- 1International Center for Agricultural Research in the Dry Areas, Rabat, Morocco
- 2Laboratory of Zoology, University of Mons, Research Institute for Biosciences, Mons, Belgium
- 3Laboratory of Entomology, Regional Center of Agricultural Research of Kenitra, National Institute of Agricultural Research, Kenitra, Morocco
- 4Independent Consultant, Edmonton, AB, Canada
- 5Federal Agency for Nature Conservation (BfN), Bonn, Germany
The importance of flower visitors for ecosystem resilience and crop production underscores the need to address the current decline of flower visitors worldwide. Farming Alternative Pollinators (FAP), economic and ecological benefits of fields hosting various marketable habitat enhancement plants, developed for flower visitors protection in low- and middle-income countries, showed multiple benefits for farmers of pollinator-dependent crops, but potential benefits of FAP for production of pollinator-independent crops have not yet been assessed. Therefore, we conducted in 2021 FAP trials with wheat (Triticum aestivum) as the main crop in two regions of Morocco where cereals are mainly grown in monocultures in field sizes ranging from 2 to 5 ha. We tested the effects of fields adding marketable habitat enhancement plants (MHEP; coriander and canola) versus control fields on pests, natural enemies, flower visitors, and net income. We found significantly lower abundance and diversity of pests in wheat fields using MHEP, but no effect on natural enemy presence or net income. The strips of MHEP attracted a high number of flower visitors in both regions (Settat and Sidi Slimane), they supported flower visitor communities by providing plant resources and alternative habitat in monocultural landscapes extremely degraded for flower visitors.
Introduction
Monocultures and associated increased use of pesticides and fertilizers are serious threats to beneficial insects like pollinators and natural enemies of crop pests (Aizen et al., 2019; Cole et al., 2020), which are important for yield in many crops (Christmann et al., 2021b; Raven and Wagner, 2021). Furthermore, nesting habitats and foraging resources for pollinators and other flower visiting insects have severely decreased due to agricultural intensification (Abudulai et al., 2010; Lasway et al., 2022; Raven and Wagner, 2021).
The loss of pollinators raises concerns about the future of pollination services (Deguines et al., 2014), crop production, and associated negative economic impacts (Potts et al., 2016). Eighty-seven percent of flowering plants depend on animal pollinators for sexual reproduction (Ollerton et al., 2011). Wild bees, regarded as the most effective pollinators (Potts et al., 2016), are a diverse group with more than 20,000 described species (Michener, 2007). Morocco is home to at least 1016 bee species—almost half the number of species found in Europe (2138 species) (Ghisbain et al., 2023; Lhomme et al., 2020; Reverté et al., 2023; Wood, 2023). Other important flower visiting insect such as pollinators in Morocco include 148 syrphid fly species and 45 butterfly species (Sahib et al., 2020; Verovnik et al., 2018). However, Morocco’s pollinator fauna, including variuos flower visitors, is still poorly known compared to many high-income countries (Lhomme et al., 2020; Potts et al., 2016).
Several strategies have been implemented in agro-ecosystems in Europe to mitigate the decline of pollinator insects and pollination services (Defra, 2015; 2016; Ministry of Agriculture, N. and F. Q, 2018; Stout, 2020). Agri-environmental schemes (AES) have been one of the most important strategies to protect and improve biodiversity in agricultural lands (Lécuyer et al., 2022). Sowing wildflower strips (WFS) is one of the most recommended approaches to enhance wild bee and pollination services in intensively managed landscapes (Blaauw and Isaacs, 2014; 2015; Campbell et al., 2017a; b; Feltham et al., 2015; Ganser et al., 2018; Rundlöf et al., 2018; Potts et al., 2016). In low- and middle-income countries, due to the perception of wildflowers as weeds, the high priority of food security and the absence of external incentives through agroecological schemes, farmers reject seeding them (Christmann et al., 2017). For Morocco and other low- and middle-income countries, AES are not affordable (Christmann, 2020b). Furthermore, farmers in these countries often reject this method due to opportunity costs and the fear of spreading weeds (Christmann et al., 2021a). Approaches where increasing plant diversity supports both pest management (Gurr et al., 2016; 2017), pollinators and flower visiting insects might have a higher chance of being adopted than approaches highlighting pollinator protection only. Research by Wan et al. (2020) found that enhancing crop diversity (intercropping) within cereals had positive effects on natural-enemy abundance; floral resources attracted a higher diversity of predators and reduced pest densities. In another study, enhanced crop diversity supported pest predators such as spiders (Boetzl et al., 2023). Undersown (intercrop) plant mixtures have the potential to enhance both natural pest control and soil health, thereby reducing the need for fertilizers and pesticides, minimizing yield losses due to pests, and ultimately offsetting the costs of additional seeds and labor (Clough et al., 2011; Gurr et al., 2016; 2017; Wan et al., 2020).
