- 1Nutritional Epidemiology Program, Jean Mayer U.S. Department of Agriculture Human Nutrition Research Center on Aging, Tufts University, Boston, MA, United States
- 2Division Of Endocrinology, Metabolism, and Nutrition, Department of Medicine, Duke University School of Medicine, Durham, NC, United States
- 3Cardiovascular Nutrition Laboratory, Jean Mayer U.S. Department of Agriculture Human Nutrition Research Center on Aging, Tufts University, Boston, MA, United States
- 4Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, United States
- 5Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, United States
The consumption of sugar-sweetened beverages (SSB), which includes soft drinks, fruit drinks, and other energy drinks, is associated with excess energy intake and increased risk for chronic metabolic disease among children and adults. Thus, reducing SSB consumption is an important strategy to prevent the onset of chronic diseases, and achieve and maintain a healthy body weight. The mechanisms by which excessive SSB consumption may contribute to complex chronic diseases may partially depend on an individual’s genetic predisposition. Gene–SSB interaction investigations, either limited to single genetic loci or including multiple genetic variants, aim to use genomic information to define mechanistic pathways linking added sugar consumption from SSBs to those complex diseases. The purpose of this review is to summarize the available gene-SSB interaction studies investigating the relationships between genetics, SSB consumption, and various health outcomes. Current evidence suggests there are genetic predispositions for an association between SSB intake and adiposity; evidence for a genetic predisposition between SSB and type 2 diabetes or cardiovascular disease is limited.
Introduction
Sugar-sweetened beverages (SSBs), such as sodas, fruit-flavored drinks, and sports drinks, are a significant source of dietary added sugars and a major contributor to excess energy intake (1). Global averages of SSB consumption range up to one 8-ounce serving/day (2), and contribute between 3 and 10% of daily energy consumption (3–6). Observational data suggest that higher SSB consumption is linked to a host of chronic diseases, including cardiovascular disease (CVD), type 2 diabetes (T2D), obesity, non-alcoholic fatty liver disease (NAFLD), and gout (7–11). Consequently, dietary guidance consistently recommends limiting added sugar consumption, particularly from SSBs (1, 12, 13). Although secular trends in dietary behavior suggest a decline in SSB consumption in recent years, national surveys suggest that >50% of US and European youth and adults continue to consume at least one serving of SSB daily (3, 5, 6, 14–16). Thus, SSB consumption continues to be a major public health concern globally.
