- 1 Department of Exploitation and Protection of the Agricultural and Forestry Resources, Università di Torino, Grugliasco, Italy
- 2 Koiné – Environmental Consulting, Parma, Italy
Wolbachia pipientis is a widespread endosymbiont of filarial nematodes and arthropods. While in worms the symbiosis is obligate, in arthropods Wolbachia induces several reproductive manipulations (i.e., cytoplasmic incompatibility, parthenogenesis, feminization of genetic males, and male-killing) in order to increase the number of infected females. These various phenotypic effects may be linked to differences in host physiology, and in particular to endocrine-related processes governing growth, development, and reproduction. Indeed, a number of evidences links Wolbachia symbiosis to insulin and ecdysteroid signaling, two multilayered pathways known to work antagonistically, jointly or even independently for the regulation of different molecular networks. At present it is not clear whether Wolbachia manipulates one pathway, thus affecting other related metabolic networks, or if it targets both pathways, even interacting at several points in each of them. Interestingly, in view of the interplay between hormone signaling and epigenetic machinery, a direct influence of the “infection” on hormonal signaling involving ecdysteroids might be achievable through the manipulation of the host’s epigenetic pathways.
Introduction
The maternally transmitted alfa-Proteobacterium Wolbachia pipientis (Rickettsiales) is a widespread endosymbiont of filarial nematodes and arthropods, including crustaceans, mites, spiders, scorpions, and especially insects, where it is estimated to infect up to 66% of the species (Werren et al., 2008).
While in worms Wolbachia are obligate symbionts, in arthropods they induce several reproductive manipulations in order to increase the number of infected females. Effects of the infection include cytoplasmic incompatibility, parthenogenesis, feminization of genetic males, and male-killing, as well as influences on host longevity and fecundity (Stouthamer et al., 1999; Werren et al., 2008). Such a host phenotypic variability is generally linked to the high genome plasticity of Wolbachia. However, experimental data also suggest a role for the symbiont in modulating the host sexual phenotypes by interaction with the hormonal signaling pathway. In particular, the various phenotypic effects may be due to differences in host physiology, and in particular to endocrine-related processes governing growth, development, and reproductive behavior which display a high variability in insects. In particular, a number of evidence links Wolbachia symbiosis to insulin and steroid (i.e., ecdysteroid) signaling. Many studies report antagonistic or cooperative relationships between steroid hormones and insulin. Indeed, an extensive crosstalk between the two hormonal signaling pathways has been demonstrated in regulating metabolism, development, and reproduction. For example, in the fly Drosophila melanogaster there is a mutual antagonistic relationship between these metabolic networks for nutrient homeostasis (Colombani et al., 2005). At a molecular level, for example, the expression of the ecdysone receptor (EcR) co-activator DOR (which is misregulated in diabetic mammals) is controlled by insulin signaling via the forkhead transcription factor FOXO (Francis et al., 2010). The two hormonal signaling pathways may also cooperate: in mosquitoes they act in combination for the yolk protein precursor gene expression required for vitellogenesis (Roy et al., 2007). In the larval prothoracic gland, the insulin-signaling pathway modulates ecdysone release and influences both the duration and rate of larval growth (Shingleton, 2005); in adults, insulin-like peptides may trigger steroid synthesis by the follicular cells in insect ovaries (Wu and Brown, 2006). A parallel regulation by ecdysone and insulin has also been demonstrated in the Drosophila’s ovary where the hormones modulate the proliferation and self-renewal of germ-line stem cells independently (Ables and Drummond-Barbosa, 2010).
In the following sections, data on the involvement of Wolbachia in modulating both host hormonal pathways are discussed. Due to the complexity of such metabolic pathways, at present, it is not clear whether Wolbachia operates by attacking one pathway, thus affecting other related metabolic networks, or if the symbiont targets both pathways, even interacting at several points in each of them.
Wolbachia and Insulin Signaling
The insulin/IGF signaling (IIS) pathway plays key roles in growth, metabolism, reproduction, and longevity in different organisms. Recently, a specific interaction between IIS and Wolbachia has been demonstrated in D. melanogaster, where insulin-like peptide mutants display an extended lifespan if they harbor the symbiont (Grönke et al., 2010). Another research involves Drosophila insulin receptor mutants, characterized by a reduction in IIS signaling with pleiotropic effects on many traits, including extreme dwarfism, sterility, increased fat levels, and shortened lifespan: interestingly, in presence of Wolbachia the IIS-related mutant phenotypes resulted in significant moderate effects (e.g., reduced fecundity and extended lifespan), suggesting that the symbiont acts to increase insulin signaling itself (Ikeya et al., 2009).
Wolbachia also seems to interact with chico, a gene encoding an insulin receptor substrate (Böhni et al., 1999). Drosophila carrying homozygous chico1 alleles are sterile, but in presence of Wolbachia the females produce progeny, even if significantly smaller than their heterozygous siblings (Clark et al., 2005; Richard et al., 2005).
