- 1Department of Endocrinology, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
- 2Endocrinology Clinic, Cluj County Emergency Clinical Hospital, Cluj-Napoca, Romania
PubMed, Scopus, and Web of Science Core Collection databases were systematically searched for studies reporting synchronous double or multiple pituitary adenomas (MPA), a rare clinical condition, with a vague pathogenesis. Multiple adenomas of the pituitary gland are referred to as morphologically and/or immunocytochemically distinct tumors that are frequently small-sized and hormonally non-functional, to account for the low detection rate. There is no general agreement on how to classify MPA, various criteria, such as tumor contiguity, immunoreactivity, and clonality analysis are being used. Among the component tumors, prolactin (PRL)-immunopositive adenomas are highly prevalent, albeit mute in the majority of cases. The most frequent clinical presentation of MPA is Cushing’s syndrome, given the fact that in more than 50% of reported cases at least one lesion stains for adrenocorticotrophic hormone (ACTH). Plurihormonal hyperactivity may be diagnosed in a patient with MPA when more than one tumor is clinically active (e.g., ACTH and PRL) or in cases with at least one composite tumor (e.g., GH and PRL), to complicate the clinical scenario. Specific challenges associated with MPA include high surgical failure rates, enforcing second-look surgery in certain cases, and difficult preoperative neuroradiological imaging evaluation, with an overall sensitivity of only 25% for magnetic resonance imaging to detect distinct multiple tumors. Alternatively, minor pituitary imaging abnormalities may raise suspicion, as these are not uncommon. Postoperative immunohistochemistry is mandatory and in conjunction to electron microscopy scanning and testing for transcription factors (i.e., Pit-1, T-pit, and SF-1) accurately define and classify the distinct cytodifferentiation of MPA.
Introduction
Up to 10–20% of people harbor a pituitary tumor, mostly in the form of a benign, sporadic, or familial (1), monoclonal, and slow-growing micro- or macroadenoma while aggressive or true malignant tumors constitute a rare finding (2). Pituitary adenomas provide up to 90% of the sellar and parasellar region masses (3), generally featuring a solitary tumor associating clinical traits attributed to pituitary hormones dysfunction and/or local mass effects. To a great extent, small pituitary adenomas have a “quiet” presentation and may behave as silent, asymptomatic tumors, in spite of positive immunohistochemistry (IHC), while true null-cell adenomas are infrequent (4).
A body of clinical case reports published over the past quarter of century point out to clinically relevant, concurrent double, or multiple adenomas of the hypophysis. Multiple pituitary adenomas (MPA) represent simultaneous, but otherwise morphologically and IHC distinct masses of the pituitary gland (5) and need to be distinguished from mixt, multihormone-secreting tumors as such term a single pituitary lesion expressing two or several hormones at IHC. Albeit rare in the clinical setting, MPA are increasingly suspected when there is an incidental finding upon preoperative magnetic resonance imaging (MRI) evaluation of the pituitary gland. As they may pose a challenging clinical scenario with regard to imaging localization and optimal first-choice approach, occasionally enforcing thorough surgical exploration of the pituitary gland to achieve complete removal of functional adenomatous tissue, MPA deserve attention as a distinctive pituitary pathology.
By screening the literature for isolated cases or case series reports, we completed a systematic review to gain insight into current knowledge concerning the diagnosis and management of MPA. Particularly, clinically relevant aspects were highlighted.
Study Methodology
A systematic search of the literature was performed in United States National Library of Medicine PubMed, Scopus, and Web of Science Core Collection databases with “multiple pituitary adenoma/tumor,” “double pituitary adenoma/tumor,” “pituitary magnetic resonance imaging,” and “pituitary immunohistochemistry” as key words. All identified clinical cases or case series were evaluated to be included in the analysis. Of these, only reports recording the patients’ clinical data, endocrine evaluation, and surgery outcome with IHC exam were retained. Case reports presenting metachronous MPA (6) or in which multiple concurrent tumors were not clearly documented in spite of a high index of suspicion (7) were excluded. Clinical cases reported on two different occasions were considered only once (8, 9). Autopsy studies lacking clinical and endocrine documentation (10) were not included in the analysis but discussed throughout the manuscript. However, three cases (two bearing prolactinomas and one non-functional adenoma), in which distinct double pituitary adenoma was confirmed by MRI, were not subjected to surgery (11–13). Double pituitary masses of different origin (e.g., adenoma and craniopharyngioma) were not considered (14). Ultimately, 63 patients harboring 129 (60 double and three triple) synchronous pituitary adenomas fulfilled the study criteria.
Prevalence of MPA: The Size of the Problem
Autopsy Reports
Since the first report on multiple pituitary tumors by Kraus in 1914 (15), estimates provided by autopsy studies on the prevalence of incidentally detected MPA varied widely, ranging from 0.01 to 9% of the examined pituitary glands (10, 16–25). Furthermore, cases with double, triple, and even 10 multiple tumors within one pituitary gland were recognized (17). In a large retrospective review of more than 9000 autopsies samples from St. Michael’s Hospital records and the Mayo Clinic Tissue Registry, Kontogeorgeos et al. described 20 tumor-transformed pituitary glands harboring multiple adenomas (i.e., 16 double and four triple), in total 44 tumors; size was measured in 30 tumors, all of which were microadenomas, the majority measuring <3 mm (10). Consistently, subsequent studies showed that 55–99% of incidentally found adenomas in autopsy materials measure <3 mm (21–23). When Kontogeorgeos et al. restricted their study to 470 randomly autopsied pituitary glands, a prevalence of 0.9% MPA was found, in fact 8.9% of adenomatous glands exhibiting multiple tumors (10). As stated by the authors themselves, the global prevalence of pituitary adenomas in the work of Kontogeorgeos et al. (10.4%) was twofold to threefold lower compared to the reports based on the examination of serial sections of the gland (10). Indeed, single-section examination allowed detection of pituitary adenomas in 2.7–13% of evaluated glands, whereas with serial sectioning of the specimens, the incidence reached 8.4–27% (17, 21–23, 25–27).
