- Department of Women’s and Children’s Health, Uppsala University, Uppsala, Sweden
Thyroid disease often causes menstrual disturbances and infertility problems. Thyroid hormone (TH) acts through its receptors, transcription factors present in most cell types in the body. Thyroid stimulating hormone (TSH) stimulates TH synthesis in the thyroid gland, but seems to have other functions as well in the female reproductive tract. The receptors of both TH and TSH increase in the receptive endometrium, suggesting that they are important for implantation, possible by influencing inflammatory mediators such as leukemia inhibitory factor. The roles of these receptors in the ovary need further studies. However, it is likely that the thyroid system is important for both follicular and embryo development. The association between thyroid disease and infertility indicate that TH and TSH affect the endometrium and ovary on the paracrine level.
Introduction
It is well known that thyroid hormones (THs) are related to several aspects of human reproduction. Hypo- and hyperthyroidism affect metabolism of sex steroids and ovarian function in women, which is often seen as menstrual irregularities (Norman and Litwack, 1987; Krassas et al., 1994, 1999). The role of TH in infertility is less understood, although TH deficiency is more common in women with polycystic ovarian syndrome (PCOS) and to some extent also in women with infertility of unknown cause. Very few studies exist on the paracrine effect and regulation of THs and its receptors in the female reproductive tract. The aim of this mini review is to summarize possible mechanisms of TH action in female fertility.
Physiology of Thyroid Hormone Regulation
Thyroid Hormones
Thyroid hormones produced by the thyroid gland are involved in several processes such as growth, development, differentiation, and metabolism throughout the body. There are two major types of THs, thyroxine (T4), the main secretory product and pro-hormone for and triiodothyronine (T3), the most biologically active form (Song et al., 2011). T4 is converted to T3 using thyroglobulin (TG), a glycoprotein and deiodinases (DOI), selene-containing proteins that occurs in three isoforms. DOI1 and DOI2 are involved in the conversion from T4 to T3 while DOI3 catalyzes the conversion of T4 to an inactive form of T3 (rT3; Song et al., 2011).
Thyroid peroxidase or thyroperoxidase (TPO) is a membrane bound hemoprotein that plays an essential role in TH synthesis (Ris-Stalpers and Bikker, 2010). TH synthesis is stimulated by the pituitary hormone thyroid stimulating hormone (TSH) that acts through the G-protein-coupled TSH receptor (TSHR; Ris-Stalpers and Bikker, 2010).
Thyroid Hormone Receptors
Thyroid hormones regulate through binding to their nuclear receptors (TRs), which have been identified in many tissues and organs of the human body (Yen, 2001). TH receptors (TRs) belong to the steroid/retinoid/TH nuclear receptor family all having a similar structure involving a, enhancing N-terminal transactivation domain, a DNA-binding domain, and a carboxyl-binding ligand binding domain (Yen, 2001). The DNA-binding domain contain cysteine–zinc fingers which binds to TH response elements on the target genes, and the ligand binding domain is responsible for TH binding and dimerization (Yen, 2001). Binding of THs leads to conformational change of the receptors, chromatin modification, and activation of transcription (Yen, 2001). In the absence of TH, the receptors can be bound to TRE and repress transcription (Yen, 2001). The transcriptional activity of TRs is regulated through various mechanism such as diversity of TR isoforms, several TREs, different heterodimeric partners, a number of co-regulators, and the cellular location of TS (Song et al., 2011).
There are two major types of TRs, TRα, and TRβ, transcribed from different chromosomes, number 17 and number 3 respectively (Lazar, 1993). Both TR isoforms mediate TH-regulated gene expression after binding to T3. Alternative splicing of TRα gene give rise to the either TRα1 or TRα2 or TRα3, which differ in their carboxyl terminus due to alternative splicing (Song et al., 2011). TRα1 binds T3 to either activate or repress target genes while the other two splicing variants lose T3 binding capacity and antagonize T3 action (Izumo and Mahdavi, 1988; Mitsuhashi et al., 1988; Macchia et al., 2001). The TRβ gene exists in three isoforms, TRβ1, TRβ2, and TRβ1, which differ in their amino acid terminus. All three are able to bind T3 with high affinity to mediate transcription (Hodin et al., 1989). The TRα and TRβ isoforms are ubiquitously expressed in rat and human tissues, the distribution regulate TH action in the target tissue (Song et al., 2011; Hodin et al., 1990; Shahrara et al., 1999).
Non-genomic actions of THs are associated with secondary messenger signaling pathways, and are generally occurring within a few minutes (Losel and Wehling, 2003; Tang et al., 2004). These effects are promoted by T4 binding to the cell surface thereby activating MAPK complexes with TRβ1 (Davis et al., 2000).
Although THs affect a wide range of cells and tissues in the body, they do not seem to be strictly necessary for life and only TRβ seem to be important for fertility in mice (Forrest et al., 1996; Wikstrom et al., 1998). TRα1 or TRβ knock-out mice are fertile while females rarely became pregnant and were deficient in nurturing any pups born (Forrest et al., 1996; Wikstrom et al., 1998), which suggest that female reproductive processes in mice depend on common pathways regulated by both TRα1 and TRβ.
Circulating Thyroid Hormone
Thyroxine-binding globulin (TBG) is responsible for transportation of in circulation. It has been assumed that 75% of T4 is bound to TBG, the rest is bound to transthyretin or albumin or is transported as a free fraction (Tahboub and Arafah, 2009). Endogenous estrogen (during pregnancy) or exogenous estrogen increase serum TBG concentration as well as sex steroid binding globulin (SHBG) while progesterone does not seem to have this effect (Tahboub and Arafah, 2009).
Hormonal Regulation of Thyroid Hormone Secretion
Hormonal regulation of TH synthesis involves stimulation by thyrotropin releasing hormone (TRH) from the hypothalamus to synthesize TSH from the pituitary anterior pituitary gland (Wolf, 2002). TSH is regulated through TR homodimer of heterodimerization with 9-cis-retinoic acid-activated retinoid X receptor binding to the TSH gene, thereby regulating the expression of the TSH gene (Wolf, 2002). TSH stimulates thyroglobulin hydrolysis and increase of iodine metabolism leading to the secretion of THs from the thyroid gland (Yen, 2001). The short- and long-term actions of TSH in the primary regulator of T3 and T4 release and secretion and plays a critical role in thyroid growth and development (Norman and Litwack, 1987). Increased serum levels of THs leads a negative feedback on the hypothalamus and pituitary leading to reduced TRH and TSH secretion (Yen, 2001).