Sustainable agricultural practices include the use of integrated pest management to reduce the need for pesticides and contribute to farm productivity. However, agricultural intensification with larger scale plantings of monocultures includes excessive use of chemical pesticides and fertilizers (Cole et al., 2020). Lack of habitat, toxicity of pesticides, and negative impacts of fertilizers, heavy machinery and tillage can negatively affect soil biodiversity, the presence of natural enemies and reduce their role in pest management (Giri and Varma, 2020). Sowing wildflowers within the main crop helps mitigate loss of habitat and negative impacts of expanded pesticide use that can harm beneficial insect populations (Griffiths-Lee et al., 2023; Gurr et al., 2017), but this approach is rarely adopted by farmers (Kleijn et al., 2019).
Diversification of agricultural systems by increasing diversity of crops has been an implemented solution to increase sustainability of agricultural production (Blaauw and Isaacs, 2015; Bommarco et al., 2013; Sardiñas and Kremen, 2015). Farming with Alternative Pollinators (FAP) (Christmann, 2019; 2020b; Christmann et al., 2017; 2021a; b; Christmann and Aw-Hassan, 2012) is a socio-economic agro-ecological farming approach focusing on better human understanding and economic sustainability and scalability of flower visitor protection in low- and middle-income countries (Christmann et al., 2021a; b). By planting so-called marketable habitat enhancement plants (MHEP) along certain parts of the fields, FAP benefits flower visitors (pollinators and natural enemies) and reduces pests instead of using insecticides (Christmann et al., 2021b). In pollinator-dependent crops it can thus ultimately increase income, providing a method-inherent and performance-related economic incentive for farmers to adopt the approach (Christmann et al., 2017; 2021a; b). FAP has been tested for pollinator-dependent crops in smallholder fields (300 m2) (Bencharki et al., 2022; Christmann et al., 2017; 2021a; b; Sentil et al., 2021; 2022a; b) and large fields (at least 1 ha) (Bencharki et al., 2024). However, the efficiency of this practice has not been tested yet on non-entomophilous crops like wheat.
Wheat (Triticum aestivum) is one of the most important crops in Morocco, with a total production in 2021 of 26,335 tons (FAO, 2021). The proteins in wheat seeds are a major source of protein in the human diet (Benitez-Cardoza et al., 2007). Its nutritional and economic value add to wheat’s importance in food security for the region.
Here, for the first time, we tested FAP in a pollinator-independent crop, wheat, in large fields with four objectives: 1) Assess the diversity and abundance of natural enemies and pests in FAP wheat fields (FAP fields) versus monocultural wheat fields (control fields); 2) compare FAP and control fields in terms of the diversity and abundance of pests and natural enemies in the main crop; 3) describe the diversity and abundance of flower visitors in FAP and control fields 4) compare the yield and the income of these FAP and control fields.
Materials and methods
Study sites
We developed our experiments in two Moroccan regions: Settat, situated in the semi-arid region, is characterized by cereal production, with wheat (Triticum aestivum L.) being the primary crop. About 90% of the arable land in Settat is used for intensive cereal cultivation, influenced by the area’s climatic conditions, which include low and unpredictable rainfall, high evaporation rates, and frequent droughts. These challenges require agricultural practices that focus on maximizing yield efficiency, often leading to a heavy reliance on monoculture, synthetic fertilizers, and pesticides to maintain production under these difficult environment conditions. Sidi Slimane, located in the Rabat-Salé-Kenitra region, presents a striking contrast to Settat, primary due to its sub-humid climate, which features more consistent rainfall and milder temperatures. This advantageous climate fosters intensive horticultural production, where large-scale monocultures of fruits and vegetables dominate the agricultural landscape. The region is recognized for its high-input farming practices, including the extensive use of irrigation, fertilizers and pesticides to sustain high yields and satisfy the demands of both local and export markets. The prevalence of intensive monoculture farming has caused considerable ecological challenges, such as soil degradation, decreased habitat diversity, and declining populations of wild pollinators. These issues make both regions ideal for exploring the potential of the FAP approach. This strategy not only seeks to support biodiversity but also aims to boost agricultural productivity and farmer income, particularly in a context where environmental sustainability is increasingly threatened.
In November 2020, we established a FAP-trial in both regions with wheat (Triticum aestivum) as the main crop and coriander (Coriandrum sativum) and canola (Brassica napus) as marketable habitat enhancement plants (MHEP) (Figure 1). Eight fields (5 FAP fields and 3 control fields) were studied in two provinces of each region (Ouled Said and Ouled Sghir in Settat; Ouled ben Hammadi and Haouafate in Sidi Slimane (16 fields in total). All the fields, either with the main crop and MHEP or only the main crop, were managed using similar farming practices concerning tillage, fertilizer and fungicide application. The irrigation system for the fields was rainfed. Each field was 20,300 m2, with 1 m x 100 m strips of each MHEP planted every 100 m on the edges of FAP fields (Figure 1). Two strips of coriander were planted on the two lateral edges, and one strip of canola was planted in the middle. This provided enough space for the farmers to use their equipment for planting and harvesting. Study fields were at least 1 km apart to ensure independent results. Since the field sizes in both regions ranged from 2 to 5 ha, the study fields measured 2.03 ha constituted entire fields, while others were trial areas within larger monocultures.
