- Department of Endocrinology, Catholic University of Rome, Rome, Italy
Objective: Thyroid-related emergencies are caused by overt dysfunction of the gland which are so severe that require admission to intensive care units (ICU) frequently. Nonetheless, in the ICU setting, it is crucial to differentiate patients with non-thyroidal illness and alterations in thyroid function tests from those with intrinsic thyroid disease. This review presents and discusses the main etiopathogenetical and clinical aspects of hypothyroid coma (HC) and thyrotoxic storm (TS), including therapeutic strategy flow-charts. Furthermore, a special chapter is dedicated to the approach to massive goiter, which represents a surgical thyroid emergency.
Data Source: We searched the electronic MEDLINE database on September 2013.
Data Selection and Data Extraction: Reviews, original articles, and case reports on “myxedematous coma,” “HC,” “thyroid storm,” “TS,” “massive goiter,” “huge goiter,” “prevalence,” “etiology,” “diagnosis,” “therapy,” and “prognosis” were selected.
Data Synthesis and Conclusion: Severe excess or defect of thyroid hormone is rare conditions, which jeopardize the life of patients in most cases. Both HC and TS are triggered by precipitating factors, which occur in patients with severe hypothyroidism or thyrotoxicosis, respectively. The pillars of HC therapy are high-dose l-thyroxine and/or tri-iodothyroinine; i.v. glucocorticoids; treatment of hydro-electrolyte imbalance (mainly, hyponatraemia); treatment of hypothermia; often, endotracheal intubation and assisted mechanic ventilation are needed. Therapy of TS is based on beta-blockers, thyrostatics, and i.v. glucocorticoids; eventually, high-dose of iodide compounds or lithium carbonate may be of benefit. Surgery represents the gold standard treatment in patients with euthyroid massive nodular goiter, although new techniques – e.g., percutaneous laser ablation – are helpful in subjects at high surgical risk or refusing operation.
Introduction
Emergencies related to thyroid gland diseases are infrequently observed in the clinical practice (1–3). They are caused by either overt dysfunction (4, 5) or marked enlargement of the gland (6) that jeopardize the life of patients, and require admission to intensive care units (ICU) in most cases.
The present paper reviews hypothyroid coma (HC), thyrotoxic storm (TS), and massive goiter, discussing related etiopathogenesis, clinical aspects, diagnostic challenges, and prognosis, and finally presenting therapeutic strategy flow-charts.
Review Criteria
The content of the present review is based on the previous reviews, original articles, and case reports on thyroid emergencies. We searched the electronic MEDLINE database on September 2013 for a combination of the following search terms: “myxedematous coma,” “hypothyroid coma,” “thyroid storm,” “thyrotoxic storm,” “massive goiter,” “huge goiter,” “prevalence,” “etiology,” “diagnosis,” “therapy,” and “prognosis.” Only papers written in the English language were included in this review.
Emergency Related to Thyroid Hormone Excess or Deficiency
Either excess or deficiency of thyroid hormones is caused by several thyroid disorders. Whatever is the underlying cause, both thyrotoxicosis and hypothyroidism deeply impact on cardiovascular and digestive systems, basal metabolism, neuro-psychological functions, muscles, and cutis. Rarely, the excess or the defect of thyroid hormone is so severe that jeopardize the life of patients, who should be promptly referred to the emergency department.
Boxes 1 and 2 summarize “classical” definitions of thyrotoxicosis and hypothyroidism and thyroid diseases predisposing to TS and HC, respectively. Really, such definitions easily apply to patients in the ambulatory setting, but do not reflect what commonly happens in critically ill subjects. Yet, it is crucial to establish a correct diagnosis in order to differentiate patients with non-thyroidal illness – i.e., affected by the so-called “euthyroid sick syndrome” (Box 3) – by those truly affected by severe thyroid disorders, as the therapeutic approach changes profoundly.
Box 1. Definition of thyrotoxicosis. Thyroid diseases causing thyrotoxicosis and predisposing to thyrotoxic storm (TS).
In this review, “subclinical thyrotoxicosis” was defined as a condition characterized by low-undetectable serum TSH with normal free thyroid hormone concentrations (7). “Overt thyrotoxicosis” was defined as a condition with suppressed serum thyrotropin (TSH) and elevated free thyroid hormone concentrations (8).
A cut-off limit of serum thyroid hormone concentrations precipitating TS cannot be established, as serum free-thyroxine (FT4) and free tri-iodothyronine (FT3) concentrations in thyrotoxic storm (TS) patients are as high as in hyperthyroid ones without TS.
The following thyroid diseases have been described in association with TS:
Graves’ disease; iodine- and amiodarone-induced thyrotoxicosis; toxic nodular goiter; cytokine- and tyrosine-kinase inhibitors-induced hyperthyroidism; subacute de Quervain’s thyroiditis; post-partum thyroiditis; and radiation thyroiditis.
Factitious (voluntary) intake of high-dose l-Thyroxine has been reported as a cause of TS, too.
Box 2. Definition of hypothyroidism. Thyroid diseases causing hypothyroidism and predisposing to hypothyroid coma (HC).
In this review, “subclinical hypothyroidism” was defined as a condition characterized by normal thyroid hormone levels and high serum TSH concentrations (9–11). “Overt hypothyroidism” was defined as a condition with serum TSH concentrations exceeding the normal range associated to low serum free thyroid hormone level (12).
Chronic (Hashimoto’s) thyroiditis and cytokine- and tyrosine-kinase inhibitors-induced hypothyroidism have been so far reported in association with hypothyroid coma (HC).
Total thyroidectomy; neck external beam radiotherapy;131I treatment of hyperthyroidism; use of anti-thyroid drugs (methimazole, propylthiouracyle), sedatives, and analgesics have been also reported as a cause of HC.
Box 3. The euthyroid sick syndrome (ESS).
Patients affected by critical illness frequently manifest alterations in serum thyroid hormone concentrations, although they do not really suffer from thyroid disease (13). This condition is known as “euthyroid sick syndrome” (ESS), “non-thyroidal illness,” or – less commonly at the moment – “low T3 syndrome” (14).
ESS is the direct consequence of changes in the function of hypothalamus-pituitary-thyroid axis, in thyroid hormone metabolism and even in thyroid gland function per se, occurring in critical illness and sometimes induced by drugs (15). So far published studies have reported alterations in deiodinase activity, TSH secretion, hormone binding to serum proteins, thyroid hormone transport into tissues, and the nuclear thyroid hormone receptors.
Starvation and sepsis are the conditions that physician working in the intensive care units (ICU) most commonly deal with and represent the paradigms for ESS (16). The laboratory hallmark of ESS, at least in its early phase, is low serum T3 concentration, typically associated to TSH levels in the low-normal range, so representing a variant of secondary or tertiary hypothyroidism (17). Concurrent low serum T4 concentrations represent a prognostically unfavorable sign (18).
Decreased binding to carrier proteins may occur in ESS (19), supporting the value of free rather than total thyroid hormone measurement in this setting (20). The use of drugs – e.g., furosemide, heparin, non-steroidal anti-inflammatory drugs, amiodarone, anti-epileptic drugs, dopamine, dobutamine, glucocorticoids, and somatostatin – might also have a significant impact on thyroid hormone and TSH levels (21, 22). Thus, TSH measurement alone should be avoided in the context of ICU, as in the absence of thyroid function tests it might be misleading and induce physicians to treat patients for hyperthyroidism.
During the recovery phase from ESS, transient elevation – usually not exceeding 20 mIU/L – in serum TSH concentrations may occur (23), resembling the recovery phase from subacute “de Quervain” thyroiditis. At this point in time, ESS should be accurately differentiated by subclinical hypothyroidism. Serum TSH concentrations above 20 mIU/l, high anti-thyroglobulin and anti-thyroperoxidase autoantibody levels, and/or a diffusely hypoechoic aspect of the thyroid gland on US are in favor of primary hypothyroidism, and should suggest to start levothyroxine substitution therapy. Conversely, studies so far conducted in the literature have demonstrated that levothyroxine substitution therapy is not indicated in patients with ESS (24).
Hypothyroid Coma
Hypothyroid (or myxedematous) coma (HC) is the result of a very severe, as yet untreated, hypothyroidism (25). It represents an endocrine emergency that should be handled in ICU.
