- 1Department of Oral Medicine, Peking University Hospital of Stomatology, Beijing, China
- 2Department of Oral Medicine West China School of Stomatology, Sichuan University, Chengdu, China
Background: The objective of this study was to systemically evaluate the association between oral lichen planus (OLP) and thyroid disease.
Method: Eleven electronic databases were searched for any clinical study published up until August 2016 that explored an association between OLP and thyroid disease, complemented with manual searching. The titles and abstracts of all studies identified from the electronic searches were then assessed independently to determine inclusion of the study. The population of interest was patients with OLP, and the exposure was the presence of thyroid disease in OLP patients. Statistical analyses were conducted using Review Manager 5.2. The odds ratios (OR) and 95% confidence intervals (CIs) were calculated to evaluate the association between OLP and thyroid disease.
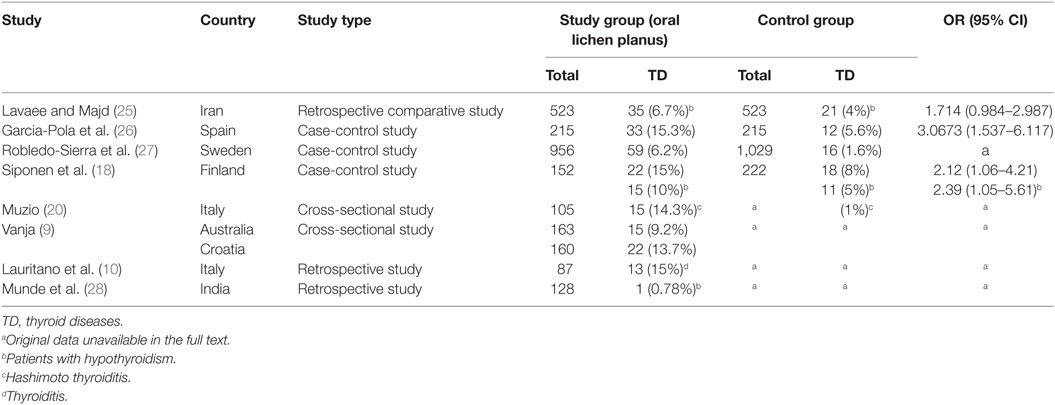
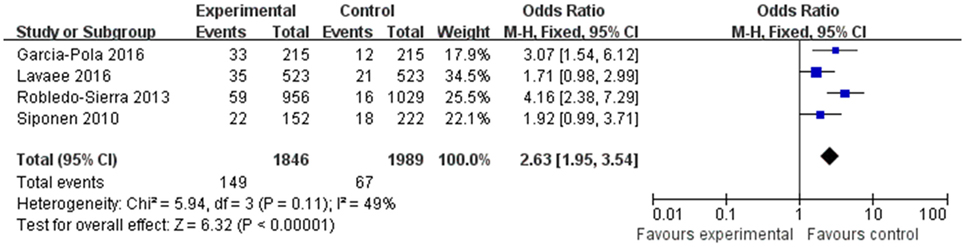
Results: Eight studies were included for review, and of these, four case-control studies were included in the final meta-analysis. The mean Newcastle–Ottawa Scale score evaluating the methodological quality of the four studies was 6.5. The OR was 2.10 (95% CI: 1.47–3.01), indicating a statistically significant difference in the prevalence of thyroid disease between the OLP and control groups. Heterogeneity was satisfactory (I2 = 0%, <25%). An approximately symmetrical funnel plot suggested no obvious publication bias. Two articles showed a higher correlation between OLP and hypothyroidism (OR 1.83, 95% CI: 1.16–2.89), with satisfactory heterogeneity (I2 = 0%, <25%).
Conclusion: This meta-analysis indicated a significantly high prevalence of thyroid disease among OLP patients compared with controls, suggesting that routine screening for thyroid disease could be beneficial to OLP patients. However, due to the small number of studies included, further studies are needed to confirm the results.