Additional data on the possible interaction between the symbiont and host IIS are provided by studies on crustaceans. Wolbachia is known to infect several species of crustaceans, and especially (but not exclusively) terrestrial isopods where the symbiont can induce the feminization of genetical males through an interaction with host hormonal signaling pathways (Bouchon et al., 2008). Crustacea are by default females, and male sex differentiation is triggered by an androgenic hormone (AH) secreted by the androgenic gland (AG; Legrand et al., 1987; Sagi and Khalaila, 2001). The current hypothesis about the feminizing action of Wolbachia is that the symbiont interacts either with the AG differentiation process or the AH receptors (Rigaud and Juchault, 1998; Bouchon et al., 2008). However, if Wolbachia bacteria are experimentally inoculated in adult males, the host soon develops female structures, despite the presence of the AG, which becomes even hypertrophic (Martin et al., 1973, 1999). This suggests that the AH receptors are no longer functional, favoring the hypothesis that Wolbachia induces feminization by targeting the receptor of the AH. Interestingly, the AH has been proven to be an insulin-like peptide (Manor et al., 2007): in vivo silencing of the gene induces an arrest of spermatogenesis, prevents the regeneration of male secondary sexual characteristics, and also induces a lag in molt and a growth reduction (Ventura et al., 2009). In sequential hermaphrodites, the silencing of the AG insulin-like factor induces the feminization of male-related phenotypes too (Rosen et al., 2010).
Wolbachia and Ecdysone Signaling
Among invertebrates Wolbachia is known to infect exclusively (!) Arthropoda and Nematoda, two Phyla belonging to the Ecdysozoa, a clade of animals which share the ability to replace the exoskeleton. This process is called ecdysis and is controlled hormonally by a class of steroids called ecdysteroids (Ewer, 2005).
In filarial worms, antibiotic curing of Wolbachia “infection” inhibits nematode fertility and development, suggesting a specific role for the symbiont in host oogenesis, embryogenesis, and molting (Casiraghi et al., 2002; Arumugam et al., 2008; Frank et al., 2010). In arthropods, several data suggest that the phenotypic effects induced by Wolbachia may be linked to steroid-related processes. As it is well known, insect steroids play key roles in the coordination of multiple developmental processes, and in adults they control important aspects of reproduction.
In particular, during development, insect molting is induced by the systemic hormone 20-hydroxyecdysone (20E). 20E acts on members of an evolutionarily conserved family of nuclear receptors: it binds to the heterodimeric EcR/Usp receptor composed of EcR and USP (ultraspiracle, homologous to the vertebrate retinoid-X receptor), which shares many commonalities with the human thyroid hormone receptor. Then the EcR/USP complex activates the transcriptional processes underlying the cellular and morphogenetic molting cascade events (Gilbert et al., 2002). A biological action of 20E binding of un-partnered EcR has also been demonstrated (Spindler et al., 2009).
Despite the fact that ecdysteroids are present throughout the entire life of insects, their role in adults is quite elusive. Ecdysteroids, for example, may have a role in lifespan (Tricoire et al., 2009; Schwedes et al., 2011) or in stress responses, such as nutritional shortage, high temperature, dry starvation, and oxidative stress (Hirashima et al., 2000; Terashima et al., 2005; Ishimoto and Kitamoto, 2011). In adult flies ecdysone-mediated signaling is also involved in stressful social interactions and in homeostatic sleep regulation (Ishimoto and Kitamoto, 2011). Another unconventional role of the “molting” hormone is the control of important aspects of reproduction, including ovarian development and oogenesis (Raikhel et al., 2005). In many insect species 20E is directly involved in the regulation of vitellogenin biosynthesis by the female fat body, a metabolic tissue functionally analogous to the vertebrate liver; and it can also induce vitellogenin synthesis in males (Huybrechts and De Loof, 1977; Bownes et al., 1983; Zhu et al., 2007). The 20E has also been shown to affect sexual behavior (Ganter et al., 2007).
De Loof (2006) proposes that ecdysteroids may also act as sex hormones. In particular, 20E secreted by the follicle cells of the insect ovary could be the physiological equivalent of vertebrate estrogens, while E – the precursor of the active molting hormone 20E – should act as a distinct hormone, being the physiological equivalent of vertebrate testosterone (De Loof and Huybrechts, 1998; De Loof, 2006). Indeed, E can regulate a set of genes that are distinct from those controlled by 20E, thus confirming that it may exert different biological functions from 20E (Beckstead et al., 2007). However, in insects the existence of sex hormones is under debate, as the differentiation of primary and secondary sexual characteristics is generally considered under the exclusive control of the genotype of each single cell (Steinmann-Zwicky et al., 1989; Schütt and Nöthiger, 2000). However recent data demonstrate that in insects, as well as in vertebrates, non-autonomous (=hormonal) sex determination controls sex dimorphism (DeFalco et al., 2008; Casper and Van Doren, 2009). Thus, if ecdysteroids function as molting and sex hormones, this could explain why Wolbachia interferes with insect development and reproduction, as discussed in the following section.
Last but not least, it is worth noting that Wolbachia establish themselves in many host steroidogenic tissues, including the fat body and the ovarian follicular epithelium, as demonstrated in many insect species and shown in Figure 1 (Sacchi et al., 2010; Gonella et al., 2011; Negri and Pellecchia, in press).
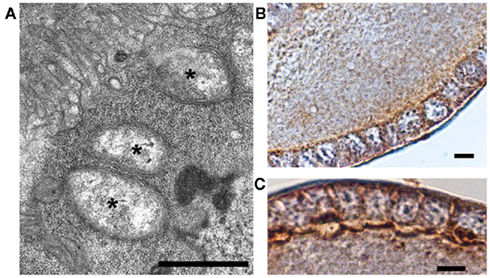
Figure 1. Wolbachia’s localization in the host’s follicular epithelium of the gonad. (A) TEM micrograph of a Wolbachia-infected Zyginidia pullula (Hemiptera, Cicadellidae) follicle cell filled with bacteria (asterisks; bar = 0.9 μm); (B,C) Immuno-histochemical reactions showing strong positivity (brown) to anti-wsp (Wolbachia surface protein) antibody in the leafhopper’s follicular epithelium (bars = 10 μm). Modified from Negri and Pellecchia(in press).
