
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 13 May 2015
Sec. Cancer Endocrinology
Volume 6 - 2015 | https://doi.org/10.3389/fendo.2015.00074
This article is part of the Research Topic The role of the Insulin/IGFs system in cancer: critical points in dysregulation, targeting problems and new blocking strategies View all 17 articles
 Cecilia Garofalo1
Cecilia Garofalo1 Mariantonietta Capristo1
Mariantonietta Capristo1 Caterina Mancarella1
Caterina Mancarella1 Hadas Reunevi2
Hadas Reunevi2 Piero Picci1
Piero Picci1 Katia Scotlandi1*
Katia Scotlandi1*Osteosarcoma (OS) is the most common primary bone tumor in children and young adults. Several studies have confirmed the involvement of the insulin-like growth factor (IGF) system in the regulation of OS cell proliferation and differentiation as well as in the protection of cells from chemotherapy. Insulin receptor substrate (IRS)-1 is a critical mediator of IGF-1R signaling, and we recently reported that its overexpression in OS cells increases proliferation, migration, and metastasis both in vitro and in vivo. In this study, we evaluated the efficacy of NT157, a selective inhibitor of IRS-1/2, in a panel of OS cells. A strong dose-dependent inhibition of growth was observed in the MG-63, OS-19, and U-2OS OS cell lines, displaying IC50 values at sub-micromolar doses after 72 h of treatment. Exposure to NT157 elicited dose- and time-dependent decreases in IRS-1 levels. Moreover, a protein analysis showed that the degradation of IRS-1 inhibited the activation of principal downstream mediators of the IGF pathway. NT157 significantly affected the cells’ migratory ability, as confirmed by a wound-healing assay. The inhibitor induced cytostatic effects, as evidenced by G2/M cell cycle arrest, and did not affect apoptosis. Consequently, NT157 was combined with drugs used to treat OS in order to capitalize on its therapeutic potential. Simultaneous treatments were made in association with chemotherapeutic agents in a fixed ratio for 72 h and cell proliferation was determined by MTT assay. Synergistic or addictive effects with respect to single agents are expressed as the combination index. Significant synergistic effects were obtained with several targeted drugs, such as Everolimus, a mammalian target of rapamycin (mTOR) inhibitor, and NVP-BEZ235, a dual inhibitor of PI-3K/mTOR. Overall, these findings provide evidence for the effectiveness of a selected inhibitor of IRS-1/2 NT157 in OS cells, displaying a promising approach based on the targeting of IRS-1 combined with other therapies for the treatment of this pediatric solid tumor.
The activation of the insulin-like growth factor (IGF) system regulates several aspects of the malignant phenotype, including the development and progression of cancer and metastasis (1, 2). The IGF family consists of circulating ligands (IGF-1, IGF-II, insulin), at least four receptors [IGF-1R, M6P/IGF-IIR, insulin receptor (IR), and hybrid receptors], and six binding proteins (IGF-BPs). Although multiple proteins are involved in IGF signal transduction, the insulin receptor substrate (IRS) molecules are the primary family of adaptor proteins that function as intermediates of IR and IGF-IR (3). Six IRS proteins have been identified, but only IRS-1 and IRS-2 are widely expressed in normal tissue (4). A number of different physiologic pathways involved in both mitogenic and metabolic responses, such as steroids, hormones, cytokines, and integrins, can regulate IRS protein expression (5–8). Importantly, many of these effector-signaling pathways have been implicated in tumorigenesis and cancer progression. Although IRS-1 is most often related to tumor growth and proliferation, IRS-2 is most frequently associated with tumor motility and invasion. The tyrosine phosphorylation of IRS proteins induces the phosphorylation of mitogen-activated protein kinase (MAPK) and subsequently increases proliferation. This tyrosine phosphorylation also activates the p110 subunit of phosphatidylinositol 3-kinase (PI-3K), leading to a decreased apoptosis, and modulates the mammalian target of rapamycin (mTOR), resulting in translational adaptation (4). IRS-1 is constitutively activated in a variety of solid tumors, including breast cancers, leiomyomas, Wilms’ tumors, rhabdomyosarcomas, liposarcomas, leiomyosarcomas, and adrenal cortical carcinomas (9). In addition to their canonical function as cytosolic signal transduction molecules, IRS proteins can be shuttled to the nucleus and may contribute to the process of malignant transformation. In 3T3 fibroblasts, IGF-1R, the known oncogene SV40T and v-src caused IRS-1 nuclear translocation (10, 11), while other authors demonstrated growth in soft agar and tumorigenicity in nude mice induced by nuclear IRS-1, independently of the oncogene SV40 T-antigen (12). Similarly, the overexpression of nuclear IRS-1 was observed in 32D cells that express either human IGF-IR or SV40T (13). Once in the nucleus, IRS-1 can interact with transcription factors, such as β-catenin, ER-α, and the androgen receptor (AR), to modulate the promoter activity of several genes involved in malignant transformation (14–17).
Osteosarcoma (OS) is the most frequent primary malignant tumor of bone and predominately affects adolescents and young adults (18). The estimated incidence rate is two to three cases/million/year, and it is most common between 10 and 20 years of age. Although modern treatment protocols combine chemotherapy, surgery, and radiotherapy, the 5-year survival rate for non-metastasizing patients remains 60–70%, and this rate decreases to less than 30% for OS patients with metastases or relapsed disease (19, 20). Thus, novel clinical strategies are needed to improve the survival of these patients. IGFs are important regulator of growth and development in normal bone and play an important role in basal bone – cell proliferation. Because IGF-1 mediates the regulation of many growth hormone (GH) functions, the dysregulation of the GH/IGFs axis may favor the pathogenesis of OS (21). Although the expression levels of IGF-1 and IGF-II increased during normal osteoblastic terminal differentiation in vitro, the expression of IGF-1R progressively decreased (22), suggesting that the upregulation of the receptor but not the ligands is the aberrant condition in OS. Despite several in vitro and in vivo studies that have demonstrated the effectiveness of therapies against IGF-1R in enhancing the antitumor response in OS (23, 24), this targeted therapy has been of limited benefit to patients with recurrent or refractory bone and soft tissue sarcomas, including OS (25). This failure may be attributed to changes in other signaling pathways of downstream components that are independent of the expression of the receptor, such as Akt, mTOR, and IRS-1. Recently, Contaldo et al. (26) showed the influence of IRS-1 to sustain tumorigenicity of OS; indeed, in vitro and in vivo data showed that the overexpression of IRS-1 in OS increased tumor proliferation, motility capacity, and anchorage-independent growth compared with parental cells.