The hypothalamus produces gonadotropin-releasing hormone (GnRH), which stimulates the pituitary gland to produce luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which in turn control the production of estradiol and progesterone. The hypothalamic–pituitary–thyroid axis interact with the GnRH–LH/FSH–estradiol/progesterone (Yen, 2001).
Estrogens also have the ability to increase the number of TRH receptors in the pituitary (Uribe et al., 2009). Because of increased TRH production in hypothyroidism, hyperprolactinemia, and altered GnRH pulsatile secretion can lead to a delay in LH response and inadequate corpus luteum function (Longcope et al., 1990).
Thyroid Stimulating Hormone Receptor
Thyroid stimulating hormone binds to the TSHR, which is a trans-membrane receptor that belongs to the G-protein-coupled receptor super family. TSHR is a key regulator of TH function (Davies et al., 2010). Binding of TSH leads to a conformational change, which activates the α-subunit of the heterotrimeric G-protein thereby activating activates cAMP production and activation of proteinkinase A, which mediates most TSH effects, especially its short-term actions (Weiss et al., 1984; Davies et al., 2002; Dremier et al., 2002).
Thyroid stimulating hormone receptor is not only located in human thyroid tissue (Mizukami et al., 1994), extra-thyroidal TSHR expression has been noticed in a variety of cell types such as kidney, thymus, cardiac muscle, and adipose tissue cells (Davies et al., 2002). TSH treatment leads to up-regulation of THSR mRNA (Huber et al., 1991, 1992).
Thyroid Hormone Disease
Hypothyreosis
The incidence of thyroid disorders increases with age and is more common in women than in men (Premawardhana and Lazarus, 2006). In developed countries, autoimmune thyroid disease (AITD), which includes subclinical hypothyroidism mainly Hashimoto’s thyroiditis and overt hypothyroidism, is the most common cause of thyroid deficiency and affects 5–15% among the women in reproductive age (Vanderpump et al., 1995; Jacobson et al., 1997; Wang and Crapo, 1997). AITD is characterized by the presence of autoantibodies against thyroglobulin (TG) or TPO. Hypothyroidism is seen as an increase in serum TSH levels and decrease in serum T4 concentration as a result of negative feedback. Thyroxin is the standard treatment of these women. Hypothyroidism it is demonstrated by menorrhagia or oligomenorrhea, pregnancy loss and/or infertility (Krassas et al., 1999), the severity correlated with THS concentration in serum (Krassas et al., 1999).
There are suggestions on restricting the upper limit of normal serum TSH to TSH to 2.5 mIU/L (Poppe et al., 2007; Dittrich et al., 2009), however there is no consensus among thyroidologists regarding the most appropriate upper limit of normal for serum TSH. A recent study involving 1054 IVF cycles, no differences in the rates of clinical pregnancy, delivery, or miscarriage were observed (Reh et al., 2010).
Infertility
The prevalence of AITD is higher among infertile women compared to fertile women (Joshi et al., 1993; Kaider et al., 1999; Grassi et al., 2001; Poppe et al., 2002; Premawardhana and Lazarus, 2006; Krassas et al., 2010), and a screening for thyroid dysfunction has been suggested (Poppe et al., 2008). However, most studies fail to demonstrate a connection between AITD and infertility (Roussev et al., 1996; Lincoln et al., 1999; Kutteh et al., 1999; Arojoki et al., 2000; Reimand et al., 2001), which might be due to higher prevalence of subclinical hypothyreosis without thyroid antibodies in often seen in patients with infertility (Negro et al., 2005; Abalovich et al., 2007), and that treatment with levothyroxine does not increase pregnancy or delivery rate (Negro et al., 2005). A Finnish study including 335 infertile women demonstrated highest TSH levels in women with unexplained infertility (Arojoki et al., 2000).
Considering that significantly higher serum TSH and the incidence of TPO antibodies is higher in infertile women compared to fertile controls (Poppe and Velkeniers, 2003), which indicate that AITD predispose for infertility disorders such as PCOS and endometriosis.
Polycystic Ovarian Syndrome
The prevalence of AITD is higher in women with PCOS compared with healthy fertile women (Gerhard et al., 1991; Poppe and Velkeniers, 2003; Janssen et al., 2004; Kachuei et al., 2012). Mueller et al. (2009) found a significant association between thyroid function, as reflected by TSH ≥2 mIU/L, and insulin resistance in patients with PCOS. Polymorphism in TH associated protein, a co-factor in TR regulation, could also offer an explanation to the relation between PCOS and the thyroid system (Goodarzi et al., 2012). High anti-TPO resulted in clomiphene citrate resistance and poor treatment response during fertility treatment of PCOS patients (Ott et al., 2010).
Endometriosis
Poppe et al. (2002) showed in a prospective study that the relative risk of endometriosis was significantly increased in women positive for TPO antibodies, while other studies fail to show a connection between AITD and endometriosis (Petta et al., 2007). TRH is reduced in endometrium from women with endometriosis compared to health controls, and epigenetic changes, e.g., methylation pattern and transcriptional repression influence TH pathway by activation of TH in the endometrium from women with endometriosis (Aghajanova and Giudice, 2011; Zelenko et al., 2012).
Hyperthyroidism
Hyperthyroidism is characterized by suppressed TSH and elevated T3 and T4, most commonly seen in women of reproductive age is Grave’s disease. The prevalence of Grave’s disease is 0.4–1.6% in developed countries (Furszyfer et al., 1972; Tunbridge et al., 1977; Jacobson et al., 1997; Aghini-Lombardi et al., 1999) The exact impact on Grave’s disease remains ill-defined as most data derives from retrospective cohort studies (Krassas et al., 1999), genetic variation in the TSHR gene has been associated with Grave’s disease (Davies et al., 2010). Like hypothyroidism, hyperthyroidism also leads to menstrual disturbances, often seen as oligomenorrhea (Goldsmith et al., 1952).
