
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 09 April 2012
Sec. Systems Endocrinology
Volume 3 - 2012 | https://doi.org/10.3389/fendo.2012.00052
This article is part of the Research Topic Estrogenic control of hypothalamic GnRH neurons View all 11 articles
Reproductive function is regulated by the secretion of luteinizing hormone (LH) and follicle-stimulating hormone from the pituitary and the steroid hormones from the gonads. The dynamic changes in the levels of the reproductive hormones regulate secondary sex characteristics, gametogenesis, cellular function, and behavior. Hypothalamic GnRH neurons, with cell bodies located in the basal hypothalamus, represent the final common pathway for neuronally derived signals to the pituitary. As such, they serve as integrators of a dizzying array of signals including sensory inputs mediating information about circadian, seasonal, behavioral, pheromonal, and emotional cues. Additionally, information about peripheral physiological function may also be included in the integrative signal to the GnRH neuron. These signals may communicate information about metabolic status, disease, or infection. Gonadal steroid hormones arguably exert the most important effects on GnRH neuronal function. In both males and females, the gonadal steroid hormones exert negative feedback regulation on axis activity at both the level of the pituitary and the hypothalamus. These negative feedback loops regulate homeostasis of steroid hormone levels. In females, a cyclic reversal of estrogen feedback produces a positive feedback loop at both the hypothalamic and pituitary levels. Central positive feedback results in a dramatic increase in GnRH secretion (Moenter et al., 1992; Xia et al., 1992; Clarke, 1993; Sisk et al., 2001). This is coupled with an increase in pituitary sensitivity to GnRH (Savoy-Moore et al., 1980; Turzillo et al., 1995), which produces the massive surge in secretion of LH that triggers ovulation. While feedback regulation of the axis in males is in part mediated by estrogen receptors (ER), there is not a clear consensus as to the relative role of ER versus AR signaling in males (Lindzey et al., 1998; Wersinger et al., 1999). Therefore, this review will focus on estrogenic signaling in the female.
Estrogen has a bimodal effect on the hypothalamus with both an inhibitory and stimulatory influence on GnRH secretion. The stimulatory effect of estrogen is seen at the end of the follicular phase where estrogen triggers the preovulatory GnRH surge (Sarkar et al., 1976; Karsch et al., 1997; Levine, 1997; Herbison, 1998; Simerly, 2002). The ability of estrogen to induce a surge declines with age in female rodents and women (Wise, 1982; Shaw et al., 2011) This appears to be due in large part to a decrease in estrogen induction of GnRH neurons, particularly in rodents (Downs and Wise, 2009), although a decline in pituitary responsiveness to GnRH contributing to attenuated luteinizing hormone (LH) surges has also been proposed (Shaw et al., 2009).
The inhibitory effect of estrogen on GnRH secretion and GnRH gene expression has been shown in in vivo studies in several mammalian species (Zoeller et al., 1988; Petersen et al., 1995). Studies in ewes indicate that estrogen inhibits GnRH pulse amplitude in the early follicular and luteal phase of the cycle (Caraty et al., 1989; Chongthammakun and Terasawa, 1993). In vivo and in vitro studies in the rat hypothalamus (Sarkar and Fink, 1980; Spratt and Herbison, 1997) indicate that estrogen inhibits GnRH mRNA expression and that this effect is localized to the rostral preoptic area of the hypothalamus. The inhibitory effect of estrogen seems to involve different anatomical sites in the hypothalamus than those associated with the stimulatory effect of estrogen on GnRH (Shander and Barraclough, 1980; Wiegand et al., 1980; Wray et al., 1989; Gibson et al., 1997; Caraty et al., 1998), which had indicated that the inhibitory and stimulatory effects may occur independently from one another. Negative feedback was localized to the arcuate and median eminence of the medial basal hypothalamus in these studies while positive feedback was mapped to the preoptic and suprachiasmatic nucleus. It is proposed that the biological substrate for these effects is kisspeptin, the hypothalamic protein previously found to be essential in pubertal onset (de Roux et al., 2003; Seminara et al., 2003). Estrogen has been shown to mediate a decrease in kisspeptin in the arcuate nucleus in contrast to increasing expression in the AVPV (Discussed below: Smith et al., 2006; Dungan et al., 2007).
In vitro evidence of negative estrogen regulation of rat GnRH gene expression includes transfection studies in both placental JEG-3 cells (Wierman et al., 1992) and in GT1-7 GnRH-expressing neuronal cells co-transfected with the estrogen receptor alpha (ER)α cDNA (Kepa et al., 1992). These in vitro studies indicated that estrogen decreases expression of the rat GnRH gene promoter. Studies of the-human GnRH promoter in transient transfection experiments in JEG-3 cells co-transfected with ERα cDNA indicate estrogen-mediated regulation of the-human GnRH promoter (Radovick et al., 1991; Dong et al., 1996). Studies performed by Roy et al. (1999) demonstrated a decrease in GnRH mRNA levels in the GnRH-expressing neuronal cell line, GT1-7, treated with 17β-estradiol over a 48-h time course, starting as early as 12 h. Our laboratory has confirmed and extended these findings and observed a decrease in GnRH expression and secretion by estradiol in both the GN11 and GT1-7 GnRH-expressing cell lines and determined that these effects were primarily mediated by ERα in GT1-7 cells and by both ERα and ERβ in GN11 cells (Ng et al., 2009).
In addition, we have shown that estrogen down-regulates GnRH gene expression in a castration–estrogen replacement paradigm using a transgenic mouse model. This model was developed by targeting GnRH neurons with construct containing the GnRH gene promoter fused to a luciferase reporter gene (Radovick et al., 1994; Wolfe et al., 1995; Kim et al., 2007; −3446/+5-luc mice). These GnRH promoter elements were shown to specifically and reproducibly target hypothalamic GnRH neurons in transgenic mice. After treatment with estradiol, gonadectomized female mice exhibited an 80% reduction in hypothalamic luciferase expression. Although these in vivo studies do not prove that ER directly regulates GnRH expression in the hypothalamus, they do establish that GnRH gene expression is negatively regulated by estrogen at a transcriptional level.
Estrogen is known to exert its effect through binding and activation of ERs. The ER is a member of the superfamily of nuclear receptors (Beato et al., 1995; Mangelsdorf et al., 1995), and is involved in transcriptional regulation of target genes. Two isoforms of ER (ERα and ERβ), with variable tissue distribution, have been described (White et al., 1987; Couse et al., 1997; Tremblay et al., 1997). ER isoforms share a common structural organization that includes six distinct functional domains, A to F, characteristic of members of the superfamily of nuclear receptors (Beato et al., 1995; Mangelsdorf et al., 1995). The A/B domain located at the N-terminal portion of the receptor contains a weak constitutive ligand-independent transcription activating function-l (AF-1); the C domain contains zinc finger-like motifs that are involved in binding to an estrogen response element (ERE); the D domain contains a hinge region that modulates DNA-binding; and the E/F domains contain the ligand-binding domain and a strong ligand-dependent activating function-2 (AF-2). Mouse ERα and ERβ share considerable homology in their DNA and ligand-binding domains (97 and 60% respectively), but display no sequence homology in their amino terminal domains (Tremblay et al., 1997). Mouse ERα has a slightly higher affinity for binding to a consensus ERE when compared to ERβ (Tremblay et al., 1997). Both ER isoforms, however, activate natural estrogen-responsive promoters to a similar extent in transient transfection studies.
The classic ER signaling pathway involves estrogen binding to ERs (Katzenellenbogen et al., 1993; Beato et al., 1995; Mangelsdorf et al., 1995) inducing a conformational change leading to receptor dimerization (Wang et al., 1995; Pettersson et al., 1997; Ogawa et al., 1998) and subsequent binding to an ERE located on promoter regions of target genes (Beato et al., 1995; Mangelsdorf et al., 1995). Binding of the ER complex to DNA activates gene transcription through its activating function domains, AF-1 and AF-2. AF-l and AF-2 can act independently, or synergize with one another to stimulate positive regulation of gene transcription (Tora et al., 1989; Berry et al., 1990). The ability of AF-1 and AF-2 to contribute to ER transcriptional activity seems to be cell- and promoter-specific (Tora et al., 1989; Tzukerman et al., 1994), and involves binding of AF-l and/or AF-2 to cofactors (Tzukerman et al., 1994; Shibata et al., 1997; Tremblay et al., 1999). Co-activators of ER include a family of related proteins known as the p160s: the SRC family, pCIP, and others (Onate et al., 1995; Horwitz et al., 1996; McKenna et al., 1999; Xu and O’Malley, 2002; Smith and O’Malley, 2004); and recruitment of p160 cofactors is sufficient for activation of the ER (Shang et al., 2000). p160s, in turn, interact with other co-activator proteins such as CREB-binding protein (CBP; Chakravarti et al., 1996), p300, and CBP-associated factor (P/CAF; Korzus et al., 1998). Other ER co-activators include RIP140 (Cavailles et al., 1995). Co-repressors of ER have also been described, including SHP (Seol et al., 1998; Johansson et al., 1999), NcoR (Lavinsky et al., 1998), and SMRT (Misiti et al., 1998).
