
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 27 August 2014
Sec. Experimental Endocrinology
Volume 5 - 2014 | https://doi.org/10.3389/fendo.2014.00139
Besides the well-known function of thyroid hormones (THs) for regulating metabolism, it has recently been discovered that THs are also involved in testicular development in mammalian and non-mammalian species. THs, in combination with follicle stimulating hormone, lead to androgen synthesis in Danio rerio, which results in the onset of spermatogenesis in the testis, potentially relating the hypothalamic–pituitary–thyroid (HPT) gland to the hypothalamic–pituitary–gonadal (HPG) axes. Furthermore, studies in non-mammalian species have suggested that by stimulating the thyroid-stimulating hormone (TSH), THs can be induced by corticotropin-releasing hormone. This suggests that the hypothalamic–pituitary–adrenal/interrenal gland (HPA) axis might influence the HPT axis. Additionally, it was shown that hormones pertaining to both HPT and HPA could also influence the HPG endocrine axis. For example, high levels of androgens were observed in the testis in Odonthestes bonariensis during a period of stress-induced sex-determination, which suggests that stress hormones influence the gonadal fate toward masculinization. Thus, this review highlights the hormonal interactions observed between the HPT, HPA, and HPG axes using a comparative approach in order to better understand how these endocrine systems could interact with each other to influence the development of testes.
Thyroid hormones (THs) have been implicated in a plethora of physiologic actions, such as metabolism, development, growth, and reproduction [reviewed in Ref. (1–5)]. In the last years, the influence of THs in gonadal development has been intensively studied in rodent species (2, 6–10); however, data remains scarce on the roles of THs in non-mammalian reproduction [reviewed in Ref. (2, 6–12)]. As endocrine axes are well conserved among vertebrates, a comparative approach to review TH function and regulation in gonadal development would help to better understand non-mammalian endocrine systems. Thus, this paper provides a comprehensive review of existing literature on the effects of THs in testicular development in non-mammalian species, highlights the interaction of the hypothalamic–pituitary–thyroid (HPT) gland, –adrenal/interrenal (HPA), and –gonadal (HPG) axes (Table 1), and identifies key areas for future research.
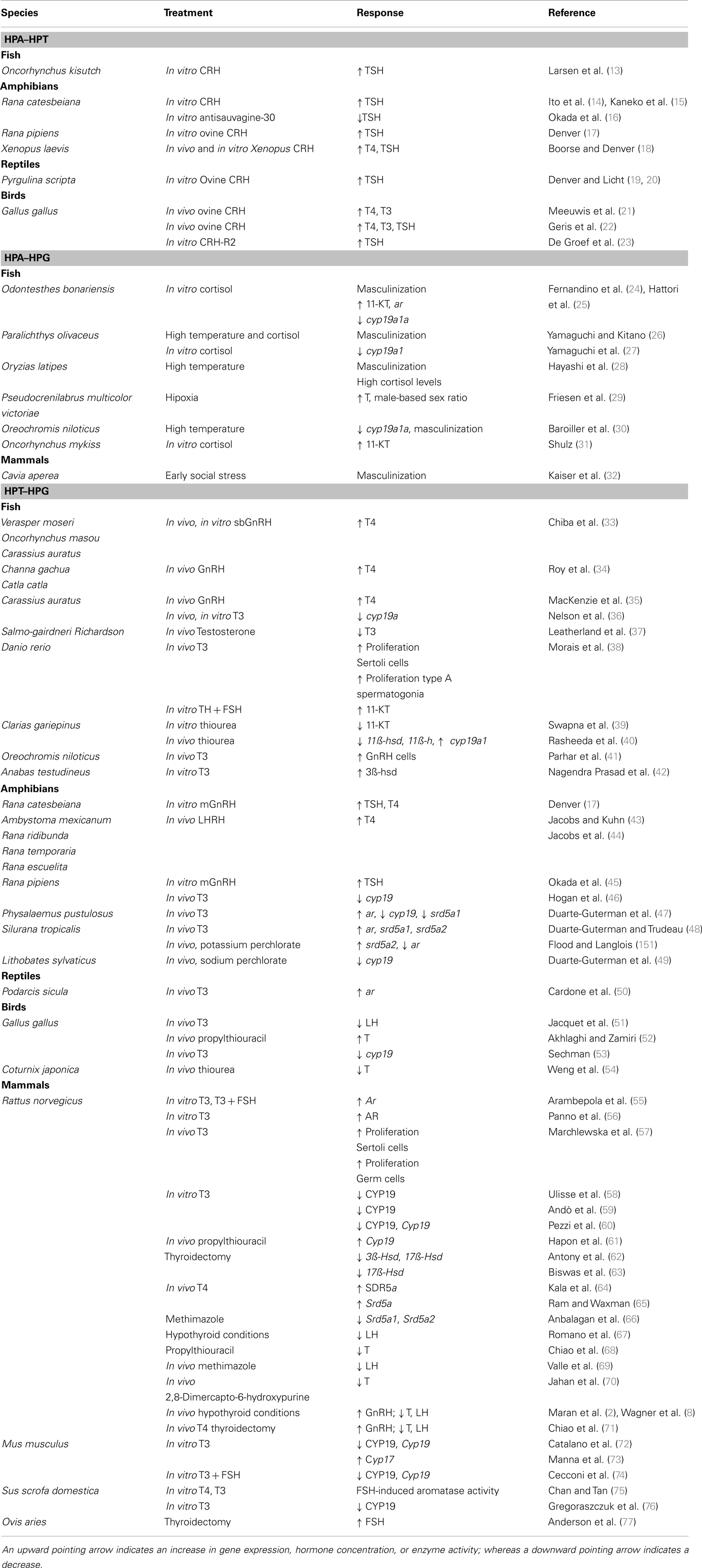
Table 1. Summary of studies that shows the interaction between the hypothalamic–pituitary–adrenal/interrenal and thyroid gland axes (HPA–HPT), –adrenal/interrenal and –gonadal axes (HPA–HPG), and –thyroid gland and –gonadal axes (HPT–HPG).
The central nervous system (CNS) is stimulated by environmental factors to regulate TH homeostasis. Thus, the hypothalamic tripeptide thyrotropin-releasing hormone (TRH) stimulates the anterior pituitary to synthesize and secrete the thyroid-stimulating hormone (TSH; Figure 1). The action of TRH has been confirmed in tetrapods [reviewed in Ref. (78, 79)]; however, in fish, mixed effects have been found. In bighead carp (Aristichthys nobilis) and Japanese eel (Anguilla japonica), TRH was shown to increase hypophyseal tsh-β expression (80, 81), while in coho salmon (Oncorhynchus kisutch), TRH-treatment did not stimulate TSH release (13). Furthermore, teleost fish have no portal systems that connect the CNS and the pituitary, in which hypothalamic neurons terminate very close to adenohypophysial cells (79). These findings suggest that TRH is not a major TSH-releasing factor in fish.
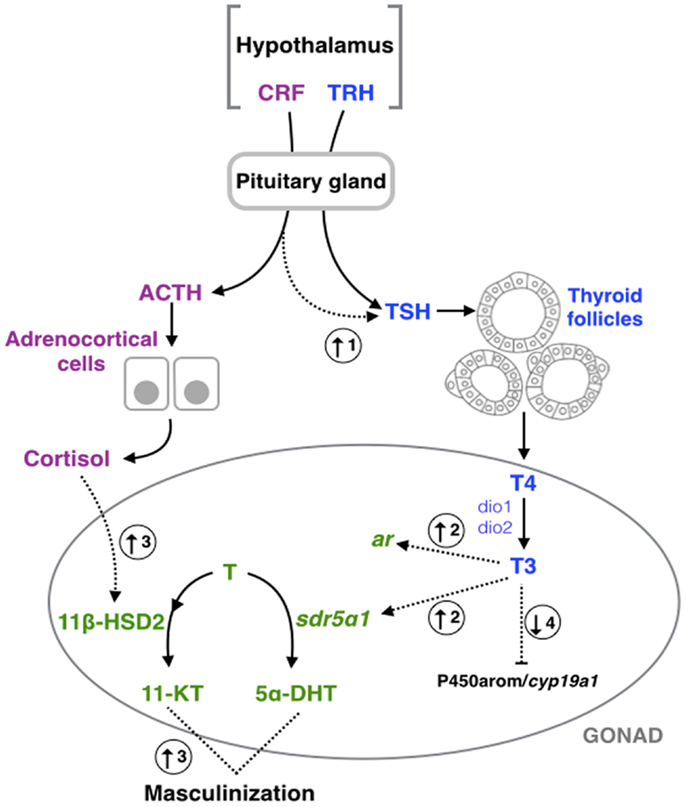
Figure 1. Schematic representation of hypothalamic–pituitary– thyroid gland (blue), –adrenal/interrenal (purple), and –gonadal interactions (green). Dashed arrows represent the points of interaction between the different axes highlighted in this review. (1) Corticotropin- releasing factor (CRF) could induce the pituitary–thyroid stimulating hormone (TSH) secretion in fish (13), amphibians (15, 16, 82), and birds (23). (2) Triiodothyronine (T3) could increase the expression of type a1 steroid 5-alpha-reductase type 1 (sdr5α1) and androgen receptor (ar) in amphibians (83). (3) Exposure to cortisol results in an increase of the androgen-related machinery and subsequent masculinization in fish (25–27, 84), and mammals (32). (4) Exposition of thyroid hormones could inhibit the aromatase (P450arom/cyp19a1) activity or expression in fish (36, 85), amphibians (46, 47), and mammals (58, 59, 75). TRH, thyrotropin-releasing hormone; Dio1, deiodinase type 1; Dio2, deiodinase type 2; T4, thyroxine; ACTH, pituitary adrenocorticotropic hormone; T (testosterone) 11β-HSD2, type 2 isozyme of 11β-hydroxysteroid dehydrogenase; 11-KT, 11-ketotestosterone; 5α-DHT, 5α-dihydrotestosterone.
