
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 18 June 2018
Sec. Thyroid Endocrinology
Volume 9 - 2018 | https://doi.org/10.3389/fendo.2018.00330
Background: A possible relationship between oral lichen planus (OLP) and thyroid disease has received attention in recent years.
Objective: This study aimed to evaluate the correlation between OLP and thyroid diseases in Chinese ethnic patients.
Methods: 192 OLP patients, 123 patients with oral lichenoid lesions (OLLs), and 162 controls were recruited in this case–control study. All participants received screening for thyroid function and underwent ultrasound. Sex and age of the patients in the three groups were matched. The prevalence of thyroid diseases in the subjects was analyzed. Using logistic regression, the odds ratio (OR) with 95% confidence intervals was appraised for associations between OLP, OLL, and different types of thyroid diseases [Hashimoto’s thyroiditis (HT), hypothyroidism, and thyroid nodule].
Results: The prevalence of thyroid diseases in the OLP group (72.4%) and OLL group (68.3%) was higher than the control group (49.4%) with statistical significance. The OR of HT was 3.16 (1.87–5.33) for OLP, 2.09 (1.18–3.70) for OLL, while the OR of thyroid nodule was 2.31 (1.30–4.09) for OLP.
Conclusion: Our study suggested a close relationship between OLP/OLL and HT and thyroid nodule in a Chinese population. The possible mechanism behind this association warrants further investigation.
Oral lichen planus (OLP) is a chronic autoimmune oral mucosa disease with a prevalence of 1–2% in the general population, which most commonly affects middle-aged and elderly female (1, 2). It has been defined as a potentially malignant disorder of the oral cavity (OPMD) (3). According to the World Health Organization (WHO), the estimated malignant transformation rate ranges from 1.09 to 1.14% (4). Despite extensive studies, the etiology and pathogenesis of OLP remains unknown. Various factors such as genetic predisposition, psychological factors, and infectious precipitants may be involved in the development of OLP (5, 6). Moreover, some systemic diseases such as hypertension, diabetes, and thyroid disorders may increase the risk of OLP (7–9).
Oral lichenoid lesions (OLLs) share some common clinical and histological features with OLP, which are often indistinguishable in their manifestation. The etiological factors for OLL have been identified in clinic and are commonly related to specific drugs, dental material, or graft-versus-host disease (10). But there were no special inducing factors mentioned above in most OLL patients.
Thyroid disease is a common endocrine systemic disease and causes serious public health problems. The prevalence of goiter is near 80% in severe iodine deficiency areas. The neonatal morbidity of congenital hypothyroidism can be 1/3,500–4,000 which is also the primary risk of mental retardation (11). Other thyroid disorders such as hyperthyroidism, thyroid cancer, and autoimmune thyroiditis have a diverse prevalence ranging from 0.5 to 1% in different countries (12). In China, over two billion people suffer from thyroid diseases, where the prevalence of hypothyroidism and hyperthyroidism has been estimated to be 6.5 and 1.3%, respectively, according to an epidemiological survey conducted in 2013. The prevalence of thyroid nodules was 12.8%, but this may be underestimated due to low sensitivity of the ultrasonographic equipment (13). In 2016, a study on the prevalence of thyroid diseases in China was conducted by Chinese Society of Endocrinology revealed that more than 40% of the population have thyroid diseases (14). An increasing number of studies have investigated the possible relationship between thyroid disease and OLP in other populations (15–17). In our clinical practice, we also observed that many OLP and OLL patients often have a diagnosis of thyroid diseases. However, there is no robust evidence regarding this association in the Chinese ethnic population in the literature to date.
Therefore, we conducted this case–control study to assess the possible relationship between OLP, OLL, and thyroid diseases in Chinese ethnic population.
This study was approved by the Peking University Institutional Review Board, China [PKUSS IRB _AF 01 _v2.0 (2014-10-01)], and all methods were performed in accordance with the relevant guidelines and regulations. Each subject agreed and provided written informed consent before the study.
There were all together 477 patients referred to the Department of Oral Medicine, Peking University School and Hospital of Stomatology, China from October 10th, 2014 to April 30th, 2017, were recruited to the case–control study, including OLP patients, OLL patients, and controls with other diseases including burning mouth syndrome, recurrent aphthous ulcers, and geographic tongue. The controls also included some healthy person without any oral mucosal diseases. All participants were matched for age and sex.
