
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 31 July 2017
Sec. Bone Research
Volume 8 - 2017 | https://doi.org/10.3389/fendo.2017.00183
Background: Osteocalcin (OC) is an intriguing hormone, concomitantly being the most abundant non-collagenous peptide found in the mineralized matrix of bone, and expanding the endocrine function of the skeleton with far-reaching extra-osseous effects. A new line of enquiry between OC and vascular calcification has emerged in response to observations that the mechanism of vascular calcification resembles that of bone mineralisation. To date, studies have reported mixed results. This systematic review and meta-analysis aimed to identify any association between OC and vascular calcification and atherosclerosis.
Methods and results: Databases were searched for original, peer reviewed human studies. A total of 1,453 articles were retrieved, of which 46 met the eligibility criteria. Overall 26 positive, 17 negative, and 29 neutral relationships were reported for assessments between OC (either concentration in blood, presence of OC-positive cells, or histological staining for OC) and extent of calcification or atherosclerosis. Studies that measured OC-positive cells or histological staining for OC reported positive relationships (11 studies). A higher percentage of Asian studies found a negative relationship (36%) in contrast to European studies (6%). Studies examining carboxylated and undercarboxylated forms of OC in the blood failed to report consistent results. The meta-analysis found no significant difference between OC concentration in the blood between patients with “atherosclerosis” and control (p = 0.13, n = 1,197).
Conclusion: No definitive association was determined between OC and vascular calcification or atherosclerosis; however, the presence of OC-positive cells and histological staining had a consistent positive correlation with calcification or atherosclerosis. The review highlighted several themes, which may influence OC within differing populations leading to inconclusive results. Large, longitudinal studies are required to further current understanding of the clinical relevance of OC in vascular calcification and atherosclerosis.
Vascular calcification is a known major risk factor for mortality and morbidity and is an independent risk factor for cardiovascular disease (1–3). Vascular calcification, long believed to be a passive part of aging and “wear and tear,” is now considered an active, cell-mediated complex process that is regulated but not yet fully understood. Osteocalcin (OC) [also known as bone glutamic acid protein (BGLAP)] is an intriguing hormone produced by osteoblasts in bone that has been recently linked with an increasing number of extra-osseous biological roles and effects (4–8). One candidate thread of enquiry is its interaction with the vascular system, and its putative role in the process of vascular calcification or atherosclerosis. OC is not only produced by bone but is expressed by vascular smooth muscle cells (VSMCs) displaying an osteoblast-like phenotype (9).
Osteocalcin is the most abundant, non-collagenous component in the mineralized matrix of bone (10). The presence of three glutamic acid (Gla) residues allows for posttranslational γ-carboxylation at positions 17, 21, and 24. Between 60 and 90% of carboxylated OC (cOC) is deposited in the bone matrix; however, it can also be released into the circulation (11). OC can be undercarboxylated (ucOC) to differing degrees (from 0 to 2 carboxyl groups) due to decarboxylation, low activity of the vitamin K-dependent carboxylase enzyme, or vitamin K deficiency. ucOC has less affinity to hydroxyapatite and is more readily released into the circulation than cOC (12, 13).
ucOC has recently been appointed a predictor and potential therapeutic target of a number of diseases including diabetes and is believed to be the active form of OC (14). Studies have shown ucOC to be a regulator of pancreatic β cell and adipocyte gene expression, glucose metabolism and to increase insulin sensitivity in humans (14). Structural inconsistencies between ucOC and cOC have been explored, but discrepancies in reports are numerous and it is unknown the extent to which structural differences may play in their biological functions (15). It is hypothesized that ucOC may be the active form of OC involved in vascular calcification, but this has yet to be investigated.
Having established roles of other Gla containing proteins, such as Matrix Gla protein, in vascular calcification, many researchers have begun to explore the role of OC. Idelevich et al. investigated the effects of OC overexpressing mice cell lines (chondrocytes and VSMCs) (16). They showed that OC stimulates VSMC mineralization and differentiation, in particular through HIF-1α activation, surmising that OC fuels glucose utilization in VSMCs and promotes osteochondrogenic differentiation resulting in calcification. However, further studies are greatly lacking.
The aim of this systematic review and meta-analysis was to investigate and critically appraise the available literature linking OC to calcification and atherosclerosis in humans.
The systematic review was carried out in accordance with the Meta-analysis Of Observational Studies in Epidemiology group proposal for reporting (17). A systematic and comprehensive search of PubMed and EMBASE (including Medline) was conducted to extract all articles examining an association between OC and vascular calcification or atherosclerosis. Identical search terms were used for both databases and included: “Osteocalcin AND Vascular Calcification,” “Osteocalcin AND Atherosclerosis,” “Osteocalcin AND Arterial Stiffness,” “Bone Gla Protein AND Vascular Calcification,” “Bone Gla Protein AND Atherosclerosis,” “Bone Gla Protein AND Arterial Stiffness,” “BGLAP AND Vascular Calcification,” “BGLAP AND Atherosclerosis,” “BGLAP AND Arterial Stiffness,” “Bone gamma-carboxyglutamic acid protein AND Vascular Calcification,” “Bone gamma-carboxyglutamic acid protein AND Atherosclerosis,” “Bone gamma-carboxyglutamic acid protein AND Arterial Stiffness,” “BGP AND Vascular Calcification,” “BGP AND Atherosclerosis,” “BGP AND Arterial Stiffness.” The searches were limited to include only human studies; with no restrictions on publication year, language, population or article type. Articles were subsequently excluded if the full text could not be found in English (n = 4). The searches were carried out by the 25/05/2017 with no year restrictions.
The titles and abstracts for returned items were examined, and inappropriate articles were rejected. The criteria for inclusion was such that the article was an original, peer reviewed paper involving either longitudinal or cross-sectional human studies that investigated the relationship between OC and calcification or atherosclerosis. A further requirement of each study was that a form of OC must have been measured within their sample population. An endpoint relating specifically to the degree or severity of calcification or atherosclerosis was required to have been measured and reported. Studies that used assumptions of an increased risk of cardiovascular disease (CVD), e.g., by age and weight within their sample populations were excluded. All searches were conducted independently by two reviewers and compared. Where differing opinions on study eligibility existed (n = 6), they were discussed with the study principle investigator.