In addition to TH regulation, it has been suggested that HPT is also involved with the HPA axis [O. kisutch, Rana catesbeiana, Rana pipiens, Xenopus laevis, Pyrgulina scripta, Gallus gallus (see Table 1)]. It is well known that the corticotropin-releasing hormone (CRH, also known as the corticotropin-releasing factor or CRF) is a potent stimulator of the pituitary adrenocorticotropic hormone (ACTH), which stimulates the synthesis and secretion of cortisol, the main stress hormone in vertebrates (86–88). A decade ago, De Groef et al. (23) observed that CRH can induce pituitary TSH secretion in chicken (G. gallus) through the CRH type 2 receptor (CRH-R2) expressed on pituitary thyrotrope cells, linking both of these endocrine axes (Figure 1). Similar results have been observed in fish, amphibians, reptiles, and other bird species [Table 1; reviewed in Ref. (13, 82, 89–91)]. The dual hypophysiotropic action of CRH has several effects on the peripheral hormonal function of the HPT axis. In amphibians, metamorphosis is dependent on THs; however, changes in CRH molecular machinery have been observed during this period of development. For example, the expression of both crh and crh-r2 increase significantly throughout frog metamorphosis (92). Noteworthy, crh transcripts start being detected earlier than crh-r2, i.e., during premetamorphosis, while the expression of crh-r2 only begins to be detected later during prometamorphosis (92). Furthermore, it has been observed that treatment with corticosteroids synergizes with THs, leading to an accelerated metamorphosis (93). Thus, Denver (91) hypothesized that both CRH and corticosteroids act on THs in order that tadpoles may respond quickly to environmental cues early in development and metamorphose according to their environment. This crosstalk between HPA and HPT allows frogs to escape from and survive in habitat desiccation and crowding, or food restriction during mid- to late prometamorphosis (91). Similar to fish, CRH-like peptide treatment lead to a significant concentration-dependent increase in TSH secretion of salmonids pituitary culture (13, 94). During smoltification of Atlantic salmon (Salmo salar), a critical period of midlife transition from freshwater to seawater with morphological, physiological, and behavioral modifications (95), the increase in THs induced a positive-feedback in the maturation of the CRF neurons [CRF neurogenesis; (96)]. Also, during early development of fish, chronological correlation between ACTH and TSH production has been observed in the pituitary of European sea bass (Dicentrarchus labrax) larvae (97). Together, this data suggest that stressor-challenge drives the THs to play both fundamental and modulatory roles in the stress response [reviewed in Ref. (89, 90)]. Moreover, a reduction in basal plasma cortisol levels was observed in hyperthyroidism-induced Cyprinus carpio (98). Thus, from the crosstalk between HPA and HPT axes, three main observations can be deduced: (i) CRH acts as a common neuroregulator of the thyroidal and adrenal/interrenal axes in non-mammalian species; (ii) the HPA and HPT axes perform concerted actions on energy metabolism and development; and (iii) the regulation, inhibition, or stimulation of CRH on the TH axis could be dependent on both stage of life and the nature of the tissues being analyzed.
The HPG axis controls signaling and biosynthesis by the sex steroids. The hypothalamic peptide gonadotropin-releasing hormone (GnRH) regulates the biosynthesis and secretion of both gonadotropins; luteinizing hormone (LH) and follicle stimulating hormone (FSH). Besides the well-known function of GnRH in regulating gonadotropins, GnRH treatment has been shown to moderately increase TSH secretion in amphibians (17, 45), suggesting that GnRH can modulate THs at the pituitary level. Several studies have also observed that GnRH can increase thyroxine (3,5,3′,5′-l-tetra-iodothyronine or T4) levels in fish (33, 34) and in amphibians (44, 99). However, no changes in triiodothyronine (3, 3′, 5-triiodo-l-thyronine or T3) concentrations were observed in plasma after injections of a superactive analog of GnRH in goldfish [Carassius auratus; (35)]. Thus, additional work should investigate the possible targets of GnRH in the TH axis.
Luteinizing hormone and FSH are the main regulators of various physiological processes related to formation and maintenance of the gonadal structures (12, 100). In males, FSH is involved in the paracrine control and the structural and nutritional support of germ cell development of the Sertoli cells, while LH regulates androgen production in the Leydig cells (101, 102). The level of both gonadotropins, as well as related gene expression, can be altered by hyper- and hypothyroidic conditions in Mus musculus (8, 9, 68, 71). Moreover, studies have shown that THs can interfere with the regulatory activity of FSH, influencing the rate of proliferation and the functioning of Sertoli cells of Rattus norvegicus (57, 103) and Danio rerio (38). The Sertoli cells are found within the seminiferous tubules and are responsible for spermatogenesis (104). The initiation of spermatogenesis requires several hormones, including FSH and androgens (105–107). For example, thyroidectomized rams (Ovis aries) – during their seasonal testicular regression – show an increase in blood FSH concentration and a faster testis growth (77, 108). Moreover, in a testis tissue culture of D. rerio, T3 in combination with FSH increases 11-ketotestosterone (11-KT) synthesis (38); the main bioactive androgen in fish (Figure 2). Thus, it has been proposed that FSH partially mediates the effects of THs in male sexual development in D. rerio.
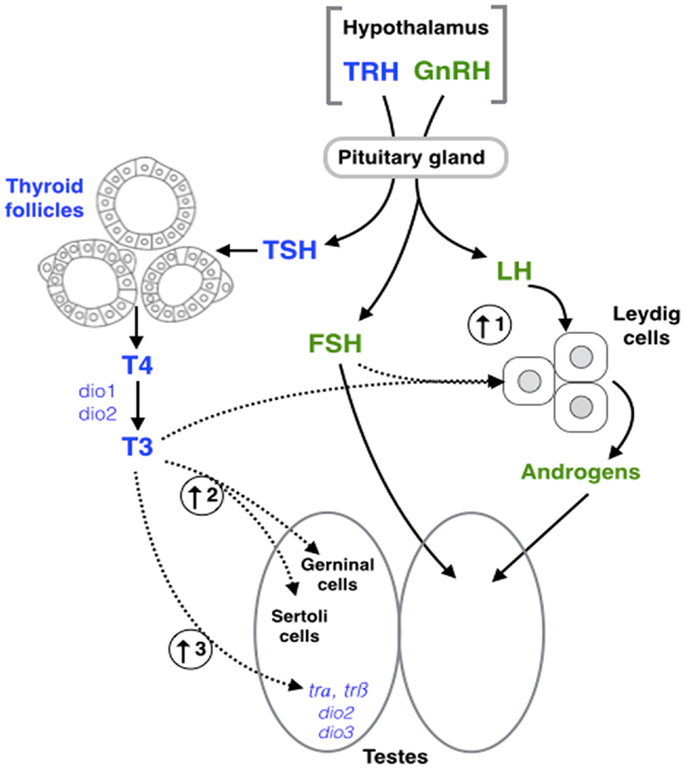
Figure 2. Schematic representation of hypothalamic–pituitary– thyroid (blue) and gonadal (green) axes interaction. Dashed arrows represent the points of interaction between the different axes highlighted in this review. (1) Triiodothyronine (T3) in combination with follicle stimulating hormone (FSH) increase 11-ketotestosterone (11-KT) synthesis in fish (38). (2) T3 exposure results in an increase of Sertoli and germ cell (GC) proliferation in fish (38), and mammals (57). (3) T3 increases the expression of thyroid receptor α (trα), thyroid receptor β (trβ), deiodinase type 2 (dio2), and deiodinase type 3 (dio3) in amphibians (83). GnRH, gonadotropin- releasing hormone; LH, luteinizing hormone; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone; T4, thyroxine; Dio1, deiodinase type 1.
Fluctuations in circulating TH levels lead to subsequent changes in the synthesis, secretion, circulation levels, metabolism, and physiological action of androgens. LH induces steroidogenesis in the Leydig cells, which are responsible for the production of androgens. Like FSH, the biosynthesis of LH is subject to the influence of THs. Hypothyroid conditions decrease circulating LH concentrations or LH bioactivity in several vertebrates [e.g., cockerel [G. gallus; (51)], rat [R. norvegicus; (67)]] as well as the level of testosterone (T) [e.g., R. norvegicus; (67, 68, 71)]. Similarly, severe hypothyroidism in R. norvegicus decreases proliferation of Leydig cells (109) and increases morphology alterations in the human testes (4, 110). Together, these studies demonstrate that fluctuations in THs can directly modulate gonadotropin actions and provide an indirect mechanism of action in which THs can impact Leydig cell proliferation, androgen biosynthesis, and ultimately, spermatogenesis. The crosstalk between both gonadotropins and THs suggests the existence of a vertebrate-wide interaction between the HPT and HPG axes.