Feminizing Wolbachia: The Induction of Host Feminine Sex Differentiation Despite Masculine Sex Determination
Feminization, that is the development of genetical males into females, deals with sex differentiation much more directly than the other Wolbachia-induced phenotypes, thus offering the opportunity to shed light on the processes governing arthropod sex development, and on the involvement of the endosymbiont in such processes.
Until now, in insects, feminization induced by Wolbachia has been demonstrated in lepidopteran and hemipteran species (Hiroki et al., 2002; Kageyama and Traut, 2003; Negri et al., 2006; Sakamoto et al., 2007). In the butterfly Eurema hecabe, it has been demonstrated that the Wolbachia feminizing effect acts continuously throughout the larval development for the maintenance of the female phenotype (Narita et al., 2007). Accordingly, this suggests that the bacterium acts on the insect sex differentiation rather than sex determination, and ecdysteroids are the best candidate for such an interaction. Evidences are in fact provided by the effects of incomplete Wolbachia suppression by antibiotic treatments during lepidopteran larval stages. In particular some tetracycline-treated individuals show larval/pupal molting defects, and others do not pupate: the dissection of dead pupae reveals that many of them failed to escape from the pupal case (Narita et al., 2007). Similar molting defects may be obtained, for example, in ecdysone receptor knock-out individuals of Blattella Germanica and D. melanogaster EcR-mutants (Davis et al., 2005; Cruz et al., 2006). Moreover, antibiotic treatments in infected E. hecabe often induce also sexually intermediate traits in wings, gonads, and genitalia (Narita et al., 2007). Notably, in Lepidoptera a role for the ecdysteroid titer in regulating sexual dimorphism, including sex specific wing development, has been proved (Lobbia et al., 2003) strengthening the hypothesis of a link between Wolbachia and ecdysteroid signaling. In particular, we cannot exclude a host/symbiont co-adaptation where a partial symbiont removal leads to biological imbalance. This may also explain the origin of intersex individuals in E. hecabe, Ostrinia scapulalis, and O. furnacalis partially cured by feminizing Wolbachia (Kageyama and Traut, 2003; Sakamoto et al., 2007). Intersexes are specimens characterized by a genetically homogeneous genotype (male in this case), but a mixture of male and female phenotypes (i.e., feminized tissues in this case): the appearance of these phenotypes may be the result of a partial but evident conflict between male and female sex hormones and/or receptors.
In Ostrinia species, in addition, a complete feminization is fatal and genetical males die during the larval development (Kageyama and Traut, 2003; Sakamoto et al., 2007), while in other species the male-killing action of Wolbachia occurs during embryogenesis (Werren et al., 2008). As discussed above, the role played by ecdysteroids during the whole developmental cycle of insects is crucial. Embryogenesis, in particular, takes place in a steroid hormone-enriched environment, where steroids act for the coordination of morphogenetic movements (Kozlova and Thummel, 2003; Gaziova et al., 2004). Thus, if male-killing Wolbachia interacts with the host hormonal pathway involving ecdysteroids, this could affect the processes required for a normal development of males.
A sex-specific action of ecdysteroids during insect embryogenesis and development has been demonstrated in some studies concerning the effects of endocrine disrupting chemicals. Indeed, ecdysteroid agonists and antagonists (e.g., bisphenol A, tebufenozide, and ethinyl estradiol) are responsible for female-biased sex ratios in the treated populations (Hahn et al., 2001; Biddinger et al., 2006; Lee and Choi, 2007; Izumi et al., 2008). According to some authors, the observed sex-specific effect could be well explained by considering insect steroids as sex hormones. In particular, larval or embryo males die because they are subjected to an unsuitable, i.e., female, hormonal environment (Hahn et al., 2001).
Wolbachia, Host Oogenesis Defect Rescuing and Fecundity Enhancement
In some cases Wolbachia is essential for insect reproduction, as in absence of the “infection” the host is not able to perform a normal oogenesis. For example, in the hymenopteran Asobara tabida the symbiosis with Wolbachia involves interference with the programmed cell death of nurse cells that is significantly higher in Wolbachia-cured insects (where the ovary completely lacks mature oocytes) than in naturally infected specimens (Dedeine et al., 2001; Pannebakker et al., 2007). The role of ecdysone in regulating cell apoptosis, a process required for insect development, is well known: during metamorphosis, for example, the steroid is a primary regulator of cell death in larval tissues which are destroyed or remodeled into an “adult” form (Tsuzuki et al., 2001; Mottier et al., 2004). Interestingly, in adults a higher level of 20E causes apoptosis of nurse cells which blocks the oogenesis process (McCall, 2004; Terashima et al., 2005; Ishimoto and Kitamoto, 2011). Therefore, it would be interesting to verify if Wolbachia interacts by modulating ecdysone signaling in A. tabida, thus influencing programmed cell death pathways occurring during host oogenesis.
In D. melanogaster, partial loss of function mutants of sex-lethal, the master regulator gene of the fly sex determination cascade, are sterile due to overproliferation of undifferentiated germ cells. In the infected line, Wolbachia is able to rescue oogenesis defects leading to partially fertile specimens (Starr and Cline, 2002). Stem cell behavior is regulated by intrinsic factors, signals from their niches and systemic hormones. Ecdysone is known to affect stem cell proliferation, also confirming current hypotheses of an involvement of steroids in cancer: in particular, altered steroids signaling, as well as extensive molecular crosstalk between steroid and insulin/insulin-like growth factors, are commonly associated with cancer (Ables and Drummond-Barbosa, 2010). Accordingly, we may speculate that the occurrence of “cancer” germ cells could be due to a misregulation of ecdysone signaling in mutant flies that is rescued by Wolbachia infection.
