
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol., 28 April 2015
Sec. Endocrinology of Aging
Volume 6 - 2015 | https://doi.org/10.3389/fendo.2015.00065
This article is part of the Research TopicOxytocin: control of bone and fat mass and metabolismView all 7 articles
Centrally acting oxytocin (OT) is known to terminate food consumption in response to excessive stomach distension, increase in salt loading, and presence of toxins. Hypothalamic-hindbrain OT pathways facilitate these aspects of OT-induced hypophagia. However, recent discoveries have implicated OT in modifications of feeding via reward circuits: OT has been found to differentially affect consumption of individual macronutrients in choice and no-choice paradigms. In this mini-review, we focus on presenting and interpreting evidence that defines OT as a key component of mechanisms that reduce eating for pleasure and shape macronutrient preferences. We also provide remarks on challenges in integrating the knowledge on physiological and pathophysiological states in which both OT activity and macronutrient preferences are affected.
Macronutrient composition of ingested food affects functioning of the organism during various physiological and pathophysiological challenges, such as pregnancy, lactation, and aging. A dynamic endocrine balance facilitates the coupling of mechanisms that link appetite regulation, metabolism, and cellular/tissue-specific responses, and one of the key hormonal regulators is oxytocin (OT) (1, 2). OT affects peripheral tissues directly by binding its G protein-coupled receptor localized in, to name a few, the mammary gland, ovary, uterus and bone. OT’s action at peripheral OT receptors, interplay between OT and other hormones, as well as functional relationships with metabolic regulators, have been thoroughly studied in relation to mechanisms essential for health (1, 3). However, OT affects organism’s functioning also by regulating intake of specific macronutrients. Those mechanisms are predominantly mediated via the OT receptor localized in the brain, and our knowledge of them has expanded rapidly in the past several years. We dedicate this mini review to synthesizing currently available information on the role of central OT circuits in eating behavior, with particular emphasis on shaping food preferences. Our final remarks pertain to delineating perspectives and challenges of linking seemingly unrelated outcomes of OT’s action in the brain and in the periphery.
From the very early stages of experimental work, it became apparent that OT promotes termination of feeding associated with generalized satiation as well as stemming from consumption-related adverse phenomena that jeopardize homeostasis (4–7).
Neuroanatomical studies have shown that most OT neurons are localized in the hypothalamus, and its paraventricular nucleus (PVN) is the main source of OT fibers innervating central targets, most prominently, the dorsal vagal complex in the brain stem (8–12). Aside from the parvocellular PVN neurons, OT is also released centrally via somatodendritic projections of magnocellular OT subpopulations in the supraoptic nucleus (SON) and PVN (13, 14). Lesioning of the PVN and disruption of the PVN-hindbrain pathways lead to increased food intake and body weight in rats (15–17). Release of OT and increased activity of OT neurons coincide with satiation-associated termination of feeding in laboratory animals (6, 18–20). Arletti et al. were first to report that intracerebroventricular (ICV) injection of OT causes a marked reduction in deprivation-induced food intake in rats (18). Many authors have confirmed the finding and, by using intraparenchymal OT receptor ligand injections or employing OT receptor-specific cytotoxins, they identified the hindbrain (particularly the dorsal vagal complex) as the area through which OT-driven feeding inhibitory mechanisms are executed (21).
A number of satiety inducing neuropeptides have been shown to affect appetite, at least partially, by acting via OT containing pathways. Those peptides include – among others – alpha melanocyte-stimulating hormone (alpha-MSH) and glucagon-like peptide-1 (GLP-1), key components of the brainstem-hypothalamic appetite circuit (22, 23). Furthermore, hyperphagia and obesity occur in mutations that lead to insufficiencies in OT PVN neuronal population development, such as that observed in the single-minded-1 (sim-1) mouse model, and these negative symptoms can be reversed by OT treatment (24). A reduction in the number of OT neurons has been reported for Prader–Willi syndrome patients exhibiting extreme overeating (25, 26). Recently, the ventromedial hypothalamic (VMH) nucleus has been identified as a hypothalamic site through which OT causes early meal termination in free feeding and fasted rats (27).
It should be emphasized that the intake of a sufficient amount of energy does not appear to be the main or the necessary factor that induces OT neuronal activity underlying termination of ingestive behavior. In fact, OT neuronal activity and release coinciding with termination of feeding occur upon changes in calorie-independent parameters associated with consumption. Those parameters include excessive stomach distension and elevated plasma osmolality (28–30). In addition, central OT inhibits consumption of toxin-tainted foods and supports long-term avoidance of those by acting through not only the brain stem but also the amygdala (31).
Though the protection of internal milieu during consumption appears to be the key neuroregulatory function of OT within the CNS, its importance for facilitating important competing behaviors, particularly with regard to reproductive and social behaviors, should not be disregarded. Sexual intercourse, lactation, and bonding within family and non-family groups are well-known stimuli to cause OT secretion in various species (32–38). Interruption of these processes by the drive to consume food, under certain conditions, might not be evolutionarily advantageous. Therefore, the anorexigenic function of OT should be seen from a broader point of adjusting/balancing physiological and behavioral responses to both internal and external challenges.