Thus, we herein investigated the preclinical efficacy of NT157, a novel small-molecule that specifically targets IRS protein, in OS cells. NT157 is a small-molecule inhibitor that induces Ser-phosphorylation and consequently the degradation of IRS-1 and IRS-2. The destruction of IRS-1/2 lead to the long-term dysregulation of IGF-1R signaling, which is responsible for the anti-proliferative activity in several cancers (27, 28). Here, we demonstrated that this compound inhibits tumor growth, cell cycle, and the motility of OS cells via the downregulation of IRS-1/IRS-2 proteins and their downstream mediators. In addition, in vitro combination studies were conducted to identify the best drug interaction between NT157 and therapies that are currently used to treat this tumor.
The small-molecule inhibitor of IRS-1/2, NT157, was kindly provided by TyrNovo Ltd. (Israel) (27). Briefly, NT157 was dissolved in dimethyl sulfoxide (DMSO) to generate a 10-mM stock solution, which was stored at −80°C. Doxorubicin was purchased from Sigma (St. Louis, MO, USA), cisplatin was obtained from TEVA (Italy), and methotrexate was obtained from Pfizer (Italy). The signal transduction inhibitor that targets mTOR, Everolimus, was purchased from Sequoia Research Products (Pangbourne, UK). The PI-3K/mTOR dual inhibitor NVP-BEZ235 was kindly provided by Novartis (Basel, Switzerland). Working dilutions of all drugs were prepared immediately before use.
The human OS cell lines U-2OS and MG-63 were provided by the American Type Culture Collection (ATCC). The IOR/OS-19 cell line was obtained from the Experimental Oncology Lab at the Rizzoli Institute (Bologna, Italy) and was previously described (29). All cell lines have recently been authenticated by STR analysis using genRESVR MPX-2 and genRESVR MPX-3 kits (serac, Bad Homburg, Germany). The following loci were verified: D16S539, D18S51, D19S433, D21S11, D2S1338, D3S1358, D5S818, D8S1179, FGA, SE33, TH01, and TPOX VWA. The last control was performed in November 2012. These cell lines were all tested for mycoplasma contamination every 3 months (last control, December 2014) using a MycoAlert mycoplasma detection set (Lonza, Nottingham, Ltd.). The cultures were maintained in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with penicillin (20 U/ml), streptomycin (100 μg/ml) (Sigma), and 10% heat-inactivated FBS (Lonza) at 37°C in a humidified 5% CO2 atmosphere.
To assess cellular growth, cells were seeded on 6-well plates (2 × 105 cells/well) in IMDM plus 10% FBS. After 24 h, various concentration of NT157 (0.3–3 μM) were added, and the cells were exposed to this drug for up to 72 h. A dose–response proliferation was evaluated on harvested cells by Trypan Blue vital cell count.
For the combined treatment, cells were plated into 96-well plates (range 2,500–5,000 cells/well) and treated for 72 h with NT157 alone (control) or combined with fixed ratios of DXR (10:1), CDDP (1:10), MTX (100:1), NVP-BEZ235 (10:1), or Everolimus (1:10). Cell proliferation was determined with an MTT assay (Roche, Indianapolis, IN, USA) according to manufacturer’s instructions.
After 48 h of treatment with NT157 alone (1–3 μM) or in combination with NVP-BEZ235 (50 nM), the cell cultures were incubated with 10 μmol/L bromodeoxyuridine (Sigma) for 1 h in a 5% CO2 atmosphere at 37°C. The harvested cells were fixed in 70% ethanol for 30 min. After DNA denaturation with 2 N HCl, 1 × 106 cells were processed for indirect immunofluorescence staining using a-bromodeoxyuridine monoclonal antibody diluted 1:8 as a primary antibody (Becton Dickinson, San Jose, CA, USA). The cells were then analyzed by flow cytometry (FACSCalibur, Becton Dickinson). To analyze the DNA content, cells were fixed with cold 70% ethanol, treated with 0.5 mg/mL RNAse, and stained with 20 μg/mL propidium iodide.
The motility assay was conducted using Transwell chambers (Costar, Cambridge, MA, USA) with an 8-μm pore size, polyvinylpyrrolidone-free, polycarbonate filters (Nucleopore, Pleasanton, CA, USA). IMDM plus 10% FBS was placed in the lower compartment of the chamber. MG-63 and U-2OS OS cells (105) were re-suspended in IMDM plus 10% FBS with or without NT157 (range 1–3 μM) and then seeded in the upper compartment. The chambers were incubated at 37°C in a humidified atmosphere containing 5% CO2 for 18 h. The cells that migrated toward the filter to reach the lower chamber base were counted after Giemsa staining. All experiments were performed in triplicate.
The cell motility was also assessed with a wound-healing assay. Briefly, MG-63 and U-2OS OS cells were plated into 60-mm cell culture plates and allowed to grow to confluence in 10% FBS containing IMDM medium. A 1-mm wide scratch was made across the cell layer using a sterile pipette tip. The medium was changed to remove floating or damaged cells. After 5, 8, and 24 h of treatment with or without NT157 (1–3 μM), the cells that had migrated over the denuded area were observed, and pictures were taken at specific time points.
Cells were treated with NT157 (0.3–1.3 μM) for 48 h or left untreated, and cell lysates were prepared and processed as previously described (30). The membranes were incubated overnight with the following primary antibodies: anti-Shc clone PG-797, anti GAPDH, anti-β-actin (Santa Cruz Biotechnology, San Diego, CA, USA), anti-phospho-Akt (Ser473) clone 736E11, anti-Akt, anti-ERK (Cell Signaling Technology, Beverly, MA, USA), anti-phospho-ERK (Tyr202/Tyr204) (Covance, Princeton, NJ, USA), anti-IRS-1 (Upstate Biotechnology, Temecula, CA, USA), anti-IRS-2 (Abcam, Cambridge, UK), and phospho-IRS-1 (Tyr612) (Invitrogen, USA); anti-rabbit or anti-mouse antibodies conjugated to horseradish peroxidase (GE Healthcare, Piscataway, NJ, USA) were used as secondary antibodies.
IC50 values were calculated from the linear transformations of the dose–response curves. To define drug–drug interactions (in terms of synergism, additivity, or antagonism), the combination index (CI) of each two-drug treatment was calculated with the isobologram equation (31) using the CalcuSyn software (Biososoft, Ferguson, MO, USA).