The subjects were recruited according to the following criteria. OLP and OLL patients who were diagnosed clinically and histologically according to WHO diagnostic criteria (18). Briefly for the diagnosis of OLP, the clinical criteria included the presence of bilateral, more or less symmetric lesions; the lesions could be lacelike network of gray–white lines, plaque, atrophic, bullous, or erosive type. The histological criteria included the presence of well-defined band-like zone of cellular infiltration confined to the superficial part of the connective tissue, sign of liquefaction degeneration in the basal cell layer and the absence of epithelial dysplasia. Patients who were clinically or/and histologically compatible with OLP were diagnosed as OLL.
According to the guideline of diagnosis and treatment of thyroid diseases in China (19), the criteria for Hashimoto’s thyroiditis (HT) included the positive detection of thyroid peroxidase antibody (TPOAb) and thyroglobulin (TGAb), with or without local and systemic manifestations including dysphonia, dyspnea, and constipation (20). For hypothyroidism, it included the higher value of thyroid-stimulating hormone (TSH), the lower value of total thyroxine (TT4) and free thyroxine (FT4). For thyroid nodules, it included one or more lump in the thyroid gland detected by B ultrasound. For hyperthyroidism, it included the lower value of TSH, the higher value of TT4, FT4, total triiodothyronine (TT3), and free triiodothyronine (FT3), with or without clinical symptoms and signs of clinical hypermetabolism, such as increased heart rate.
Patients under 18 years old or who were pregnant; patients with a history of action capability restricting or life-threatening systemic diseases like uremia, or other autoimmune diseases which seriously affects the quality of life, such as psoriasis, vitiligo, Behcet disease, or bullous diseases.
The demographic and clinical information from the patients was recorded including age, gender, site of oral lesion, medication history, and general condition.
All subjects were instructed to undergo a thyroid examination including thyroid function (TT3, TT4, FT3, FT4, and TSH), thyroid related antibodies (TPOAb and TGAb). These indicators were detected by chemiluminescence immunoassay (CLIA). Patients also received a B ultrasound of the thyroid gland (19, 21).
Results of the thyroid examination were determined by two senior endocrinologists of Peking University First Hospital to confirm the diagnosis of HT, hypothyroidism, thyroid nodule, and hyperthyroidism accordingly. The thyroid diseases of the subjects were newly diagnosed.
IBM SPSS statistical software (version 19.0, IBM Crop, Armonk, New York, NY, USA) was used for data recording and analyzing.
The prevalence of thyroid diseases in the three groups was calculated. The differences were evaluated by the chi-square test. Furthermore, we calculated the odds ratio (OR) with 95% confidence intervals (CI) for associations between OLP, OLL, and different types of thyroid diseases (HT, hypothyroidism, and thyroid nodule) using logistic regression adjusted for matched age and sex. The significance level was set as P < 0.05.
192 OLP patients (24.45% male and 75.5% female; mean age: 49.53 ± 9.93 years), 123 OLL patients (23.6% male and 76.4% female, mean age: 50.46 ± 9.38 years), and 162 control subjects (19.75% male and 80.25% female; mean age: 50.96 ± 8.96 years) were assessed in our study. The subjects were appropriately age and sex matched. The information of OLP/OLL and control group patients is shown in Table 1.
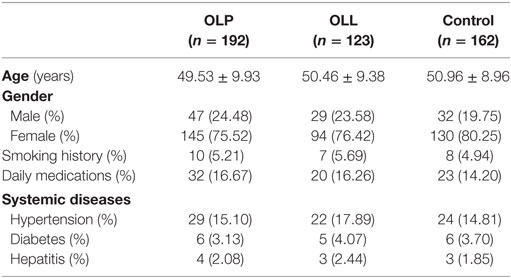
Table 1. Demographics information of the oral lichen planus (OLP)/oral lichenoid lesion (OLL) and control groups.
About 2/3 OLP and OLL patients are non-erosive in clinical type, and the condition of lesion location is shown in Table 2.