In a background of thyroid hormone deficiency, HC is usually triggered by precipitating factors, as low outside temperatures, systemic (mainly, pulmonary) infections, congestive heart failure (CHF), labor, cerebrovascular events, intake of anesthetics, depressants, neuroleptics, or large liquid amounts (25–31). It manifests rarely, with a prevalence approaching 0.1% of hospitalized hypothyroid patients, and particularly affects female subjects aged >60 years (32). Even if l-thyroxine replacement therapy is quickly and appropriately given, about 15–20% of patients finally die (1).
Etiology
Most patients referred to the hospital for HC are already followed by the endocrinologist or the general practitioner because of hypothyroidism consequent to autoimmune chronic thyroiditis, thyroidectomy or Graves’ disease treated by radioiodine (131I). Usually, these subjects have previously been taking l-thyroxine substitution therapy, unless they have subsequently withdrawn it on their own initiative. Rarely, the cause of HC is not of primary thyroid origin, but is because of reduced TSH excretion by the pituitary gland (e.g., hypopituitarism) (33).
Patients presenting with secondary hypothyroidism have been previously submitted to surgery or radiotherapy because of pituitary adenoma, or are affected by pituitary macroadenoma overwhelming TSH-producing pituitary cells. Some drugs – e.g., amiodarone and lithium – may directly cause hypothyroidism and HC (22–24). Amiodarone is an anti-arrhythmic drug containing high (about 37 mg) iodine amounts. Approximately 15% of patients taking amiodarone develop hypothyroidism or thyrotoxicosis, mainly depending on concurrent thyroid disease (e.g., autoimmune or nodular thyroid disease) and daily iodine intake (21). Amiodarone might cause hypothyroidism inducing inhibition of 5′-deiodinase activity and Wolff–Chaikoff effect. Lithium is commonly used to treat bipolar disorders, inhibits thyroid hormone release from the thyroid gland, and increases thyroid autoimmunity if present before therapy (30, 34, 35). Severe hypothyroidism as a result of impaired iodine uptake by thyrocytes has been described in neoplastic patients treated with tyrosine-kinase selective inhibitors (36). Finally, the case of a Chinese patient referred to the hospital with HC provoked by ingestion of raw bok choy has been reported (37). It has been demonstrated that plants of the family of Brassicaceae contain glucosinolate, a sulfur-containing organic anions bonded to glucose that is hydrolyzed to thiocyanate, a compound known for its competition with iodine. Ingestion of large amounts of Brassicaceae induces goiter and hypothyroidism (38).
Diagnosis
The diagnosis of HC is based on (1) the history of previous thyroid disease and progressive lethargy; (2) peculiar signs and symptoms (Table 1); (3) serum free T4 (FT4) and free T3 (FT3) concentrations below the normal reference range; and (4) serum TSH concentrations far exceeding the normal reference range. With regard to TSH values, they usually exceed 100 mIU/L in most severe and long-lasting cases; however, in patients with hypopituitarism (where serum TSH concentrations are typically low-normal) (5, 39) and in those with recent-onset hypothyroidism, serum TSH concentrations may not be so high.
The diagnosis of HC is relatively simple. However, because serum thyroid hormone and TSH measurement commonly take hours, once arrived at the hospital, besides serum TSH, FT4, and FT3 concentration measurement, patients should undergo further (i.e., first level) examinations:
- Arterial hemogasanalysis, to eventually detect hypoxemia, hypercapnia, and respiratory acidosis.
- Labanalysis, which may reveal anemia, hyponatraemia, hypoglycemia, high serum creatine kinase, lactate dehydrogenase, transaminases, creatinine, and cholesterol concentrations. Potential mechanisms underlying hyponatraemia in HC patients are represented by increased serum anti-diuretic hormone (ADH) (40) and impaired water excretion and urine output due to reduced delivery of water to the distal nephron (41). The atony of urinary bladder with the consequent urine retention and the high serum creatine kinase concentrations due to rhabdomyolysis may lead to renal failure (15, 42).
- Electrocardiography, usually demonstrating sinus bradycardia, low voltages (related to pericardial effusion), Q–T prolongation and flattened or inverted T waves (consequent to myocardial ischemia).
- Echocardiography, which may disclose a pericardial effusion associated to cardiomegaly, increased thickness of all cardiac walls and reduced cardiac output.
- Computed tomography (CT) of the brain that is normal in most patients with primary hypothyroidism (although long-lasting hypothyroidism promotes atherogenesis and, then, ischemic encephalopathy), and generally detects pituitary macroadenoma or an empty sella in patients with secondary hypothyroidism.
Treatment
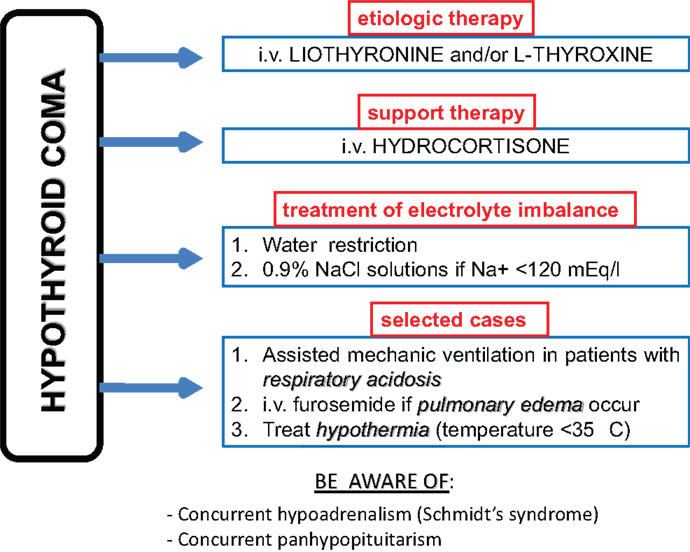
The pillar of HC therapy is l-thyroxine or l-thyroxine plus liothyronine substitution therapy preferably administered intravenously, owing to the poor intestinal absorption related to severe hypothyroidism (Figure 1). Because of the rarity of HC, prospective studies recruiting large series of patients and evaluating the best therapeutic regimens are lacking in the literature. Therefore, this topic is still controversial. The major matter of debate concerns the starting dose of substitution therapy. Some authors (43) recommend starting with high-dose (300–400 mcg/daily) of T4 i.v., but sudden cardiac death has been reported and most recent literature does not support such a regimen. Rapid correction of hypothyroidism may be tolerated by young otherwise fit adults, but these are not the usual patients presenting with HC. Other authors (15, 44) suggest to give liothyronine 10–20 mcg i.v. as bolus initially, followed by 10 mcg every 4 h for the first 24 h and every 6 h for days 2–3, and then to start oral administration if feasible. Alternatively, a T3 plus T4 approach might be used: an initial dose of 250 mcg of intravenous T4 might be administered, followed by 100 mcg 24 h later and 50 mcg per day (i.v. or by mouth) after that. Simultaneously with the first T4 bolus (day 1), a 10 mcg T3 as i.v. bolus is given followed by 10 mcg i.v. every 8–12 h until the patients is conscious and taking maintenance T4 (15).
Beyond thyroid hormone substitution therapy, hydrocortisone 100 mg every 6 h intravenously and treatment of hydro-electrolyte imbalance should be administered. In particular, hyponatraemia should be first corrected through water restriction. 0.9% NaCl solutions (slow at 12 mmol/l/24 h) should be administered to patients with severe hyponatraemia (<120 mEq/L) only. Hypertonic saline should be used cautiously to avoid osmotic demyelinization syndrome (1, 15). The use of diuretics is generally suggested after the administration of hypertonic saline in order to promote a water diuresis (15).
Hypothermia is to be treated through passive and gradual heating (e.g., by blankets); in contrast, the active body heating is not warranted because of the risk of vasodilatation and shock. Endotracheal intubation and assisted mechanic ventilation with constant monitoring of haemogasanalysis parameters are needed in case of severe respiratory acidosis.
At the same time, treatment of precipitating factors, if any, is needed. For instance, large spectrum antibiotics must be administered to patients with pneumonia, after samples for urine and blood cultures have been previously collected awaiting for laboratory report (3). Furthermore, particular attention must be paid to concurrent diseases in the context of autoimmune polyendocrine syndromes. In particular, hypoadrenalism due to Schmidt’s syndrome (association of autoimmune adrenalitis and Hashimoto’s thyroiditis) should be ruled out (45, 46). Nonetheless, hypoadrenalism might occur as the consequence of panhypopituitarism. In any case, until results of hypothalamus–pituitary–adrenal axis study will be available, intravenous glucocorticoids administration is always mandatory together with l-thyroxine therapy, to avoid the occurrence of acute adrenal insufficiency (45). Hypoglycemia is a serious and common complication of HC and should be recognized and treated as soon as possible by intravenous glucose solutions.