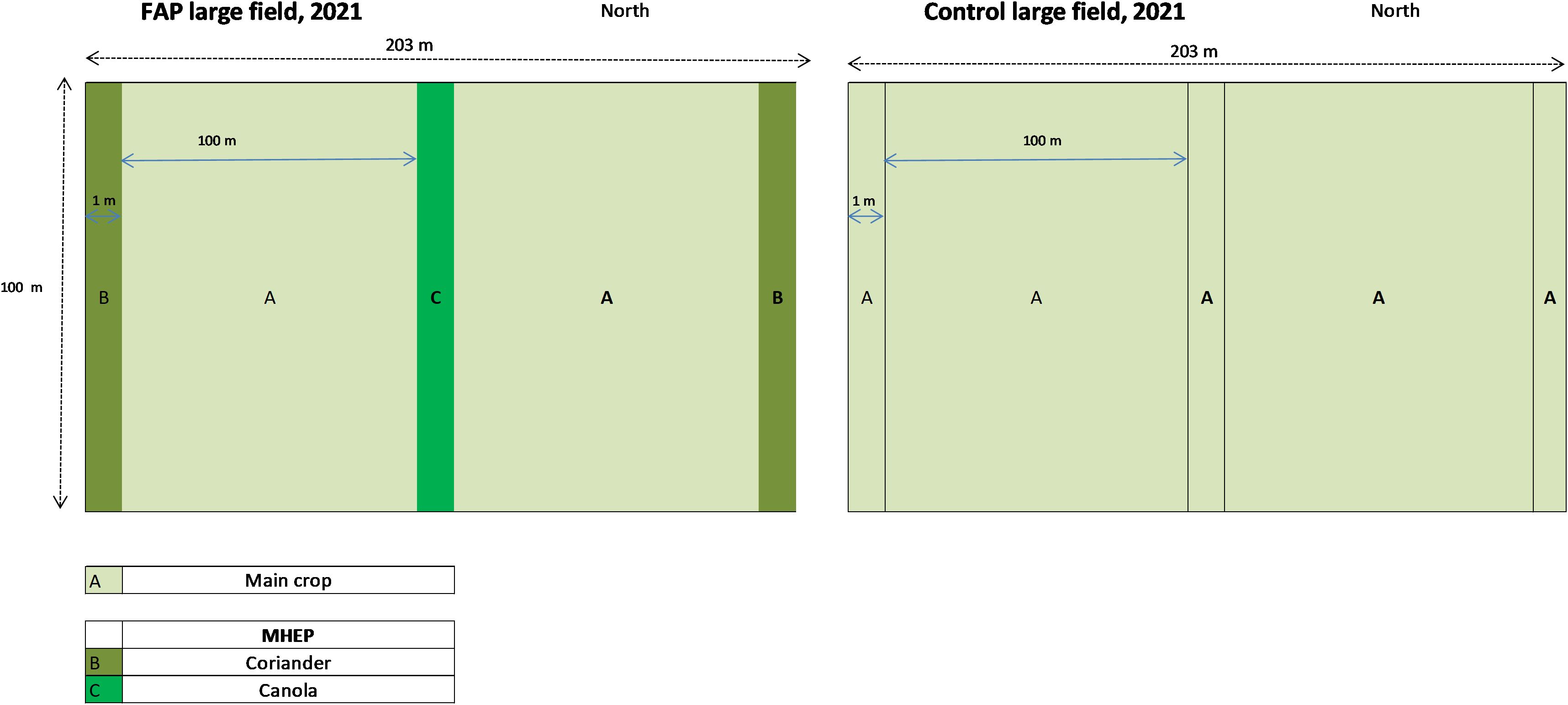
Figure 1. Farming with Alternative Pollinators (FAP) experimental designs of wheat (Triticum aestivum) for Settat (semi-arid) and Sidi Slimane (sub-humid) regions. A, main crop area with wheat; B, Marketable Habitat Enhancement Plants (MHEP) area with coriander; C, MHEP area with canola.
Marketable habitat enhancement plants
During farmer selection in the Settat region, we discovered that many farm workers had become aware of the importance of pollinators, other insects, and the benefits they provide to crops due to many FAP-trials with smallholders in 2018-2019 (Christmann et al., 2021b). We selected farmers who already knew about the importance of pollinators for many crops, non-crop plants, and wildlife. In the previous trials, participating smallholders were able to provide feedback on pollinator abundance and the variety of flower-visitors per MHEP. To select the appropriate MHEP, we involved large-scale farmers in the decision-making process, considering their knowledge about seeding, flowering times, and water demands. Farmers’ inputs were particularly important in considering the market potential of specific MHEP at the targeted harvesting time. Based on criteria such as potential income, different flower types, flowering time, and seeding time, we selected two plants as MHEP: canola (Brassica napus) and coriander (Coriandrum sativum). These plants have been shown to attract flower visitors and natural enemies in previous studies (Christmann et al., 2021b; Sentil et al., 2021; 2022). We agreed on three practices that the farmers would be responsible for: establishing the plant strips, managing the MHEP, and harvesting the crops (main crop and MHEP).