While the involvement of OT in the “homeostatic” regulation of food intake has been a widely recognized phenomenon, the past several years have brought exciting discoveries that strongly suggest an implication of central OT in another aspect of consumption: macronutrient preferences and feeding reward. These discoveries have capitalized on linking evidence pertaining to neuroanatomy and functional significance of the OT system outside the realm of classical satiety/feeding termination mechanisms, and they have refined our understanding of OT as not just a “homeostasis rescue molecule,” but also as a neuroregulator of intricate and complex dietary choice processes. Numerous reports have shown widespread distribution of the OT receptor throughout the brain and, importantly, specific sites involved in reward processing, such as the nucleus accumbens and the ventral tegmental area, appear to be prominent central targets of OT signaling (39, 40). PVN OT neuronal projections form somatic and axodendritic synapses with mesolimbic neurons (41, 42). Data from human and laboratory animal studies link OT receptor activation/availability with modifications in non-feeding rewards (from natural rewards, such as social and reproductive behaviors to administration of drugs of abuse). For example, Jarrett and colleagues found that cocaine treatment changes OT receptor binding density in the bed nucleus of the stria terminalis in female rats (43). Baracz et al. reported that direct intraparenchymal administration of OT in the core of the nucleus accumbens dose-dependently decreases methamphetamine-seeking behavior (44). The same group of investigators found also that intra-accumbens core OT attenuates methamphetamine-induced conditioned place preference in rats (45). In a recently published set of experiments employing OT receptor ligand injections in the nucleus accumbens and lentiviral-mediated overexpression of the OT receptor in this site, Bahi showed that OT attenuates the development, maintenance, and primed reinstatement of ethanol-induced conditioned place preference (46). Intracranial infusions of OT in female mice promote the development of a conditioned social preference (47). Damiano et al. showed by using functional magnetic resonance imaging (fMRI) that certain single nucleotide polymorphisms in the OT receptor gene are associated with a differential response of the mesolimbic system during anticipation of monetary rewards in healthy human subjects (48). Neurochemical studies have pointed to a relationship between OT and dopamine in modification of perceived rewards; for example, in mice central administration of OT has been found to reduce methamphetamine-elicited dopamine release in the striatum and nucleus accumbens (39) and promote a concomitant decrease in glutamate release and increase in extracellular presence of γ-aminobutyric acid (GABA) in the medial prefrontal cortex (49).
Neuroendocrine and behavioral processes governing food intake and addiction show a partial overlap. For example, an appetite stimulating hormone, ghrelin (50), activates the VTA dopamine circuit and promotes consumption of palatable food over “bland” diets (51), increases ethanol intake (52) and facilitates cocaine-induced conditioned place preference in rodents (53). Injections of anorexogenic leptin decrease self-administration of drugs of abuse (54), whereas food restriction has an opposite effect (55). Finally, sugar preference is associated with increased ethanol responsiveness (56), self-administration of cocaine (57), and amphetamine (58) in rats. Therefore, considering the link between central OT and addictive-like behaviors, an intuitive question arose as to whether this relationship might expand onto feeding reward.
Pioneering studies were performed on OT knockout (KO) mice. Amico and colleagues found that genetic deletion of OT leads to the enhanced initial and sustained intake of palatable sucrose solutions in the KO mice compared to the wild-type (WT) counterparts (59). The effect of the OT-null genotype on sucrose consumption could be observed in both dark and light phase of the 24-h cycle, and it persisted even in animals subjected to periods of stress induced in the platform shaker stress model (60). OT KO mice and their background strain tested in a progressive ratio operant licking paradigm display a similar motivational drive to consume sucrose (61). Sclafani et al. found that OT KOs given a choice between two tastants (water served as a control ingestant), exhibit a heightened preference not just for sucrose, but for palatable isocaloric carbohydrate solutions regardless of their sweetness (e.g., Polycose and cornstarch). Interestingly, a non-caloric non-carbohydrate sweetener, saccharin, was also overconsumed by the KOs (60), which is in line with the notion that OT affects feeding reward. Interestingly, the propensity to overconsume palatable tastants in OT KO mice does not generalize to fat. Two-bottle preference tests in which mice could choose between water and a palatable lipid emulsion, Intralipid, showed a similar fat preference profile between KO and WT cohorts (61). In order to further examine the issue of preference to fat, Miedlar et al. (62) employed a similar paradigm as the one used by Amico et al. in the initial study on sucrose intake in OT Kos; however, instead of the sugar water, the animals were given Intralipid. While OT KO mice drank more Intralipid during the first day of having access to the tastant (which may be related to altered neophobic or stress-related processing), on subsequent days they were found to consume the same amount of Intralipid as WT controls.
The OT KO model findings are largely in agreement with the results of experiments on laboratory animals without genetic modifications in the OT system. Gene expression analysis with real-time PCR showed upregulation of OT mRNA levels in the hypothalami of rats eating scheduled, volume-unrestricted, high-sugar diet compared to standard food (63). An increase in OT transcript content has been also found in mice given 48-h ad libitum access to a 10% sucrose solution versus animals consuming isocaloric Intralipid during that time (64). Herisson et al. studied hypothalamic OT gene expression in mice given short-term access to sucrose, cornstarch, or saccharin (on top of the standard diet) and determined that exposure to carbohydrates but not to saccharin elevated OT mRNA above control values; notably, a higher level of significance was detected after sucrose intake (65). Furthermore, the comparison of hypothalamic OT neuronal activity levels induced by consumption of sucrose or Intralipid (equivalent volumes) shows a much greater number of Fos-positive OT cells in the sucrose group. It should be noted, however, that even in the case of mice ingesting fat, OT neuronal activity is higher at the end than at the beginning of a meal, which reflects the role of central OT as a general satiety mediator and the phenomenon of elevated OT neuronal activation and OT release coinciding with feeding termination is seen regardless of a diet type and palatability (66). Diet composition does, however, affect the magnitude of the OT system’s response at the end of a meal (66–68).