Regarding MPA, the emphasis should be on their prevalence among adenomatous pituitaries, which clearly shows that MPA is not an infrequent pituitary pathology. Evaluation of serial 1 mm sections of autopsied pituitaries showed a MPA prevalence of 18.6 and 34.3% in the study of Costello (17) and Burrow (22), respectively. About 8.3% double tumors in a small series of 24 adenomatous glands were found by Tomita et al. (28), while more recently Buurman and Saeger reported 17 (5.4%) multiple tumors out of 316 affected pituitaries (25). Large autopsy series pointed to rates of MPA of 7–10.5% of tumoral glands (17, 21–24, 29), the majority of (≈90%) presenting as double adenomas and only rarely as three tumors (10, 25). Ectopic tumors (30) or combination forms, such as anterior pituitary adenoma and posterior pituitary granular cell myoblastoma (31), have been reported.
Surgical Specimens
Prevalence data in surgical specimens is rather scarce, mostly originating from isolated case reports or specific patient databases (Table 1) (5, 29, 32–35).
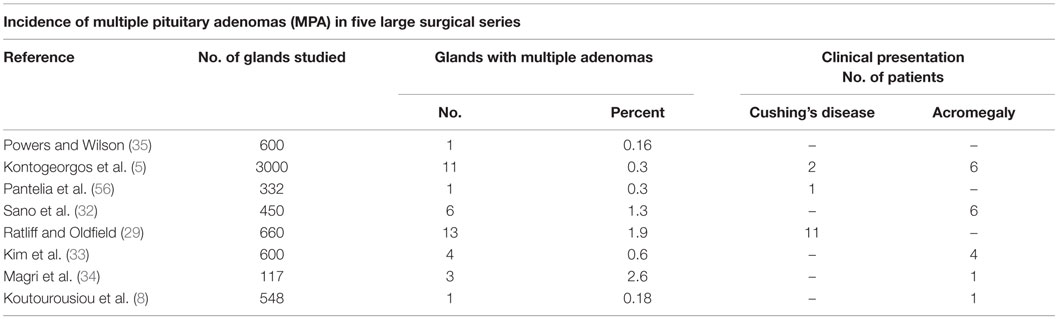
Table 1. MPA incidence from five large surgical series, with specifications regarding most frequent clinical presentation.
Overall, the reported prevalence of MPA according to surgical data varies from 0.37% detected by Kontogeorgeos et al. in an unselected series of 3000 cases of pituitary adenomas (5) to 1.3% observed by Sano in a surgical specimens review of 450 cases (32) and 2.6% by Magri in 117 unselected pituitary adenoma patients (34) but is highly likely to be underestimated. In a series of 600 operated patients referred for pituitary adenoma, Kim et al. reported four (0.67%) cases (33), while Ratliff documented distinct double pituitary tumors in 1.9% of resected specimens (29). Variables including the tumor size or the size and quality of the surgical specimen submitted to the pathological exam, particularly on second-look interventions, might have impact on the estimates. In a small series of Cushing’s disease patients, incidentally identified adenomas had an average maximum diameter of 3 ± 4 mm (median 2 mm) (29). Detection of a second tumor is more difficult in the presence of a macroadenoma (e.g., GH-producing tumors), often associated with compression of surrounding pituitary tissue, potentially masking a second tumor located within the compressed gland (29, 36). Furthermore, clinically non-active, intrasellar adenomas, lacking a tumor mass effect, even if confirmed by MRI might not represent an indication for surgery (13). Hence, the prevalence of MPA might be underestimated.
Classification and Morphology: The Clue to the Unknown
Synchronous MPA are classified upon macroscopic aspect into clearly distinct tumors, recognized as such by neuroradiological imaging and/or surgery (37, 38), and contiguous (collision) tumors, which are resected as a single tumor and confirmed as MPA by the pathologist (9, 32, 34, 39, 40). An interpolated section of non-tumorous pituitary parenchyma should be distinctively recognized in individualized adenomas as a histological criterion. In the contiguous form, specific cytology, IHC and ultrastructure features, in addition to a well-delineated border between the tumors prove existence of different cell populations. In spite of that, discrete morphological differences between cells may also feature single pituitary adenomas (39); vice versa, similar patterns do not exclude distinct immunoreactivity. However, some authors strictly defined MPA based on the evidence of distinct tumors (32).
Evaluation of multiple adenomas in autopsy materials most commonly demonstrated immune-positivity for PRL (10, 28); however, tumors staining for PRL are rather mute in the setting of MPA (10). Apart from PRL-immunopositive tumors, the majority of incidentally discovered multiple tumors is represented by FSH-secreting and null cell adenomas.
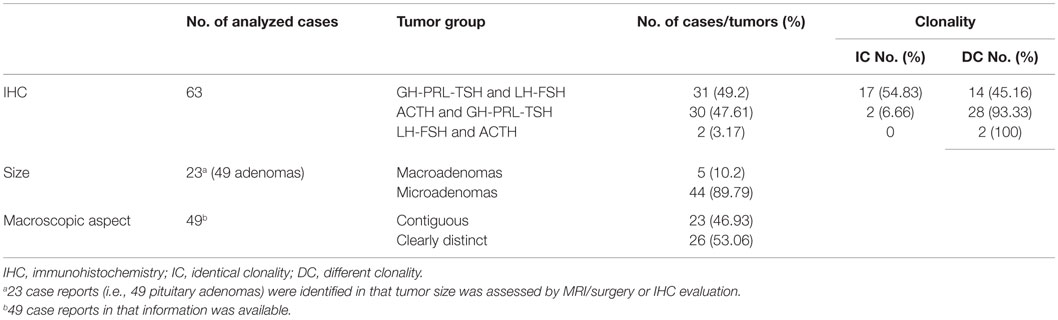
In surgical samples, earlier studies reported GH-immunopositive tumors as being most prevalent (10, 12, 32). Systematically, analyzing the 63 MPA case reports with IHC data, we observed a 50.7% chance that at least one tumor is adrenocorticotrophic hormone (ACTH)-immunopositive, which is in accordance to Cushing’s disease as the most prevalent clinical presentation (Table 2). Silent cell PRL-secreting adenomas or functional prolactinomas coexist, most frequently, with either GH- or ACTH-secreting tumors (Table 3). When excluding MPA assigned to genetic forms (i.e., MEN-1 syndrome), double GH- and PRL-secreting tumors appear to be the most prevalent combination. Indeed, higher rates of mixt ACTH- and PRL-secreting adenomas are typically seen in patients harboring the MEN-1 mutation, otherwise they are regarded as a rather rare clinical finding. Nonetheless, in the setting of MPA, PRL-secreting tumors were the most common incidental lesion coexisting with ACTH-secreting adenomas (Table 2) (7, 29), which potentially points toward a genetic common background. However, this was not systematically investigated. Other reports indicated combinations of ACTH-producing tumors to gonadotrophic tumors (36, 41, 42).
