
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 28 February 2018
Sec. Pediatric Endocrinology
Volume 9 - 2018 | https://doi.org/10.3389/fendo.2018.00054
This article is part of the Research Topic Hot topics of debate on Turner syndrome: growth, puberty, cardiovascular risks, fertility and psychosocial development View all 11 articles
Background: Whether children with chromosomal disorders of growth and puberty are affected by secular trends (STs) as observed in the general population remains unanswered, but this question has relevance for expectations of spontaneous development and treatment responses.
Objectives: The aim of the study was to evaluate STs in birth parameters, growth, and pubertal development in girls with Turner syndrome (TS).
Study design: Retrospective analysis of KIGS data (Pfizer International Growth Database). We included all TS patients who entered KIGS between 1987 and 2012 and were born from 1975 to 2004, who were prepubertal and growth treatment naïve at first entry (total number: 7,219). Pretreatment height and ages at the start of treatment were compared across 5-year birth year groups, with subgroup analyses stratified by induced or spontaneous puberty start.
Results: We observed significant STs across the birth year groups for birth weight [+0.18 SD score (SDS), p < 0.001], pretreatment height at mean age 8 years (+0.73 SDS, p < 0.001), height at the start of growth hormone (GH) therapy (+0.38 SDS, p < 0.001) and start of puberty (+0.42 SDS, p < 0.001). Spontaneous puberty onset increased from 15 to 30% (p < 0.001). Mean age at the start of GH treatment decreased from 10.8 to 7.4 years (−3.4 years; p < 0.001), and substantial declines were seen in ages at onset of spontaneous and induced puberty (−2.0 years; p < 0.001) and menarche (−2.1 years; p < 0.001).
Conclusion: Environmental changes leading to increased height and earlier and also more common, spontaneous puberty are applicable in TS as in normal girls. In addition, greater awareness for TS may underlie trends to earlier start of GH therapy and induction of puberty at a more physiological age.
Secular trends (STs) in birth parameters (1–3), growth (4, 5), and timing of puberty (6–8) are observed in normal populations in various settings. Changes in nutrition, better access to health care, and other environmental factors have been implicated as causative factors for these changes (4). Whether STs that affect normal populations also modulate growth and puberty of children with genetic or chromosomal disturbances that inherently affect growth and puberty remains unanswered.
Turner syndrome (TS) is caused by structural abnormalities in or complete loss of an X chromosome. It affects approximately 1 in 2,500 live-born female girls. The clinical phenotype of TS varies substantially, but in the majority of subjects includes short stature and ovarian failure, leading to hypogonadism and infertility (9).
In subjects with TS, haploinsufficiency of the SHOX gene has been proposed as an important cause of the growth phenotype in TS, since patients with heterozygous mutations in SHOX exhibit Leri-Weill syndrome, a bone dysplasia associated with short stature (10), whereas homozygous mutations with loss of both SHOX gene copies lead to a rare severe osteodysplasia (11). However, haploinsufficiency for the SHOX gene does not fully explain the growth phenotype and its variation in TS subjects. Probably, other factors such as estrogen deficiency (12), loss of additional X-chromosomal genes, or more general aneuploidy effects might be implicated in TS-associated short stature (13).
Although the majority of girls with TS have normal birth parameters, the frequency of TS in newborns with low birth weight (BW) and length is higher than expected (14, 15). This has been explained in part by loss or altered expression of X-chromosomal genes that are involved in fetal growth (16). Data analyzing the evolution of birth parameters in a sufficiently sized TS cohort over time to identify a ST in BW and length are lacking.
Girls with TS frequently exhibit delayed or absent pubertal development due to early ovarian failure. The exact molecular mechanisms leading to ovarian dysfunction in TS remain obscure. In a minority of patients (5–20%), puberty starts spontaneously and may even lead to spontaneous menarche in few subjects (17, 18). This seems to occur more frequently in subjects with a higher degree of mosaicism. As for auxological parameters, data on the presence or absence of an ST on spontaneous or induced puberty in TS are not available.
To assess STs on birth parameters, spontaneous growth and pubertal development in patients with TS and to evaluate whether clinical management of girls with TS has changed over time.