We previously demonstrated that the IGF system, including its critical mediator IRS-1, is involved in the regulation of OS cell proliferation (26). Thus, the efficacy of the selective inhibitor of IRS-1/2 NT157 was investigated in three representative OS cell lines. Growing MG-63, OS-19, and U-2OS cells were treated with different concentrations of the compound (0.3–3 μM) for up to 72 h (Figure 1A), and a dose–response proliferation was assessed by Trypan Blue cell counting assay. A strong dose-dependent inhibition of growth was observed in all cell lines tested, showing IC50 values at sub-micromolar doses (ranging from 0.3 to 0.8 μM) after 72 h of treatment. NT157 reportedly acts via the downregulation of IRS-1 (27). Our cellular models expressed high basal levels of IRS-1 protein. The exposure of three OS cell lines to NT157 elicited dose- and time-dependent decreases in the IRS-1 protein levels. The maximal activity was reached already after 24 h of treatment (Figure 1B), confirming that the inhibitory effects on tumor growth in OS were related to destruction of IRS-1.
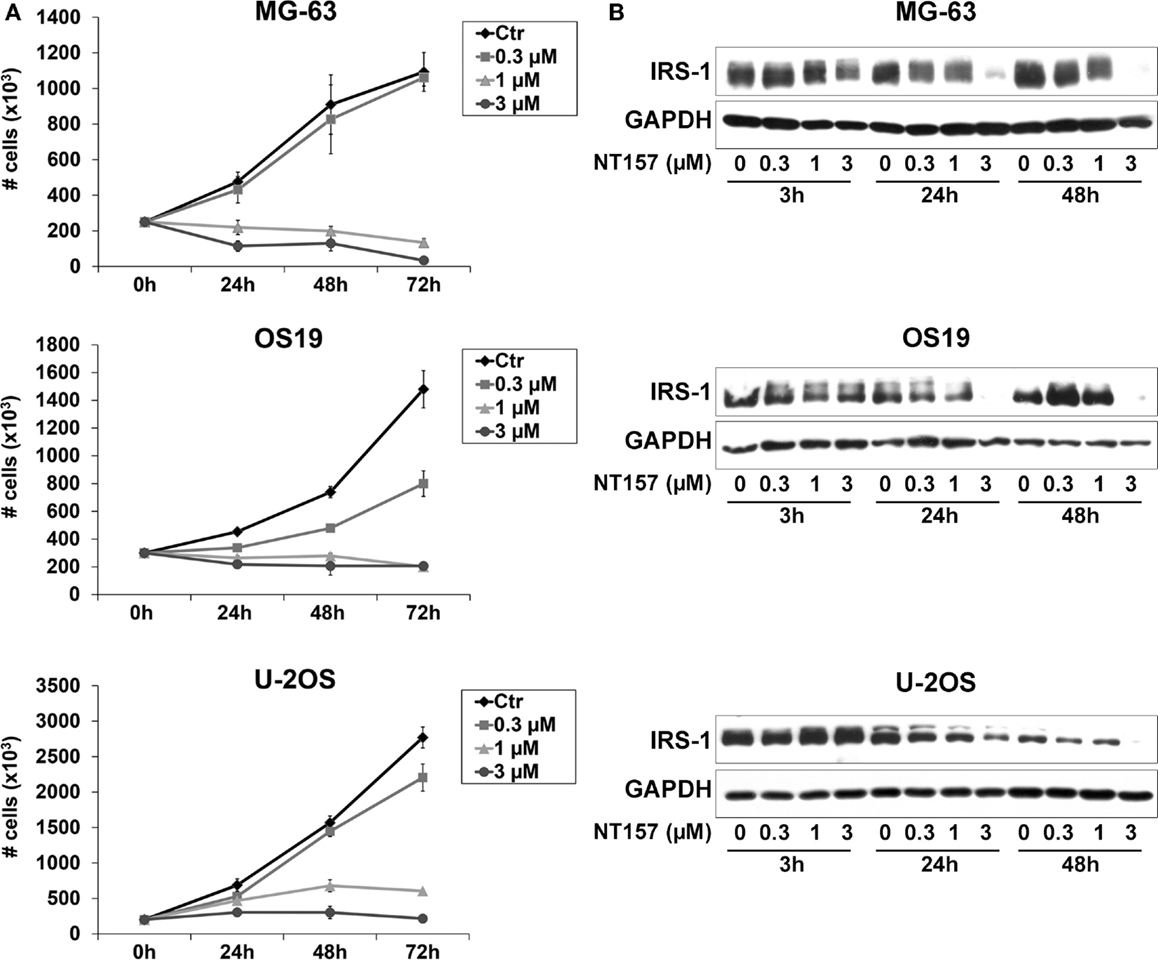
Figure 1. (A) The in vitro sensitivity to a selected inhibitor of IRS-1/2, NT157, for a panel of OS cell lines. Cell growth was assessed by staining cells with Trypan Blue and counting viable cells after up to 72 h of exposure to NT157 (0.3–3 μM) in MG-63, OS-19, and U-2OS cells. Points indicate three independent experiments; bars indicate the SE. (B) Downregulation of IRS-1 protein level in OS cells in response to NT157. Growing MG-63, OS-19, and U-2OS cells were treated with or without NT157 (0.3–3 μM) for 3–48 h. The expression of IRS-1 was determined by western blotting using 40 μg of total protein cell lysate. GAPDH was used as a loading control. The figure shows data representative of two independent experiments.
Because OS is a highly metastatic tumor, the effect of NT157 on cellular migration was also evaluated. MG-63 and U-2OS cells were pre-incubated with inhibitor (1–3 μM) for 24 h, and a motility assay was performed using Transwell chambers. Both cell lines displayed a significant reduction in motility (p < 0.05) compared with the control in response to 3 μM NT157 (Figure 2A); this effect was also confirmed with a wound-healing assay (Figure 2B). This effect could be attributed to the inhibition of IRS-2 protein (27), which is known to be essential for tumor metastasis (32), by NT157 (Figure 2C).