Regarding the immunoreactivity, it resulted that 17.82% (23/129) adenomas exhibited a pluriendocrine, mixt secretory pattern. The most frequent combination immunoprofile was represented by mixt GH and PRL secretion, in fact, resembling the high incidence of composite GH-PRL secretion encountered in single pituitary adenomas. In a review of double pituitary adenomas, including tumors with pathological report albeit incomplete endocrine evaluation, Iacovazzo et al. identified coexistent ACTH- and PRL-secreting synchronous tumors in 33% of cases, closely followed by combined non-functional and GH-secreting pituitary adenomas (24%), GH- and PRL-secreting tumors (10%), and non-functional adenomas combined with either ACTH-secreting (7%) or PRL-secreting tumors (7%). In most of the cases, tumors staining for PRL were silent (7).
Based on tumor immunoreactivity profile and assigned clonality, MPA should be classified into three main patterns (9): (1) GH-PRL-TSH-secreting adenomas combined with FSH-LH-secreting tumors; (2) GH-PRL-TSH producing adenomas combined with ACTH-secreting tumors; and (3) ACTH-secreting adenomas combined with FSH-LH-producing tumors, with the first two subgroups being identified as the most prevalent ones (Table 3).
Specifically, each of the tumors can secrete one or several hormones; hence, making different combinations of pituitary adenomas possible.
Pathogenetic Considerations in MPA
A number of genetic (e.g., oncogenes, tumor suppressor genes, and cell cycle abnormalities) or epigenetic (e.g., CpG dinucleotides methylation, expression of micro-RNAs and large non-coding-RNAs, and modification of histone tails) events appear to be involved in pituitary tumor genesis. However, limited information is available with regard to oncogenesis of multiple adenomas of the pituitary gland.
The Multiple-Hit (Multicentricity) Theory
Coincidental monoclonal expansion of two distinct genetically mutated pituitary cell types could result in the development of synchronous tumors. Evidence of distinct tumor capsules at microdissection of surgical specimens, recognized even in the contiguous tumor phenotype (29), favors the concept of a multicentric origin of MPA.
The Transdifferentiation Theory
Alternatively, cells of one already constituted pituitary adenoma could transdifferentiate into another cell type, with different morphologic and phenotypic characteristics including secretory features and local behavior.
Insight to the pathogenesis of MPA was gained by immunohistochemical localization of transcription factors, which allows classification of pituitary tumors according to the lineage of cell differentiation irrespective of the tumor hormone substance. Occasionally, genetic analysis for clonality has practical value as the demonstration of true lineage infidelity in the pathology specimen readily suggests multiple adenomas (40), although single plurihormonal adenomas exhibiting heterogeneous cell populations of different clonal origin were also recognized (43). As such, combination patterns in that tumors releasing secretory products consistent with multiple clonal proliferations were certified by genetic analysis in few cases of composite pituitary adenomas (43, 44). Transcription factors immunoprofiling revealed in two of three cases of double pituitary adenomas lineages of distinct origin, with Pit-1 expression in PRL-secreting cells and the hormone-negative cell component expressing SF-1 (34). Likewise, Pit-1 was expressed in PRL-secreting or pluriendocrine and T-pit in ACTH-secreting adenoma, while double adenoma combining T-pit and SF-1 expressing cells resulted in development of ACTH- and gonadotroph-cell adenomas, as shown by Jastania et al. (40). Double tumors with different clonality rather support the true multiple adenomas theory (45), whereas transdifferentiation might be more plausible in cases of MPA sharing immunoreactivity to any of the hormones secreted by one of the three different lineages into which pluripotent progenitor cells from the normal pituitary gland differentiate (42).
Pituitary tumor-expressed growth factors and hormone receptors may participate in tumor development through auto- or paracrine mechanisms (46), and this hypothesis could apply for MPA as well. The particular condition of GH-secreting adenomas, which release mitogenic GH and IGF-1 to stimulate development of a second tumor, is prototypical (5).
Genetic abnormalities might play key roles in tumor genesis in patients with MPA as underpinned by positivity for MEN-1 mutation in 4/63 (6.34%) MPA cases included here, which are about 2.3-fold more frequent than expected in adult patients with pituitary adenoma (47). In addition to that, MEN-1 gene mutation was also confirmed in one of the patients with hyperprolactinemia harboring two distinct lesions on pituitary MRI (11), not included in our analysis. Notwithstanding, specific testing for genetic causes related to pituitary tumors was not offered to all patients, which in addition to the small study population may impair data accuracy, thus requiring further studies. In MEN-1 syndrome, the prevalence of tumors with more than one lineage is significantly higher compared to non-MEN-1 cases, with ACTH- and PRL-producing tumors as the most prevalent combination (48). Furthermore, Kim et al. (33) were able to report two cases of familial-isolated pituitary double GH-secreting adenomas (FIDPA), unrelated to MEN-1.