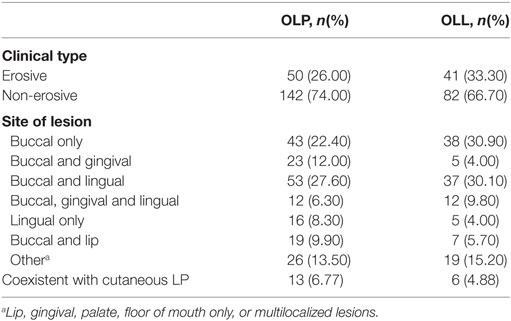
Table 2. Clinical characteristics of oral lichen planus (OLP)/oral lichenoid lesion (OLL) group patients.
The prevalence rate of thyroid disease in OLP and OLL groups was remarkably higher than control group (Table 3). In particular, HT was significantly connected to OLP and OLL; moreover, thyroid nodules were positively associated with OLP but not OLL. Hypothyroidism showed no significant association with OLP/OLL (Table 4). Thyroid hormones and autoantibodies level of OLP/OLL and control are shown in Table 5.

Table 3. Prevalence of thyroid diseases in oral lichen planus (OLP), oral lichenoid lesion (OLL), and control group.
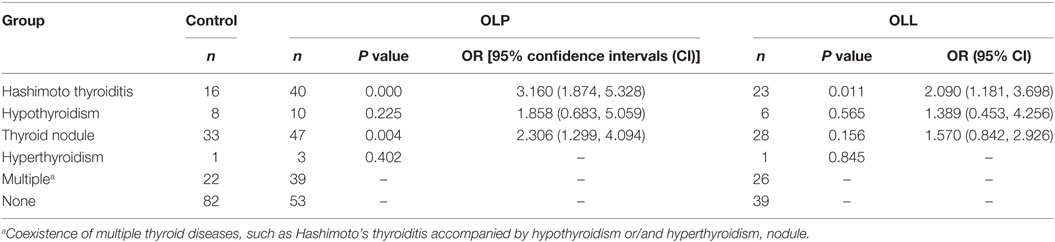
Table 4. Thyroid diseases in oral lichen planus (OLP) and oral lichenoid lesion (OLL) patients and control group by risk factor analysis.
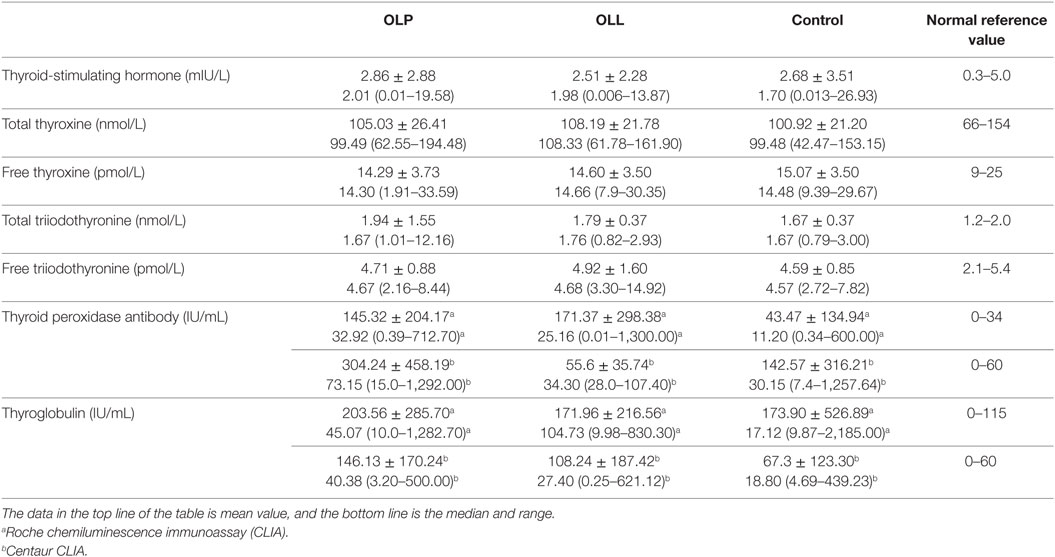
Table 5. Thyroid hormones and autoantibodies level of oral lichen planus (OLP)/oral lichenoid lesion (OLL) and control group.