Hyperthyreosis leads to increased levels of SHBG and an increase in total estradiol without change in free estradiol levels (Akande and Hockaday, 1972). The conversion ratio of the androgens androstenedione and testosterone to estrone and estradiol respectively is increased in hyperthyroid women (Southren et al., 1974; Krassas and Pontikides, 2005). In addition, the patients also have altered GnRH-induced LH secretion, which is shown by that serum levels of LH is increased and the pulsatile pattern of LH seem to be disturbed, leading to changes in menstrual pattern (Akande, 1974).
Thyroid Disorders and Miscarriage
The presence of AITD increases the incidence of first trimester pregnancy loss after IVF treatment (Bussen et al., 2000; Negro et al., 2005). Thyroid treatment is beneficial for women with suffering from recurrent miscarriage (Vaquero et al., 2006). The mechanism behind AITD and recurrent pregnancy has been debated. One theory is that AITD does not have a direct effect, it is just a marker of a general autoimmune imbalance responsible for rejection of the fetal graft (Bussen et al., 2000). A second theory is that AITD reflects a thyroid dysfunction with decreased ability to adapt to the increased requirements of pregnancy (Krassas et al., 1999; Bussen et al., 2000). The third is that women with AITD have an underlying fertility problem leading to conception at a later age, thereby increasing the risk of pregnancy loss (Bussen et al., 2000). A recent study found that although TSH was well below the conventional upper cut-off levels, less than 1.5 μIU/ml, inadequate adaptation to the increased thyroid requirement might result in recurrent miscarriage (Dal Lago et al., 2011).
Genetic Variation and Fertility
CT60 cytotoxic T lymphocyte antigen-4 gene polymorphism was shown to be associated with thyroid autoantibody production in patients with Hashimoto’s and postpartum thyroiditis (Zaletel et al., 2010).
Single nucleotide polymorphism rs4704397 in the phosphodiesterase 8B (PDE8B) gene associated with altered serum TSH concentrations was not associated with offspring birth weight or gestational age at delivery (Shields et al., 2009). TRα1 mutation results in repressed peroxisome proliferator-activated receptor gamma (PPARgamma; Ying et al., 2007), which might impair trophoblast invasiveness and placentation (Fournier et al., 2011).
Environmental Factors
Endocrine disruptors act with agonistic or antagonistic effects on steroid hormone receptors and may alter reproductive function and/or cause feminization by binding to estrogen or androgen receptors. Increasing evidence show that environmental factors such as endocrine disruptors influence reproduction in mammals, fish, birds, reptiles, aquatic invertebrates; the effect in humans being less studied. It has been discussed that PCBs, phthalates, bisphenol A, brominated flame retardants, and perfluorinated chemicals may have thyroid disrupting properties (Boas et al., 2011). Phthalates adversely affected the level of reproductive hormones (LH, free testosterone, sex hormone-binding globulin), anogenital distance and thyroid function (Jurewicz and Hanke, 2011), and perchlorate given to lactating rhesus monkeys suppressed iodine uptake (Ozpinar et al., 2011). However, bisphenol A in patients were significantly higher in women with recurrent miscarriage than control women, without direct effect on thyroid function (Sugiura-Ogasawara et al., 2005). The increase in infertility rate seen in the western world might not only be influenced by the fact that women tend to have their first child later in life, environmental factors influencing fertility might also play a role.
Expression of Thyroid Hormone Receptors in Reproductive Tissue
Endometrium
TRα1 and TRβ1 and TSHR are expressed in human endometrium, the highest level is seen in the receptive endometrium (Aghajanova et al., 2011). Several paracrine factors are of importance for successful embryo implantation, leukemia inhibitory factor (LIF), and Leptin being among the most studied (Stewart et al., 1992; Chehab et al., 1996; Antczak and Van Blerkom, 1997; Cioffi et al., 1997; Gonzalez et al., 2000, 2004; Kawamura et al., 2002; Aghajanova, 2004). TSH increase LIF and LIF receptor expression in endometrial epithelial ishikawa cells and in thyroid cells (Ren et al., 1999; Aghajanova et al., 2011). TSH releases leptin in human adipose tissue culture (Menendez et al., 2003), and it is possible that this effect is present also in the human endometrium, thereby playing a role for successful implantation (Figure 1).
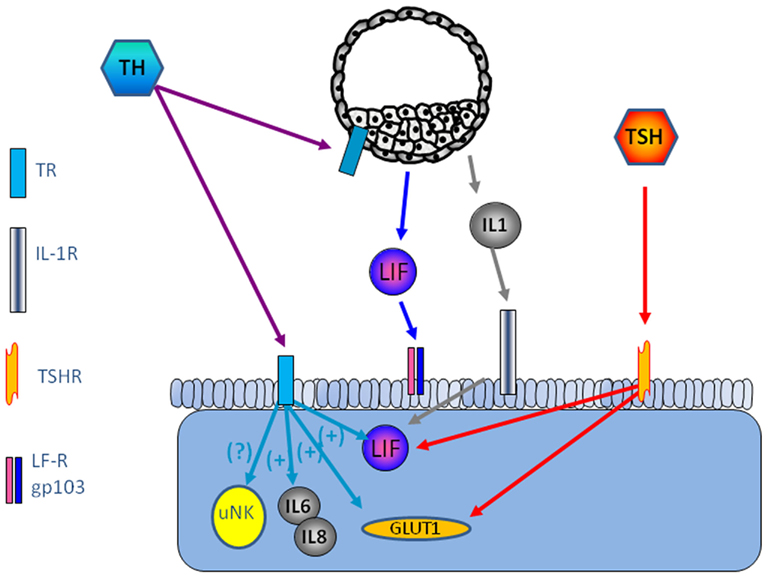
Figure 1. Possible interaction between the embryo and endometrium is shown. The thyroid system is involved in the regulation of LIF and interleukins, key factors for successful implantation.
The expression of TSH receptors leads to the question if there is a possibility that TSH induce TH secretion from other cells than the thyroid gland. Our recent study show increased secretion of free triiodothyronine (T3) and total thyroxin (T4) by Ishikawa cells compared with non-stimulated cells (Aghajanova et al., 2011), and a previous study show that progesterone is involved in TH signaling such as type II iodothyronine deiodinase and TRs, TPO, and TG in the endometrium. Transcripts required for TH synthesis such as thyroid peroxidase (TPO) and thyroglobulin (TG), which further suggest that the endometrium may be a site of thyroxin production (Catalano et al., 2007) (Figure 1).