Introduction
Oral lichen planus (OLP) is a chronic inflammatory mucocutaneous disease most commonly affecting middle-aged females. It is estimated to affect 0.5–2.0% of the general population (1–3). Some researchers believe a complex series of immune-modulated events is responsible for the pathogenesis of OLP (4); however, the factor that initially triggers OLP remains unknown. The few predisposing factors currently thought to have a role in its pathogenesis include the patient’s genetic background (5) and psychological factors such as periods of psychological stress and anxiety (6–8). In some populations, OLP has been reported to be associated with certain systemic diseases, including hepatitis C virus, hypertension, diabetes mellitus, and thyroid disease (2, 3, 9, 10).
The thyroid is a secretory organ secreting thyroid hormones, which control many aspects of growth, development, regeneration, and metabolism. Deviations from normal thyroid hormone levels in either direction occur quite frequently and are very unpleasant for patients (11). The most common thyroid disorders include thyroid nodules, hyperthyroidism, autoimmune thyroid diseases (AITDs) caused by abnormal autoantibodies, and so on. The human AITDs broadly include Graves’ disease and Hashimoto’s thyroiditis (HT), which are the most common causes of thyroid gland dysfunction and nonendemic goiter (12). HT, the most common cause of hypothyroidism, involves antibodies that directly bind to thyroid antigens (13). Graves’ disease, on the other hand, involves the binding of autoantibodies to the TSH receptor, which leads to stimulation, and is the most common cause of thyrotoxicosis (14). In 2012, a review summarized studies of autoimmune thyroid disease incidence and prevalence since 1950, found high prevalence and incidence of hypothyroidism and prevalence for TPO antibodies in Denmark, Finland, Norway, and other European countries (15). Hypothyroidism and hyperthyroidism affect renal function by direct renal effects as well as systemic hemodynamic, metabolic, and cardiovascular effects (16). However, the pathogenesis of AITDs remains unclear.
A correlation between thyroid disease and OLP was first identified in a case report in 1994 (17), and several studies exploring this correlation have been conducted since. One study found that OLP patients have a higher prevalence of thyroid disease, especially hypothyroidism, compared with controls (18). In 2009, a study conducted in Taiwan by Sun et al. found that 21.3 and 24.4% of Chinese OLP patients had significantly higher levels of serum antithyroglobulin autoantibodies and antithyroid microsomal autoantibodies, respectively, compared with healthy controls (19). Another study published in 2013 indicated a higher prevalence of HT in an OLP group relative to the general population (20). However, this relationship between OLP and thyroid disease still remains controversial. The aim of this study was to systemically analyze the relationship between OLP and thyroid disease.
Methods
This review was performed following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (21).
Criteria for Selection
Articles based on clinical studies that explored the relationship between OLP and thyroid disease were considered for inclusion. Any type of study was considered, including case-control, cross-sectional, and cohort studies, either with or without a control group. Studies involving patients clinically diagnosed with OLP, with or without a pathological diagnosis, were included. No restrictions on age, sex, race, or country were set. The exposure of interest was the presence of thyroid disease, and the main outcome indicator was an odds ratio (OR) for OLP and thyroid disease. Studies including a control group were identified, so that their data could be merged to calculate the ORs and 95% confidence intervals (CIs). Studies that did not include a control group were retained for literature review.
Search Strategy
Studies in English or Chinese that met the above criteria were collected by searching the following databases: PubMed, MEDLINE, Cochrane Library, Science Direct, Web of Science, Embase, China National Knowledge Infrastructure, China Science and Technology Journal Database (CQVIP), Wanfang Data, Chinese Biomedical Literature Database, and the Chinese Science Citation Database. The MeSH terms (“oral lichen planus” OR “lichen planus” OR “OLP” OR “LP”) AND (“thyroid disease” OR “hypothyroidism” OR “thyroid nodule” OR “hyperthyroidism” OR “Hashimoto disease” OR “Hashimoto’s thyroiditis” OR “thyroiditis”) were searched, complemented with manual searching, for publications listed up until August 2016. The searches were conducted by two independent investigators (Dan Li and Jin Li).
Study Selection
Two of the authors independently assessed the titles and abstracts of all identified studies. Full reports were obtained for those studies that appeared to meet the inclusion criteria or those for which a clear decision could not be made from the title and abstract alone. Where disagreements occurred, they were resolved by discussion or by consulting a third author (Chunlei Li and Hong Hua).