Wolbachia has also been implicated in improving the fitness of several insect hosts (Dedeine et al., 2003).
New insights into the mechanisms underlying host fecundity enhancement are provided by a recent study on D. mauritania: Wolbachia improves fecundity both by enhancing stem cell proliferation and reducing programmed cell death in the germarium (Fast et al., 2011). The authors hypothesize that the presence of the bacterium in the germ-line stem cell niche modulates stem cell activity, although a contribution from systemic or stem cell intrinsic signals cannot be ruled out. It remains unclear, however, whether stem cells themselves sense and respond to Wolbachia. According to us, the role of ecdysone might be of primary importance, as the systemic hormone is known to stimulate directly germ-line stem cells in order to promote their self-renewal and activity (Ables and Drummond-Barbosa, 2010), with Wolbachia as the director of the scene.
Interplay between Steroid Signaling and Epigenetic Pathways
A growing body of data suggests a role for hormones in modulating epigenetic changes. In mammals, for example, steroids are able to induce epigenetic differences necessary for a correct sex differentiation of the brain (Nugent and McCarthy, 2011), and in adults they actively maintain DNA methylation patterns (Auger et al., 2011). An interaction between steroid/thyroid receptors and the epigenetic machinery (e.g., histone modifying enzymes and DNA methyltransferases) has been proposed too (Tsai et al., 2009; Haddad et al., 2010; Pathak et al., 2010). Novel insights into the mechanisms underlying such an interaction are provided by studies on nuclear receptor co-regulators (NRCs; i.e., co-activators and co-repressors). Strikingly, NRCs are key epigenetic regulators and utilize enzymatic activities to modify epigenetically the DNA and chromatin (Mahajan and Samuels, 2000; Rosenfeld et al., 2006; Hsia et al., 2010).
Thanks to studies on Drosophila, we now have compelling evidences of a direct interaction between steroids (specifically ecdysone) and epigenetic factors. For example, during fly development, neural circuit sculpting is due to cooperation between EcR and histone modifying enzymes (Kirilly et al., 2011). In addition, in the fly’s ovary, ecdysone interacts with chromatin remodeling factors for modulating the proliferation and self-renewal of germ-line stem cells (Ables and Drummond-Barbosa, 2010). Ecdysone receptor signaling also needs direct cooperation with nucleosome remodeling complexes, and many EcR co-activators and co-repressors that contribute to the epigenetic memory have been identified and characterized (Kimura et al., 2008; Sawatsubashi et al., 2010; Kugler et al., 2011).
Interestingly, recent data demonstrate that Wolbachia infection is able to modulate the host genomic imprinting through methylation of the DNA (Negri et al., 2009a,b). In the leafhopper Z. pullula, Wolbachia-infected genetic males develop into intersexes with a female phenotype. In particular, two kinds of intersexes are described: “intersex females” which are feminized males with ovaries, even able to produce progeny; and “intersex males” which bear testes and are characterized by a very low Wolbachia density (Negri et al., 2009a). Remarkably, Wolbachia-infected “intersex females” possess the same imprinting pattern of uninfected females, thus demonstrating that the infection disrupts the male imprinting (Negri et al., 2009a,b). In addition, the alteration occurs only if the bacterium exceeds a density threshold, as “intersex males” maintain a male genome-methylation pattern (Negri et al., 2009a). The epigenetic modifications affect the expression of genes involved in sex determination and development (Negri, unpublished data), thus avoiding the need for Wolbachia to interfere with each single gene separately.
In view of the interplay between hormone signaling and epigenetic machinery, data on the whole suggest that the manipulation of the host’s epigenetic pathways might be achievable through a direct influence of Wolbachia on hormonal signaling involving ecdysteroids.
The model proposed in Figure 2 tries to explain possible interactions. Once 20E is biosynthesized, it binds the nuclear receptor EcR which heterodimerizes with USP. The EcR/USP complex binds DNA and complexes with nuclear NRCs. Then, NRCs catalyze DNA methyltransferases for a correct methylation pattern of differentially methylated regions or directly function as histone modifying enzymes, thus activating proper selective transcriptional programs. In infected insects, Wolbachia may interact with the ecdysone pathway by synthesizing products competing with 20E or function as/interfere with NRCs. As a result, the EcR binding to DNA or the recruitment of DNA methyltransferases and/or histone modifying enzymes should be affected.
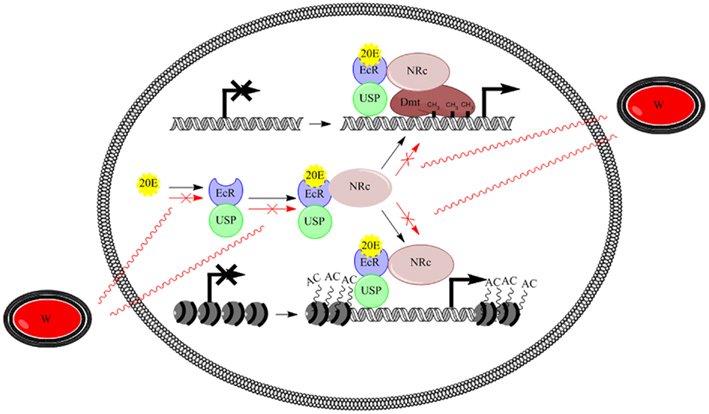
Figure 2. Model illustrating the possible interplay between ecdysone signaling and epigenetic regulation, and Wolbachia action. 20E binds the nuclear receptor EcR which heterodimerizes with USP. Then, the EcR/USP complex binds DNA and complexes with nuclear receptor co-regulators, which catalyze DNA methyltransferases or directly function as histone modifying enzymes, thus activating proper selective transcriptional programs. Wolbachia may interact by synthesizing products competing with 20E or function as/interfere with nuclear receptor co-regulators, respectively. 20E, 20-hydroxyecdysone; EcR, ecdysone receptor; USP, ultraspiracle; NRc, nuclear receptor co-regulator; Dmt, DNA-methyltransferase; W, Wolbachia bacteria
Accordingly, studies on Wolbachia–host interactions should give great attention for example to substances with an antagonist action on the ecdysone nuclear receptor; selective nuclear receptor modulators; or co-regulators of nuclear receptors, in view of their emerging role in integrating the transcriptional co-regulation with the epigenetic regulation (Rosenfeld et al., 2006; Kato et al., 2011).