Sampling of pests and natural enemies
In March and April 2021, we conducted three samplings, with each lasting two days (four fields per day) and ten days between them. Each field was visited once for one hour to collect flower visitors, pests and natural enemies. To collect natural enemies and pests in the fields, we used funnels – 180 mm diameter and 2 ml Eppendorf tubes. For sampling of pests/natural enemies, in FAP fields we selected 10 plants randomly in each MHEP (i.e., 10 coriander plants and 10 canola plants) and 10 in the main crop to be beaten, whereas in control fields we sampled 30 plants of the main crop from different places to be beaten. In both types of fields, we conducted beating 5 times on each selected plant to collect crop natural enemies/pests. The samples were put into plastic bags and labeled with the date, field, and management. In the lab, all collected insects were sorted using dissecting microscopes and conserved in 2 ml Eppendorf tubes containing 70% ethanol. Collected specimens were identified to the lowest possible taxonomic level using microscopes and identification keys (Bonsignore and Vacante, 2012). The data collected was pooled in two different ways to compare the abundance and diversity of pests and natural enemies on (a) the whole field and for comparability in (b), we randomly selected 10 wheat plants from the 30 sampled wheat plants to compare with the 10 wheat plants from the control main crop area.
Flower-visitor sampling
To characterize the flower visitor communities in MHEP strips, we performed three samplings in March and April of 2021 in all fields. For each sampling over two consecutive days, we collected flower visitors in FAP and control fields. Although wheat is pollinator-independent, we sampled wheat and MHEP flower visitors. We used two methods to sample flower visitors: netting along transects and pan traps, because in most studies, pan traps and net transect were the most recommended methods for sampling flower visitors. In each field, we used both methods to collect the flower visitors. After collecting the data, we merged the data from each field and then summed the control and FAP fields to calculate the abundance. During MHEP bloom, we did transect sampling in the main crop for 10 minutes, randomly selecting 5 minutes in each area of 10,000 m2 of the main crop. In FAP fields, we also sampled the MHEP strips (i.e. area of 3x100 m2), for 10 minutes (5 minutes for each MHEP, 5 min in the canola strip and 2.5 min in each coriander strip). 1.5% area of each monoculture field was likewise sampled for 10 minutes (10 min was divided between the three strips) (Supplementary Figure 1).
To limit sampling bias between the fields, we conducted sampling during warm, calm, and sunny days between 10 am and 4 pm. Honeybees (Apis mellifera), carpenter bees (Xylocopa pubescens), and bumblebees (Bombus terrestris) were not collected but identified visually in the field. All other bees were collected and identified in the lab.
For pan-trap sampling, we placed bowl traps (volume of 500 ml, diameter of 145 mm, depth of 45 mm) of three different colors (yellow, white, and blue) on both sides of the wheat fields to collect flower visitors in FAP and control fields. In each (10,000 m2) main crop area, we placed one set of three bowls towards each end of the transect line (Supplementary Figure 1). The traps were left in the fields for 30 hours, starting at 10 am on the first day, and were collected at 4 pm on the second day. Bees were identified to the genus level in the lab (following key of Michez et al., 2019) and sent to different taxonomists to determine the species level (see Acknowledgements).
Economic assessments
Economic assessments were calculated based on entire field harvests, using machines for the wheat harvest. We measured the total weight of wheat from each field and used the market price per kilogram to determine the income from the wheat crop. For the FAP and control fields, the total area of the main crop (i.e. 98.5% of the field) were weighed and calculated based on seeds market price. Regarding 1.5% area, in FAP fields, MHEP seeds were weighed and also calculated per field, and for control fields the total weight of 1.5% wheat area was considered and calculated per field. Income from MHEP was also calculated by multiplying the total weight by the market price per kilogram. Additionally, we deducted the investment costs for seeds and additional labor costs for harvesting MHEP in the 1.5% of study fields (estimated at 200 MAD, which is equivalent to 3 person-days per field). We did not consider labor costs for harvesting the 1.5% zones in control fields, as they can be quickly harvested together with the main crop.
In our economic assessments of net return, different inputs and additional labor times were recognized. Expense items were calculated based on actual producer investments and production weights, which are presented in Supplementary Material Table 1; Table 1.