The patients studied had received recombinant growth hormone (GH, Genotropin®, Pfizer Inc.) as part of the pharmacoepidemiologic survey known as KIGS® (Pfizer International Growth Database). KIGS was established in 1987 as a worldwide observational registry to monitor outcomes and safety of Genotropin (somatropin, Pfizer Inc., New York, NY, USA) treatment in children with short stature. The KIGS survey was conducted in accordance with the Declaration of Helsinki (19).
As of June 2012, TS patients who entered the KIGS registry between 1987 and 2012 were included (total number: 7,219). Only patients who were at the prepubertal stage and naïve to any growth treatment at first entry were included. Birth years ranged from 1975 to 2004. The diagnosis of TS was made according to standard clinical practice and was confirmed by karyotype by the treating physicians. Patients’ characteristics are depicted in Table 1.
The main objective of this study was to assess STs on birth parameters, spontaneous growth, and pubertal development in patients with TS and to evaluate whether clinical management of girls with TS had changed over time. We had the following hypotheses:
– There is a positive ST for birth parameters.
– There is a positive ST for height before initiation of GH treatment.
– There is a positive ST for onset of puberty in the total TS cohort.
In order to address the outlined objectives, we analyzed data from three subcohorts derived from the KIGS database.
To assess STs, KIGS data on BW, birth length (BL), height SD score (SDS) at 8 years of age (chronological age 7.0–9.0 years) before initiation of any treatment, height SDS, and age at the start of GH therapy and at the start of puberty were analyzed in time intervals which were defined by year of birth (before 1980, 1980–1984, 1985–1989; 1990–1994; 1995–1999; and 2000–2004).
Cohort 1a was used to assess trends both in pretreatment height and in the age at the start of GH therapy. Inclusion required sufficient pretreatment data without exposure to therapies affecting growth (GH, oxandrolone, and sex steroids). To assess trends in pretreatment height, TS girls born before 1980 were included for analyses regarding BL/BW and pretreatment height at 8 years (unless they started recombinant GH before 1985). To analyze trends in age at the start of GH therapy, TS girls born before 1980 were excluded (as the approval of TS as an indication for GH therapy occurred beyond this birth cohort and this group, by definition, was relatively old at the start of GH). For this analysis, we also excluded the last group born between 2000 and 2004 as their maximum age was only 11 years old in 2012 when KIGS data collection ended. Data included in statistical analysis of trends are shaded gray in Tables 2 and 3.
We divided data in three categories:
(a) Displayed and tested (presented as shaded data in Tables 2 and 3).
(b) Displayed but not tested (Ht and age), since these data are relevant where sufficient data are available but are likely prone to bias. Thus, data are displayed as they are still informative but are not tested.
(c) Not displayed and not tested (puberty), since only insufficient data are available.
Since age at the start of GH treatment changed over time, we additionally compared subgroups from each cohort who had pretreatment measurements at a comparable age of 8 years (between 7.0 and 9.0 years; cohort 1b). Mean exact age at this measurement did not differ across the birth year groups.
Cohort 2 was used to assess trends in puberty timing. It included only those TS subjects with data during the age period when puberty was expected to occur. Therefore, we excluded the last group born between 2000 and 2004 as their maximum age was only 11 years old in 2012 when KIGS data collection ended.
Height was converted to SDS using both the height reference for healthy children of Prader (20) and the reference for TS of Ranke et al. (21). To calculate weight SDS, the normal population reference of Freeman et al. was used (22). To calculate body mass index SDS, the normal population reference of Cole was used (23). BW and BL for gestational age SDS were calculated using the reference of Niklasson et al. (24). The midparental height SDS was calculated as follows: (father’s height SDS + mother’s height SDS)/1.61 (25).
The onset of puberty was defined by the visit at which either spontaneous breast development (Tanner stage > B1) was first observed or the date at which estrogen replacement therapy was initiated. The assessment of the qualitative and quantitative aspects of estrogen replacement was done by the treating physicians. Furthermore, available data were stratified into whether pubertal development started spontaneously or was pharmacologically induced. The group with spontaneous start of puberty included patients with spontaneous progression of puberty until menarche as well as those who later required sex steroid substitution before menarche.
Statistical analyses [descriptive data analysis, calculation of SDS, and analysis of variance (ANOVA)] were carried out using SAS software (SAS Version 9.2, SAS Institute, Cary NC, USA). ANOVA models, F-tests, were applied to determine if there are any statistical mean differences between the groups based on year of birth. A p-value < 0.05 was considered to indicate statistical significance.