Injection studies utilizing a blood–brain barrier (BBB) penetrant OT receptor antagonist, L-368,899, in both choice and no-choice feeding paradigms (68), have consistently produced elevation of carbohydrate intake in laboratory animals, whereas consumption of Intralipid has not been affected (64, 65). When a choice between carbohydrates is given in a two-bottle test, OT receptor blockade by systemic administration of L-368,899 appears to have a preferential stimulatory effect on sucrose consumption, which can either reflect a special functional relationship between central OT and appetite for this particular carbohydrate, or – in the light of recent studies showing the presence of the OT receptor in taste buds – can at least partially (via peripheral interactions of the systemically injected agent) stem from altered taste perception (64, 65, 69).
Mullis and colleagues have recently reported an important piece of evidence linking OT to feeding reward (70). They equipped rats with a cannula aimed at the ventral tegmental area and found that OT infusion in this site decreases deprivation-induced chow intake as well as palatability-driven sucrose consumption. These effects are abolished by a pretreatment with an OT receptor antagonist, L-368,899. Importantly, when L-368,899 or another OT receptor antagonist, (d(CH2)5(1),Tyr(Me)(2),Orn(8))-OT, were injected alone in the ventral tegmental area, they stimulated sugar intake, but they failed to induce chow consumption (70). This is consistent with the earlier findings showing that when animals are given a choice between sucrose and fat diets, systemic administration of a BBB-penetrant OT receptor blocker shifts preference toward sugar without affecting total energy consumption (64). Human research on the effects of OT on eating for reward is still very much in its infancy. It is known that in pregnant women, food cravings diminish as OT levels increase gradually during gestation (71). Over the course of the menstrual cycle, food intake (including sugar) is low during ovulation (high OT levels) and it increases during the luteal phase (low OT levels) (72, 73). The 2013 paper by Ott and colleagues (74) outlined the effects of intranasally administered OT on ingestive behavior, with special emphasis on rewarding aspects of consumption. OT was found to markedly decrease snack consumption (chocolate cookies, rice waffles, and salt crackers were offered to the subjects) during the postprandial snack test administered shortly after a full buffet-style breakfast. Noteworthy, the reduction in total snack intake was driven primarily by restraining by 25% the consumption of high-sugar chocolate cookies; hence, the initial human data support the notion coined through laboratory animal experiments that there is a functional link between OT and sugary food-driven reward. Obviously, one of the key issues that warrants caution in how we interpret the currently available body of evidence (especially related to human observations) is that while both dendtritic and hypophyseal relaease of OT can occur simultaneously, peripheral levels of OT do not always correlate with central secretion (75–78). Furthermore, McCullough and colleagues have recently provided a comprehensive analysis of pitfalls associated with typically used research techniques and strategies aimed at determining concentration of peripheral OT, and urged a particularly careful approach to construing peripheral OT data (79).
Intriguingly, while central OT suppresses certain types of feeding reward (especially those related to carbohydrate consumption) and contributes to a reduction of intake and a transient shift in dietary choices, it appears that orexigenic opioid receptor ligands (and possibly also other neuromediators of feeding for pleasure) diminish meal-end activity of hypothalamic OT neurons thereby likely promoting continuation of ingestive behavior. For example, butorphanol tartrate at a dose that promotes overeating of sugary foods dampens OT PVN neuronal activity in rats that have consumed the amount of high-sucrose powder diet that is satiating for saline-treated controls (80). In rats, opioid receptor agonists have also been shown to decrease OT neuronal activity in response to noxious stimuli, whereas an antagonist, naloxone, potentiates anorexigenic effects of emetic agents (81, 82). Finally, Mitra et al. showed that daily habitual intake of high-sucrose diets in rats reduces c-Fos expression in OT neurons after a high-sucrose or low-sucrose meal compared to rats receiving daily low-sugar food (83). This suggests that regular sugar consumption might reduce the meal-end activity of OT neurons in response to any food regardless of its composition. Hence, the balance of evidence suggests that while endogenous OT appears to reduce the consumption of palatable sugary foods, habitual ingestion of such foods – typically associated with enhanced activity in reward circuits – may dampen responsiveness of OT neurons to physiological parameters that would otherwise be conducive to termination of food intake.
Evidence indicates that, aside from facilitating generalized feeding termination, central OT under certain circumstances plays a role of a carbohydrate (especially, sugar)-specific satiety mediator and, therefore, it may influence the composition characteristics of a freely selected daily diet (a schematic overview of key anatomical components of OT-dependent feeding mechanism are depicted in Figure 1). Thus, changes in OT system’s activity that modify selection of foods due to their macronutrient content may affect health status of the organism.