Thyroid hormones have been suggested at modulators of immune response such as influence on cytokines and NK-cells (De Vito et al., 2011). CD56 positive NK cell level has been shown to be significantly higher in women with miscarriage (Kim et al., 2011). Interleukins are important factors in endometrial development and implantation. In tears from patients with thyroid-associated ophthalmopathy, the interleukins IL-1β, IL-6, and IL-8 were significantly higher than healthy controls (Huang et al., 2011). It is not known if TH is involved n the regulation of inflammatory mediators in the endometrium (Figure 1).
Furthermore, as TSH and THs regulate glucose transport via glucose transporters (GLUT), in particular GLUT1 (Hosaka et al., 1992; Weinstein and Haber, 1993), which makes TH as a candidate for regulation of GLUT1 in decidualized endometrium, mouse blastocysts (Pantaleon et al., 2001), and morula–blastocyst transition in mice (Leppens-Luisier et al., 2001) (Figure 1).
Leptin has been suggested to influence the thyroidal axis by regulating the expression of TSH (Sanchez et al., 2004). Leptin and TSH share the same pulsatile pattern reaching the highest levels during morning hours, which might explain why some individuals with leptin deficiency have abnormal TSH secretion pattern (Mantzoros et al., 2001). However, most individuals with leptin deficiency have normal TSH levels (Legradi et al., 1997; Paz-Filho et al., 2009).
Ovary
Thyroid hormones are present in follicular fluid and TRs are present in granulosa cells (Wakim et al., 1993, 1994; Aghajanova et al., 2009) and in primordial, primary, and secondary follicles (Aghajanova et al., 2009). Mature (MII) oocytes from women undergoing IVF demonstrate the presence of TRα1, TRα2, TRβ1, and TRβ2 mRNA, supporting the hypothesis that T3 has a direct effect on the human oocyte (Zhang et al., 1997). Our study show TSHR, TRα1, and TRβ1 and deiodinases types 2 and 3 in combination with activation of cAMP by TSH indicates a possibility of conversion of peripheral T4 on ovarian tissue (Aghajanova et al., 2009). Failure of oocytes to get fertilized during IVF treatment was correlated with increased TSH in serum (Cramer et al., 2003). Development of bovine embryos was shown to benefit from TH-treatment (Ashkar et al., 2010). TH-treatment of embryos increased bovine blastocyst formation and hatching rates as well post-cryopreservation viability (Ashkar et al., 2010). Despite this, no difference in TR was seen during development of human embryo indicating that THs are important throughout development (Zhang et al., 2009).
In cases of hyperthyroidism, the ultrasonography appearance of the ovaries showed an abnormal size, form, and number of follicle. The appearance became normal after treatment (Skjoldebrand Sparre et al., 2002). Multicystic ovaries during profound hypothyroidism has also been noticed (Van Voorhis et al., 1994), which suggest a direct effect of THs on the ovary (Maruo et al., 1992). High TSH was also seen in women with ovulatory dysfunction compared with tubal infertility and male infertility (Arojoki et al., 2000).
Thyroid stimulating hormone receptor in the ovary is up-regulated by gonadotropin-driven cascade and down-regulated by estradiol. The relatively recently discovered glycoprotein thyrostimulin is expressed in the ovary and was shown to activate ovarian TSH receptor expression, suggesting a paracrine regulation of thyroid function in the rat ovary (Sun et al., 2010).
Conclusion
Thyroid hormone as well as TSH seem to involved in regulation of the endometrium to a receptive phase and is thereby also likely to play a role during embryo implantation. Of special interest is the role of THs on inflammatory mediators such as LIF, one of the most important cytokines in female reproduction. Furthermore, TH as well as TSH seem to play an important role in normal ovarian function, which clinically evident in the relation between PCOS and thyroid disease.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abalovich, M., Mitelberg, L., Allami, C., Gutierrez, S., Alcaraz, G., Otero, P., and Levalle, O. (2007). Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. Gynecol. Endocrinol. 23, 279–283.
Aghajanova, L. (2004). Leukemia inhibitory factor and human embryo implantation. Ann. N. Y. Acad. Sci. 1034, 176–183.
Aghajanova, L., and Giudice, L. C. (2011). Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod. Sci. 18, 229–251.
Aghajanova, L., Lindeberg, M., Carlsson, I. B., Stavreus-Evers, A., Zhang, P., Scott, J. E., Hovatta, O., and Skjöldebrand-Sparre, L. (2009). Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod. Biomed. Online 18, 337–347.
Aghajanova, L., Stavreus-Evers, A., Lindeberg, M., Landgren, B. M., Sparre, L. S., and Hovatta, O. (2011). Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil. Steril. 95, 230–237, 237.e1–237.e2.
Aghini-Lombardi, F., Antonangeli, L., Martino, E., Vitti, P., Maccherini, D., Leoli, F., Rago, T., Grasso, L., Valeriano, R., Balestrieri, A., and Pinchera, A. (1999). The spectrum of thyroid disorders in an iodine-deficient community: the Pescopagano survey. J. Clin. Endocrinol. Metab. 84, 561–566.
Akande, E. O. (1974). The effect of oestrogen on plasma levels of luteinizing hormone in euthyroid and thyrotoxic postmenopausal women. J. Obstet. Gynaecol. Br. Commonw. 81, 795–803.
Akande, E. O., and Hockaday, T. D. (1972). Plasma oestrogen and luteinizing hormone concentrations in thyrotoxic menstrual disturbance. Proc. R. Soc. Med. 65, 789–790.
Antczak, M., and Van Blerkom, J. (1997). Oocyte influences on early development: the regulatory proteins leptin and STAT3 are polarized in mouse and human oocytes and differentially distributed within the cells of the preimplantation stage embryo. Mol. Hum. Reprod. 3, 1067–1086.
Arojoki, M., Jokimaa, V., Juuti, A., Koskinen, P., Irjala, K., and Anttila, L. (2000). Hypothyroidism among infertile women in Finland. Gynecol. Endocrinol. 14, 127–131.
Ashkar, F. A., Semple, E., Schmidt, C. H., St John, E., Bartlewski, P. M., and King, W. A. (2010). Thyroid hormone supplementation improves bovine embryo development in vitro. Hum. Reprod. 25, 334–344.