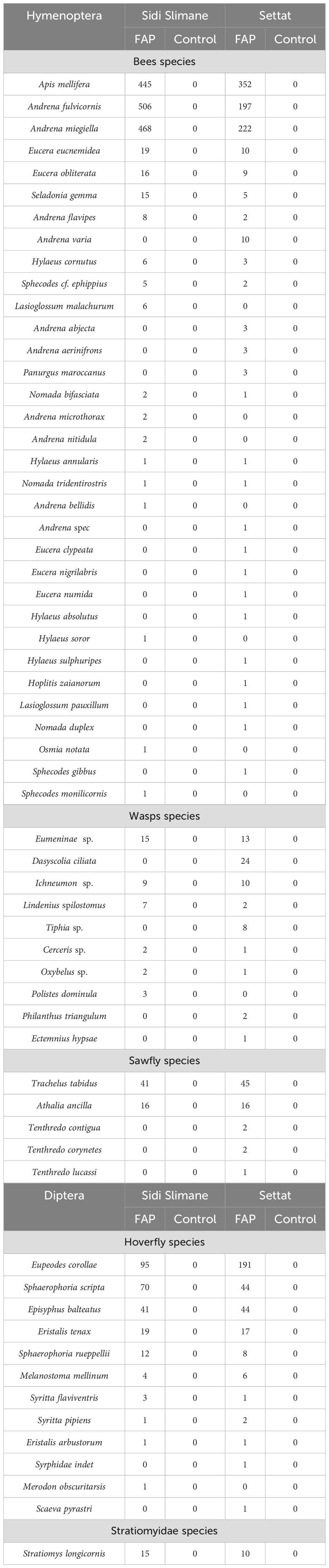
Table 1. Most abundant bee species, wasps species, sawflies species and diptera (hoverfly and Stratiomyidae species) visiting MHEP.
Statistical analysis
To evaluate the impact of management type (FAP vs. control) and arthropod category (natural enemies vs. pests) on arthropod abundance and species richness, we conducted a series of statistical analyses. We first cleaned and organized the data to ensure completeness and generated descriptive statistics to summarize abundance and species richness across the different management types. We assessed the normality of residuals using the Shapiro-Wilk test and checked for homogeneity of variances using Levene’s test. A one-way ANOVA was performed to examine the main effects and interactions between management type and arthropod categories (natural enemies and pests) regarding abundance and species richness. Significant interactions and main effects were further investigated through post hoc pairwise comparisons with Bonferroni corrections. All statistical tests were conducted using R (Version 4.4.0; R Development Core Team, 2023), utilizing relevant packages for data manipulation, visualization, and analysis. The results were visualized with boxplots annotated for statistical significance, aiding in the interpretation of group differences.
