- 1Department of Obstetrics and Gynecology, Division of Maternal-Fetal Medicine Perinatal Research, The University of Texas Medical Branch, Galveston, TX, United States
- 2Department of Reproductive Biology and Obstetrics and Gynecology, Case Western Reserve University, Cleveland, OH, United States
- 3Department of Obstetrics and Gynecology, Wake Forest School of Medicine, Winston-Salem, NC, United States
Human parturition is an inflammatory process that involves both fetal and maternal compartments. The precise immune cell interactions have not been well delineated in human uterine tissues during parturition, but insights into human labor initiation have been informed by studies in animal models. Unfortunately, the timing of parturition relative to fetal maturation varies among viviparous species—indicative of different phylogenetic clocks and alarms—but what is clear is that important common pathways must converge to control the birth process. Herein, we hypothesize a novel signaling mechanism initiated by human fetal membrane aging and senescence-associated inflammation. Programmed events of fetal membrane aging coincide with fetal growth and organ maturation. Mechanistically, senescence involves in telomere shortening and activation of p38 mitogen-activated signaling kinase resulting in aging-associated phenotypic transition. Senescent tissues release inflammatory signals that are propagated via exosomes to cause functional changes in maternal uterine tissues. In vitro, oxidative stress causes increased release of inflammatory mediators (senescence-associated secretory phenotype and damage-associated molecular pattern markers) that can be packaged inside the exosomes. These exosomes traverse through tissues layers, reach maternal tissues to increase overall inflammatory load transitioning them from a quiescent to active state. Animal model studies have shown that fetal exosomes can travel from fetal to the maternal side. Thus, aging fetal membranes and membrane-derived exosomes cargo fetal signals to the uterus and cervix and may trigger parturition. This review highlights a novel hypothesis in human parturition research based on data from ongoing research using human fetal membrane model system.
Preterm birth (delivery before the 37th week of gestation) has increased globally nearly 30% in the last 25 years despite improvements in perinatal care (1). To address PTB, a clear understanding of the signals that initiate labor is needed (2). Both preterm, specifically spontaneous preterm, and term parturition share common terminal pathways that involve intrauterine inflammation and oxidative stress (OS), resulting in myometrial contractions and cervical remodeling (2, 3). Significant knowledge gaps exist, but over the past decade, we have come to realize that fetal endocrine signals, particularly those derived from the adrenal axis (i.e., CRH, ACTH) function as a biologic clock, concomitantly triggering organ maturation and labor and delivery at term (4). We hypothesize, however, that endocrine signals alone are not sufficient to disrupt the homeostatic balance that maintains uterine quiescence. Inflammation and OS of the amniochorionic (fetal) membranes at the feto-maternal interface are postulated as signals that perturb the relaxed uterine state (3). In this brief review, we introduce the concept and provide circumstantial evidence that in utero aging of the fetal membranes generates inflammatory proteins and prostanoids that trigger parturition.
Aging
The amniochorionic membranes surround the intrauterine cavity, providing a structural barrier to contain amniotic fluid for the growing fetus (5). The aging of these membranes is now recognized as a contributor of labor inducing signals (6). Like all tissues, the fetal membranes undergo aging, a natural, biologic phenomenon of random stochastic changes that result in altered molecular structure and function. Aging in utero is associated with the development of the fetus, with acceleration of this process projected to have long-term programming consequences in later life, potentially predisposing to adult-onset diseases. We posit that aging of the fetal membranes developmentally synchronize with the fetus to dictate the duration of pregnancy. As we have reported, progressive reductions in telomere length of fetal membrane cell chromosomes parallel those in fetal leukocytes (7). Herein, we hypothesize that fetal membrane aging is associated with sterile inflammatory changes (Figure 1) propagated via exosomes (30–100 nm spherical microvesicles) from amniochorionic cells to maternal tissues. Accelerated fetal membrane aging, manifested as telomere length reduction, is influenced by biochemical mediators of OS generated within fetal organs as they mature (8). OS fluctuates throughout pregnancy and maximum OS is seen at term (9–17). This is partly due to increased metabolic demand from the fetus, reduced maternal supply of substrate, no change in antioxidant status in uterine tissues creating an imbalance in redox state and increased stretching of membranes. This increased OS accelerates telomere attrition as Guanine nucleotide base-rich telomere are highly susceptible to OS induced DNA damage.