Additionally, somatic mutations (e.g., mutation of Gs protein) extended to more than one pituitary adenoma might be involved in the process of multiple tumor formation and have been demonstrated, particularly in GH-producing adenoma. Transcriptional studies may reveal whether coexisting tumors originate from one cell type (33). About 40% of GH-secreting adenomas harbor a Gs protein mutation (49), implying this mutation has a determinant tumorigenic role (50). A somatic mutation in the ubiquitin-specific protease 8 (USP8) gene (51) was reported in about one-thirds to two-thirds of ACTH-secreting adenomas, which is the most prevalent functional tumor found in MPA. Nevertheless, none of the cases with USP8 mutations exhibited double or multiple adenomas.
Clinical Management of MPA
Of all cases, 45/63 (71.42%) beard hormonally active tumors in our analysis; therefore, recognition of double or multiple pituitary tumors within one pituitary gland is prerequisite to avoid failure of surgery by missing the causative lesion (29, 41, 52, 53).
It still is a matter of debate, which type of endocrine dysfunction essentially unravels the disease in patients harboring MPA, some case series reports favoring acromegaly (54), whereas others argue for Cushing’s disease (7).
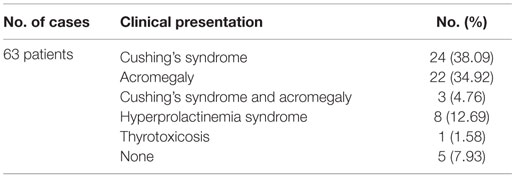
Of 63 fully documented clinical cases reported until now, 24 presented as Cushing’s syndrome (5, 29, 30, 56, 36, 41, 42, 54–58), closely followed by acromegaly with 22 cases (5, 9, 32, 33, 39, 41, 50, 53, 54, 58–63). Of note, synchronous ACTH- and GH-producing tumors were confirmed by IHC in seven patients; three of these cases expressed clinically as Cushing’s syndrome, one as acromegaly, and three were diagnosed with both hyperadrenalism and acromegaly (54, 58, 59) (Table 3). However, particularly incipient disease clinical features in acromegaly might be mild; according to Endocrine Society guidelines, IGF-1 screening in each patient with confirmed pituitary mass is recommended (64). Hyperprolactinemia in acromegaly patients points out to either mixt GH and PRL tumor release or pituitary stalk compression in the setting of suprasellar macroadenoma but raises also the possibility of two independent GH- and PRL-secreting adenomas. In agreement to this, Tolis et al. reported a patient with acromegaly and galactorrhea–amenorrhea syndrome caused by two separate adenomas, one producing GH and the other PRL (53). Furthermore, failure of dopamine agonists’ effect in prolactinoma may suggest double pituitary adenoma in certain cases (65).
Apparently, about 1.6–3.3% of Cushing’s disease patients exhibit double or multiple pituitary tumors (29). Three cases of double pituitary lesions were reported by Meij et al. in patients with Cushing’s disease (42). Additionally, two out of three patients with double pituitary tumors reported by McKelvie et al. presented ACTH-dependent hypercortisolemia (41). By screening a Cushing’s syndrome database, Ratliff and Oldfield found double pituitary tumors in 13 of 660 operated pituitaries, prospectively evaluated (29). Up to the moment, only two clinical cases featuring clearly separated pituitary adenomas, both ACTH-secreting, were reported (30, 43). Cushing’s disease may be subclinical (59) and easily missed (7, 66).
The source of ACTH hypersecretion in ACTH-immunoreactive forms of MPA is mainly eutopic due to hyperfunctional pituitary corticotroph adenoma cells; however, ACTH may also derive from ectopic corticotroph tumor cells located in the parasellar area, the neurohypohysis, or the pituitary stalk (30, 67). Corticotroph cell nodular hyperplasia has been suggested in a small number of patients with Cushing’s disease (68), including MPA (42, 55).
Lack of disease remission after effective transsphenoidal surgery in a patient with Cushing’s disease supports, in theory, the possibility of residual tumor or MPA in that the incidental non-functional tumor was removed. Nevertheless, clear distinction between persistent disease after unsuccessful surgery and disease recurrence should be made (69), as the latter is also increasingly reported in cured pituitary ACTH-secreting adenoma (70).
Higher rates of Cushing’s disease among case reports possibly reflect more aggressive management enforced by the need to cure adrenal hyperfunction. However, variability between studies is relatively high and the small numbers of cases significantly impairs accuracy.
Imaging Detection of MPA
Multiple adenomas of the pituitary gland are traditionally known as difficult to be detected at preoperative imaging investigation (5, 29, 42, 54), the widely accepted reason being the small size of at least one of tumors, in several cases <3 mm. Older studies reported low accuracy for MRI diagnosis of MPA, such as in the case series of Ratliff, where none of the seven cases harboring clearly separated double pituitary tumors identified by surgery had been previously detected by MRI as such (29). Nevertheless, a detailed overview of all MPA clinical case reports clearly shows that MRI was able to detect two or even three (56) pituitary lesions in about 25% of cases. Except for the five ACTH-secreting adenomas in the series of Ratliff, where MRI was non-diagnostic (29), a finding which is in agreement to up to about 40% of cases of Cushing’s disease having normal MRI (71), a single tumor image or, alternatively, ill-defined abnormalities, such as an asymmetric, inhomogeneous appearance of the gland (55) or a global increase in the pituitary volume (30) were noted in most patients, which might prompt the neurosurgeon to careful exploration of the gland. Most challenging situations remain contiguous MPA, resulting in single tumor appearance, even on high-resolution MRI exam (34) or macroadenomas masking small coexisting tumors (36, 57). As true MPA are rare, false-positive findings of double or multiple tumors on pituitary MRI might be detected; thus, diagnosis is confirmed following pathological exam.
High-resolution MRI techniques need to be employed in hormonally active tumors with non-diagnostic imaging tests (54). Thin-sliced and dynamic MRI represents a readily accessible option to improve resolution and increasingly detect multiple lesions. Alternatively, 3.0-T MRI could detect pituitary microadenomas not identified by 1.5-T MRI imaging (72). On the other hand, the higher sensitivity attributed to the higher field strength in >3.0-T MRI devices may also increase the likelihood of false positive results, and images have to be interpreted cautiously. Use of spoiled gradient recalled acquisition (SPGR) sequences improves detection of ACTH-producing adenomas (73, 74). Recently, more advanced techniques, such as MET-PET/3.0-T MRI, showed higher sensitivity in the detection of ACTH-secreting pituitary microadenomas compared to traditional and dynamic MRI (75, 76).