The recent description of a patient with idiopathic hypogonadotropic hypogonadism with a mutation in a G-protein coupled receptor, referred to as GPR54, has added to our knowledge of signaling pathways involved in this complex system (de Roux et al., 2003; Seminara et al., 2003). GPR54 binds kisspeptin (kiss1) to regulate GnRH secretion (Kotani et al., 2001; Smith et al., 2005, 2006; d’Anglemont de Tassigny et al., 2008). Several studies have confirmed that GPR54 co-localizes with GnRH neurons (Irwig et al., 2004; Parhar et al., 2004; Han et al., 2005; Messager et al., 2005; Novaira et al., 2009). Central or systemic administration of kiss1 leads to GnRH and gonadotropin secretion in both prepubertal and adult animals (Gottsch et al., 2004; Matsui et al., 2004; Messager et al., 2005; Navarro et al., 2005; Shahab et al., 2005; Zhang et al., 2008). Confirming that kiss1 lies upstream of the GnRH neuron, mice who have a knock-out of GPR54, as well as humans that have mutations in GPR54, respond normally to GnRH.
In rats, ER isoform selective ligands have provided evidence that ERα is the predominant receptor isoform mediating negative feedback (Sanchez-Criado et al., 2006). However E2 is known to regulate gene transcription by binding to high affinity receptor dimers and by facilitating dimer formation between ERs (Katzenellenbogen et al., 1993). In fact, ERα and ERβ can form heterodimers in tissues where they are co-expressed (Pace et al., 1997; Powell et al., 2010) and the DBD was sufficient to allow for heterodimerization (Pace et al., 1997). This might explain the higher LH levels observed in ERα and ERβ double gene knock-out (KO) mice compared to ERα KO mice (Couse et al., 2003). The studies demonstrating the key role for ERα in negative feedback build on previous studies demonstrating an important role for kisspeptin for LH secretion during the entire estrous cycle despite dynamic changes in estrogen levels (Roa et al., 2006). Kiss1 neurons are activated during the preovulatory period (Smith et al., 2006), and blockade of kiss1 secretion results in a lack of LH surge generation (Kinoshita et al., 2005). Two principal populations of kiss1 neurons are described in the hypothalamus; one in the arcuate nucleus and one in the AVPV which match well with the anatomical loci of estrogen positive and negative feedback discussed above (model is summarized in Figure 1). The arcuate population has been proposed to mediate estrogen negative feedback while the specific subset of neurons in the AVPV have been shown to be critical intermediates in transducing estradiol positive feedback required to induce ovulation (Popolow et al., 1981; Wiegand and Terasawa, 1982; Simerly and Swanson, 1987; Gu and Simerly, 1997). Within the AVPV of female mice, Kiss1-expressing neurons are activated by estrogen (Smith et al., 2005, 2006). Interestingly, perinatal treatment with the environmental estrogenic compound bisphenol A (BPA) produces males with female-like development of the kisspeptin neurons in the AVPV that correlates with the ability of BPA treated male mice to generate LH surges in response to high levels of estrogen (Bai et al., 2011). Kiss1 neurons have been shown to express ERα (and some ERβ), and kiss1 neurons in the AVPV secrete kiss1 in response to estrogen treatment. Since these neurons contact GnRH neurons, it has been proposed that kiss1 neurons in the AVPV are the locus of estrogen positive feedback leading to the gonadotropin surge. This complements studies from Wintermantel et al. (2006) that have shown neuronal ERα was required for generation of the LH surge in mice. The identity of these AVPV neurons is not entirely clear. While some have proposed that both ARC and AVPC kiss1 neurons may be GABA neurons (Petersen et al., 2003; Cravo et al., 2011) or glutaminergic neurons (Cravo et al., 2011) recent compelling evidence exists that a subset of both the ARC and AVPV kiss1 neurons also express galanin (Porteous et al., 2011; Kallo et al., 2012) and that a large number of ARC kiss1, and even some AVPV kiss1 neurons, co-express neurokinin B and dynorphin (Navarro et al., 2009). Whether kisspeptin is permissive or active in regulating GnRH neurons at the time of the LH surge is unknown (Dungan et al., 2007). Nonetheless, GnRH neuronal activity is increased by kiss1 and modulated by changes in estradiol levels (Pielecka-Fortuna et al., 2008).
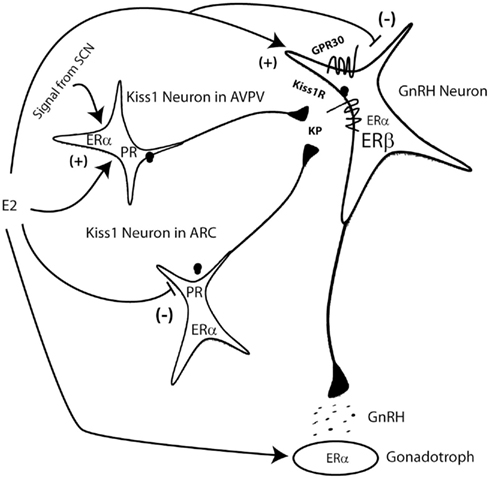
Figure 1. Model of estrogenic regulation of the GnRH neuron. Estrogen (E2) from the ovary regulates the pituitary gonadotroph, the kiss1 neurons in the AVPV and the arcuate (ARC) nuclei and can also directly regulate the GnRH neuron. E2 regulation of kiss1 in both nuclei is regulated by ERα and progesterone receptor (PR). A daily signal from the SCN to the kiss1 neurons of the AVPV helps coordinate surge generation. Direct effects at the level of the GnRH neuron can be mediated by ERα, ERβ, or the membrane associated GPR30. Kiss1 (KP) regulates GnRH neurons by binding to and activating the kiss1 receptor (also known as GPR54).
Although the presence of ERs in the gonadotroph is well accepted and hence estrogen action on the gonadotroph is direct. The mechanism by which estrogen regulates GnRH neurons is not well understood. The more prevalent hypothesis is that the influence of estrogen on GnRH is not direct but is conveyed to GnRH neurons via presynaptic afferents from adjacent cells that express ER (now thought to be kiss1 neurons). This hypothesis is based on several in vivo double-labeling immunohistochemical studies examining the hypothalamus of many mammalian species where co-expression of GnRH and ER was not demonstrated (Shivers et al., 1983; Herbison and Theodosis, 1992; Watson et al., 1992; Herbison et al., 1993, 1995; Kalra, 1993; Lehman and Karsch, 1993; Navas et al., 1995; Sullivan et al., 1995; Lopez et al., 1996; Warembourg et al., 1998; Kelly et al., 2003; Garcia-Segura and McCarthy, 2004). Results from these studies indicate that, although ERs are highly expressed in the hypothalamus, few if any GnRH neurons express ER. Additionally then, the effects of estrogen on GnRH neurons would occur in an indirect manner by ERα and/or ERβ expressing neurons, or glial cells (Rage et al., 1997; Smith and Jennes, 2001; Prevot, 2002; Petersen et al., 2003). However, the original studies may have been hampered by an inability to detect a lower concentration of ERs in tissues, or by the castration paradigms that were used, which could alter ER expression levels.
An accumulating body of in vitro evidence currently indicates the presence of functional ERs in GnRH neurons. A number of these studies have used immortalized GnRH-expressing neuronal cell lines and have shown that they express ERα and/or ERβ (Shen et al., 1998; Butler et al., 1999; Roy et al., 1999; Hrabovszky et al., 2000; Kallo et al., 2001; Martinez-Morales et al., 2001) and respond to estrogen treatment by increasing galanin (Shen et al., 1998) and androgen receptor gene expression (Poletti et al., 1994). These studies show evidence for a direct estrogen effect on GnRH neurons, and suggest the presence of a functional ER signaling pathways in these neurons. ERβ may be the predominant ER in GnRH neurons (Hrabovszky et al., 2001; Kallo et al., 2001; Legan and Tsai, 2003; Petersen et al., 2003; Skinner and Dufourny, 2005); however, a separate immunohistochemistry study of female rats found ERα transcripts in GnRH neurons in the preoptic area (Butler et al., 1999). Prenatal GnRH neurons have been shown to express ERβ transcripts, however, the number of cells expressing ERβ decreased over time (Sharifi et al., 2002). These studies led to the most recent evidence in humans that ERβ colocalized with GnRH (Hrabovszky et al., 2007). With mounting evidence that GnRH neurons contain ERs, the functional significance remains to be determined as well as the intracellular signaling processes used. However, a direct, transcription-dependent mechanism on GnRH has been shown (Temple et al., 2004).