Distribution of BW and BL of all TS patients in whom birth parameters were available (n = 6372) are described in Table 1. Throughout the birth year cohorts “before 1980” until “1990–1994”, we observed a small ST for BW SDS with subsequent stabilization, with an increase of 0.18 SD over time, corresponding to about 157–180 g (depending on the gestational age; Table 2). In addition, a positive ST was observed in midparental height SDS.
Height SDS at the start of GH therapy at the standardized age of 8 years (range between 7.0 and 9.0 years, see Methods) showed a positive ST between before 1980 and 2000–2004, for both Prader and Ranke height SDS statistics (Table 2) (Figure 1 for Ranke height SDS results). In addition, positive STs for height SDS (Prader and Ranke height SDS statistics) could also be observed both at the start of GH therapy (Table 2) and at the start of puberty (thelarche) (Table 3). Comparable to the ST in height SDS at 8 years of age, an ST in midparental height was also observed (+0.5 SD).
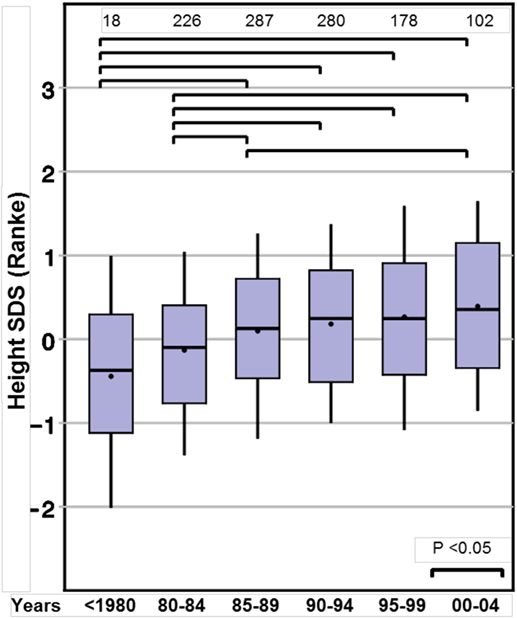
Figure 1. Secular trends on height in 8-year-old girls with Turner syndrome, height SD score (Ranke).
Age at the start of GH therapy declined substantially, from 10.8 years in the birth cohort 1980–1984 to 7.4 years in the birth cohort from 1995 to 1999. As described in Section “Methods”, the even more extreme mean ages at the start of GH in the before 1980 and 2000–2004 cohorts are likely artifactual, due to selection biases.
Age at the start of puberty (whether induced or spontaneous) declined from 14.4 years in the before 1980 birth cohort to 12.4 years in the 1995–1999 cohort (p < 0.001). When stratified by spontaneous or induced puberty, STs toward an earlier start of puberty were evident in both subgroups, and the proportion with spontaneous puberty onset increased from 15% in those born before 1980 to 30% in those born 1995–1999 (Table 3).
To determine whether age at spontaneous puberty and age at the start of GH in patients with TS are associated, we performed a correlation analysis, revealing a highly significant correlation between age at the start of GH treatment and age at pubertal onset [correlation coefficient 0.65 (p-value < 0.0001)], which explained 32% of the variability in spontaneous puberty with age at GH start [intercept = 10.3 years (p < 0.0001), slope = 0.31 (p < 0.0001)].
Age at menarche also declined substantially from 16.0 to 13.9 years (p < 0.001). Again, this observation remained significant in the two subgroups with either spontaneous or induced puberty (Table 3; Figure 2).
To the best of our knowledge, this is the first study analyzing whether TS girls display the same STs for auxological and pubertal development as those observed in the normal population. We found a highly significant ST for height at 8 years of age (+0.7 SDS). In addition, STs for height were present at the start of GH treatment and at the start of puberty. However, for these latter time points, the mean age differed greatly between the birth year groups, limiting direct comparisons. At the age of 8 years—before either the start of sex steroids or GH treatment may have influenced growth—the TS girls born after 2004 were more than half an SD taller compared to those born before 1980. Reported STs in height in normal populations differ between countries. Eastern and developing countries still show marked positive STs (26, 27), while conversely Western countries show only small (28) or even negative STs in growth (29). In contrast, our large group of TS girls included a wide variety of ethnicities, yet the positive ST in height SDS at the age of 8 years was observed for all, irrespective from their country of residence (data not shown). The remarkable positive ST of more than half an SDS, which corresponds to about 3 cm gain in height even without any endocrine treatment, surpasses contemporary estimates in the normal population. A height gain of 0.5 cm/decade or less would be expected for the corresponding birth year cohorts in most Western countries (30). Thus, the TS girls in this study born between 1975 and 2004 exhibited the same degree of ST on height at age 8 years as was observed one generation earlier in their parents.