The included articles were analyzed, and data were collated using an extraction form. The extracted data included the following: the population characteristics (age, sample size, ethnicity, and health status); type and method of OC measured; endpoint measurements; results of outcome and exposure measures; and the overall conclusions of the article and any key limitations or bias. A risk of bias assessment was performed according to the Cochrane Collaboration’s tool for assessing risk of bias (18).
A meta-analysis was performed on those studies that provided OC concentration in blood samples from an “increased vascular calcification/atherosclerosis group” and from a “control/healthy group.” Data were analyzed as forest plots using the Cochrane Review Manager software (Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and as funnel plots using Stata (StataCorp., 2009. Stat Statistical Software: Release 11. College Station, TX, USA). Funnel plot asymmetry (publication bias) was tested by Egger’s test (19). Since heterogeneity was expected between study protocols (different population characteristics, different methods of defining calcification or atherosclerosis, different specificity of assessment methods of OC) random-effect models were used. The results of continuous data of OC concentration are expressed as mean and SD. Where studies did not provide mean and SD, authors were contacted for data. In cases where no response from authors was obtained (n = 8), they could not be included in the statistical analysis (20–27). One author declined to supply requested information (28). Studies were weighted by sample size, and statistical significance was set at p < 0.05. The results are expressed as mean difference as all studies reported OC concentrations in the same units. It was not possible to perform further analyses due to large heterogeneity between studies.
The initial search yielded 1,453 records from which 374 abstracts were reviewed and 46 articles met the inclusion criteria (Figure 1). A description of each study is provided in Table 1. Of the 46 studies included in this review, 26 (56%) were designed specifically to examine the relationship between OC and markers of calcification or atherosclerosis (20, 22, 24–27, 29–47). The other 20 studies evaluated OC among a number of measurements, as a covariate, or in secondary analyses (21, 23, 28, 48–61). Forty-four out of the 46 studies were cross-sectional in design. Twenty-four studies did not make adjustments for any potential confounding variables, and 22 conducted multivariate analyses (Table 1). Ten studies had a sample size greater than 300 (20, 24, 25, 27, 30, 33, 35, 43, 45, 62).
Thirty-three studies (72%) measured OC by enzyme-linked immunosorbent assay, electrogenerated chemiluminescence, or radioimmunoassay, while the remaining studies used flow cytometry or fluorescence activated cell sorting methods, or examined OC by histological immunostaining (Table 1). OC was measured using luminometry in one study (63). ucOC was measured in five studies (22, 31, 32, 41, 44), cOC in one study (44), and total OC was measured in the remaining studies (Table 1). OC positive mononuclear cells, endothelial progenitor cells (EPCs), or osteoprogenitor cells (OPCs) were examined by seven studies (37, 38, 40, 42, 52, 61, 62).
Methods of calcification or atherosclerosis measurements used in OC analyses varied and ranged from calcification scoring methods (n = 22), intima-media thickness measurements (n = 14), pulse wave velocity (PWV) measurements (n = 4), plaque presence (n = 2), and coronary angiography or echocardiography (n = 6; Table 1). One study used 18F-Sodium Fluoride uptake as a marker of calcification (54).
Results of the risk of bias assessment for all 46 studies are presented in Figure 2. Due to the majority of studies being cross-sectional in design and limitations on sample sizes, only one study randomly selected participants. Since all the included studies were observational cohort studies, no risk of bias assessment for “Allocation concealment” could be performed. Forty-six percent of studies included a component of blinding. None of the studies were reported with high risk of attrition bias or other bias. Four studies were reported with high risk of reporting bias. Overall, most information was from studies at low risk of bias.
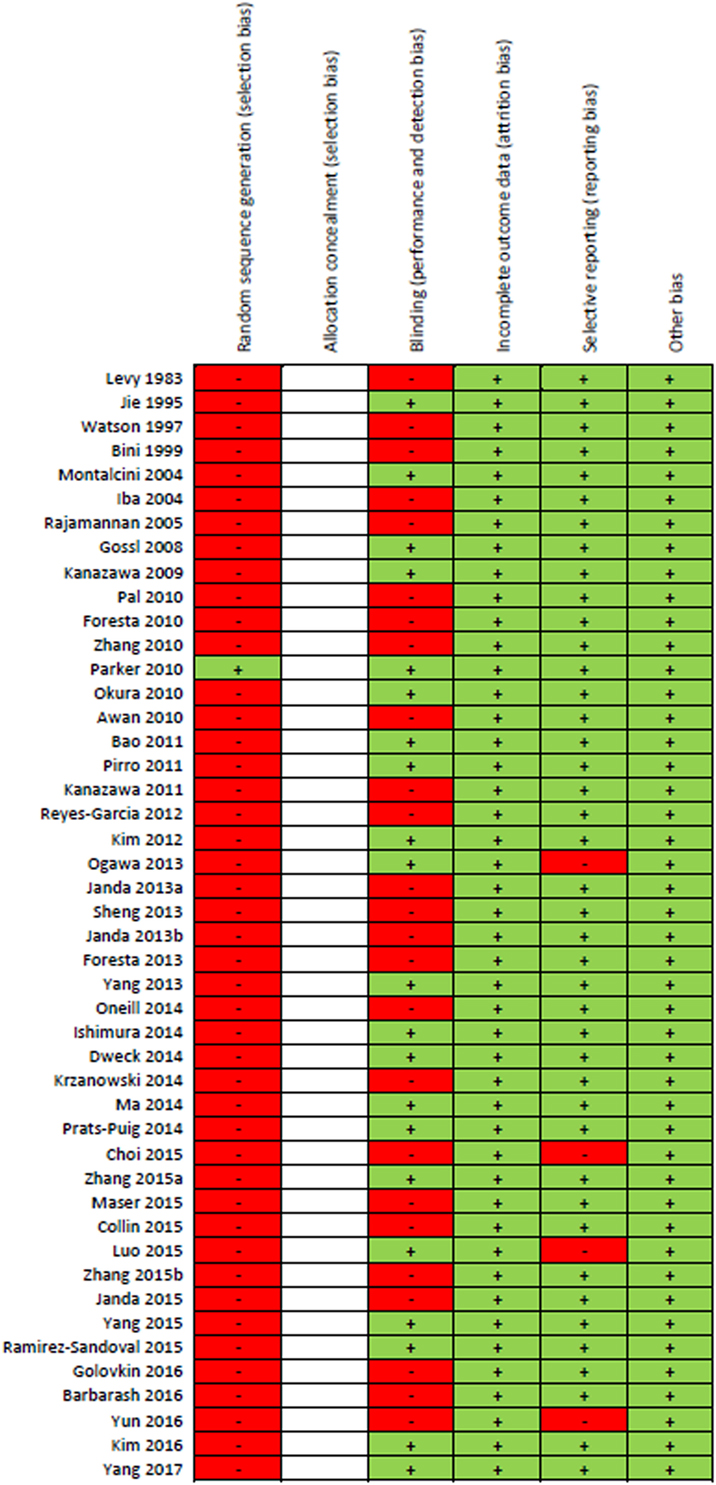
Figure 2. “Risk of bias” summary: green (+) indicates low-bias risk and red (–) indicates high-bias risk. The studies included in this review were all observational in study design and thus the risk of bias for the item “allocation concealment” was not performed and spaces were left blank.