Studies from mice with generalized ERα disruption (ERαKO) indicate that ERα plays a major role in regulating the reproductive neuroendocrine–gonadal system (Lubahn et al., 1993; Couse et al., 1999; Schomberg et al., 1999; Dupont et al., 2000). ERα is widely expressed throughout the reproductive axis, including the hypothalamus, pituitary, gonads (both ovaries and testis), and uterus. Therefore, a generalized disruption of ERα would be predicted to affect the axis at various levels centrally and peripherally (Lubahn et al., 1993; Schomberg et al., 1999; Dupont et al., 2000), rendering the isolation of the effect of estrogen at any level impossible. Homozygous ERα KO mice display significant gonadal defects and impaired feedback regulation of the neuroendocrine axis. Homozygous ERα KO females were also infertile and were found to have hypoplastic uteri, large hemorrhagic cysts, and absent corpus luteum in their ovaries (Couse et al., 1995; Korach et al., 1996; Schomberg et al., 1999; Dupont et al., 2000).
In addition, studies in homozygous ERα KO females indicate that they exhibit 10-fold higher serum estrogen level than wild type controls. LH levels were also significantly increased in intact ERαKO females compared to those in WT females (Lindzey et al., 1998; Wersinger et al., 1999). Estrogen feedback regulation is thought to occur both at the level of the hypothalamus and at the level of the pituitary. Thus, elevated LH levels may indirectly reflect elevated GnRH levels due to loss of negative estrogen regulation at the level of the hypothalamus or may also reflect loss of negative estrogen regulation at the level of the pituitary. Similarly, LH and follicle-stimulating hormone (FSH) mRNA levels were higher in intact ERα KO compared to those in intact WT females, and were comparable to LH and FSH mRNA levels in ovariectomized, non-replaced WT females (Scully et al., 1997; Lindzey et al., 1998). These results suggest a role for estrogen in negative regulation of LH and FSH, but do not localize the effect to the level of the hypothalamus, and/or the pituitary.
Initial studies of ERβKO mice failed to identify a significant reproductive phenotype. However, it was noted later that ER knock-out mice produced by insertion of an ER allele containing a neomycin resistance gene could produce a chimeric protein that is partially estrogen-responsive (Couse et al., 1995; Antal et al., 2008; Chen et al., 2009). Complete ERβKO mice demonstrated subfertility (Krege et al., 1998; Dupont et al., 2000; Antal et al., 2008) though gonadotropin levels appear to be unchanged compared to control mice. These data suggest that ERβ may not be important for central E2 negative feedback of the axis. However, gonadotropin levels are less elevated in ERαKO versus the combined ERαβ KO mice, indicating that ERβ may have a negative feedback role in the axis (Couse et al., 2000). Interestingly, the role of ERβ may be to increase progesterone mediated effects at the level of the hypothalamus to produce the gonadotropin surge (Chappell and Levine, 2000).
Additional studies in ERαKO mice indicated a role for ERα in the positive regulation of LH secretion by the pituitary and inferred that negative regulation occurs at the hypothalamic level (Lindzey et al., 2006). Recently, ERα expression in neuronal cells was shown to be required for positive feedback of estradiol (Wintermantel et al., 2006). Furthermore, a pituitary-specific ERα KO mouse model, although being infertile, was shown to have normal levels of LH, suggesting that positive feedback regulation was impaired but negative regulation by estrogen was intact (Gieske et al., 2008). In contrast, we also generated a pituitary-specific KO of ERα and find elevated serum LH levels (Singh et al., 2009). It is not clear why these two models differ in regards to LH levels and may reflect differences in sampling or LH assay methodologies.
In the classical, or genomic pathway, ERs bind to an ERE on DNA to alter the transcription of genes (O’Malley and Tsai, 1992; Arbogast, 2008). However, ERα has been shown to signal through ERE-independent, non-classical, as well as non-genomic pathways (McEwen and Alves, 1999; Kousteni et al., 2001) through protein–protein interactions by tethering to other transcription factors such as AP-1 and NF-kB and the transcriptional complex (Stein and Yang, 1995; Ray et al., 1997; Kushner et al., 2000; Pedram et al., 2002). Furthermore, ERα has been shown to display antagonistic effects on ERα mediated transcription by interfering with recruitment of an AP-1 protein complex on DNA (Matthews et al., 2006). ER mediated, ligand-dependent signaling has been shown to involve cross-talk with growth factor mediated pathways through phosphorylation by MAPK activation (Kato et al., 1995; Couse and Korach, 1999; Feng et al., 2001; Masuhiro et al., 2005), and ERα has also been shown to induce rapid membrane-initiated signaling (Levin, 2005; Revankar et al., 2005; Pedram et al., 2006; Vasudevan and Pfaff, 2007).
Non-genomic effects of estradiol have been reported in several studies. For example, estradiol increased the phosphorylation of cAMP response element binding protein (CREB) in GnRH neurons (Abraham et al., 2003) as well as calcium oscillations (Abe et al., 2008) and potassium currents (DeFazio and Moenter, 2002; Farkas et al., 2007; Roepke et al., 2007; Zhang et al., 2007). Immunocytochemical localization was performed for ER isoforms in GT1-7 cells, localizing both receptors at the cell membrane to some degree (Navarro et al., 2003) and providing some evidence that the ERα isoform was responsible for norepinephrine responsiveness (Morales et al., 2007). Rapid signaling effects of estradiol have also been linked in hypothalamic neurons to activated protein kinase C pathways (Qiu et al., 2003). A membrane ER that is distinct from either ERα or ERβ has recently been identified. The GPR30 orphan receptor, a member of the G-protein coupled family of receptors, has been identified as a putative ER. GPR30 located in the endoplasmic reticulum, Golgi apparatus, and nuclear membranes have been shown to be activated by estradiol and other estrogen agonists (Filardo, 2002; Brailoiu et al., 2007; Prossnitz et al., 2008). GPR30 has been found in primate and mouse GnRH neurons (Noel et al., 2009; Sun et al., 2010) and is proposed to be located at the plasma membrane as membrane impermeable BSA conjugated E2 can exert rapid effects on GnRH function (Noel et al., 2009). Further complexity has recently been added with the report of a novel ER, acting as a G-protein coupled receptor in the hypothalamus (Qiu et al., 2008).
The preovulatory release of GnRH consists of a 2- to 4-h increase in the overall amount of GnRH secreted, occurring between 1600 and 2000 hours on the afternoon of proestrus (Levine and Ramirez, 1982). Classical neuroendocrine studies demonstrated that the CNS mechanisms that mediate the release of a GnRH surge require the integration of two obligatory signals – the preovulatory estrogen surge, and a daily neural signal that is synaptically conveyed from the circadian clock resident in the suprachiasmatic nucleus (Levine, 1997). The major action of estrogen is to “couple” the daily neural signal to the neuronal circuitries that mediate release of GnRH surges (Karsch and Foster, 1975; Legan and Karsch, 1975). In the absence of a sufficient elevation of estrogen, the daily signal is not communicated to neurons controlling the release of GnRH, and no surge takes place; with exposure of the hypothalamus to a sufficient estrogen stimulus, the pathways that convey the daily neural signal for the surge are rendered patent, and the GnRH is released into the hypophysial portal vasculature. At the same time, estrogen greatly enhances the responsiveness of the gonadotrophs to this burst of GnRH (Taga et al., 1982; Bauer-Dantoin et al., 1995; Shupnik, 1996). Both of these processes, along with the ability of GnRH to “self-prime” the estrogen-exposed pituitary to its own actions (Kamel et al., 1987) culminate in the release of a massive, but transient increase of LH on the afternoon of proestrus, which in turn triggers ovulation on the following morning of estrous.
How does estrogen couple the daily neuronal signal to the neurons responsible for the release of GnRH surges? One major locus of this integrative activity is the AVPV nucleus of the hypothalamus, where estrogen appears to activate ERα in neurons that receive afferents from the SCN and to project to GnRH neurons (possibly kiss1 neurons; Van der Beek et al., 1997; Tsukahara et al., 2008). We have examined the cellular actions of estrogen that may mediate these effects, focusing on the roles that PRs may play. Estrogen induces the expression of both isoforms of PR, PRB, and the N-terminally truncated PRA, in the AVPV, as well as other hypothalamic and preoptic nuclei. We have determined that induction of PRs is obligatory for the successful release of GnRH surges; thus, GnRH and LH surges are absent in ovarian intact and estrogen-treated PR gene knock-out (PRKO) mice (Chappell et al., 1997, 1999), and in rats treated with a progesterone receptor antagonist or intra-cerebroventricular PR antisense oligonucleotides (Chappell and Levine, 2000). We have proposed a model for the release of GnRH surges that includes (1) the induction of PRs by estrogen in AVPV neurons, (2) delivery of the daily neural signal from the SCN to AVPV neurons by neurotransmitter circuitries, and (3) the neurotransmitter-mediated activation of intracellular second messenger production that in turn transactivates the PRs in a ligand-independent manner; thereafter, (4) induces signals to kiss1 neurons that evoke the neurosecretion for the GnRH surge, which is (5) further amplified by ovarian progesterone release in response to the LH surge. The net result of all of these integrated physiological events is the release of a robust GnRH surge that is timed to trigger ovulation in concert with behavioral heat and maximal wakefulness, and uterine proliferation and differentiation thereby maximizing the chances for successful fertilization and implantation. The downstream targets of PR signaling that in turn initiate the GnRH surge process are not known, although we have provided evidence that one such target may be the neuropeptide Y (NPY) gene (Bauer-Dantoin et al., 1993). It is not clear how activated PRs may regulate NPY transcription, as there appear to be no classical palindromic PRE/GRE sites in the promoter of the preproNPY gene.