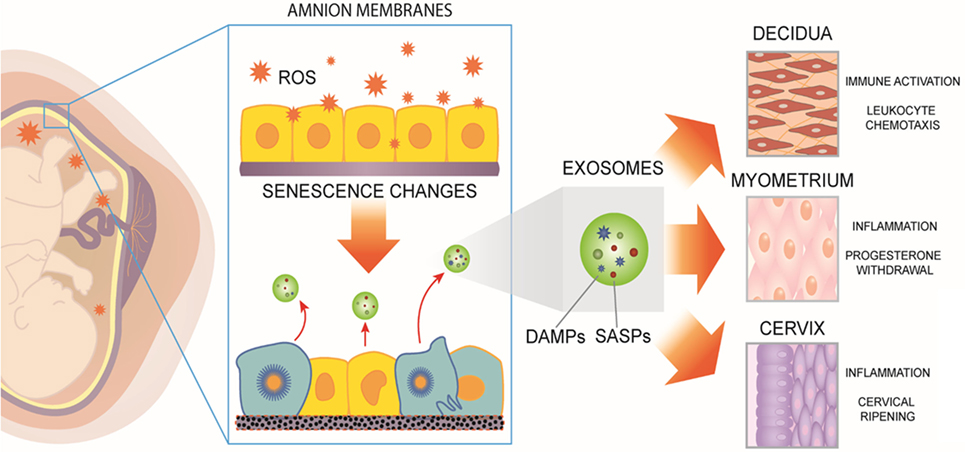
Figure 1. Human fetal membranes undergo cumulative oxidative stress during gestation. Reactive oxygen species (ROS) can lead to telomere-dependent, p38-mediated amnion cell senescence. Aging within fetal membranes coincides with fetal growth and organ maturation and, therefore, senescence-associated molecular signals can be hypothesized as proxies for fetal parturition signals. Senescent fetal cells release signals in the form of senescence-associated secretory phenotype and damage-associated molecular pattern (DAMP) markers [senescence-associated secretory proteins (SASPs) and DAMPs]. They can be collectively considered as sterile inflammatory mediators of parturition. SASPs and DAMPs can be packaged inside exosomes and propagated to maternal uterine tissues. In decidua, myometrium, and cervix, fetal-derived exosomes fuse with maternal target cells and deliver their cargo, increasing a localized inflammatory load. When inflammation reaches a threshold, quiescent uterine tissues transition to an active laboring state. Thus, fetal exosomes serve as signals of fetal readiness for parturition. In summary, fetal tissue derived exosomes that can be isolated from maternal blood could serve as biomarkers of fetal maturation at term. In preterm labor, fetal exosome cargo content may reflect pathophysiologic derangements and serve as a biomarker indicative of imminent delivery.
Mechanisms of Fetal Membrane Aging
Aging is an inevitable process in the life of all cells and organisms (18). OS plays a major role in regulating aging process (19, 20), especially in intrauterine tissues during pregnancy (21–23). OS builds up in part by an increase in fetal energy utilization as gestation progresses, but also due to the limited maternal supply of metabolic substrates and low antioxidant reserves (11). The peroxidation of cellular proteins, lipids, and DNA activate the p38 mitogen-activated protein kinase (MAPK) signaling pathway (24, 25), a pluripotent stress mediator that induces p16Ink4 and p19arf intermediates (26, 27), leading to cell cycle arrest and senescence (28).
Consequences of Fetal Membrane Senescence
Fetal membranes undergo telomere-dependent aging during gestation, with the shortest telomere lengths detected post-term (~42 weeks’ gestation) (3, 29). Progressive telomere length reduction during gestation is a feature of oxidative damage to highly vulnerable G-rich repeats within the telomere DNA sequence (30). OS-induced p38MAPK activation causes ultrastructural changes characteristic of senescence, manifested by enlargement of cell and organelle (ER and mitochondria) volumes as well as damage to nuclear and plasma membranes. Unlike the phenomena of apoptosis and autophagy, clearing of senescent cells by immunological mechanisms has not been reported during pregnancy and their persistence may instigate a unique inflammatory response (31) although senescence surveillance by immune cells has been reported in other fields (32). The uterine environment senses fetal membrane aging based on its recognition of inflammatory mediators known as senescence-associated secretory proteins (SASPs) (31, 33). SASP include cytokines, chemokines, growth factors, matrix degrading enzymes, and enzymes that generate prostanoids. Many of the SASP factors are already reported to be associated with both term and preterm labor (34–42). SASPs are released from senescent fetal membrane cells and their release can be reduced by treating fetal membrane cells with p38MAPK inhibitor, suggesting the role for this stress signaler in fetal membrane aging and inflammation.
We also want to acknowledge that fetal membranes are not the only tissues that undergo aging. Multiple reports have suggested decidual aging and its association with term and preterm parturition (43–45). These reports have suggested the mechanistic role of p53, a prosenescence and apoptosis promoter, in murine decidual tissues. Although our group reports no p53-mediated senescence activation in human fetal membranes, it is likely that distinct mechanisms of senescence activation in feto-maternal compartments. All these processes may synergize to cause parturition as effecter molecules and their activation processes are likely regulated differently in various tissues. Placental aging associated with adverse pregnancy outcomes has been reviewed recently by Cox and Redman (46). Similarly, unexplained anteparturm still births have been linked to placental aging and other adverse outcomes (17, 47, 48). The rest of the review is focused on our work using fetal membrane senescence.