Observational and experimental evidence linking SSB consumption to heritable metabolic risk factors and disease risk, i.e., those with underlying genetic predispositions, have paved the way for gene–SSB interaction studies (17–19). These gene–diet interaction studies may provide insight into the molecular mechanisms by which SSB consumption influences disease risk. From a public health perspective, this knowledge could be used to develop personalized dietary recommendations for the primary prevention and treatments of chronic diseases, and may provide motivation for patients to adhere to lifestyle guidance (20). The purpose of this review is to summarize current gene–SSB interaction studies on chronic disease risk factors and disease outcomes.
SSB Consumption, Genetics, and Health Outcomes
We conducted a comprehensive PubMed search for literature on SSB consumption and genetic interactions for publications through July 2017. Our search strategy combined terms for SSB consumption [“sugar-sweetened beverage(s),” “soda(s),” “sugar,” “beverage(s),” “drink(s)”], diet (“diet,” “dietary,” “food”), genetics [keywords: “gene(s),” “genome,” “genetic”], and/or interactions [“interaction(s),” “modify(ies),” “modification(s)”]. We also cross-referenced recovered studies and relevant reviews to identify additional studies. We identified nine published studies linking SSB to a variety of health outcomes with consideration for underlying genetic predisposition (Table 1). Studies included cross-sectional and longitudinal analyses of population-based cohort studies, and meta-analytic data from population-based cohort studies. The genetic variants investigated were either limited to single loci or expanded multiple loci (Table 2).
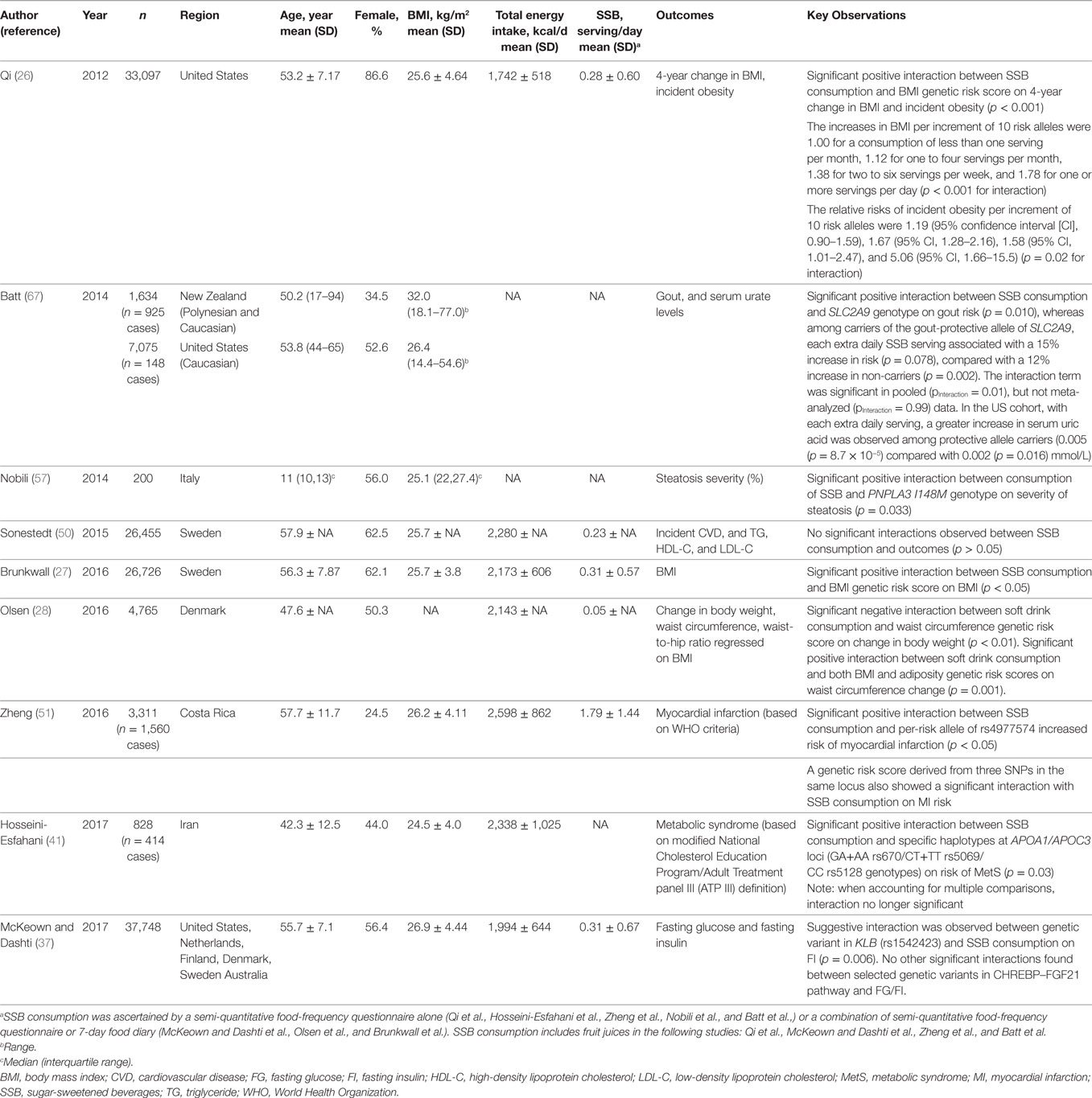
Table 1. Population-based studies of the interaction between genetics and sugar-sweetened beverage (SSB) consumption on various health outcomes.
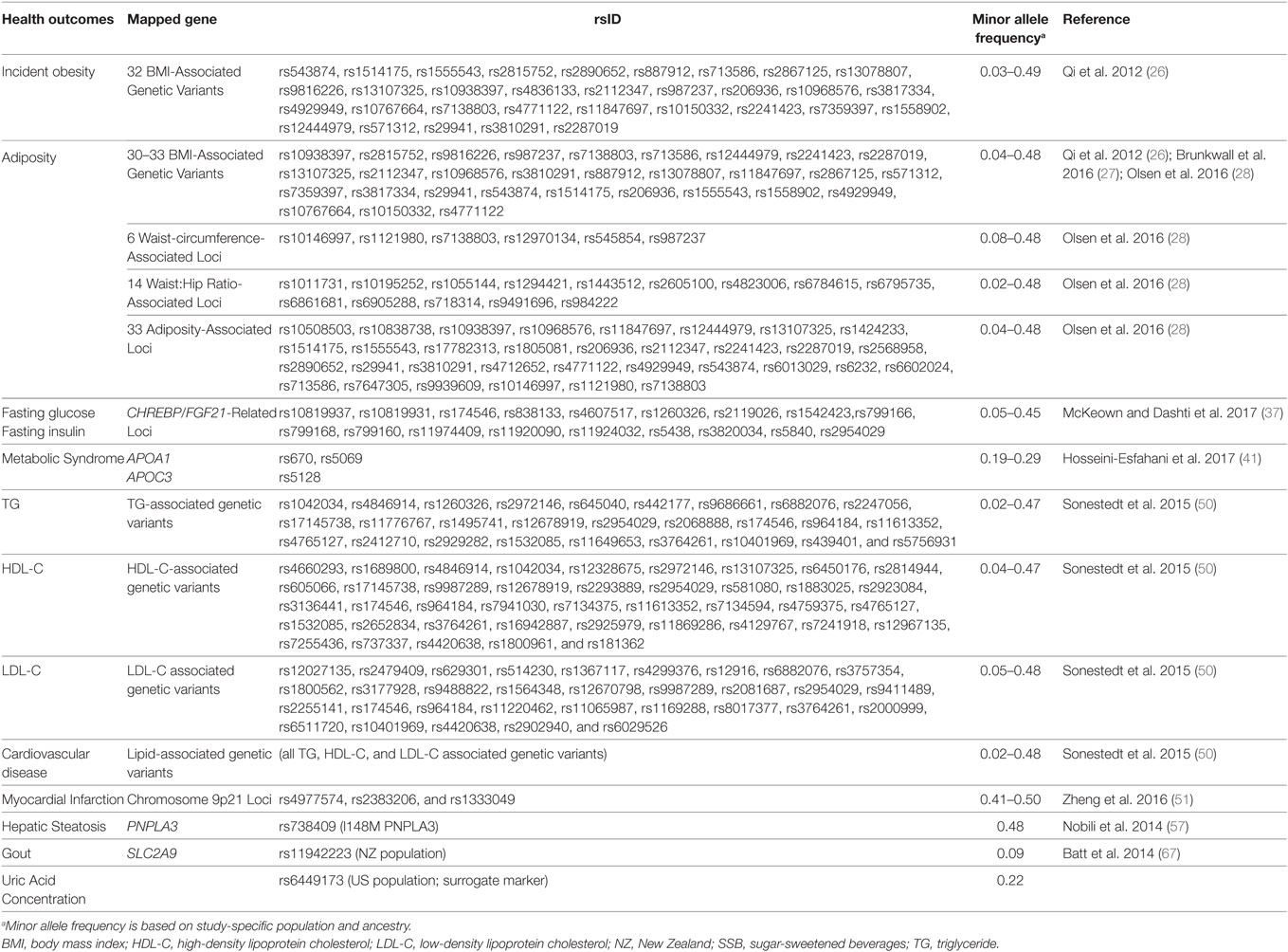
Table 2. Health outcomes that have been associated with increased SSB consumption and have been studied in regard to SSB by gene interaction.