Estrogen’s positive feedback actions also include a massive stimulation of pituitary responsiveness to GnRH stimulation. That E2-induced PR expression is obligatory for the manifestation of GnRH self-priming was demonstrated by the finding that this phenomenon is absent in PRKO animals (Chappell et al., 1999). The ability of progesterone to induce the GnRH self-priming response is rapid and depends upon mRNA and protein synthesis, but the cellular targets of PRs, whether activated by GnRH or progesterone, remain unclear.
Progesterone receptor in the ARC also likely contributes to negative feedback of the axis. Microimplants of the progesterone antagonist RU486 in the ARC, but not in the POA, blunt the negative feedback effects of progesterone in ewes (Goodman et al., 2011). The locus of action of the progesterone is likely the kisspeptin/dynorphin/neurokinin B expressing neurons in the arcuate (Goodman et al., 2004, 2011; Navarro et al., 2011).
Rapid effects of P have been documented in a variety of tissues, each potentially being mediated by one or more of several “non-classical” signaling mechanisms. Some of the rapid actions of P have been attributed to the ability of bound PRA and PRB to interact with the Src tyrosine kinase localized to the plasma membrane, which in turn prompts cellular responses via activation of the Src/Ras/Raf-1/MAPK signaling pathway (Faivre et al., 2008). At least three PRA/B-independent pathways have also been identified that may mediate the effects of P in a variety of tissues and cell types. Progesterone is known to be rapidly metabolized in the brain to several neurosteroids, including allopregnanolone (3α-hydroxy-5α-pregnan-20-one; 3α5αTHP), which has been shown to modulate GABAA receptors in the brain (Majewska et al., 1986). Secondly, a progesterone membrane binding protein, progesterone receptor membrane component 1 (PGRMC1), has been suggested to mediate the ability of P to activate protein kinase G or other rapid signaling mechanisms in certain cells (Falkenstein et al., 1996).
The involvement of PRs in the positive feedback actions of E2, nevertheless, is unequivocal, as both GnRH surges and GnRH self-priming mechanisms are inoperative in PRKO mice.
In summary, studies on estrogen effects on GnRH neuronal activity and secretion are wrought with complexity due to the various ER isoforms, direct versus indirect effects, classical versus non-classical estrogen signaling and the role of other hormones, such as testosterone (Eagleson et al., 2000; McGee et al., 2012) and progesterone, in modulating estrogen action on GnRH neurons. To overcome these difficulties, investigators have used a wide array of in vitro models, pharmacological tools, anatomical mapping, and transgenic and knock-out models. While these studies have helped clarify the role and mechanisms of estrogen action on GnRH neurons, many questions remain to be answered.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abe, H., Keen, K. L., and Terasawa, E. (2008). Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology 149, 1155–1162.
Abraham, I. M., Han, S. K., Todman, M. G., Korach, K. S., and Herbison, A. E. (2003). Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J. Neurosci. 23, 5771–5777.
Antal, M. C., Krust, A., Chambon, P., and Mark, M. (2008). Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc. Natl. Acad. Sci. U.S.A. 105, 2433–2438.
Arbogast, L. A. (2008). Estrogen genomic and membrane actions at an intersection. Trends Endocrinol. Metab. 19, 1–2.
Bai, Y., Chang, F., Zhou, R., Jin, P. P., Matsumoto, H., Sokabe, M., and Chen, L. (2011). Increase of anteroventral periventricular kisspeptin neurons and generation of E2-induced LH-surge system in male rats exposed perinatally to environmental dose of bisphenol-A. Endocrinology 152, 1562–1571.
Bauer-Dantoin, A. C., Tabesh, B., Norgle, J. R., and Levine, J. E. (1993). RU486 administration blocks neuropeptide Y potentiation of luteinizing hormone (LH)-releasing hormone-induced LH surges in proestrous rats. Endocrinology 133, 2418–2423.
Bauer-Dantoin, A. C., Weiss, J., and Jameson, J. L. (1995). Roles of estrogen, progesterone, and gonadotropin-releasing hormone (GnRH) in the control of pituitary GnRH receptor gene expression at the time of the preovulatory gonadotropin surges. Endocrinology 136, 1014–1019.
Beato, M., Herrlich, P., and Schutz, G. (1995). Steroid hormone receptors: many actors in search of a plot. Cell 83, 851–857.
Berry, M., Metzger, D., and Chambon, P. (1990). Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 9, 2811–2818.
Brailoiu, E., Dun, S. L., Brailoiu, G. C., Mizuo, K., Sklar, L. A., Oprea, T. I., Prossnitz, E. R., and Dun, N. J. (2007). Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol. 193, 311–321.
Butler, J. A., Sjoberg, M., and Coen, C. W. (1999). Evidence for oestrogen receptor alpha-immunoreactivity in gonadotropin-releasing hormone-expressing neurones. J. Neuroendocrinol. 11, 331–335.
Caraty, A., Fabre-Nys, C., Delaleu, B., Locatelli, A., Bruneau, G., Karsch, F. J., and Herbison, A. (1998). Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology 139, 1752–1760.
Caraty, A., Locatelli, A., and Martin, G. B. (1989). Biphasic response in the secretion of gonadotropin-releasing hormone in ovariectomized ewes injected with oestradiol. J. Endocrinol. 123, 375–382.
Cavailles, V., Dauvois, S., L’Horset, F., Lopez, G., Hoare, S., Kushner, P. J., and Parker, M. G. (1995). Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 14, 3741–3751.
Chakravarti, D., LaMorte, V. J., Nelson, M. C., Nakajima, T., Schulman, I. G., Juguilon, H., Montminy, M., and Evans, R. M. (1996). Role of CBP/P300 in nuclear receptor signalling. Nature 383, 99–103.
Chappell, P. E., and Levine, J. E. (2000). Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology 141, 1477–1485.
Chappell, P. E., Lydon, J. P., Conneely, O. M., O’Malley, B. W., and Levine, J. E. (1997). Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology 138, 4147–4152.
Chappell, P. E., Schneider, J. S., Kim, P., Xu, M., Lydon, J. P., O’Malley, B. W., and Levine, J. E. (1999). Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology 140, 3653–3658.
Chen, M., Wolfe, A., Wang, X., Chang, C., Yeh, S., and Radovick, S. (2009). Generation and characterization of a complete null estrogen receptor alpha mouse using Cre/LoxP technology. Mol. Cell. Biochem. 321, 145–153.
Chongthammakun, S., and Terasawa, E. (1993). Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology 132, 735–743.
Clarke, I. J. (1993). Variable patterns of gonadotropin-releasing hormone secretion during the estrogen-induced luteinizing hormone surge in ovariectomized ewes. Endocrinology 133, 1624–1632.
Couse, J. F., Curtis, S. W., Washburn, T. F., Lindzey, J., Golding, T. S., Lubahn, D. B., Smithies, O., and Korach, K. S. (1995). Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 9, 1441–1454.
Couse, J. F., Curtis Hewitt, S., and Korach, K. S. (2000). Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. J. Steroid Biochem. Mol. Biol. 74, 287–296.
Couse, J. F., Hewitt, S. C., Bunch, D. O., Sar, M., Walker, V. R., Davis, B. J., and Korach, K. S. (1999). Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science 286, 2328–2331.
Couse, J. F., and Korach, K. S. (1999). Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 20, 358–417.
Couse, J. F., Lindzey, J., Grandien, K., Gustafsson, J. A., and Korach, K. S. (1997). Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 138, 4613–4621.
Couse, J. F., Yates, M. M., Walker, V. R., and Korach, K. S. (2003). Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol. Endocrinol. 17, 1039–1053.
Cravo, R. M., Margatho, L. O., Osborne-Lawrence, S., Donato, J. Jr., Atkin, S., Bookout, A. L., Rovinsky, S., Frazão, R., Lee, C. E., Gautron, L., Zigman, J. M., and Eliasa, C. F. (2011). Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173, 37–56.
d’Anglemont de Tassigny, X., Fagg, L. A., Carlton, M. B., and Colledge, W. H. (2008). Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149, 3926–3932.
de Roux, N., Genin, E., Carel, J. C., Matsuda, F., Chaussain, J. L., and Milgrom, E. (2003). Hypogonadotropic hypogonadism due to loss of function of the Kiss1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. U.S.A. 100, 10972–10976.
DeFazio, R. A., and Moenter, S. M. (2002). Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol. Endocrinol. 16, 2255–2265.
Dong, K. W., Chen, Z. G., Cheng, K. W., and Yu, K. L. (1996). Evidence for estrogen receptor-mediated regulation of human gonadotropin-releasing hormone promoter activity in human placental cells. Mol. Cell. Endocrinol. 117, 241–246.