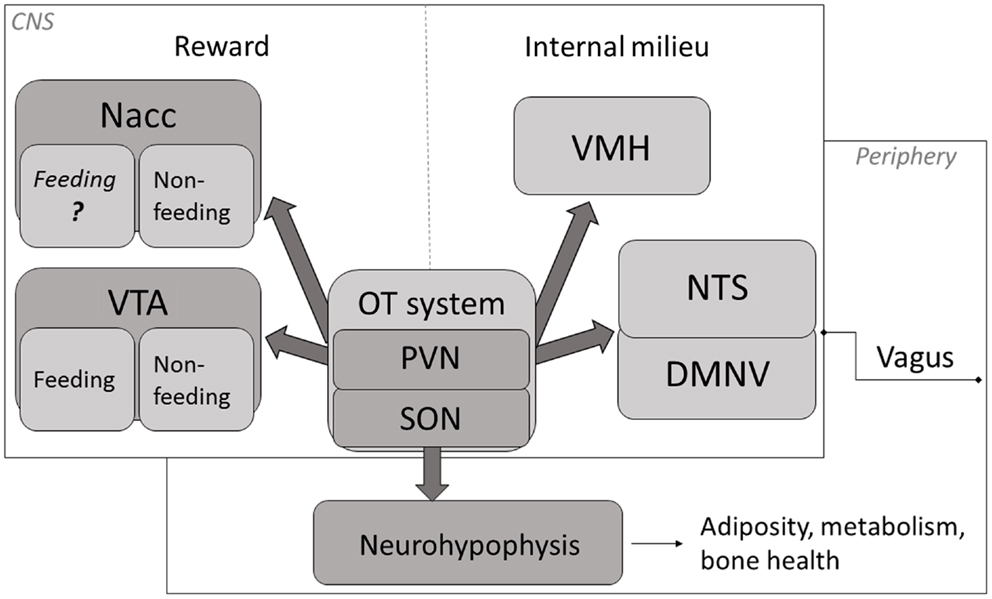
Figure 1. A schematic representation of key mechanisms through which OT affects appetite. OT neuronal activity has been associated with termination of food intake (84–87). The magnitude of this response is modified by the integrated peripheral signals (mediated largely via the vagus and the brainstem relay circuit) and by the rewarding value of a meal (74, 84, 88, 89). Release of OT in the CNS promotes termination of consumption when internal milieu is jeopardized (energy imbalance, abnormal GI and/or osmotic parameters) as well as part of intricate reward processing. Neurohypophyseal OT participates in the regulation of mechanisms related to metabolism, adiposity and bone tissue status. Nacc, nucleus accumbens; VTA, ventral tegmental area; PVN, paraventricular nucleus; SON, supraoptic nucleus; VMH, ventromedial hypothalamus; NTS, nucleus of the solitary tract; DMNV, dorsal motor nucleus of the vagus.
Aging is associated with disturbances in food intake, notably with a decrease in energy consumption and anhedonia (90), and those have been attributed to psychosocial and pathophysiological causes. Data generated through human observations and laboratory animal models reflect the age-related decline in food intake and the dysregulation of energy balance (91–94). Importantly, it has been shown that in human beings (95) and in rodents (96), fat preference is greatly diminished, whereas the percentage intake of carbohydrate-derived calories is elevated (97, 98). While the shift in macronutrient preferences likely reflects changed metabolic needs, it may simultaneously contribute to susceptibility to the development of age-related pathologies: potential consequences of alterations in macronutrient intake are extremely broad and may be conducive to energy imbalance (99), fat mass changes (100–103), and osteoporosis (104–106).
One of the greatest challenges is to identify in which of the many physiological and pathophysiological states associated both with shifts in dietary preferences and changes in OT system’s activity, the modified OT tone serves as the causative factor of undesirable modifications in a consumption profile. Unfortunately, our knowledge of changes in the OT system in aging, especially those within the central nervous system, is far from being systematized. The relatively few studies published thus far have presented conflicting evidence in regard to the density of the OT receptor, number of OT neurons, and functional outcomes of exogenous and endogenous OT during the aging process. For example, Fliers and Swaab (107) reported an age-related increase in OT secretion in the PVN, but not in the SON, whereas OT plasma levels were similar in young versus old male rats (107). Keck et al (90) found a decrease in stress-induced intra-PVN OT secretion in male rats, while Zbuzek et al (108) and Melis et al (109) did not detect differences in hypothalamic OT levels (90, 108, 109). An age-related decrease in OT concentration was shown in the septum and hippocampus, and a decrease in OT receptor binding, in the caudate putamen, olfactory tubercle, and ventromedial hypothalamic nucleus in male rats (109). In Rhesus monkeys, CSF OT levels were positively correlated with adult female age (110). Several authors did not find correlation between age and changes in the OT system and those reports mentioned comparable social memory and anti-depressant effect of OT injections and similar OT fiber density in the rat and a similar number of OT cells in the PVN in the human being (111–113). Considering a growing interest in elucidating neuroendocrine bases of behavioral modifications that occur during aging that are detrimental to the general health status, a thorough investigation of age-related neural changes – including those pertaining to the OT system – are of critical importance. We therefore stress the need to accelerate research on in-depth identification of age-related changes of central OT pathways as well as the functional link between central OT and alterations in macronutrient-driven reward in the aging process in order to aid in conceptualizing new diagnostic markers of pathophysiology of aging and in devising novel treatment strategies that integrate multiple functional facets of OT.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the Royal Society of New Zealand Marsden grant.
1. Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology (1991) 129:785–91. doi: 10.1210/endo-129-2-785
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Lee HJ, Macbeth AH, Pagani JH, Young WS III. Oxytocin: the great facilitator of life. Prog Neurobiol (2009) 88:127–51. doi:10.1016/j.pneurobio.2009.04.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Tom N, Assinder SJ. Oxytocin in health and disease. Int J Biochem Cell Biol (2010) 42:202–5. doi:10.1016/j.biocel.2009.10.008
4. Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav (1990) 48:825–30. doi:10.1016/0031-9384(90)90234-U
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Benelli A, Bertolini A, Arletti R. Oxytocin-induced inhibition of feeding and drinking: no sexual dimorphism in rats. Neuropeptides (1991) 20(1):57–62. doi:10.1016/0143-4179(91)90040-P
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides (1991) 12:113–8. doi:10.1016/0196-9781(91)90176-P
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res (1992) 578:256–60. doi:10.1016/0006-8993(92)90255-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Sagar SM, Price KJ, Kasting NW, Sharp FR. Anatomic patterns of Fos immunostaining in rat brain following systemic endotoxin administration. Brain Res Bull (1995) 36:381–92. doi:10.1016/0361-9230(94)00217-O
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Huang W, Sved AF, Stricker EM. Vasopressin and oxytocin release evoked by NaCl loads are selectively blunted by area postrema lesions. Am J Physiol Regul Integr Comp Physiol (2000) 278:R732–40.