Obesity
Several observational studies have observed a positive relationship between SSB consumption and adiposity (21–23), including a recent meta-analysis which found a 0.22 kg annual weight gain per additional serving of daily SSB consumption (9). Longitudinal analyses of 1,003 participants from the Framingham Heart Study (24) observed 29% greater increase in visceral, a risk factor for T2D and CVD (25), but not subcutaneous adipose tissue, among daily consumers SSB compared with non-consumers over a 6-year period.
Evidence from three separate investigations, encompassing a total of 50,000+ participants from cohorts in the U.S. and Northern Europe and using similar analytic approaches, found that greater consumption of SSB exacerbated the association between obesity-related genes and BMI (26–28). These studies used weighted genetic risk scores aggregating over 30 obesity-related genetic variants identified from genome-wide association studies to quantify genetic predisposition to obesity (Table 2). Qi and colleagues observed consistent findings across three US cohorts whereby genetic predisposition to obesity was exacerbated with greater SSB consumption (26). In a pooled analysis of two cohorts, the increase in BMI per each 10 BMI-related risk-allele increment was 1.78 among the highest SSB consumers (≥1 serving/day), compared to only 1.00 among the lowest SSB consumers (<1 serving/month) (Pinteraction = < 0.001) (26). Consistent with BMI, the relative risk of incident obesity per increment of 10 risk alleles was 5.06 (95% CI 1.66–15.5) among the highest SSB consumers (≥ 1 serving/day), compared to only 1.19 (95% CI 0.90–1.59) among the lowest SSB consumers (<1 serving/month) (Pinteraction = 0.02). The study replicated the trend in a third independent cohort (26). Brunkwall and colleagues observed similar interactions in a meta-analysis of cross-sectional data from two Swedish cohorts (27). The increase in BMI, per each 10 BMI-related risk-allele increment, was 1.28 kg/m2 (SE = 0.17) among the highest SSB consumers compared to 0.82 kg/m2 (SE = 0.11) among seldom SSB consumers (Pinteraction = 0.03) (27). Olsen and colleagues generated four genetic risk scores including variants associated with BMI, waist circumference, waist-to-hip ratio, and a combined score, and assessed SSB consumption in relation to annual changes in body weight, waist circumference, and waist circumference adjusted for BMI for three cohort studies (28). Consistent with previous reports, yet with a smaller effect size, higher genetic risk score exacerbated the association between SSB consumption and change in waist circumference, whereas with each risk allele increase in genetic risk score (for BMI-related variants or the complete score) the annual change in waist circumference per each additional serving of SSB per day was 0.05 cm [genetic risk score for BMI-related variants adjusted for BMI: 0.05 (0.02, 0.09) cm per year; p = 0.001; complete genetic risk score (variants associated with BMI, waist circumference, and waist-to-hip ratio): 0.05 (0.02, 0.07) cm per year; p = 0.001] higher. Interestingly, additional analyses in this study provided the first evidence that a genetic predisposition to a high waist circumference may attenuate the association between SSB consumption and body weight gain. For each waist circumference-related risk allele, change in annual body weight was lower with each additional serving of SSB per day [−0.06 (−0.10, −0.02) kg per year; p = 0.001].
T2D and Metabolic Syndrome (MetS)
Evidence from ecological, cross-sectional, and prospective studies suggest that higher SSB consumption and greater consumption of added sugars are dietary exposures linked to greater T2D risk (29). Indeed, several large investigations of prospective cohort studies have corroborated these associations (8, 30–32), and were summarized in a recent meta-analysis which indicated a 13% greater risk of T2D with each additional serving of SSB consumption (32). Higher SSB consumption has also been linked to increased risk of insulin resistance (HOMA-IR) (33, 34), higher rate of impaired fasting glucose (35), and greater risk of MetS (36).
We have recently investigated how these relationships may be inconsistently associated with variants involved in fructose metabolism and the ChREBP–FGF21 pathway by selecting genetic variants that are critical determinants of hepatic glucose metabolism, regulation of ChREBP and plasma TG concentrations, or the metabolic hormone FGF21 and its obligate receptor beta-klotho (KLB) (37). In a meta-analysis of 11 cohorts from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium including up to 34,748 adults of European descent, we first replicated earlier observations that higher SSB consumption was positively associated with fasting insulin and glucose concentrations. In addition, we identified a suggestive interaction between a genetic variant (rs1542423) in the KLB locus on fasting insulin. However the interaction finding did not replicate upon further investigation in replication cohorts. In a small case-control study, Hosseini-Esfahani and colleagues also investigated the potential interaction between SSB consumption and three genetic variants (rs670, rs5069, and rs5128) in the APOA1/APOC3 locus, previously associated with dyslipidemia (38–40), on prevalence of MetS in approximately 800 residents of Iran (41). Haplotype analysis identified an interaction between SSB consumption and the combination of two genotypes at the locus of interest (GA+AA rs670/CT+TT rs5069/CC rs5128) on MetS risk (Pinteraction = 0.03). Carriers of the genotype and those who were the highest consumers of SSB had nine times higher odds of MetS compared to those with the same genotype but were the lowest SSB consumers. Among those with other combinations of genotypes, the observed increase in MetS risk with higher SSB consumption was blunted. Given the large number of statistical tests, the selected 2-sided p-value threshold of <0.05 applied in this study is not sufficiently conservative.