Downs, J. L., and Wise, P. M. (2009). The role of the brain in female reproductive aging. Mol. Cell. Endocrinol. 299, 32–38.
Dungan, H. M., Gottsch, M. L., Zeng, H., Gragerov, A., Bergmann, J. E., Vassilatis, D. K., Clifton, D. K., and Steiner, R. A. (2007). The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J. Neurosci. 27, 12088–12095.
Dupont, S., Krust, A., Gansmuller, A., Dierich, A., Chambon, P., and Mark, M. (2000). Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127, 4277–4291.
Eagleson, C. A., Gingrich, M. B., Pastor, C. L., Arora, T. K., Burt, C. M., Evans, W. S., and Marshall, J. C. (2000). Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J. Clin. Endocrinol. Metab. 85, 4047–4052.
Faivre, E. J., Daniel, A. R., Hillard, C. J., and Lange, C. A. (2008). Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol. Endocrinol. 22, 823–837.
Falkenstein, E., Meyer, C., Eisen, C., Scriba, P. C., and Wehling, M. (1996). Full-length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 229, 86–89.
Farkas, I., Varju, P., and Liposits, Z. (2007). Estrogen modulates potassium currents and expression of the Kv4.2 subunit in GT1-7 cells. Neurochem. Int. 50, 619–627.
Feng, W., Webb, P., Nguyen, P., Liu, X., Li, J., Karin, M., and Kushner, P. J. (2001). Potentiation of estrogen receptor activation function 1 (AF-1) by Src/JNK through a serine 118-independent pathway. Mol. Endocrinol. 15, 32–45.
Filardo, E. J. (2002). Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J. Steroid Biochem. Mol. Biol. 80, 231–238.
Garcia-Segura, L. M., and McCarthy, M. M. (2004). Minireview: role of glia in neuroendocrine function. Endocrinology 145, 1082–1086.
Gibson, M. J., Wu, T. J., Miller, G. M., and Silverman, A. J. (1997). What nature’s knockout teaches us about GnRH activity: hypogonadal mice and neuronal grafts. Horm. Behav. 31, 212–220.
Gieske, M. C., Kim, H. J., Legan, S. J., Koo, Y., Krust, A., Chambon, P., and Ko, C. (2008). Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females. Endocrinology 149, 20–27.
Goodman, R. L., Coolen, L. M., Anderson, G. M., Hardy, S. L., Valent, M., Connors, J. M., Fitzgerald, M. E., and Lehman, M. N. (2004). Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145, 2959–2967.
Goodman, R. L., Holaskova, I., Nestor, C. C., Connors, J. M., Billings, H. J., Valent, M., Lehman, M. N., and Hileman, S. M. (2011). Evidence that the arcuate nucleus is an important site of progesterone negative feedback in the ewe. Endocrinology 152, 3451–3460.
Gottsch, M. L., Cunningham, M. J., Smith, J. T., Popa, S. M., Acohido, B. V., Crowley, W. F., Seminara, S., Clifton, D. K., and Steiner, R. A. (2004). A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145, 4073–4077
Gu, G. B., and Simerly, R. B. (1997). Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J. Comp. Neurol. 384, 142–164.
Han, S. K., Gottsch, M. L., Lee, K. J., Popa, S. M., Smith, J. T., Jakawich, S. K., Clifton, D. K., Steiner, R. A., and Herbison, A. E. (2005). Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 25, 11349–11356.
Herbison, A. E. (1998). Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr. Rev. 19, 302–330.
Herbison, A. E., Horvath, T. L., Naftolin, F., and Leranth, C. (1995). Distribution of estrogen receptor-immunoreactive cells in monkey hypothalamus: relationship to neurones containing luteinizing hormone-releasing hormone and tyrosine hydroxylase. Neuroendocrinology 61, 1–10.
Herbison, A. E., Robinson, J. E., and Skinner, D. C. (1993). Distribution of estrogen receptor-immunoreactive cells in the preoptic area of the ewe: co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone. Neuroendocrinology 57, 751–759.
Herbison, A. E., and Theodosis, D. T. (1992). Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience 50, 283–298.
Horwitz, K. B., Jackson, T. A., Bain, D. L., Richer, J. K., Takimoto, G. S., and Tung, L. (1996). Nuclear receptor coactivators and corepressors. Mol. Endocrinol. 10, 1167–1177.
Hrabovszky, E., Kallo, I., Szlavik, N., Keller, E., Merchenthaler, I., and Liposits, Z. (2007). Gonadotropin-releasing hormone neurons express estrogen receptor-beta. J. Clin. Endocrinol. Metab. 92, 2827–2830.
Hrabovszky, E., Shughrue, P. J., Merchenthaler, I., Hajszan, T., Carpenter, C. D., Liposits, Z., and Petersen, S. L. (2000). Detection of estrogen receptor-beta messenger ribonucleic acid and 125I- estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 141, 3506–3509
Hrabovszky, E., Steinhauser, A., Barabas, K., Shughrue, P. J., Petersen, S. L., Merchenthaler, I., and Liposits, Z. (2001). Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142, 3261–3264.
Irwig, M. S., Fraley, G. S., Smith, J. T., Acohido, B. V., Popa, S. M., Cunningham, M. J., Gottsch, M. L., Clifton, D. K., and Steiner, R. A. (2004). Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of Kiss-1 mRNA in the male rat. Neuroendocrinology 80, 264–272.
Johansson, L., Thomsen, J. S., Damdimopoulos, A. E., Spyrou, G., Gustafsson, J. A., and Treuter, E. (1999). The orphan nuclear receptor SHP inhibits agonist-dependent transcriptional activity of estrogen receptors ERalpha and ERbeta. J. Biol. Chem. 274, 345–353.
Kallo, I., Butler, J. A., Barkovics-Kallo, M., Goubillon, M. L., and Coen, C. W. (2001). Oestrogen receptor beta-immunoreactivity in gonadotropin releasing hormone-expressing neurones: regulation by oestrogen. J. Neuroendocrinol. 13, 741–748.
Kallo, I., Vida, B., Deli, L., Molnar, C. S., Hrabovszky, E., Caraty, A., Ciofi, P., Coen, C. W., and Liposits, Z. (2012). Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J. Neuroendocrinol. 24, 464–476.
Kalra, S. P. (1993). Mandatory neuropeptide-steroid signaling for the preovulatory luteinizing hormone-releasing hormone discharge. Endocr. Rev. 14, 507–538.
Kamel, F., Balz, J. A., Kubajak, C. L., and Schneider, V. A. (1987). Effects of luteinizing hormone (LH)-releasing hormone pulse amplitude and frequency on LH secretion by perifused rat anterior pituitary cells. Endocrinology 120, 1644–1650.
Karsch, F. J., Bowen, J. M., Caraty, A., Evans, N. P., and Moenter, S. M. (1997). Gonadotropin-releasing hormone requirements for ovulation. Biol. Reprod. 56, 303–309.
Karsch, F. J., and Foster, D. L. (1975). Sexual differentiation of the mechanism controlling the preovulatory discharge of luteinizing hormone in sheep. Endocrinology 97, 373–379.
Kato, S., Endoh, H., Masuhiro, Y., Kitamoto, T., Uchiyama, S., Sasaki, H., Masushige, S., Gotoh, Y., Nishida, E., Kawashima, H., Metzger, D., and Chambon, P. (1995). Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270, 1491–1494.
Katzenellenbogen, B. S., Bhardwaj, B., Fang, H., Ince, B. A., Pakdel, F., Reese, J. C., Schodin, D., and Wrenn, C. K. (1993). Hormone binding and transcription activation by estrogen receptors: analyses using mammalian and yeast systems. J. Steroid Biochem. Mol. Biol. 47, 39–48.
Kelly, M. J., Qiu, J., and Ronnekleiv, O. K. (2003). Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann. N. Y. Acad. Sci. 1007, 6–16.
Kepa, J. K., Wang, C., Neeley, C. I., Raynolds, M. V., Gordon, D. F., Wood, W. M., and Wierman, M. E. (1992). Structure of the rat gonadotropin-releasing hormone (rGnRH) gene promoter and functional analysis in hypothalamic cells. Nucleic Acid Res. 20, 1393–1399.
Kim, H. H., Wolfe, A., Cohen, R. N., Eames, S. C., Johnson, A. L., Wieland, C. N., and Radovick, S. (2007). In vivo identification of a 107-base pair promoter element mediating neuron-specific expression of mouse gonadotropin-releasing hormone. Mol. Endocrinol. 21, 457–471.
Kinoshita, M., Tsukamura, H., Adachi, S., Matsui, H., Uenoyama, Y., Iwata, K., Yamada, S., Inoue, K., Ohtaki, T., Matsumoto, H., and Maeda, K. I. (2005). Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146, 4431–4436.
Korach, K. S., Couse, J. F., Curtis, S. W., Washburn, T. F., Lindzey, J., Kimbro, K. S., Eddy, E. M., Migliaccio, S., Snedeker, S. M., Lubahn, D. B., Schomberg, D. W., and Smith, E. P. (1996). Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog. Horm. Res. 51, 159–186; discussion 186–188.
