbardella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Graziadio, Y2hpYXJhZ3JhemlhZGlvQGxpdmUuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.