Conclusion
Many experimental data support the role of Wolbachia in modulating the insect sexual phenotypes by interaction with the host hormonal signaling pathway. Indeed, the various phenotypic effects observed may be due to differences in host physiology and in particular to endocrine-related processes governing growth, development, and reproductive behavior.
In particular, a number of evidences links Wolbachia symbiosis to insulin and ecdysteroid signaling.
Several studies report an extensive crosstalk between the two hormonal signaling pathways, which may work antagonistically, jointly or even independently. Like many other symbiotic bacteria, Wolbachia could operate by attacking one crucial pathway in their hosts, thus affecting other metabolic networks, or by targeting both pathways, even interacting at several points in each of them for its own benefit (that is the manipulation of host reproduction and development in order to increase the number of infected females). In view of the interplay between hormone signaling and epigenetic machinery, a direct influence of the “infection” on hormonal signaling involving ecdysteroids might be achievable through the manipulation of host epigenetic pathways.
Although further work is needed to fully clarify the genetic and molecular bases of such an interaction, new work hypotheses have been now offered for the study of the mechanisms used by symbionts to dialog with their hosts. Likewise, the Wolbachia–host interaction should become an emerging model system for the study of hormone signaling orchestration made by microbial symbionts playing with nuclear receptors, and for shedding light on the role of NRCs in integrating the transcriptional co-regulation with the epigenetic regulation.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ables, E. T., and Drummond-Barbosa, D. (2010). The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell 7, 581–592.
Arumugam, S., Pfarr, K. M., and Hoerauf, A. (2008). Infection of the intermediate mite host with Wolbachia-depleted Litomosoides sigmodontis microfilariae: impaired L1 to L3 development and subsequent sex-ratio distortion in adult worms. Int. J. Parasitol. 38, 981–987.
Auger, C. J., Coss, D., Auger, A. P., and Forbes-Lorman, R. M. (2011). Epigenetic control of vasopressin expression is maintained by steroid hormones in the adult male rat brain. Proc. Natl. Acad. Sci. U.S.A. 108, 4242–4247.
Beckstead, R. B., Lam, G., and Thummel, C. S. (2007). Specific transcriptional responses to juvenile hormone and ecdysone in Drosophila. Insect Biochem. Mol. Biol. 37, 570–578.
Biddinger, D., Hull, L., Huang, H., McPheron, B., and Loyer, M. (2006). Sublethal effects of chronic exposure to tebufenozide on the development, survival and reproduction of the tufted apple bud moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 99, 834–842.
Böhni, R., Riesgo-Escovar, J., Oldham, S., Brogiolo, W., Stocker, H., Andruss, B. F., Beckingham, K., and Hafen, E. (1999). Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97, 865–875.
Bouchon, D., Cordaux, R., and Grève, P. (2008). “Feminizing Wolbachia and the evolution of sex determination in isopods,” in Insect Symbiosis, eds K. Bourtzis and T. Miller (Boca Raton, FL: Taylor and Francis Group LLC), 273–294.
Bownes, M., Blair, M., Kozma, R., and Dempster, M. (1983). 20E stimulates tissue-specific yolk-protein gene transcription in both male and female Drosophila. J. Embryol. Exp. Morphol. 78, 249–263.
Casiraghi, M., McCall, J. W., Simoncini, L., Kramer, L. H., Sacchi, L., Genchi, C., Werren, J. H., and Bandi, C. (2002). Tetracycline treatment and sex-ratio distortion: a role for Wolbachia in the moulting of filarial nematodes? Int. J. Parasitol. 32, 1457–1468.
Casper, A. L., and Van Doren, M. (2009). The establishment of sexual identity in the Drosophila germline. Development 136, 3821–3830.
Clark, M. E., Anderson, C. L., Cande, J., and Karr, T. L. (2005). Widespread prevalence of Wolbachia in laboratory stocks and the implications for Drosophila research. Genetics 170, 1667–1675.
Colombani, J., Bianchini, L., Layalle, S., Pondeville, E., Dauphin-Villemant, C., Antoniewski, C., Carré, C., Noselli, S., and Léopold, P. (2005). Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310, 667–670.
Cruz, J., Mané Padròs, D., Belleés, X., and Martin, D. (2006). Functions of the ecdysone receptor isoform-A in the hemimetabolous insect Blattella germanica revealed by systemic RNAi in vivo. Dev. Biol. 297, 158–171.
Davis, M. B., Carney, G. E., Robertson, A. E., and Bender, M. (2005). Phenotypic analysis of EcR-A mutants suggests that EcR isoforms have unique functions during Drosophila development. Dev. Biol. 282, 385–396.
De Loof, A. (2006). Ecdysteroids: the overlooked sex steroids of insect? Males: the black box. Insect Sci. 13, 325–338.