Surgery and Postoperative Evaluation
Confirmed microadenoma at initial pituitary exploration prompts to the careful inspection of the anterior and lateral surfaces of the gland for a second tumor (29, 52) as more than 50% of small tumors, particularly GH- and PRL-secreting adenomas, present on the surface of the gland (5). Exploratory pituitary surgery should be reserved only to cases with high suspicion or during second-look surgery as well as for ACTH-secreting tumors with normal MRI and non-diagnostic inferior petrosal sinus sampling (IPSS). Intraoperative pituitary imaging [iUltrasonography (iUS)/iMRI] seems be useful to assure complete tumor removal in certain cases. Residual tumor detection is facilitated with iMRI, particularly when located in the para- or suprasellar area (77), to achieve rates of resection of up to 96% in unselected series (78); however, the sensitivity of the method significantly decreases when remnant lesions are smaller than 3 mm (79). Few studies reported on the use of iUS to allow targeted resection of small, hyperechoic pituitary adenomas, even in MRI-negative cases, as in the typical condition of Cushing’s disease (80, 81), although limited experience is available. Immunohistochemistry allows the exclusion of false double pituitary lesions by detecting distinct immunochemical signatures in surgical specimens.
Isolated case reports evoke Cushing’s disease patients in whom the surgeon removed a PRL- or GH-secreting adenoma while the ACTH-secreting tumor was identified and resected at reoperation (29, 41). Apart from absent ACTH stain on IHC, lack of postsurgical hypoadrenalism is highly suggestive of tumor persistence in operated Cushing’s disease patients (57). Even if both tumors are correctly removed, unequivocal diagnosis of MPA is difficult when adenomas show identical immunoreactivity; in these cases, variability in cells size, conventional staining, and cytoplasm appearance (36, 55) or presence of a thin lamellae of compressed normal pituitary tissue separating the tumors might be of help. Electron microscopy scanning may provide additional information (e.g., degree of granularity, changes in Golgi apparatus, or presence or absence of fibrous bodies) and should be considered when available (32, 33, 39, 40, 54, 65). Furthermore, quantitation of O-methylguanine-DNA methyltransferase (MGMT) immunoexpression, the Ki-67 nuclear labeling index, or supplemental transcription factors (82) may show significant differences among tumors in patients suspected for multiple adenomas.
Conclusion
Autopsy series indicate a prevalence of 7–10% MPA of all adenomatous pituitary glands, which is highly discordant to exceedingly rare clinical and/or operative reports (i.e., 1–1.5% of pituitary adenomas), thus suggesting the many cases of MPA that remain undiagnosed, since only rarely these multiple tumors are clinically relevant. Notwithstanding, double lesion images or equivalent abnormalities on high-resolution MRI scan are not rare in patients harboring MPA and should always point to careful surgical pituitary exploration, particularly in Cushing’s syndrome or acromegaly patients. However, definitive diagnosis should be concluded based upon pathological examinations. Persistence of endocrine disturbances after pituitary surgery leads to a high index of suspicion for MPA. Up to this moment, the etiopathogeny of MPA remains elusive, in spite of various theoretical concepts and higher prevalence of MEN-1 mutation among genotyped cases. Further, molecular analysis will provide new insights into the pathogenesis of pituitary adenomas and the mechanisms of multidirectional phenotypic differentiation.
Author Contributions
RMB collected data and drafted the manuscript. CEG is responsible for the design of the work, contributed to data analysis, and wrote the review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Igreja S, Chahal HS, King P, Bolger GB, Srirangalingam U, Guasti L, et al. Characterization of aryl hydrocarbon receptor interacting protein (AIP) mutations in familial isolated pituitary adenoma families. Hum Mutat (2010) 31(8):950–60. doi:10.1002/humu.21292
2. Sav A, Rotondo F, Syro LV, Di Ieva A, Cusimano MD, Kovacs K. Invasive, atypical and aggressive pituitary adenomas and carcinomas. Endocrinol Metab Clin North Am (2015) 44:99–104. doi:10.1016/j.ecl.2014.10.008
3. Gsponer J, De Tribolet N, Deruaz JP, Janzer R, Uské A, Mirimanoff RO, et al. Diagnosis, treatment, and outcome of pituitary tumors and other abnormal intrasellar masses. Retrospective analysis of 353 patients. Medicine (1999) 78:236–69.
4. Asa SL, Kovacs K, Stefaneanu L, Horvath E, Billestrup N, Gonzalez-Manchon C, et al. Pituitary adenomas in mice transgenic for growth hormone-releasing hormone. Endocrinology (1992) 131:2083–9. doi:10.1210/endo.131.5.1425411
5. Kontogeorgos G, Scheithauer BW, Horvath E, Kovacs K, Lloyd RV, Smyth HS, et al. Double adenomas of the pituitary: a clinicopathological study of 11 tumors. Neurosurgery (1992) 31:840–9. doi:10.1097/00006123-199211000-00003
6. Thodou E, Kontogeorgos G, Horvath E, Kovacs K, Smyth HS, Ezzat S. Asynchronous pituitary adenomas with differing morphology. Arch Pathol Lab Med (1995) 119(8):748–50.
7. Iacovazzo D, Bianchi A, Lugli F, Milardi D, Giampietro A, Lucci-Cordisco E, et al. Double pituitary adenomas. Endocrine (2013) 43(2):452–7. doi:10.1007/s12020-013-9876-3
8. Koutourousiou M, Kontogeorgos G, Wesseling P, Grotenhuis AJ, Seretis A. Collision sellar lesions: experience with eight cases and review of the literature. Pituitary (2010) 13:8–17. doi:10.1007/s11102-009-0190-2
9. Kannuki S, Matsumoto K, Sano T, Shintani Y, Bando H, Saito S. Double pituitary adenoma – two case reports. Neurol Med Chir (1996) 36:818–21. doi:10.2176/nmc.36.818
10. Kontogeorgos G, Kovacs K, Horvath E, Scheithauer BW. Multiple adenomas of the human pituitary. A retrospective autopsy study with clinical implications. J Neurosurg (1991) 74(2):243–7. doi:10.3171/jns.1991.74.2.0243
11. Sahdev A, Jager R. Bilateral pituitary adenomas occurring with multiple neoplasia type one. AJNR Am J Neuroradiol (2000) 21:1067–9.