Distribution of TH-related machinery in gonadal tissue is highly sex-specific. TSH stimulates the thyroid gland to synthetize and secrete T4, which is mainly converted into T3 by different types of deiodinases [Dios; (111–113)]. Thus, deiodinases (type 1, 2, and 3) play a major role in achieving the levels of intracellular T3 in target tissues by the deiodination of T4. Deiodinases have been identified in the testes of vertebrate species [e.g., rainbow trout, Oncorhynchus mykiss (114), Western clawed frog, Silurana tropicalis (48), G. gallus (115), and R. norvegicus (116)]. The roles of deiodinases in the mammalian testis have been reviewed in detail (9). In developing R. norvegicus, the activity of Dio1 and Dio2 is higher in the testes than in the ovaries, whereas Dio3 activity is greater in the ovary tissue (116). Moreover, deiodinase activity (Dio1, Dio2, and Dio3) is predominant during developmental periods (neonatal and weanling), and subsequently declines in the adult life of R. norvegicus (116). Similar observations have been confirmed in teleosts. For example, testes of striped parrotfish (Scarus iseri) are characterized by higher levels of dio2 and dio3 mRNA than in ovaries (117). The transcripts encoding dio2 mRNA in O. mykiss reach their highest levels in the testes during stage II (beginning of spermatogenesis); a period characterized by the differentiation of somatic testicular cells, active proliferation of spermatogonia, and the formation of spermatocysts. At this point, dio2 expression progressively decreases to later stages of spermatogenesis (114). These results support the idea that TH availability is highly regulated in testicular development and during spermatogenesis by deiodinase activity.
Other important components of the HPT axis are the thyroid receptors (TRs). THs mediate TR signaling and are crucial for testis development and function. The expression of trs in testicular tissue and the physiological implications in mammalian species have been reviewed thoroughly (118, 119). Thus, trα and trβ code for a number of tr-isoforms, including: trα1, trα2, trα3, trβ1, trβ2, and trβ3, which have been identified in the testes of several vertebrates: fish (114, 117), amphibians (47, 83, 120), reptiles (50), and mammals (104, 121–125). In all vertebrate classes, TRs have been localized in Sertoli cells indicating that this cell-type is an evolutionary-conserved target for THs (38, 126); however, the presence of TRs in other types of testicular cells has been debated (8, 126). For example, both Leydig and Sertoli cells have been shown to express trβ in D. rerio; whereas trα was only observed in Sertoli cells (38). In R. norvegicus testes, trα mRNA was detected at all testis stages, while trβ could not be amplified at any of the stages studied (127, 128). Moreover, the fetal and prepubertal periods represent the highest expression of trs in mammals, predominantly trα1 (123), coinciding with high levels of dio2 expression during these particular periods of testis development (116).
The expression of trs in testes is dependent on circulating TH concentrations. Recent studies in S. iseri and R. norvegicus demonstrated that tr mRNA levels fluctuate with TH production within gonadal tissues (117, 129). Moreover, the analysis of the promoter of TRα and TRβ showed putative thyroid response elements (TREs) in mice (M. musculus) and medaka (Oryzias latipes) (12), reinforcing the auto-regulation of TRs by THs. Also, it has been found that trα and trβ transcript levels vary in testis tissue of the Brook trout (Salvelinus fontinalis) according to the seasons, with constant expression throughout spermatogenesis, and higher mRNA levels after spawning season (130). In addition, extra-thyroidal expression of TSH-receptors and TRH-receptors has been identified in the testes [D. labrax (131); fathead minnow, Pimephales promelas (132); Japanese quail, Coturnix japonica (133); M. musculus; R. norvegicus; Guinea pig, Cavia porcellus; and O. aries and Homo sapiens [reviewed in Ref. (125)]]. However, the regulatory role of TSH and TRH-receptors in the male gonad remains unclear.
Transmembrane transport of THs in the gonads is facilitated by the monocarboxylate transporter (Mct) family, specifically the solute carrier family 16 member 2 (Scl16a2 or Mct8) and the solute carrier family 16 member 10 (Scl16a10 or Mct10) (134–136). Muzzio et al. (137) found gender differences in transmembrane transporters, specifically mct8, in the gonads of the fathead minnow (P. promelas). The ovarian mct8 mRNA levels were nearly twofold higher than testicular levels. However, mct8 presented an antagonistic response with the goitrogen methimazole and T3 treatments. Similarly, in P. promelas, hypothyroid-induced condition up-regulates the expression of mct8; whereas hyperthyroidism condition decreases mct8 transcripts (137). Therefore, it is important to include the regulation of the transmembrane proteins when studying the roles of THs in male reproduction.
Thyroid hormones modulate androgen biosynthesis through direct and indirect regulation of the expression and activity of the steroidogenic enzymes involved in their synthesis [reviewed in-depth by Ref. (2, 6–12, 122)]. Recently, Flood et al. (12) performed an in silico analysis of the promoter of several enzymes and receptors involved in both the androgen and TH axes. It was found that several putative TREs and androgen responsive elements (AREs) were present in all of the androgen and TH-related genes studied. This reinforces the hypothesis of a potential direct crosstalk between these two endocrine axes and is supported by experimental approaches in several vertebrates. For example, in air-breathing catfish males (Clarias gariepinus), thiourea-treatment (TH inhibitor) led to selective down-regulation on the expression of the 11β-hydroxylase gene (cyp11b1) and 11β-hydroxysteroid dehydrogenase (hsd11b2); whereas no other alterations were observed for 3β-hsd, 20β-hsd, and cyp17 (cytochrome P-450c17alpha) mRNA levels (40). In the same species, hypothyroidism-induction resulted in a reduction of 11-KT levels in serum and testis tissue (39). Moreover, in a D. rerio testis tissue culture, T3 alone stimulated the proliferation of both Sertoli cells and type A undifferentiated spermatogonia, resulting in newly formed spermatogonial cysts (38). However, T3 exposure alone produces no change in release of 11-KT; whereas when exposed to T3 in combination with FSH, a significant increase in 11-KT synthesis was observed (38). These results support the existence of a cross-regulation between THs (HPT axis) and androgens (HPG axis).
Thyroid hormone availability in the testes can be modulated at different levels of the HPG axis. Aforementioned, GnRH treatment increased TSH and T4 secretions in fish and amphibians (17, 33, 44, 45, 99); however, no changes in T3 were observed in C. auratus (35). These discrepancies in TH responses suggest that GnRH and gonadotropins can modulate the baseline of TH levels in plasma, but deiodinase activity would have to be stimulated in order to increase the concentration of the active T3. Thus, the expression of dios has been shown to respond to androgen signaling. Treatment with flutamide (an androgen receptor antagonist) produced a down-regulation of trβ in testes of P. promelas males (138). Additionally, androgens modulate TH synthesis and peripheral metabolism in fish. In O. mykiss, it was observed that T treatment had no effect on the plasma concentrations of T4, but reduced the levels of T3 (139). In tetrapods, androgen receptors (ar) have been identified in the thyroid gland of reptiles [American alligator, Alligator mississippiensis; (140)], and several mammals (141–143). These observations reinforce the idea that a direct crosstalk between HPG and HPT is possible.
Thyroid hormones have considerable influence in the sexual ontogeny of male vertebrates, through direct interactions with genes involved in sex-determination and gonadal development in the HPG axis (12). It is known that THs play an important role in testicular development and function. In mammals, the genomic and non-genomic actions of THs during testicular development have been extensively reviewed (8, 10, 12). As described above, THs regulate proliferation and differentiation for both Sertoli and Leydig cells (104, 144). In rodent neonates, hypothyroidism and hyperthyroidism conditions affect the number of Sertoli cells by either extending or shortening their period of proliferation, respectively (145–149). Additionally in testes, TH-related machinery has distinct patterns of spatiotemporal expression with developmental stages. The expressions of trs and dio2 decrease with gonadal maturation, suggesting that THs play a crucial role in early testis development and that cessation of TH signaling could be responsible for testis maturation [O. mykiss (114); D. rerio (150); S. tropicalis (83, 151); and R. norvegicus (121–123, 127, 152)]. Interestingly, in situ hybridization studies in D. rerio have shown that dio1 and dio2 mRNA levels were highest and concentrated at the rostral and caudal regions in the somite stages 6 through 18 (153), which are the stages at which gonadal development starts (154). The expression of dio3 was first found in the 6-somite stage, with an increasing area and intensity through 22–24 h post-fertilization – the period at which sex differentiation occurs (153, 154). Altogether, these results demonstrate that maintenance of a baseline level of active T3 by deiodinases, as well as the TH machinery, could be necessary to vertebrate testis development.