Results of the meta-analysis examining OC concentrations between groups with normal vascular parameters and those presenting with markers of calcification/atherosclerosis are detailed in Figure 3. There was no significant overall difference between OC concentration (total, ucOC, or cOC) in patients with “atherosclerosis” and control, though a trend toward lower OC concentrations was seen in the control group [overall mean difference 0.93 ng/mL (95% CI −0.28, 2.15), p = 0.13]. There was significant statistical heterogeneity, I2 88%, p < 0.00001. Egger’s test showed no publication bias present (p = 0.279, Figure 4).
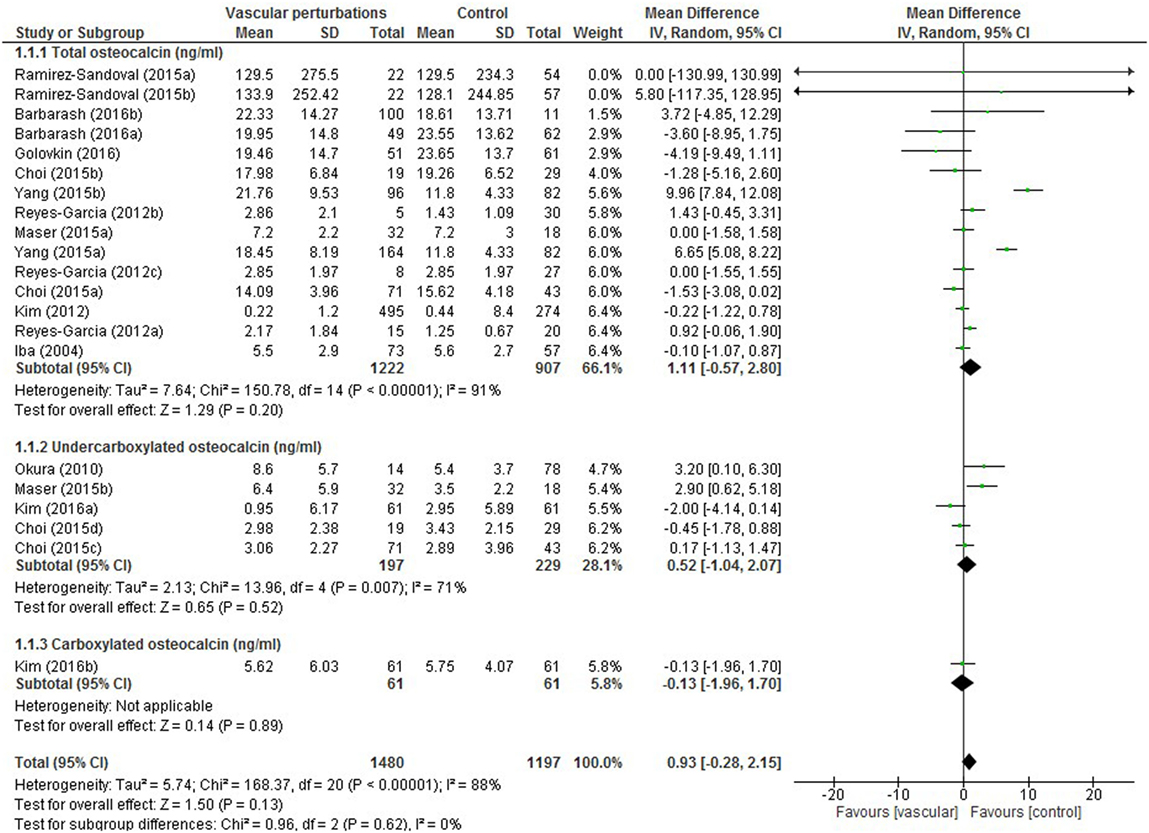
Figure 3. Meta-analysis examining osteocalcin (OC) concentration (nanograms per millilitre) differences between groups with and without vascular perturbations (markers of calcification or atherosclerosis).
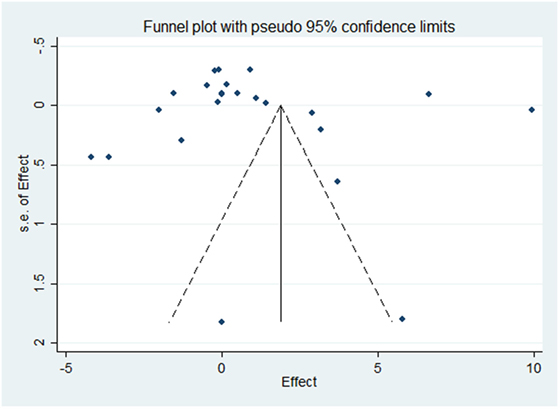
Figure 4. Funnel plot evaluating publication bias on the effect of OC concentration on atherosclerosis or calcification. The SE of the mean difference in osteocalcin concentration for each study is plotted against its effect size (horizontal axis). Although the distribution of the studies within the funnel plot does not appear symmetrical, there was no statistical evidence of publication bias (Egger’s statistic p = 0.279).