Signals from Aging Fetal Membranes
Senescence-associated secretory proteins include cytokines, chemokines, angiogenic and other growth factors, matrix degrading enzymes as well as their endogenous inhibitors, cell adhesion molecules, proapoptotic receptors, and their ligands (33). Many of these factors have previously been identified in fetal and maternal tissues from term and spontaneous preterm births (31). SASPs perpetuate cellular inflammation and injury by causing the release of damage-associated molecular patterns (DAMPs) (49). Unlike inflammatory cytokines or chemokines released as specific responses to cellular stress, DAMPs are molecules with other defined intracellular signaling functions, but when leaked into the extracellular space they elicit a powerful inflammatory response (50). DAMPs from senescent fetal membrane cells include high mobility group box (HMGB)-1, a non-histone nuclear protein, HSP70, fragments of cell-free DNA, telomere repeat sequences, and uric acid (51). DAMPs are recognized by pattern recognition receptors located in the plasma membranes and on endosomes of nearby cells (52, 53). Their ubiquitous expression enables most cells to recognize and ligate DAMPs, causing inflammation, complement activation, and cell necrosis (52).
DAMPs in a Feed Forward Loop Cause Fetal Senescence to Signal Parturition
As mentioned above, SASP markers are well reported to be associated with human parturition. Several studies have reported a higher concentration of pro-inflammatory cytokines and chemokines at term labor compared to term not in labor (54–56). Similarly, several reports have compared them between term and spontaneous preterm birth with and without preterm rupture of the membranes (34, 39, 57, 58). This surge in inflammatory markers at term labor is theorized as changes initiated by endocrine disruptions (59), vascular changes (60), and leukocyte migration and activation (61, 62). In adverse pregnancies, the inflammatory response is associated with either infectious or other risk exposures (2).
Functional role for DAMPs, specifically HMGB1 (50, 63–65), uric acid (66, 67) and cell-free fetal DNA have also been reported in term and preterm parturition (68–71). In vitro experiments have shown that HMGB1 released from senescent fetal cells in a feedforward loop cause increased expression of TLR2 and TLR4, cause p38MAPK-mediated senescence and inflammatory cytokine release in amnion epithelial cells. Both senescence activation and inflammatory cytokine release were inhibited by p38MAPK inhibitor SB 203580. This suggests the activation of pathway mediated by p38AMPK (72). Gomez-Lopez et al. have reported that intraperitoneal injection of HMGB1 into pregnant B6 mice leads to spontaneous preterm birth and high rate of pup mortality (73). Thus, HMGB1, a normal nuclear component, can function as a pro-parturition inflammatory mediator. Telomere fragments are released from senescent fetal cells and they are seen in high abundance in the amniotic fluid of women at term labor compared to term not in labor (74). These cell-free fetal telomere fragments are also DAMPs with immunological functions. Using cell-free telomere fragment mimics (TTAGG2 repeats) as a stimulant, Polettini et al. showed amnion cells undergo p38MAPK-mediated senescence and inflammatory cytokine release (8). Like in HMGB1 experiments, this effect was inhibited by p38MAPK inhibitor SB203580, confirming the role of this signaler in inducing this pathway. In addition, Polettini et al. showed that intraamniotic injection of telomere fragments into CD1 mice could cause OS, p38MAPK activation, senescence, and low birth weight and prematurity (8). Thus, multiple pieces of evidence indicate that DAMPs, such as SASPs, have a functional role in preterm and term parturition. It is unclear how these proinflammatory mediators reach from senescent fetal tissues to maternal compartments to increase an overall inflammatory load.
SASPs and DAMPs as Exosome-Encapsulated Signals
Localized effects within the fetal membranes are insufficient to promote robust uterine contractions, but SASP and DAMP signals can be propagated across the feto-maternal interface through two different paths: (1) direct chemical diffusion to adjacent tissue layers or (2) encapsulated within exosomes, which can be transported to sites of functional activity in the myometrium, decidua, or cervix. One of the limiting steps in the former transport approach is that SASPs and DAMPs, including free HMGB1, are modified by acetylation or oxidization rendering very short half-lives in biologic fluids due to proteolytic degradation (75). By contrast, encapsulation in exosomes protects their cargo and increases the stability of potential signals by several fold. Exosomes are bioactive, spherical, cell-derived vesicles (30–100 nm) secreted during the process of exocytosis, which have been reported to increase in number as a function of duration of pregnancy (76–78). In addition to common membrane and cytosolic molecules, exosomes harbor unique, cell-specific subsets of proteins, such as HMGB1, cell-free fetal DNA, and telomere fragments. Exosomes afford a low intraluminal ambient pH, shielding contents, such as HMGB1 from oxidation, and conferring secure transport to distant sites (79). Exosomes contain molecular constituents of their cell of origin, including proteins and RNA that reflect the physiological state of the cell source and, hence, can serve as a source of representative biomarkers (80–84). Recent report by Sheller et al. has shown that exosomes from amnion epithelial cell grown under normal and OS conditions had specific markers reflective of physiologic changes (85). Similarly, in their review, Cuffe et al. found that placental OS generates biomarkers that are packaged in exosomes (86), reflecting cellular physiologic status. These placental-specific exosomes can be isolated from maternal liquid biopsies and can be used as biomarkers to determine placental function.