Data Extraction and Management
Data extraction and management were performed independently by two reviewers (Dan Li and Jin Li). The following data were recorded from each study: authors, country of origin, study type, the main outcome measures, and the outcomes.
Statistical Analysis
Statistical analyses were conducted using Review Manager 5.2 (Cochrane Collaboration). The estimates of the association between OLP and thyroid disease were expressed as ORs and 95% CIs and plotted on a forest plot. The I2 test was performed to evaluate the heterogeneity among the studies, with possible values of 0–100%; I2 values of 25 and 50% were used as cutoffs for modest and high heterogeneity (22). If no significant heterogeneity was found, a fixed-effects model was used to calculate the combined OR. Otherwise, a random-effects model was used.
Risk of Bias
The methodological quality of the included studies was assessed using the Newcastle–Ottawa Scale for the case-control studies (23). Funnel plots were used to evaluate underlying publication bias, and a sensitivity analysis was performed to identify the influence of the individual studies on the combined OR.
Results
Study Selection and Characteristics
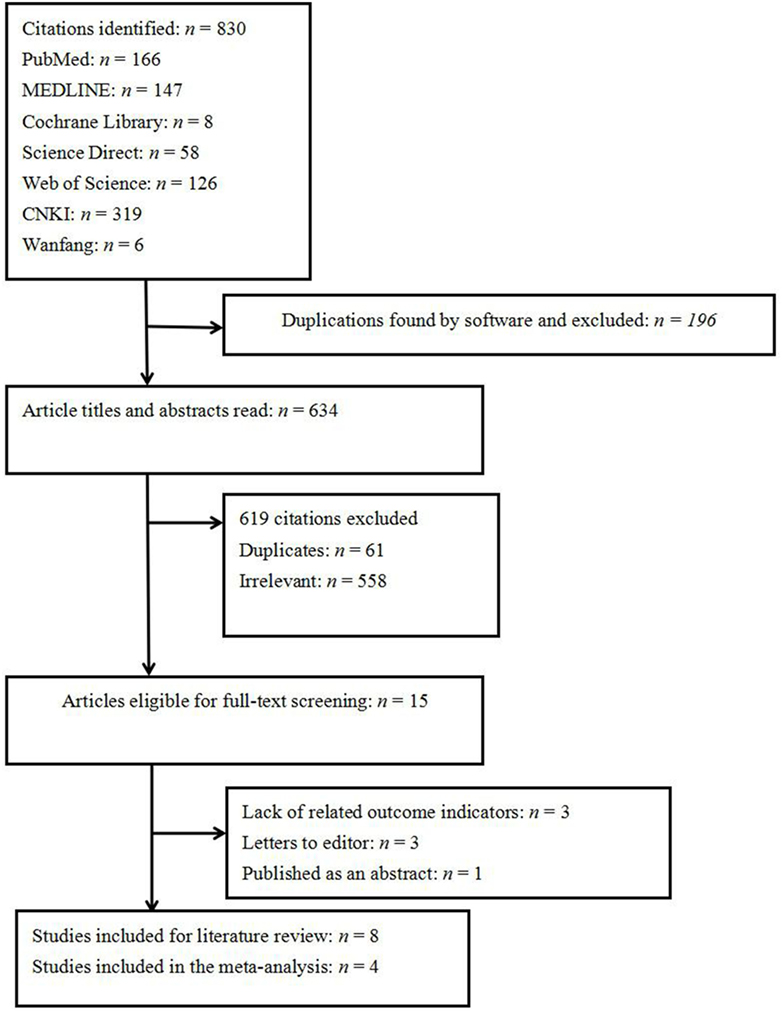
A total of 830 papers were initially identified. Of these, 765 citations were excluded because they were duplicates, had irrelevant contents, or did not meet the inclusion criteria. Of the 15 eligible full-text studies, 3 were excluded because of a lack of related outcome indicators and outcomes, 1 abstract published in a supplemental journal of oral disease was excluded because of no full text (24), and 3 letters to journal editors were also excluded (Figure 1). Finally, eight studies were eligible for inclusion (Table 1). These studies covered a broad geographical range, with five conducted in Europe, two in Asia, and one in both Oceania and Europe. Four of the eight included studies did not feature a control group, and thus the OR could not be estimated. The four remaining studies, with a total of 1,846 OLP patients, were included in the meta-analysis.