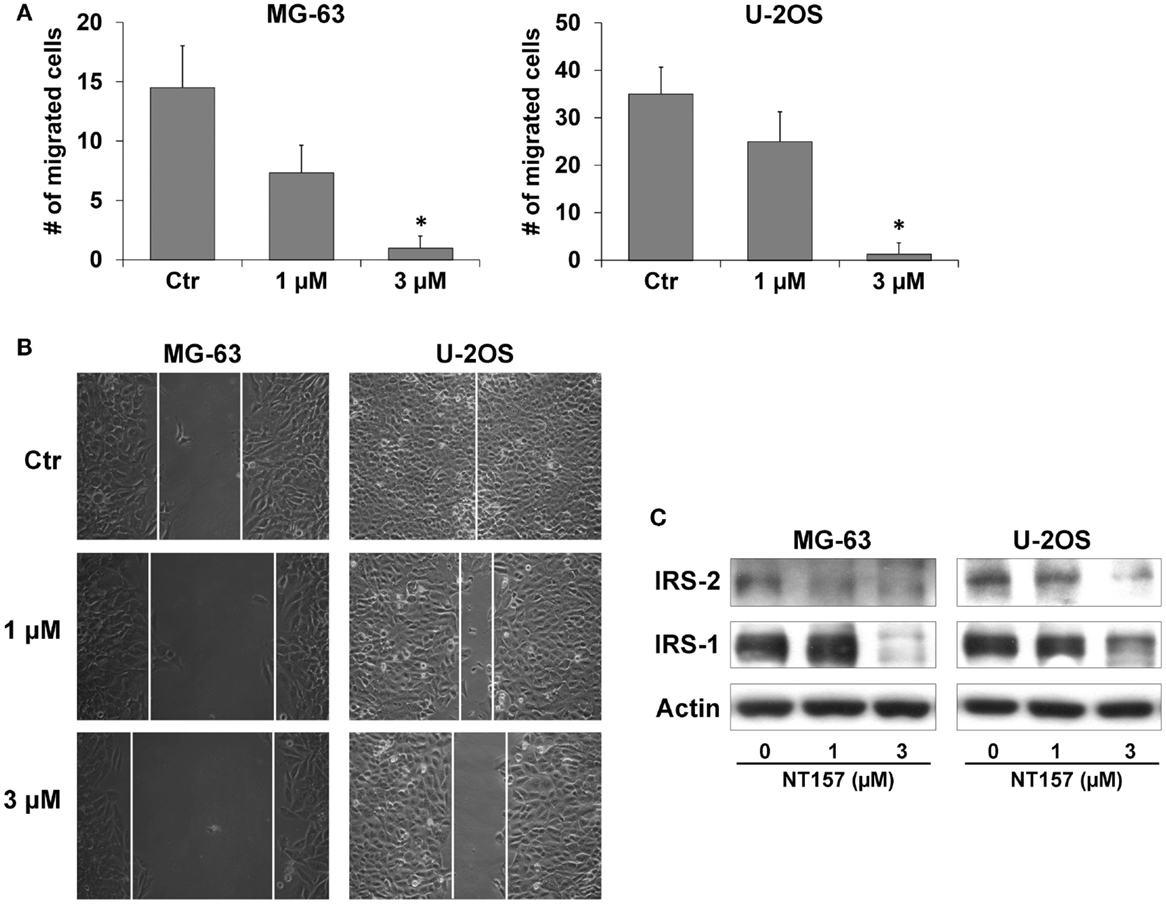
Figure 2. NT157 inhibits migration ability of OS cell lines. (A) Cell migration of MG-63 and U-2OS cells after treatment with NT157 for 18 h. Columns show the mean of three independent experiments: bars indicate the SE. *p < 0.05, Student’s t-test. (B) Wound-healing assay in MG-63 and U-2OS cells. Representative pictures were taken after 24 h of treatment with NT157 (1–3 μM). Magnification 100×. (C) Inhibition of cell motility is mediated by downregulation of IRS-2 and IRS-1 in MG-63 and U-2OS cells after 24 h of treatment with NT157 (1–3 μM). β-actin was used as loading control.
The effect on proliferation was related to a modification of the cellular content. Treatment with 1–3 μM NT157 for 48 h substantially increased the percentage of cells in the G2/M phase (Figure 3A), which inhibited cell cycle progression, in keeping with observed in other cancer cell lines (28). In addition, the manual counting of viable cells revealed that NT157 significantly decreased the number of viable cells without inducing apoptotic cell death (data not shown). This finding demonstrated the cytostatic rather than cytotoxic activity of NT157 in this tumor histotype.
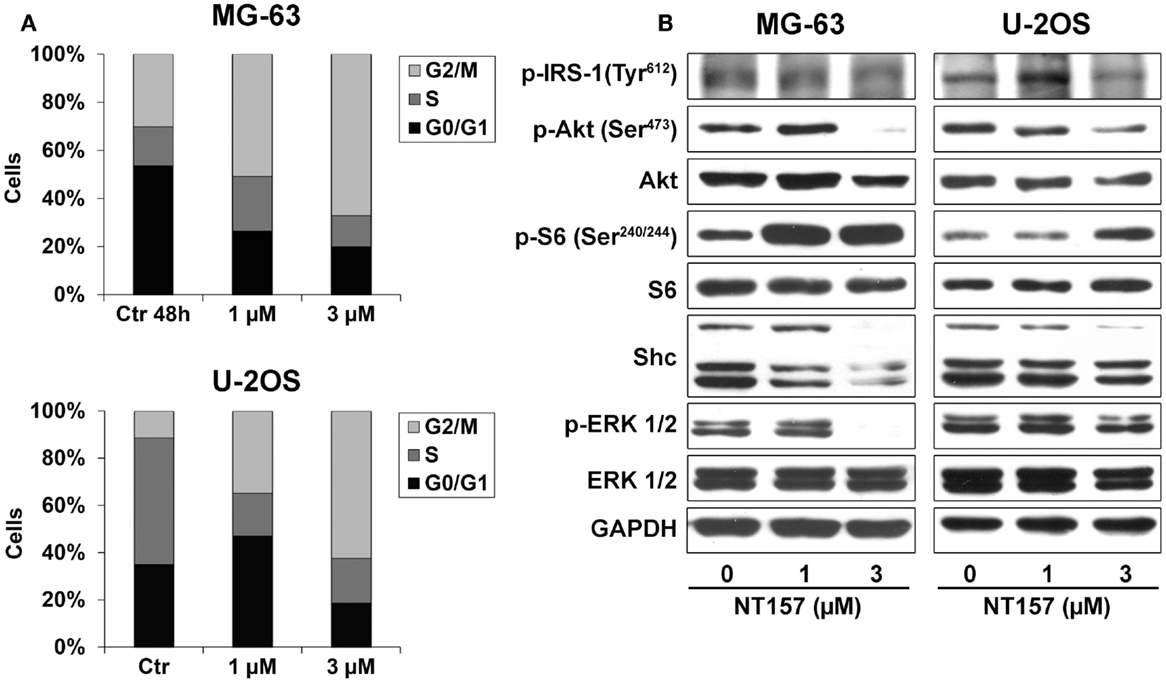
Figure 3. (A) Analysis of NT157 effects on cell cycle after 48 h of treatment (1–3 μM) in MG-63 and U-2OS OS cell lines. Columns show the mean percentage of cells in different cell cycle phases as measured by flow cytometry. (B) Analysis of major downstream signaling of IRS-1 after treatment with or without NT157 (1–3 μM) by western blotting using 40 μg of total protein cell lysate. GAPDH was used as a loading control. The figure shows data representative of two independent experiments.