It is important to notice that this positive ST in height could result in a delay of diagnosis because TS patients at the age of eight are nowadays nearly in the normal range or “less short.” However, our data show a constant shift toward an earlier start in GH treatment (reflecting probably the age of diagnosis) between the before 1980 and the post-2000 birth cohorts. Thus, although still a significant number of TS subjects seem to be diagnosed late, the awareness of TS seems to have improved despite less apparent phenotypical features.
We found a small positive ST for BW (+0.18 SD), but only a minor variation in BL. These findings are in line with data on healthy term infants who show a ST only for BW but not for BL. Comparison of recent growth curves and birth parameters (1, 24, 31) with historical data (2) showed a higher average BW for infants born at term. In contrast to that, BL remained constant in almost all the industrialized Western countries, and no change in this variable has been detected over the last 40 years. Higher maternal body weight and pregnancy weight gain (32) in recent years may be a reason for the observed gain in BW. Since both maternal and paternal genes contribute to infants’ birth parameters, the observed ST in midparental height might contribute to the observed changes in birth parameters. Furthermore, the ST in maternal height might be associated with alterations of the intrauterine environment, which again could be linked to the observed ST in birth parameters.
In recent years, epidemiological data from USA (33) and Denmark (8) showed STs toward earlier start of puberty in girls. We therefore were interested whether the same trend could be detected in TS patients in whom spontaneous puberty can be observed in about one third. We found a comparable decline in the age at spontaneous thelarche of about 2 years between those born before 1980 to those born in 2000–2004. As the time interval between visits was usually 6 months, the correct age at thelarche was likely earlier than that which we recorded. However, this limitation applied similarly for each of the five birth year groups with no expectation of bias.
The reasons behind the earlier spontaneous thelarche in TS subjects are unclear. We initially hypothesized that a lower threshold to karyotyping and a broader access to modern genetic diagnostics might have led to an increased proportion of patients with mosaicism, thereby explaining the doubling in the prevalence of spontaneous puberty and the decline in age at thelarche. However, as depicted in Table 3, the prevalence of patients with monosomy X did not change across the birth year groups. As in healthy girls, one can speculate that the increase in weight SDS of about one SD in the TS girls with spontaneous thelarche might have influenced the age at thelarche. However, in other settings the increase in weight did not fully explain the ST on puberty in healthy Danish girls (8, 34). Aksglaede and coworkers suggested that factors other than weight, such as changes in living conditions, nutrition during fetal development and childhood, and the wide distribution of endocrine disrupting chemicals (EDCs) (8, 35) might provoke the observed secular change. Probably, TS girls are exposed to the same changes in environmental conditions. Therefore, if EDCs are really causally related to the reported pubertal changes, these might also be related to the even more pronounced decrease in thelarche in TS girls.
As already reported in patients with idiopathic GH deficiency, age at puberty start correlated with age at the start of GH treatment in our study (36). Although the highly significant correlation between age at commencement of GH treatment and onset of spontaneous puberty is only an association and not proof of a causal relation, one can speculate that exposure to elevated GH concentrations might affect gonadotroph function, either through a direct or indirect effect (e.g., mediated by IGF-I). In this context, several in vitro studies have demonstrated that IGF-I is able to directly stimulate gonadotropin synthesis and secretion (37), so that a GH-induced increase in circulating IGF-I levels might contribute to the observed decline in age at the start of puberty. However, in a previous study in Italian TS patients, neither age at start nor prevalence of spontaneous puberty differed between GH-treated patients and a small non-GH treated control group who received androgen treatment (18). Furthermore, in our study, age at the start of GH treatment explains only 32% of the observed variability in spontaneous puberty, indicating that additional factors are probably involved in the physiology of earlier age at start and increased prevalence of spontaneous puberty.