Cardiovascular Disease
Mounting evidence suggests a link between SSB consumption and CVD (19). Each additional serving of SSB consumption was estimated to associate with a 22% increased risk for myocardial infarction (7) and a 13% increased risk for stroke (42). Consistent with those results, higher SSB consumption associates with intermediate CVD risk factors including obesity (9, 21–23), hypertension (43–45), and dyslipidemia (35, 46–49). These associations prompted initial efforts to test whether genetic variation might interact with SSB consumption to influence CVD risk. One prospective cohort study in 26,455 Swedish adults investigated whether genetic risk for dyslipidemia [weighted genetic risk score for 80 known genetic variants associated with triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), or low-density lipoprotein cholesterol (LDL-C) concentrations] interacted with SSB consumption to influence incident ischemic CVD and plasma lipid concentrations (TG, HDL-C, and LDL-C). The study did not observe significant interactions (50). By contrast, another study took a candidate gene approach for a locus on chromosome 9p21 famously known for its robust association with CVD. In 3,311 Hispanic adults, Zheng and colleagues observed a 48% increased risk of myocardial infarction per each risk allele of rs4977574 (in the 9p21 region) in participants with high SSB consumption (>2 serving/day) than in those with low SSB consumption (<1 serving/day) (Pinteraction = 0.005) (51). No significant interactions were observed for the two other variants tested from the same region (rs2383206 and rs1333049).
Non-Alcoholic Fatty Liver Disease
Evidence also suggests a positive relationship between SSB consumption and NAFLD (52). In a cross-sectional study of 2,634 U.S. adults, daily SSB consumers were found to be 1.5 times as likely to have NAFLD compared to SSB non-consumers (10). The strongest genetic determinant of NAFLD is the PNPLA3 locus (53, 54), which has been consistently associated with increased liver fat synthesis (steatosis) in genome-wide association studies (54–56). In a sample of 200 Italian youths (10–13 years) at high risk for NAFLD, Nobili and colleagues examined whether PNPLA3 interacted with SSB consumption to influence the severity of hepatic steatosis (57). They observed that the PNPLA3 genetic variant was more strongly associated with severity of hepatic steatosis among those reporting drinking SSB at least once weekly compared to less frequent consumption (p = 0.03).
Gout
Gout is a disease in which elevated circulating uric acid may crystallize, and these crystals deposit in joints, tendons and surrounding tissues, causing inflammation and joint pain. The disorder has been associated with increased CVD risk (58). SSB consumption associates with risk for gout in both observational and experimental studies (59–63). Batt and colleagues investigated whether SSB consumption may differentially influence gout risk and serum uric acid levels with variants in the SLC2A9 locus among 1,634 Polynesian and Caucasian individuals living in New Zealand (N.Z.) [a region with higher prevalence of gout (64)] and 7,075 Caucasians living in the U.S. SLC2A9 encodes the GLUT9 facilitative transporter which is a high affinity transporter for uric acid (65). The SLC2A9 variant of interest is known to explain ~4% of the variance in serum urate levels (66, 67). Upon pooling individual level data from the N.Z. and U.S. cohorts, with each additional daily SSB serving there was a 12% increased gout risk among non-carriers of the protective C allele, whereas no increase in risk among carriers of the gout-protective C allele (Pinteraction = 0.01). No statistically significant interactions were observed for plasma uric acid levels. In the U.S. cohort there was a trend (Pinteraction = 0.062) toward higher serum uric acid levels with each additional daily SSB serving among carriers of the gout-protective C allele [change in serum urate per increase in category of SSB intake (95% CI): 0.005 (0.003–0.007) mmol/L].
Discussion
In this review, we have summarized the limited body of evidence describing how genes implicated in various diseases may interact with SSB consumption to modify cardiometabolic health and chronic disease risk. To date, the strongest evidence for interaction between genes and SSB consumption is for obesity in three independent studies that consistently support a link between a genetic risk for obesity and SSB consumption on changes in adiposity (26–28). The majority of current evidence suggests that the adverse effects of SSB consumption on health may be strongest among subgroups with a genetic predisposition for the adverse metabolic outcomes, with some exceptions. In two studies, one related to changes in body weight (28) and one related to gout (67), the genetic predisposition seems to attenuate or possibly mask the association between SSB intake and the outcome.
The public health implications of these findings should be carefully interpreted both in context of the effect size of the interaction and in the frequency of the effect variant. For impactful personalized nutrition, the effect of the interaction should be clinically meaningful and the frequency of the effect variant common among the population. Such an interaction may be helpful in serving as a motivational tool to encourage compliance with guidance on lifestyle modification for select individuals (20). In the current review, effect sizes of interactions ranged from larger interactions with likely impactful effect sizes (26, 51) to smaller effect sizes (28, 37). While interactions with smaller effect sizes may have unclear clinical and translational impact, they may provide unique biological insight and help frame subsequent research questions.
Inferring potential mechanisms by which genetics and SSB consumption may influence disease onset is more feasible in interaction studies involving genetic variants with known functions. Batt and colleagues present an example of a gene by SSB consumption interaction study with the potential to provide insight into the molecular origin of increased risk of gout for some individuals (67). It is known that SLC2A9 encodes the GLUT9 transporter, a renal uric acid transporter (59, 65, 68), and genetic variants of the transporter associate with blood levels of uric acid and risk of gout (66, 69–71). SSB are composed of 50% fructose, and a major end product of fructose metabolism is uric acid. Genetic variants associated with a reduced ability to clear the increased uric acid produced during fructose metabolism may be one mechanism by which increased SSB consumption could lead to increased blood levels of uric acid in some individuals (59, 72). Thus, it is possible that individuals with a SLC2A9 mutation that leads to a defective GLUT9 transporter may have decreased ability to eliminate high uric acid loads produced from high-fructose metabolism following high SSB consumers. Thus, these individuals may generally have higher blood uric acid levels, so even a small increase in mean uric acid levels may lead to an increase in gout risk. Potential for mechanistic insights such as this cannot be determined from studies of genes with limited knowledge of their biological function or a host of pleiotropic genes.