In D. rerio testes, T3 in combination with FSH results in newly formed spermatogonial cysts and induces an increase in the synthesis of 11-KT (38). Moreover, it was observed in pejerrey fish (Odontesthes bonariensis), Japanese flounder (Paralichthys olivaceus), and O. latipes that environmental stressors, and/or cortisol treatment, induce 11-KT synthesis (25, 27, 28). It was suggested that the measured elevation of 11-KT could be explained through different mechanisms of action, including: the up-regulation of hsd11b2 transcript [gene that codes for 11β-HSD; (84)], the inhibition of aromatase [enzyme that converts T to estradiol; (27)], and/or through the hepatic catabolism of cortisol (31, 155). Thus, the elevation of cortisol increases androgen biosynthesis with the concomitant masculinization of larvae (156). In summary, the crosstalk between HPA and HPG in the environmental sex-determination of fish has been heavily studied; however, due to the potential for interaction between HPT, HPA, and HPG axes, further studies are needed to clarify the role of the THs in the environmental sex-determination process.
This review on the interaction of HPT, HPA, and HPG axes illustrates our present understanding on the relationship between these endocrine axes and testicular development in different species of vertebrates, although it is necessary to confirm this hypothesis in other species (Figure 3). Some key points can be highlighted: (i) THs could have an important influence in gonadal development, especially on reproduction; (ii) there could be a relationship between T3, in combination with FSH, and induced androgen production, which is required to initiate spermatogenesis; (iii) the availability of deiodinases and TRs during testicular and early developmental stages could be crucial to exert TH action and to regulate testicular development; and (iv) the dual action of CRH on HPT and HPA axes could explain, at least in part, the high levels of androgens during the period of environmental sex-determination. Thus, we hypothesize that these hormonal axis interactions direct the gonadal fate toward masculinization.
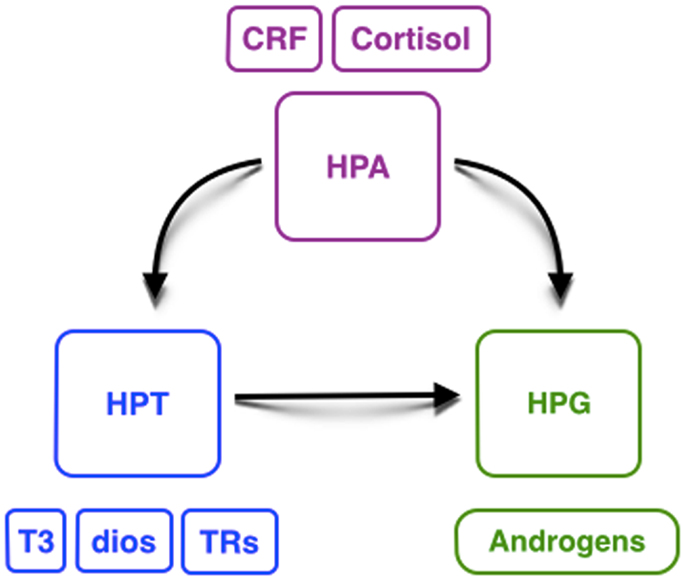
Figure 3. Hypothetical interaction between the hypothalamic– pituitary–thyroid gland (HPT, blue), adrenal/interrenal (HPA, purple), and gonadal (HPG, green) axes. CRF, corticotropin-releasing factor; T3, triiodothyronine; Dios, deiodinases; TRs, thyroid receptors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to Valerie S. Langlois, and CONICET Grant D731, and Agencia Nacional de Promoción Científica y Tecnológica Grant 2012 No 0366 to Juan I. Fernandino. Also, Juan I. Fernandino is a member of the scientific researchers at the CONICET.
1. Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol (2000) 62:439–66. doi: 10.1146/annurev.physiol.62.1.439
2. Maran RRM. Thyroid hormones: their role in testicular steroidogenesis. Arch Androl (2003) 49:375–88. doi:10.1080/01485010390204968
3. Blanton ML, Specker JL. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction. Crit Rev Toxicol (2007) 37:97–115. doi:10.1080/10408440601123529
4. Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev (2010) 31:702–55. doi:10.1210/er.2009-0041
5. Yoshimura T. Thyroid hormone and seasonal regulation of reproduction. Front Neuroendocrinol (2013) 34:157–66. doi:10.1016/j.yfrne.2013.04.002
6. Cooke PS, Holsberger DR, Witorsch RJ, Sylvester PW, Meredith JM, Treinen KA, et al. Thyroid hormone, glucocorticoids, and prolactin at the nexus of physiology, reproduction, and toxicology. Toxicol Appl Pharmacol (2004) 194:309–35. doi:10.1016/j.taap.2003.09.016
7. Swapna I, Senthilkumaran B. Thyroid hormones modulate the hypothalamo–hypophyseal–gonadal axis in teleosts: molecular insights. Fish Physiol Biochem (2007) 33:335–45. doi:10.1007/s10695-007-9165-2
8. Wagner MS, Wajner SM, Maia AL. The role of thyroid hormone in testicular development and function. J Endocrinol (2008) 199:351–65. doi:10.1677/JOE-08-0218
9. Wagner MS, Wajner SM, Maia AL. Is there a role for thyroid hormone on spermatogenesis? Microsc Res Tech (2009) 72:796–808. doi:10.1002/jemt.20759
10. Zamoner A, Pessoa-Pureur R, Silva FR. Membrane-initiated actions of thyroid hormones on the male reproductive system. Life Sci (2011) 89:507–14. doi:10.1016/j.lfs.2011.04.006
11. Habibi HR, Nelson ER, Allan ERO. New insights into thyroid hormone function and modulation of reproduction in goldfish. Gen Comp Endocrinol (2012) 175:19–26. doi:10.1016/j.ygcen.2011.11.003
12. Flood DEK, Fernandino JI, Langlois VS. Thyroid hormones in male reproductive development: evidence for direct crosstalk between the androgen and thyroid hormone axes. Gen Comp Endocrinol (2013) 192:2–14. doi:10.1016/j.ygcen.2013.02.038
13. Larsen DA, Swanson P, Dickey JT, Rivier J, Dickhoff WW. In vitro thyrotropin-releasing activity of corticotropin-releasing hormone-family peptides in coho salmon, Oncorhynchus kisutch. Gen Comp Endocrinol (1998) 109:276–85. doi:10.1006/gcen.1997.7031
14. Ito Y, Okada R, Mochida H, Hayashi H, Yamamoto K, Kikuyama S. Molecular cloning of bullfrog corticotropin-releasing factor (SCRF): eVect of homologous CRF on the release of TSH from pituitary cells in vitro. Gen Comp Endocrinol (2004) 138:218–27. doi:10.1016/j.ygcen.2004.06.006
15. Kaneko M, Fujisawa H, Okada R, Yamamoto K, Nakamura M, Kikuyama S. Thyroid hormones inhibit frog corticotropin-releasing factor-induced thyrotropin release from the bullfrog pituitary in vitro. Gen Comp Endocrinol (2005) 144:122–7. doi:10.1016/j.ygcen.2005.05.003
16. Okada R, Miller MF, Yamamoto K, De Groef B, Denver RJ, Kikuyama S. Involvement of the corticotropin-releasing factor (CRF) type 2 receptor in CRF-induced thyrotropin release by the amphibian pituitary gland. Gen Comp Endocrinol (2007) 150:437–44. doi:10.1016/j.ygcen.2006.11.002
17. Denver RJ. Several hypothalamic peptides stimulate in vitro thyrotropin secretion by pituitaries of anuran amphibians. Gen Comp Endocrinol (1988) 72:383–93. doi:10.1016/0016-6480(88)90160-8
18. Boorse GC, Denver RJ. Expression and hypophysiotropic actions of corticotrophin-releasing factor in Xenopus laevis. Gen Comp Endocrinol (2004) 137:272–82. doi:10.1016/j.ygcen.2004.04.001
19. Denver RJ, Licht P. Neuropeptides influencing pituitary hormone secretion in hatchling turtles. J Exp Zool (1989) 251:306–15. doi:10.1002/jez.1402510307
20. Denver RJ, Licht P. Modulation of neuropeptide-stimulated pituitary hormone secretion in hatchling turtles. Gen Comp Endocrinol (1990) 77:107–15. doi:10.1016/0016-6480(90)90211-4
21. Meeuwis R, Michielsen R, Decuypere E, Kühn ER. Thyrotropic activity of the ovine corticotropin-releasing factor in the chick embryo. Gen Comp Endocrinol (1989) 76:357–63. doi:10.1016/0016-6480(89)90130-5
22. Geris KL, Kotanen SP, Berghman LR, Kühn ER, Darras VM. Evidence of a thyrotropin-releasing activity of ovine corticotropin-releasing factor in the domestic fowl (Gallus domesticus). Gen Comp Endocrinol (1996) 104:139–46. doi:10.1006/gcen.1996.0156
23. De Groef B, Goris N, Arckens L, Kühn ER, Darras VM. Corticotropin-releasing hormone (CRH)-induced thyrotropin release is directly mediated through CRH receptor type 2 on thyrotropes. Endocrinology (2003) 144:5537–44. doi:10.1210/en.2003-0526