Korzus, E., Torchia, J., Rose, D. W., Xu, L., Kurokawa, R., McInerney, E. M., Mullen, T. M., Glass, C. K., and Rosenfeld, M. G. (1998). Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science 279, 703–707.
Kotani, M., Detheux, M., Vandenbogaerde, A., Communi, D., Vanderwinden, J. M., Le Poul, E., Brezillon, S., Tyldesley, R., Suarez-Huerta, N., Vandeput, F., Blanpain, C., Schiffmann, S. N., Vassart, G., and Parmentier, M. (2001). The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 276, 34631–34636.
Kousteni, S., Bellido, T., Plotkin, L. I., O’Brien, C. A., Bodenner, D. L., Han, L., Han, K., Han, K., DiGregorio, G. B., Katzenellenbogen, J. A., Katzenellenbogen, B. S., Roberson, P. K., Weinstein, R. S., Jilka, R. L., and Manolagas, S. C. (2001). Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104, 719–730.
Krege, J. H., Hodgin, J. B., Couse, J. F., Enmark, E., Warner, M., Mahler, J. F., Sar, M., Korach, K. S., Gustafsson, J. A., and Smithies, O. (1998). Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. U.S.A. 95, 15677–15682.
Kushner, P. J., Agard, D. A., Greene, G. L., Scanlan, T. S., Shiau, A. K., Uht, R. M., and Webb, P. (2000). Estrogen receptor pathways to AP-1. J. Steroid Biochem. Mol. Biol. 74, 311–317.
Lavinsky, R. M., Jepsen, K., Heinzel, T., Torchia, J., Mullen, T. M., Schiff, R., Del-Rio, A. L., Ricote, M., Ngo, S., Gemsch, J., Susan, G., Hilsenbeck, S. G., Osborne, C. K., Glass, C. K., Rosenfeld, M. G., and Rose, D. W. (1998). Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc. Natl. Acad. Sci. U S.A. 95, 2920–2925.
Legan, S. J., and Karsch, F. J. (1975). A daily signal for the LH surge in the rat. Endocrinology 96, 57–62.
Legan, S. J., and Tsai, H. W. (2003). Oestrogen receptor-alpha and -beta immunoreactivity in gonadotropin-releasing hormone neurones after ovariectomy and chronic exposure to oestradiol. J. Neuroendocrinol. 15, 1164–1170.
Lehman, M. N., and Karsch, F. J. (1993). Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology 133, 887–895.
Levin, E. R. (2005). Integration of the extranuclear and nuclear actions of estrogen. Mol. Endocrinol. 19, 1951–1959.
Levine, J. E. (1997). New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol. Reprod. 56, 293–302.
Levine, J. E., and Ramirez, V. D. (1982). Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology 111, 1439–1448.
Lindzey, J., Wetsel, W. C., Couse, J. F., Stoker, T., Cooper, R., and Korach, K. S. (1998). Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-alpha knockout mice. Endocrinology 139, 4092–4101.
Lindzey, J., Jayes, F. L., Yates, M. M., Couse, J. F., and Korach, K. S. (2006). The bi-modal effects of estradiol on gonadotropin synthesis and secretion in female mice are dependent on estrogen receptor alpha. J. Endocrinol. 191, 309–317.
Lopez, F. J., Merchenthaler, I., Liposits, Z., and Negro-Vilar, A. (1996). Steroid imprinting and modulation of sexual dimorphism in the luteinizing hormone-releasing hormone neuronal system. Cell. Mol. Neurobiol. 16, 129–141.
Lubahn, D. B., Moyer, J. S., Golding, T. S., Couse, J. F., Korach, K. S., and Smithies, O. (1993). Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. U.S.A. 90, 11162–11166
Majewska, M. D., Harrison, N. L., Schwartz, R. D., Barker, J. L., and Paul, S. M. (1986). Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232, 1004–1007.
Mangelsdorf, D. J., Thummel, C., Beato, M., Herrlich, P., Schutz, G., Umesono, K., Blumberg, B., Kastner, P., Mark, M., Chambon, P., and Evan, R. M. (1995). The nuclear receptor superfamily: the second decade. Cell 83, 835–839.
Martinez-Morales, J. R., Morales, A., Marin, R., Hernandez-Jimenez, J. G., Acevedo, A., Guerra, B., Hernandez, G., Lopez-Coviella, I., Prieto, L., and Alonso, R. (2001). Estrogen modulates norepinephrine-induced accumulation of adenosine cyclic monophosphate in a subpopulation of immortalized luteinizing hormone-releasing hormone secreting neurons from the mouse hypothalamus. Neurosci. Lett. 298, 61–64.
Masuhiro, Y., Mezaki, Y., Sakari, M., Takeyama, K., Yoshida, T., Inoue, K., Yanagisawa, J., Hanazawa, S., O’Malley, B. W., and Kato, S. (2005). Splicing potentiation by growth factor signals via estrogen receptor phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 102, 8126–8131.
Matsui, H., Takatsu, Y., Kumano, S., Matsumoto, H., and Ohtaki, T. (2004). Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem. Biophys. Res. Commun. 320, 383–388.
Matthews, J., Wihlen, B., Tujague, M., Wan, J., Strom, A., and Gustafsson, J. A. (2006). Estrogen receptor (ER) beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol. Endocrinol. 20, 534–543.
McEwen, B. S., and Alves, S. E. (1999). Estrogen actions in the central nervous system. Endocr. Rev. 20, 279–307.
McGee, W. K., Bishop, C. V., Bahar, A., Pohl, C. R., Chang, R. J., Marshall, J. C., Pau, F. K., Stouffer, R. L., and Cameron, J. L. (2012). Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum. Reprod. 27, 531–540.
McKenna, N. J., Lanz, R. B., and O’Malley, B. W. (1999). Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20, 321–344.
Messager, S., Chatzidaki, E. E., Ma, D., Hendrick, A. G., Zahn, D., Dixon, J., Thresher, R. R., Isabelle Malinge, I., Lomet, D., Carlton, M. B. L., Colledg, W. H., Caraty, A., and Aparicio, S. A. J. R. (2005). Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. U.S.A. 102, 1761–1766.
Misiti, S., Schomburg, L., Yen, P. M., and Chin, W. W. (1998). Expression and hormonal regulation of coactivator and corepressor genes. Endocrinology 139, 2493–2500.
Moenter, S. M., Brand, R. C., and Karsch, F. J. (1992). Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 130, 2978–2984.
Morales, A., Gonzalez, M., Marin, R., Diaz, M., and Alonso, R. (2007). Estrogen inhibition of norepinephrine responsiveness is initiated at the plasma membrane of GnRH-producing GT1-7 cells. J. Endocrinol. 194, 193–200.
Navarro, C. E., Abdul Saeed, S., Murdock, C., Martinez-Fuentes, A. J., Arora, K. K., Krsmanovic, L. Z., and Catt, K. J. (2003). Regulation of cyclic adenosine 3′, 5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotropin-releasing hormone neurons. Mol. Endocrinol. 17, 1792–1804.
Navarro, V. M., Castellano, J. M., Fernandez-Fernandez, R., Tovar, S., Roa, J., Mayen, A., Barreiro, M. L., Casanueva, F. F., Aguilar, E., Dieguez, C., Pinilla, L., and Tena-Sempere, M. (2005). Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology 146, 1689–1697.
Navarro, V. M., Gottsch, M. L., Chavkin, C., Okamura, H., Clifton, D. K., and Steiner, R. A. (2009). Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 29, 11859–11866.
Navarro, V. M., Gottsch, M. L., Wu, M., Garcia-Galiano, D., Hobbs, S. J., Bosch, M. A., Pinilla, L., Clifton, D. K., Dearth, A., Ronnekleiv, O. K., Braun, R. E., Palmiter, R. D., Tena-Sempere, M., Alreja, M., and Steiner, R. A. (2011). Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 152, 4265–4275.
Navas, J. M., Anglade, I., Bailhache, T., Pakdel, F., Breton, B., Jego, P., and Kah, O. (1995). Do gonadotropin-releasing hormone neurons express estrogen receptors in the rainbow trout? A double immunohistochemical study. J. Comp. Neurol. 363, 461–474.
Ng, Y., Wolfe, A., Novaira, H. J., and Radovick, S. (2009). Estrogen regulation of gene expression in GnRH neurons. Mol. Cell. Endocrinol. 303, 25–33.
Noel, S. D., Keen, K. L., Baumann, D. I., Filardo, E. J., and Terasawa, E. (2009). Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol. Endocrinol. 23, 349–359.
Novaira, H. J., Ng, Y., Wolfe, A., and Radovick, S. (2009). Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol. Cell. Endocrinol. 311, 126–134.
Ogawa, S., Inoue, S., Watanabe, T., Hiroi, H., Orimo, A., Hosoi, T., Ouchi, Y., and Muramatsu, M. (1998). The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem. Biophys. Res. Commun. 243, 122–126.
