- 1Rheumatology Unit, University of Pisa, Pisa, Italy
- 2Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
Sjögren’s syndrome (SS) and autoimmune thyroid diseases (AITD) may frequently coexist in clinical practice, resulting in a complex overlapping disorder that represents a particular example of the expression of heterogeneity in patients with autoimmune disorders. Objective of this review was to describe the prevalence of the SS–AITD association in the most recent literature, exploring in particular to what extent the presence of AITD might influence the clinical expression of SS and vice versa. Moreover, we summarized some of the proposed genetic, biologic, and molecular mechanisms implied in the pathogenesis of AITD–SS association. Finally, we explored risk factors for lymphoma development in both AITD and SS. We performed a Medline search of English language articles published in the PubMed database in order to provide a critical overview of the recent literature on pathogenesis and clinical features of AITD–SS overlapping disease. All the articles were critically analyzed to select the most relevant contributions.
Introduction
Sjögren’s syndrome (SS) is a complex and heterogeneous autoimmune disease that frequently co-occurs with both organ-specific and non-organ specific, systemic rheumatic diseases (i.e., rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis) offering a unique opportunity to investigate pathogenesis, long-term-evolution, and outcome of different autoimmune phenotypes and subsets (1–5).
The association of SS and autoimmune thyroid diseases (AITD) has been largely documented, suggesting that AITD could be overrepresented in patient with SS with respect to general population and vice versa (6). Interestingly, Rojas-Villarraga et al. (7) analyzing prevalence of multiple autoimmune syndromes in 1,083 patients belonging to four autoimmune disease cohorts described AITD and SS as the most frequent coexisting autoimmune disorders in single patients. Overall, then, it has been hypothesized that common genetic, immunologic, and biologic factors may be implied in SS and AITD, leading to the coexistence of these two conditions (8). Moreover, both SS and AITD may lead to the development of non-Hodgkin’s lymphoma (NHL) highlighting the relationship between similar autoimmunity pathways and lymphoproliferation (9, 10). Although the majority of data support an increased prevalence of AITD in SS patients, some authors have suggested that this observation might be related to the age and sex distribution of SS and AITD rather than representing a true association. However, literature data are difficult to compare due to the fact that different SS classification criteria and AITD definitions have been utilized over the years, making it difficult to define the exact prevalence of SS–AITD association (11, 12).
In this review, we aimed at describing the frequency of the SS–AITD association in the most recent literature, exploring, in particular, to what extent the presence of AITD may influence the clinical expression of SS. Moreover, we will summarize some of the proposed pathogenetic mechanisms for the coincidence of SS and AITD and the common biologic factors involved into lymphoproliferative complications.
Prevalence of SS in AITD: A Clinical Spectrum Ranging From Isolated Sicca Symptoms to Full-Blown SS
Autoimmune thyroid diseases, namely, Hashimoto’s thyroiditis (HT) and Graves’ disease (GD), are organ-specific autoimmune disorders, essentially resulting from a T cell-mediated immune attack of the thyroid resulting into lymphocytic infiltration of the thyroid parenchyma (13). The association between AITD and other organ specific (polyglandular autoimmune syndromes), or systemic rheumatic disorders has been widely described. SS is perhaps the most frequently rheumatic autoimmune disease associated with AITD, and particularly with HT. Several authors have reported that a full-blown SS could be 10 times higher in AITD than in the general population (14). In fact, the prevalence of SS in AITD has been assessed by several heterogeneous studies over the time and apparently varies from 3% up to 32% (15–17).
In 1992, Warfvinge et al. (18), analyzing different degrees of morphologic and functional salivary gland changes in AITD, found that 11 patients out of 19 presented xerostomia, a compromised unstimulated salivary flow and alterations of their lower lip biopsy, and/or parotid scintigraphy. Six of them presented a true SS. Similarly, Tektonidou (16) in a cohort of 58 patients with antinuclear antibodies positive AITD found that 9% fulfilled the criteria for SS. These data on small cohorts were confirmed also in large population studies over the years. For example, Biro et al. (19) evaluating the prevalence of SS in 426 patients with HT or GD found that SS had a prevalence of 17% in HT and of 5% in GD. Similarly, Lu et al. (20) observed that the risk of SS in patients with thyroiditis was 3.6 times higher than in individuals without thyroiditis.