Due to multiple end points and assessments within studies, results are reported in terms of total outcomes to avoid bias reporting of overall positive, negative or neutral findings per study in the semiquantitative analysis. Among the studies, the relationship between OC and markers of atherosclerosis or calcification was reported as positive for 26 outcomes and negative for 17 outcomes, while no relationship was established for 29 outcomes.
No significant relationship was established for cOC, which was measured by only one study (44). In seven studies that measured ucOC and markers of atherosclerosis or calcification, two positive outcomes were reported (31, 32), three negative outcomes (22, 29), and four non-significant outcomes (41, 44, 60). Fifty-four percent of studies did not adjust for any confounding variables (Table 1). Of these, 17 positive outcomes between OC and markers of atherosclerosis or calcification were reported, 5 negative outcomes, and 12 non-significant outcomes. Within the other 22 studies adjusting for age and/or other confounding variables including CVD risk factors, 8 positive outcomes were reported between OC and markers of atherosclerosis or calcification, 11 negative outcomes were reported, and 17 outcomes were non-significant.
All 13 studies measuring OC positive mononuclear cells, EPCs, or OPCs, or histological staining for OC, reported a positive relationship between OC and markers of atherosclerosis.
A number of different outcomes were reported within the same studies, depending on gender, type of OC measured, or type of calcification or atherosclerosis measurement. Ogawa-Furuya et al. (29) and Kanazawa et al. (35) found a negative association between OC and markers of atherosclerosis within men but no significant association in women (29, 35). Prats-Puig et al. (32) found a positive association with ucOC, but no significant association was reported for total OC (32). Reyes-Garcia et al. (34) found a positive association in women only. Ma et al. (24) found an association between OC and plaque scores within men with normal glucose tolerance, but no association was reported with C-IMT (24). Choi et al. (41) found a positive association in men with total OC but no association in women or with ucOC (41). Yun et al. (43) found a negative association at low levels of OC only (43), and Zhang et al. (42) found an association in unstable angina pectoris and acute myocardial infarction patients but not in pectoris patients with stable angina (42). The single longitudinal study by Kanazawa et al. (47) showed conflicting results as baseline measurements demonstrated that OC was significantly and positively correlated with plaque score; however, OC was then negatively correlated with changes in plaque score at the end of the study (47).
Overall, in studies that conducted gender sub-analyses, more positive relationship outcomes between OC and measurements of atherosclerosis or calcification were observed within males than negative outcomes, while the reverse was reported within females. A neutral outcome was the most common finding overall for males and females. For the remaining studies when the study population was analyzed as a total, a positive outcome was the most common finding.
Thirty-six percent of outcomes from studies conducted in Asia found a negative relationship between OC and markers of atherosclerosis or calcification, in contrast to 6% in European studies and 5% in American, Canadian, Mexican, or Australian studies. Outcomes examined by population characteristics, e.g., chronic kidney disease patients, healthy adults, vascular problems (vascular dysfunction/coronary heart disease/atherosclerosis) were mixed. When examining studies that only used blood samples to measure OC (n = 33), i.e., excluding the OC positive cell studies and histological studies, no trend became apparent.
No trend was observed for differing methods of measuring calcification or atherosclerosis, and results were similar when examining total studies and those that used blood samples to assess OC. Overall, the method of calcification scoring to assess calcification or atherosclerosis resulted in the most non-significant outcomes with OC measurements.
This review aimed to uncover whether there was a conclusive association between OC and vascular calcification or atherosclerosis in humans by performing a systematic review of the current literature. In total, 33 studies measuring blood OC concentrations and 13 studies measuring OC positive cells or histological staining of OC were found through the literature searches. Overall, no clear association could be made between OC and extent of calcification or atherosclerosis, which was confirmed by meta-analysis. However, all studies measuring OC positive cells or histological staining of OC showed a positive relationship with calcification or atherosclerosis. Some potential reasons for discrepancies in results were explored during the synthesis, including the method of OC measurement, variability in population characteristics and ethnicity, gender, and method of measuring calcification or atherosclerosis.
The majority of studies measured blood concentrations of total OC, ucOC, or both, while only one study measured cOC. No significant relationship was established for cOC, and the studies measuring total OC and/or ucOC resulted in a combination of positively and negatively correlated associations, as well as non-significant outcomes. Within the current review, the role of the different forms of OC in the vasculature, i.e., ucOC or cOC, could not be determined as too few studies examined OC in its different presentations. Total OC may not be a valuable measurement for risk of vascular calcification as it is suggested that ucOC is the biologically active form. Future research may benefit from focusing on the various types of OC to ascertain whether there is a relationship present. However, there has been difficulty in measuring ucOC and cOC as few assays exist and it is unclear which assay system provides the most accurate measurements due to problems with comparability and heterogeneity of OC (14, 66–68). OC also displays a circadian rhythmicity with a nocturnal peak and thus timing of blood sampling may also contribute to variations in results (69, 70).
Circulating mononuclear cells, EPCs, and OPCs expressing OC were used by studies included in this review as a form of OC measurement. These cells are found in the bloodstream and released by bone marrow. EPCs can differentiate into endothelial cells and play a role in angiogenesis (71). It has been hypothesized that OC positive EPCs are involved in the mechanism of calcification by mediating abnormal vascular repair. This is thought to be as a result of the activation of osteogenic genes within the EPCs. EPCs are considered to be part of the initial response to vessel damage; however, instead of promoting normal repair they express an “osteogenic transcriptosome” which promotes calcification. This is further supported by gene expression analyses of CD34+ cells showing expression of bone mineralization related proteins such as Runx2 and BMP-2. It has been proven that OC is expressed by atherosclerotic plaques and VSMCs, which have differentiated as part of the process of calcification already (72) and so studying the association between cells expressing OC and calcification could provide further direction for future studies. All the articles reviewed in this study found a significant, positive correlation between OC positive cells and increased calcification or atherosclerosis.