We used a t-test to compare economic outcomes between FAP and control fields in terms of yield (weight) and income. It was hypothesized that yield and income in FAP fields were not significantly different from those of the control fields (considered as the null hypothesis for the t-test). To test this hypothesis, a t-test for independent samples was conducted.
Results
Diversity and abundance of pests and natural enemies in the entire field in both regions
In the Sidi Slimane region, across 8 wheat fields, we collected a total of 244 specimens of natural enemies and 689 pest specimens, highlighting the diversity and abundance of both beneficial and harmful insect populations in this area (Table 2). Similarly, in the Settat region, from 8 different wheat fields, we gathered a total of 219 specimens of natural enemies, while the number of pest specimens reached 598, providing valuable comparative data on the interactions between pests and their natural enemies across regions with differing environmental conditions (Table 2).
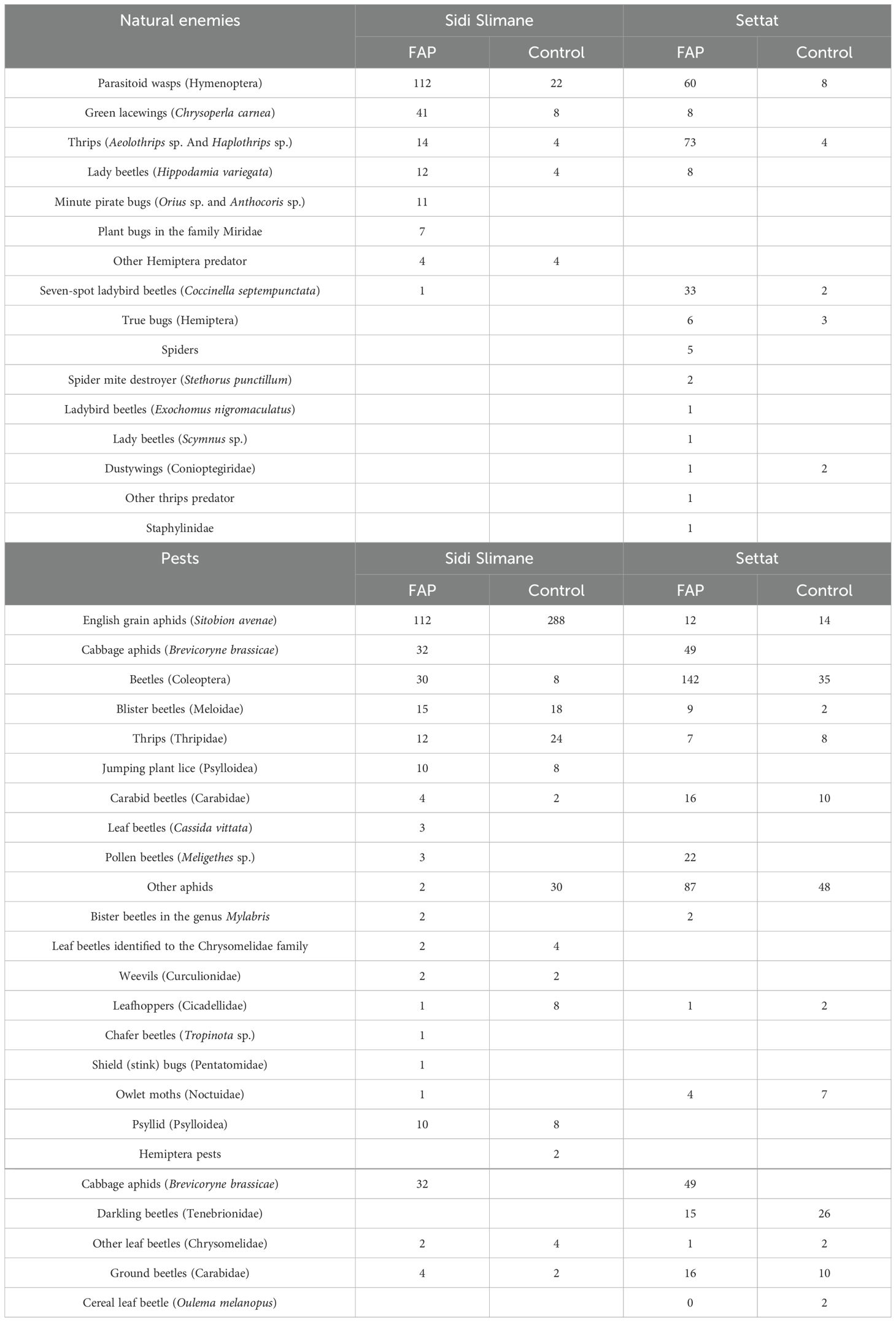
Table 2. Total abundance of natural enemies and pests representing in control and FAP fields in Settat and Sidi Slimane regions.
Impact of FAP approach on the abundance and diversity of pests and natural enemies at full field level