The spectrum of sicca symptoms in patients with AITD that do not fulfill the criteria for SS is even more common. More specifically, Coll et al. (21) in a cohort of 176 patients with AITD found that 19 of 52 (37%) patients presented isolated xerostomia and 39/170 (23%) isolated keratoconjunctivitis sicca. Other authors described an isolated positivity for antinuclear antibodies in 25–55% of patients with AITD. The real implication of antinuclear antibodies positivity in AITD patients has to be clarified, but it is generally believed that they may reflect polyclonal activation and antibody production that over time may trigger the development of concomitant systemic autoimmune diseases including SS (22). In clinical settings, patients with AITD and a positivity for antinuclear antibodies should then be monitored more closely for the development of SS during the follow-up.
Overall, the available literature suggests that both a full blown SS and “incomplete” subsets of SS may be quite common among patients with AITD. Among these incomplete forms, an important differential diagnosis is represented by patients with sicca symptoms, affected by fibromyalgia. Patients with fibromyalgia often present dry eyes and dry mouth and noteworthy, they may present AITD or a positivity for thyroid auto antibodies as well. In the study by Mavragani et al. (23), fibromyalgia patients with sicca symptoms presented thyroid auto antibodies with a prevalence of 60%. Besides fibromyalgia, a number of other systemic disorders associated with AITD also involves salivary glands and should be considered as well in the differential diagnosis. Infectious diseases like hepatitis C virus and Ebstein B virus infections are among the major mimickers of SS in AITD patients, especially due to viral lymphotropism and epithelial tropism (24, 25). Immune disorders including sarcoidosis and IgG4 disease may be considered as well. The latter represents a novel entity characterized by high serum IgG4 and IgG4 plasma cell-mediated fibro-inflammatory lesions that may involve not only thyroid but also lung, pancreas, kidney, and salivary and lacrymal glands (26). More specifically, IgG4 has been linked to four types of thyroid diseases including: Riedel’s thyroiditis, fibrosing variant of Hashimoto’s thyroiditis, IgG4-related Hashimoto’s thyroiditis, and GD with elevated IgG4 levels (27). The pathogenetic role of IgG4 in these IgG4-related thyroid diseases, however, still remains poorly understood. Those patients may often present with an SS-like subacute diffuse enlargement of lacrymal and salivary glands and sicca symptoms. A minor salivary gland biopsy is often necessary to distinguish IgG4 disease from SS (28, 29).
Prevalence of AITD in SS: A Specific Subset of the Disease?
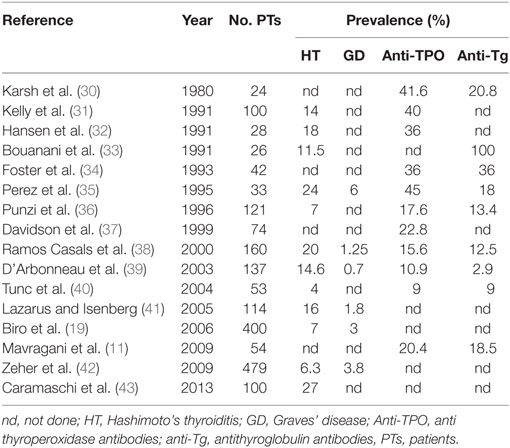
Several uncontrolled studies have described the presence of AITD in SS with frequencies ranging between 10 and 30% (8). Table 1 summarizes the most important studies reporting frequency of AITD in SS. The differences observed among these studies might be linked to the different ethnic origin of the patients or to the diagnostic criteria adopted for both SS and HT classification.
As far as the clinical manifestation of AITD in SS, three studies are particularly relevant. In the study by Lazarus and Isenberg (41), 16% of the SS patients developed AITD. The most common clinical manifestation of AITD in SS was hypothyroidism, even though subclinical AITD was probably more common. In the vast majority of the cases, hypothyroidism had been diagnosed before the diagnosis of SS: similarly, Jara et al. (6) in a cohort of 160 primary SS patients found evidence of thyroid disease in 36% of patients: 20% were diagnosed as AITD and 16% were diagnosed as non-AITD. The clinical pattern in more than half of primary SS patients was subclinical hypothyroidism. Finally, in the study by Zeher et al. (42) 479 patients with pSS were investigated with regard to several types of thyroid disease. Overall, thyroid dysfunction was found in 95 patients (21.25%). Apparently, the diagnosis of HT and GD may be present either before or after the onset of SS but, differently from the study by Lazarus and Isenberg (41), both HT and GD were more likely to follow SS. The authors found that in 50% of the cases SS preceded HT by 5.5 years. Interestingly, D’Arbonneau et al. (39) showed that the presence of thyroid-related autoantibodies represented a risk factor for development of AITD during follow-up.