Boas, M., Feldt-Rasmussen, U., and Main, K. M. (2011). Thyroid effects of endocrine disrupting chemicals. Mol. Cell Endocrinol. [Epub ahead of print].
Bussen, S., Steck, T., and Dietl, J. (2000). Increased prevalence of thyroid antibodies in euthyroid women with a history of recurrent in-vitro fertilization failure. Hum. Reprod. 15, 545–548.
Catalano, R. D., Critchley, H. O., Heikinheimo, O., Baird, D. T., Hapangama, D., Sherwin, J. R., Charnock-Jones, D. S., Smith, S. K., and Sharkey, A. M. (2007). Mifepristone induced progesterone withdrawal reveals novel regulatory pathways in human endometrium. Mol. Hum. Reprod. 13, 641–654.
Chehab, F. F., Lim, M. E., and Lu, R. (1996). Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 12, 318–320.
Cioffi, J. A., Van Blerkom, J., Antczak, M., Shafer, A., Wittmer, S., and Snodgrass, H. R. (1997). The expression of leptin and its receptors in pre-ovulatory human follicles. Mol. Hum. Reprod. 3, 467–472.
Cramer, D. W., Sluss, P. M., Powers, R. D., McShane, P., Ginsburgs, E. S., Hornstein, M. D., Vitonis, A. F., and Barbieri, R. L. (2003). Serum prolactin and TSH in an in vitro fertilization population: is there a link between fertilization and thyroid function? J. Assist. Reprod. Genet. 20, 210–215.
Dal Lago, A., Vaquero, E., Pasqualetti, P., Lazzarin, N., De Carolis, C., Perricone, R., and Moretti, C. (2011). Prediction of early pregnancy maternal thyroid impairment in women affected with unexplained recurrent miscarriage. Hum. Reprod. 26, 1324–1330.
Davies, T., Marians, R., and Latif, R. (2002). The TSH receptor reveals itself. J. Clin. Invest. 110, 161–164.
Davies, T. F., Yin, X., and Latif, R. (2010). The genetics of the thyroid stimulating hormone receptor: history and relevance. Thyroid 20, 727–736.
Davis, P. J., Shih, A., Lin, H. Y., Martino, L. J., and Davis, F. B. (2000). Thyroxine promotes association of mitogen-activated protein kinase and nuclear thyroid hormone receptor (TR) and causes serine phosphorylation of TR. J. Biol. Chem. 275, 38032–38039.
De Vito, P., Incerpi, S., Pedersen, J. Z., Luly, P., Davis, F. B., and Davis, P. J. (2011). Thyroid hormones as modulators of immune activities at the cellular level. Thyroid 21, 879–890.
Dittrich, R., Kajaia, N., Cupisti, S., Hoffmann, I., Beckmann, M. W., and Mueller, A. (2009). Association of thyroid-stimulating hormone with insulin resistance and androgen parameters in women with PCOS. Reprod. Biomed. Online 19, 319–325.
Dremier, S., Coulonval, K., Perpete, S., Vandeput, F., Fortemaison, N., Van Keymeulen, A., Deleu, S., Ledent, C., Clément, S., Schurmans, S., Dumont, J. E., Lamy, F., Roger, P. P., and Maenhaut, C. (2002). The role of cyclic AMP and its effect on protein kinase A in the mitogenic action of thyrotropin on the thyroid cell. Ann. N. Y. Acad. Sci. 968, 106–121.
Forrest, D., Hanebuth, E., Smeyne, R. J., Everds, N., Stewart, C. L., Wehner, J. M., and Curran, T. (1996). Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. EMBO J. 15, 3006–3015.
Fournier, T., Guibourdenche, J., Handschuh, K., Tsatsaris, V., Rauwel, B., Davrinche, C., and Evain-Brion, D. (2011). PPARgamma and human trophoblast differentiation. J. Reprod. Immunol. 90, 41–49.
Furszyfer, J., Kurland, L. T., McConahey, W. M., Woolner, L. B., and Elveback, L. R. (1972). Epidemiologic aspects of Hashimoto’s thyroiditis and Graves’ disease in Rochester, Minnesota (1935–1967), with special reference to temporal trends. Metab. Clin. Exp. 21, 197–204.
Gerhard, I., Becker, T., Eggert-Kruse, W., Klinga, K., and Runnebaum, B. (1991). Thyroid and ovarian function in infertile women. Hum. Reprod. 6, 338–345.
Goldsmith, R. E., Sturgis, S. H., Lerman, J., and Stanbury, J. B. (1952). The menstrual pattern in thyroid disease. J. Clin. Endocrinol. Metab. 12, 846–855.
Gonzalez, R. R., Caballero-Campo, P., Jasper, M., Mercader, A., Devoto, L., Pellicer, A., and Simon, C. (2000). Leptin and leptin receptor are expressed in the human endometrium and endometrial leptin secretion is regulated by the human blastocyst. J. Clin. Endocrinol. Metab. 85, 4883–4888.
Gonzalez, R. R., Rueda, B. R., Ramos, M. P., Littell, R. D., Glasser, S., and Leavis, P. C. (2004). Leptin-induced increase in leukemia inhibitory factor and its receptor by human endometrium is partially mediated by interleukin 1 receptor signaling. Endocrinology 145, 3850–3857.
Goodarzi, M. O., Jones, M. R., Li, X., Chua, A. K., Garcia, O. A., Chen, Y. D., Krauss, R. M., Rotter, J. I., Ankener, W., Legro, R. S., Azziz, R., Strauss, J. F. III, Dunaif, A., and Urbanek, M. (2012). Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J. Med. Genet. 49, 90–95.
Grassi, G., Balsamo, A., Ansaldi, C., Balbo, A., Massobrio, M., and Benedetto, C. (2001). Thyroid autoimmunity and infertility. Gynecol. Endocrinol. 15, 389–396.
Hodin, R. A., Lazar, M. A., and Chin, W. W. (1990). Differential and tissue-specific regulation of the multiple rat c-erbA messenger RNA species by thyroid hormone. J. Clin. Invest. 85, 101–105.