24. Fernandino JI, Popesku J, Paul-Prasanth B, Xiong H, Hattori RS, Oura M, et al. Analysis of sexually dimorphic expression of genes at early gonadogenesis of pejerrey Odontesthes bonariensis using a heterologous microarray. Sex Dev (2011) 5:89–101. doi:10.1159/000324423
25. Hattori RS, Fernandino JI, Kishii A, Kimura H, Kinno T, Oura M, et al. Cortisol-induced masculinization: does thermal stress affect gonadal fate in pejerrey, a teleost fish with temperature-dependent sex determination? PLoS One (2009) 4:e6548. doi:10.1371/journal.pone.0006548
26. Yamaguchi T, Kitano T. High temperature induces cyp26b1 mRNA expression and delays meiotic initiation of germ cells by increasing cortisol levels during gonadal sex differentiation in Japanese flounder. Biochem Biophys Res Commun (2012) 419:287–92. doi:10.1016/j.bbrc.2012.02.012
27. Yamaguchi T, Yoshinaga N, Yazawa T, Gen K, Kitano T. Cortisol is involved in temperature-dependent sex determination in the Japanese flounder. Endocrinology (2010) 151:3900–8. doi:10.1210/en.2010-0228
28. Hayashi Y, Kobira H, Yamaguchi T, Shiraishi E, Yazawa T, Hirai T, et al. High temperature causes masculinization of genetically female medaka by elevation of cortisol. Mol Reprod Dev (2010) 77:679–686. doi:10.1002/mrd.21203
29. Friesen CN, Aubin-Horth N, Chapman LJ. The effect of hypoxia on sex hormones in an African cichlid Pseudocrenilabrus multicolor victoriae. Comp Biochem Physiol A Mol Integr Physiol (2012) 162:22–30. doi:10.1016/j.cbpa.2012.01.019
30. Baroiller JF, Chourrout D, Fostier A, Jalabert B. Temperature and sex chromosomes govern sex ratios of the mouthbrooding Cichlid fish Oreochromis niloticus. J Exp Zool (1995) 273:216–23. doi:10.1002/jez.1402730306
31. Schulz RW. In vitro metabolism of steroid hormones in the liver and in blood cells of male rainbow trout (Salmo gairdneri Richardson). Gen Comp Endocrinol (1986) 64:312–9. doi:10.1016/0016-6480(86)90019-5
32. Kaiser S, Kruijve FPM, Swaab DF, Sachser N. Early social stress in female guinea pigs induces a masculinization of adult behavior and corresponding changes in brain and neuroendocrine function. Behav Brain Res (2003) 144:199–210. doi:10.1016/S0166-4328(03)00077-9
33. Chiba H, Amano M, Yamada H, Fujimoto Y, Ojima D, Okuzawa K, et al. Involvement of gonadotropin-releasing hormone in thyroxine release in three different forms of Teleost Fish: Barfin Founder, Masu Salmon and Goldfish. Fish Physiol Biochem (2004) 30:267–73. doi:10.1007/s10695-005-8676-y
34. Roy P, Datta M, Dasgupta S, Bhattacharya S. Gonadotropin-releasing hormone stimulates thyroid activity in a freshwater murrel, Channa gachua (Ham.), and Carps, Catla catla (Ham.) and Cirrhinus mrigala (Ham.). Gen Comp Endocrinol (2000) 117:456–63. doi:10.1006/gcen.1999.7432
35. MacKenzie DS, Sokolowska M, Peter RE, Breton B. Increased gonadotropin levels in goldfish do not result in alterations in circulating thyroid hormone levels. Gen Comp Endocrinol (1987) 67:202–13. doi:10.1016/0016-6480(87)90149-3
36. Nelson ER, Allan ER, Pang FY, Habibi HR. Thyroid hormone and reproduction: regulation of estrogen receptors in goldfish gonads. Mol Reprod Dev (2010) 77:784–94. doi:10.1002/mrd.21219
37. Leatherland JF, Li M, Barkataki S. Stressors, glucocorticoids and ovarian function in teleosts. J Fish Biol (2010) 76:86–111. doi:10.1111/j.1095-8649.2009.02514.x
38. Morais RD, Nobrega RH, Gomez-Gonzalez NE, Schmidt R, Bogerd J, Franca LR, et al. Thyroid hormone stimulates the proliferation of Sertoli cells and single type A spermatogonia in adult zebrafish (Danio rerio) testis. Endocrinology (2013) 154:4365–76. doi:10.1210/en.2013-1308
39. Swapna I, Rajasekhar M, Supriya A, Raghuveer K, Sreenivasulu G, Rasheeda MK, et al. Thiourea-induced thyroid hormone depletion impairs testicular recrudescence in the air-breathing catfish, Clarias gariepinus. Comp Biochem Physiol A Mol Integr Physiol (2006) 144:1–10. doi:10.1016/j.cbpa.2006.01.017
40. Rasheeda MK, Sreenivasulu G, Swapna I, Raghuveer K, Wang DS, Thangaraj K, et al. Thiourea-induced alteration in the expression patterns of some steroidogenic enzymes in the air-breathing catfish Clarias gariepinus. Fish Physiol Biochem (2005) 31:275–9. doi:10.1007/s10695-006-0036-z
41. Parhar IS, Soga T, Sakuma Y. Thyroid hormone and estrogen regulate brain region specific messenger ribonucleic acids encoding three gonadotropin-releasing hormone genes in sexually immature male fish, Oreochromis niloticus. Endocrinology (2000) 141:1618–26. doi:10.1210/endo.141.5.7460
42. Nagendra Prasad RJ, Datta M, Bhattacharya S. Differential regulation of Leydig cell 3beta-hydroxysteroid dehydrogenase/delta5-delta4-isomerase activity by gonadotropin and thyroid hormone in a fresh water perch, Anabas testudineus. Comp Biochem Physiol C (1999) 124:165–73.
43. Jacobs GFM, Kuhn ER. TRH injection induces thyroxine release in the metamorphosed but not in the neotenic Axolotl, Ambystoma mexicanum. Gen Comp Endocrinol (1987) 66:502–5.
44. Jacobs GFM, Goyvaerts MP, Vandorpe G, Quaghebeur AML, Kuhn ER. Luteinizing hormone-releasing hormone as a potent stimulator of the thyroidal axis in ranid frogs. Gen Comp Endocrinol (1988) 70:274–83. doi:10.1016/0016-6480(88)90147-5
45. Okada R, Yamamoto K, Koda A, Ito Y, Hayashi H, Tanaka S, et al. Development of radioimmunoassay for bullfrog thyroid-stimulating hormone (TSH): effects of hypothalamic releasing hormones on the release of TSH from the pituitary in vitro. Gen Comp Endocrinol (2004) 135:42–50. doi:10.1016/j.ygcen.2003.09.001
46. Hogan NS, Crump KL, Duarte P, Lean DR, Trudeau VL. Hormone cross-regulation in the tadpole brain: developmental expression profiles and effect of T3 exposure on thyroid hormone- and estrogen-responsive genes in Rana pipiens. Gen Comp Endocrinol (2007) 154:5–15. doi:10.1016/j.ygcen.2007.02.011
47. Duarte-Guterman P, Ryan MJ, Trudeau VL. Developmental expression of sex steroid- and thyroid hormone-related genes and their regulation by triiodothyronine in the gonad-mesonephros of a Neotropical frog Physalaemus pustulosus. Gen Comp Endocrinol (2012) 177:195–204. doi:10.1016/j.ygcen.2012.03.011
48. Duarte-Guterman P, Trudeau VL. Transcript profiles and triiodothyronine regulation of sex steroid- and thyroid hormone-related genes in the gonad-mesonephros complex of Silurana tropicalis. Mol Cell Endocrinol (2011) 331:143–9. doi:10.1016/j.mce.2010.09.004
49. Duarte-Guterman P, Navarro-Martín L, Trudeau VL. Mechanisms of crosstalk between endocrine systems: regulation of sex steroid hormone synthesis and action by thyroid hormones. Gen Comp Endocrinol (2014). doi:10.1016/j.ygcen.2014.03.015
50. Cardone A, Angelini F, Esposito T, Comitato R, Varriale B. The expression of androgen receptor messenger RNA is regulated by tri-iodothyronine in lizard testis. J Steroid Biochem Mol Biol (2000) 72:133–41. doi:10.1016/S0960-0760(00)00021-2
51. Jacquet JM, Seigneurin F, De Reviers M. Effect of thyroxine on testicular function, circulating luteinising hormone and pituitary sensitivity to luteinising hormone-releasing hormone in the cockerel (Gallus domesticus). Br Poult Sci (1993) 34:803–14. doi:10.1080/00071669308417639
52. Akhlaghi A, Zamiri MJ. Effect of transient prepubertal hypothyroidism on serum testosterone level and seminal characteristics of chickens. Iran J Vet Res (2007) 8:23–31.