Finally, regarding the clinical impact of AITD on the clinical course, literature data are quite heterogeneous especially due to different control groups that have been included (i.e., SS–AITD versus SS without AITD; SS–AITD versus AITD) and to the classification criteria adopted to define SS. However, Caramaschi et al. (43) demonstrated that the association of AITD in patients suffering from SS defined a subset of patients with milder disease and normal complement 4 levels. More specifically, patients with AITD–SS had less evidence of cryoglobulins, palpable purpura, peripheral neuropathy, and lymphoma. This observation has been confirmed by several additional studies. However, it is nowadays widely accepted that AITD–SS patients are at a greater risk of developing additional autoimmune diseases such as autoimmune liver diseases, inflammatory bowel diseases, and systemic lupus erythematosus and should be closely monitored over the follow-up due to their tendency to present widespread disorders of their immune systems.
SS and AITD: Common Pathogenetic Mechanisms From Epithelitis to Non-Hodgkin Lymphoma
A large amount of data have highlighted that AITD and SS can be considered as pathogenetically correlated. First of all, these two disorders are characterized by similar histological features with a tissue infiltrate that consists primarily of CD4+ T lymphocytes and the possible formation of germinal center-like structures unrevealing B cell activation (8). Second, from a genetic point of view, the two conditions present a similar background with thyroid and epithelial cells expressing the same HLA molecules class II: HLA-B8 and HLA-DR3 (8). In particular, HLA-B8 and -DR3 haplotypes have been reported with a higher frequency in both primary SS and AITD whereas cytotoxic T lymphocytic antigen 4 gene polymorphisms have been reported more frequently in patients with AITD and other autoimmune diseases including rheumatoid arthritis (15).
A third point to take into account is the existence of animal models that spontaneously develop both SS and AITD. Thyroiditis and SS in NOD.H-2h4 mice are chronic autoimmune diseases that develop relatively early in life and persist for the life of the animal making them an excellent model to test therapeutic protocols over a long period of time (44).
Another point reinforcing the pathogenetical link between AITD and SS is represented by the crucial role of the epithelial cells in orchestrating the tissue inflammation and the involvement of several regulatory chemokines, such as the IFN-γ-inducible protein 10 (IP-10/CXCL10) in initiating and perpetuating the autoimmune process. CXCL10 exerts its function through binding to chemokine (C-X-C motif) receptor 3 (CXCR3), and its production is stimulated by IFN-γ and TNF-α, whose production in turn is provided by T helper 1 cells (Th1) (45).
In AITD, it has been postulated that CXCL10 could be a marker of a stronger and more aggressive inflammatory response, subsequently leading to thyroid destruction and hypothyroidism (45). Similarly, epithelial cells from SS patients apparently produce CXCL9 and CXCL10 as well, while most of the CD3+ lymphocytes in periductal foci express CXCR3, thus contributing to salivary gland damage (46).
Another cytokine that exerts a fundamental role in the pathogenesis of both AITD and SS is B-cell activating factor that seems to be especially important for the survival of autoreactive B cells (47). Serum B-cell activating factor concentrations were described as significantly higher in GD and correlated with serum antithyroglobulin antibodies (48). B-cell activating factor has been also correlated to disease activity and severity in SS exerting a key role in lymphoproliferative complications (49–52).
Not surprisingly, both AITD and SS may evolve into B-cell NHLs of salivary glands and thyroid, with a prevalence of 0.5 and 5%, respectively (9). Intriguingly cases of patients with AITD, SS, and NHL have been described even if the vast majority of the studies seem to support the evidence that AITD–SS patients have a lower risk of developing NHL compared to SS patients without AITD (53–56). It remains a matter of debate whether the presence of SS in AITD patients may increase the risk of thyroid lymphoma in AITD patients.
The striking association between SS, AITD, and NHL has provided valuable insights into the relationship between autoimmunity and lymphoproliferation. In this multistep process, key players are represented by chronic inflammation and sustained antigenic stimulation that are apparently able to promote B-cell activation and proliferation. Immune deregulation, moreover, sometimes promotes B cell proliferation mediated by infectious agents (i.e., suppression/dysregulation of T-cells leading to EBV-driven B-cell proliferation) (57, 58). Ultimately, SS and AITD seem to be characterized by a wide spectrum of genetic and molecular abnormalities that culminate in uncontrolled B-cell activation, proliferation, and neoplastic transformation.