Trafficking and Functional Changes Induced by Exosomes at Distant Sites
Trafficking of exosomes, delivery of cargo at specific sites and their functional role have not been well reported during pregnancy. Recent findings by Chang et al. reported expression and trafficking of placental microRNAs at the feto-maternal interface. In a model of humanized mouse, authors report expression of 160-kb human 19 miRNA cluster (C19MC) locus or lentivirally express C19MC miRNA members selectively in the placenta of mouse (87). Pregnancy caused elevated expression of C19MC miRNA in the placenta of transgenic mice that resembled C19MC miRNAs patterns in humans. The authors further report that placental miRNA traffic primarily to the maternal circulation, suggesting a paracrine mode of signaling between the fetus and the mother (87).
In vitro experiments have shown that oxidatively stressed fetal membrane cells secrete exosomes richer in inflammatory mediators than cells grown under control conditions (85). Exosomes are increased at term in maternal plasma samples, and particularly so during certain pregnancy complications, and are more prevalent during labor (76, 81, 88). When fetal membrane cell-derived exosomes were injected into the intraamniotic cavity of mice, they were shown to traverse across the placental layers and accumulate within maternal tissues, including the myometrium and kidneys (89). Exosomes used for these studies were isolated from human amnion cells using ultracentrifugation and size exclusion chromatography were 50–120 nm in size and exhibited tetraspanin and endosomal sorting complexes required for transport markers. In addition, amnion-derived exosomes also expressed NANOG, a stem-cell-specific marker, expressed in amnion and chorion cells but in other uterine derived cells. In our animal model study, we were able to show two key modes of propagation of exosomes: (1) diffusion of exosome through tissue layers from fetal side to maternal side of the placenta and uterus, and (2) systemic propagation of exosomes through blood to various maternal organ system. Although, we were able to determine the propagation of exosomes, we are yet to determine their functional effect on maternal side in situ. In human amnion cells exposed to OS in vitro, exosomes were secreted into the conditioned media and could be passively transferred to human myometrial cell cultures, where they fused with the uterine cells and activated host cell COX-2, connexin-43, and cytokine mRNA expression. Salomon et al. have also shown the functional effect of exosomes under different oxygen tension (90). These combined results provide a proof of concept that the fetal membranes can propagate and traffic a parturition signal to the uterus via exosomes.
Exosomes as Biomarkers
Because exosome contents are specific to the derivative cell, they constitute a real-time “fingerprint” of their cell of origin (91). Thus, exosomes could potentially contribute as biomarkers of the physiologic state of fetal membrane cells during pregnancy and parturition (76, 88). Exosomes exhibit several advantages over classical soluble or “free” biomarkers present in biofluids (83, 91, 92). In particular, the stability of molecules packaged into exosomes is enhanced since they are protected from degradation in vivo and during storage (79).
Aging Starts In Utero
In summary, our thesis provides a new concept of the mechanisms underlying human parturition. We emphasize that along with its embryonic development program, the process of organismal aging of any mammal, including the human, starts in utero, at the time of fertilization. In utero programming involves both embryonic and extraembryonic tissue longevity, ultimately creating a homeostatic, stable environment preparing the fetus for independent extrauterine existence as a neonate. However, given their strategic layering between the fetus and the maternal decidua and myometrium, it appears that the fetal membranes tissues monitor the timing of gestation and promote expulsion of the intrauterine contents via an outburst of sterile inflammatory mediators. These mediators are propagated vectorially, from the fetal-to-maternal direction, promoting labor-associated changes in the myometrium. Concomitant with fetal maturation, tissues of the fetal membranes age and senesce, generating exosomes that carry molecules that are transformed into a uterotonic payload (3, 6, 74). As the interaction of SASPs and DAMPs reach a threshold, myometrial activation is initiated and the birthing process is launched.
If these concepts and our preliminary data are confirmed and supported by continuing investigation, several testable hypotheses are self-evident:
1. p38MAPK inhibitors could be used to prevent or even reverse cellular damage, providing a potential therapeutic strategy to mitigate fetal membrane aging and PTB (93).
2. Exosomes expressing selective fetal membrane-specific antigens could be sampled from maternal blood as cell-specific, non-invasive “liquid biopsies” to longitudinally monitor amniochorionic membrane aging during pregnancy.
3. Given the physical characteristics of exosomes, they are suitable to a variety of mechanical separation methods that currently confound standard “OMICs” approaches to the quantification of “free” biomarkers. Moreover, enhanced stability of exosomes in biologic fluids is likely to enhance biomarker sensitivity and assay performance.