Relationship between OLP and Thyroid Disorders
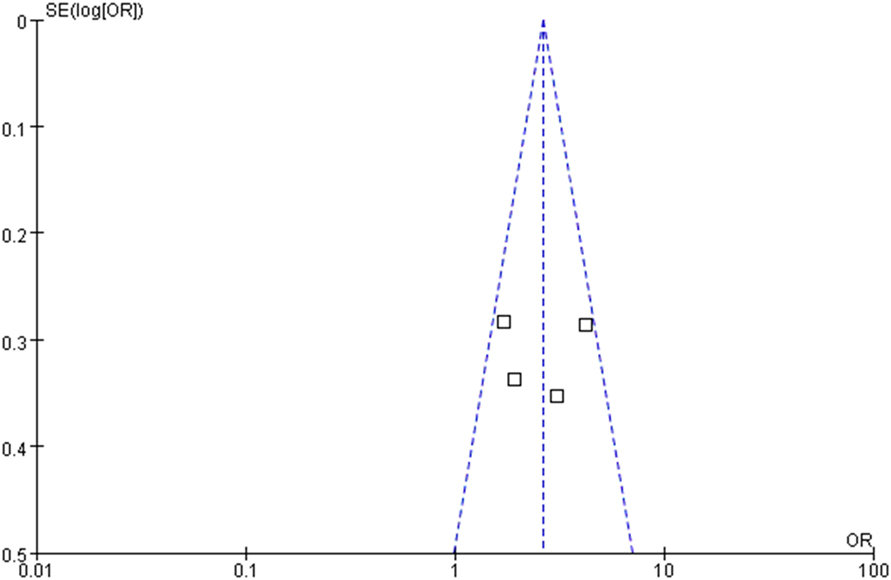
The OR for the association between OLP and thyroid disease varied from 1.71 (95% CI: 0.98–2.99) to 4.16 (95% CI: 2.38–7.29), demonstrating a statistically significant difference in the prevalence of thyroid disease between OLP patients and controls. The heterogeneity among the included studies (I2) was 49% (>50%, Figure 2), representing critical heterogeneity; thus, a fixed-effect model was used for the meta-analysis. The pooled OR was 2.63 (95% CI: 1.95–3.54). Following the sensitivity analysis, heterogeneity was more satisfactory at I2 = 0% (<25%, Figure 3), with a combined OR among the three studies of 2.10 (95% CI: 1.47–3.01). The methodological quality of the four included studies was evaluated, with a mean score of 6.5 (Table 2). The funnel plot was approximately symmetrical, suggesting no obvious publication bias (Figure 4).
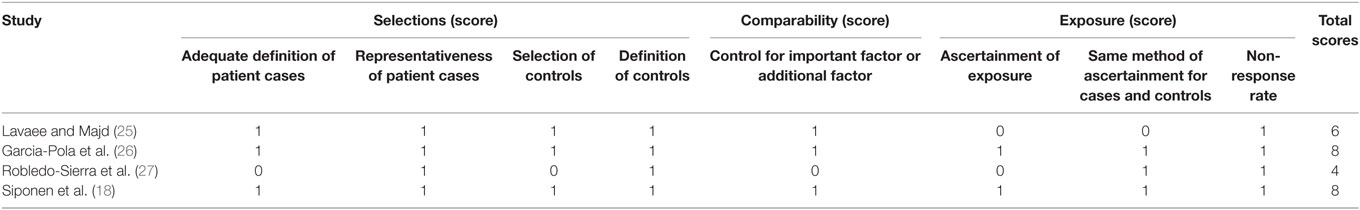
Table 2. Methodological quality of the included studies based on the Newcastle–Ottawa Scale for assessing the quality of case-control studies.
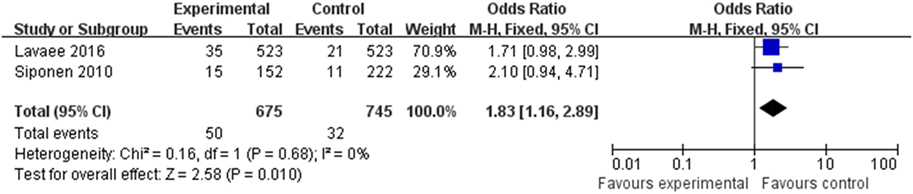
Relationship between OLP and Hypothyroidism
Two of the eight articles provided data pertaining to hypothyroidism. The data were merged (Figure 5) and revealed a correlation between OLP and hypothyroidism (OR 1.83, 95% CI: 1.16–2.89), with satisfactory heterogeneity (I2 = 0%, <25%).