Natural enemy abundance was more than one time higher in FAP than in control fields but there was no statistically significant difference between FAP and control fields (Figure 2A), while pest abundance was more than two times higher in control than in FAP fields (Figure 2C). Whereas in natural enemy diversity between FAP vs. control fields while there was no difference in natural enemy diversity between FAP vs. control fields (Figure 2B), pest diversity was significantly higher in control fields (Figure 2D) Table 3.
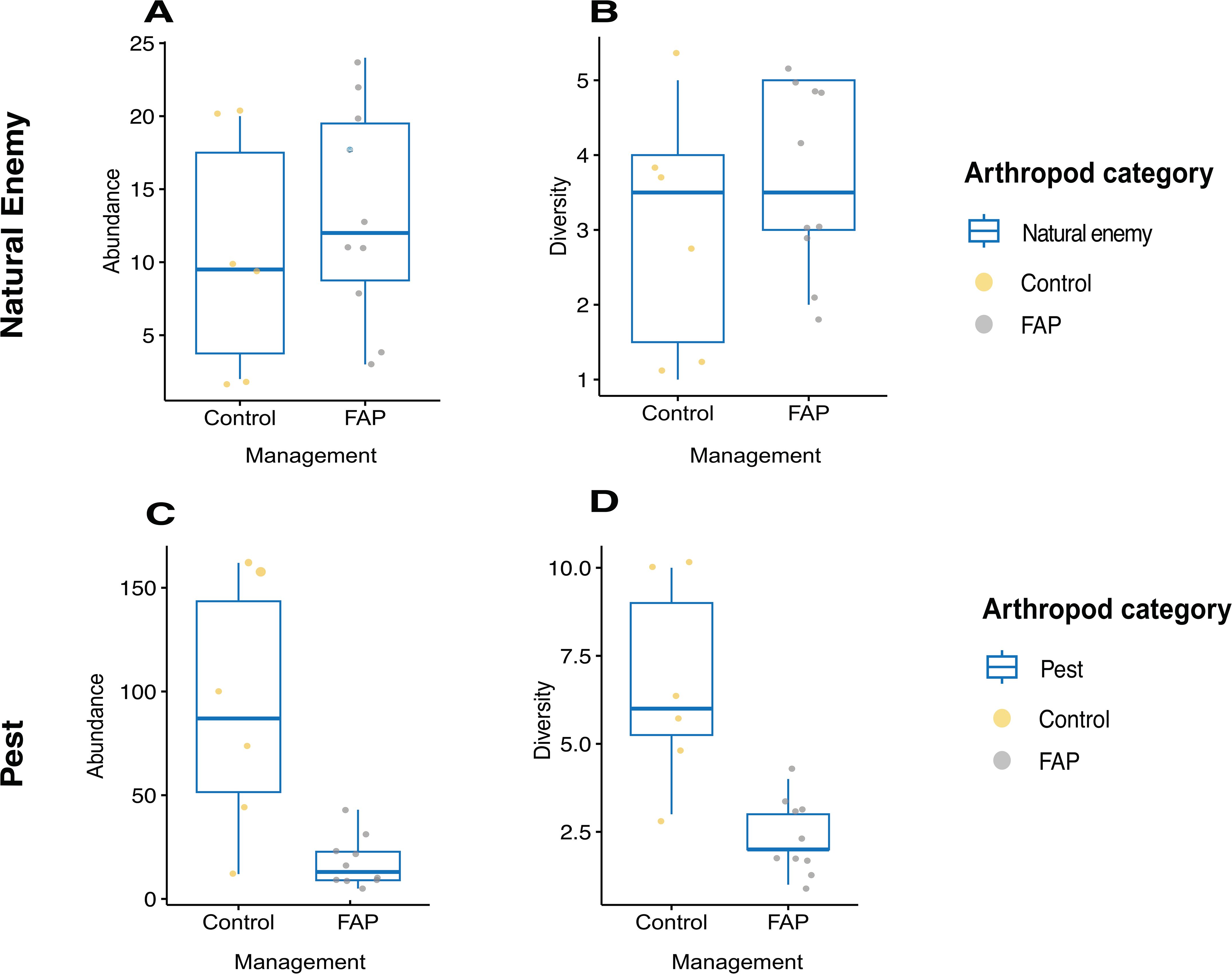
Figure 2. Abundance (A, C) and diversity (B, D) of natural enemies (A, B) and pests (C, D) of 10 Farming with Alternative Pollinators (FAP) (100% FAP field areas) and 6 control (100%control field areas) fields.
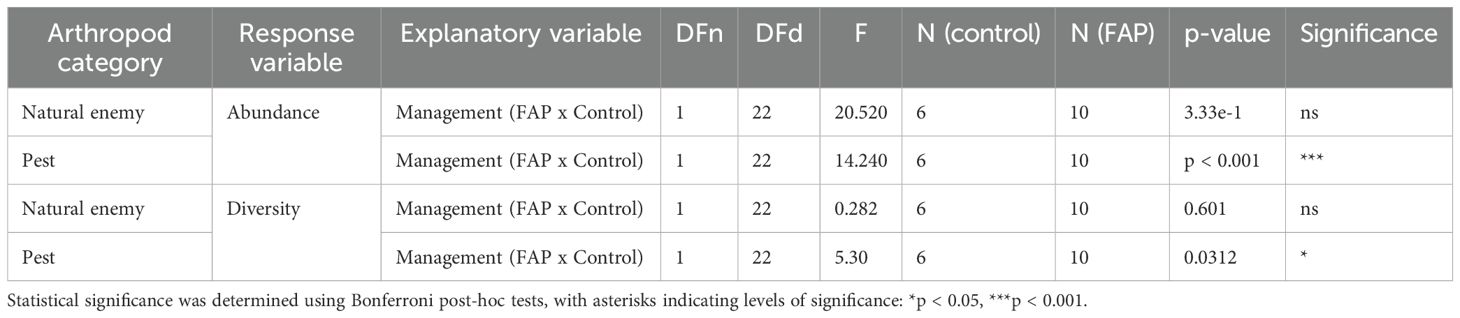
Table 3. ANOVA calculation for total abundance and diversity of natural enemies and pests at full field level.
Impact of FAP approach on the abundance and diversity of pests and natural enemies in the main crop
Natural enemy abundance was approximately the same in both managements, statistically was no difference in the abundance of natural enemies between FAP and control fields (Figure 3A), whereas pest densities were more than one time higher in control than in FAP fields (Figure 3C). Regarding the diversity of the pests and natural enemies in the main crop, there was highly significant difference between control and FAP fields only of the pests (Figure 3D), but not of the natural enemies (Figure 3B) Table 4.