O’Malley, B. W., and Tsai, M. J. (1992). Molecular pathways of steroid receptor action. Biol. Reprod. 46, 163–167.
Onate, S. A., Tsai, S. Y., Tsai, M. J., and O’Malley, B. W. (1995). Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270, 1354–1357
Pace, P., Taylor, J., Suntharalingam, S., Coombes, R. C., and Ali, S. (1997). Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. J. Biol. Chem. 272, 25832–25838.
Parhar, I. S., Ogawa, S., and Sakuma, Y. (2004). Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinology 145, 3613–3618.
Pedram, A., Razandi, M., Aitkenhead, M., Hughes, C. C., and Levin, E. R. (2002). Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J. Biol. Chem. 277, 50768–50775.
Pedram, A., Razandi, M., and Levin, E. R. (2006). Nature of functional estrogen receptors at the plasma membrane. Mol. Endocrinol. 20, 1996–2009.
Petersen, S. L., McCrone, S., Keller, M., and Shores, S. (1995). Effects of estrogen and progesterone on luteinizing hormone-releasing hormone messenger ribonucleic acid levels: consideration of temporal and neuroanatomical variables. Endocrinology 136, 3604–3610.
Petersen, S. L., Ottem, E. N., and Carpenter, C. D. (2003). Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol. Reprod. 69, 1771–1778.
Pettersson, K., Grandien, K., Kuiper, G. G., and Gustafsson, J. A. (1997). Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol. Endocrinol. 11, 1486–1496.
Pielecka-Fortuna, J., Chu, Z., and Moenter, S. M. (2008). Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149, 1979–1986
Poletti, A., Melcangi, R. C., Negri-Cesi, P., Maggi, R., and Martini, L. (1994). Steroid binding and metabolism in the luteinizing hormone- releasing hormone-producing neuronal cell line GT1-1. Endocrinology 135, 2623–2628
Popolow, H. B., King, J. C., and Gerall, A. A. (1981). Rostral medial preoptic area lesions’ influence on female estrous processes and LHRH distribution. Physiol. Behav. 27, 855–861.
Porteous, R., Petersen, S. L., Yeo, S. H., Bhattarai, J. P., Ciofi, P., de Tassigny, X. D., Colledge, W. H., Caraty, A., and Herbison, A. E. (2011). Kisspeptin neurons co-express met-enkephalin and galanin in the rostral periventricular region of the female mouse hypothalamus. J. Comp. Neurol. 519, 3456–3469.
Powell, E., Wang, Y., Shapiro, D. J., and Xu, W. (2010). Differential requirements of Hsp90 and DNA for the formation of estrogen receptor homodimers and heterodimers. J. Biol. Chem. 285, 16125–16134.
Prevot, V. (2002). Glial-neuronal-endothelial interactions are involved in the control of GnRH secretion. J. Neuroendocrinol. 14, 247–255.
Prossnitz, E. R., Arterburn, J. B., Smith, H. O., Oprea, T. I., Sklar, L. A., and Hathaway, H. J. (2008). Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu. Rev. Physiol. 70, 165–190.
Qiu, J., Bosch, M. A., Tobias, S. C., Grandy, D. K., Scanlan, T. S., Ronnekleiv, O. K., and Kelly, M. J. (2003). Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 23, 9529–9540.
Qiu, J., Ronnekleiv, O. K., and Kelly, M. J. (2008). Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids 73, 985–991.
Radovick, S., Ticknor, C. M., Nakayama, Y., Notides, A. C., Rahman, A., Weintraub, B. D., Cutler, G. B. Jr., and Wondisford, F. E. (1991). Evidence for direct estrogen regulation of the human gonadotropin-releasing hormone gene. J. Clin. Invest. 88, 1649–1655.
Radovick, S., Wray, S., Muglia, L., Westphal, H., Olsen, B., Smith, E., Patriquin, E., and Wondisford, F. E. (1994). Steroid hormone regulation and tissue-specific expression of the human GnRH gene in cell culture and transgenic animals. Horm. Behav. 28, 520–529.
Rage, F., Lee, B. J., Ma, Y. J., and Ojeda, S. R. (1997). Estradiol enhances prostaglandin E2 receptor gene expression in luteinizing hormone-releasing hormone (LHRH) neurons and facilitates the LHRH response to PGE2 by activating a glia-to-neuron signaling pathway. J. Neurosci. 17, 9145–9156.
Ray, P., Ghosh, S. K., Zhang, D. H., and Ray, A. (1997). Repression of interleukin-6 gene expression by 17 beta-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett. 409, 79–85
Revankar, C. M., Cimino, D. F., Sklar, L. A., Arterburn, J. B., and Prossnitz, E. R. (2005). A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630.
Roa, J., Vigo, E., Castellano, J. M., Navarro, V. M., Fernandez-Fernandez, R., Casanueva, F. F., Dieguez, C., Aguilar, E., Pinilla, L., and Tena-Sempere, M. (2006). Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology 147, 2864–2878.
Roepke, T. A., Malyala, A., Bosch, M. A., Kelly, M. J., and Ronnekleiv, O. K. (2007). Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology 148, 4937–4951.
Roy, D., Angelini, N. L., and Belsham, D. D. (1999). Estrogen directly respresses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-alpha (ERalpha)- and ERbeta-expressing GT1-7 GnRH neurons. Endocrinology 140, 5045–5053.
Sanchez-Criado, J. E., de Las Mulas, J. M., Bellido, C., Navarro, V. M., Aguilar, R., Garrido-Gracia, J. C., Malagon, M. M., Tena-Sempere, M., and Blanco, A. (2006). Gonadotropin-secreting cells in ovariectomized rats treated with different oestrogen receptor ligands: a modulatory role for ERbeta in the gonadotrope? J. Endocrinol. 188, 167–177.
Sarkar, D. K., Chiappa, S. A., Fink, G., and Sherwood, N. M. (1976). Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 264, 461–463.
Sarkar, D. K., and Fink, G. (1980). Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: effects of steroids. J. Endocrinol. 86, 511–524.
Savoy-Moore, R. T., Schwartz, N. B., Duncan, J. A., and Marshall, J. C. (1980). Pituitary gonadotropin-releasing hormone receptors during the rat estrous cycle. Science 209, 942–944.
Schomberg, D. W., Couse, J. F., Mukherjee, A., Lubahn, D. B., Sar, M., Mayo, K. E., and Korach, K. S. (1999). Targeted disruption of the estrogen receptor-alpha gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology 140, 2733–2744.
Scully, K. M., Gleiberman, A. S., Lindzey, J., Lubahn, D. B., Korach, K. S., and Rosenfeld, M. G. (1997). Role of estrogen receptor-alpha in the anterior pituitary gland. Mol. Endocrinol. 11, 674–681.
Seminara, S. B., Messager, S., Chatzidaki, E. E., Thresher, R. R., Acierno, J. S. Jr., Shagoury, J. K., Bo-Abbas, Y., Kuohung, W., Kristine, M., Schwinof, M. A., Hendrick, A. G., Zahn, D., Dixon, J. B. A., Kaiser, U. B., Slaugenhaupt, S. A., Gusella, J. F., O’Rahilly, S., Carlton, M. B. L., Crowley, W. F. Jr., Samuel, A. J. R., Aparicio, B. M., and Colledge, W. H. (2003). The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 349, 1614–1627.
Seol, W., Hanstein, B., Brown, M., and Moore, D. D. (1998). Inhibition of estrogen receptor action by the orphan receptor SHP (short heterodimer partner). Mol. Endocrinol. 12, 1551–1557.
Shahab, M., Mastronardi, C., Seminara, S. B., Crowley, W. F., Ojeda, S. R., and Plant, T. M. (2005). Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc. Natl. Acad. Sci. U.S.A. 102, 2129–2134.
Shander, D., and Barraclough, C. A. (1980). Role of the preoptic brain in the regulation of preovulatory gonadotropin surges in the hamster. Exp. Brain Res. 40, 123–130.
Shang, Y., Hu, X., DiRenzo, J., Lazar, M. A., and Brown, M. (2000). Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103, 843–852.
Sharifi, N., Reuss, A. E., and Wray, S. (2002). Prenatal LHRH neurons in nasal explant cultures express estrogen receptor beta transcript. Endocrinology 143, 2503–2507
Shaw, N. D., Srouji, S. S., Histed, S. N., and Hall, J. E. (2011). Differential effects of aging on estrogen negative and positive feedback. Am. J. Physiol. Endocrinol. Metab. 301, E351–E355.
Shaw, N. D., Srouji, S. S., Histed, S. N., McCurnin, K. E., and Hall, J. E. (2009). Aging attenuates the pituitary response to gonadotropin-releasing hormone. J. Clin. Endocrinol. Metab. 94, 3259–3264.
Shen, E. S., Meade, E. H., Perez, M. C., Deecher, D. C., Negro-Vilar, A., and Lopez, F. J. (1998). Expression of functional estrogen receptors and galanin messenger ribonucleic acid in immortalized luteinizing hormone-releasing hormone neurons: estrogenic control of galanin gene expression. Endocrinology 139, 939–948.