Hodin, R. A., Lazar, M. A., Wintman, B. I., Darling, D. S., Koenig, R. J., Larsen, P. R., Moore, D. D., and Chin, W. W. (1989). Identification of a thyroid hormone receptor that is pituitary-specific. Science 244, 76–79.
Hosaka, Y., Tawata, M., Kurihara, A., Ohtaka, M., Endo, T., and Onaya, T. (1992). The regulation of two distinct glucose transporter (GLUT1 and GLUT4) gene expressions in cultured rat thyroid cells by thyrotropin. Endocrinology 131, 159–165.
Huang, D., Xu, N., Song, Y., Wang, P., and Yang, H. (2011). Inflammatory cytokine profiles in the tears of thyroid-associated ophthalmopathy. Graefes Arch. Clin. Exp. Ophthalmol.
Huber, G. K., Concepcion, E. S., Graves, P. N., and Davies, T. F. (1991). Positive regulation of human thyrotropin receptor mRNA by thyrotropin. J. Clin. Endocrinol. Metab. 72, 1394–1396.
Huber, G. K., Weinstein, S. P., Graves, P. N., and Davies, T. F. (1992). The positive regulation of human thyrotropin (TSH) receptor messenger ribonucleic acid by recombinant human TSH is at the intranuclear level. Endocrinology 130, 2858–2864.
Izumo, S., and Mahdavi, V. (1988). Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature 334, 539–542.
Jacobson, D. L., Gange, S. J., Rose, N. R., and Graham, N. M. (1997). Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 84, 223–243.
Janssen, O. E., Mehlmauer, N., Hahn, S., Offner, A. H., and Gartner, R. (2004). High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur. J. Endocrinol. 150, 363–369.
Joshi, J. V., Bhandarkar, S. D., Chadha, M., Balaiah, D., and Shah, R. (1993). Menstrual irregularities and lactation failure may precede thyroid dysfunction or goitre. J. Postgrad. Med. 39, 137–141.
Jurewicz, J., and Hanke, W. (2011). Exposure to phthalates: reproductive outcome and children health. A review of epidemiological studies. Int. J. Occup. Med. Environ. Health 24, 115–141.
Kachuei, M., Jafari, F., Kachuei, A., and Keshteli, A. H. (2012). Prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Arch. Gynecol. Obstet. 285, 853–856.
Kaider, A. S., Kaider, B. D., Janowicz, P. B., and Roussev, R. G. (1999). Immunodiagnostic evaluation in women with reproductive failure. Am. J. Reprod. Immunol. 42, 335–346.
Kawamura, K., Sato, N., Fukuda, J., Kodama, H., Kumagai, J., Tanikawa, H., Nakamura, A., and Tanaka, T. (2002). Leptin promotes the development of mouse preimplantation embryos in vitro. Endocrinology 143, 1922–1931.
Kim, N. Y., Cho, H. J., Kim, H. Y., Yang, K. M., Ahn, H. K., Thornton, S., Park, J. C., Beaman, K., Gilman-Sachs, A., and Kwak-Kim, J. (2011). Thyroid autoimmunity and its association with cellular and humoral immunity in women with reproductive failures. Am. J. Reprod. Immunol. 65, 78–87.
Krassas, G. E., and Pontikides, N. (2005). Gonadal effect of radiation from 131I in male patients with thyroid carcinoma. Arch. Androl. 51, 171–175.
Krassas, G. E., Pontikides, N., Kaltsas, T., Papadopoulou, P., and Batrinos, M. (1994). Menstrual disturbances in thyrotoxicosis. Clin. Endocrinol. (Oxf.) 40, 641–644.
Krassas, G. E., Pontikides, N., Kaltsas, T., Papadopoulou, P., Paunkovic, J., Paunkovic, N., and Duntas, L. H. (1999). Disturbances of menstruation in hypothyroidism. Clin. Endocrinol. (Oxf.) 50, 655–659.
Krassas, G. E., Poppe, K., and Glinoer, D. (2010). Thyroid function and human reproductive health. Endocr. Rev. 31, 702–755.
Kutteh, W. H., Schoolcraft, W. B., and Scott, R. T. Jr. (1999). Antithyroid antibodies do not affect pregnancy outcome in women undergoing assisted reproduction. Hum. Reprod. 14, 2886–90.
Lazar, M. A. (1993). Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr. Rev. 14, 184–193.
Legradi, G., Emerson, C. H., Ahima, R. S., Flier, J. S., and Lechan, R. M. (1997). Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology 138, 2569–2576.
Leppens-Luisier, G., Urner, F., and Sakkas, D. (2001). Facilitated glucose transporters play a crucial role throughout mouse preimplantation embryo development. Hum. Reprod. 16, 1229–1236.
Lincoln, S. R., Ke, R. W., and Kutteh, W. H. (1999). Screening for hypothyroidism in infertile women. J. Reprod. Med. 44, 455–457.
Longcope, C., Abend, S., Braverman, L. E., and Emerson, C. H. (1990). Androstenedione and estrone dynamics in hypothyroid women. J. Clin. Endocrinol. Metab. 70, 903–907.
Losel, R., and Wehling, M. (2003). Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 4, 46–56.
Macchia, P. E., Takeuchi, Y., Kawai, T., Cua, K., Gauthier, K., Chassande, O., Seo, H., Hayashi, Y., Samarut, J., Murata, Y., Weiss, R. E., and Refetoff, S. (2001). Increased sensitivity to thyroid hormone in mice with complete deficiency of thyroid hormone receptor alpha. Proc. Natl. Acad. Sci. U.S.A. 98, 349–354.
Mantzoros, C. S., Ozata, M., Negrao, A. B., Suchard, M. A., Ziotopoulou, M., Caglayan, S., Elashoff, R. M., Cogswell, R. J., Negro, P., Liberty, V., Wong, M. L., Veldhuis, J., Ozdemir, I. C., Gold, P. W., Flier, J. S., and Licinio, J. (2001). Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J. Clin. Endocrinol. Metab. 86, 3284–3291.
Maruo, T., Hiramatsu, S., Otani, T., Hayashi, M., and Mochizuki, M. (1992). Increase in the expression of thyroid hormone receptors in porcine granulosa cells early in follicular maturation. Acta Endocrinol. 127, 152–160.