There are general differences in the methods used to select genetic variants among the studies described here. Single genetic variants previously known to be strongly associated with the outcomes of interest from genome-wide association studies were selected for T2D, CVD, NAFLD, and gout, while an aggregate score, also related to the outcome, was selected for obesity and MetS. In our research with respect to glycemic traits (37), we have selected genetic variants related to both the exposure and outcome. In the case of the adiposity genetic risk score analyses, as the functionality of several BMI-related loci remain largely unknown, it is difficult to pinpoint mechanisms by which the score aggregating several of these variants interacts with SSB consumption. Thus, individual variant interaction tests may be insightful as single variants can be more readily mapped to a biological function. Both Qi and colleagues (26) and Brunkwall and colleagues (27) conducted follow-up analyses and provided evidence for modest effects of each individual variant by SSB consumption interaction on BMI. While nominal significance was observed for a few individual SNPs [Qi and colleagues: rs543874 in SEC16B (p = 0.0003) and Brunkwall and colleagues: rs1555543 in PTBP2 (p = 0.02)], these findings do not show significance when accounting for multiple testing. On the contrary, Olsen and colleagues provide evidence that GPRC5B rs12444979 individual interaction with SSB consumption for change in waist circumference, which warrants further follow-up. Current knowledge regarding genetic variants related to variability in SSB consumption is limited. A genome-wide association analysis of macronutrient intake has identified one locus that was associated with carbohydrate intake (73). However, efforts to disclose genetic variants related to single foods and beverages are ongoing and are made possible by large biobanks with genome-wide genetic data and dietary intake data (https://biobankengine.stanford.edu/coding/INI1309).
Direct-to-consumer genetic testing companies that offer nutrition recommendations currently do not offer personalized recommendations related to SSB consumption as they do not account for the interactions related to SSB consumption described in this Review. While they may not include these recommendations simply because of the greater risk of chronic diseases with higher SSB consumption in the general population, it may also, in part, be driven by various limitations in the current evidence. With the exception of obesity, the described studies are of limited sample sizes and have not been replicated. For example, the studies related to NAFLD and MetS had modest sample sizes of 200 and 828, respectively. In the case of obesity, effort has been placed on replicating interaction findings first reported by Qi et al. in non-U.S. populations and despite minor differences in findings, the consistency of the results across longitudinal studies with multiple sampling of diet increased confidence in the finding. Attempting replication of other recent interaction findings in larger and more diverse populations, with prospective designs, is warranted to corroborate initial findings in this field as has been recently conducted for T2D (74). For the purpose of generalizability of findings, replication attempts should include populations of different age groups to account for variability in SSB consumption with age (i.e., higher consumption among younger individuals) (26, 67), and cohorts from various countries to account for differences in SSB formulation (i.e., Europe SSB are sweetened with sucrose, which is composed of 50% fructose and 50% glucose, whereas a higher proportion of SSB in the U.S. are sweetened with high-fructose corn syrup composed of 55% fructose and 45% glucose). Other considerations for future research include differences in the exposure, SSB consumption, both in terms of dietary assessment methodology (i.e., semi-quantitative food-frequency questionnaire or 7-day food records) and in SSB consumption definition (i.e., inclusion of fruit juice). Including fruit juice in SSB definition may contribute to different results (51).
In summary, the detrimental effects of SSB consumption on disease risk is of public health concern, regardless of genetic predisposition. Imamura and colleagues estimated that SSB consumption could contribute to 1.8 million T2D events in the US over 10 years, and to 2.6 million events in the UK, even after accounting for obesity status (32). The gene by SSB consumption interaction data to-date are interesting and may suggest that some individuals who consume greater SSB may be more susceptible to greater risk of adiposity; however, further studies are needed on the effect of sugars in those with genetic predisposition for diabetes or CVD. In the meantime, there is a continued need to develop public health interventions that reduce the consumption of SSB globally.
Author Contributions
HD contributed to the conception and design of the research; and DH and HD contributed to the acquisition, analysis, and interpretation of the data. All authors drafted the manuscript, critically revised the manuscript, agreed to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation, though no other collaboration, with one of the authors HD.
Funding
This work is supported by NIH 5T32HL069772-15 (DH), AHA 16CSA28590003 (MH, NM, and DH), NIH R01DK100425 (MH), and USDA ARS agreement No. 58-1950-4-003 (NM) and 588-1950-9-001 (AL).
Abbreviations
CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NAFLD, non-alcoholic fatty liver disease; SSB, sugar-sweetened beverages; TG, triglyceride; T2D, type 2 diabetes.
References
1. U.S. Department of Health and Human Services, U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 8th ed. (2015). Available from: http://health.gov/dietaryguidelines/2015/guidelines/
2. Singh GM, Micha R, Khatibzadeh S, Lim S, Ezzati M, Mozaffarian D. Estimated global, regional, and national disease burdens related to sugar-sweetened beverage consumption in 2010. Circulation (2015) 132:639–66. doi:10.1161/CIRCULATIONAHA.114.010636
3. Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened beverage consumption among U.S. adults, 2011–2014. NCHS Data Brief, no 270. Hyattsville, MD: National Center for Health Statistics (2017):1–8.
4. Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened beverage consumption among U.S. youth, 2011–2014. NCHS Data Brief, no 271. Hyattsville, MD: National Center for Health Statistics (2017):1–8.
5. Brand-Miller JC, Barclay AW. Declining consumption of added sugars and sugar-sweetened beverages in Australia: a challenge for obesity prevention. Am J Clin Nutr (2017) 105:854–63. doi:10.3945/ajcn.116.145318
6. Duffey KJ, Huybrechts I, Mouratidou T, Libuda L, Kersting M, De Vriendt T, et al. Beverage consumption among European adolescents in the HELENA study. Eur J Clin Nutr (2012) 66:244–52. doi:10.1038/ejcn.2011.166
7. Narain A, Kwok CS, Mamas MA. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Clin Pract (2016) 70:791–805. doi:10.1111/ijcp.12841
8. Schwingshackl L, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Schwedhelm C, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol (2017) 32:363–75. doi:10.1007/s10654-017-0246-y
9. Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr (2013) 98:1084–102. doi:10.3945/ajcn.113.058362
10. Ma J, Fox CS, Jacques PF, Speliotes EK, Hoffmann U, Smith CE, et al. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol (2015) 63:462–9. doi:10.1016/j.jhep.2015.03.032
11. Choi JWJ, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum (2008) 59:109–16. doi:10.1002/art.23245
12. Vos MB, Kaar JL, Welsh JA, Horn LVV, Feig DI, Anderson CAM, et al. Added sugars and cardiovascular disease risk in children: a scientific statement from the American Heart Association. Circulation (2017) 135:e1017–34. doi:10.1161/CIR.0000000000000439
13. Guideline: Sugars Intake for Adults and Children. Geneva: World Health Organization (2015). Available from: http://www.ncbi.nlm.nih.gov/books/NBK285537/
14. Miller G, Merlo C, Demissie Z, Sliwa S, Park S. Trends in beverage consumption among high school students – United States, 2007–2015. MMWR Morb Mortal Wkly Rep (2017) 66:112–6. doi:10.15585/mmwr.mm6604a5
15. Mesirow MSC, Welsh JA. Changing beverage consumption patterns have resulted in fewer liquid calories in the diets of US children: national health and nutrition examination survey 2001–2010. J Acad Nutr Diet (2015) 115:559–66.e4. doi:10.1016/j.jand.2014.09.004
16. Stea TH, Øverby NC, Klepp K-I, Bere E. Changes in beverage consumption in Norwegian children from 2001 to 2008. Public Health Nutr (2012) 15:379–85. doi:10.1017/S1368980011001959
17. Malik VS, Popkin BM, Bray GA, Després J-P, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation (2010) 121:1356–64. doi:10.1161/CIRCULATIONAHA.109.876185
18. Malik VS, Hu FB. Sweeteners and risk of obesity and type 2 diabetes: the role of sugar-sweetened beverages. Curr Diab Rep (2012) 12:195–203. doi:10.1007/s11892-012-0259-6
19. Malik VS. Sugar sweetened beverages and cardiometabolic health. Curr Opin Cardiol (2017) 32:572–9. doi:10.1097/HCO.0000000000000439
20. Stewart KFJ, Wesselius A, Schreurs MAC, Schols AMWJ, Zeegers MP. Behavioural changes, sharing behaviour and psychological responses after receiving direct-to-consumer genetic test results: a systematic review and meta-analysis. J Community Genet (2017):1–18. doi:10.1007/s12687-017-0310-z
21. Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ (2013) 346:e7492. doi:10.1136/bmj.e7492
22. Forshee RA, Anderson PA, Storey ML. Sugar-sweetened beverages and body mass index in children and adolescents: a meta-analysis. Am J Clin Nutr (2008) 87:1662–71.
23. Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr (2009) 89:438–9. doi:10.3945/ajcn.2008.26980
24. Ma J, McKeown NM, Hwang S-J, Hoffmann U, Jacques PF, Fox CS. Sugar-sweetened beverage consumption is associated with change of visceral adipose tissue over 6 years of follow-UpCLINICAL PERSPECTIVE. Circulation (2016) 133:370–7. doi:10.1161/CIRCULATIONAHA.115.018704
25. Hwang Y-C, Fujimoto WY, Hayashi T, Kahn SE, Leonetti DL, Boyko EJ. Increased visceral adipose tissue is an independent predictor for future development of atherogenic dyslipidemia. J Clin Endocrinol Metab (2016) 101:678–85. doi:10.1210/jc.2015-3246
26. Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med (2012) 367:1387–96. doi:10.1056/NEJMoa1203039
27. Brunkwall L, Chen Y, Hindy G, Rukh G, Ericson U, Barroso I, et al. Sugar-sweetened beverage consumption and genetic predisposition to obesity in 2 Swedish cohorts. Am J Clin Nutr (2016) 104:809–15. doi:10.3945/ajcn.115.126052
28. Olsen NJ, Ängquist L, Larsen SC, Linneberg A, Skaaby T, Husemoen LLN, et al. Interactions between genetic variants associated with adiposity traits and soft drinks in relation to longitudinal changes in body weight and waist circumference. Am J Clin Nutr (2016) 104:816–26. doi:10.3945/ajcn.115.122820
29. Basu S, Yoffe P, Hills N, Lustig RH. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. PLoS One (2013) 8:e57873. doi:10.1371/journal.pone.0057873
30. Greenwood DC, Threapleton DE, Evans CEL, Cleghorn CL, Nykjaer C, Woodhead C, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose–response meta-analysis of prospective studies. Br J Nutr (2014) 112:725–34. doi:10.1017/S0007114514001329
31. Malik VS, Popkin BM, Bray GA, Després J-P, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care (2010) 33:2477–83. doi:10.2337/dc10-1079
32. Imamura F, O’Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ (2015) 351:h2376. doi:10.1136/bmj.h3576
33. Lana A, Rodríguez-Artalejo F, Lopez-Garcia E. Consumption of sugar-sweetened beverages is positively related to insulin resistance and higher plasma leptin concentrations in men and nonoverweight women. J Nutr (2014) 144:1099–105. doi:10.3945/jn.114.195230