Histological staining for OC resulted in similarly positive findings. The positive correlation found in all these studies between OC and calcification supports the hypothesis that OC expressed by these cells may contribute to the initial calcification of the vessels. This is comparable to Idelevich et al. (16) whose observations suggest OC is an active contributor to the mineralization process and stimulates differentiation of chondrocytes and VSMCs (16). These observations indicate the potential clinical implications of OC in detecting subclinical atherosclerosis and spotty calcifications. Conflicting results arise only when OC is measured in blood samples, suggesting a need for ucOC and cOC to be measured separately with reliable reproducible assays to disentangle their functions.
All the studies examined in this systematic review, except two, were cross-sectional observational studies. These, although useful, are also limited in their interpretation as a cause–effect relationship cannot be concluded from the results. The mechanism behind calcification remains very much unresolved and so the role, if any, of OC in the process is difficult to identify. The longitudinal study by Kanazawa et al. showed conflicting results between baseline measurements and final measurements. These results demonstrated that initially, total OC was significantly and positively correlated with carotid plaque score (47). However, OC was negatively correlated with changes in plaque score even after adjustment with atherosclerosis-related risk factors at the end of the study. This suggested that OC was relevant to calcification at both extremes, forming a U-shaped association. It was therefore hypothesized that atherosclerotic plaques may initially promote OC secretion but eventually the increased level of OC may suppress the progression of atherosclerosis or calcification. Furthermore, the longitudinal study by Yang et al. (62) reported that very high numbers of early circulating OC positive EPCs tended to be associated with to the risk of all-cause mortality (62). Further studies in a similar prospective longitudinal style should be carried out to confirm the hypotheses suggested. This may provide a reason for the numerous conflicting cross-sectional studies that have studied populations at different phases of disease.
No clear trends could be seen as a result of gender, although it can be noted more negative than positive outcomes between OC and calcification or atherosclerosis were observed in men, while the opposite was observed for women. Gender differences in OC actions have been reported elsewhere, for example in diabetes and fertility (5, 73). Due to the variety of population characteristics included the studies reviewed, associations could not be concluded between particular populations and study results. Most participants were over 50 years of age (data not shown), and this may seem reasonable as the risk of vascular calcification and atherosclerosis increases with age; however, with an increase in age also comes an increase in comorbidities, which could have influenced the results found in some studies. In addition, OC concentrations are influenced by medication including glucocorticoid therapy, antiresorptive agents and vitamin D treatment (74). Not all the studies accounted for these being potential confounding factors.
An interesting study by Namba et al. (75) examined the effect on bone metabolism markers and atherosclerosis measures in patients with atrial fibrillation when switching from warfarin (a vitamin K antagonist) to rivaroxaban (75). This study found ucOC concentrations to decrease after 6 months of rivaroxaban treatment as vitamin K was no longer prohibited. Concomitantly, osteopontin (an atherosclerosis-related marker) was decreased, bone alkaline phosphatase (a bone formation marker) was increased and PWV and augmentation index were significantly decreased. The availability of vitamin K allows for γ-carboxylation of ucOC to cOCN, and the reported improvements in atherosclerosis markers suggest and allude to the importance and potential clinical relevance of the differing presentations of OC, and their usefulness to detect at risk populations.
Ethnicity may play a role in the conflicting results of the studies in this review. Thirty-seven percent of studies conducted in Asia reported negative relationships between OC and calcification or atherosclerosis, compared to 6% of European studies. Studies in both populations used a combination of endpoints measuring calcification or atherosclerosis, showing that this variation did not affect this comparison. Within 10 studies that had a sample size >300, 1 reported a positive outcome, 7 reported negative outcomes, with the remaining two finding no significant outcomes. Eight of these larger studies were conducted in Asia, thus the negative outcomes may be reflective of higher statistical power or ethnicity or a combination of both.
The meta-analysis performed providing adequate data on OC concentration confirmed findings from the qualitative component of this systematic review. The large heterogeneity reported again questions the reliability of serum or plasma measurements of OC concentration, the accuracy of methods of measurement of total OC and its undercarboxylated and carboxylated forms, and the need for well-defined studies with a primary aim of assessing OC’s role in vascular calcification and atherosclerosis. The heterogeneity present in the meta-analysis can be further explained by the variety of study populations (kidney disease, diabetes or glucose intolerance, postmenopausal women, and hypertension) and the different methods employed to distinguish between those with and without vascular calcification or atherosclerosis and varying severities therein.
There are a few limitations in this review, which should be considered. Despite a thorough search of the two databases chosen, the addition of more databases may have widened the search to increase the number of results and hence improve the reliability and validity of the findings. However, the review was carried out by two independent reviewers, and searches generated were analyzed separately and then compared. Only one study analyzed cOC, which limits the results reported here. Furthermore, due to the observational nature of the studies included, only associations can be drawn and no causal relationships can be concluded.
In conclusion, no clear association can be made between OC and vascular calcification or atherosclerosis from the currently available published research. This review has highlighted themes, which may influence OC within differing populations leading to inconclusive results. In addition, the various forms of circulating OC should be separately measured and considered in future studies. Longitudinal studies may provide more insightful results as to the potential pathological effects of OC.
SM and SO: substantial contributions to the conception or design of the work. All the authors: the analysis and interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported and that there is no other financial or other potential conflict of interest.
The reviewer, KH, and handling editor declared their shared affiliation, and the handling editor states that the process nevertheless met the standards of a fair and objective review.
This research was supported by the BBSRC doctoral training partnership (grant no. BB/I024291/1).