Conclusion
The coexistence of SS and AITD occurs frequently in clinical practice probably due to common pathogenetic mechanisms shared by these two conditions. From a practical point of view, it is important to screen patients with SS for AITD and vice versa because the presence of the two disorders may influence patients’ clinical presentation and long-term outcome. It is widely accepted that AITD–SS patients may have a milder phenotype of SS with a lower risk for lymphoma development. However, it remains unclear whether a concomitant diagnosis of SS may increase or not the risk for thyroid lymphoproliferative complications in AITD patients. Intriguingly, AITD–SS patients over the follow up frequently present additional autoimmune diseases and should be closely monitored especially for liver autoimmunity. Further studies are warranted to explore genetic, biologic, and molecular factors underlying the SS–AITD association in order to provide further insights for the comprehension of this complex and varied subset of autoimmunity.
Author Contributions
CB, FF, MM, PF, and AA gave substantial contribution in the conception and design of the work, in the literature research and in writing the paper; gave the final approval of the version to be published; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CB, AA, and PF revised it critically.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer DL and handling Editor declared their shared affiliation.
Funding
Funding provided by the University of Pisa.
References
1. Brito-Zeron P, Baldini C, Bootsma H, Bowman SJ, Jonsson R, Mariette X, et al. Sjogren syndrome. Nat Rev Dis Primers (2016) 2:16047. doi:10.1038/nrdp.2016.47
2. Baldini C, Pepe P, Quartuccio L, Priori R, Bartoloni E, Alunno A, et al. Primary Sjogren’s syndrome as a multi-organ disease: impact of the serological profile on the clinical presentation of the disease in a large cohort of Italian patients. Rheumatology (Oxford) (2014) 53(5):839–44. doi:10.1093/rheumatology/ket427
3. Barone F, Colafrancesco S. Sjogren’s syndrome: from pathogenesis to novel therapeutic targets. Clin Exp Rheumatol (2016) 34(4 Suppl 98):58–62.
4. Luciano N, Valentini V, Calabro A, Elefante E, Vitale A, Baldini C, et al. One year in review 2015: Sjogren’s syndrome. Clin Exp Rheumatol (2015) 33(2):259–71.
5. Baldini C, Mosca M, Della Rossa A, Pepe P, Notarstefano C, Ferro F, et al. Overlap of ACA-positive systemic sclerosis and Sjogren’s syndrome: a distinct clinical entity with mild organ involvement but at high risk of lymphoma. Clin Exp Rheumatol (2013) 31(2):272–80.
6. Jara LJ, Navarro C, Brito-Zeron Mdel P, Garcia-Carrasco M, Escarcega RO, Ramos-Casals M. Thyroid disease in Sjogren’s syndrome. Clin Rheumatol (2007) 26(10):1601–6. doi:10.1007/s10067-007-0638-6
7. Rojas-Villarraga A, Amaya-Amaya J, Rodriguez-Rodriguez A, Mantilla RD, Anaya JM. Introducing polyautoimmunity: secondary autoimmune diseases no longer exist. Autoimmune Dis (2012) 2012:254319. doi:10.1155/2012/254319
8. Amador-Patarroyo MJ, Arbelaez JG, Mantilla RD, Rodriguez-Rodriguez A, Cardenas-Roldan J, Pineda-Tamayo R, et al. Sjogren’s syndrome at the crossroad of polyautoimmunity. J Autoimmun (2012) 39(3):199–205. doi:10.1016/j.jaut.2012.05.008
9. Teixeira Mendes LS, Wotherspoon A. Marginal zone lymphoma: associated autoimmunity and auto-immune disorders. Best Pract Res Clin Haematol (2017) 30(1–2):65–76. doi:10.1016/j.beha.2016.07.006
10. Baldini C, Pepe P, Luciano N, Ferro F, Talarico R, Grossi S, et al. A clinical prediction rule for lymphoma development in primary Sjogren’s syndrome. J Rheumatol (2012) 39(4):804–8. doi:10.3899/jrheum.110754