De Loof, A., and Huybrechts, R. (1998). “Insects do not have sex hormones”: a myth? Gen. Comp. Endocrinol. 111, 245–260.
Dedeine, F., Bandi, C., Bouletreau, M., and Kramer, L. H. (2003). “Insights into Wolbachia obligatory symbiosis,” in Insect Symbiosis, eds K. Bourtzis and T. Miller (Boca Raton, FL: Taylor and Francis Group LLC), 267–282.
Dedeine, F., Vavre, F., Fleury, F., Loppin, B., Hochberg, M. E., and Bouletreau, M. (2001). Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl. Acad. Sci. U.S.A. 98, 6247–6252.
DeFalco, T., Camara, N., Le Bras, S., and Van Doren, M. (2008). Non-autonomous sex determination controls sexually dimorphic development of the Drosophila gonad. Dev. Cell 14, 275–286.
Ewer, J. (2005). How the ecdysozoan changed its coat. PLoS Biol. 3, e349. doi:10.1371/journal.pbio.0030349
Fast, E., Toomey, M., Panaram, K., Desjardins, D., Kolaczyk, E., and Frydman, H. M. (2011). Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science 334, 990–992.
Francis, V. A., Zorzano, A., and Teleman, A. A. (2010). dDOR is an EcR coactivator that forms a feed-forward loop connecting insulin and ecdysone signalling. Curr. Biol. 20, 1799–1808.
Frank, K., Frank, K., and Heald, R. D. (2010). The emerging role of Wolbachia species in heartworm disease. Compend. Contin. Educ. Vet. 32, E1–E5.
Ganter, G. K., Walton, K. L., Merriman, J. O., Salmon, M. V., Brooks, K. M., Maddula, S., and Kravitz, E. A. (2007). Increased male male courtship in ecdysone receptor deficient adult flies. Behav. Genet. 37, 507–512.
Gaziova, I., Bonnette, P. C., Henrich, V. C., and Jindra, M. (2004). Cell-autonomous roles of the ecdysoneless gene in Drosophila development and oogenesis. Development 131, 2715–2725.
Gilbert, L. I., Rybczynski, R., and Warren, J. T. (2002). Control and biochemical nature of the ecdysteroidogenic pathway. Annu. Rev. Entomol. 47, 883–916.
Gonella, E., Negri, I., Marzorati, M., Mandrioli, M., Sacchi, L., Pajoro, M., Crotti, E., Rizzi, A., Clementi, E., Tedeschi, R., Bandi, C., Alma, A., and Daffonchio, D. (2011). Bacterial endosymbiont localization in Hyalesthes obsoletus, the insect vector of bois noir in Vitis vinifera. Appl. Environ. Microbiol. 77, 1423–1435.
Grönke, S., Clarke, D. F., Broughton, S., Andrews, T. D., and Partridge, L. (2010). Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 6, e1000857. doi:10.1371/journal.pgen.1000857
Haddad, F., Jiang, W., Bodell, P. W., Qin, A. X., and Baldwin, K. M. (2010). Cardiac myosin heavy chain gene regulation by thyroid hormone involves altered histone modifications. Am. J. Physiol. Heart Circ. Physiol. 299, H1968–H1980.
Hahn, T., Liess, M., and Schulz, R. (2001). Effects of the hormone mimetic insecticide tebufenozide on Chironomus riparius larvae in two different exposure setups. Ecotoxicol. Environ. Saf. 49, 171–178.
Hirashima, A., Rauschenbach, I. Y., and Sukhanova, M. J. (2000). Ecdysteroids in stress responsive and nonresponsive Drosophila virilis lines under stress conditions. Biosci. Biotechnol. Biochem. 64, 2657–2662.
Hiroki, M., Kato, Y., Kamito, T., and Miura, K. (2002). Feminizationof genetic males by a simbiotic bacterium in a butterfly, Eurema hecabe (Lepidoptera: Pieridae). Naturwissenschaften 89, 67–70.
Hsia, E. Y., Goodson, M. L., Zou, J. X., Privalsky, M. L., and Chen, H. W. (2010). Nuclear receptor coregulators as a new paradigm for therapeutic targeting. Adv. Drug Deliv. Rev. 62, 1227–1237.
Huybrechts, R., and De Loof, A. (1977). Induction of vitellogenin synthesis in male Sarcophaga bullata by ecdysterone. J. Insect Physiol. 23, 1359–1362.
Ikeya, T., Broughton, S., Alic, N., Grandison, R., and Partridge, L. (2009). The endosymbiont Wolbachia increases insulin/IGF-like signalling in Drosophila. Proc. Biol. Sci. 276, 3799–3807.
Ishimoto, H., and Kitamoto, T. (2011). Beyond molting – roles of the steroid molting hormone ecdysone in regulation of memory and sleep in adult Drosophila. Fly (Austin) 5, 215–220.
Izumi, N., Yanagibori, R., Shigeno, S., and Sajiki, J. (2008). Effects of bisphenol A on the development, growth and sex ratio of the housefly Musca domestica. Environ. Toxicol. Chem. 27, 1343–1353.
Kageyama, D., and Traut, W. (2003). Opposite sex-specific effects of Wolbachia and interference with the sex determination of its host Ostrinia scapulalis. Proc. Biol. Sci. 271, 251–258.
Kato, S., Yokoyama, A., and Fujiki, R. (2011). Nuclear receptor coregulators merge transcriptional coregulation with epigenetic regulation. Trends Biochem. Sci. 36, 272–281.