34. Ma J, Jacques PF, Meigs JB, Fox CS, Rogers GT, Smith CE, et al. Sugar-sweetened beverage but not diet soda consumption is positively associated with progression of insulin resistance and prediabetes. J Nutr (2016) 146:2544–50. doi:10.3945/jn.116.234047
35. Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation (2007) 116:480–8. doi:10.1161/CIRCULATIONAHA.107.689935
36. Narain A, Kwok CS, Mamas MA. Soft drink intake and the risk of metabolic syndrome: a systematic review and meta-analysis. Int J Clin Pract (2017) 71:e12927. doi:10.1111/ijcp.12927
37. McKeown NM, Dashti HS, Ma J, Haslam DE, Jong JCK, Smith CE, et al. Sugar-sweetened beverage intake associations with fasting glucose and insulin concentrations are not modified by selected genetic variants in a ChREBP-FGF21 pathway: a meta-analysis. Diabetologia (2017):1–14. doi:10.1007/s00125-017-4475-0
38. Chien K-L, Chen M-F, Hsu H-C, Su T-C, Chang W-T, Lee C-M, et al. Genetic association study of APOA1/C3/A4/A5 gene cluster and haplotypes on triglyceride and HDL cholesterol in a community-based population. Clin Chim Acta (2008) 388:78–83. doi:10.1016/j.cca.2007.10.006
39. Talmud PJ, Hawe E, Martin S, Olivier M, Miller GJ, Rubin EM, et al. Relative contribution of variation within the APOC3/A4/A5 gene cluster in determining plasma triglycerides. Hum Mol Genet (2002) 11:3039–46. doi:10.1093/hmg/11.24.3039
40. Eichenbaum-Voline S, Olivier M, Jones EL, Naoumova RP, Jones B, Gau B, et al. Linkage and association between distinct variants of the APOA1/C3/A4/A5 gene cluster and familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol (2004) 24:167–74. doi:10.1161/01.ATV.0000099881.83261.D4
41. Hosseini-Esfahani F, Mirmiran P, Daneshpour MS, Mottaghi A, Azizi F. The effect of interactions of single nucleotide polymorphisms of APOA1/APOC3 with food group intakes on the risk of metabolic syndrome. Avicenna J Med Biotechnol (2017) 9(2):94–103.
42. Bernstein AM, de Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr (2012) 95:1190–9. doi:10.3945/ajcn.111.030205
43. Brown IJ, Stamler J, Horn LV, Robertson CE, Chan Q, Dyer AR, et al. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure. Hypertension (2011) 57:695–701. doi:10.1161/HYPERTENSIONAHA.110.165456
44. Chen L, Caballero B, Mitchell DC, Loria C, Lin P-H, Champagne CM, et al. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure. Circulation (2010) 121:2398–406. doi:10.1161/CIRCULATIONAHA.109.911164
45. Jayalath VH, Sievenpiper JL, de Souza RJ, Ha V, Mirrahimi A, Santaren ID, et al. Total fructose intake and risk of hypertension: a systematic review and meta-analysis of prospective cohorts. J Am Coll Nutr (2014) 33:328–39. doi:10.1080/07315724.2014.916237
46. Hert KA, Fisk IIPS, Rhee YS, Brunt AR. Decreased consumption of sugar-sweetened beverages improved selected biomarkers of chronic disease risk among US adults: 1999 to 2010. Nutr Res (2014) 34:58–65. doi:10.1016/j.nutres.2013.10.005
47. Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA (2010) 303:1490–7. doi:10.1001/jama.2010.449
48. Welsh JA, Sharma A, Cunningham SA, Vos MB. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents clinical perspective. Circulation (2011) 123:249–57. doi:10.1161/CIRCULATIONAHA.110.972166
49. de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation (2012) 125(1735–1741):S1. doi:10.1161/CIRCULATIONAHA.111.067017
50. Sonestedt E, Hellstrand S, Schulz C-A, Wallström P, Drake I, Ericson U, et al. The association between carbohydrate-rich foods and risk of cardiovascular disease is not modified by genetic susceptibility to dyslipidemia as determined by 80 validated variants. PLoS One (2015) 10:e0126104. doi:10.1371/journal.pone.0126104
51. Zheng Y, Li Y, Huang T, Cheng H-L, Campos H, Qi L. Sugar-sweetened beverage intake, chromosome 9p21 variants, and risk of myocardial infarction in Hispanics. Am J Clin Nutr (2016) 103:1179–84. doi:10.3945/ajcn.115.107177
52. Zelber-Sagi S, Godos J, Salomone F. Lifestyle changes for the treatment of nonalcoholic fatty liver disease: a review of observational studies and intervention trials. Ther Adv Gastroenterol (2016) 9:392. doi:10.1177/1756283X16638830
53. Kawaguchi T, Sumida Y, Umemura A, Matsuo K, Takahashi M, Takamura T, et al. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PLoS One (2012) 7:e38322. doi:10.1371/journal.pone.0038322
54. Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet (2011) 7:e1001324. doi:10.1371/journal.pgen.1001324
55. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet (2008) 40:1461–5. doi:10.1038/ng.257
56. Kitamoto T, Kitamoto A, Yoneda M, Hyogo H, Ochi H, Nakamura T, et al. Genome-wide scan revealed that polymorphisms in the PNPLA3, SAMM50, and PARVB genes are associated with development and progression of nonalcoholic fatty liver disease in Japan. Hum Genet (2013) 132:783–92. doi:10.1007/s00439-013-1294-3
57. Nobili V, Liccardo D, Bedogni G, Salvatori G, Gnani D, Bersani I, et al. Influence of dietary pattern, physical activity, and I148M PNPLA3 on steatosis severity in at-risk adolescents. Genes Nutr (2014) 9:392. doi:10.1007/s12263-014-0392-8
58. Abeles AM. Hyperuricemia, gout, and cardiovascular disease: an update. Curr Rheumatol Rep (2015) 17:13. doi:10.1007/s11926-015-0495-2
59. Merriman TR, Dalbeth N, Johnson RJ. Sugar-sweetened beverages, urate, gout and genetic interaction. Pac Health Dialog (2014) 20(1):31–8.