12. De Oliveira Andrade LJ, Santos França L, Santos França L, Cordeiro de Almeida MA. Double pituitary prolactinoma. J Clin Endocrinol Metab (2010) 95:4848–9. doi:10.1210/jc.2010-1412
13. Oner AY, Tokgoz N, Erbas G, Tali ET. Magnetic resonance imaging findings of simultaneous double pituitary adenoma: a case report and review of the literature. Riv Neuroradiol (2004) 17(1):113–5. doi:10.1177/197140090401700115
14. Tzonos T, Pfingst E. [On a rare double tumor in the pituitary region]. Zentralbl Neurochir (1964) 25:108–11.
15. Kraus EJ. Die beziehungen der Zellen des Vordedappens der menschlichen Hypophyse zueinander unter normalen Verhaltnissen und in Tumoren. Beitr Pathol Anat Allg Pathol (1914) 58:159–210.
19. Haugen OA. Pituitary adenomas and the histology of the prostate in elderly men. An analysis in an autopsy series. Acta Path Microbiol Scand A (1973) A81:425–34.
21. Kovacs K, Horvath E, Ryan N, Ezrin C. Null cell adenoma of the human pituitary. Virchows Arch A Pathol Anat Histol (1980) 387(2):165–74. doi:10.1007/BF00430697
22. Burrow GN, Wortzman G, Rewcastle NB, Holgate RC, Kovacs K. Microadenomas of the pituitary and abnormal sellar tomograms in an unselected autopsy series. N Engl J Med (1981) 304(3):156–8. doi:10.1056/NEJM198101153040306
23. Parent A, Bebin J, Smith RR. Incidental pituitary adenomas. J Neurosurg (1981) 54:228–31. doi:10.3171/jns.1981.54.2.0228
24. McComb DJ, Ryan N, Horvath E, Kovacs K. Subclinical adenomas of the human pituitary. New light on old problems. Arch Pathol Lab Med (1983) 107(9):488–91.
25. Buurman H, Saeger W. Subclinical adenomas in postmortem pituitaries: classification and correlations to clinical data. Eur J Endocrinol (2006) 154(5):753–8. doi:10.1530/eje.1.02107
26. Parent AD, Brown B, Smith EE. Incidental pituitary adenomas: a retrospective study. Surgery (1982) 92(5):880–3.
27. Hardy J. Transsphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg (1969) 16:185–217.
28. Tomita T, Gates E. Pituitary adenomas and granular cell tumors. Incidence, cell type, and location in 100 pituitary glands at autopsy. Am J Clin Pathol (1999) 111:817–25.
29. Ratliff JK, Oldfield EH. Multiple pituitary adenomas in Cushing’s disease. J Neurosurg (2000) 93:753–61. doi:10.3171/jns.2000.93.5.0753
30. Mendola M, Dolci A, Piscopello L, Tomei G, Bauer D, Corbetta S, et al. Rare case of Cushing’s disease due to double ACTH-producing adenomas, one located in the pituitary gland and one into the stalk. Hormones (2014) 13(4):574–8. doi:10.14310/horm.2002.1503
31. Tomita T, Kuziez M, Watanabe I. Double tumors of anterior and posterior pituitary gland. Acta Neuropathol (1981) 54(2):161–4. doi:10.1007/BF00689411
32. Sano T, Horiguchi H, Xu B, Li C, Hino A, Sakaki M, et al. Double pituitary adenomas: six surgical cases. Pituitary (1999) 1:243–50. doi:10.1023/A:1009994123582
33. Kim K, Yamada S, Usui M, Sano T. Preoperative identification of clearly separated double pituitary adenomas. Clin Endocrinol (Oxf) (2004) 61(1):26–30. doi:10.1111/j.1365-2265.2004.02055.x
34. Magri F, Villa C, Locatelli D, Scagnelli P, Lagonigro MS, Morbini P, et al. Prevalence of double pituitary adenomas in a surgical series: clinical, histological and genetic features. J Endocrinol Invest (2010) 33:325–31. doi:10.3275/6716
35. Powers SK, Wilson CB. Simultaneously occurring prolactinomas. Case report. J Neurosurg (1981) 55:124–6. doi:10.3171/jns.1981.55.1.0124
36. Rotondo F, Khatun N, Scheithauer BW, Horvath E, Marotta TR, Cusimano M, et al. Unusual double pituitary adenoma: a case report. Pathol Int (2011) 61:42–6. doi:10.1111/j.1440-1827.2010.02613.x
37. Cannavò S, Curtò L, Lania A, Saccomanno K, Salpietro FM, Trimarchi F. Unusual MRI finding of multiple adenomas in the pituitary gland: a case report and review of the literature. Magn Reson Imaging (1999) 17:633–6.