Kimura, S., Sawatsubashi, S., Ito, S., Kouzmenko, A., Suzuki, E., Zhao, Y., Yamagata, K., Tanabe, M., Ueda, T., Fujiyama, S., Murata, T., Matsukawa, H., Takeyama, K., Yaegashi, N., and Kato, S. (2008). Drosophila arginine methyltransferase 1 (DART1) is an ecdysone receptor co-repressor. Biochem. Biophys. Res Commun. 371, 889–893.
Kirilly, D., Wong, J. J., Lim, E. K., Wang, Y., Zhang, H., Wang, C., Liao, Q., Wang, H., Liou, Y. C., Wang, H., and Yu, F. (2011). Intrinsic epigenetic factors cooperate with the steroid hormone ecdysone to govern dendrite pruning in Drosophila. Neuron 72, 86–100.
Kozlova, T., and Thummel, C. S. (2003). Essential roles for ecdysone signaling during Drosophila mid-embryonic development. Science 301, 1911–1914.
Kugler, S. J., Gehring, E. M., Wallkamm, V., Krüger, V., and Nagel, A. C. (2011). The Putzig-NURF nucleosome remodeling complex is required for ecdysone receptor signaling and innate immunity in Drosophila melanogaster. Genetics 188, 127–139.
Lee, S.-B., and Choi, J. (2007). Effects of bisphenol A and ethynyl estradiol exposure on enzyme activities, growth and development in the fourth instar larvae of Chironomus riparius (Diptera, Chironomidae). Ecotoxicol. Environ. Saf. 68, 84–90.
Legrand, J. J., Legrand-Hamelin, E., and Juchault, P. (1987). Sex determination in Crustacea. Biol. Rev. 62, 439–470.
Lobbia, S., Niitsu, S., and Fujiwara, H. (2003). Female-specific wing degeneration caused by ecdysteroid in the tussock moth, Orgyia recens: hormonal and developmental regulation of sexual dimorphism. J. Insect Sci. 3, 1–7.
Mahajan, M. A., and Samuels, H. H. (2000). A new family of nuclear receptor coregulators that integrate nuclear receptor signaling through CREB-binding protein. Mol. Cell. Biol. 20, 5048–5063.
Manor, R., Weil, S., Oren, S., Glazer, L., Aflalo, E. D., Ventura, T., Chalifa-Caspi, V., Lapidot, M., and Sagi, A. (2007). Insulin and gender: an insulin-like gene expressed exclusively in the androgenic gland of the male crayfish. Gen. Comp. Endocrinol. 150, 326–336.
Martin, G., Juchault, P., and Legrand, J. J. (1973). Mise en evidence d’un micro-organisme intracytoplasmique symbiote de l’oniscoïde Armadillidium vulgare Latreille dont la présence accompagne l’intersexualité ou la féminisation totale des mâles génétiques de la lignée thélygène. C. R. Acad. Sci. Paris 276, 2313–2316.
Martin, G., Sorokine, O., Moniatte, M., Bulet, P., Hetru, C., and Van Dorsselaer, A. (1999). The structure of a glycosylated protein hormone responsible for sex determination in the isopod, Armadillidium vulgare. Eur. J. Biochem. 262, 727–736.
Mottier, V., Siaussat, D., Bozzolan, F., Auzoux-Bordenave, S., Porcheron, P., and Debernard, S. (2004). The 20-hydroxyecdysone-induced cellular arrest in G2 phase is preceded by an inhibition of cyclin expression. Insect Biochem. Mol. Biol. 34, 51–60.
Narita, S., Kageyama, D., Nomura, M., and Fukatsu, T. (2007). Unexpected mechanism of symbiont-induced reversal of insect sex: feminizing Wolbachia continuously acts on the butterfly Eurema hecabe during larval development. Appl. Environ. Microbiol. 73, 4332–4341.
Negri, I., Franchini, A., Gonella, E., Daffonchio, D., Mazzoglio, P. J., Mandrioli, M., and Alma, A. (2009a). Unravelling the Wolbachia evolutionary role: the reprogramming of the host genomic imprinting. Proc. Biol. Sci. 276, 2485–2491.
Negri, I., Mazzoglio, P. J., Franchini, A., Mandrioli, M., and Alma, A. (2009b). Male or female? The epigenetic conflict between a feminizing bacterium and its insect host. Commun. Integr. Biol. 2, 1–2.
Negri, I., and Pellecchia, M. (in press). “Sex steroids in insects and the role of the endosymbiont Wolbachia: a new perspective,” in Sex Hormones, ed R. H. Dubey (InTech Publisher).
Negri, I., Pellecchia, M., Mazzoglio, P. J., Patetta, A., and Alma, A. (2006). Feminizing Wolbachia in Zyginidia pullula (Insecta, Hemiptera), a leafhopper with an XX/X0 sex-determination system. Proc. Biol. Sci. 273, 2409–2416.
Nugent, B. M., and McCarthy, M. M. (2011). Epigenetic underpinnings of developmental sex differences in the brain. Neuroendocrinology 93, 150–158.
Pannebakker, B. A., Loppin, B., Elemans, C. P., Humblot, L., and Vavre, F. (2007). Parasitic inhibition of cell death facilitates symbiosis. Proc. Natl. Acad. Sci. U.S.A. 104, 213–215.
Pathak, S., D’Souza, R., Ankolkar, M., Gaonkar, R., and Balasinor, N. H. (2010). Potential role of estrogen in regulation of the Insulin-like growth factor2–H19 locus in the rat testis. Mol. Cell. Endocrinol. 314, 110–117.
Raikhel, A. S., Brown, M., and Belles, X. (2005). “Hormonal control of reproductive processes,” in Comprehensive Insect Physiology, Biochemistry, Pharmacology and Molecular Biology, eds L. Gilbert, S. Gill, and K. Iatrou (Boston: Elsevier Press), 433–491.