The age at pharmacological induction of puberty decreased comparably to the age at spontaneous start of puberty. This phenomenon might be explained in particular by two factors: first, the decrease in age at the start of GH therapy (and probably age at diagnosis) might allow puberty induction at a more physiological age range. Second, the awareness of physicians for the psychosocial and physical sequelae of delayed puberty induction might have improved over time. In this context, one study has reported an even more improved height outcome for the early use of very low-dose estrogens in TS girls (38), and another study reported no significant influence of early start of low-dose estrogens on height development in TS girls (39). Together with the negative impact on bone health due to late estrogen exposure, these data argue strongly against a delay and in favor of an earlier starting age of puberty induction.
Although a ST in thelarche is found in several populations, all recent epidemiological studies (40) showed that the timing of menarche remained mostly unchanged with a consecutively longer interval between thelarche to menarche. In this study, we observed a significant reduction in age at menarche, both in TS girls with spontaneous as well as in those induced puberty. Whereas in TS girls with induced puberty probably the same explanations as for earlier age at thelarche might lead to the decrease in age at menarche, the reasons for the decrease in age at menarche in TS girls with spontaneous puberty remain unclear. Since the group with spontaneous start of puberty included subjects with spontaneous start but later requirement of sex steroid substitution before menarche, we speculate that the earlier menarche in this group is related to earlier sex steroid replacement therapy, comparable to the earlier start of pharmacologically induced puberty.
A major strength of this study is the unprecedented large study sample of girls with TS, allowing a comparison of growth and pubertal development over time in still sufficiently sized birth year cohorts. However, data from post-marketing studies such as KIGS have some important shortcomings. Therefore, the database contains no data from untreated TS subjects, which would allow determining whether the STs at birth, age 8 years or at the start of GH treatment translate to differences in adult height. Furthermore, several authors have speculated on the influence of socioeconomic factors as causative factors for the STs on height, which are not available in the database. Interobserver differences in a multicenter database in determining height and pubertal status are a potential weakness, but are probably balanced out by the large cohort size.
In summary, we find that trends toward increased childhood height and earlier pubertal onset operate not only in normal populations, but also in TS subjects, who also showed a doubling in the prevalence in spontaneous puberty onset between before 1980 to 1995–1999. In addition to these environment-related trends, awareness for TS seems to have improved, leading to earlier ages at the start of GH and pharmacological induction of puberty.
The patients studied had received recombinant GH (Genotropin®, Pfizer Inc.) as part of the pharmacoepidemiologic survey known as KIGS® (Pfizer International Growth Database). KIGS was established in 1987 as a worldwide observational registry to monitor outcomes and safety of Genotropin (somatropin, Pfizer Inc., New York, NY, USA) treatment in children with short stature. The KIGS survey was conducted in accordance with the Declaration of Helsinki.
KIGS is sponsored by Pfizer Inc.
FA, BG, AL, and JW developed the study design. FA, CC-H, BG, KO, AL, and JW discussed the findings. JW and BG wrote the first draft of the paper. All the authors contributed to draft revisions and approved the final manuscript.
JW received grant support from Pfizer and Ipsen, and also lecture honoraria from Pfizer, Novo Nordisk, and Ipsen. AL was and FA and CC-H are Pfizer full-time employees. KO received honoraria from Pfizer as a member of the KIGS steering committee. Hypothesis development, analysis, interpretation, and conclusion contained in this study are those of the authors’ alone.
Many thanks to all investigators, patients, and families who have contributed data to the KIGS database.
BL, birth length; BMI, body mass index; BW, birth weight; GH, growth hormone; ST, secular trend; TS, Turner syndrome.
1. Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics (2010) 125(2):e214–24. doi:10.1542/peds.2009-0913
2. Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from liveborn birth-weight data at 24 to 42 weeks of gestation. Pediatrics (1963) 32:793–800.