38. Eytan S, Kim KY, Bleich D, Raghuwanshi M, Eloy JA, Liu JK. Isolated double pituitary adenomas: a silent corticotroph adenoma and a microprolactinoma. J Clin Neurosci (2015) 22(10):1676–8. doi:10.1016/j.jocn.2015.03.040
39. Syro LV, Horvath E, Kovacs K. Double adenoma of the pituitary: a somatotroph adenoma colliding with a gonadotroph adenoma. J Endocrinol Invest (2000) 23:37–41. doi:10.1007/BF03343674
40. Jastania RA, Alsaad KO, Al-Shraim M, Kovacs K, Asa SL. Double adenomas of the pituitary: transcription factors Pit-1, T-pit, and SF-1 identify cytogenesis and differentiation. Endocr Pathol (2005) 16:187–94. doi:10.1385/EP:16:3:187
41. McKelvie PA, McNeill P. Double pituitary adenomas: a series of three patients. Pathology (2002) 34:57–60. doi:10.1080/00313020120105651
42. Meij BP, Lopes MB, Vance ML, Thorner MO, Laws ER Jr. Double pituitary lesions in three patients with Cushing’s disease. Pituitary (2000) 3:159–68. doi:10.1023/A:1011499609096
43. Tahara S, Kurotani R, Ishii Y, Sanno N, Teramoto A, Osamura RY. A case of Cushing’s disease caused by pituitary adenoma producing adrenocorticotropic hormone and growth hormone concomitantly: aberrant expression of transcription factors NeuroD1 and Pit-1 as a proposed mechanism. Mod Pathol (2002) 15(10):1102–5. doi:10.1097/01.MP.0000030451.28828.00
44. Mazarakis N, Kontogeorgos G, Kovacs K, Horvath E, Borboli N, Piaditis G. Composite somatotroph-ACTH-immunoreactive pituitary adenoma with transformation of hyperplasia to adenoma. Pituitary (2001) 4(4):215–21. doi:10.1023/A:1020764013137
45. Asa SL, Ezzat S. Molecular basis of pituitary development and cytogenesis. Front Horm Res (2004) 32:1–19. doi:10.1159/000079035
46. Melmed S. Pathogenesis of pituitary tumors. Nat Rev Endocrinol (2011) 7:257–66. doi:10.1038/nrendo.2011.40
47. Scheithauer BW, Laws ER Jr, Kovacs K, Horvath E, Randall RV, Carney JA. Pituitary adenomas of the multiple endocrine neoplasia type I syndrome. Semin Diagn Pathol (1987) 4(3):205–11.
48. Trouillas J, Labat-Moleur F, Sturm N, Kujas M, Heymann MF, Figarella-Branger D, et al. A case-control study in a series of 77 patients versus 2509 non-MEN1 patients. Am J Surg Pathol (2008) 32:534–43. doi:10.1097/PAS.0b013e31815ade45
49. Lyons J, Landis CA, Harsh G, Vallar L, Grunewald K, Feichtinger H, et al. Two G protein oncogenes in human endocrine tumors. Science (1990) 249:655–9. doi:10.1126/science.2116665
50. Shintani Y, Yoshimoto K, Horie H, Sano T, Kanesaki Y, Hosoi E, et al. Two different pituitary adenomas in a patient with multiple endocrine neoplasia type 1 associated with growth hormone releasing hormone-producing pancreatic tumor: clinical and genetic features. Endocr J (1995) 42:331–40. doi:10.1507/endocrj.42.331
51. Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet (2015) 47(1):31–8. doi:10.1038/ng.3166
52. Woosley RE. Multiple secreting microadenomas as a possible cause of selective transsphenoidal adenomectomy failure. Case report. J Neurosurg (1983) 58:267–9. doi:10.3171/jns.1983.58.2.0267
53. Tolis G, Bertrand G, Carpenter S, McKenzie JM. Acromegaly and galactorrhea-amenorrhea with two pituitary adenomas secreting growth hormone or prolactin. Ann Intern Med (1978) 89(3):345–8. doi:10.7326/0003-4819-89-3-345
54. Zieliński G, Maksymowicz M, Podgórski J, Olszewski WT. Double, synchronous pituitary adenomas causing acromegaly and Cushing’s disease. A case report and review of literature. Endocr Pathol (2013) 24:92–9. doi:10.1007/s12022-013-9237-z
55. Andrioli M, Pecori Giraldi F, Losa M, Terreni M, Invitti C, Cavagnini F. Cushing’s disease due to double pituitary ACTH secreting adenomas: the first case report. Endocr J (2010) 57:833–7. doi:10.1507/endocrj.K10E-140
56. Pantelia E, Kontogeorgos G, Piaditis G, Rologis D. Triple adenoma in Cushing’s disease: case report. Acta Neurochir (1998) 140:190–3. doi:10.1007/s007010050083
57. Oyama K, Yamada S, Hukuhara N, Hiramatsu R, Taguchi M, Yazawa M, et al. FSH-producing macroadenoma associated in a patient with Cushing’s disease. Neuro Endocrinol Lett (2006) 27:733–6.
58. Blevins LS Jr, Hall GS, Madoff DH, Laws ER Jr, Wand GS. Case report: acromegaly and Cushing’s disease in a patient with synchronous pituitary adenomas. Am J Med Sci (1992) 304:294–7. doi:10.1097/00000441-199211000-00005
59. Kobayashi Y, Takei M, Ohkubo Y, Kakizawa Y, Matoba H, Kumagai M, et al. A somatotropin-producing pituitary adenoma with an isolated adrenocorticotropin-producing pituitary adenoma in a female patient with acromegaly, subclinical Cushing’s disease and a left adrenal tumor. Endocr J (2014) 61(6):589–95. doi:10.1507/endocrj.EJ14-0093
60. Shimizu C, Koike T, Sawamura Y. Double pituitary adenomas with distinct histological features and immunophenotypes. J Neurol Neurosurg Psychiatry (2004) 75:140.