Richard, D. S., Rybczynski, R., Wilson, T. G., Wang, Y., Wayne, M. L., Zhou, Y., Partridge, L., and Harshman, L. G. (2005). Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J. Insect Physiol. 51, 455–464.
Rigaud, T., and Juchault, P. (1998). Sterile intersexuality in an isopod induced by the interaction between a bacterium (Wolbachia) and the environment. Can. J. Zool. 76, 493–499.
Rosen, O., Manor, R., Weil, S., Gafni, O., Linial, A., Aflalo, E. D., Ventura, T., and Sagi, A. (2010). A sexual shift induced by silencing of a single insulin-like gene in crayfish: ovarian upregulation and testicular degeneration. PLoS ONE 5, e15281. doi:10.1371/journal.pone.0015281
Rosenfeld, M. G., Lunyak, V. V., and Glass, C. K. (2006). Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 20, 1405–1428.
Roy, S. G., Hansen, I. A., and Raikhel, A. S. (2007). Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 37, 1317–1326.
Sacchi, L., Genchi, M., Clementi, E., Negri, I., Alma, A., Ohler, S., Sassera, D., Bourtzis, K., and Bandi, C. (2010). Bacteriocyte-like cells harbour Wolbachia in the ovary of Drosophila melanogaster (Insecta, Diptera) and Zyginidia pullula (Insecta, Hemiptera). Tissue Cell 42, 328–333.
Sagi, A., and Khalaila, I. (2001). The crustacean androgen: a hormone in an isopod and androgenic activity in decapods. Am. Zool. 41, 477–484.
Sakamoto, H., Kageyama, D., Hoshizaki, S., and Yshikawa, Y. (2007). Sex specific death in the Asian corn borer moth (Ostrinia furnacalis) infected by Wolbachia occurs across larval development. Genome 50, 645–652.
Sawatsubashi, S., Murata, T., Lim, J., Fujiki, R., Ito, S., Suzuki, E., Tanabe, M., Zhao, Y., Kimura, S., Fujiyama, S., Ueda, T., Umetsu, D., Ito, T., Takeyama, K., and Kato, S. (2010). A histone chaperone, DEK, transcriptionally coactivates a nuclear receptor. Genes Dev. 24, 159–170.
Schütt, C., and Nöthiger, R. (2000). Structure, function and evolution of sex-determining systems in Dipteran insects. Development 127, 667–677.
Schwedes, C., Tulsiani, S., and Carney, G. E. (2011). Ecdysone receptor expression and activity in adult Drosophila melanogaster. J. Insect Physiol. 57, 899–907.
Shingleton, A. W. (2005). Body-size regulation: combining genetics and physiology. Curr. Biol. 15, R825–R827.
Spindler, K. D., Hönl, C., Tremmel, Ch., Braun, S., Ruff, H., and Spindler-Barth, M. (2009). Ecdysteroid hormone action. Cell. Mol. Life Sci. 66, 3837–3850.
Starr, D. J., and Cline, T. W. (2002). A host parasite interaction rescues Drosophila oogenesis defects. Nature 418, 76–79.
Steinmann-Zwicky, M., Schmid, H., and Nöthiger, R. (1989). Cell-autonomous and inductive signals can determine the sex of the germ line of Drosophila by regulating the gene Sxl. Cell 57, 157–166.
Stouthamer, R., Breeuwer, J. A., and Hurst, G. D. (1999). Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53, 71–102.
Terashima, J., Takaki, K., Sakurai, S., and Bownes, M. (2005). Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. J. Endocrinol. 187, 69–79.
Tricoire, H., Battisti, V., Trannoy, S., Lasbleiz, C., Pret, A. M., and Monnier, V. (2009). The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech. Ageing Dev. 130, 547–552.
Tsai, H.-W., Grant, P. A., and Rissman, E. F. (2009). Sex differences in histone modifications in the neonatal mouse brain. Epigenetics 4, 47–53.
Tsuzuki, S., Iwami, M., and Sakurai, S. (2001). Ecdysteroid-inducible genes in the programmed cell death during insect metamorphosis. Insect Biochem. Mol. Biol. 31, 321–331.
Ventura, T., Manor, R., Aflalo, E. D., Weil, S., Raviv, S., Glazer, L., and Sagi, A. (2009). Temporal silencing of an androgenic gland-specific insulin-like gene affecting phenotypical gender differences and spermatogenesis. Endocrinology 150, 1278–1286.
Werren, J. H., Baldo, L., and Clark, M. E. (2008). Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741–751.
Wu, Q., and Brown, M. R. (2006). Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 51, 1–24.
Keywords: Wolbachia, insulin, ecdysone, nuclear receptors, epigenetic
Citation: Negri I (2012) Wolbachia as an “infectious” extrinsic factor manipulating host signaling pathways. Front. Endocrin. 2:115. doi: 10.3389/fendo.2011.00115
Received: 09 November 2011;
Paper pending published: 30 November 2011;
Accepted: 21 December 2011;
Published online: 09 January 2012.
Edited by:
Joe Hull, USDA Agricultural Research Service, USAReviewed by:
Sergio Polakof, French National Institute for Agricultural Research, FranceKostas Bourtzis, University of Western Greece, Greece
Copyright: © 2012 Negri. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Ilaria Negri, Department of Exploitation and Protection of the Agricultural and Forestry Resources, Università di Torino, Via L. da Vinci, 44, 10095 Grugliasco, Italy. e-mail:SWxhcmlhLm5lZ3JpQHVuaXRvLml0