To assess if the effect on cell cycle was mediated by the inhibition of principal pathways downstream of IRS-1, cells were treated with different concentrations of NT157 for 48 h. A dose-dependent experiment showed that NT157 could downregulate the Tyr-phosphorylation of IRS-1 and, in turn, phosphorylation of Akt and ERK in the MG-63 and U-2OS cell lines (Figure 3B). The shc protein level was only weakly downregulated in MG-63 cells and remained unchanged in U-2OS cells, suggesting that the inhibition of IRS-1 by NT157 did not elicit significant compensatory effects on other signaling adaptors. Interestingly, blocking the PI-3K/Akt pathways resulted in significant S6K phosphorylation following treatment with the IRS-1 inhibitor. A number of works showed that p70S6K, which is downstream of mTOR, could phosphorylate serine residues on IRS-1 to result in proteasomal degradation (33, 34). Because NT compounds induce strong Ser-phosphorylation of the IRS protein, their inhibitory activity could be amplified by feedback that involves p70S6K.
Several studies indicated that the pharmacologic inhibition of mTOR, which leads to the downregulation of IRS-1, results in the compensatory upregulation of AKT activity via increased levels of IGF-1R and IRS-1 (35). Thus, NT157 administration in association with therapies that target mTOR may be advantageous, which agrees with our findings. In particular, the combination of Everolimus and NVP-BEZ235 has been studied in OS cell lines. Everolimus is an mTOR inhibitor, while NVP-BEZ235 is a dual inhibitor of PI-3K/mTOR signaling. Both drugs have been reported to be active in OS models (36, 37). Simultaneous treatments were made in association with chemotherapeutic agents in a fixed ratio for 72 h and cell viability was determined by MTT assay. Synergistic or addictive effects with respect to single agents are expressed as the CI [Synergism: CI <0.9; additive: 0.9 ≤ CI ≥ 1.10 according to Chou et al. (31)]. The effectiveness of Everolimus and NVP-BEZ235 significantly increased in the combination regimens compared to treatment with these drugs alone, demonstrating a synergistic effect [synergism CI ≤ 0.9 (Table 1)]. Conversely, combined treatment with methotrexate, cisplatin, and doxorubicin, the main drugs used to treat sarcoma patients, showed that NT157 produced only modest additive effects, except for the association with doxorubicin in MG-63 cells, which also demonstrated a synergistic increase in efficacy (Table 1).
The advantageous effect of the NT157 and NVP-BEZ235 combination treatment was also confirmed with a cell cycle analysis. Both the percentages of G2/M phase cells and G0/G1 cells increased in response to NT157/NVP-BEZ235 combination treatment in the MG-63 and U-2OS cell lines (Figure 4).
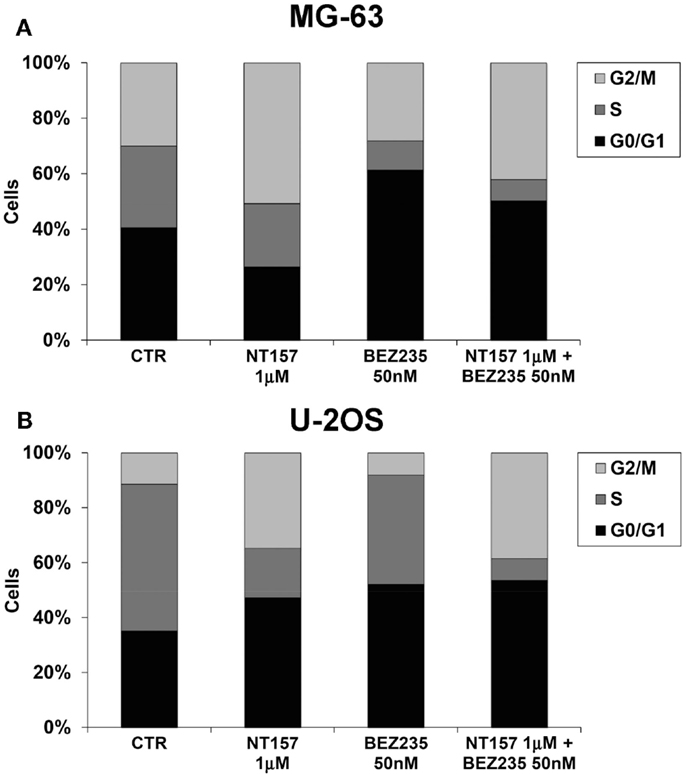
Figure 4. Inhibition MG-63 (A) and U-2OS (B) cell cycle by NT157 (1 μM) in association with PI-3K/mTOR inhibitor NVP-BEZ235 (50 nM). Columns show the mean percentage of cells in different cell cycle phases as measured by flow cytometry.
The prognosis of patients with recurrent and metastatic OS remains poor. Thus, developing new strategies to block pathways that are essential for tumor growth and metastasis provide possible alternatives to improve the outcomes for these patients. IGF signaling is a central player in the induction/maintenance of the epithelial mesenchymal transition (EMT) and cell stemness, two strictly related programs that play a key role in metastatic spread and resistance to cancer treatments (2). Accumulating evidence has indicated that the IGF-1 signaling pathway is dysregulated in OS (38). A recent study of genome-wide gene expression and subsequent gene set analysis in OS cell lines and biopsies demonstrated increased IGF signaling in high-grade OS compared with OS progenitors (39). Several reports demonstrated that the expression levels of adaptor protein IRSs, which signaling from upstream activators, like IGF-IR and IR, to multiple downstream effectors to modulate normal growth, metabolism, survival, and differentiation (4), are increased and hyper-activated in many human tumors (40). In OS, altered IRS-1 expression inhibits osteoblastic differentiation and enhances tumor malignancy (26).
In this study, we reported the preclinical antitumor activity of NT157, a selective inhibitor of IRS-1/IRS-2 in OS. The in vitro and in vivo efficacy of NT157 was recently reported in several tumors (27, 28). Treatment with NT compounds in vivo significantly inhibited the growth of vemurafenib-resistant melanoma and displayed potent antitumor effects in ovarian and prostate cancer (28). In particular, in androgen-dependent and -independent prostate carcinoma, NT157 decreases the expression of IRS proteins and downregulates IGF-1R-mediated AKT activation, leading to cell cycle arrest, apoptosis, and a delay of castrate-resistant prostate cancer progression in xenografts (28). We have demonstrated that in vitro NT157 can inhibit proliferation, cell cycle progression, and motility in different OS cell lines. Importantly, short-term exposure to NT compounds has been demonstrated to be sufficient to gain long-lasting antitumoral effects (27). From a clinical point of view, this attribute allows for treatment relatively infrequent treatments, which should reduce side effects.