10. Siaud P, Puech R, Assenmacher I, Alonso G. Microinjection of oxytocin into the dorsal vagal complex decreases pancreatic insulin secretion. Brain Res (1991) 546(2):190–4. doi:10.1016/0006-8993(91)91480-O
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Hornby PJ. Central neurocircuitry associated with emesis. Am J Med (2001) 111(Suppl 8A):106S–12S. doi:10.1016/S0002-9343(01)00849-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Charpak S, Armstrong WE, Muhlethaler M, Dreifuss JJ. Stimulatory action of oxytocin on neurones of the dorsal motor nucleus of the vagus nerve. Brain Res (1984) 300:83–9. doi:10.1016/0006-8993(84)91342-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience (1989) 32:435–9. doi:10.1016/0306-4522(89)90091-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Miyagawa A, Okamura H, Ibata Y. Coexistence of oxytocin and NADPH-diaphorase in magnocellular neurons of the paraventricular and the supraoptic nuclei of the rat hypothalamus. Neurosci Lett (1994) 171:13–6. doi:10.1016/0304-3940(94)90592-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav (1981) 27:1031–40. doi:10.1016/0031-9384(81)90366-8
16. Sims JS, Lorden JF. Effect of paraventricular nucleus lesions on body weight, food intake and insulin levels. Behav Brain Res (1986) 22:265–81. doi:10.1016/0166-4328(86)90071-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Shor-Posner G, Azar AP, Insinga S, Leibowitz SF. Deficits in the control of food intake after hypothalamic paraventricular nucleus lesions. Physiol Behav (1985) 35:883–90. doi:10.1016/0031-9384(85)90255-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides (1989) 10:89–93. doi:10.1016/0196-9781(89)90082-X
19. Deblon N, Veyrat-Durebex C, Bourgoin L, Caillon A, Bussier AL, Petrosino S, et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One (2011) 6:e25565. doi:10.1371/journal.pone.0025565
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Sabatier N, Leng G, Menzies J. Oxytocin, feeding, and satiety. Front Endocrinol (Lausanne) (2013) 4:35. doi:10.3389/fendo.2013.00035
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Baskin DG, Kim F, Gelling RW, Russell BJ, Schwartz MW, Morton GJ, et al. A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology (2010) 151:4207–13. doi:10.1210/en.2010-0295
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Katsurada K, Maejima Y, Nakata M, Kodaira M, Suyama S, Iwasaki Y, et al. Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem Biophys Res Commun (2014) 451:276–81. doi:10.1016/j.bbrc.2014.07.116
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Olszewski PK, Wirth MM, Shaw TJ, Grace MK, Billington CJ, Giraudo SQ, et al. Role of alpha-MSH in the regulation of consummatory behavior: immunohistochemical evidence. Am J Physiol Regul Integr Comp Physiol (2001) 281:R673–80.
24. Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol (2008) 22:1723–34. doi:10.1210/me.2008-0067
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metab (1995) 80:573–9. doi:10.1210/jcem.80.2.7852523
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Martin A, State M, Anderson GM, Kaye WM, Hanchett JM, McConaha CW, et al. Cerebrospinal fluid levels of oxytocin in Prader-Willi syndrome: a preliminary report. Biol Psychiatry (1998) 44:1349–52. doi:10.1016/S0006-3223(98)00190-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Noble EE, Billington CJ, Kotz CM, Wang C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am J Physiol Regul Integr Comp Physiol (2014) 307:R737–45. doi:10.1152/ajpregu.00118.2014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Calatayud S, Quintana E, Esplugues J, Barrachina MD. Role of central oxytocin in the inhibition by endotoxin of distension-stimulated gastric acid secretion. Naunyn Schmiedebergs Arch Pharmacol (1999) 360:676–82. doi:10.1007/s002109900085
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am J Physiol (1987) 253:R661–5.
30. Nelson EE, Alberts JR, Tian Y, Verbalis JG. Oxytocin is elevated in plasma of 10-day-old rats following gastric distension. Brain Res Dev Brain Res (1998) 111:301–3. doi:10.1016/S0165-3806(98)00147-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Olszewski PK, Waas JR, Brooks LL, Herisson F, Levine AS. Oxytocin receptor blockade reduces acquisition but not retrieval of taste aversion and blunts responsiveness of amygdala neurons to an aversive stimulus. Peptides (2013) 50:36–41. doi:10.1016/j.peptides.2013.09.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Borrow AP, Cameron NM. The role of oxytocin in mating and pregnancy. Horm Behav (2012) 61:266–76. doi:10.1016/j.yhbeh.2011.11.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab (1987) 64:27–31. doi:10.1210/jcem-64-1-27
34. Garrison JL, Macosko EZ, Bernstein S, Pokala N, Albrecht DR, Bargmann CI. Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science (2012) 338:540–3. doi:10.1126/science.1226201
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Oldfield RG, Hofmann HA. Neuropeptide regulation of social behavior in a monogamous cichlid fish. Physiol Behav (2011) 102:296–303. doi:10.1016/j.physbeh.2010.11.022
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Ophir AG, Gessel A, Zheng D-JJ, Phelps SM. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm Behav (2012) 61:445–53. doi:10.1016/j.yhbeh.2012.01.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Poletini MO, McKee DNT, Szawka RE, Bertram R, Helena CVV, Freeman ME. Cervical stimulation activates A1 and locus coeruleus neurons that project to the paraventricular nucleus of the hypothalamus. Brain Res Bull (2012) 88:566–73. doi:10.1016/j.brainresbull.2012.06.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res (2008) 170:261–76. doi:10.1016/S0079-6123(08)00422-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch Pharmacol (2008) 376:441–8. doi:10.1007/s00210-007-0245-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Gould BR, Zingg HH. Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor-LacZ reporter mouse. Neuroscience (2003) 122:155–67. doi:10.1016/S0306-4522(03)00283-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Succu S, Sanna F, Cocco C, Melis T, Boi A, Ferri GL, et al. Oxytocin induces penile erection when injected into the ventral tegmental area of male rats: role of nitric oxide and cyclic GMP. Eur J Neurosci (2008) 28:813–21. doi:10.1111/j.1460-9568.2008.06385.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Sofroniew MV. Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat and human. J Histochem Cytochem (1980) 28:475–8. doi:10.1177/28.5.7381192