4. Custom exosomes could be used as therapeutic delivery vehicles that contain cargo that promotes uterine quiescence.
In summary, we propose a novel concept of parturition in humans mediated by paracrine factors generated by natural and physiologic aging of fetal cells through senescence. The aging trajectory of fetal membranes and placenta are likely reflections of fetal growth and maturation. Aging, an inflammatory condition, generate inflammatory mediators, including DAMPs and well characterized uterotonins and propagate them to various feto-maternal tissues through exosomes. These communication channels reflect physiologic status of their cells of origin. Thus, exosome cargo contents reflect pregnancy status and, therefore, can function as potential biomarkers. Ongoing research has successfully isolated placental-derived exosomes from maternal plasma during normal and abnormal pregnancies (81). Future research in this area is expected to provide novel insights into fetal signaling during pregnancy and parturition.
Author Contributions
RM, SM, and RT conceived the idea and drafted the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, GR, and handling editor declared their shared affiliation, and the handling editor states that the process nevertheless met the standards of a fair and objective review.
Funding
This review was supported by NIH/NICHD grants 1R01HD084532-01A1 and R03HD067446 to RM.
References
1. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ (2010) 88:31–8. doi:10.2471/BLT.08.062554
2. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science (2014) 345:760–5. doi:10.1126/science.1251816
3. Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update (2016) 22:535–60. doi:10.1093/humupd/dmw022
4. Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept (2002) 108:159–64. doi:10.1016/S0167-0115(02)00105-2
5. Bryant-Greenwood GD. The extracellular matrix of the human fetal membranes: structure and function. Placenta (1998) 19:1–11. doi:10.1016/S0143-4004(98)90092-3
6. Menon R. Human fetal membranes at term: dead tissue or signalers of parturition? Placenta (2016) 44:1–5. doi:10.1016/j.placenta.2016.05.013
7. Menon R, Yu J, Basanta-Henry P, Brou L, Berga SL, Fortunato SJ, et al. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One (2012) 7(2):e31136. doi:10.1371/journal.pone.0031136
8. Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere fragment induced amnion cell senescence: a contributor to parturition? PLoS One (2015) 10:e0137188. doi:10.1371/journal.pone.0137188
9. Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol (2011) 25:287–99. doi:10.1016/j.bpobgyn.2010.10.016
10. Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, et al. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am J Pathol (2007) 171:1168–79. doi:10.2353/ajpath.2007.070528
11. Dennery PA. Oxidative stress in development: nature or nurture? Free Radic Biol Med (2010) 49:1147–51. doi:10.1016/j.freeradbiomed.2010.07.011
12. Jones ML, Mark PJ, Lewis JL, Mori TA, Keelan JA, Waddell BJ. Antioxidant defenses in the rat placenta in late gestation: increased labyrinthine expression of superoxide dismutases, glutathione peroxidase 3, and uncoupling protein 2. Biol Reprod (2010) 83:254–60. doi:10.1095/biolreprod.110.083907
13. Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA, et al. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol (2014) 184:1740–51. doi:10.1016/j.ajpath.2014.02.011
14. Menon R, Fortunato SJ, Milne GL, Brou L, Carnevale C, Sanchez SC, et al. Amniotic fluid eicosanoids in preterm and term births: effects of risk factors for spontaneous preterm labor. Obstet Gynecol (2011) 118:121–34. doi:10.1097/AOG.0b013e3182204eaa
15. Micle O, Muresan M, Antal L, Bodog F, Bodog A. The influence of homocysteine and oxidative stress on pregnancy outcome. J Med Life (2012) 5:68–73.
16. Wisdom SJ, Wilson R, McKillop JH, Walker JJ. Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. Am J Obstet Gynecol (1991) 165:1701–4. doi:10.1016/0002-9378(91)90018-M
17. Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol (2017) 77. doi:10.1111/aji.12653
18. Xue H, Xian B, Dong D, Xia K, Zhu S, Zhang Z, et al. A modular network model of aging. Mol Syst Biol (2007) 3:147. doi:10.1038/msb4100189
19. Campisi J. From cells to organisms: can we learn about aging from cells in culture? Exp Gerontol (2001) 36:607–18. doi:10.1016/S0531-5565(00)00230-8
20. Serrano M, Blasco MA. Putting the stress on senescence. Curr Opin Cell Biol (2001) 13:748–53. doi:10.1016/S0955-0674(00)00278-7
21. Miranda J, Romero R, Korzeniewski SJ, Schwartz AG, Chaemsaithong P, Stampalija T, et al. The anti-aging factor alpha-klotho during human pregnancy and its expression in pregnancies complicated by small-for-gestational-age neonates and/or preeclampsia. J Matern Fetal Neonatal Med (2014) 27:449–57. doi:10.3109/14767058.2013.818652
22. Biron-Shental T, Sukenik-Halevy R, Sharon Y, Goldberg-Bittman L, Kidron D, Fejgin MD, et al. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol (2010) 202(381):e1–7. doi:10.1016/j.ajog.2010.01.036
23. De Felice B, Nappi C, Zizolfi B, Guida M, Di Spiezio Sardo A, Bifulco G, et al. Telomere shortening in women resident close to waste landfill sites. Gene (2012) 500:101–6. doi:10.1016/j.gene.2012.03.040
24. Bracchitta G, Catalfo A, Martineau S, Sage E, De Guidi G, Girard PM. Investigation of the phototoxicity and cytotoxicity of naproxen, a non-steroidal anti-inflammatory drug, in human fibroblasts. Photochem Photobiol Sci (2013) 12:911–22. doi:10.1039/c3pp25326k
25. Akasaka E, Takekoshi S, Horikoshi Y, Toriumi K, Ikoma N, Mabuchi T, et al. Protein oxidative damage and heme oxygenase in sunlight-exposed human skin: roles of MAPK responses to oxidative stress. Tokai J Exp Clin Med (2010) 35:152–64.
26. Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CW, et al. Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat Genet (2004) 36:343–50. doi:10.1038/ng1317
27. Tie G, Messina KE, Yan J, Messina JA, Messina LM. Hypercholesterolemia induces oxidant stress that accelerates the ageing of hematopoietic stem cells. J Am Heart Assoc (2014) 3:e000241. doi:10.1161/JAHA.113.000241
28. Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J (2011) 30:1536–48. doi:10.1038/emboj.2011.69
29. Bonney EA, Krebs K, Saade G, Kechichian T, Trivedi J, Huaizhi Y, et al. Differential senescence in feto-maternal tissues during mouse pregnancy. Placenta (2016) 43:26–34. doi:10.1016/j.placenta.2016.04.018
30. Szalai VA, Singer MJ, Thorp HH. Site-specific probing of oxidative reactivity and telomerase function using 7,8-dihydro-8-oxoguanine in telomeric DNA. J Am Chem Soc (2002) 124:1625–31. doi:10.1021/ja0119651
31. Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ, et al. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol (2015) 213(3):.e1–16. doi:10.1016/j.ajog.2015.05.041
32. Hoenicke L, Zender L. Immune surveillance of senescent cells – biological significance in cancer- and non-cancer pathologies. Carcinogenesis (2012) 33:1123–6. doi:10.1093/carcin/bgs124
33. Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol (2010) 5:99–118. doi:10.1146/annurev-pathol-121808-102144
34. Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am (1997) 11:135–76. doi:10.1016/S0891-5520(05)70347-0
35. Mitchell MD, Dudley DJ, Edwin SS, Schiller SL. Interleukin-6 stimulates prostaglandin production by human amnion and decidual cells 82. Eur J Pharmacol (1991) 192:189–91. doi:10.1016/0014-2999(91)90090-D
36. Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol (1991) 165:969–71. doi:10.1016/0002-9378(91)90450-6
37. Bennett PR, Rose MP, Myatt L, Elder MG. Preterm labor: stimulation of arachidonic acid metabolism in human amnion cells by bacterial products. Am J Obstet Gynecol (1987) 156:649–55. doi:10.1016/0002-9378(87)90070-6
38. Park JS, Park CW, Lockwood CJ, Norwitz ER. Role of cytokines in preterm labor and birth. Minerva Ginecol (2005) 57:349–66.
39. Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol (1989) 161:336–41. doi:10.1016/0002-9378(89)90409-2
40. Peltier MR. Immunology of term and preterm labor. Reprod Biol Endocrinol (2003) 1:122. doi:10.1186/1477-7827-1-122
41. Weiss A, Goldman S, Shalev E. The matrix metalloproteinases (MMPS) in the decidua and fetal membranes. Front Biosci (2007) 12:649–59. doi:10.2741/2089
42. Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury 1. Nutr Rev (2007) 65:S194–202. doi:10.1301/nr.2007.dec.S194-S202
43. Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest (2010) 120:803–15. doi:10.1172/JCI40051
44. Hirota Y, Cha J, Yoshie M, Daikoku T, Dey SK. Heightened uterine mammalian target of rapamycin complex 1 (mTORC1) signaling provokes preterm birth in mice. Proc Natl Acad Sci U S A (2011) 108:18073–8. doi:10.1073/pnas.1108180108
45. Burnum KE, Hirota Y, Baker ES, Yoshie M, Ibrahim YM, Monroe ME, et al. Uterine deletion of Trp53 compromises antioxidant responses in the mouse decidua. Endocrinology (2012) 153:4568–79. doi:10.1210/en.2012-1335
46. Cox LS, Redman C. The role of cellular senescence in ageing of the placenta. Placenta (2017) 52:139–45. doi:10.1016/j.placenta.2017.01.116
47. Ferrari F, Facchinetti F, Saade G, Menon R. Placental telomere shortening in stillbirth: a sign of premature senescence? J Matern Fetal Neonatal Med (2016) 29:1283–8. doi:10.3109/14767058.2015.1046045
48. Smith R, Maiti K, Aitken RJ. Unexplained antepartum stillbirth: a consequence of placental aging? Placenta (2013) 34:310–3. doi:10.1016/j.placenta.2013.01.015
49. Huang J, Xie Y, Sun X, Zeh HJ III, Kang R, Lotze MT, et al. DAMPs, ageing, and cancer: the ‘DAMP Hypothesis’. Ageing Res Rev (2015) 24:3–16. doi:10.1016/j.arr.2014.10.004
50. Romero R, Chaiworapongsa T, Alpay SZ, Xu Y, Hussein Y, Dong Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med (2011) 24:1444–55. doi:10.3109/14767058.2011.591460
51. Baumbusch MA, Buhimschi CS, Oliver EA, Zhao G, Thung S, Rood K, et al. High mobility group-box 1 (HMGB1) levels are increased in amniotic fluid of women with intra-amniotic inflammation-determined preterm birth, and the source may be the damaged fetal membranes. Cytokine (2016) 81:82–7. doi:10.1016/j.cyto.2016.02.013