Potential inhibitors of the PI-3K–AKT–mTOR pathway, which is frequently dysregulated in cancer (41), are expected to have therapeutic utility in many tumors, and several of these inhibitors are under current investigation as therapeutic agents for cancer (42). In the last decade, particular attention in sarcoma treatment has been focused on the blockade of mTOR by rapamycin and derivatives, which were reported to inhibit the growth of OS cells lines in vitro and in xenografts (43–46). However, a recent phase I study of pediatric solid cancer demonstrated no objective response to temsirolimus, an analog of rapamycin, in OS patients (47). This discrepancy between preclinical and clinical results is explained by the presence of negative feedback that activates IGF-IR downstream signaling and protects against mTOR inhibition (43). The inhibition of mTOR results in the hyperphosphorylation and activation of Akt, leading to resistance to apoptosis and increased cell growth. This effect is abrogated by the inhibition of IGF-1R (43). In vitro and in vivo studies showed that combined anti-IGF-1R antibody and mTOR inhibitor treatment decreased pAKT activation (24, 43, 48, 49). Based on these findings, recent clinical trials showed that this combination was effective in patients with sarcomas (50, 51). Here, we have demonstrated that NT157 exhibits a strong synergistic effect in OS cells when combined with Everolimus, an orally administered rapamycin analog, suggesting that combination therapy based on mTOR and IRS-1 inhibitors may be an appropriate strategy to enhance mTOR-targeted anticancer therapy in this tumor. Pignochino et al. recently reported that the combination of Everolimus and sorafenib, a multikinase inhibitor, inhibited OS cell lines (52), showing that the mTORC2 upregulation observed in sorafenib-treated OS may represent the escape mechanism from this targeted therapy. Combining sorafenib with the mTOR inhibitor Everolimus, fully blocked both mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), which enhanced the antitumor, antimetastatic, and antiangiogenic activities of this treatment. Although the combination of sorafenib and Everolimus was efficacious for patients with advanced or unresectable OS, a phase 2 clinical trial showed that 45% of patients were free from progression at 6 months, suggesting that this strategy should be further studied, either by modulating the same drugs or improving the inhibitory specificity with novel targeted therapies (53). In this context, combined treatment of NT157 with rapamycin analogs will represent an advantageous therapeutic alternative for OS patients. Interestingly, our combination experiments have also demonstrated that NT157 exerted synergistic effect with NVP-BEZ235, a dual class I PI-3K/mTOR inhibitor (54). NVP-BEZ235 has shown promising therapeutic activity in carcinomas (55, 56) and lymphomas (57). Several reports demonstrated its effectiveness for the treatment of bone sarcoma and more specifically, for OS (36, 58, 59). Manara et al. (36) reported that the combination of NVP-BEZ235 with the TK inhibitor NVP-AEW541 synergistically affected the U-2OS cell line. Combined with these findings, our results further support that the combination of IRS-1/2 inhibitor NT157 with NVP-BEZ235 is applicable for the treatment of OS.
The NT-mediated suppression of IRS-1 and IRS-2 has an important clinical implication for overcoming drug resistance. The inhibition of IRS proteins following NT157 treatment clearly improves the response of prostate cancer xenografts to docetaxel (28). In addition, recent studies (27, 60) demonstrated that acquired resistance to the B-RAFV600E/K inhibitor in melanoma is mediated by increased levels of IGF-1R and IRS-1, and this resistance can be effectively reversed by treatment with NT157. Other targeted therapies specifically block IGF-1R and induce a compensatory activation of IR via IGF-II, which leads to drug resistance (61–63). In this context, NT157 disrupts signaling downstream of both IGF-1R and IR and reduces the probability of drug resistance. Finally, short-term exposure to NT compounds has been demonstrated to be sufficient to gain long-lasting antitumoral effects. From a clinical point of view, this attribute allows for relatively infrequent treatments, which should lead to reduced side effects.
Overall, our data provide evidence that the docking protein IRS-1 is a potential target for treating OS. Due to the lack of apoptotic activity, NT157 is a promising adjuvant drug for bone sarcomas. These results suggest the need for future testing of the combination therapy of mTOR inhibitors and NT compounds in a clinical setting for the treatment of patients with chemorefractory, advanced OS.
Conception and design: CG, KS. Acquisition, analysis, or interpretation of data: CG, MC, CM. Writing, review, and/or revision of the manuscript: CG, HR, PP, KS. Final approval of the version to be published: KS, PP.
Dr. Hadas Reuveni has a patent entitled “Novel protein kinase modulators and therapeutic uses thereof” with royalties paid to TyrNovo, and a patent entitled “Combinations of insulin receptor substrate modulators and protein kinase modulators for treating cancer” with royalties paid. The other co-authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The Specialty Chief Editor Antonino Belfiore declares that, despite having collaborated with author Cecilia Garofalo, the review process was handled objectively and no conflict of interest exists.
We would like to thank Cristina Ghinelli for editing the manuscript. This work was financially supported by the Italian Association for Cancer Research (KS – AIRC Project N.14049; CG – MFAG N.11584); The Italian Ministry of Research and Instruction (F.I.R.B. project number:RBAP11884M 005), and 5‰ contributions to the Rizzoli Institute (Project N. 5736 to CG).
1. Samani AA, Yakar S, Leroith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev (2007) 28:20–47. doi: 10.1210/er.2006-0001
2. Malaguarnera R, Belfiore A. The emerging role of insulin and insulin-like growth factor signaling in cancer stem cells. Front Endocrinol (2014) 5:10. doi:10.3389/fendo.2014.00010
3. Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, et al. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature (1991) 352:73–7. doi:10.1038/352073a0
4. Dearth RK, Cui X, Kim HJ, Hadsell DL, Lee AV. Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle (2007) 6:705–13. doi:10.4161/cc.6.6.4035
5. White MF. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol Cell Biochem (1998) 182:3–11. doi:10.1023/A:1006806722619
6. Dearth RK, Cui X, Kim HJ, Kuiatse I, Lawrence NA, Zhang X, et al. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol Cell Biol (2006) 26:9302–14. doi:10.1128/MCB.00260-06
7. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol (2006) 7:85–96. doi:10.1038/nrm1837
8. Xu J, Messina JL. Crosstalk between growth hormone and insulin signaling. Vitam Horm (2009) 80:125–53. doi:10.1016/S0083-6729(08)00606-7
9. Chang Q, Li Y, White MF, Fletcher JA, Xiao S. Constitutive activation of insulin receptor substrate 1 is a frequent event in human tumors: therapeutic implications. Cancer Res (2002) 62:6035–8.