53. Sechman A. The role of thyroid hormones in regulation of chicken ovarian steroidogenesis. Gen Comp Endocrinol (2013) 190:68–75. doi:10.1016/j.ygcen.2013.04.012
54. Weng Q, Saita E, Watanabe G, Takahashi S, Sedqyar M, Suzuki AK, et al. Effect of methimazole-induced hypothyroidism on adrenal and gonadal functions in male Japanese quail (Coturnix japonica). J Reprod Dev (2007) 53:1335–41. doi:10.1262/jrd.19081
55. Arambepola NK, Bunick D, Cooke PS. Thyroid hormone effects on androgen receptor messenger RNA expression in rat Sertoli and peritubular cells. J Endocrinol (1998) 156:43–50. doi:10.1677/joe.0.1560043
56. Panno ML, Sisci D, Salerno M, Lanzino M, Pezzi V, Morrone EG, et al. Thyroid hormone modulates androgen and oestrogen receptor content in the Sertoli cells of peripubertal rats. J Endocrinol (1996) 148:43–50. doi:10.1677/joe.0.1480043
57. Marchlewska K, Kula K, Walczak-Jedrzejowska R, Oszukowska E, Orkisz S, Slowikowska-Hilczer J. Triiodothyronine modulates initiation of spermatogenesis in rats depending on treatment timing and blood level of the hormone. Mol Cel Endocrinol (2011) 341:25–34. doi:10.1016/j.mce.2011.04.022
58. Ulisse S, Jannini EA, Carosa E, Piersanti D, Graziano FM, D’Armiento M. Inhibition of aromatase activity in rat Sertoli cells by thyroid hormone. J Endocrinol (1994) 140:431–6. doi:10.1677/joe.0.1400431
59. Andò S, Sirianni R, Forastieri P, Casaburi I, Lanzino M, Rago V, et al. Aromatase expression in prepuberal Sertoli cells: effect of thyroid hormone. Mol Cell Endocrinol (2001) 178:11–21. doi:10.1016/S0303-7207(01)00443-9
60. Pezzi V, Panno ML, Sirianni R, Forastieri P, Casaburi I, Lanzino M, et al. Effects of tri-iodothyronine on alternative splicing events in the coding region of cytochrome P450 aromatase in immature rat Sertoli cells. J Endocrinol (2001) 170:381–93. doi:10.1677/joe.0.1700381
61. Hapon MB, Gamarra-Luques C, Jahn GA. Short term hypothyroidism affects ovarian function in the cycling rat. Reprod Biol Endocrinol (2010) 8:14. doi:10.1186/1477-7827-8-14
62. Antony FF, Aruldhas MM, Udhayakumar RCR, Maran RRM, Govindarajulu P. Inhibition of Leydig-cell activity in-vivo and in-vitro in hypothyroid rats. J Endocrinol (1995) 144:293–300. doi:10.1677/joe.0.1440293
63. Biswas NM, Ghosh PK, Biswas R, Ghosh D. Effect of thyroidectomy, and thyroxine and alpha (2u)-globulin replacement therapy on testicular steroidogenic and gametogenic activities in rats. J Endocrinol (1994) 140:343–7. doi:10.1677/joe.0.1400343
64. Kala N, Ravisankar B, Govindarajulu P, Aruldhas MM. Impact of foetal-onset hypothyroidism on the epididymis of mature rats. Int J Androl (2002) 25:139–48. doi:10.1046/j.1365-2605.2002.00338.x
65. Ram PA, Waxman DJ. Pretranslational control by thyroid hormone of rat liver steroid 5 alpha-reductase and comparison to the thyroid dependence of two growth hormone-regulated CYP2C mRNAs. J Biol Chem (1990) 265:19223–9.
66. Anbalagan J, Sashi AM, Vengatesh G, Stanley JA, Neelamohan R, Aruldhas MM. Mechanism underlying transient gestational-onset hypothyroidism-induced impairment of posttesticular sperm maturation in adult rats. Fertil Steril (2010) 93:2491–7. doi:10.1016/j.fertnstert.2010.02.005
67. Romano RM, Bargi-Souza P, Brunetto EL, Goulart-Silva F, Avellar MCW, Oliveira CA, et al. Hypothyroidism in adult male rats alters posttranscriptional mechanisms of luteinizing hormone biosynthesis. Thyroid (2013) 23:497–505. doi:10.1089/thy.2011.0514
68. Chiao YC, Cho WL, Wang PS. Inhibition of testosterone production by propylthiouracil in rat Leydig cells. Biol Reprod (2002) 67:416–22. doi:10.1095/biolreprod67.2.416
69. Valle LBS, Oliveirafilho RM, Romaldini JH, Lara PF. Pituitary testicular axis abnormalities in immature male hypothyroid rats. J Steroid Biochem Mol Biol (1985) 23:253–7. doi:10.1016/0022-4731(85)90402-9
70. Jahan S, Ahmed S, Emanuel E, Fatima I, Ahmed H. Effect of an anti-thyroid drug, 2,8-dimercapto-6-hydroxy purine on reproduction in male rats. Pak J Pharm Sci (2012) 25:401–6.
71. Chiao YC, Lee HY, Wang SW, Hwang JJ, Chien CH, Huang SW, et al. Regulation of thyroid hormones on the production of testosterone in rats. J Cell Biochem (1999) 73:554–62. doi:10.1002/(SICI)1097-4644(19990615)
72. Catalano S, Pezzi V, Chimento A, Giordano C, Carpino A, Young M, et al. Triiodothyronine decreases the activity of the proximal promoter (PII) of the aromatase gene in the mouse Sertoli cell line,TM4. Mol Endocrinol (2003) 17:923–34. doi:10.1210/me.2002-0102
73. Manna PR, Kero J, Tena-Sempere M, Pakarinen P, Stocco DM, Huhtaniemi IT. Assessment of mechanisms of thyroid hormone action in mouse Leydig cells: regulation of the steroidogenic acute regulatory protein, steroidogenesis, and luteinizing hormone receptor function. Endocrinology (2001) 142:319–31. doi:10.1210/endo.142.1.7900
74. Cecconi S, Rucci N, Scaldaferri ML, Masciulli MP, Rossi G, Moretti C, et al. Thyroid hormone effects on mouse oocyte maturation and granulosa cell aromatase activity. Endocrinology (1999) 140:1783–8. doi:10.1210/endo.140.4.6635
75. Chan WK, Tan CH. Inhibition of follicle-stimulating hormone induction of aromatase activity in porcine granulosa cells by thyroxine and triiodothyronine. Endocrinology (1986) 119:2353–9. doi:10.1210/endo-119-5-2353
76. Gregoraszczuk EL, Slomczynska M, Wilk R. Thyroid hormone inhibits aromatase activity in porcine thecal cells cultured alone and in coculture with granulosa cells. Thyroid (1998) 8:1157–63. doi:10.1089/thy.1998.8.1157
77. Anderson GM, Lapwood KR, Knight PG, Parkinson TJ. The reproductive response of rams to thyroidectomy: mediation by impaired inhibin feedback rather than a change in LH pulsatility. Reproduction (2003) 126:353–64. doi:10.1530/rep.0.1260353
78. Galasa L, Raoulta E, Tononb M-C, Okadad R, Jenkse BG, Castañof JP, et al. TRH acts as a multifunctional hypophysiotropic factor in vertebrates. Gen Comp Endocrinol (2009) 164:40–50. doi:10.1016/j.ygcen.2009.05.003
79. Cerdá-Reverter JM, Canosa LF. Neuroendocrine systems of the fish brain. In: Farrell AP, Brauner CG, Van Der Kraak G, Bernier N, editors. The Fish Neuroendocrinology. (Vol. 28), San Diego, CA: Academic Press (2009). p. 3–74.
80. Chatterjee A, Hsieh YL, Yu JY. Molecular cloning of cDNA encoding thyroid stimulating hormone beta subunit of bighead carp Aristichthys nobilis and regulation of its gene expression. Mol Cell Endocrinol (2001) 174:1–9. doi:10.1016/S0303-7207(01)00392-6
81. Han YS, Liao IC, Tzeng WN, Yu JY. Cloning of the cDNA for thyroid stimulating hormone beta subunit and changes in activity of the pituitary-thyroid axis during silvering of the Japanese eel, Anguilla japonica. J Mol Endocrinol (2004) 32:179–94. doi:10.1677/jme.0.0320179
82. Okada R, Kobayashi T, Yamamoto K, Nakakura T, Tanaka S, Vaudry H, et al. Neuroendocrine regulation of thyroid-stimulating hormone secretion in amphibians. Ann N Y Acad Sci (2009) 1163:262–70. doi:10.1111/j.1749-6632.2008.03662.x
83. Duarte-Guterman P, Langlois VS, Pauli BD, Trudeau VL. Expression and T3 regulation of thyroid hormone- and sex steroid-related genes during Silurana (Xenopus) tropicalis early development. Gen Comp Endocrinol (2010) 166:428–35. doi:10.1016/j.ygcen.2009.12.008
84. Fernandino JI, Hattori RS, Kishi A, Strüssmann CA, Somoza GM. The cortisol and androgen pathways cross talk in high-temperature induced masculinization: 11β-hydroxysteroid dehydrogenase as a key enzyme. Endocrinology (2012) 153:6003–11. doi:10.1210/en.2012-1517
85. Supriya A, Raghuveer K, Swapna I, Rasheeda MK, Kobayashi T, Nagahama Y, et al. Thyroid hormone modulation of ovarian recrudescence of air-breathing catfish Clarias gariepinus. Fish Physiol Biochem (2005) 31:267–70. doi:10.1007/s10695-006-0034-1
87. Flik G, Klaren PH, Van Den Burg EH, Metz JR, Huising MO. CRF and stress in fish. Gen Comp Endocrinol (2006) 146:36–44. doi:10.1016/j.ygcen.2005.11.005
88. Denver RJ. Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann N Y Acad Sci (2009) 1163:1–16. doi:10.1111/j.1749-6632.2009.04433.x
89. De Groef B, Van Der Geyten S, Darras VM, Kühn ER. Role of corticotropin-releasing hormone as a thyrotropin-releasing factor in non-mammalian vertebrates. Gen Comp Endocrinol (2006) 146:62–8. doi:10.1016/j.ygcen.2005.10.014
90. Peter MCS. The role of thyroid hormones in stress response of fish. Gen Comp Endocrinol (2011) 172:198–210. doi:10.1016/j.ygcen.2011.02.023
91. Denver RJ. Chapter seven – neuroendocrinology of amphibian metamorphosis. In: Yun-Bo S, editor. Current Topics in Developmental Biology. New York, NY: Academic Press (2013). p. 195–227.