Discussion
Oral lichen planus is recognized as an autoimmune mucocutaneous disease and is more common among females. Multiple factors have been identified as potentially contributing to its pathogenesis. The prevailing theory is that a complex series of immune-modulated events is responsible for OLP pathogenesis, but the main cause of OLP remains unknown. In recent years, the association between OLP and thyroid disorders has drawn attention. A study from 2010 showed a correlation between OLP and thyroid disease in a Finnish population (OR 2.12, 95% CI: 1.06–4.21) (18). There are other reports linking OLP and thyroid disease (25–28), but until now, the correlation between OLP and thyroid diseases has been questionable. Hence, we performed this review and meta-analysis.
We ultimately included eight articles that met the inclusion criteria, and data from four of these eight were combined for meta-analysis. Following a sensitivity analysis, we found that patients with OLP were significantly more likely to have thyroid disease than controls (OR 2.10, 95% CI: 1.47–3.01). There was no obvious publication bias, and three of the four studies had a high methodological quality score. Heterogeneity was satisfactory at I2 = 0%, after excluding the data from the study by Robledo-Sierra et al. (27), which had relatively low methodological quality. The results lead us to conclude that there is a strong link between OLP and the presence of thyroid disease, consistent with most previous studies.
Hypothyroidism and HT were the most frequently identified thyroid diseases in the studies. A possible link between HT and OLP was reported in an article by Lo et al. in 2013, who reported a prevalence of HT of 14.3% in a group of patients with OLP. The prevalence of HT-related hypothyroidism in the general population was 1%; thus, the difference was statistically significant (OR 14.29, 95% CI: 1.9–16.2; P < 0.0003) (20). Two of the eight articles provided data on hypothyroidism alone, and the combined data demonstrated a correlation between OLP and hypothyroidism (OR 1.83, 95% CI: 1.16–2.89). This is in contrast to the finding of Lavaee and Majd, with result showed no significant association between hypothyroidism and OLP (OR 1.714, 95% CI: 0.984–2.987) (25), and further research is needed to validate the results.
The causes of OLP and HT-related hypothyroidism are similar. OLP is considered to be a T-cell-mediated autoimmune disease in which auto-cytotoxic CD8 + T cells trigger apoptosis of the basal cells of the oral epithelium (2). HT is an organ-specific autoimmune disease, and its pathogenesis involves release of perforin by CD8+ cytotoxic T cells, and particle enzymes are considered the main cause of thyroid cell damage, eventually leading to hypothyroidism (29, 30). Both OLP and thyroid disease are more common in women than in men according to previous reports. In 2008, age-standardized prevalences of 1.27% overall, 0.96% in men, and 1.57% in women were calculated from a review article that extracted data from valid studies reporting the prevalence of OLP (31). A retrospective study evaluated the incidence of OLP in southeast Serbia over a 10-year period; women were found to be significantly more likely to have OLP (P < 0.001) (32). In the OLP groups of the four case-control studies included in our analysis, 65.4–74% were women, consistent with previous reports. A cohort study published in 1995 reported a higher incidence of thyroid disease in women than in men in a 20-year follow-up survey (12).
The Mechanism between OLP and thyroid diseases need to be further elucidated. From this study, this association between OLP and HT-related hypothyroidism suggests that thyroid disease may be involved in the pathogenesis of OLP. A molecular mimicry hypothesis was proposed for both lichen planus and HT, in which structural similarities between microbial antigens and human autoimmune reactions can turn a defensive immune reaction into an autoimmune reaction (33). However, the thyroid diseases in the articles analyzed in this study were not confined to hypothyroidism and HT. We suggest that OLP patients, especially women, should undergo routine screening for thyroid disease. Serological levels of thyroid stimulating hormone, T3 and T4, are indicators of thyroid function, and antithyroid peroxidase autoantibody and antithyroglobulin antibodies may be detected in AITDs. B-scan ultrasonography can be used to detect lesions such as thyroid nodules or as a secondary test for HT (34).