Although the comprehensive pathogenesis of OLP remains unclear, it has been reported that several systemic disorders are connected to this disorder, including metabolic syndrome, mental health disorders, and autoimmune diseases (1, 2). Of these, thyroid disease has gained an increasing interest in this field more recently. A meta-analysis has been conducted by our team and reported a close relationship between thyroid disease and OLP in many countries (9). However, the situation in Chinese patients is lacking evidence, hence the study concept presented here.
All patients in three groups come from different parts of the country, mostly north of China, such as Beijing, Hebei Province, Shandong Province, and Shanxi Provence. Since 1993, China has been popularizing the consumption of iodized salt. In 2002, the coverage rate of iodized salt in China has reached 98.72%, the iodine deficiency was largely eliminated (22). But it may probably because of the excess iodine or other reasons, there is a high prevalence of thyroid diseases in Chinese general population. In 2016, a study on the prevalence of thyroid diseases in China was conducted by Chinese Society of Endocrinology revealed that more than 40% of the population have thyroid problem (14). In this study, the prevalence of thyroid disease in OLP (72.4%) and OLL (68.8%) patients was significantly higher than the control group (49.4%), demonstrating a positive relationship between these variables.
Among all the thyroid disease, HT and hypothyroidism were most commonly identified types in OLP patients. In accordance with previous studies (23–25), we demonstrated that OLP (OR = 3.16, 95% CI = 1.87–5.33) and OLL (OR = 2.09, 95% CI = 1.18–3.70) patients showed an increased risk of HT. However, hypothyroidism showed no significant association with OLP/OLL. Interestingly, we also found a close association between thyroid nodules and OLP (OR = 2.31; 95% CI = 1.30–4.09) but not OLL (OR = 1.57; 95% CI = 0.84–2.93). These co-existing conditions have not been commonly reported in previous studies.
Hashimoto’s thyroiditis is a prevalent autoimmune endocrine diseases with a prevalence of 5–10% in the general population and is characterized by lymphocytic infiltration and fibrosis of the thyroid gland (26). This pathological characteristic seems to be very similar to the hallmark features of OLP, where there is necrosis of the basal cell layer and dense lymphocyte infiltration in the adjacent connective tissue (27). The pathogenesis of HT is far from understood. It is well accepted that the development of HT depends on an immune defect within the individual where there is genetic susceptibility in combination with causative environmental factors. Evidence indicates that environmental factors such as infections and xenobiotics play important role in the pathogenesis of HT (28). Similar as for OLP, T-cell and B-cell mediated immune responses play key roles in HT, especially the former cell type (1, 29, 30). Although the reasons for comorbidity of OLP and HT are still not well understood, it seems that OLP and HT share some common immunological triggers and pathogenic processes.
Several authors have suggested that there is a common genetic susceptibility for LP and thyroid autoimmune disease by analyzing the links between specific alleles of the HLA genes in these disorders (15). Hawkins et al. found that HLA-DRw9 was closely associated with some autoimmune diseases including Graves’ disease and HT in Chinese populations (31). Similarly, a recent work conducted by Lin and Sun reported the remarkably higher frequencies of occurrences of HLA-DRw9 in Chinese OLP patients (32).
Oral lichen planus and HT are both T-cell-mediated autoimmune diseases that self-antigen or exogenous agents might be the triggers of the immune response (29, 33, 34). In genetically predisposed subjects, a defensive immune reaction could be changed into an autoimmune reaction because of structural comparability between microbial antigens and human autoantigens (35). Benvenga and Guarneri have found that molecular mimicry played important role in a better understanding of AITD (36). The main infectious agents that have been linked to OLP are hepatitis C virus and Helicobacter pylori (37, 38), which has been reportedly linked to AITD (39–41). Some other viruses such as EB virus and cytomegalovirus have been mentioned in OLP, but these appear to be anecdotal observations (42). Further research on infection and the associated mechanisms behind this are needed.
Hypothyroidism was the most commonly reported comorbidity disease in OLP in previous studies (43, 44). However, a negative connection between OLP or OLL and hypothyroidism was observed in this study. This may be partly due to the different methodology (retrospective study versus prospective study). Previous studies have indicated that HT was the most common cause of hypothyroidism (45), but in our study, the autoantibodies level of patients classified to hypothyroidism group was normal, so the different diagnostic criteria for hypothyroidism, which might explain the different findings (16, 44) to some extent.