92. Manzon RG, Denver RJ. Regulation of pituitary thyrotropin gene expression during Xenopus metamorphosis: negative feedback is functional throughout metamorphosis. J Endocrinol (2004) 182:273–85. doi:10.1677/joe.0.1820273
93. Bonett RM, Hoopfer ED, Denver RJ. Molecular mechanisms of corticosteroid synergy with thyroid hormone during tadpole metamorphosis. Gen Comp Endocrinol (2010) 168:209–19. doi:10.1016/j.ygcen.2010.03.014
94. Baker BI, Bird DJ, Buckingham JC. In the trout, CRH and AVT synergize to stimulate ACTH release. Regul Pept (1996) 67:207–10. doi:10.1016/S0167-0115(96)00130-9
95. Dickhoff WW. Hormones, metamorphosis, and smolting. In: Schreibman MP, Scanes CG, Pang PKT, editors. The Endocrinology of Growth, Development, and Metabolism in Vertebrates. San Diego, CA: Academic Press (1993). p. 519–40.
96. Ebbesson LO, Nilsen TO, Helvik JV, Tronci V, Stefansson SO. Corticotropin-releasing factor neurogenesis during midlife development in salmon: genetic, environmental and thyroid hormone regulation. J Neuroendocrinol (2011) 23:733–41. doi:10.1111/j.1365-2826.2011.02164.x
97. Cambré M, Mareels G, Corneillie S, Moons L, Ollevier F, Vandesande F. Chronological appearance of the different hypophysial hormones in the pituitary of bass larvae (Dicentrarchus labrax) during their early development: an immunocytochemical demonstration. Gen Comp Endocrinol (1990) 77:408–15. doi:10.1016/0016-6480(90)90231-A
98. Geven EJW, Verkaar F, Flik G, Klaren PHM. Experimental hyperthyroidism and central mediators of stress axis and thyroid axis activity in common carp (Cyprinus carpio L.). J Mol Endocrinol (2006) 37:443–52. doi:10.1677/jme.1.02144
99. Jacobs GF, Michielsen RP, Kuhn ER. Thyroxine and triiodothyronine in plasma and thyroids of the neotenic and metamorphosed axolotl Ambystoma mexicanum: influence of TRH injections. Gen Comp Endocrinol (1988) 70:145–51. doi:10.1016/0016-6480(88)90103-7
100. Schulz RW, De França LR, Lareyre JJ, Legac F, Chiarini-Garcia H, Nobrega RH, et al. Spermatogenesis in fish. Gen Comp Endocrinol (2010) 165:390–411. doi:10.1016/j.ygcen.2009.02.013
101. Huhtaniemi I. Focus on gonadotrophin signalling. Reproduction (2005) 130:261–2. doi:10.1530/rep.1.00886
102. Huhtaniemi IT, Themmen APN. Mutations in human gonadotropin and gonadotropin-receptor genes. Endocrine (2005) 26:207–17. doi:10.1385/ENDO:26:3:207
103. Atanassova NN, Walker M, Mckinnell C, Fisher JS, Sharpe RM. Evidence that androgens and oestrogens, as well as follicle-stimulating hormone, can alter Sertoli cell number in the neonatal rat. J Endocrinol (2005) 184:107–17. doi:10.1677/joe.1.05884
104. Holsberger DR, Cooke PS. Understanding the role of thyroid hormone in Sertoli cell development: a mechanistic hypothesis. Cell Tissue Res (2005) 322:133–40. doi:10.1007/s00441-005-1082-z
105. Russell LD, Alger LE, Nequin LG. Hormonal control of pubertal spermatogenesis. Endocrinology (1987) 120:1615–32. doi:10.1210/endo-120-4-1615
106. Almiron I, Chemes H. Spermatogenic onset. II. FSH modulates mitotic activity of germ and Sertoli cells in immature rats. Int J Androl (1988) 11:235–46. doi:10.1111/j.1365-2605.1988.tb00998.x
107. Campbell B, Dickey JT, Swanson P. Endocrine changes during onset of puberty in male spring Chinook salmon, Oncorhynchus tshawytscha. Biol Reprod (2003) 69:2109–17. doi:10.1095/biolreprod.103.020560
108. Parkinson TJ, Douthwaite JA, Follett BK. Responses of prepubertal and mature rams to thyroidectomy. J Reprod Fertil (1995) 104:51–6. doi:10.1530/jrf.0.1040051
109. Cristovao FC, Bisi H, Mendonca BB, Bianco AC, Bloise W. Severe and mild neonatal hypothyroidism mediate opposite effects on Leydig cells of rats. Thyroid (2002) 12:13–8. doi:10.1089/105072502753451913
110. Krassas GE, Papadopoulou F, Tziomalos K, Zeginiadou T, Pontikides N. Hypothyroidism has an adverse effect on human spermatogenesis: a prospective, controlled study. Thyroid (2008) 18:1255–9. doi:10.1089/thy.2008.0257
111. Orozco A, Valverde RC. Thyroid hormone deiodination in fish. Thyroid (2005) 15:799–813. doi:10.1089/thy.2005.15.799
112. St Germain DL, Galton VA, Hernandez A. Minireview: defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology (2009) 150:1097–107. doi:10.1210/en.2008-1588
113. Dentice M, Salvatore D. Deiodinases: the balance of thyroid hormone: local impact of thyroid hormone inactivation. J Endocrinol (2011) 209:273–82. doi:10.1530/joe-11-0002
114. Sambroni E, Gutieres S, Cauty C, Guiguen Y, Breton B, Lareyre JJ. Type II iodothyronine deiodinase is preferentially expressed in rainbow trout (Oncorhynchus mykiss) liver and gonads. Mol Reprod Dev (2001) 60:338–50. doi:10.1002/mrd.1096
115. Van der Geyten S, Van den Eynde I, Segers IB, Kuhn ER, Darras VM. Differential expression of iodothyronine deiodinases in chicken tissues during the last week of embryonic development. Gen Comp Endocrinol (2002) 128:65–73. doi:10.1016/S0016-6480(02)00065-5
116. Bates JM, St Germain DL, Galton VA. Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology (1999) 140:844–51. doi:10.1210/endo.140.2.6537
117. Johnson KM, Lema SC. Tissue-specific thyroid hormone regulation of gene transcripts encoding iodothyronine deiodinases and thyroid hormone receptors in striped parrotfish (Scarus iseri). Gen Comp Endocrinol (2011) 172:505–17. doi:10.1016/j.ygcen.2011.04.022
118. Valadares NF, Polikarpov I, Garratt RC. Ligand induced interaction of thyroid hormone receptor beta with its coregulators. J Steroid Biochem Mol Biol (2008) 112:205–12. doi:10.1016/j.jsbmb.2008.10.006
119. Dittrich R, Beckmann MW, Oppelt PG, Hoffmann I, Lotz L, Kuwert T, et al. Thyroid hormone receptors and reproduction. J Reprod Immunol (2011) 90:58–66. doi:10.1016/j.jri.2011.02.009
120. Arukwe A, Jenssen BM. Differential organ expression patterns of thyroid hormone receptor isoform genes in p,p′-DDE-treated adult male common frog, Rana temporaria. Environ Toxicol Pharmacol (2005) 20(3):485–92. doi:10.1016/j.etap.2005.05.008
121. Jannini EA, Olivieri M, Francavilla S, Gulino A, Ziparo E, D’Armiento M. Ontogenesis of the nuclear 3,5,3′-triiodothyronine receptor in the rat testis. Endocrinology (1990) 126:2521–6. doi:10.1210/endo-126-5-2521
122. Jannini EA, Carosa E, Rucci N, Screponi E, D’Armiento M. Ontogeny and regulation of variant thyroid hormone receptor isoforms in developing rat testis. J Endocrinol Invest (1999) 22:843–8. doi:10.1111/j.1743-6109.2009.01701.x
123. Buzzard JJ, Morrison JR, O’Bryan MK, Song Q, Wreford NG. Developmental expression of thyroid hormone receptors in the rat testis. Biol Reprod (2000) 62:664–9. doi:10.1095/biolreprod62.3.664
124. Williams GR. Cloning and characterization of two novel thyroid hormone receptor beta isoforms. Mol Cell Biol (2000) 20:8329–42. doi:10.1128/MCB.20.22.8329-8342.2000
125. Williams GR. Extrathyroidal expression of TSH receptor. Ann Endocrinol (Paris) (2011) 72:68–73. doi:10.1016/j.ando.2011.03.006
126. Singh SR, Burnicka-Turek O, Chauhan C, Hou SX. Spermatogonial stem cells, infertility and testicular cancer. J Cell Mol Med (2011) 15:468–83. doi:10.1111/j.1582-4934.2010.01242.x