3. Rosenberg M. Birth weights in three Norwegian cities, 1860-1984. Secular trends and influencing factors. Ann Hum Biol (1988) 15(4):275–88. doi:10.1080/03014468800009751
4. Gohlke B, Woelfle J. Growth and puberty in German children: is there still a positive secular trend? Dtsch Arztebl Int (2009) 106(23):377–82. doi:10.3238/arztebl.2009.0377
5. Cole TJ. Secular trends in growth. Proc Nutr Soc (2000) 59(2):317–24. doi:10.1017/S0029665100000355
6. Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, et al. Secondary sexual characteristics in boys: data from the pediatric research in office settings network. Pediatrics (2012) 130(5):e1058–68. doi:10.1542/peds.2011-3291
7. Ong KK, Ahmed ML, Dunger DB. Lessons from large population studies on timing and tempo of puberty (secular trends and relation to body size): the European trend. Mol Cell Endocrinol (2006) 25(4–255):8–12. doi:10.1016/j.mce.2006.04.018
8. Aksglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the Copenhagen puberty study. Pediatrics (2009) 123(5):e932–9. doi:10.1542/peds.2008-2491
9. Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab (2006) 91(10):3897–902. doi:10.1210/jc.2006-0558
10. Rao E, Weiss B, Fukami M, Rump A, Niesler B, Mertz A, et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet (1997) 16(1):54–63. doi:10.1038/ng0597-54
11. Zinn AR, Wei F, Zhang L, Elder FF, Scott CI, Marttila P, et al. Complete SHOX deficiency causes Langer mesomelic dysplasia. Am J Med Genet (2002) 110(2):158–63. doi:10.1002/ajmg.10422
12. Klein KO, Baron J, Colli MJ, McDonnell DP, Cutler GB. Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. J Clin Invest (1994) 94(6):2475–80. doi:10.1172/JCI117616
13. Haverkamp F, Wolfle J, Zerres K, Butenandt O, Amendt P, Hauffa BP, et al. Growth retardation in Turner syndrome: aneuploidy, rather than specific gene loss, may explain growth failure. J Clin Endocrinol Metab (1999) 84(12):4578–82. doi:10.1210/jcem.84.12.6200
14. Hagman A, Wennerholm U-B, Kallen K, Barrenas M-L, Landin-Wilhelmsen K, Hanson C, et al. Women who gave birth to girls with Turner syndrome: maternal and neonatal characteristics. Hum Reprod (2010) 25(6):1553–60. doi:10.1093/humrep/deq060
15. Even L, Cohen A, Marbach N, Brand M, Kauli R, Sippell W, et al. Longitudinal analysis of growth over the first 3 years of life in Turner’s syndrome. J Pediatr (2000) 137(4):460–4. doi:10.1067/mpd.2000.109110
16. Wisniewski A, Milde K, Stupnicki R, Szufladowicz-Wozniak J. Weight deficit at birth and Turner’s syndrome. J Pediatr Endocrinol Metab (2007) 20(5):607–13. doi:10.1515/JPEM.2007.20.5.607
17. Massa G, Vanderschueren-Lodeweyckx M, Malvaux P. Linear growth in patients with Turner syndrome: influence of spontaneous puberty and parental height. Eur J Pediatr (1990) 149(4):246–50. doi:10.1007/BF02106283
18. Pasquino AM, Passeri F, Pucarelli I, Segni M, Municchi G. Spontaneous pubertal development in Turner’s syndrome. Italian study group for Turner’s syndrome. J Clin Endocrinol Metab (1997) 82(6):1810–3. doi:10.1210/jcem.82.6.3970
19. Riis P. Thirty years of bioethics: the Helsinki declaration 1964-2003. New Rev Bioeth (2003) 1(1):15–25. doi:10.1080/1740028032000131396
20. Prader A, Largo RH, Molinari L, Issler C. Physical growth of Swiss children from birth to 20 years of age. First Zurich longitudinal study of growth and development. Helv Paediatr Acta Suppl (1989) 52:1–125.
21. Ranke MB, Stubbe P, Majewski F, Bierich JR. Spontaneous growth in Turner’s syndrome. Acta Paediatr Scand Suppl (1988) 343:22–30.
22. Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child (1995) 73(1):17–24. doi:10.1136/adc.73.1.17
23. Cole TJ. A chart to link child centiles of body mass index, weight and height. Eur J Clin Nutr (2002) 56(12):1194–9. doi:10.1038/sj.ejcn.1601473
24. Niklasson A, Albertsson-Wikland K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr (2008) 8:8. doi:10.1186/1471-2431-8-8
25. Ranke MB. Towards a consensus on the definition of idiopathic short stature. Horm Res (1996) 45(Suppl 2):64–6. doi:10.1159/000184851
26. Zong X-N, Li H, Wu H-H, Zhang Y-Q. Socioeconomic development and secular trend in height in China. Econ Hum Biol (2015) 19:258–64. doi:10.1016/j.ehb.2015.09.006
27. dos Santos FK, Maia JAR, Gomes TNQF, Daca T, Madeira A, Katzmarzyk PT, et al. Secular trends in growth and nutritional status of Mozambican school-aged children and adolescents. PLoS One (2014) 9(12):e114068. doi:10.1371/journal.pone.0114068
28. Bonthuis M, van Stralen KJ, Verrina E, Edefonti A, Molchanova EA, Hokken-Koelega ACS, et al. Use of national and international growth charts for studying height in European children: development of up-to-date European height-for-age charts. PLoS One (2012) 7(8):e42506. doi:10.1371/journal.pone.0042506
29. Komlos J, Lauderdale BE. The mysterious trend in American heights in the 20th century. Ann Hum Biol (2007) 34(2):206–15. doi:10.1080/03014460601116803
30. Hauspie RC, Vercauteren M, Susanne C. Secular changes in growth and maturation: an update. Acta Paediatr Suppl (1997) 423:20–7.
31. Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics (2001) 108(2):E35. doi:10.1542/peds.108.2.e35
32. Yeh J, Shelton JA. Increasing prepregnancy body mass index: analysis of trends and contributing variables. Am J Obstet Gynecol (2005) 193(6):1994–8. doi:10.1016/j.ajog.2005.05.001
33. Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics (2002) 110(5):911–9. doi:10.1542/peds.110.5.911
34. Aksglaede L, Juul A, Olsen LW, Sorensen TIA. Age at puberty and the emerging obesity epidemic. PLoS One (2009) 4(12):e8450. doi:10.1371/journal.pone.0008450
35. Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. Role of environmental factors in the timing of puberty. Pediatrics (2008) 121(Suppl 3):S167–71. doi:10.1542/peds.2007-1813C
36. Price DA. Puberty in children with idiopathic growth hormone deficiency on growth hormone treatment: preliminary analysis of the data from the Kabi Pharmacia International Growth Study. Acta Paediatr Scand Suppl (1991) 379:117–24. doi:10.1111/j.1651-2227.1991.tb12061.x
37. Adam CL, Gadd TS, Findlay PA, Wathes DC. IGF-I stimulation of luteinizing hormone secretion, IGF-binding proteins (IGFBPs) and expression of mRNAs for IGFs, IGF receptors and IGFBPs in the ovine pituitary gland. J Endocrinol (2000) 166(2):247–54. doi:10.1677/joe.0.1660247
38. Ross JL, Quigley CA, Cao D, Feuillan P, Kowal K, Chipman JJ, et al. Growth hormone plus childhood low-dose estrogen in Turner’s syndrome. N Engl J Med (2011) 364(13):1230–42. doi:10.1056/NEJMoa1005669
39. Massa G, Heinrichs C, Verlinde S, Thomas M, Bourguignon JP, Craen M, et al. Late or delayed induced or spontaneous puberty in girls with Turner syndrome treated with growth hormone does not affect final height. J Clin Endocrinol Metab (2003) 88(9):4168–74. doi:10.1210/jc.2002-022040
40. Kahl H, Schaffrath Rosario A, Schlaud M. [Sexual maturation of children and adolescents in Germany. Results of the German health interview and examination survey for children and adolescents (KiGGS)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz (2007) 50(5–6):677–85. doi:10.1007/s00103-007-0229-3
Keywords: Turner syndrome, height, growth, birth weight, birth length, puberty, secular trend
Citation: Woelfle J, Lindberg A, Aydin F, Ong KK, Camacho-Hubner C and Gohlke B (2018) Secular Trends on Birth Parameters, Growth, and Pubertal Timing in Girls with Turner Syndrome. Front. Endocrinol. 9:54. doi: 10.3389/fendo.2018.00054
Received: 01 December 2017; Accepted: 05 February 2018;
Published: 28 February 2018
Edited by:
Ahmet Uçar, University of Health Sciences, TurkeyReviewed by:
Andrew Whatmore, University of Manchester, United KingdomCopyright: © 2018 Woelfle, Lindberg, Aydin, Ong, Camacho-Hubner and Gohlke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joachim Woelfle, am9hY2hpbS53b2VsZmxlQHVrYi51bmktYm9ubi5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.