Menendez, C., Baldelli, R., Camina, J. P., Escudero, B., Peino, R., Dieguez, C., and Casanueva, F. F. (2003). TSH stimulates leptin secretion by a direct effect on adipocytes. J. Endocrinol. 176, 7–12.
Mitsuhashi, T., Tennyson, G. E., and Nikodem, V. M. (1988). Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc. Natl. Acad. Sci. U.S.A. 85, 5804–5808.
Mizukami, Y., Hashimoto, T., Nonomura, A., Michigishi, T., Nakamura, S., Noguchi, M., and Matsukawa, S. (1994). Immunohistochemical demonstration of thyrotropin (TSH)-receptor in normal and diseased human thyroid tissues using monoclonal antibody against recombinant human TSH-receptor protein. J. Clin. Endocrinol. Metab. 79, 616–619.
Mueller, A., Schofl, C., Dittrich, R., Cupisti, S., Oppelt, P. G., Schild, R. L., Beckmann, M. W., and Häberle, L. (2009). Thyroid-stimulating hormone is associated with insulin resistance independently of body mass index and age in women with polycystic ovary syndrome. Hum. Reprod. 24, 2924–2930.
Negro, R., Mangieri, T., Coppola, L., Presicce, G., Casavola, E. C., Gismondi, R., Locorotondo, G., Caroli, P., Pezzarossa, A., Dazzi, D., and Hassan, H. (2005). Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Hum. Reprod. 20, 1529–1533.
Ott, J., Aust, S., Kurz, C., Nouri, K., Wirth, S., Huber, J. C., and Mayerhofer, K. (2010). Elevated antithyroid peroxidase antibodies indicating Hashimoto’s thyroiditis are associated with the treatment response in infertile women with polycystic ovary syndrome. Fertil. Steril. 94, 2895–2897.
Ozpinar, A., Golub, M. S., Poppenga, R. H., Blount, B. C., and Gillespie, J. R. (2011). Thyroid status of female rhesus monkeys and preliminary information on impact of perchlorate administration. Lab. Anim. (NY) 45, 209–214.
Pantaleon, M., Ryan, J. P., Gil, M., and Kaye, P. L. (2001). An unusual subcellular localization of GLUT1 and link with metabolism in oocytes and preimplantation mouse embryos. Biol. Reprod. 64, 1247–1254.
Paz-Filho, G., Delibasi, T., Erol, H. K., Wong, M. L., and Licinio, J. (2009). Congenital leptin deficiency and thyroid function. Thyroid Res. 2, 11.
Petta, C. A., Arruda, M. S., Zantut-Wittmann, D. E., and Benetti-Pinto, C. L. (2007). Thyroid autoimmunity and thyroid dysfunction in women with endometriosis. Hum. Reprod. 22, 2693–2697.
Poppe, K., Glinoer, D., Van Steirteghem, A., Tournaye, H., Devroey, P., Schiettecatte, J., and Velkeniers, B. (2002). Thyroid dysfunction and autoimmunity in infertile women. Thyroid 12, 997–1001.
Poppe, K., and Velkeniers, B. (2003). Thyroid disorders in infertile women. Ann. Endocrinol. (Paris) 64, 45–50.
Poppe, K., Velkeniers, B., and Glinoer, D. (2007). Thyroid disease and female reproduction. Clin. Endocrinol. (Oxf.) 66, 309–321.
Poppe, K., Velkeniers, B., and Glinoer, D. (2008). The role of thyroid autoimmunity in fertility and pregnancy. Nat. Clin. Pract. Endocrinol. Metab. 4, 394–405.
Premawardhana, L. D., and Lazarus, J. H. (2006). Management of thyroid disorders. Postgrad. Med. J. 82, 552–558.
Reh, A., Grifo, J., and Danoff, A. (2010). What is a normal thyroid-stimulating hormone (TSH) level? Effects of stricter TSH thresholds on pregnancy outcomes after in vitro fertilization. Fertil. Steril. 94, 2920–2922.
Reimand, K., Talja, I., Metskula, K., Kadastik, U., Matt, K., and Uibo, R. (2001). Autoantibody studies of female patients with reproductive failure. J. Reprod. Immunol. 51, 167–176.
Ren, S. G., Seliktar, J., Li, X., Hershman, J. M., Braunstein, G. D., and Melmed, S. (1999). In vivo and in vitro regulation of thyroid leukemia inhibitory factor (LIF): marker of hypothyroidism. J. Clin. Endocrinol. Metab. 84, 2883–2887.
Ris-Stalpers, C., and Bikker, H. (2010). Genetics and phenomics of hypothyroidism and goiter due to TPO mutations. Mol. Cell. Endocrinol. 322, 38–43.
Roussev, R. G., Kaider, B. D., Price, D. E., and Coulam, C. B. (1996). Laboratory evaluation of women experiencing reproductive failure. Am. J. Reprod. Immunol. 35, 415–420.
Sanchez, V. C., Goldstein, J., Stuart, R. C., Hovanesian, V., Huo, L., Munzberg, H., Friedman, T. C., Bjorbaek, C., and Nillni, E. A. (2004). Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J. Clin. Invest. 114, 357–369.
Shahrara, S., Drvota, V., and Sylven, C. (1999). Organ specific expression of thyroid hormone receptor mRNA and protein in different human tissues. Biol. Pharm. Bull. 22, 1027–1033.
Shields, B. M., Freathy, R. M., Knight, B. A., Hill, A., Weedon, M. N., Frayling, T. M., Hattersley, A. T., and Vaidya, B. (2009). Phosphodiesterase 8B gene polymorphism is associated with subclinical hypothyroidism in pregnancy. J. Clin. Endocrinol. Metab. 94, 4608–4612.
Skjoldebrand Sparre, L., Kollind, M., and Carlstrom, K. (2002). Ovarian ultrasound and ovarian and adrenal hormones before and after treatment for hyperthyroidism. Gynecol. Obstet. Invest. 54, 50–55.
Song, Y., Yao, X., and Ying, H. (2011). Thyroid hormone action in metabolic regulation. Protein Cell 2, 358–368.
Southren, A. L., Olivo, J., Gordon, G. G., Vittek, J., Brener, J., and Rafii, F. (1974). The conversion of androgens to estrogens in hyperthyroidism. J. Clin. Endocrinol. Metab. 38, 207–214.