127. Canale D, Agostini M, Giorgilli G, Caglieresi C, Scartabelli G, Nardini V, et al. Thyroid hormone receptors in neonatal, prepubertal, and adult rat testis. J Androl (2001) 22:284–8. doi:10.1002/j.1939-4640.2001.tb02182.x
128. Rao JN, Liang JY, Chakraborti P, Feng P. Effect of thyroid hormone on the development and gene expression of hormone receptors in rat testes in vivo. J Endocrinol Invest (2003) 26:435–43. doi:10.1007/BF03345199
129. De Paul AL, Mukdsi JH, Pellizas CG, Montesinos M, Gutierrez S, Susperreguy S, et al. Thyroid hormone receptor alpha 1-beta 1 expression in epididymal epithelium from euthyroid and hypothyroid rats. Histochem Cell Biol (2008) 129:631–42. doi:10.1007/s00418-008-0397-8
130. de Montgolfier B, Faye A, Audet C, Cyr DG. Seasonal variations in testicular connexin levels and their regulation in the brook trout, Salvelinus fontinalis. Gen Comp Endocrinol (2009) 162:276–85. doi:10.1016/j.ygcen.2009.03.025
131. Rocha A, Gomez A, Galay-Burgos M, Zanuy S, Sweeney GE, Carrillo M. Molecular characterization and seasonal changes in gonadal expression of a thyrotropin receptor in the European sea bass. Gen Comp Endocrinol (2007) 152:89–101. doi:10.1016/j.ygcen.2007.03.001
132. Lema SC, Dickey JT, Schultz IR, Swanson P. Thyroid hormone regulation of mRNAs encoding thyrotropin beta-subunit, glycoprotein alpha-subunit, and thyroid hormone receptors alpha and beta in brain, pituitary gland, liver, and gonads of an adult teleost, Pimephales promelas. J Endocrinol (2009) 202:43–54. doi:10.1677/JOE-08-0472
133. Catena ML, Porter TE, Mcnabb FM, Ottinger MA. Cloning of a partial cDNA for Japanese quail thyroid-stimulating hormone and effects of methimazole on the thyroid and reproductive axes. Poult Sci (2003) 82:381–7. doi:10.1093/ps/82.3.381
134. Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch (2004) 447:619–28. doi:10.1007/s00424-003-1067-2
135. Bernal J. Thyroid hormone transport in developing brain. Curr Opin Endocrinol Diabetes Obes (2011) 18:295–9. doi:10.1097/MED.0b013e32834a78b3
136. Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol (2011) 25:1–14. doi:10.1210/me.2010-0095
137. Muzzio AM, Noyes PD, Stapleton HM, Lema SC. Tissue distribution and thyroid hormone effects on mRNA abundance for membrane transporters Mct8, Mct10, and organic anion-transporting polypeptides (Oatps) in a teleost fish. Comp Biochem Physiol A Mol Integr Physiol (2014) 167:77–89. doi:10.1016/j.cbpa.2013.09.019
138. Filby AL, Thorpe KL, Maack G, Tyler CR. Gene expression profiles revealing the mechanisms of anti-androgen- and estrogen-induced feminization in fish. Aquat Toxicol (2007) 81:219–31. doi:10.1016/j.aquatox.2006.12.003
139. Leatherland JF. Effects of 17 beta-estradiol and methyl testosterone on the activity of the thyroid gland in rainbow trout, Salmo gairdneri Richardson. Gen Comp Endocrinol (1985) 60:343–52. doi:10.1016/0016-6480(85)90067-X
140. Bermudez DS, Skotko JP, Ohta Y, Boggs AS, Iguchi T, Guillette LJ Jr. Sex steroid and thyroid hormone receptor expressions in the thyroid of the American alligator (Alligator mississippiensis) during different life stages. J Morphol (2011) 272:698–703. doi:10.1002/jmor.10936
141. Pelletier G. Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol (2000) 15:1261–70.
142. Zhai Q-H, Ruebel K, Thompson G, Lloyd R. Androgen receptor expression in C-cells and in medullary thyroid carcinoma. Endocr Pathol (2003) 4:159–65. doi:10.1385/EP:14:2:159
143. Magri F, Capelli V, Rotondi M, Leporati P, La Manna L, Ruggiero R, et al. Expression of estrogen and androgen receptors in differentiated thyroid cancer: an additional criterion to assess the patient’s risk. Endocr Relat Cancer (2012) 19:463–71. doi:10.1530/erc-11-0389
144. Mendis-Handagama SM, Siril Ariyaratne HB. Leydig cells, thyroid hormones and steroidogenesis. Indian J Exp Biol (2005) 43:939–62.
145. Joyce KL, Porcelli J, Cooke PS. Neonatal goitrogen treatment increases adult testis size and sperm production in the mouse. J Androl (1993) 14:448–55. doi:10.1002/j.1939-4640.1993.tb03261.x
146. Cooke PS, Zhao YD, Bunick D. Triiodothyronine inhibits proliferation and stimulates differentiation of cultured neonatal Sertoli cells: possible mechanism for increased adult testis weight and sperm production induced by neonatal goitrogen treatment. Biol Reprod (1994) 51:1000–5. doi:10.1095/biolreprod51.5.1000
147. De Franca LR, Hess RA, Cooke PS, Russell LD. Neonatal hypothyroidism causes delayed Sertoli cell maturation in rats treated with propylthiouracil: evidence that the Sertoli cell controls testis growth. Anat Rec (1995) 242:57–69. doi:10.1002/ar.1092420108
148. Holsberger DR, Kiesewetter SE, Cooke PS. Regulation of neonatal Sertoli cell development by thyroid hormone receptor alpha1. Biol Reprod (2005) 73:396–403. doi:10.1095/biolreprod.105.041426
149. Auharek SA, de Franca LR. Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period. J Anat (2010) 216:577–88. doi:10.1111/j.1469-7580.2010.01219.x
150. Takayama S, Hostick U, Haendel M, Eisen J, Darimont B. An F-domain introduced by alternative splicing regulates activity of the zebrafish thyroid hormone receptor alpha. Gen Comp Endocrinol (2008) 155:176–89. doi:10.1016/j.ygcen.2007.04.012
151. Flood DEK, Langlois VS. Crosstalk between the thyroid hormone and androgen axes during reproductive development in Silurana tropicalis. Gen Comp Endocrinol (2014). doi:10.1016/j.ygcen.2014.03.037
152. Jannini EA, Ulisse S, D’Armiento M. Thyroid hormone and male gonadal function. Endocr Rev (1995) 16:443–59. doi:10.1210/edrv-16-4-443
153. Dong W, Macaulay LJ, Kwok KW, Hinton DE, Stapleton HM. Using whole mount in situ hybridization to examine thyroid hormone deiodinase expression in embryonic and larval zebrafish: a tool for examining OH-BDE toxicity to early life stages. Aquat Toxicol (2013) 13(2–133):190–9. doi:10.1016/j.aquatox.2013.02.008
154. von Hofsten J, Olsson P-E. Zebrafish sex determination and differentiation: involvement of FTZ-F1 genes. Reprod Biol Endocrinol (2005) 3:1–11. doi:10.1186/1477-7827-3-63
155. Kime DE. The hepatic catabolism of cortisol in teleost fish – adrenal origin of 11-oxotestosterone precursors. Gen Comp Endocrinol (1978) 35:322–8. doi:10.1016/0016-6480(78)90078-3
Keywords: thyroid hormone, corticotropin-releasing hormone, gonadotropins, androgen, testis, fish, amphibians
Citation: Castañeda Cortés DC, Langlois VS and Fernandino JI (2014) Crossover of the hypothalamic pituitary–adrenal/interrenal, –thyroid, and –gonadal axes in testicular development. Front. Endocrinol. 5:139. doi: 10.3389/fendo.2014.00139
Received: 28 March 2014; Accepted: 11 August 2014;
Published online: 27 August 2014.
Edited by:
Fátima Regina Mena Barreto Silva, Universidade Federal de Santa Catarina, BrazilCopyright: © 2014 Castañeda Cortés, Langlois and Fernandino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valerie S. Langlois, Chemistry and Chemical Engineering Department, Royal Military College of Canada, P. O. Box 17 000, Stn Forces, Kingston, ON K7K 7B4, Canada e-mail:dmFsZXJpZS5sYW5nbG9pc0BybWMuY2E=;
Juan I. Fernandino, Int. Marino Km. 8.200, Chascomús, Buenos Aires Province B7130IWA, Argentina e-mail:ZmVybmFuZGlub0BpbnRlY2guZ292LmFy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.