Conclusion
The etiology of OLP is unclear; however, we found a positive and statistically significant correlation between OLP and thyroid disease. From this, we can assume that thyroid disease may be involved in the pathogenesis of OLP, or that OLP is a clinical manifestation of thyroid disease. To verify this conclusion, more reliable clinical studies are needed. Routine screening for thyroid disease as part of therapeutic intervention for OLP is suggested. This may also help elucidate the pathogenesis of, as well as identify new treatment strategies, for OLP.
Author Contributions
HH and QC conceived and designed the research and critically revised the manuscript. DL and CL drafted the protocol. DL and JL participated in the search, screening, and analysis of the literature and in writing the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SF and handling editor declared their shared affiliation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number: 81730030).
References
1. Eisen D, Carrozzo M, Bagan SJ, Thongprasom K. Number V oral lichen planus: clinical features and management. Oral Dis (2005) 11:338–49. doi:10.1111/j.1601-0825.2005.01142.x
2. Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol (2015) 60:222–9. doi:10.4103/0019-5154.156315
3. Alrashdan MS, Cirillo N, McCullough M. Oral lichen planus: a literature review and update. Arch Dermatol Res (2016) 308:539–51. doi:10.1007/s00403-016-1667-2
4. Olson MA, Rogers RR, Bruce AJ. Oral lichen planus. Clin Dermatol (2016) 34:495–504. doi:10.1016/j.clindermatol.2016.02.023
5. Bermejo-Fenoll A, Lopez-Jornet P. Familial oral lichen planus: presentation of six families. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2006) 102:e12–5. doi:10.1016/j.tripleo.2006.03.016
6. Soto AM, Rojas AG, Esguep A. Association between psychological disorders and the presence of oral lichen planus, burning mouth syndrome and recurrent aphthous stomatitis. Med Oral (2004) 9:1–7.
7. Rojo-Moreno JL, Bagan JV, Rojo-Moreno J, Donat JS, Milian MA, Jimenez Y. Psychologic factors and oral lichen planus. A psychometric evaluation of 100 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (1998) 86:687–91. doi:10.1016/S1079-2104(98)90205-0
8. Ivanovski K, Nakova M, Warburton G, Pesevska S, Filipovska A, Nares S, et al. Psychological profile in oral lichen planus. J Clin Periodontol (2005) 32:1034–40. doi:10.1111/j.1600-051X.2005.00829.x
9. Vucicevic BV, Savage NW, Brailo V, Skrinjar I, Valter K, Alajbeg I, et al. The significance of oral and systemic factors in Australian and Croatian patients with oral lichen planus. Acta Dermatovenerol Croat (2014) 22:97–102.
10. Lauritano D, Arrica M, Lucchese A, Valente M, Pannone G, Lajolo C, et al. Oral lichen planus clinical characteristics in Italian patients: a retrospective analysis. Head Face Med (2016) 12:18. doi:10.1186/s13005-016-0115-z
11. Mondal S, Raja K, Schweizer U, Mugesh G. Chemistry and biology in the biosynthesis and action of thyroid hormones. Angew Chem Int Ed Engl (2016) 55:7606–30. doi:10.1002/anie.201601116
12. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) (1995) 43:55–68. doi:10.1111/j.1365-2265.1995.tb01894.x
13. Iddah MA, Macharia BN, Ng Wena AG, Keter A, Ofulla AV. Thyroid hormones and hematological indices levels in thyroid disorder patients at Moi Teaching and Referral Hospital, Western Kenya. ISRN Endocrinol (2013):1–6. doi:10.1155/2013/385940
14. Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves’ disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab (2001) 86:930–4. doi:10.1210/jc.86.2.930
15. Mcleod D, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine (2012) 42:252–65. doi:10.1007/s12020-012-9703-2
16. Iglesias P, Bajo MA, Selgas R, Diez JJ. Thyroid dysfunction and kidney disease: an update. Rev Endocr Metab Disord (2017) 18:131–44. doi:10.1007/s11154-016-9395-7
17. Kurgansky D, Burnett JW. Widespread lichen planus in association with Turner’s syndrome and multiple endocrinopathies. Cutis (1994) 54:108–10.