Thyroid nodule is a common thyroid disorder with a high prevalence in the general population. The percentages have been shown to vary depending on the mode of discovery, e.g., 2–6% (palpation), 19–35% (ultrasound), and 8–65% (autopsy data) (46–48). In the majority of cases, various stages of nodule formation and degeneration could be reflected in the thyroid nodule history, including adenoma, cyst, or colloid nodule (49). Iodine deficiency and external radiation are the most frequent factors contributing to the development of nodule. Other etiological precipitants include smoking, genetic factors, and inflammation, which are also thought to be associated with thyroid nodule (50). The association between OLP and thyroid nodule has not been commonly mentioned in previous studies, further studies are necessary to confirm this connection and fully understand the potential mechanism.
In summary, this study demonstrated that OLP was closely associated with HT and thyroid nodule but not hypothyroidism. The possible mechanism behind this association is still unresolved and merits further investigation. Considering the high prevalence of thyroid disease in OLP and OLL patients, it would be useful for middle-aged women affected by OLP to be screened for thyroid diseases. As for the related mechanism of OLP and thyroid disease, it may be possible to consider the molecular simulation theory as a research hypothesis.
This study was approved by the Peking University Institutional Review Board, China [PKUSS IRB _AF 01 _v2.0 (2014-10-01)], and all methods were performed in accordance with the relevant guidelines and regulations. Each subject agreed and provided written informed consent before the study.
HH and TZ developed the project and implementation scheme. TZ, DL, and HH recruited and collected clinical cases. DL and CL performed the statistical analysis of relevant data. TZ, CL, and DL participated in the writing and modification of the article. CL, QC, and HH critically revised the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This manuscript was edited by the native English speaking scientists of Elixigen Company (Huntington Beach, CA, USA).
This study was supported by the National Natural Science Foundation of China [grant number 81730030] and State Key Laboratory of Oral Diseases Open Fund [grant number SKLOD2017OF10].
1. Srinivas K, Aravinda K, Ratnakar P, Nigam N, Gupta S. Oral lichen planus – review on etiopathogenesis. Natl J Maxillofac Surg (2011) 2:15–6. doi:10.4103/0975-5950.85847
2. Eisen D, Carrozzo M, Bagan SJ, Thongprasom K. Number V oral lichen planus: clinical features and management. Oral Dis (2005) 11:338–49. doi:10.1111/j.1601-0825.2005.01142.x
3. Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med (2007) 36:575–80. doi:10.1111/j.1600-0714.2007.00582.x
4. Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc (2014) 145:45–56. doi:10.14219/jada.2013.10
5. Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill Y, Griffiths M, et al. Update on oral lichen planus: etiopathogenesis and management. Crit Rev Oral Biol Med (1998) 9:86–122. doi:10.1177/10454411980090010501
6. Lodi G, Scully C, Carrozzo M, Griffiths M, Sugerman PB, Thongprasom K. Current controversies in oral lichen planus: report of an international consensus meeting. Part 2. Clinical management and malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2005) 100:164–78. doi:10.1016/j.tripleo.2004.06.076
7. Mozaffari HR, Sharifi R, Sadeghi M. Prevalence of oral lichen planus in diabetes mellitus: a meta-analysis study. Acta Inform Med (2016) 24:390–3. doi:10.5455/aim.2016.24.390-393
8. Lauritano D, Arrica M, Lucchese A, Valente M, Pannone G, Lajolo C, et al. Oral lichen planus clinical characteristics in Italian patients: a retrospective analysis. Head Face Med (2016) 12:18. doi:10.1186/s13005-016-0115-z
9. Li D, Li J, Li C, Chen Q, Hua H. The association of thyroid disease and oral lichen planus: a literature review and meta-analysis. Front Endocrinol (2017) 8:310. doi:10.3389/fendo.2017.00310
10. Dudhia BB, Dudhia SB, Patel PS, Jani YV. Oral lichen planus to oral lichenoid lesions: evolution or revolution. J Oral Maxillofac Pathol (2015) 19:364–70. doi:10.4103/0973-029X.174632
11. Gruters A, Krude H. Update on the management of congenital hypothyroidism. Horm Res (2007) 68(Suppl 5):107–11. doi:10.1159/000110591
12. Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull (2011) 99:39–51. doi:10.1093/bmb/ldr030