1. Wayhs R, Zelinger A, Raggi P. High coronary artery calcium scores pose an extremely elevated risk for hard events. J Am Coll Cardiol (2002) 39(2):225–30. doi:10.1016/S0735-1097(01)01737-5
2. Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol (2000) 36(4):1253–60. doi:10.1016/S0735-1097(00)00872-X
3. Bowman MAH, McNally EM. Genetic pathways of vascular calcification. Trends Cardiovasc Med (2012) 22(4):93–8. doi:10.1016/j.tcm.2012.07.002
4. Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell (2013) 155(1):228–41. doi:10.1016/j.cell.2013.08.042
5. Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell (2011) 144(5):796–809. doi:10.1016/j.cell.2011.02.004
6. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell (2007) 130(3):456–69. doi:10.1016/j.cell.2007.05.047
7. Yeap BB, Chubb SA, Flicker L, McCaul KA, Ebeling PR, Beilby JP, et al. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur J Endocrinol (2010) 163(2):265–72. doi:10.1530/EJE-10-0414
8. Kanazawa I, Tanaka K, Ogawa N, Yamauchi M, Yamaguchi T, Sugimoto T. Undercarboxylated osteocalcin is positively associated with free testosterone in male patients with type 2 diabetes mellitus. Osteoporos Int (2013) 24(3):1115–9. doi:10.1007/s00198-012-2017-7
9. Evrard S, Delanaye P, Kamel S, Cristol JP, Cavalier E; SFBC/SN Joined Working Group on Vascular Calcifications. Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta (2015) 438:401–14. doi:10.1016/j.cca.2014.08.034
10. Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev (1989) 69(3):990–1047.
11. Neve A, Corrado A, Cantatore FP. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res (2011) 343(2):289–302. doi:10.1007/s00441-010-1086-1
12. Plantalech L, Guillaumont M, Vergnaud P, Leclercq M, Delmas PD. Impairment of gamma carboxylation of circulating osteocalcin (bone Gla protein) in elderly women. J Bone Miner Res (1991) 6(11):1211–6. doi:10.1002/jbmr.5650061111
13. Cairns JR, Price PA. Direct demonstration that the vitamin K-dependent bone Gla protein is incompletely gamma-carboxylated in humans. J Bone Miner Res (1994) 9(12):1989–97. doi:10.1002/jbmr.5650091220
14. Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia (2011) 54(6):1291–7. doi:10.1007/s00125-011-2155-z
15. Li J, Zhang H, Yang C, Li Y, Dai Z. An overview of osteocalcin progress. J Bone Miner Metab (2016) 34(4):367–79. doi:10.1007/s00774-015-0734-7
16. Idelevich A, Rais Y, Monsonego-Ornan E. Bone Gla protein increases HIF-1alpha-dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler Thromb Vasc Biol (2011) 31(9):e55–71. doi:10.1161/ATVBAHA.111.230904
17. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA (2000) 283(15):2008–12. doi:10.1001/jama.283.15.2008
18. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi:10.1136/bmj.d5928
19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi:10.1136/bmj.315.7109.629
20. Yang R, Ma X, Dou J, Wang F, Luo Y, Li D, et al. Relationship between serum osteocalcin levels and carotid intima-media thickness in Chinese postmenopausal women. Menopause (2013) 20(11):1194–9. doi:10.1097/GME.0b013e31828aa32d
21. Janda K, Krzanowski M, Gajda M, Dumnicka P, Fedak D, Lis GJ, et al. Cardiovascular risk in chronic kidney disease patients: intima-media thickness predicts the incidence and severity of histologically assessed medial calcification in radial arteries. J Biomed Sci (2015) 16:2275. doi:10.1186/s12882-015-0067-8
22. Zhang M, Ni Z, Zhou W, Qian J. Undercarboxylated osteocalcin as a biomarker of subclinical atherosclerosis in non-dialysis patients with chronic kidney disease. J Biomed Sci (2015) 22:75. doi:10.1186/s12929-015-0183-6
23. Janda K, Krzanowski M, Gajda M, Dumnicka P, Fedak D, Lis GJ, et al. Impaired fasting glucose and diabetes as predictors for radial artery calcification in end stage renal disease patients. Int J Endocrinol (2013) 2013:969038. doi:10.1155/2013/969038
24. Ma H, Lin H, Hu Y, Li X, He W, Jin X, et al. Serum levels of osteocalcin in relation to glucose metabolism and carotid atherosclerosis in Chinese middle-aged and elderly male adults: the Shanghai Changfeng study. Eur J Intern Med (2014) 25(3):259–64. doi:10.1016/j.ejim.2014.01.017
25. Zhang Y, Qi L, Gu W, Yan Q, Dai M, Shi J, et al. Relation of serum osteocalcin level to risk of coronary heart disease in Chinese adults. Am J Cardiol (2010) 106(10):1461–5. doi:10.1016/j.amjcard.2010.07.013
26. Bao Y, Zhou M, Lu Z, Li H, Wang Y, Sun L, et al. Serum levels of osteocalcin are inversely associated with the metabolic syndrome and the severity of coronary artery disease in Chinese men. Clin Endocrinol (Oxf) (2011) 75(2):196–201. doi:10.1111/j.1365-2265.2011.04065.x
27. Parker BD, Bauer DC, Ensrud KE, Ix JH. Association of osteocalcin and abdominal aortic calcification in older women: the study of osteoporotic fractures. Calcif Tissue Int (2010) 86(3):185–91. doi:10.1007/s00223-010-9332-9
28. Ishimura E, Okuno S, Okazaki H, Norimine K, Yamakawa K, Yamakawa T, et al. Significant association between bone-specific alkaline phosphatase and vascular calcification of the hand arteries in male hemodialysis patients. Kidney Blood Press Res (2014) 39(4):299–307. doi:10.1159/000355807
29. Ogawa-Furuya N, Yamaguchi T, Yamamoto M, Kanazawa I, Sugimoto T. Serum osteocalcin levels are inversely associated with abdominal aortic calcification in men with type 2 diabetes mellitus. Osteoporos Int (2013) 24(8):2223–30. doi:10.1007/s00198-013-2289-6