61. Tosaka M, Kohga H, Kobayashi S, Zama A, Tamura M, Murakami M, et al. Double pituitary adenomas detected on preoperative magnetic resonance images. Case illustration. J Neurosurg (2000) 92:361. doi:10.3171/jns.2000.92.2.0361
62. Mohammed S, Cusimano MD, Scheithauer BW, Rotondo F, Horvath E, Kovacs K. O-Methylguanine-DNA methyltransferase immunoexpression in a double pituitary adenoma: case report. Neurosurgery (2010) 66:421–2. doi:10.1227/01.NEU.0000363852.77126.AD
63. Rahman M, Jusue-Torres I, Alkabbani A, Salvatori R, Rodriguez FJ, Quinones-Hinojosa A. Synchronous GH- and prolactin-secreting pituitary adenomas. Endocrinol Diabetes Metab Case Rep (2014) 2014:140052. doi:10.1530/EDM-14-0052
64. Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, et al. Endocrine Society. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2014) 99(11):3933–51. doi:10.1210/jc.2014-2700
65. Coire CI, Smyth HS, Rosso D, Horvath E, Kovacs K. A double pituitary adenoma presenting as a prolactin secreting tumor with partial response to medical therapy. Case report. Endocr Pathol (2010) 21:135–8. doi:10.1007/s12022-009-9104-0
66. Wynne AG, Scheithauer BW, Young WF Jr, Kovacs K, Ebersold MJ, Horvath E. Coexisting corticotroph and lactotroph adenomas: case report with reference to the relationship of corticotropin and prolactin excess. Neurosurgery (1992) 30:919–23. doi:10.1097/00006123-199206000-00018
67. Vassiliadi D, Tsagarakis S. Unusual causes of Cushing’s syndrome. Arq Bras Endocrinol Metabol (2007) 51(8):1245–52. doi:10.1590/S0004-27302007000800010
68. Haap M, Gallwitz B, Meyermann R, Mittelbronn M. Cushing’s disease associated with both pituitary microadenoma and corticotroph hyperplasia. Exp Clin Endocrinol Diabetes (2009) 117(6):289–93. doi:10.1055/s-0028-1085997
69. Fleşeriu M. Recent advances in the medical treatment of Cushing’s disease. F1000Prime Rep (2014) 6:18. doi:10.12703/P6-18
70. Bou Khalil R, Baudry C, Guignat L, Carrasco C, Guibourdenche J, Gaillard S, et al. Sequential hormonal changes in 21 patients with recurrent Cushing’s disease after successful pituitary surgery. Eur J Endocrinol (2011) 165(5):729–37. doi:10.1530/EJE-11-0424
71. Escourolle H, Abecassis JP, Bertagna X, Guilhaume B, Pariente D, Derome P, et al. Comparison of computerized tomography and magnetic resonance imaging for the examination of the pituitary gland in patients with Cushing’s disease. Clin Endocrinol (Oxf) (1993) 39:307–13. doi:10.1111/j.1365-2265.1993.tb02370.x
72. Stobo DB, Lindsay RS, Connell JM, Dunn L, Forbes KP. Initial experience of 3 Tesla versus conventional field strength magnetic resonance imaging of small functioning pituitary tumours. Clin Endocrinol (Oxf) (2011) 75(5):673–7. doi:10.1111/j.1365-2265.2011.04098.x
73. Patronas N, Bulakbasi N, Stratakis CA, Lafferty A, Oldfield EH, Doppman J, et al. Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J Clin Endocrinol Metab (2003) 88:1565–9. doi:10.1210/jc.2002-021438
74. Chittiboina P, Montgomery BK, Millo C, Herscovitch P, Lonser RR. High-resolution(18)F-fluorodeoxyglucose positron emission tomography and magnetic resonance imaging for pituitary adenoma detection in Cushing disease. J Neurosurg (2015) 122(4):791–7. doi:10.3171/2014.10.JNS14911
75. Alzahrani AS, Farhat R, Al-Arifi A, Al-Kahtani N, Kanaan I, Abouzied M. The diagnostic value of fused positron emission tomography/computed tomography in the localization of adrenocorticotropin-secreting pituitary adenoma in Cushing’s disease. Pituitary (2009) 12(4):309–14. doi:10.1007/s11102-009-0180-4
76. Ikeda H. Demonstration of improved surgical outcome in patients with both ACTH and GH secreting adenomas diagnosed by a new sensitive method of MET-PET fusion 3T-MRI. Endocrinol Metab Syndr (2013) 2:3. doi:10.4172/2161-1017.S1.002
77. Berkmann S, Schlaffer S, Nimsky C, Fahlbusch R, Buchfelder M. Follow-up and long-term outcome of nonfunctioning pituitary adenoma operated by transsphenoidal surgery with intraoperative high-giled magnetic resonance imaging. Acta Neurochir (Wien) (2014) 156(12):2233–43. doi:10.1007/s00701-014-2210-x
78. Vitaz TW, Inkabi KE, Carrubba CJ. Intraoperative MRI for transphenoidal procedures: short-term outcome for 100 consecutive cases. Clin Neurol Neurosurg (2011) 113(9):731–5. doi:10.1016/j.clineuro.2011.07.025
79. Paterno V, Fahlbusch R. High-filed iMRI in transsphenoidal pituitary adenoma surgery with special respect to typical localization of residual tumor. Acta Neurochir (Wien) (2014) 156(3):463–74. doi:10.1007/s00701-013-1978-4
80. Knappe UJ, Engelbach M, Konz K, Lakomek HJ, Saeger W, Schonmayr R, et al. Ultrasound-assisted microsurgery for Cushing’s disease. Exp Clin Endocrinol Diabetes (2011) 119(4):191–200. doi:10.1055/s-0029-1241207
81. Watson JC, Shawker TH, Nieman LK, DeVroom HL, Doppman JL, Oldfield EH. Localization of pituitary adenomas by using intraoperative ultrasound pituitary in patients with Cushing’s disease and no demonstrable tumor on magnetic resonance imaging. J Neurosurg (1998) 89(6):927–32. doi:10.3171/jns.1998.89.6.0927
Keywords: double pituitary adenomas, multiple pituitary adenomas, Cushing’s disease, acromegaly, immunohistochemistry, magnetic resonance imaging, pituitary transcription factor
Citation: Budan RM and Georgescu CE (2016) Multiple Pituitary Adenomas: A Systematic Review. Front. Endocrinol. 7:1. doi: 10.3389/fendo.2016.00001
Received: 15 November 2015; Accepted: 08 January 2016;
Published: 01 February 2016
Edited by:
Marek Bolanowski, Wroclaw Medical University, PolandReviewed by:
Leandro Kasuki, Federal University of Rio de Janeiro, BrazilEsra Hatipoglu, Liv Hospital, Turkey
Copyright: © 2016 Budan and Georgescu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carmen E. Georgescu, Y19lX2dlb3JnZXNjdUB5YWhvby5jb20=
 Renata M. Budan
Renata M. Budan Carmen E. Georgescu
Carmen E. Georgescu