52. Santoni G, Cardinali C, Morelli MB, Santoni M, Nabissi M, Amantini C. Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J Neuroinflammation (2015) 12:21. doi:10.1186/s12974-015-0239-2
53. Said-Sadier N, Ojcius DM. Alarmins, inflammasomes and immunity. Biomed J (2012) 35:437–49. doi:10.4103/2319-4170.104408
54. Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med (2006) 11:317–26. doi:10.1016/j.siny.2006.05.001
55. Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol (1994) 32:108–13. doi:10.1111/j.1600-0897.1994.tb01101.x
56. Stephen GL, Lui S, Hamilton SA, Tower CL, Harris LK, Stevens A, et al. Transcriptomic profiling of human choriodecidua during term labor: inflammation as a key driver of labor. Am J Reprod Immunol (2015) 73:36–55. doi:10.1111/aji.12328
57. Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol (1995) 22:281–342.
58. Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol (2010) 63:73–92. doi:10.1111/j.1600-0897.2009.00791.x
59. Dudley DJ. Immunoendocrinology of preterm labor: the link between corticotropin-releasing hormone and inflammation. Am J Obstet Gynecol (1999) 180:S251–6. doi:10.1016/S0002-9378(99)70711-8
60. Wadhwa PD, Culhane JF, Rauh V, Barve SS. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J (2001) 5:119–25. doi:10.1023/A:1011353216619
61. Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod (1999) 14:229–36. doi:10.1093/humrep/15.1.229
62. Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol (2009) 80:122–31. doi:10.1016/j.jri.2009.01.002
63. Buhimschi CS, Baumbusch MA, Dulay AT, Oliver EA, Lee S, Zhao G, et al. Characterization of RAGE, HMGB1, and S100beta in inflammation-induced preterm birth and fetal tissue injury. Am J Pathol (2009) 175:958–75. doi:10.2353/ajpath.2009.090156
64. Dubicke A, Andersson P, Fransson E, Andersson E, Sioutas A, Malmstrom A, et al. High-mobility group box protein 1 and its signalling receptors in human preterm and term cervix. J Reprod Immunol (2010) 84:86–94. doi:10.1016/j.jri.2009.09.010
65. Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med (2012) 25:558–67. doi:10.3109/14767058.2011.599083
66. Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, et al. Uric acid induces trophoblast IL-1beta production via the inflammasome: implications for the pathogenesis of preeclampsia 3. Am J Reprod Immunol (2011) 65:542–8. doi:10.1111/j.1600-0897.2010.00960.x
67. Girard S, Heazell AE, Derricott H, Allan SM, Sibley CP, Abrahams VM, et al. Circulating cytokines and alarmins associated with placental inflammation in high-risk pregnancies. Am J Reprod Immunol (2014) 72:422–34. doi:10.1111/aji.12274
68. Jakobsen TR, Clausen FB, Rode L, Dziegiel MH, Tabor A. High levels of fetal DNA are associated with increased risk of spontaneous preterm delivery. Prenat Diagn (2012) 32:840–5.
69. Farina A, LeShane ES, Romero R, Gomez R, Chaiworapongsa T, Rizzo N, et al. High levels of fetal cell-free DNA in maternal serum: a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol (2005) 193:421–5. doi:10.1016/j.ajog.2004.12.023
70. Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, et al. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion (2001) 41:1524–30. doi:10.1046/j.1537-2995.2001.41121524.x
71. Phillippe M. Cell-free fetal DNA, telomeres, and the spontaneous onset of parturition. Reprod Sci (2015) 22:1186–201. doi:10.1177/1933719115592714
72. Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR, et al. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS One (2014) 9:e113799. doi:10.1371/journal.pone.0113799
73. Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, et al. Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am J Reprod Immunol (2016) 75:3–7. doi:10.1111/aji.12443
74. Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY) (2016) 8:216–30. doi:10.18632/aging.100891
75. Zandarashvili L, Sahu D, Lee K, Lee YS, Singh P, Rajarathnam K, et al. Real-time kinetics of high-mobility group box 1 (HMGB1) oxidation in extracellular fluids studied by in situ protein NMR spectroscopy. J Biol Chem (2013) 288:11621–7. doi:10.1074/jbc.M113.449942
76. Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE, et al. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med (2014) 12:204. doi:10.1186/1479-5876-12-204