18. Siponen M, Huuskonen L, Laara E, Salo T. Association of oral lichen planus with thyroid disease in a Finnish population: a retrospective case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2010) 110:319–24. doi:10.1016/j.tripleo.2010.04.001
19. Chang JY, Chiang CP, Hsiao CK, Sun A. Significantly higher frequencies of presence of serum autoantibodies in Chinese patients with oral lichen planus. J Oral Pathol Med (2009) 38:48–54. doi:10.1111/j.1600-0714.2008.00686.x
20. Lo ML, Santarelli A, Campisi G, Lacaita M, Favia G. Possible link between Hashimoto’s thyroiditis and oral lichen planus: a novel association found. Clin Oral Investig (2013) 17:333–6. doi:10.1007/s00784-012-0767-4
21. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (2015) 349:g7647. doi:10.1136/bmj.g7647
22. Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract (2008) 14:951–7. doi:10.1111/j.1365-2753.2008.00986.x
23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi:10.1007/s10654-010-9491-z
24. Kalogirou E, Chrysomali E, Piperi E, Stefaniotis T, Tsiklakis K, Sklacounou A. Thyroid disease frequency in Greek patients with oral lichen planus. Oral Dis (2014) 20:14.
25. Lavaee F, Majd M. Evaluation of the association between oral lichen planus and hypothyroidism: a retrospective comparative study. J Dent (Shiraz) (2016) 17:38–42.
26. Garcia-Pola MJ, Llorente-Pendas S, Seoane-Romero JM, Berasaluce MJ, Garcia-Martin JM. Thyroid disease and oral lichen planus as comorbidity: a prospective case-control study. Dermatology (2016) 232:214–9. doi:10.1159/000442438
27. Robledo-Sierra J, Mattsson U, Jontell M. Use of systemic medication in patients with oral lichen planus – a possible association with hypothyroidism. Oral Dis (2013) 19:313–9. doi:10.1111/odi.12009
28. Munde AD, Karle RR, Wankhede PK, Shaikh SS, Kulkurni M. Demographic and clinical profile of oral lichen planus: a retrospective study. Contemp Clin Dent (2013) 4:181–5. doi:10.4103/0976-237X.114873
29. Hasham A, Tomer Y. Genetic and epigenetic mechanisms in thyroid autoimmunity. Immunol Res (2012) 54:204–13. doi:10.1007/s12026-012-8302-x
30. Gao Y, Liu M-M, Xiu R-J. The immunological pathogenesis of Hashimoto thyroiditis. J Intern Med Concept Pract (2013) 6:392–6.
31. McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med (2008) 37:447–53. doi:10.1111/j.1600-0714.2008.00662.x
32. Kesić L, Obradović R, Mihailović D, Radicević G, Stanković S, Todorović K. Incidence and treatment outcome of oral lichen planus in Southeast Serbia in a 10-year period (1997-2007). Vojnosanit Pregl (2009) 66:435–9.
33. Guarneri F, Giuffrida R, Di Bari F, Cannavò SP, Benvenga S. Thyroid autoimmunity and lichen. Front Endocrinol (2017) 8:146. doi:10.3389/fendo.2017.00146
Keywords: thyroid disease, oral lichen planus, hypothyroidism, hashimoto thyroiditis, pathogenesis, meta-analysis
Citation: Li D, Li J, Li C, Chen Q and Hua H (2017) The Association of Thyroid Disease and Oral Lichen Planus: A Literature Review and Meta-analysis. Front. Endocrinol. 8:310. doi: 10.3389/fendo.2017.00310
Received: 30 August 2017; Accepted: 25 October 2017;
Published: 09 November 2017
Edited by:
Alessandro Antonelli, University of Pisa, ItalyReviewed by:
Adele Zingoni, Università degli Studi di Torino, ItalySilvia Martina Ferrari, University of Pisa, Italy
Copyright: © 2017 Li, Li, Li, Chen and Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Hua, aG9uZ2h1YTE5NjhAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work.
 Dan Li
Dan Li Jin Li
Jin Li Chunlei Li1
Chunlei Li1