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Jarrett TM, McMurray MS, Walker CH, Johns JM. Cocaine treatment alters oxytocin receptor binding but not mRNA production in postpartum rat dams. Neuropeptides (2006) 40:161–7. doi:10.1016/j.npep.2006.03.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
44. Baracz SJ, Everett NA, McGregor IS, Cornish JL. Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats. Addict Biol (2014). doi:10.1111/adb.12198
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behav Brain Res (2012) 228:185–93. doi:10.1016/j.bbr.2011.11.038
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
46. Bahi A. The oxytocin receptor impairs ethanol reward in mice. Physiol Behav (2015) 139:321–7. doi:10.1016/j.physbeh.2014.11.046
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
47. Kent K, Arientyl V, Khachatryan MM, Wood RI. Oxytocin induces a conditioned social preference in female mice. J Neuroendocrinol (2013) 25:803–10. doi:10.1111/jne.12075
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
48. Damiano CR, Aloi J, Dunlap K, Burrus CJ, Mosner MG, Kozink RV, et al. Association between the oxytocin receptor (OXTR) gene and mesolimbic responses to rewards. Mol Autism (2014) 5:7. doi:10.1186/2040-2392-5-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Qi J, Han WY, Yang JY, Wang LH, Dong YX, Wang F, et al. Oxytocin regulates changes of extracellular glutamate and GABA levels induced by methamphetamine in the mouse brain. Addict Biol (2012) 17:758–69. doi:10.1111/j.1369-1600.2012.00439.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
50. Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature (2001) 409:194–8. doi:10.1038/35051587
51. Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, et al. Ghrelin increases intake of rewarding food in rodents. Addict Biol (2010) 15:304–11. doi:10.1111/j.1369-1600.2010.00216.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
52. Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci USA (2009) 106:11318–23. doi:10.1073/pnas.0812809106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
53. Davis KW, Wellman PJ, Clifford PS. Augmented cocaine conditioned place preference in rats pretreated with systemic ghrelin. Regul Pept (2007) 140:148–52. doi:10.1016/j.regpep.2006.12.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
54. Shalev U, Yap J, Shaham Y. Leptin attenuates acute food deprivation-induced relapse to heroin seeking. J Neurosci (2001) 21:RC129.
55. Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther (1981) 217:241–7.
56. Woods JE II, McKay PF, Masters J, Seyoum R, Chen A, La Duff L, et al. Differential responding for brain stimulation reward and sucrose in high-alcohol-drinking (HAD) and low-alcohol-drinking (LAD) rats. Alcohol Clin Exp Res (2003) 27:926–36. doi:10.1111/j.1530-0277.2003.tb04417.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Gosnell BA. Sucrose intake predicts rate of acquisition of cocaine self-administration. Psychopharmacology (2000) 149:286–92. doi:10.1007/s002130000375
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
58. DeSousa NJ, Bush DE, Vaccarino FJ. Self-administration of intravenous amphetamine is predicted by individual differences in sucrose feeding in rats. Psychopharmacology (2000) 148:52–8. doi:10.1007/s002130050024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
59. Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol (2005) 289:R1798–806. doi:10.1152/ajpregu.00558.2005
60. Billings LB, Spero JA, Vollmer RR, Amico JA. Oxytocin null mice ingest enhanced amounts of sweet solutions during light and dark cycles and during repeated shaker stress. Behav Brain Res (2006) 171:134–41. doi:10.1016/j.bbr.2006.03.028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
61. Sclafani A, Rinaman L, Vollmer RR, Amico JA. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol (2007) 292:R1828–33. doi:10.1152/ajpregu.00826.2006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
62. Miedlar JA, Rinaman L, Vollmer RR, Amico JA. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am J Physiol Regul Integr Comp Physiol (2007) 293:R1063–8. doi:10.1152/ajpregu.00228.2007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
63. Olszewski PK, Shaw TJ, Grace MK, Hoglund CE, Fredriksson R, Schioth HB, et al. Complexity of neural mechanisms underlying overconsumption of sugar in scheduled feeding: involvement of opioids, orexin, oxytocin and NPY. Peptides (2009) 30:226–33. doi:10.1016/j.peptides.2008.10.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
64. Olszewski PK, Klockars A, Olszewska AM, Fredriksson R, Schioth HB, Levine AS. Molecular, immunohistochemical, and pharmacological evidence of oxytocin’s role as inhibitor of carbohydrate but not fat intake. Endocrinology (2010) 151:4736–44. doi:10.1210/en.2010-0151
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
65. Herisson FM, Brooks LL, Waas JR, Levine AS, Olszewski PK. Functional relationship between oxytocin and appetite for carbohydrates versus saccharin. Neuroreport (2014) 25:909–14. doi:10.1097/WNR.0000000000000201
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
66. Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab (2006) 4:313–21. doi:10.1016/j.cmet.2006.08.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