10. Tu X, Batta P, Innocent N, Prisco M, Casaburi I, Belletti B, et al. Nuclear translocation of insulin receptor substrate-1 by oncogenes and Igf-I. Effect on ribosomal RNA synthesis. J Biol Chem (2002) 277:44357–65. doi:10.1074/jbc.M208001200
11. Wu A, Sciacca L, Baserga R. Nuclear translocation of insulin receptor substrate-1 by the insulin receptor in mouse embryo fibroblasts. J Cell Physiol (2003) 195:453–60. doi:10.1002/jcp.10261
12. Ito T, Sasaki Y, Wands JR. Overexpression of human insulin receptor substrate 1 induces cellular transformation with activation of mitogen-activated protein kinases. Mol Cell Biol (1996) 16:943–51.
13. Prisco M, Santini F, Baffa R, Liu M, Drakas R, Wu A, et al. Nuclear translocation of insulin receptor substrate-1 by the simian virus 40 T antigen and the activated type 1 insulin-like growth factor receptor. J Biol Chem (2002) 277:32078–85. doi:10.1074/jbc.M204658200
14. Morelli C, Garofalo C, Sisci D, Del Rincon S, Cascio S, Tu X, et al. Nuclear insulin receptor substrate 1 interacts with estrogen receptor alpha at ERE promoters. Oncogene (2004) 23:7517–26. doi:10.1038/sj.onc.1208014
15. Chen J, Wu A, Sun H, Drakas R, Garofalo C, Cascio S, et al. Functional significance of type 1 insulin-like growth factor-mediated nuclear translocation of the insulin receptor substrate-1 and beta-catenin. J Biol Chem (2005) 280:29912–20. doi:10.1074/jbc.M504516200
16. Wu A, Chen J, Baserga R. Nuclear insulin receptor substrate-1 activates promoters of cell cycle progression genes. Oncogene (2008) 27:397–403. doi:10.1038/sj.onc.1210636
17. Lanzino M, Garofalo C, Morelli C, Le Pera M, Casaburi I, Mcphaul MJ, et al. Insulin receptor substrate 1 modulates the transcriptional activity and the stability of androgen receptor in breast cancer cells. Breast Cancer Res Treat (2009) 115:297–306. doi:10.1007/s10549-008-0079-1
18. Bielack S, Carrle D, Casali PG. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol (2009) 20(Suppl 4):137–9. doi:10.1093/annonc/mdp154
19. Bernthal NM, Federman N, Eilber FR, Nelson SD, Eckardt JJ, Eilber FC, et al. Long-term results (>25 years) of a randomized, prospective clinical trial evaluating chemotherapy in patients with high-grade, operable osteosarcoma. Cancer (2012) 118:5888–93. doi:10.1002/cncr.27651
20. Zhou W, Hao M, Du X, Chen K, Wang G, Yang J. Advances in targeted therapy for osteosarcoma. Discov Med (2014) 17:301–7.
21. Scotlandi K, Picci P. Targeting insulin-like growth factor 1 receptor in sarcomas. Curr Opin Oncol (2008) 20:419–27. doi:10.1097/CCO.0b013e328302edab
22. Viereck V, Siggelkow H, Pannem R, Braulke T, Scharf JG, Kubler B. Alteration of the insulin-like growth factor axis during in vitro differentiation of the human osteosarcoma cell line HOS 58. J Cell Biochem (2007) 102:28–40. doi:10.1002/jcb.21274
23. Dong J, Demarest SJ, Sereno A, Tamraz S, Langley E, Doern A, et al. Combination of two insulin-like growth factor-I receptor inhibitory antibodies targeting distinct epitopes leads to an enhanced antitumor response. Mol Cancer Ther (2010) 9:2593–604. doi:10.1158/1535-7163.MCT-09-1018
24. Kolb EA, Kamara D, Zhang W, Lin J, Hingorani P, Baker L, et al. R1507, a fully human monoclonal antibody targeting IGF-1R, is effective alone and in combination with rapamycin in inhibiting growth of osteosarcoma xenografts. Pediatr Blood Cancer (2010) 55:67–75. doi:10.1002/pbc.22479
25. Pappo AS, Vassal G, Crowley JJ, Bolejack V, Hogendoorn PC, Chugh R, et al. A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: results of a sarcoma alliance for research through collaboration study. Cancer (2014) 120:2448–56. doi:10.1002/cncr.28728
26. Contaldo C, Myers TJ, Zucchini C, Manara MC, Chiodoni C, Colombo MP, et al. Expression levels of insulin receptor substrate-1 modulate the osteoblastic differentiation of mesenchymal stem cells and osteosarcoma cells. Growth Factors (2014) 32:41–52. doi:10.3109/08977194.2013.870168
27. Reuveni H, Flashner-Abramson E, Steiner L, Makedonski K, Song R, Shir A, et al. Therapeutic destruction of insulin receptor substrates for cancer treatment. Cancer Res (2013) 73:4383–94. doi:10.1158/0008-5472.CAN-12-3385
28. Ibuki N, Ghaffari M, Reuveni H, Pandey M, Fazli L, Azuma H, et al. The tyrphostin NT157 suppresses insulin receptor substrates and augments therapeutic response of prostate cancer. Mol Cancer Ther (2014) 13:2827–39. doi:10.1158/1535-7163.MCT-13-0842
29. Benini S, Baldini N, Manara MC, Chano T, Serra M, Rizzi S, et al. Redundancy of autocrine loops in human osteosarcoma cells. Int J Cancer (1999) 80:581–8. doi:10.1002/(SICI)1097-0215(19990209)80:4<581::AID-IJC16>3.3.CO;2-F
30. Scotlandi K, Manara MC, Nicoletti G, Lollini PL, Lukas S, Benini S, et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res (2005) 65:3868–76. doi:10.1158/0008-5472.CAN-04-3192
31. Chou TC, Motzer RJ, Tong Y, Bosl GJ. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. J Natl Cancer Inst (1994) 86:1517–24. doi:10.1093/jnci/86.20.1517
32. Hoang CD, Zhang X, Scott PD, Guillaume TJ, Maddaus MA, Yee D, et al. Selective activation of insulin receptor substrate-1 and -2 in pleural mesothelioma cells: association with distinct malignant phenotypes. Cancer Res (2004) 64:7479–85. doi:10.1158/0008-5472.CAN-04-1898
33. Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, et al. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol (2000) 14:783–94. doi:10.1210/mend.14.6.0446
34. Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol (2004) 167:399–403. doi:10.1083/jcb.200408161
35. Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther (2005) 4:1533–40. doi:10.1158/1535-7163.MCT-05-0068
36. Manara MC, Nicoletti G, Zambelli D, Ventura S, Guerzoni C, Landuzzi L, et al. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin Cancer Res (2010) 16:530–40. doi:10.1158/1078-0432.CCR-09-0816