60. Beyl RN, Hughes L, Morgan S. Update on importance of diet in gout. Am J Med (2016) 129:1153–8. doi:10.1016/j.amjmed.2016.06.040
61. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ (2008) 336:309–12. doi:10.1136/bmj.39449.819271.BE
62. Zgaga L, Theodoratou E, Kyle J, Farrington SM, Agakov F, Tenesa A, et al. The association of dietary intake of purine-rich vegetables, sugar-sweetened beverages and dairy with plasma urate, in a cross-sectional study. PLoS One (2012) 7:e38123. doi:10.1371/journal.pone.0038123
63. Dalbeth N, House ME, Gamble GD, Horne A, Pool B, Purvis L, et al. Population-specific influence of SLC2A9 genotype on the acute hyperuricaemic response to a fructose load. Ann Rheum Dis (2013) 72:1868–73. doi:10.1136/annrheumdis-2012-202732
64. Hollis-Moffatt JE, Xu X, Dalbeth N, Merriman ME, Topless R, Waddell C, et al. Role of the urate transporter SLC2A9 gene in susceptibility to gout in New Zealand Māori, Pacific Island, and Caucasian case–control sample sets. Arthritis Rheum (2009) 60:3485–92. doi:10.1002/art.24938
65. Caulfield MJ, Munroe PB, O’Neill D, Witkowska K, Charchar FJ, Doblado M, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med (2008) 5:e197. doi:10.1371/journal.pmed.0050197
66. Köttgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet (2013) 45:145–54. doi:10.1038/ng.2500
67. Batt C, Phipps-Green AJ, Black MA, Cadzow M, Merriman ME, Topless R, et al. Sugar-sweetened beverage consumption: a risk factor for prevalent gout with SLC2A9 genotype-specific effects on serum urate and risk of gout. Ann Rheum Dis (2014) 73:2101–6. doi:10.1136/annrheumdis-2013-203600
68. Wright AF, Rudan I, Hastie ND, Campbell H. A ‘complexity’ of urate transporters. Kidney Int (2010) 78:446–52. doi:10.1038/ki.2010.206
69. Dehghan A, Köttgen A, Yang Q, Hwang S-J, Linda Kao WH, Rivadeneira F, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet (2008) 372:1953–61. doi:10.1016/S0140-6736(08)61343-4
70. Charles BA, Shriner D, Doumatey A, Chen G, Zhou J, Huang H, et al. A genome-wide association study of serum uric acid in African Americans. BMC Med Genomics (2011) 4:17. doi:10.1186/1755-8794-4-17
71. Matsuo H, Yamamoto K, Nakaoka H, Nakayama A, Sakiyama M, Chiba T, et al. Genome-wide association study of clinically defined gout identifies multiple risk loci and its association with clinical subtypes. Ann Rheum Dis (2016) 75:652–9. doi:10.1136/annrheumdis-2014-206191
72. Lin W-T, Chan T-F, Huang H-L, Lee C-Y, Tsai S, Wu P-W, et al. Fructose-rich beverage intake and central adiposity, uric acid, and pediatric insulin resistance. J Pediatr (2016) 171:90–6.e1. doi:10.1016/j.jpeds.2015.12.061
73. Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum Mol Genet (2013) 22:1895–902. doi:10.1093/hmg/ddt032
74. Li SX, Imamura F, Ye Z, Schulze MB, Zheng J, Ardanaz E, et al. Interaction between genes and macronutrient intake on the risk of developing type 2 diabetes: systematic review and findings from European Prospective Investigation into Cancer (EPIC)-InterAct. Am J Clin Nutr (2017) 106:263–75. doi:10.3945/ajcn.116.150094
Keywords: carbohydrate metabolism, observational studies, genetics, diet, type 2 diabetes, sugar-sweetened beverages
Citation: Haslam DE, McKeown NM, Herman MA, Lichtenstein AH and Dashti HS (2018) Interactions between Genetics and Sugar-Sweetened Beverage Consumption on Health Outcomes: A Review of Gene–Diet Interaction Studies. Front. Endocrinol. 8:368. doi: 10.3389/fendo.2017.00368
Received: 15 October 2017; Accepted: 15 December 2017;
Published: 08 January 2018
Edited by:
Shafqat Ahmad, Harvard University, United StatesReviewed by:
Maximilian Zeyda, Medical University of Vienna, AustriaConsolato Sergi, University of Alberta Hospital, Canada
Copyright: © 2018 Haslam, McKeown, Herman, Lichtenstein and Dashti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hassan S. Dashti, aGFzc2FuLmRhc2h0aSYjeDAwMDQwO21naC5oYXJ2YXJkLmVkdQ==
 Danielle E. Haslam
Danielle E. Haslam Nicola M. McKeown1
Nicola M. McKeown1 Hassan S. Dashti
Hassan S. Dashti