13. Yang K. The number of thyroid patients in China is over 200 million. Shanghai Med Pharm (2013) 34:44.
14. Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: a cross-sectional study in 10 cities. Thyroid (2016) 26:1125–30. doi:10.1089/thy.2015.0613
15. Guarneri F, Giuffrida R, Di Bari F, Cannavo SP, Benvenga S. Thyroid autoimmunity and lichen. Front Endocrinol (2017) 8:146. doi:10.3389/fendo.2017.00146
16. Garcia-Pola MJ, Llorente-Pendas S, Seoane-Romero JM, Berasaluce MJ, Garcia-Martin JM. Thyroid disease and oral lichen planus as comorbidity: a prospective case-control study. Dermatology (2016) 232:214–9. doi:10.1159/000442438
17. Lavaee F, Majd M. Evaluation of the association between oral lichen planus and hypothyroidism: a retrospective comparative study. J Dent (2016) 17:38–42.
18. Van Der Meij EH, Van Der Waal I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med (2003) 32:507–12. doi:10.1034/j.1600-0714.2003.00125.x
19. Chinese Society of Endocrinology. A guide to the diagnosis and treatment of thyroid diseases in China – laboratory and auxiliary examinations for thyroid diseases. Chin J Intern Med (2007) 46:697–702.
20. Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev (2014) 13:391–7. doi:10.1016/j.autrev.2014.01.007
21. Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid (2003) 13:3–126. doi:10.1089/105072503321086962
22. Cao X, Zheng Q, Wang J, Xu J, Gu Y, Wang H, et al. Study on national iodized salt monitoring in 2011. Chin J Control Endem Dis (2013) 1(36–38):45.
23. Chang JY, Chiang CP, Hsiao CK, Sun A. Significantly higher frequencies of presence of serum autoantibodies in Chinese patients with oral lichen planus. J Oral Pathol Med (2009) 38:48–54. doi:10.1111/j.1600-0714.2008.00686.x
24. Ebrahimi M, Lundqvist L, Wahlin YB, Nylander E. Mucosal lichen planus, a systemic disease requiring multidisciplinary care: a cross-sectional clinical review from a multidisciplinary perspective. J Low Genit Tract Dis (2012) 16:377–80. doi:10.1097/LGT.0b013e318247a907
25. Lo ML, Santarelli A, Campisi G, Lacaita M, Favia G. Possible link between Hashimoto’s thyroiditis and oral lichen planus: a novel association found. Clin Oral Investig (2013) 17:333–6. doi:10.1007/s00784-012-0767-4
26. Usha MV, Sundaram KR, Unnikrishnan AG, Jayakumar RV, Nair V, Kumar H. High prevalence of undetected thyroid disorders in an iodine sufficient adult south Indian population. J Indian Med Assoc (2009) 107:72–7.
27. Rossi S, Ciarrocca K. Oral lichen planus and lichenoid mucositis. Dent Clin North Am (2014) 58:299–313. doi:10.1016/j.cden.2014.01.001
28. Benvenga S, Antonelli A, Vita R. Thyroid nodules and thyroid autoimmunity in the context of environmental pollution. Rev Endocr Metab Disord (2015) 16:319–40. doi:10.1007/s11154-016-9327-6
29. Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci (2007) 49:89–106. doi:10.2334/josnusd.49.89
30. Erdogan M, Erdem N, Cetinkalp S, Ozgen AG, Saygili F, Yilmaz C, et al. Demographic, clinical, laboratory, ultrasonographic, and cytological features of patients with Hashimoto’s thyroiditis: results of a university hospital of 769 patients in Turkey. Endocrine (2009) 36:486–90. doi:10.1007/s12020-009-9258-z
31. Hawkins BR, Lam KS, Ma JT, Wang C, Yeung RT. Strong association between HLA DRw9 and Hashimoto’s thyroiditis in southern Chinese. Acta Endocrinol (1987) 114:543–6.