Death is caused by respiratory and/or cardiac (arrhythmias, acute myocardial infarction, acute pulmonary edema, and cardiac shock) complications (47, 48). Although both right and left heart failure may occur in HC, the former is a clue to hypothyroidism. Reduced stroke volume should be consequent to either reduced myocardial contractility per se or cardiac tamponade. In patients with pulmonary edema, diuretics (particularly, i.v. furosemide) must be added to the standard HC therapy. Torsades de pointes ventricular tachycardia might be the result of prolonged Q–T interval, and therefore patients should be carefully and constantly monitored with particular regard to this life-threatening event. Use of digoxin is not indicated in subjects with tamponade, and should generally be very cautious in HC patients because of prolonged half-life and decreased volume of distribution. If a treatment with digoxin is started, serum digoxin levels should be monitored regularly and dosage should be administered very slowly increasing. Myocardial infarction is not uncommon in subjects with HC and can be precipitated by too high l-thyroxine doses needed to treat HC in patient with underlying ischemic cardiomyopathy. Owing to impaired consciousness, clinical alert related to ischemic events is lacking in HC patients, and therefore cardiac enzymes – also in the absence of typical electrocardiographic features of myocardial infarction – should be routinely measured in such condition.
Importantly, HC patients are at high risk of bleeding caused by an acquired von Willebrand syndrome and reduction in coagulation factors V, VII, VIII, IX, and X (15, 49). Such a risk is reversible with L-T4 substitution therapy (49).
Thyrotoxic Storm
Thyrotoxic storm represents the extreme consequence of a severe thyrotoxicosis (4, 50). As HC, TS is frequently triggered by typical precipitating factors that occur when patients are already affected by overt (occasionally, subclinical) hyperthyroidism (51).
The main triggering factors so far reported are summarized in Table 2.
Fortunately, TS is a rare event mostly affecting hyperthyroid patients who have not been adequately treated or have withdrawn thyrostatic therapy on their own initiative or have undergone surgery (52–54). Its prevalence averages 1% of hospitalized subjects with hyperthyroidism (53) and is more frequent in the female gender, with a female to male ratio of about 3 to 1 (55). In a recent study, an incidence of TS cases in Japan was estimated to be 0.2 persons/100,000 Japanese population/year, accounting for 0.22% of all thyrotoxic patients and 5.4% of thyrotoxic patients admitted to the hospital (55). In the same series, the mean age of TS patients was 45 years, ranging 6–87 years, without differences between men and women (55).
Prognosis is unfavorable in many cases, unless an adequate treatment is quickly done (56). Indeed, adequate therapy reduces TS mortality to 10% cases approximately (56, 57). Death occurs because of multi-organ failure in about 25% of patients and because of CHF in 1 out of 5 cases (55); respiratory failure, arrhythmia, disseminated intravascular coagulation, gastro-intestinal tract perforation, and sepsis represent the cause of death in the remaining cases (55).
Etiology
Why serum FT4 and FT3 concentrations in TS patients are as high as in hyperthyroid ones without TS, is still a matter of debate. Indeed, the clinical picture characteristic of TS is not related to thyroid hormone levels (55). Nonetheless, patients presenting with TS have a larger amount of catecholamine binding sites ubiquitously than hyperthyroid subjects who do not develop it.
In accordance with the long-standing evidence on the interrelations between thyroid hormone and catecholamines (58), the most agreed etiopathogenetic hypothesis is that, in presence of both a larger availability of adrenergic receptors and a reduction of thyroid hormone binding to TBG (thyroid hormone binding globulin), the leak of catecholamine provoked by an acute event (i.e., triggering factor) finally precipitates TS.
Pregnancy and the post-partum period are both at high risk of TS occurrence (59–61). Indeed, on the one hand, the altered coagulation state peculiar to pregnancy might be worsened by thyrotoxicosis (see Diagnosis section); on the other hand, immune system modulation – and “re-modulation” in the post-natal phase – may give origin to a new-onset autoimmune hyperthyroidism, or reawaken a previously present (i.e., pre-conception) one (59–62).
Recent radioiodine treatment of severely hyperthyroid patients (i.e., subjects manifesting marked signs and symptoms of thyrotoxicosis, suppressed TSH, markedly elevated free T4 and/or free T3, and elevated radioactive iodine uptake) represents a risk factor for the development of TS (63–67). However, it has been demonstrated that it is safe to administer I-131 to patients who are severely hyperthyroid without fear of TS, provided beta blockade drugs are used to control the signs and symptoms (67).
Furthermore, external radiation therapy for neck neoplasm might cause thyroid follicle rupture and, consequently, the leakage of large amounts of pre-formed thyroid hormones into the systemic blood circulation, finally precipitating TS (67). The same mechanism underlies severe thyrotoxicosis and thyrotoxic coma seldom provoked by acute (68) or subacute (69, 70) thyroiditis. In contrast, the etiopathogenesis of the TS occurring in patients with partial hydatiform mole (71) can be ascribed to thyroid hyperfunction with high 24-h RAIU, following beta-HCG follicular cell stimulation.
Anecdotal cases of accidental (72) or voluntary (73) thyroid hormone ingestion unleashing TS have been reported. Finally, analogously to what happens in HC, the use of tyrosine-kinase inhibitors, particularly sorafenib (74), may trigger TS in cancer patients.
Diagnosis
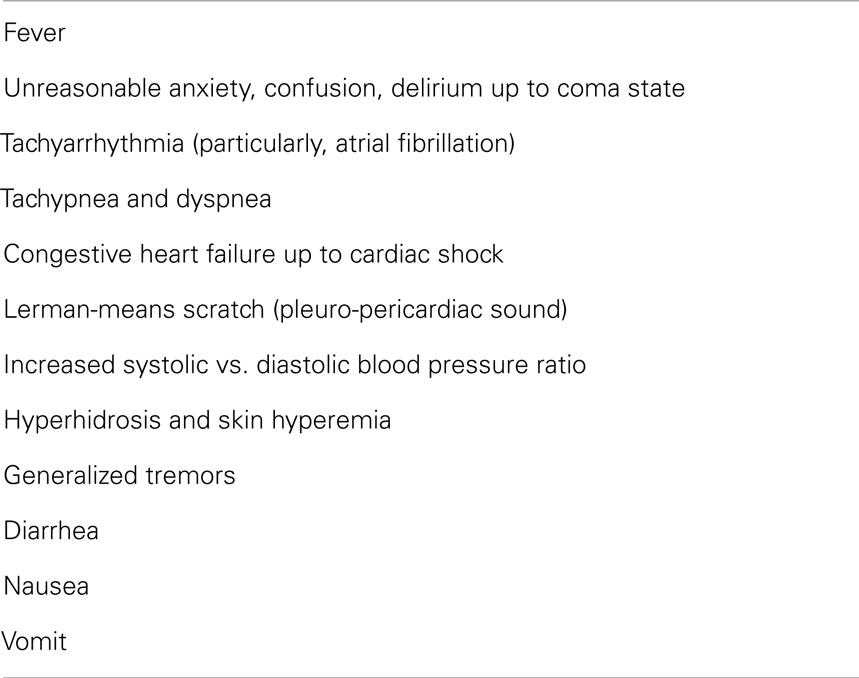
Clinically, TS presents as multi-organ failure in most cases (75, 76). The organs mainly affected by thyroid hormone excess are the heart – tachyarrhythmia ranging from sinus tachycardia to atrial fibrillation is invariably present (77–79), the nervous system (80, 81), the gastro-intestinal tract (82, 83), and the liver (79, 84). Fever is also a frequent event (85, 86).
History of recent traumas in the neck area should always be searched for in patients without history of thyroid disease (87–90). Occasionally, the clinical picture of apathetic thyrotoxicosis occurs, in particular in older patients (91).
The diagnosis of TS is based on: (1) history of thyroid disease and eventual triggering factors; (2) typical signs and symptoms (Table 3); (3) serum FT4 and FT3 concentrations exceeding the normal reference range and undetectable (<0.1 mIU/L) TSH levels (92).
Besides altered thyroid function tests, elevation of serum bilirubin levels (seldom associated to jaundice) and transaminase (93), hyperglycemia, low total cholesterol values and electrolyte imbalance (in Asian men, hypokalemia and associated periodic paralysis) are frequently detected. Total serum bilirubin level concentrations >3 mg/dl have been associated to high risk of death in TS patients, independently of jaundice (55).