67. Lucio-Oliveira F, Franci CR. Effect of the interaction between food state and the action of estrogen on oxytocinergic system activity. J Endocrinol (2012) 212:129–38. doi:10.1530/JOE-11-0272
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
68. Singru PS, Wittmann G, Farkas E, Zseli G, Fekete C, Lechan RM. Refeeding-activated glutamatergic neurons in the hypothalamic paraventricular nucleus (PVN) mediate effects of melanocortin signaling in the nucleus tractus solitarius (NTS). Endocrinology (2012) 153:3804–14. doi:10.1210/en.2012-1235
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
69. Sinclair MS, Perea-Martinez I, Dvoryanchikov G, Yoshida M, Nishimori K, Roper SD, et al. Oxytocin signaling in mouse taste buds. PLoS One (2010) 5:e11980. doi:10.1371/journal.pone.0011980
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
70. Mullis K, Kay K, Williams DL. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res (2013) 1513:85–91. doi:10.1016/j.brainres.2013.03.026
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
71. Rosso P. Regulation of food intake during pregnancy and lactation. Ann N Y Acad Sci (1987) 499:191–6. doi:10.1111/j.1749-6632.1987.tb36210.x
72. Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, et al. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav (2005) 47:164–9. doi:10.1016/j.yhbeh.2004.10.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
73. Mitchell MD, Haynes PJ, Anderson AB, Turnbull AC. Plasma oxytocin concentrations during the menstrual cycle. Eur J Obstet Gynecol Reprod Biol (1981) 12:195–200. doi:10.1016/0028-2243(81)90077-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
74. Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, et al. Oxytocin reduces reward-driven food intake in humans. Diabetes (2013) 62:3418–25. doi:10.2337/db13-0663
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
75. Sabatier N, Caquineau C, Douglas AJ, Leng G. Oxytocin released from magnocellular dendrites: a potential modulator of alpha-melanocyte-stimulating hormone behavioral actions? Ann N Y Acad Sci (2003) 994:218–24. doi:10.1111/j.1749-6632.2003.tb03183.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
76. Ludwig M, Sabatier N, Dayanithi G, Russell JA, Leng G. The active role of dendrites in the regulation of magnocellular neurosecretory cell behavior. Prog Brain Res (2002) 139:247–56. doi:10.1016/S0079-6123(02)39021-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
77. Leng G, Caquineau C, Sabatier N. Regulation of oxytocin secretion. Vitam Horm (2005) 71:27–58. doi:10.1016/S0083-6729(05)71002-5
78. Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XMM, et al. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci (2003) 23:10351–8.
79. McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev (2013) 37:1485–92. doi:10.1016/j.neubiorev.2013.04.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
80. Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav (2007) 91:506–12. doi:10.1016/j.physbeh.2007.01.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
81. Olszewski PK, Shi Q, Billington CJ, Levine AS. Opioids affect acquisition of LiCl-induced conditioned taste aversion: involvement of OT and VP systems. Am J Physiol Regul Integr Comp Physiol (2000) 279:R1504–11.
82. Flanagan LM, Verbalis JG, Stricker EM. Naloxone potentiation of effects of cholecystokinin and lithium chloride on oxytocin secretion, gastric motility and feeding. Neuroendocrinology (1988) 48:668–73. doi:10.1159/000125080
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
83. Mitra A, Gosnell BA, Schioth HB, Grace MK, Klockars A, Olszewski PK, et al. Chronic sugar intake dampens feeding-related activity of neurons synthesizing a satiety mediator, oxytocin. Peptides (2010) 31:1346–52. doi:10.1016/j.peptides.2010.04.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
84. Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG. c-Fos expression in rat brain and brainstem nuclei in response to treatments that alter food intake and gastric motility. Mol Cell Neurosci (1993) 4:93–106. doi:10.1006/mcne.1993.1011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
85. Flanagan LM, Dohanics J, Verbalis JG, Stricker EM. Gastric motility and food intake in rats after lesions of hypothalamic paraventricular nucleus. Am J Physiol (1992) 263:R39–44.
86. Verbalis JG, Blackburn RE, Hoffman GE, Stricker EM. Establishing behavioral and physiological functions of central oxytocin: insights from studies of oxytocin and ingestive behaviors. Adv Exp Med Biol (1995) 395:209–25.
87. Verbalis JG, Blackburn RE, Olson BR, Stricker EM. Central oxytocin inhibition of food and salt ingestion: a mechanism for intake regulation of solute homeostasis. Regul Pept (1993) 45:149–54. doi:10.1016/0167-0115(93)90198-H
88. Mantella RC, Rinaman L, Vollmer RR, Amico JA. Cholecystokinin and D-fenfluramine inhibit food intake in oxytocin-deficient mice. Am J Physiol Regul Integr Comp Physiol (2003) 285:R1037–45. doi:10.1152/ajpregu.00383.2002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
89. Olszewski PK, Ulrich C, Ling N, Allen K, Levine AS. A non-peptide oxytocin receptor agonist, WAY-267,464, alleviates novelty-induced hypophagia in mice: insights into changes in c-Fos immunoreactivity. Pharmacol Biochem Behav (2014) 124:367–72. doi:10.1016/j.pbb.2014.07.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
90. Keck ME, Hatzinger M, Wotjak CT, Landgraf R, Holsboer F, Neumann ID. Ageing alters intrahypothalamic release patterns of vasopressin and oxytocin in rats. Eur J Neurosci (2000) 12:1487–94. doi:10.1046/j.1460-9568.2000.00030.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
91. Gruenewald DA, Marck BT, Matsumoto AM. Fasting-induced increases in food intake and neuropeptide Y gene expression are attenuated in aging male brown Norway rats. Endocrinology (1996) 137:4460–7. doi:10.1210/en.137.10.4460