77. Naglova H, Bucova M. HMGB1 and its physiological and pathological roles. Bratisl Lek Listy (2012) 113:163–71.
78. Kang R, Zhang Q, Zeh HJ III, Lotze MT, Tang D. HMGB1 in cancer: good, bad, or both? Clin Cancer Res (2013) 19:4046–57. doi:10.1158/1078-0432.CCR-13-0495
79. Kralj-Iglic V. Stability of membranous nanostructures: a possible key mechanism in cancer progression. Int J Nanomedicine (2012) 7:3579–96. doi:10.2147/IJN.S29076
80. Truong G, Guanzon D, Kinhal V, Elfeky O, Lai A, Longo S, et al. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells – liquid biopsies for monitoring complications of pregnancy. PLoS One (2017) 12:e0174514. doi:10.1371/journal.pone.0174514
81. Mitchell MD, Peiris HN, Kobayashi M, Koh YQ, Duncombe G, Illanes SE, et al. Placental exosomes in normal and complicated pregnancy 1. Am J Obstet Gynecol (2015) 213:S173–81. doi:10.1016/j.ajog.2015.07.001
82. Zhang J, Liu SC, Luo XH, Tao GX, Guan M, Yuan H, et al. Exosomal long noncoding RNAs are differentially expressed in the cervicovaginal lavage samples of cervical cancer patients. J Clin Lab Anal (2016) 30:1116–21. doi:10.1002/jcla.21990
83. Huebner AR, Somparn P, Benjachat T, Leelahavanichkul A, Avihingsanon Y, Fenton RA, et al. Exosomes in urine biomarker discovery 51. Adv Exp Med Biol (2015) 845:43–58. doi:10.1007/978-94-017-9523-4_5
84. Rolfo C, Castiglia M, Hong D, Alessandro R, Mertens I, Baggerman G, et al. Liquid biopsies in lung cancer: the new ambrosia of researchers 2. Biochim Biophys Acta (2014) 1846:539–46. doi:10.1016/j.bbcan.2014.10.001
85. Sheller S, Papaconstantinou J, Urrabaz-Garza R, Richardson L, Saade G, Salomon C, et al. Amnion-epithelial-cell-derived exosomes demonstrate physiologic state of cell under oxidative stress. PLoS One (2016) 11:e0157614. doi:10.1371/journal.pone.0157614
86. Cuffe JSM, Holland O, Salomon C, Rice GE, Perkins AV. Review: placental derived biomarkers of pregnancy disorders. Placenta (2017) 54:104–10. doi:10.1016/j.placenta.2017.01.119
87. Chang G, Mouillet JF, Mishima T, Chu T, Sadovsky E, Coyne CB, et al. Expression and trafficking of placental microRNAs at the feto-maternal interface. FASEB J (2017) 31(7):2760–70. doi:10.1096/fj.201601146R
88. Salomon C, Torres MJ, Kobayashi M, Scholz-Romero K, Sobrevia L, Dobierzewska A, et al. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One (2014) 9:e98667. doi:10.1371/journal.pone.0098667
89. Sheller-Miller S, Lei J, Saade G, Salomon C, Burd I, Menon R. Feto-maternal trafficking of exosomes in murine pregnancy models. Front Pharmacol (2016) 7:432. doi:10.3389/fphar.2016.00432
90. Salomon C, Kobayashi M, Ashman K, Sobrevia L, Mitchell MD, Rice GE. Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS One (2013) 8:e79636. doi:10.1371/journal.pone.0079636
91. Lin J, Li J, Huang B, Liu J, Chen X, Chen XM, et al. Exosomes: novel biomarkers for clinical diagnosis 177. ScientificWorldJournal (2015) 2015:657086. doi:10.1155/2015/657086
92. Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol (2015) 77:13–27. doi:10.1146/annurev-physiol-021014-071641
Keywords: fetal signals, parturition, exosomes, biomarker, aging, fetal membranes, p38MAPK, microvesicles
Citation: Menon R, Mesiano S and Taylor RN (2017) Programmed Fetal Membrane Senescence and Exosome-Mediated Signaling: A Mechanism Associated With Timing of Human Parturition. Front. Endocrinol. 8:196. doi: 10.3389/fendo.2017.00196
Received: 25 April 2017; Accepted: 27 July 2017;
Published: 17 August 2017
Edited by:
Carlos Salomon, The University of Queensland, AustraliaReviewed by:
Gregory Edward Rice, The University of Queensland, AustraliaJames Cuffe, Griffith University, Australia
Copyright: © 2017 Menon, Mesiano and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramkumar Menon, cmFtLm1lbm9uQHV0bWIuZWR1
 Ramkumar Menon
Ramkumar Menon Sam Mesiano2
Sam Mesiano2