Nonetheless, an altered coagulation state – in particular, antithrombin deficiency and increased levels of factor VIII – has been observed in patients with TS (94, 95), sometimes inducing disseminated intravascular coagulation (79, 96). Individuals exceptionally presenting with hypoglycemia, associated (97) or not (79) to lactic acidosis, have been described, as well.
The diagnostic criteria for TS were indicated in 1969 by Mazzaferri et al. (98) who included temperature ≥100°F (37.8°C), marked tachycardia, accentuated signs, and symptoms of thyrotoxicosis and evidence of dysfunction in one more of the central nervous, cardiovascular, or gastro-intestinal systems.
Burch and Wartofsky (56) introduced a punctual score system to identify TS patients definitely. Such score system was composed of the following items: temperature (ranging from 37.2 to >40°C); central nervous system effects (from mild agitation to coma, passing through the psychotic state); gastro-intestinal-hepatic dysfunction (including diarrhea, nausea/vomiting, abdominal pain, and unexplained jaundice); heart rate (ranging 90 to ≥140 bpm); atrial fibrillation; heart failure (low score pedal edema – high score pulmonary edema); and negative or positive precipitant history. A score of 45 or more is highly suggestive of TS; a score of 25–44 supports the diagnosis; a score below 25 makes TS unlikely.
Recently, Akamizu et al. (55) have reported the diagnostic criteria of TS based on Japanese Nationwide surveys. They utilized five symptoms – i.e., central nervous system manifestations, fever (≥38°C), tachycardia (≥130 bpm), CHF, and gastrointestinal (GI)-hepatic manifestations – to define diagnostic criteria for “definite” (TS1) or “suspected” (TS2) cases of TS. Based on the combination of thyrotoxicosis and the above mentioned symptoms, they defined TS1 patients as those presenting with thyrotoxicosis and at least three combinations of fever, tachycardia, CHF, or GI/hepatic manifestations. Suspicion for TS (TS2 cases) should raise when patients meet the diagnostic criteria for TS1, except that FT3 or FT4 values are not available but data before or after the episode suggest that they are thyrotoxic at the time of TS.
Differences in TS diagnostic criteria between Burch and Wartofsky (56) and Akamizu et al (55) are reported in Table 4. Actually, the score system for TS proposed by Akamizu neither significantly differs from, nor adds substantial advantages to, the prior Burch–Wartofsky criteria. Nevertheless, it should be highlighted that Japanese population enrolled in Akamizu et al.’s study is different from American and European populations by the genetic point of view. One of possible examples of such a difference is that, in their series, TS patients affected by Graves’ disease exceeded 95%, whereas in non-Asian series – particularly in those from Europe, where areas of mild to moderate iodine insufficiency are still present – a higher incidence of patients with toxic nodular goiter, iodine-induced hyperthyroidism, or destructive thyroiditis should be expected. Therefore, certain clinical manifestations, like as jaundice, may be expressed at different degrees in Asian compared to non-Asian patients; analogously, a difference in patient’s response to treatment should be taken into account, too.
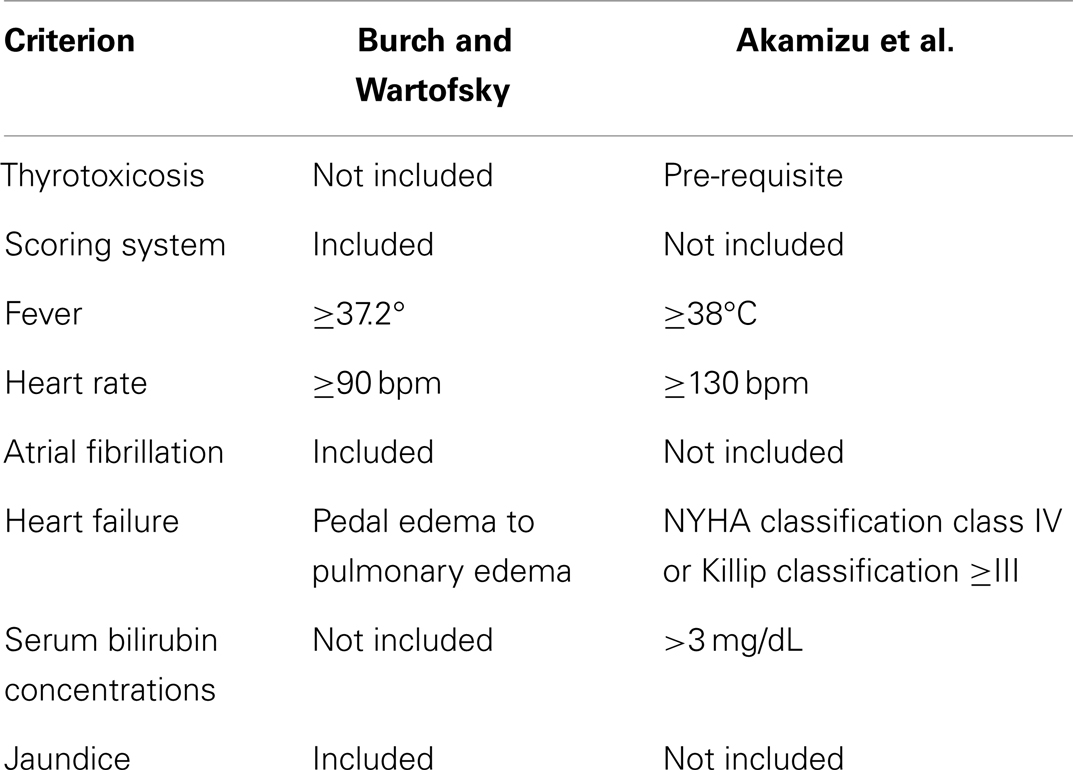
Table 4. Differences in diagnostic criteria for thyrotoxic storm between Burch and Wartofsky and the Japanese survey.
Treatment
The initial treatment of TS is resuscitation. Patients are at high risk of severe hypoxemia and tissue ischemia and need oxygen (Figure 2). Most of them need intubation and mechanic ventilation. Furthermore, it is mandatory to start fluid infusion and electrolyte correction as soon as possible.
Specific TS treatment – i.e., therapy given to reduce thyroid hormone excess in the bloodstream and its deleterious peripheral effects – relies on the following medications, in order of importance (99–101):
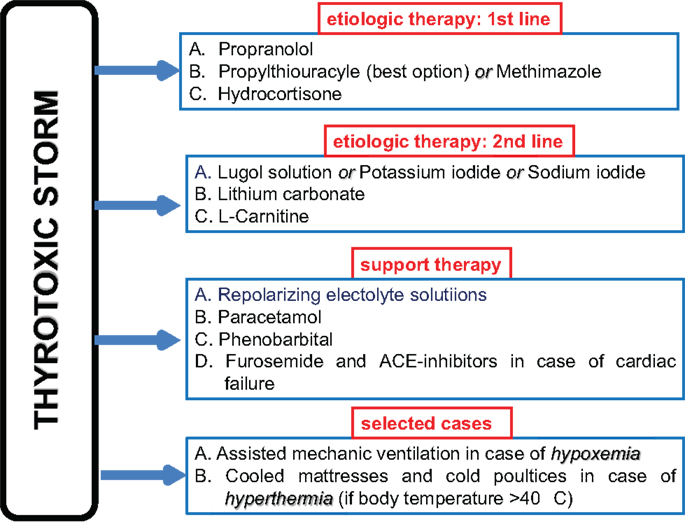
1. Beta-blockers: propranolol 1–2 mg intravenously or 40–80 mg per os every 8 h is the drug of choice because, on the one hand, contrasts the increased binding of catecholamine to beta-adrenergic receptors, on the other hand, reduces the T4 to T3 peripheral deiodination.
2. Thyrostatics: methimazole 15–20 mg every 6 h or propylthyouracyle with a loading dose of 500–1000 mg followed by 250 mg every 4 h (15, 102). The latter should be preferred because it reduces the T4 to T3 peripheral deiodination. However, methimazole may be given intravenously in case the patient is not able to swallow and a total parenteral nutrition is needed. It should be mentioned that rectal administration of both methimazole and propylthyouracyle is allowed, at a dose of 400–600 mg every 6 h and 20–40 mg every 8–6 h, respectively.
3. Large iodine amount: Lugol solution (10 drops three times daily) or saturated potassium iodide solution (5 drops three times daily) per os or sodium iodide 500–1000 mg daily intravenously inhibits thyroid hormone leakage by the thyroid gland. Iodine should be administered not sooner than 1 h after anti-thyroid drug administration.