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
92. Mattison JA, Black A, Huck J, Moscrip T, Handy A, Tilmont E, et al. Age-related decline in caloric intake and motivation for food in rhesus monkeys. Neurobiol Aging (2005) 26:1117–27. doi:10.1016/j.neurobiolaging.2004.09.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
93. Toshinai K, Mondal MS, Shimbara T, Yamaguchi H, Date Y, Kangawa K, et al. Ghrelin stimulates growth hormone secretion and food intake in aged rats. Mech Ageing Dev (2007) 128:182–6. doi:10.1016/j.mad.2006.10.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
94. Wolden-Hanson T. Mechanisms of the anorexia of aging in the Brown Norway rat. Physiol Behav (2006) 88:267–76. doi:10.1016/j.physbeh.2006.05.032
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
95. De Castro JM. Age-related changes in spontaneous food intake and hunger in humans. Appetite (1993) 21:255–72. doi:10.1006/appe.1993.1044
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
96. Veyrat-Durebex C, Alliot J. Changes in pattern of macronutrient intake during aging in male and female rats. Physiol Behav (1997) 62:1273–8. doi:10.1016/S0031-9384(97)00304-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
97. Islam AK, Beczkowska IW, Bodnar RJ. Interactions among aging, gender, and gonadectomy effects upon naloxone hypophagia in rats. Physiol Behav (1993) 54:981–92. doi:10.1016/0031-9384(93)90312-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
98. Whichelow MJ, Prevost AT. Dietary patterns and their associations with demographic, lifestyle and health variables in a random sample of British adults. Br J Nutr (1996) 76:17–30. doi:10.1079/BJN19960006
99. Schutz Y. Macronutrients and energy balance in obesity. Metabolism (1995) 44:7–11. doi:10.1016/0026-0495(95)90311-9
100. Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav (1995) 58:1067–77. doi:10.1016/0031-9384(95)02003-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
101. Pliner P, Fleming AS. Food intake, body weight, and sweetness preferences over the menstrual cycle in humans. Physiol Behav (1983) 30:663–6. doi:10.1016/0031-9384(83)90240-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
102. Dalvit-McPhillips SP. The effect of the human menstrual cycle on nutrient intake. Physiol Behav (1983) 31:209–12. doi:10.1016/0031-9384(83)90120-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
103. Bowen DJ, Grunberg NE. Variations in food preference and consumption across the menstrual cycle. Physiol Behav (1990) 47:287–91. doi:10.1016/0031-9384(90)90144-S
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
104. Chiu KM, Ju J, Mayes D, Bacchetti P, Weitz S, Arnaud CD. Changes in bone resorption during the menstrual cycle. J Bone Miner Res (1999) 14:609–15. doi:10.1359/jbmr.1999.14.4.609
105. Schlemmer A, Hassager C, Risteli J, Risteli L, Jensen SB, Christiansen C. Possible variation in bone resorption during the normal menstrual cycle. Acta Endocrinol (Copenh) (1993) 129:388–92.
106. Nielsen HK, Brixen K, Bouillon R, Mosekilde L. Changes in biochemical markers of osteoblastic activity during the menstrual cycle. J Clin Endocrinol Metab (1990) 70:1431–7. doi:10.1210/jcem-70-5-1431
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
107. Fliers E, Swaab DF. Activation of vasopressinergic and oxytocinergic neurons during aging in the Wistar rat. Peptides (1983) 4:165–70. doi:10.1016/0196-9781(83)90108-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
108. Zbuzek V, Fuchs AR, Zbuzek VK, Wu WH. Neurohypophyseal aging: differential changes in oxytocin and vasopressin release, studied in Fischer 344 and Sprague-Dawley rats. Neuroendocrinology (1988) 48:619–26. doi:10.1159/000125072
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
109. Melis MR, Stancampiano R, Fratta W, Argiolas A. Oxytocin concentration changes in different rat brain areas but not in plasma during aging. Neurobiol Aging (1992) 13:783–6. doi:10.1016/0197-4580(92)90102-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
110. Parker KJ, Hoffman CL, Hyde SA, Cummings CS, Maestripieri D. Effects of age on cerebrospinal fluid oxytocin levels in free-ranging adult female and infant rhesus macaques. Behav Neurosci (2010) 124:428–33. doi:10.1037/a0019576
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
111. Arletti R, Benelli A, Poggioli R, Luppi P, Menozzi B, Bertolini A. Aged rats are still responsive to the antidepressant and memory-improving effects of oxytocin. Neuropeptides (1995) 29:177–82. doi:10.1016/0143-4179(95)90021-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
112. Fliers E, De Vries GJ, Swaab DF. Changes with aging in the vasopressin and oxytocin innervation of the rat brain. Brain Res (1985) 348:1–8. doi:10.1016/0006-8993(85)90351-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
113. Wierda M, Goudsmit E, Van der Woude PF, Purba JS, Hofman MA, Bogte H, et al. Oxytocin cell number in the human paraventricular nucleus remains constant with aging and in Alzheimer’s disease. Neurobiol Aging (1991) 12:511–6. doi:10.1016/0197-4580(91)90081-T
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: appetite regulation, reward system, satiety, sucrose, overeating
Citation: Klockars A, Levine AS and Olszewski PK (2015) Central oxytocin and food intake: focus on macronutrient-driven reward. Front. Endocrinol. 6:65. doi: 10.3389/fendo.2015.00065
Received: 31 January 2015; Accepted: 13 April 2015;
Published: 28 April 2015
Edited by:
Ez-Zoubir Amri, CNRS University of Nice-Sophia Antipolis, FranceReviewed by:
Joseph George Verbalis, Georgetown University, USACopyright: © 2015 Klockars, Levine and Olszewski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pawel Karol Olszewski, Department of Biological Sciences, University of Waikato, Private Bag 3105, Hamilton 3240, New Zealand,cGF3ZWxAd2Fpa2F0by5hYy5ueg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.