30. Kim KJ, Kim KM, Park KH, Choi HS, Rhee Y, Lee YH, et al. Aortic calcification and bone metabolism: the relationship between aortic calcification, BMD, vertebral fracture, 25-hydroxyvitamin D, and osteocalcin. Calcif Tissue Int (2012) 91(6):370–8. doi:10.1007/s00223-012-9642-1
31. Okura T, Kurata M, Enomoto D, Jotoku M, Nagao T, Desilva VR, et al. Undercarboxylated osteocalcin is a biomarker of carotid calcification in patients with essential hypertension. Kidney Blood Press Res (2010) 33(1):66–71. doi:10.1159/000289575
32. Prats-Puig A, Osiniri I, Soriano-Rodriguez P, Carreras-Badosa G, Bunuel-Alvarez JC, Vila-Pablos C, et al. Undercarboxylated osteocalcin relates to cardiovascular risk markers in offspring of families with metabolic syndrome. Atherosclerosis (2014) 233(1):272–7. doi:10.1016/j.atherosclerosis.2014.01.002
33. Sheng L, Cao W, Cha B, Chen Z, Wang F, Liu J. Serum osteocalcin level and its association with carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol (2013) 12:22. doi:10.1186/1475-2840-12-22
34. Reyes-Garcia R, Rozas-Moreno P, Jimenez-Moleon JJ, Villoslada MJ, Garcia-Salcedo JA, Santana-Morales S, et al. Relationship between serum levels of osteocalcin and atherosclerotic disease in type 2 diabetes. Diabetes Metab (2012) 38(1):76–81. doi:10.1016/j.diabet.2011.07.008
35. Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab (2009) 94(1):45–9. doi:10.1210/jc.2008-1455
36. Jie KS, Bots ML, Vermeer C, Witteman JC, Grobbee DE. Vitamin K intake and osteocalcin levels in women with and without aortic atherosclerosis: a population-based study. Atherosclerosis (1995) 116(1):117–23. doi:10.1016/0021-9150(95)05537-7
37. Gössl M, Mödder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol (2008) 52(16):1314–25. doi:10.1016/j.jacc.2008.07.019
38. Foresta C, De Toni L, Biagioli A, Ganz F, Magagna S, Caretta N. Increased levels of osteocalcin-positive endothelial progenitor cells in patients affected by erectile dysfunction and cavernous atherosclerosis. J Sex Med (2010) 7(2 Pt 1):751–7. doi:10.1111/j.1743-6109.2009.01520.x
39. Foresta C, Strapazzon G, De Toni L, Fabris F, Grego F, Gerosa G, et al. Platelets express and release osteocalcin and co-localize in human calcified atherosclerotic plaques. J Thromb Haemost (2013) 11(2):357–65. doi:10.1111/jth.12088
40. Pal SN, Rush C, Parr A, Van Campenhout A, Golledge J. Osteocalcin positive mononuclear cells are associated with the severity of aortic calcification. Atherosclerosis (2010) 210(1):88–93. doi:10.1016/j.atherosclerosis.2009.11.001
41. Choi BH, Joo NS, Kim MJ, Kim KM, Park KC, Kim YS. Coronary artery calcification is associated with high serum concentration of undercarboxylated osteocalcin in asymptomatic Korean men. Clin Endocrinol (Oxf) (2015) 83(3):320–6. doi:10.1111/cen.12792
42. Zhang H, Wang LJ, Si DL, Wang C, Yang JC, Jiang P, et al. Correlation between osteocalcin-positive endothelial progenitor cells and spotty calcification in patients with coronary artery disease. Clin Exp Pharmacol Physiol (2015) 42(7):734–9. doi:10.1111/1440-1681.12366
43. Yun SH, Kim MJ, Choi BH, Park KC, Park KS, Kim YS. Low level of osteocalcin is related with arterial stiffness in Korean adults: an inverse J-shaped relationship. J Clin Endocrinol Metab (2016) 101(1):96–102. doi:10.1210/jc.2015-2847
44. Kim KM, Lim S, Moon JH, Jin H, Jung KY, Shin CS, et al. Lower uncarboxylated osteocalcin and higher sclerostin levels are significantly associated with coronary artery disease. Bone (2016) 83:178–83. doi:10.1016/j.bone.2015.11.008
45. Luo Y, Ma X, Hao Y, Xiong Q, Xu Y, Pan X, et al. Relationship between serum osteocalcin level and carotid intima-media thickness in a metabolically healthy Chinese population. Cardiovasc Diabetol (2015) 14:82. doi:10.1186/s12933-015-0245-9
46. Levy RJ, Gundberg C, Scheinman R. The identification of the vitamin K-dependent bone protein osteocalcin as one of the gamma-carboxyglutamic acid containing proteins present in calcified atherosclerotic plaque and mineralized heart valves. Atherosclerosis (1983) 46(1):49–56. doi:10.1016/0021-9150(83)90163-6
47. Kanazawa I, Yamaguchi T, Sugimoto T. Relationship between bone biochemical markers versus glucose/lipid metabolism and atherosclerosis; a longitudinal study in type 2 diabetes mellitus. Diabetes Res Clin Pract (2011) 92(3):393–9. doi:10.1016/j.diabres.2011.03.015
48. Janda K, Krzanowski M, Chowaniec E, Kusnierz-Cabala B, Dumnicka P, Krasniak A, et al. Osteoprotegerin as a marker of cardiovascular risk in patients on peritoneal dialysis. Pol Arch Med Wewn (2013) 123(4):149–55. doi:10.20452/pamw.1678
49. Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation (1997) 96(6):1755–60. doi:10.1161/01.CIR.96.6.1755
50. Montalcini T, Emanuele V, Ceravolo R, Gorgone G, Sesti G, Perticone F, et al. Relation of low bone mineral density and carotid atherosclerosis in postmenopausal women. Am J Cardiol (2004) 94(2):266–9. doi:10.1016/j.amjcard.2004.03.083
51. Krzanowski M, Janda K, Dumnicka P, Dubiel M, Stompor M, Kusnierz-Cabala B, et al. Relationship between aortic pulse wave velocity, selected proinflammatory cytokines, and vascular calcification parameters in peritoneal dialysis patients. J Hypertens (2014) 32(1):142–8. doi:10.1097/HJH.0b013e32836569c7
52. Collin J, Gossl M, Matsuo Y, Cilluffo RR, Flammer AJ, Loeffler D, et al. Osteogenic monocytes within the coronary circulation and their association with plaque vulnerability in patients with early atherosclerosis. Int J Cardiol (2015) 181:57–64. doi:10.1016/j.ijcard.2014.11.156