4. Glucocorticoids: hydrocortisone 100 mg intravenously every 6–8 h reduces the T4 to T3 peripheral deiodination.
5. Lithium carbonate: 300 mg every 6–8 h inhibits proteolysis of colloid, and is administered as an alternative to inorganic iodine to limit the release of pre-formed thyroid hormone into the bloodstream.
Extracorporeal plasmapheresis is an additional tool for removing circulating thyroxine in patients who do not respond quickly to conventional standard therapy (103). Besides, a support therapy including liquids (particularly, repolarizing electrolyte solutions), antipyretics (e.g., paracetamol) and phenobarbital (which plays a sedative role and reduces serum FT4 and FT3 concentrations by increasing thyroid hormone metabolism) may be of benefit. Because salicylates compete with thyroid hormone for binding to carrier proteins and may cause a transient increase in serum free T4 concentrations, their use should be avoided in the treatment of TS. l-Carnitine, a naturally occurring inhibitor of thyroid hormone nuclear uptake, was able to reverse and prevent symptoms of hyperthyroidism in a randomized, double-blind, and placebo-controlled clinical trial (104), and has been effectively used in successive thyroid storms in association with low doses of methimazole (105).
If CHF is present, not only liquid should be administered cautiously, but large amounts of diuretics (intravenous furosemide) are frequently required in association with specific cardiac support therapy (78). Indeed, cardiac insufficiency in TS patients is mostly categorized as NYHA functional class IV and/or Killip class III or IV, and many patients manifest pulmonary edema and high output-induced cardiogenic shock (55). ACE-inhibitors in association with beta-blockers are the cornerstones of therapeutic strategy in TS patients with heart failure, as much as heart rate (≥130 bpm) tachycardia occurs.
Atrial fibrillation is observed in up to 40% of TS patients (55), in whom heparin therapy is obviously indicated; however, changes occurring in patients with TS may lead to heparin resistance and pro-coagulation state (94–96).
High body temperatures must be reduced by cooled mattresses and cold poultices. Moreover, in the presence of infections, soon after blood and urine specimens have been obtained for cultures, large spectrum antibiotics should be promptly started waiting for culture results.
In the TS Japanese series, factors relevant to the mortality were presence of multi-organ failure; presence of disseminated intravascular coagulation; and presence of shock. In the same series, Glasgow Coma Scale and blood urea nitrogen values predicted irreversible damages (55). Thus, these conditions should be recognized and adequately treated.
Once the patient is stable, the differential diagnosis of thyroid disease underlying TS should be accurately investigated, with the aim of distinguishing thyroid hyperfunction, destructive thyroiditis or thyrotoxicosis factitia. Based on the patient’s history and clinical presentation, the work-up should consider the use of thyroid ultrasound, possibly including color-power imaging for evaluation of gland’s volume, echogenicity, vascularity, and presence of nodular disease. Serum anti-TSH receptor autoantibody concentrations should be measured in cases suspected, and without previous history, of Graves’ disease. If destructive thyroiditis or thyrotoxicosis factitia is suspected, serum thyroglobulin measurement and, in the latter, measurement of fecal thyroid hormone excretion should be obtained; nonetheless, in both cases Tc99m thyroid scan should detect scanty/absent radionuclide uptake in the thyroid bed.
Compressive Symptoms Caused by Huge Goiter and Aggressive Thyroid Tumors
Nowadays, patients affected by goiter – diffuse or nodular – are rarely submitted to emergency thyroidectomy or tracheotomy due to compressive symptoms on esophagus and/or trachea, such as dysphagia, dysphonia, and dyspnea. Indeed, appropriate therapeutic strategies (e.g., surgery or radioiodine therapy) are usually applied before massive goiter development.
The disease mainly affects female gender and the prognosis is usually favorable, except for malignancies (106, 107).
Etiology
Thyroid disorders eventually causing emergency due to goiter mass-effect are the following: long-lasting benign huge goiters, for which patients previously refused surgery or radioiodine therapy (6, 108–110); massive hematoma occurring within a thyroid nodule (111, 112); primary malignancies of the thyroid gland (particularly, anaplastic carcinoma) (113); metastases to thyroid (114); and fibrous Riedel’s thyroiditis (115).
Diagnosis and Therapy
The diagnosis of goiter-related compressive symptoms is based on symptoms and signs complained by patients. Imaging examinations either confirm the clinical suspicion or show the surgeon goiter’s extension toward the neighboring structures, and particularly cleavage plans. The clinical picture is characterized by dyspnea with hypoxemia and respiratory acidosis, stridor, dysphonia, dysphagia, and difficult or impossible extension/flexion of the neck (6, 108–110). Rarely, superior vena cava syndrome (116, 117) or even chylothorax (118) occur. Huge mediastinal goiter causing precordial pain has been reported in one patient (119).
Symptoms of compression of the tracheal tree might be relieved by the administration of a CPAP mask. This notwithstanding, surgery represents the gold standard treatment in patients with massive goiter (120, 121). Ultrasound, CT and magnetic resonance (MRI) of the neck represent useful diagnostic tools before surgery (122, 123). Some ultrasound aspects (hypoechogenicity, punctate calcifications, undefined margins, high intranodal vascularity, and more tall than width lesion) are peculiar to thyroid malignant nodules (122), and suggest the need of performing fine needle aspiration biopsy. Indeed, a pre-surgical diagnosis of thyroid malignancy obtained by cytological examination should induce the surgeon to perform a total rather than a decompressive only, partial thyroidectomy. On the contrary, in case of benign goiter, surgery should be more limited (e.g., sub-total or near-total thyroidectomy), to reduce the risk of damaging one or both recurrent laryngeal nerves and/or causing permanent hypoparathyroidism.
99mTc or radioiodine (123I or 131I) thyroid scan is indicated to detect hot nodules and intrathoracic goiter (109, 123). Indeed, in patients who present huge toxic multinodular goiter and are at high surgical risk and/or refuse operation, radioiodine (131I) therapy should obtain good results on compressive symptoms (109, 124). Recently, radioiodine therapy and percutaneous laser ablation have been proposed as helpful alternative methods to effectively reduce goiter size in subjects affected by euthyroid nodular goiter containing “cold” nodules on thyroid scan (125–127).
In patients affected by Riedel’s thyroiditis, a rare form of fibrosing thyroiditis, when therapy with glucocorticoids fails to improve the clinical picture and symptoms are so severe that surgery is mandatory, isthmus resection is usually enough to relieve compressive complaints (115).
Urgent tracheotomy is performed in cases of anaplastic thyroid carcinoma (113), poorly differentiated thyroid carcinoma or thyroid metastases (114), when the neoplasm is not confined to the neck or invades surrounding tissues so widely that cleavage plans are undetectable, but palliative surgery is mandatory due to acute respiratory insufficiency. Patients with massive goiter have an extremely high risk for local complications when submitted to tracheotomy: therefore, if any, this procedure should be performed in an operating room by skilled surgeons.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sarlis NJ, Gourgiotis L. Thyroid emergencies. Rev Endocr Metab Disord (2003) 4:129–36. doi: 10.1023/A:1022933918182
2. Kearney T, Dang C. Diabetic and endocrine emergencies. Postgrad Med J (2007) 83:79–86. doi:10.1136/pgmj.2006.049445
3. Burger AG, Philippe J. Thyroid emergencies. Baillieres Clin Endocrinol Metab (1992) 6:77–93. doi:10.1016/S0950-351X(05)80332-5
4. Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am (2006) 35:663–86. doi:10.1016/j.ecl.2006.09.008
5. Mitchell JM. Thyroid disease in the emergency department. Thyroid function tests and hypothyroidism and myxedema coma. Emerg Med Clin North Am (1989) 7:885–902.
7. Papi G, Pearce EN, Braverman LE, Betterle C, Roti E. A clinical and therapeutic approach to thyrotoxicosis with thyroid-stimulating hormone suppression only. Am J Med (2005) 118:349–61. doi:10.1016/j.amjmed.2005.01.004
8. Bolaert K, Franklyn JA. Thyroid hormone in health and disease. J Endocrinol (2005) 187:1–15. doi:10.1677/joe.1.06131
9. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev (2008) 29:76–131. doi:10.1210/er.2006-0043
10. Papi G, degli Uberti ED, Betterle C, Carani C, Pearce EN, Braverman LE, et al. Subclinical hypothyroidism. Curr Opin Endocrinol Diabetes Obes (2007) 14:197–208. doi:10.1097/MED.0b013e32803577e7
11. Wartofsky L, Van Nostrand D, Burman KD. Overt and “subclinical” hypothyroidism in women. Obstet Gynecol Surv (2006) 61:535–42. doi:10.1097/01.ogx.0000228778.95752.66
12. LeGrys VA, Hartmann K, Walsh JF. The clinical consequences and diagnosis of hyperthyroidism. Clin Lab Sci (2004) 17:203–8.