Shibata, H., Spencer, T. E., Onate, S. A., Jenster, G., Tsai, S. Y., Tsai, M. J., and O’Malley, B. W. (1997). Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog. Horm. Res. 52, 141–164; discussion 164–165.
Shivers, B. D., Harlan, R. E., Morrell, J. I., and Pfaff, D. W. (1983). Absence of oestradiol concentration in cell nuclei of LHRH- immunoreactive neurones. Nature 304, 345–347.
Shupnik, M. A. (1996). Gonadal hormone feedback on pituitary gonadotropin genes. Trends Endocrinol. Metab. 7, 272–276.
Simerly, R. B. (2002). Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 25, 507–536.
Simerly, R. B., and Swanson, L. W. (1987). Castration reversibly alters levels of cholecystokinin immunoreactivity within cells of three interconnected sexually dimorphic forebrain nuclei in the rat. Proc. Natl. Acad. Sci. U.S.A. 84, 2087–2091.
Singh, S. P., Wolfe, A., Ng, Y., DiVall, S. A., Buggs, C., Levine, J. E., Wondisford, F. E., and Radovick, S. (2009). Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor alpha (ESR1). Biol. Reprod. 81, 488–496.
Sisk, C. L., Richardson, H. N., Chappell, P. E., and Levine, J. E. (2001). In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology 142, 2929–2936.
Skinner, D. C., and Dufourny, L. (2005). Oestrogen receptor beta-immunoreactive neurones in the ovine hypothalamus: distribution and colocalisation with gonadotropin-releasing hormone. J. Neuroendocrinol. 17, 29–39.
Smith, C. L., and O’Malley, B. W. (2004). Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 25, 45–71.
Smith, J. T., Clifton, D. K., and Steiner, R. A. (2006). Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction 131, 623–630.
Smith, J. T., Cunningham, M. J., Rissman, E. F., Clifton, D. K., and Steiner, R. A. (2005). Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146, 3686–3692.
Smith, M. J., and Jennes, L. (2001). Neural signals that regulate GnRH neurones directly during the oestrous cycle. Reproduction 122, 1–10.
Spratt, D. P., and Herbison, A. E. (1997). Regulation of preoptic area gonadotropin-releasing hormone (GnRH) mRNA expression by gonadal steroids in the long-term gonadectomized male rat. Brain Res. Mol. Brain Res. 47, 125–133.
Stein, B., and Yang, M. X. (1995). Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol. Cell. Biol. 15, 4971–4979.
Sullivan, K. A., Witkin, J. W., Ferin, M., and Silverman, A. J. (1995). Gonadotropin-releasing hormone neurons in the rhesus macaque are not immunoreactive for the estrogen receptor. Brain Res. 685, 198–200.
Sun, J., Chu, Z., and Moenter, S. M. (2010). Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J. Neurosci. 30, 3912–3923.
Taga, M., Minaguchi, H., Kigawa, T., and Sakamoto, S. (1982). Effects of sex steroid hormones on rat anterior pituitary LH-RH receptor. Nihon Sanka Fujinka Gakkai Zasshi 34, 627–633.
Temple, J. L., Laing, E., Sunder, A., and Wray, S. (2004). Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J. Neurosci. 24, 6326–6333.
Tora, L., White, J., Brou, C., Tasset, D., Webster, N., Scheer, E., and Chambon, P. (1989). The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 59, 477–487.
Tremblay, A., Tremblay, G. B., Labrie, F., and Giguere, V. (1999). Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol. Cell 3, 513–519.
Tremblay, G. B., Tremblay, A., Copeland, N. G., Gilbert, D. J., Jenkins, N. A., Labrie, F., and Giguere, V. (1997). Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol. Endocrinol. 11, 353–365.
Tsukahara, S., Hojo, R., Kuroda, Y., and Fujimaki, H. (2008). Estrogen modulates Bcl-2 family protein expression in the sexually dimorphic nucleus of the preoptic area of postnatal rats. Neurosci. Lett. 432, 58–63.
Turzillo, A. M., DiGregorio, G. B., and Nett, T. M. (1995). Messenger ribonucleic acid for gonadotropin-releasing hormone receptor and numbers of gonadotropin-releasing hormone receptors in ovariectomized ewes after hypothalamic-pituitary disconnection and treatment with estradiol. J. Anim. Sci. 73, 1784–1788.
Tzukerman, M. T., Esty, A., Santiso-Mere, D., Danielian, P., Parker, M. G., Stein, R. B., Pike, J. W., and McDonnell, D. P. (1994). Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol. Endocrinol. 8, 21–30.
Van der Beek, E. M., Horvath, T. L., Wiegant, V. M., Van den Hurk, R., and Buijs, R. M. (1997). Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J. Comp. Neurol. 384, 569–579.
Vasudevan, N., and Pfaff, D. W. (2007). Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr. Rev. 28, 1–19.
Wang, H., Peters, G. A., Zeng, X., Tang, M., Ip, W., and Khan, S. A. (1995). Yeast two-hybrid system demonstrates that estrogen receptor dimerization is ligand-dependent in vivo. J. Biol. Chem. 270, 23322–23329.
Warembourg, M., Leroy, D., Peytevin, J., and Martinet, L. (1998). Estrogen receptor and progesterone receptor-immunoreactive cells are not co-localized with gonadotropin-releasing hormone in the brain of the female mink (Mustela vison). Cell Tissue Res. 291, 33–41.
Watson, R. E. Jr., Langub, M. C. Jr., and Landis, J. W. (1992). Further evidence that most luteinizing hormone-releasing hormone neurons are not directly estrogen-responsive: simultaneous localization of luteinizing hormone-releasing hormone and estrogen receptor immunoreactivity in the Guinea-pig brain. J. Neuroendocrinol. 4, 311–317.
Wersinger, S. R., Haisenleder, D. J., Lubahn, D. B., and Rissman, E. F. (1999). Steroid feedback on gonadotropin release and pituitary gonadotropin subunit mRNA in mice lacking a functional estrogen receptor alpha. Endocrine 11, 137–143.
White, R., Lees, J. A., Needham, M., Ham, J., and Parker, M. (1987). Structural organization and expression of the mouse estrogen receptor. Mol. Endocrinol. 1, 735–744.
Wiegand, S. J., and Terasawa, E. (1982). Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 34, 395–404.
Wiegand, S. J., Terasawa, E., Bridson, W. E., and Goy, R. W. (1980). Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 31, 147–157.
Wierman, M. E., Kepa, J. K., Sun, W., Gordon, D. F., and Wood, W. M. (1992). Estrogen negatively regulates rat gonadotropin releasing hormone (rGnRH) promoter activity in transfected placental cells. Mol. Cell. Endocrinol. 86, 1–10.
Wintermantel, T. M., Campbell, R. E., Porteous, R., Bock, D., Grone, H. J., Todman, M. G., Korach, K. S., Greiner, E., Pérez, C. A., Schütz, G., and Herbison, A. E. (2006). Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52, 271–280.
Wise, P. M. (1982). Alterations in proestrous LH, FSH, and prolactin surges in middle-aged rats. Proc. Soc. Exp. Biol. Med. 169, 348–354.
Wolfe, A. M., Wray, S., Westphal, H., and Radovick, S. (1995). Cell-specific expression of the human gonadotropin-releasing hormone gene in transgenic animals. J. Biol. Chem. 271, 20018–20023.
Wray, S., Zoeller, R. T., and Gainer, H. (1989). Differential effects of estrogen on luteinizing hormone-releasing hormone gene expression in slice explant cultures prepared from specific rat forebrain regions. Mol. Endocrinol. 3, 1197–1206.
Xia, L., Van Vugt, D., Alston, E. J., Luckhaus, J., and Ferin, M. (1992). A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology 131, 2812–2820.
Xu, J., and O’Malley, B. W. (2002). Molecular mechanisms and cellular biology of the steroid receptor coactivator (SRC) family in steroid receptor function. Rev. Endocr. Metab. Disord. 3, 185–192.
Zhang, C., Bosch, M. A., Levine, J. E., Ronnekleiv, O. K., and Kelly, M. J. (2007). Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J. Neurosci. 27, 10153–10164.
Zhang, C., Roepke, T. A., Kelly, M. J., and Ronnekleiv, O. K. (2008). Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J. Neurosci. 28, 4423–4434.
Keywords: GnRH, kisspeptin, GPR54, estrogen receptor, feedback, progesterone receptor, arcuate, AVPV
Citation: Radovick S, Levine JE and Wolfe A (2012) Estrogenic regulation of the GnRH neuron. Front. Endocrin. 3:52. doi: 10.3389/fendo.2012.00052
Received: 13 December 2011; Accepted: 16 March 2012;
Published online: 09 April 2012.
Edited by:
Henryk Urbanski, Oregon National Primate Research Center, USAReviewed by:
A. Kemal Topaloglu, Cukorova University Faculty of Medicine, TurkeyCopyright: © 2012 Radovick, Levine and Wolfe. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Andrew Wolfe, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA. e-mail:YXdvbGZlM0BqaG1pLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.