37. Blay JY. Updating progress in sarcoma therapy with mTOR inhibitors. Ann Oncol (2011) 22:280–7. doi:10.1093/annonc/mdq307
38. Gallagher EJ, LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology (2011) 152:2546–51. doi:10.1210/en.2011-0231
39. Kuijjer ML, Peterse EF, Van Den Akker BE, Briaire-De Bruijn IH, Serra M, Meza-Zepeda LA, et al. IR/IGF1R signaling as potential target for treatment of high-grade osteosarcoma. BMC Cancer (2013) 13:245. doi:10.1186/1471-2407-13-245
40. Mardilovich K, Pankratz SL, Shaw LM. Expression and function of the insulin receptor substrate proteins in cancer. Cell Commun Signal (2009) 7:14. doi:10.1186/1478-811X-7-14
41. Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer (2002) 2:489–501. doi:10.1038/nrc839
42. Yap TA, Garrett MD, Walton MI, Raynaud F, De Bono JS, Workman P. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol (2008) 8:393–412. doi:10.1016/j.coph.2008.08.004
43. Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene (2007) 26:1932–40. doi:10.1038/sj.onc.1209990
44. Houghton PJ, Morton CL, Kolb EA, Gorlick R, Lock R, Carol H, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer (2008) 50:799–805. doi:10.1002/pbc.21214
45. Gazitt Y, Kolaparthi V, Moncada K, Thomas C, Freeman J. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int J Oncol (2009) 34:551–61. doi:10.3892/ijo_00000181
46. Zhou Q, Deng Z, Zhu Y, Long H, Zhang S, Zhao J. mTOR/p70S6K signal transduction pathway contributes to osteosarcoma progression and patients’ prognosis. Med Oncol (2010) 27:1239–45. doi:10.1007/s12032-009-9365-y
47. Spunt SL, Grupp SA, Vik TA, Santana VM, Greenblatt DJ, Clancy J, et al. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J Clin Oncol (2011) 29:2933–40. doi:10.1200/JCO.2010.33.4649
48. Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res (2009) 69:7662–71. doi:10.1158/0008-5472.CAN-09-1693
49. Kolb EA, Gorlick R, Maris JM, Keir ST, Morton CL, Wu J, et al. Combination testing (stage 2) of the anti-IGF-1 receptor antibody IMC-A12 with rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer (2012) 58:729–35. doi:10.1002/pbc.23157
50. Quek R, Wang Q, Morgan JA, Shapiro GI, Butrynski JE, Ramaiya N, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res (2011) 17:871–9. doi:10.1158/1078-0432.CCR-10-2621
51. Schwartz GK, Tap WD, Qin LX, Livingston MB, Undevia SD, Chmielowski B, et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: a multicentre, open-label, phase 2 trial. Lancet Oncol (2013) 14:371–82. doi:10.1016/S1470-2045(13)70049-4
52. Pignochino Y, Dell’aglio C, Basirico M, Capozzi F, Soster M, Marchio S, et al. The combination of sorafenib and everolimus abrogates mTORC1 and mTORC2 upregulation in osteosarcoma preclinical models. Clin Cancer Res (2013) 19:2117–31. doi:10.1158/1078-0432.CCR-12-2293
53. Grignani G, Palmerini E, Ferraresi V, D’ambrosio L, Bertulli R, Asaftei SD, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol (2014) 16(1):98–107. doi:10.1016/S1470-2045(14)71136-2
54. Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther (2008) 7:1851–63. doi:10.1158/1535-7163.MCT-08-0017
55. Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci U S A (2009) 106:19503–8. doi:10.1073/pnas.0905056106
56. Santiskulvong C, Konecny GE, Fekete M, Chen KY, Karam A, Mulholland D, et al. Dual targeting of phosphoinositide 3-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach in human ovarian carcinoma. Clin Cancer Res (2011) 17:2373–84. doi:10.1158/1078-0432.CCR-10-2289
57. Bhatt AP, Bhende PM, Sin SH, Roy D, Dittmer DP, Damania B. Dual inhibition of PI3K and mTOR inhibits autocrine and paracrine proliferative loops in PI3K/Akt/mTOR-addicted lymphomas. Blood (2010) 115:4455–63. doi:10.1182/blood-2009-10-251082
58. Nanni P, Nicoletti G, Landuzzi L, Croci S, Murgo A, Palladini A, et al. High metastatic efficiency of human sarcoma cells in Rag2/gammac double knockout mice provides a powerful test system for antimetastatic targeted therapy. Eur J Cancer (2010) 46:659–68. doi:10.1016/j.ejca.2009.11.018
59. Gobin B, Battaglia S, Lanel R, Chesneau J, Amiaud J, Redini F, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, inhibits osteosarcoma cell proliferation and tumor development in vivo with an improved survival rate. Cancer Lett (2014) 344:291–8. doi:10.1016/j.canlet.2013.11.017
60. Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell (2010) 18:683–95. doi:10.1016/j.ccr.2010.11.023
61. Buck E, Gokhale PC, Koujak S, Brown E, Eyzaguirre A, Tao N, et al. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther (2010) 9:2652–64. doi:10.1158/1535-7163.MCT-10-0318
62. Garofalo C, Manara MC, Nicoletti G, Marino MT, Lollini PL, Astolfi A, et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing’s sarcoma is dependent on insulin receptor signaling. Oncogene (2011) 30:2730–40. doi:10.1038/onc.2010.640
Keywords: NT157, IRS-1, osteosarcoma, IGF system, sarcoma, chemotherapy
Citation: Garofalo C, Capristo M, Mancarella C, Reunevi H, Picci P and Scotlandi K (2015) Preclinical effectiveness of selective inhibitor of IRS-1/2 NT157 in osteosarcoma cell lines. Front. Endocrinol. 6:74. doi: 10.3389/fendo.2015.00074
Received: 13 February 2015; Accepted: 25 April 2015;
Published: 13 May 2015
Edited by:
Antonino Belfiore, University Magna Graecia of Catanzaro, ItalyReviewed by:
Roberta Malaguarnera, University “Magna Graecia” of Catanzaro, ItalyCopyright: © 2015 Garofalo, Capristo, Mancarella, Reunevi, Picci and Scotlandi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katia Scotlandi, Experimental Oncology Laboratory, CRS Development of Biomolecular Therapies, Rizzoli Institute, Via di Barbiano 1/10, Bologna 40136, Italy,a2F0aWEuc2NvdGxhbmRpQGlvci5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.