13. Farwell AP. Euthyroid sick syndrome in the intensive care unit. In: Irwin RS, Rippe JM, editors. Irwin and Rippe’s Intensive Care Medicine. Philadephia, PA: Lippincott Williams & Wilkins (2003). p. 1205–16.
14. DeGroot LJ. “Non-thyroidal illness syndrome” is functional central hypothyroidism, and if severe, hormone replacement is appropriate in light of present knowledge. J Endocrinol Invest (2003) 26:1163–70. doi:10.1007/BF03349151
15. Klubo-Gwiezdzinska J, Wartofsky L. Thyroid emergencies. Med Clin North Am (2012) 96:385–403. doi:10.1016/j.mcna.2012.01.015
16. Boelen A, Kwakkel J, Fliers E. Beyond low plasma T3: local thyroid hormone metabolism during inflammation and infection. Endocr Rev (2011) 32:670–93. doi:10.1210/er.2011-0007
17. Peeters RP. Non thyroidal illness: to treat or not to treat? Ann Endocrinol (Paris) (2007) 68:224–8. doi:10.1016/j.ando.2007.06.011
18. Chopra IJ. Clinical review 86: euthyroid sick syndrome: is it a misnomer? J Clin Endocrinol Metab (1997) 82:329–34. doi:10.1210/jcem.82.2.3745
19. Afandi B, Vera R, Shussler GC, Yap MG. Concordant decreases of thyroxine and thyroxine binding protein concentrations during sepsis. Metabolism (2000) 49:753–4. doi:10.1053/meta.2000.6239
20. Pontecorvi A, Lakshmanan M, Robbins J. Intracellular transport of 3,5,3’-triiodo-L-thyronine in rat skeletal myoblasts. Endocrinol (1987) 121:2145–52.
21. Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab (2010) 95:2529–35. doi:10.1210/jc.2010-0180
22. Van den Berghe G, de Zegher F, Lauwers P. Dopamine and the sick euthyroid syndrome in critical illness. Clin Endocrinol (Oxf) (1994) 41:731–7. doi:10.1111/j.1365-2265.1994.tb02787.x
23. Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones (Athens) (2011) 10:117–24. doi:10.14310/horm.2002.1301
24. Bello G, Palliani G, Annetta MG, Pontecorvi A, Antonelli M. Treating nonthyroidal illness syndrome in the critically ill patient: still a matter of controversy. Curr Drug Targets (2009) 10:778–87. doi:10.2174/138945009788982414
25. Chabot J. Myxedematous coma and terminal forms of untreated myxedema. Cah Coll Med Hop Paris (1964) 258:215–20.
27. Turhan NO, Kockar MC, Inegol I. Myxematous coma in a laboring woman suggested a pre-eclamptic coma: a case report. Acta Obstet Gynecol Scand (2004) 83:1089–91. doi:10.1111/j.0001-6349.2004.0122a.x
28. Lanska D, Harsch HH. Hypothermic coma associated with thioradizine in a myxedematous patient. J Clin Psychiatry (1984) 45:188–9.
29. Moore AP, Macfarlane IA, Blumhardt LD. Neuroleptic malignant syndrome and hypothyroidism. J Neurol Neurosurg Psychiatry (1990) 53:517–8. doi:10.1136/jnnp.53.6.517
30. Shaheen M. Severe congestive heart failure patient on amiodarone presenting with myxedemic coma: a case report. Indian Heart J (2009) 61:392–3.
31. Church CO, Callen EC. Myxedema coma associated with combination aripiprazole and sertraline therapy. Ann Pharmacother (2009) 43:2113–6. doi:10.1345/aph.1M369
33. Finzi G, Calamo A, Chiodera P, Ponari O. Myxedematous coma due to transitory secondary hypothyroidism. Description of a case. Ann Ital Med Int (1991) 6:248–50.
34. Barbesino G. Drugs affecting thyroid function. Thyroid (2010) 20:763–70. doi:10.1089/thy.2010.1635
35. Lazarus JH. Lithium and thyroid. Best Pract Res Clin Endocrinol Metab (2009) 23:723–33. doi:10.1016/j.beem.2009.06.002
36. Mannavola D, Coco P, Vannucchi G, Bertuelli R, Carletto M, Casali PG, et al. A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab (2007) 92:3531–4. doi:10.1210/jc.2007-0586
37. Chu M, Seltzer TF. Myxedema coma induced by ingestion of raw bok choy. N Engl J Med (2010) 362:1945–6. doi:10.1056/NEJMc0911005
38. Laurberg P, Andersen S, Knudsen N, Ovesen L, Nøhr SB, Bülow Pedersen I. Thiocyanate in food and iodine in milk: from domestic animal feeding to improved understanding of cretinism. Thyroid (2002) 12:897–902. doi:10.1089/105072502761016520
39. Spittle L. Diagnoses in opposition: thyroid storm and myxedema coma. AACN Clin Issues Crit Care Nurs (1992) 3:200–8.
40. Skowsky RH, Kikuchi TA. The role of vasopressin in the impaired water excretion of myxedema. Am J Med (1978) 64:613–21. doi:10.1016/0002-9343(78)90581-8
41. DeRubertis FR Jr, Michelis MF, Bloom ME, Mintz DH, Field JB, Davis BB. Impaired water excretion in myxedema. Am J Med (1971) 51:41–53. doi:10.1016/0002-9343(71)90322-6
42. Ardalan MR, Ghabili K, Mirnour R, Shoja MM. Hypothyroidism-induced rhabdomyolisis and renal failure. Ren Fail (2011) 33:553–4. doi:10.3109/0886022X.2011.569109
43. Holvey DN, Goodner CJ, Nikoloff JT, Dowling JT. Treatment of myxedema coma with intravenous thyroxine. Arch Intern Med (1964) 113:139–46. doi:10.1001/archinte.1964.00280070091015
44. Klubo-Gwiezdzinska J, Wartofsky L. Myxedema coma. In: Wass JAH, Stewart PM, editors. 2nd ed. Oxford Textbook of Endocrinology and Diabetes. Oxford: Oxford University Press (2011). p. 537–43.
45. Michels AW, Gottlieb PA. Autoimmune polyglandular syndromes. Nat Rev Endocrinol (2010) 6:270–7. doi:10.1038/nrendo.2010.40
46. Eipe N, Murto K. Adrenal insufficiency and thyroid replacement therapy. Paediatr Anaesth (2009) 19:422–3. doi:10.1111/j.1460-9592.2009.02946.x
47. Shuvy M, Shifman OE, Nusair S, Pappo O, Lotan C. Hypothyroidism-induced myocardial damage and heart failure: an overlooked entity. Cardiovasc Pathol (2009) 18:183–6. doi:10.1016/j.carpath.2007.12.015
48. Ringel MD. Management of hypothyroidism and hyperthyroidism in the intensive care unit. Crit Care Clin (2001) 17:59–74. doi:10.1016/S0749-0704(05)70152-4
49. Manfredi E, van Zaane B, Gerdes VE, Brandjes DP, Squizzato A. Hypothyroidism and acquired von Willebrand’s syndrome: a systematic review. Haemophilia (2008) 14:423–33. doi:10.1111/j.1365-2516.2007.01642.x
51. McKeown NJ, Tews MC, Gossain VV, Shah SM. Hyperthyroidism. Emerg Med Clin North Am (2005) 23:669–85. doi:10.1016/j.emc.2005.03.002
54. Shaked Y, Samra Y, Zwas ST. Graves’ disease presenting as pyrexia of unknown origin. Postgrad Med J (1988) 64:209–12. doi:10.1136/pgmj.64.749.209
55. Akamizu T, Satoh T, Iozaki O, Suzuki A, Wakino S, Iburi T, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid (2012) 22:661–79. doi:10.1089/thy.2011.0334
56. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am (1993) 22:263–77.
57. Trasciatti S, Prete C, Palummeri E, Foppiani L. Thyroid storm as precipitating factor in onset of coma in an elderly woman: case report and literature review. Aging Clin Exp Res (2004) 16:490–4. doi:10.1007/BF03327408
58. Waldstein SS. Thyroid-catecholamine interrelations. Annu Rev Med (1966) 17:123–32. doi:10.1146/annurev.me.17.020166.001011
59. Waltman PA, Brewer JM, Lobert S. Thyroid storm during pregnancy. A medical emergency. Crit Care Nurse (2004) 24:74–9.