32. Lin SC, Sun A. HLA-DR and DQ antigens in Chinese patients with oral lichen planus. J Oral Pathol Med (1990) 19:298–300. doi:10.1111/j.1600-0714.1990.tb00848.x
33. Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: an update on pathogenesis and treatment. J Oral Maxillofac Pathol (2011) 15:127–32. doi:10.4103/0973-029X.84474
34. Esfahanian F, Ghelich R, Rashidian H, Jadali Z. Increased levels of serum interleukin-17 in patients with Hashimoto’s thyroiditis. Indian J Endocrinol Metab (2017) 21:551–4. doi:10.4103/ijem.IJEM_412_16
35. Cusick MF, Libbey JE, Fujinami RS. Molecular mimicry as a mechanism of autoimmune disease. Clin Rev Allergy Immunol (2012) 42:102–11. doi:10.1007/s12016-011-8293-8
36. Benvenga S, Guarneri F. Molecular mimicry and autoimmune thyroid disease. Rev Endocr Metab Disord (2016) 17:485–98. doi:10.1007/s11154-016-9363-2
37. Alaizari NA, Al-Maweri SA, Al-Shamiri HM, Tarakji B, Shugaa-Addin B. Hepatitis C virus infections in oral lichen planus: a systematic review and meta-analysis. Aust Dent J (2016) 61:282–7. doi:10.1111/adj.12382
38. Kazanowska-Dygdała M, Duś I, Radwan-Oczko M. The presence of Helicobacter pylori in oral cavities of patients with leukoplakia and oral lichen planus. J Appl Oral Sci (2016) 24:18–23. doi:10.1590/1678-775720150203
39. Papamichael KX, Papaioannou G, Karga H, Roussos A, Mantzaris GJ. Helicobacter pylori infection and endocrine disorders: is there a link? World J Gastroenterol (2009) 15:2701. doi:10.3748/wjg.15.2701
40. Shukla SK, Singh G, Ahmad S, Pant P. Infections, genetic and environmental factors in pathogenesis of autoimmune thyroid diseases. Microb Pathog (2018) 116:279–88. doi:10.1016/j.micpath.2018.01.004
41. Fallahi P, Ferrari SM, Vita R, Benvenga S, Antonelli A. The role of human parvovirus B19 and hepatitis C virus in the development of thyroid disorders. Rev Endocr Metab Disord (2016) 17:529–35. doi:10.1007/s11154-016-9361-4
42. Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol (2010) 28:100–8. doi:10.1016/j.clindermatol.2009.03.004
43. Dreiher J, Shapiro J, Cohen AD. Lichen planus and dyslipidaemia: a case-control study. Br J Dermatol (2009) 161:626–9. doi:10.1111/j.1365-2133.2009.09235.x
44. Siponen M, Huuskonen L, Laara E, Salo T. Association of oral lichen planus with thyroid disease in a Finnish population: a retrospective case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2010) 110:319–24. doi:10.1016/j.tripleo.2010.04.001
45. Santoro D, Vadala C, Siligato R, Buemi M, Benvenga S. Autoimmune thyroiditis and glomerulopathies. Front Endocrinol (2017) 8:119. doi:10.3389/fendo.2017.00119
46. Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (1977) 7:481–93. doi:10.1111/j.1365-2265.1977.tb01340.x
47. Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med (2004) 351:1764–71. doi:10.1056/NEJMcp031436
48. Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab (2008) 22:901–11. doi:10.1016/j.beem.2008.09.019
49. Meier CA. Thyroid nodules: pathogenesis, diagnosis and treatment. Baillieres Best Pract Res Clin Endocrinol Metab (2000) 14:559–75. doi:10.1053/beem.2000.0103
Keywords: oral lichen planus, oral lichenoid lesion, Hashimoto’s thyroiditis, hypothyroidism, thyroid nodule, molecular mimicry
Citation: Zhou T, Li D, Chen Q, Hua H and Li C (2018) Correlation Between Oral Lichen Planus and Thyroid Disease in China: A Case–Control Study. Front. Endocrinol. 9:330. doi: 10.3389/fendo.2018.00330
Received: 13 February 2018; Accepted: 31 May 2018;
Published: 18 June 2018
Edited by:
Alessandro Antonelli, Università degli Studi di Pisa, ItalyReviewed by:
Roberto Vita, Università degli Studi di Messina, ItalyCopyright: © 2018 Zhou, Li, Chen, Hua and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunlei Li, bGljaHVubGVpMTJAaG90bWFpbC5jb20=
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.