53. Yang Z, Ying C, Zhao H, Fang Y, Chen Y, Shen W. Mineral metabolism disturbances are associated with the presence and severity of calcific aortic valve disease*. J Zhejiang Univ Sci B (2015) 16(5):362–9. doi:10.1631/jzus.B1400292
54. Dweck MR, Jenkins WS, Vesey AT, Pringle MA, Chin CW, Malley TS, et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging (2014) 7(2):371–8. doi:10.1161/CIRCIMAGING.113.001508
55. Awan Z, Alwaili K, Alshahrani A, Langsetmo L, Goltzman D, Genest J. Calcium homeostasis and skeletal integrity in individuals with familial hypercholesterolemia and aortic calcification. Clin Chem (2010) 56(10):1599–607. doi:10.1373/clinchem.2010.147066
56. Iba K, Takada J, Yamashita T. The serum level of bone-specific alkaline phosphatase activity is associated with aortic calcification in osteoporosis patients. J Bone Miner Metab (2004) 22(6):594–6. doi:10.1007/s00774-004-0528-9
57. Bini A, Mann KG, Kudryk BJ, Schoen FJ. Noncollagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol (1999) 19(8):1852–61. doi:10.1161/01.ATV.19.8.1852
58. O’Neill WC, Adams AL. Breast arterial calcification in chronic kidney disease: absence of smooth muscle apoptosis and osteogenic transdifferentiation. Kidney Int (2014) 85(3):668–76. doi:10.1038/ki.2013.351
59. Rajamannan NM, Nealis TB, Subramaniam M, Pandya S, Stock SR, Ignatiev CI, et al. Calcified rheumatic valve neoangiogenesis is associated with vascular endothelial growth factor expression and osteoblast-like bone formation. Circulation (2005) 111(24):3296–301. doi:10.1161/CIRCULATIONAHA.104.473165
60. Maser RE, Lenhard MJ, Sneider MB, Pohlig RT. Osteoprotegerin is a better serum biomarker of coronary artery calcification than osteocalcin in type 2 diabetes. Endocr Pract (2015) 21(1):14–22. doi:10.4158/EP14229.OR
61. Pirro M, Schillaci G, Mannarino MR, Scarponi AM, Manfredelli MR, Callarelli L, et al. Circulating immature osteoprogenitor cells and arterial stiffening in postmenopausal osteoporosis. Nutr Metab Cardiovasc Dis (2011) 21(9):636–42. doi:10.1016/j.numecd.2010.01.015
62. Yang SW, Hennessy RR, Khosla S, Lennon R, Loeffler D, Sun T, et al. Circulating osteogenic endothelial progenitor cell counts: new biomarker for the severity of coronary artery disease. Int J Cardiol (2017) 227:833–9. doi:10.1016/j.ijcard.2016.10.036
63. Ramirez-Sandoval JC, Casanova I, Villar A, Gomez FE, Cruz C, Correa-Rotter R. Biomarkers associated with vascular calcification in peritoneal dialysis. Perit Dial Int (2016) 36(3):262–8. doi:10.3747/pdi.2014.00250
64. Golovkin AS, Kokov AN, Masenko V, Khryachkova ON, Malyuta E, Barbarash OL. Markers of calcium and phosphate metabolism and osteopenic syndrome in patients with coronary artery disease. Panminerva Med (2016) 58(4):253–62.
65. Barbarash OL, Lebedeva NB, Kokov AN, Novitskaya AA, Hryachkova ON, Voronkina AV, et al. Decreased cathepsin K plasma level may reflect an association of osteopoenia/osteoporosis with coronary atherosclerosis and coronary artery calcification in male patients with stable angina. Heart Lung Circ (2016) 25(7):691–7. doi:10.1016/j.hlc.2016.02.002
66. Lee AJ, Hodges S, Eastell R. Measurement of osteocalcin. Ann Clin Biochem (2000) 37(Pt 4):432–46. doi:10.1177/000456320003700402
67. Nimptsch K, Hailer S, Rohrmann S, Gedrich K, Wolfram G, Linseisen J. Determinants and correlates of serum undercarboxylated osteocalcin. Ann Nutr Metab (2007) 51(6):563–70. doi:10.1159/000114211
68. Vergnaud P, Garnero P, Meunier PJ, Breart G, Kamihagi K, Delmas PD. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS study. J Clin Endocrinol Metab (1997) 82(3):719–24. doi:10.1210/jcem.82.3.3805
69. Gundberg CM, Markowitz ME, Mizruchi M, Rosen JF. Osteocalcin in human serum: a circadian rhythm. J Clin Endocrinol Metab (1985) 60(4):736–9. doi:10.1210/jcem-60-4-736
70. Terreni A, Pezzati P. Biochemical markers in the follow-up of the osteoporotic patients. Clin Cases Miner Bone Metab (2012) 9(2):80–4.
71. Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med (2012) 2(7):a006692. doi:10.1101/cshperspect.a006692
72. Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol (2001) 21(12):1998–2003. doi:10.1161/hq1201.100229
73. Rui X, Xu B, Su J, Pan C, Zhan C, Su B, et al. Differential pattern for regulating insulin secretion, insulin resistance, and lipid metabolism by osteocalcin in male and female T2DM patients. Med Sci Monit (2014) 20:711–9. doi:10.12659/MSM.890130
74. Beresford JN, Gallagher JA, Poser JW, Russell RG. Production of osteocalcin by human bone cells in vitro. Effects of 1,25(OH)2D3, 24,25(OH)2D3, parathyroid hormone, and glucocorticoids. Metab Bone Dis Relat Res (1984) 5(5):229–34.
Keywords: osteocalcin, calcification, atherosclerosis, bone hormone, vascular disease, bone glutamic acid protein
Citation: Millar SA, Patel H, Anderson SI, England TJ and O’Sullivan SE (2017) Osteocalcin, Vascular Calcification, and Atherosclerosis: A Systematic Review and Meta-analysis. Front. Endocrinol. 8:183. doi: 10.3389/fendo.2017.00183
Received: 12 June 2017; Accepted: 12 July 2017;
Published: 31 July 2017
Edited by:
Jakob Starup-Linde, Aarhus Universitetshospital, DenmarkReviewed by:
Jennifer Tickner, University of Western Australia, AustraliaCopyright: © 2017 Millar, Patel, Anderson, England and O’Sullivan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sophie A. Millar, c3R4c2FtaWxAbm90dGluZ2hhbS5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.