60. Molitch ME. Endocrine emergencies in pregnancy. Baillieres Clin Endocrinol Metab (1992) 6:167–91. doi:10.1016/S0950-351X(05)80337-4
61. Daly MJ, Wilson CM, Dolan SJ, Kennedy A, McCance DR. Reversible dilated cardiomyopathy associated with post-partum thyrotoxic storm. QJM (2009) 102:217–9. doi:10.1093/qjmed/hcn173
62. Bagtharia S, Goyal V, Chakrabarti R, Utting H. Ruptured ectopic pregnancy concealing thyroid storm. J Obstet Gynaecol (2007) 27:213–4. doi:10.1080/01443610601157828
63. Kadmon PM, Noto RB, Boney CM, Goodwin G, Gruppuso PA. Thyroid storm in a child following radioactive iodine (RAI) therapy: a consequence of RAI versus withdrawal of antithyroid medication. J Clin Endocrinol Metab (2001) 86:1865–7. doi:10.1210/jcem.86.5.7473
64. McDermott MT, Kidd GS, Dodson LE Jr, Hofeldt FD. Radioiodine-induced thyroid storm. Case report and literature review. Am J Med (1983) 75:353–9. doi:10.1016/0002-9343(83)90950-6
65. Thebault C, Leurent G, Potier J, Bedossa M, Bonnet F. A case of thryoid storm following radioiodine therapy underlying usefulness of cardiac MRI. Eur J Intern Med (2009) 20:136–7. doi:10.1016/j.ejim.2008.12.014
66. Vijayakumar V, Nusynowwitz ML, Ali S. Is it safe to treat hyperthyroid patients with I-131 without fear of thyroid storm? Ann Nucl Med (2006) 20:383–5. doi:10.1007/BF03027372
67. Diaz R, Blakey MD, Murphy PB, Cryar AK, Cmelak AJ. Thyroid storm after intensity-modulated radiation therapy: a case report and discussion. Oncologist (2009) 14:233–9. doi:10.1634/theoncologist.2008-0156
68. Al-Kordi RS, Alenizi E, Elgazzar AH. Acute suppurative thyroiditis with abscess, gas formation, and thyrotoxic crisis. Nuklearmedizin (2008) 47:N44–6.
69. Sherman SI, Ladenson PW. Subacute thyroiditis causing thyroid storm. Thyroid (2007) 17:283. doi:10.1089/thy.2007.0070
70. Swinburne JL, Kreisman SH. A rare case of subacute thyroiditis causing thyroid storm. Thyroid (2007) 17:73–6. doi:10.1089/thy.2006.0140
71. Chiniwala NU, Woolf PD, Bruno CP, Kaur S, Spector H, Yacono K. Thyroid storm caused by a partial hydatiform mole. Thyroid (2008) 18:479–81. doi:10.1089/thy.2007.0212
72. Majlesi N, Greller HA, McGuigan MA, Caraccio T, Su MK, Chan GM. Thyroid storm after pediatric levothyroxine ingestion. Pediatrics (2010) 126:e470–3. doi:10.1542/peds.2009-2138
73. Hartung B, Schott M, Daldrup T, Ritz-Timme S. Lethal thyroid storm after uncontrolled intake of liothyronine in order to lose weight. Int J Legal Med (2010) 124:637–40. doi:10.1007/s00414-010-0423-y
74. Haraldsdottir S, Li Q, Villalona-Calero MA, Olencki TE, Kendra K, Ing SW. Case of sorafenib-induced thyroid storm. J Clin Oncol (2013) 31:e262–4. doi:10.1200/JCO.2012.46.7142
75. Chong HW, See KC, Phua J. Thyroid storm with multiorgan failure. Thyroid (2010) 20:333–6. doi:10.1089/thy.2009.0181
76. Jiang YZ, Hutchinson KA, Bartelloni P, Manthous CA. Thyroid storm presenting as multiple organ dysfunction syndrome. Chest (2000) 118:877–9. doi:10.1378/chest.118.3.877
77. Martinez-Diaz GJ, Formaker C, Hsia R. Atrial fibrillation from thyroid storm. J Emerg Med (2012) 42:e7–9. doi:10.1016/j.jemermed.2008.06.023
78. Ngo AS, Lung Tan DC. Thyrotoxic heart disease. Resuscitation (2006) 70:287–90. doi:10.1016/j.resuscitation.2006.01.009
79. Kobayashi C. Severe starvation hypoglycemia and congestive heart failure induced by thyroid crisis, with accidentally induced severe liver dysfunction and disseminated intravascular coagulation. Intern Med (2005) 44:234–9. doi:10.2169/internalmedicine.44.234
80. Abbasi B, Sharif Z, Sprabery LR. Hypokalemic thyrotoxic periodic paralysis with thyrotoxic psychosis and hypercapnic respiratory failure. Am J Med Sci (2010) 340:147–53. doi:10.1097/MAJ.0b013e3181cbf567
81. Harris C. Recognizing thyroid storm in the neurologically impaired patient. J Neurosci Nurs (2007) 39:40–2. doi:10.1097/01376517-200702000-00008
82. Hsiao FC, Hung YJ, Hsieh CH, Wu LY, Shih KC, He CT. Abdominal pain and multiorgan dysfunction syndrome in a young woman. Am J Med Sci (2007) 334:399–401. doi:10.1097/MAJ.0b013e3180a7268b
83. Karanikolas M, Velissaris D, Karamouzos V, Filos KS. Thyroid storm presenting as intra-abdominal sepsis with multi-organ failure requiring intensive care. Anaesth Intensive Care (2009) 37:1005–7.
84. Choudhary AM, Roberts I. Thyroid storm presenting with liver failure. J Clin Gastroenterol (1999) 29:318–21. doi:10.1097/00004836-199912000-00004
85. McGugan EA. Hyperpyrexia in the emergency department. Emerg Med (Fremantle) (2001) 2001(13):116–20. doi:10.1046/j.1442-2026.2001.00189.x
86. Shaked Y, Samra Y, Zwas ST. Graves’ disease presenting as pyrexia of unknown origin. Postgrad Med J (1998) 64:209–12. doi:10.1136/pgmj.64.749.209
87. Delikoukos S, Mantzos F. Thyroid storm induced by blunt thyroid gland trauma. Am Surg (2007) 73:1247–9.
88. Hagiwara A, Murata A, Matsuda T, Sakaki S, Shimazaki S. Thyroid storm after blunt thyroid injury: a case report. J Trauma (2007) 63:E85–7. doi:10.1097/01.ta.0000232184.07317.56
89. Vora NM, Fedok F, Stack BC Jr. Report of a rare case of trauma-induced thyroid storm. Ear Nose Throat J (2002) 81:570–2.
90. Hughes SC, David LA, Turner R. Storm in a T-CUP: thyroid crisis following trauma. Injury (2003) 34:946–7. doi:10.1016/S0020-1383(02)00388-1
91. Ghobrial MW, Ruby EB. Coma and thyroid storm in apathetic thyrotoxicosis. South Med J (2002) 95:552–4. doi:10.1097/00007611-200205000-00019
92. Pimentel L, Hansen KN. Thyroid disease in the emergency department: a clinical and laboratory review. J Emerg Med (2005) 28:201–9. doi:10.1016/j.jemermed.2004.08.020
93. Hull K, Horenstein R, Naglieri R, Munir K, Ghany M, Celi FS. Two cases of thyroid storm-associated cholestatic jaundice. Endocr Pract (2007) 13:476–80. doi:10.4158/EP.13.5.476
94. Belchikov YG, Marotta SE. Heparin management in a patient with thyroid storm. Pharmacotherapy (2010) 30:134e–8e. doi:10.1592/phco.30.4.421
95. Boppidi H, Daram SR. Thyroid dysfunction and the coagulation system: the often ignored link. South Med J (2009) 102:132. doi:10.1097/SMJ.0b013e318186be48
96. Martin D. Disseminated intravascular coagulation precipitated by thyroid storm. South Med J (2009) 102:193–5. doi:10.1097/SMJ.0b013e318183f929
97. Izumi K, Kondo S, Okada T. A case of atypical thyroid storm with hypoglycemia and lactic acidosis. Endocr J (2009) 56:747–52. doi:10.1507/endocrj.K09E-043
98. Mazzaferri EL, Skillman TG. Thyroid storm. A review of 22 episodes with special emphasis on the use of guanethidine. Arch Intern Med (1969) 124:684–90. doi:10.1001/archinte.1969.00300220036006
99. Migneco A, Ojetti V, Testa A, De Lorenzo A, Gentiloni Silveri N. Management of thyrotoxic crisis. Eur Rev Med Pharmacol Sci (2005) 9:69–74.