11. Mavragani CP, Fragoulis GE, Moutsopoulos HM. Endocrine alterations in primary Sjogren’s syndrome: an overview. J Autoimmun (2012) 39(4):354–8. doi:10.1016/j.jaut.2012.05.011
12. Baldini C, Talarico R, Tzioufas AG, Bombardieri S. Classification criteria for Sjogren’s syndrome: a critical review. J Autoimmun (2012) 39(1–2):9–14. doi:10.1016/j.jaut.2011.12.006
13. Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev (2015) 14(2):174–80. doi:10.1016/j.autrev.2014.10.016
14. Bourji K, Gatto M, Cozzi F, Doria A, Punzi L. Rheumatic and autoimmune thyroid disorders: a causal or casual relationship? Autoimmun Rev (2015) 14(1):57–63. doi:10.1016/j.autrev.2014.10.007
15. Lazurova I, Benhatchi K, Rovensky J, Kozakova D, Wagnerova H, Tajtakova M, et al. Autoimmune thyroid disease and autoimmune rheumatic disorders: a two-sided analysis. Ann N Y Acad Sci (2009) 1173:211–6. doi:10.1111/j.1749-6632.2009.04809.x
16. Tektonidou MG. Presence of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med (2010) 123(10):e23. doi:10.1016/j.amjmed.2010.03.030
17. Soy M, Guldiken S, Arikan E, Altun BU, Tugrul A. Frequency of rheumatic diseases in patients with autoimmune thyroid disease. Rheumatol Int (2007) 27(6):575–7. doi:10.1007/s00296-006-0263-8
18. Warfvinge G, Larsson A, Henricsson V, Ericsson UB, Hansen B, Manthorpe R. Salivary gland involvement in autoimmune thyroiditis, with special reference to the degree of association with Sjogren’s syndrome. Oral Surg Oral Med Oral Pathol (1992) 74(3):288–93. doi:10.1016/0030-4220(92)90061-T
19. Biro E, Szekanecz Z, Czirjak L, Danko K, Kiss E, Szabo NA, et al. Association of systemic and thyroid autoimmune diseases. Clin Rheumatol (2006) 25(2):240–5. doi:10.1007/s10067-005-1165-y
20. Lu MC, Yin WY, Tsai TY, Koo M, Lai NS. Increased risk of primary Sjogren’s syndrome in female patients with thyroid disorders: a longitudinal population-based study in Taiwan. PLoS One (2013) 8(10):e77210. doi:10.1371/journal.pone.0077210
21. Coll J, Anglada J, Tomas S, Reth P, Goday A, Millan M, et al. High prevalence of subclinical Sjogren’s syndrome features in patients with autoimmune thyroid disease. J Rheumatol (1997) 24(9):1719–24.
22. Tektonidou MG, Anapliotou M, Vlachoyiannopoulos P, Moutsopoulos HM. Presence of systemic autoimmune disorders in patients with autoimmune thyroid diseases. Ann Rheum Dis (2004) 63(9):1159–61. doi:10.1136/ard.2004.022624
23. Mavragani CP, Skopouli FN, Moutsopoulos HM. Increased prevalence of antibodies to thyroid peroxidase in dry eyes and mouth syndrome or sicca asthenia polyalgia syndrome. J Rheumatol (2009) 36(8):1626–30. doi:10.3899/jrheum.081326
24. Dittfeld A, Gwizdek K, Michalski M, Wojnicz R. A possible link between the Epstein-Barr virus infection and autoimmune thyroid disorders. Cent Eur J Immunol (2016) 41(3):297–301. doi:10.5114/ceji.2016.63130
25. Ferri C, Ramos-Casals M, Zignego AL, Arcaini L, Roccatello D, Antonelli A, et al. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun Rev (2016) 15(12):1145–60. doi:10.1016/j.autrev.2016.09.006
26. Stone JH. IgG4-related disease: pathophysiologic insights drive emerging treatment approaches. Clin Exp Rheumatol (2016) 34(4 Suppl 98):66–8.
27. Kottahachchi D, Topliss DJ. Immunoglobulin G4-related thyroid diseases. Eur Thyroid J (2016) 5(4):231–9. doi:10.1159/000452623
28. Delle Sedie A, Baldini C, Donati V, Mosca M. Mikulicz’s disease: a long-term follow-up case report. Clin Exp Rheumatol (2012) 30(4):596.
29. Fragoulis GE, Zampeli E, Moutsopoulos HM. IgG4-related sialadenitis and Sjogren’s syndrome. Oral Dis (2016) 23(2):152–6.