Stewart, C. L., Kaspar, P., Brunet, L. J., Bhatt, H., Gadi, I., Kontgen, F., and Abbondanzo, S. J. (1992). Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79.
Sugiura-Ogasawara, M., Ozaki, Y., Sonta, S., Makino, T., and Suzumori, K. (2005). Exposure to bisphenol A is associated with recurrent miscarriage. Hum. Reprod. 20, 2325–2329.
Sun, S. C., Hsu, P. J., Wu, F. J., Li, S. H., Lu, C. H., and Luo, C. W. (2010). Thyrostimulin, but not thyroid-stimulating hormone (TSH), acts as a paracrine regulator to activate the TSH receptor in mammalian ovary. J. Biol. Chem. 285, 3758–3765.
Tahboub, R., and Arafah, B. M. (2009). Sex steroids and the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 23, 769–780.
Tang, H. Y., Lin, H. Y., Zhang, S., Davis, F. B., and Davis, P. J. (2004). Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology 145, 3265–3272.
Tunbridge, W. M., Evered, D. C., Hall, R., Appleton, D., Brewis, M., Clark, F., Evans, J. G., Young, E., Bird, T., and Smith, P. A. (1977). Lipid profiles and cardiovascular disease in the Whickham area with particular reference to thyroid failure. Clin. Endocrinol. (Oxf.) 7, 495–508.
Uribe, R. M., Zacarias, M., Corkidi, G., Cisneros, M., Charli, J. L., and Joseph-Bravo, P. (2009). 17 Beta-oestradiol indirectly inhibits thyrotrophin-releasing hormone expression in the hypothalamic paraventricular nucleus of female rats and blunts thyroid axis response to cold exposure. J. Neuroendocrinol. 21, 439–448.
Van Voorhis, B. J., Neff, T. W., Syrop, C. H., and Chapler, F. K. (1994). Primary hypothyroidism associated with multicystic ovaries and ovarian torsion in an adult. Obstet. Gynecol. 83, 885–887.
Vanderpump, M. P., Tunbridge, W. M., French, J. M., Appleton, D., Bates, D., Clark, F., Grimley Evans, J., Hasan, D. M., Rodgers, H., Tunbridge, F., and Young, E. T. (1995). The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin. Endocrinol. (Oxf.) 43, 55–68.
Vaquero, E., Lazzarin, N., Caserta, D., Valensise, H., Baldi, M., Moscarini, M., and Arduini, D. (2006). Diagnostic evaluation of women experiencing repeated in vitro fertilization failure. Eur. J. Obstet. Gynecol. Reprod. Biol. 125, 79–84.
Wakim, A. N., Paljug, W. R., Jasnosz, K. M., Alhakim, N., Brown, A. B., and Burholt, D. R. (1994). Thyroid hormone receptor messenger ribonucleic acid in human granulosa and ovarian stromal cells. Fertil. Steril. 62, 531–534.
Wakim, A. N., Polizotto, S. L., Buffo, M. J., Marrero, M. A., and Burholt, D. R. (1993). Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil. Steril. 59, 1187–1190.
Wang, C., and Crapo, L. M. (1997). The epidemiology of thyroid disease and implications for screening. Endocrinol. Metab. Clin. North Am. 26, 189–218.
Weinstein, S. P., and Haber, R. S. (1993). Glucose transport stimulation by thyroid hormone in ARL 15 cells: partial role of increased GLUT1 glucose transporter gene transcription. Thyroid 3, 135–142.
Weiss, S. J., Philp, N. J., Ambesi-Impiombato, F. S., and Grollman, E. F. (1984). Thyrotropin-stimulated iodide transport mediated by adenosine 3′,5′-monophosphate and dependent on protein synthesis. Endocrinology 114, 1099–1107.
Wikstrom, L., Johansson, C., Salto, C., Barlow, C., Campos Barros, A., Baas, F., Forrest, D., Thorén, P., and Vennström, B. (1998). Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. EMBO J. 17, 455–461.
Wolf, G. (2002). The regulation of the thyroid-stimulating hormone of the anterior pituitary gland by thyroid hormone and by 9-cis-retinoic acid. Nutr. Rev. 60, 374–377.
Yen, P. M. (2001). Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 81, 1097–1142.
Ying, H., Araki, O., Furuya, F., Kato, Y., and Cheng, S. Y. (2007). Impaired adipogenesis caused by a mutated thyroid hormone alpha1 receptor. Mol. Cell. Biol. 27, 2359–2371.
Zaletel, K., Krhin, B., Gaberscek, S., Bicek, A., Pajic, T., and Hojker, S. (2010). Association of CT60 cytotoxic T lymphocyte antigen-4 gene polymorphism with thyroid autoantibody production in patients with Hashimoto’s and postpartum thyroiditis. Clin. Exp. Immunol. 161, 41–47.
Zelenko, Z., Aghajanova, L., Irwin, J. C., and Giudice, L. C. (2012). Nuclear receptor, coregulator signaling, and chromatin remodeling pathways suggest involvement of the epigenome in the steroid hormone response of endometrium and abnormalities in endometriosis. Reprod. Sci. 19, 152–162.
Zhang, P., Zucchelli, M., Bruce, S., Hambiliki, F., Stavreus-Evers, A., Levkov, L., Skottman, H., Kerkelä, E., Kere, J., and Hovatta, O. (2009). Transcriptome profiling of human pre-implantation development. PLoS ONE 4, e7844.
Keywords: thyroid hormone, TSH, endometrium, ovary, infertility
Citation: Stavreus Evers A (2012) Paracrine interactions of thyroid hormones and thyroid stimulation hormone in the female reproductive tract have an impact on female fertility. Front. Endocrin. 3:50. doi: 10.3389/fendo.2012.00050
Received: 30 January 2012; Accepted: 13 March 2012;
Published online: 30 March 2012.
Edited by:
Angelica Lindén Hirschberg, Karolinska Institutet, SwedenReviewed by:
Alessandro Antonelli, University of Pisa, ItalyRosa Marina Melillo, University of Naples Federico II, Italy
Copyright: © 2012 Stavreus Evers. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Anneli Stavreus Evers, Department of Women’s and Children’s Health, Uppsala University, 751 85 Uppsala, Sweden. e-mail:YW5uZWxpLnN0YXZyZXVzLWV2ZXJzQGtiaC51dS5zZQ==