100. Han YY, Sun WZ. An evidence-based review on the use of corticosteroids in peri-operative and critical care. Acta Anaesthesiol Sin (2002) 40:71–9.
101. Thomas DJ, Hardy J, Sarwar R, Banner NR, Mumani S, Lemon K, et al. Thyroid storm treated with intravenous methimazole in patients with gastrointestinal dysfunction. Br J Hosp Med (Lond) (2006) 67:492–3.
102. Hampton J. Thyroid gland disorder emergencies: thyroid storm and myxedema coma. AACN Adv Crit Care (2013) 24:325–32. doi:10.1097/NCI.0b013e31829bb8c3
103. Koball S, Hickstein H, Gloger M, Hinz M, Henschel J, Stange J, et al. Treatment of thyrotoxic crisis with plasmapheresis and single pass albumin dialysis: a case report. Artif Organs (2010) 34:E55–8. doi:10.1111/j.1525-1594.2009.00924.x
104. Benvenga S, Ruggeri RM, Russo A, Lapa D, Campenni A, Trimarchi F. Usefulness of L-carnitine, a naturally occurring peripheral antagonist of thyroid hormone action, in iatrogenic hyperthyroidism: a randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab (2001) 86:3579–94. doi:10.1210/jcem.86.8.7747
105. Benvenga S, Lapa D, Cannavò S, Trimarchi F. Successive thyroid storm treated with L-carnitine and low doses of methimazole. Am J Med (2003) 115:417–8. doi:10.1016/S0002-9343(03)00399-1
106. Shaha AR, Burnett C, Alfonso A, Jaffe BM. Goiters and airway problems. Am J Surg (1989) 158:378–80. doi:10.1016/0002-9610(89)90137-2
107. Smallridge RC. Metabolic and anatomic thyroid emergencies: a review. Crit Care Med (1992) 20:276–91.
108. Ayabe H, Kawahara K, Tagawa Y, Tomita M. Upper airway obstruction from a benign goiter. Surg Today (1992) 22:88–90. doi:10.1007/BF00326133
109. Margaritora S, Cesario A, Porziella V, Granone P. Huge mediastinal goiter. Eur J Cardiothorac Surg (2003) 23:840. doi:10.1016/S1010-7940(03)00049-6
110. Xu J, Shen B, Li Y, Zhang T. Enormous goiter in posterior mediastinum: report of 2 cases and literature review. J Formos Med Assoc (2009) 108:337–43. doi:10.1016/S0929-6646(09)60075-9
111. Tsilchorozidou T, Vagropoulos I, Karagianidou C, Grigoriadis N. Huge intrathyroidal hematoma causing airway obstruction: a multidisciplinary challenge. Thyroid (2006) 16:795–9. doi:10.1089/thy.2006.16.795
112. Testini M, Gurrado A, Lissidini G, Lardo D, Poli E, Piccinni G. Emergency surgery for acute respiratory failure secondary to spontaneous thyroid hemorrhage. Int Surg (2008) 93:158–62.
113. Neff RL, Farrar WB, Kloos RT, Burman KD. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am (2008) 37:525–38. doi:10.1016/j.ecl.2008.02.003
114. Papi G, Fadda G, Corsello SM, Corrado S, Rossi ED, Radighieri E, et al. Metastases to the thyroid gland: prevalence, clinicopathological aspects and prognosis: a 10-year experience. Clin Endocrinol (Oxf) (2007) 66:565–71.
115. Papi G, LiVolsi VA. Current concepts on Riedel thyroiditis. Am J Clin Pathol (2004) 121(Suppl):S50–63.
116. Padmanabhan H. Superior vena cava syndrome: a presentation of anaplastic thyroid carcinoma. J Clin Oncol (2010) 28:e151–4. doi:10.1200/JCO.2009.24.8740
117. McKellar DP, Verazin GT, Lim KM, Spiegel JC, Block BL. Superior vena cava syndrome and tracheal obstruction due to multinodular goiter. Head Neck (1994) 16:72–4. doi:10.1002/hed.2880160114
118. Darwish BK, Kabbani SS. Giant substernal goiter with chylothorax. Asian Cardiovasc Thorac Ann (2003) 11:165–6. doi:10.1177/021849230301100218
119. Iacobellis G. Huge mediastinal goiter: an unusual cause of precordial pain. Thyroid (2004) 14:635. doi:10.1089/1050725041692855
120. Shaha AR. Surgery for benign thyroid disease causing tracheoesophageal compression. Otolaryngol Clin North Am (1990) 23:391–401.
121. McHenry CR, Piotrowski JJ. Thyroidectomy in patients with marked thyroid enlargement: airway management, morbidity, and outcome. Am Surg (1994) 60:586–91.
122. Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am (2007) 36:707–35. doi:10.1016/j.ecl.2007.04.009
123. Lahr B, Lee JK, Shih WJ. Tc-99m pertechnetate imaging and CT show bilateral huge cervical and intrathoracic goiter extending to the posterior mediastinum. Clin Nucl Med (1996) 21:905–6. doi:10.1097/00003072-199611000-00028
124. Drivas I, Mansberg R, Roberts JM, Kean AM. Massive intrathoracic toxic multinodular goiter treated with radioiodine. Clin Nucl Med (2003) 28:138–9. doi:10.1097/00003072-200302000-00014
125. Kaniuka S, Lass P, Sworczak K. Radioiodine – an attractive alternative to surgery in large non-toxic multinodular goitres. Nucl Med Rev Cent East Eur (2009) 12:23–9.
126. Papini E, Bizzarri G, Pacella CM. Percutaneous laser ablation of benign and malignant thyroid nodules. Curr Opin Endocrinol Diabetes Obes (2008) 15:434–9. doi:10.1097/MED.0b013e32830eb89a
127. Papini E, Guglielmi R, Bizzarri G, Graziano F, Bianchini A, Brufani C, et al. Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid (2007) 17:229–35. doi:10.1089/thy.2006.0204
Appendix
Key Points
• Severe excess or defect of thyroid hormone represent rare conditions jeopardizing the life of patients in most cases.
• It is crucial to differentiate patients with non-thyroidal illness – i.e., affected by the so-called “euthyroid sick syndrome” – by those truly affected by severe thyroid disorders, as the therapeutic approach changes profoundly.
• Both HC and TS are triggered by peculiar precipitating factors, which occur in patients with severe hypothyroidism or thyrotoxicosis, respectively.
• The pillars of HC’s therapy are high-dose l-thyroxine and/or tri-iodothyroinine; i.v. glucocorticoids; treatment of hydro-electrolyte imbalance (mainly, hyponatraemia); treatment of hypothermia; often, endotracheal intubation and assisted mechanic ventilation are needed.
• Therapy of thyroid storm is based on beta-blockers, thyrostatics, and i.v. glucocorticoids; eventually, high-dose of iodide compounds or lithium carbonate may be of benefit.
• Surgery represents the gold standard treatment in patients with euthyroid massive nodular goiter, although new techniques – e.g., percutaneous laser ablation – are helpful in subjects at high surgical risk or refusing operation.
Keywords: hypothyroid coma, thyrotoxic storm, hyperthyroidism, thyrotoxicosis, hypothyroidism, massive goiter
Citation: Papi G, Corsello SM and Pontecorvi A (2014) Clinical concepts on thyroid emergencies. Front. Endocrinol. 5:102. doi: 10.3389/fendo.2014.00102
Received: 28 April 2014; Accepted: 15 June 2014;
Published online: 01 July 2014.
Edited by:
Bernadette Biondi, Federico II University of Naples, ItalyReviewed by:
Maria Moreno, University of Sannio, ItalyGiovanni Vitale, Istituto Auxologico Italiano – Universita’ Degli Studi Di Milano, Italy
Fabio Orlandi, Presidio Ospedaliero Gradenigo, Italy
Copyright: © 2014 Papi, Corsello and Pontecorvi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alfredo Pontecorvi, Department of Endocrinology, Catholic University of Rome, Largo A. Gemelli 1, 00168 Rome, Italy e-mail:cG9udGVjb3J2aUBybS51bmljYXR0Lml0
 Giampaolo Papi
Giampaolo Papi Salvatore Maria Corsello
Salvatore Maria Corsello Alfredo Pontecorvi
Alfredo Pontecorvi