30. Karsh J, Pavlidis N, Weintraub BD, Moutsopoulos HM. Thyroid disease in Sjogren’s syndrome. Arthritis Rheum (1980) 23(11):1326–9. doi:10.1002/art.1780231118
31. Kelly CA, Foster H, Pal B, Gardiner P, Malcolm AJ, Charles P, et al. Primary Sjogren’s syndrome in north east England – a longitudinal study. Br J Rheumatol (1991) 30(6):437–42. doi:10.1093/rheumatology/30.6.437
32. Hansen BU, Ericsson UB, Henricsson V, Larsson A, Manthorpe R, Warfvinge G. Autoimmune thyroiditis and primary Sjogren’s syndrome: clinical and laboratory evidence of the coexistence of the two diseases. Clin Exp Rheumatol (1991) 9(2):137–41.
33. Bouanani M, Bataille R, Piechaczyk M, Salhi SL, Pau B, Bastide M. Autoimmunity to human thyroglobulin. Respective epitopic specificity patterns of anti-human thyroglobulin autoantibodies in patients with Sjogren’s syndrome and patients with Hashimoto’s thyroiditis. Arthritis Rheum (1991) 34(12):1585–93. doi:10.1002/art.1780341218
34. Foster H, Fay A, Kelly C, Charles P, Walker D, Griffiths I. Thyroid disease and other autoimmune phenomena in a family study of primary Sjogren’s syndrome. Br J Rheumatol (1993) 32(1):36–40. doi:10.1093/rheumatology/32.1.36
35. Perez B, Kraus A, Lopez G, Cifuentes M, Alarcon-Segovia D. Autoimmune thyroid disease in primary Sjogren’s syndrome. Am J Med (1995) 99(5):480–4. doi:10.1016/S0002-9343(99)80223-X
36. Punzi L, Ostuni PA, Betterle C, De Sandre P, Botsios C, Gambari PF. Thyroid gland disorders in primary Sjogren’s syndrome. Rev Rhum Engl Ed (1996) 63(11):809–14.
37. Davidson BK, Kelly CA, Griffiths ID. Primary Sjogren’s syndrome in the North East of England: a long-term follow-up study. Rheumatology (Oxford) (1999) 38(3):245–53. doi:10.1093/rheumatology/38.3.245
38. Ramos-Casals M, Garcia-Carrasco M, Cervera R, Gaya J, Halperin I, Ubieto I, et al. Thyroid disease in primary Sjogren syndrome. Study in a series of 160 patients. Medicine (Baltimore) (2000) 79(2):103–8. doi:10.1097/00005792-200003000-00004
39. D’Arbonneau F, Ansart S, Le Berre R, Dueymes M, Youinou P, Pennec YL. Thyroid dysfunction in primary Sjogren’s syndrome: a long-term followup study. Arthritis Rheum (2003) 49(6):804–9. doi:10.1002/art.11460
40. Tunc R, Gonen MS, Acbay O, Hamuryudan V, Yazici H. Autoimmune thyroiditis and anti-thyroid antibodies in primary Sjogren’s syndrome: a case-control study. Ann Rheum Dis (2004) 63(5):575–7. doi:10.1136/ard.2003.010058
41. Lazarus MN, Isenberg DA. Development of additional autoimmune diseases in a population of patients with primary Sjogren’s syndrome. Ann Rheum Dis (2005) 64(7):1062–4. doi:10.1136/ard.2004.029066
42. Zeher M, Horvath IF, Szanto A, Szodoray P. Autoimmune thyroid diseases in a large group of Hungarian patients with primary Sjogren’s syndrome. Thyroid (2009) 19(1):39–45. doi:10.1089/thy.2007.0398
43. Caramaschi P, Biasi D, Caimmi C, Scambi C, Pieropan S, Barausse G, et al. The co-occurrence of Hashimoto thyroiditis in primary Sjogren’s syndrome defines a subset of patients with milder clinical phenotype. Rheumatol Int (2013) 33(5):1271–5. doi:10.1007/s00296-012-2570-6
44. Kayes TD, Weisman GA, Camden JM, Woods LT, Bredehoeft C, Downey EF, et al. New murine model of early onset autoimmune thyroid disease/hypothyroidism and autoimmune exocrinopathy of the salivary gland. J Immunol (2016) 197(6):2119–30. doi:10.4049/jimmunol.1600133
45. Antonelli A, Ferrari SM, Giuggioli D, Ferrannini E, Ferri C, Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev (2014) 13(3):272–80. doi:10.1016/j.autrev.2013.10.010
46. Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren’s syndrome. Arthritis Rheum (2002) 46(10):2730–41. doi:10.1002/art.10577
47. Sjostrand M, Johansson A, Aqrawi L, Olsson T, Wahren-Herlenius M, Espinosa A. The Expression of BAFF is controlled by IRF transcription factors. J Immunol (2016) 196(1):91–6. doi:10.4049/jimmunol.1501061
48. Lin JD, Wang YH, Fang WF, Hsiao CJ, Chagnaadorj A, Lin YF, et al. Serum BAFF and thyroid autoantibodies in autoimmune thyroid disease. Clin Chim Acta (2016) 462:96–102. doi:10.1016/j.cca.2016.09.004
49. Varin MM, Le Pottier L, Youinou P, Saulep D, Mackay F, Pers JO. B-cell tolerance breakdown in Sjogren’s syndrome: focus on BAFF. Autoimmun Rev (2010) 9(9):604–8. doi:10.1016/j.autrev.2010.05.006
50. Daridon C, Devauchelle V, Hutin P, Le Berre R, Martins-Carvalho C, Bendaoud B, et al. Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjogren’s syndrome. Arthritis Rheum (2007) 56(4):1134–44. doi:10.1002/art.22458
51. Youinou P, Daridon C, Saraux A, Devauchelle V, Pers JO. Is B-cell the conductor of the lymphocyte orchestra in the salivary glands of patients with primary Sjogren’s syndrome. Clin Exp Rheumatol (2006) 24(5):491–2.
52. Carubbi F, Cipriani P, Di Benedetto P, Ruscitti P, Alunno A, Gerli R, et al. Persistence of focal lymphocytic sialadenitis in patients with primary Sjogren’s syndrome treated with rituximab: a possible role for glandular BAFF. Clin Exp Rheumatol (2016) 3(2):152–6.
53. Coutant G, Algayres JP, Rapp C, Jean R, Desrame C, Bechade D, et al. [Thyroid lymphoma and Gougerot-Sjogren syndrome]. Rev Med Interne (1999) 20(4):374–5. doi:10.1016/S0248-8663(99)83083-5
54. Androulaki A, Syriou V, Lazaris AC, Paterakis T, Pikazis D, Papathomas T, et al. Maltoma of the thyroid and Sjogren’s syndrome in a woman with Hashimoto’s thyroiditis. Endocr Pathol (2006) 17(1):89–94. doi:10.1385/EP:17:1:89
55. Mazur-Roszak M, Litwiniuk M, Lacka K. [Lymphoma of the thyroid in a patient with autoimmune thyroiditis and Sjogren’s syndrome – case report]. Pol Merkur Lekarski (2008) 25(146):155–7.
56. Serefhanoglu S, Tapan U, Ertenli I, Kalyoncu U, Uner A. Primary thyroid marginal zone B-cell lymphoma MALT-type in a patient with rheumatoid arthritis. Med Oncol (2010) 27(3):826–32. doi:10.1007/s12032-009-9293-x
57. Adem J, Eray M, Eeva J, Nuutinen U, Pelkonen J. Advantages of targeting B cell receptor complex to treat B-cell derived autoimmune diseases and lymphomas. Mol Immunol (2017) 88:135–7. doi:10.1016/j.molimm.2017.05.023
Keywords: Sjögren’s syndrome, autoimmune thyroid diseases, non-Hodgkin’s lymphoma, comorbidities, pathogenesis
Citation: Baldini C, Ferro F, Mosca M, Fallahi P and Antonelli A (2018) The Association of Sjögren Syndrome and Autoimmune Thyroid Disorders. Front. Endocrinol. 9:121. doi: 10.3389/fendo.2018.00121
Received: 30 July 2017; Accepted: 09 March 2018;
Published: 03 April 2018
Edited by:
Terry Francis Davies, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Bijay Vaidya, University of Exeter, United KingdomDerek LeRoith, Icahn School of Medicine at Mount Sinai, United States
Copyright: © 2018 Baldini, Ferro, Mosca, Fallahi and Antonelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Baldini, Y2hpYXJhLmJhbGRpbmk3NEBnbWFpbC5jb20=
 Chiara Baldini
Chiara Baldini Francesco Ferro1
Francesco Ferro1 Poupak Fallahi
Poupak Fallahi Alessandro Antonelli
Alessandro Antonelli