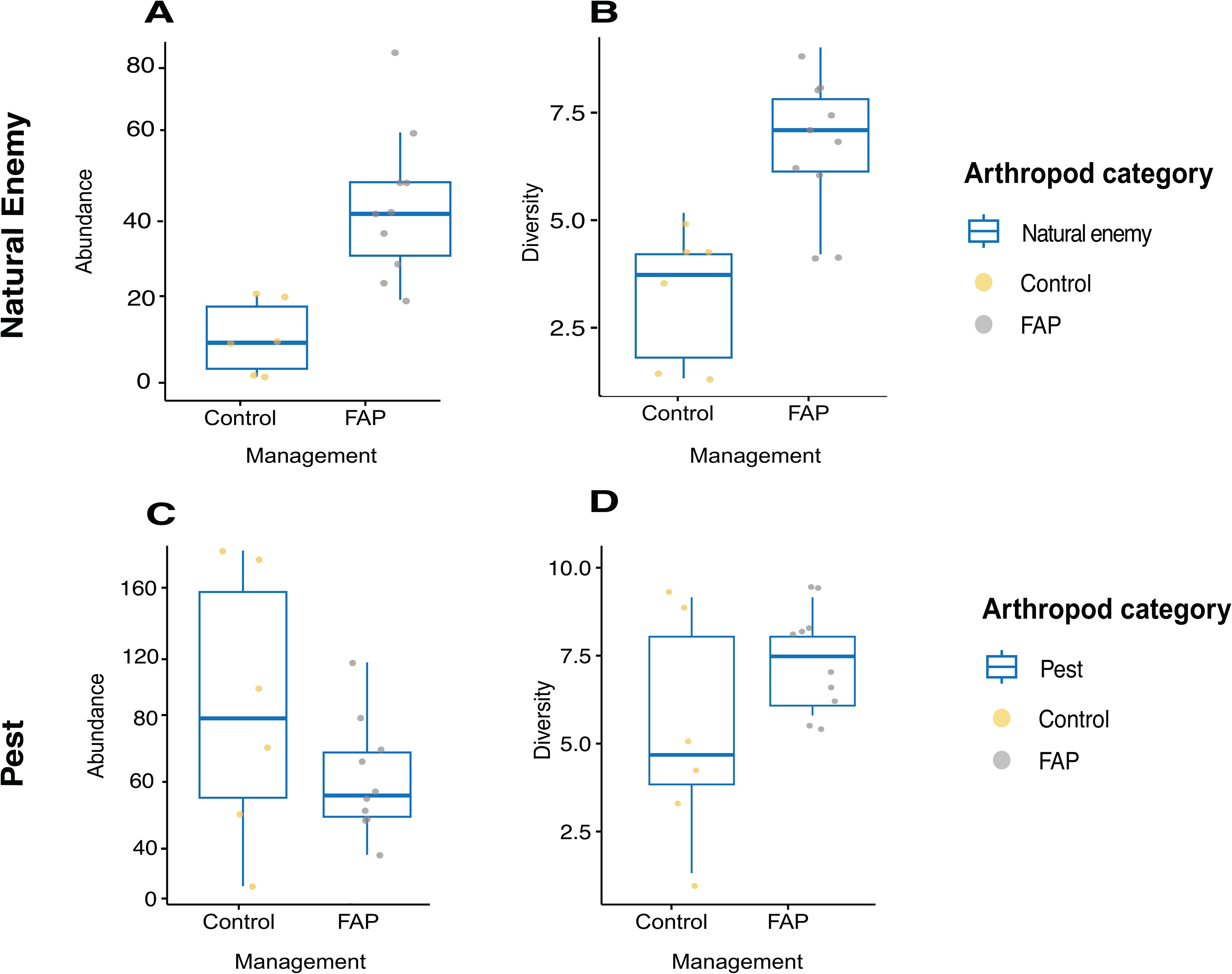
Figure 3. Abundance (A, C) and diversity (B, D) of natural enemies (A, B) and pests (C, D) in the main crop of 10 Farming with Alternative Pollinators (FAP) (98.5% FAP field areas) and 6 control (100% control field areas) fields.
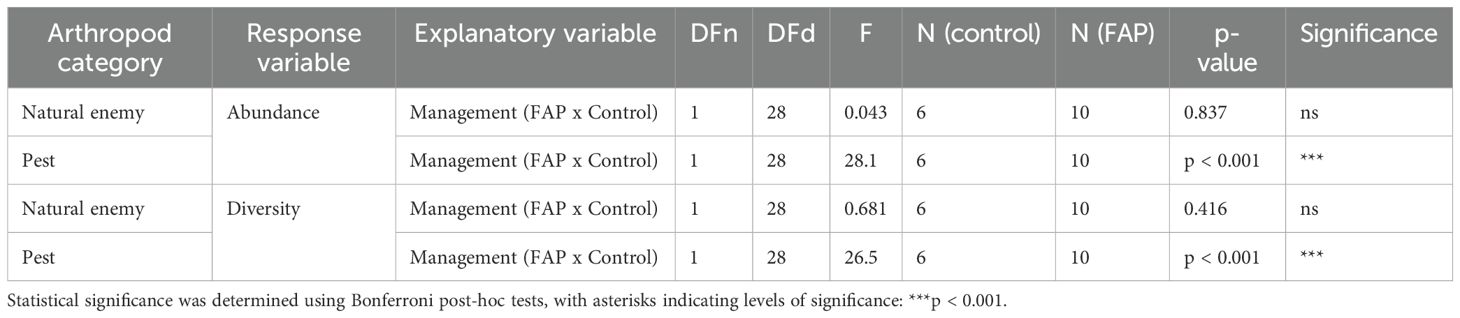
Table 4. ANOVA calculation for abundance and diversity of natural enemies and pests in the main crop.
Effect of MHEP strips on flower visitors
As wheat is a pollinator-independent crop, we did not collect any specimen of pollinators in the main crop. As expected, MHEP strips attracted many different flower visitors.
In Settat region we recorded 838 specimens of bees, we also collected 322 Syrphidae, 2 wasps, 13 other Vespidae and 107 Sawflies. Besides, In Sidi Slimane region we recorded 1,828 specimens of bees. We collected 253 Syrphidae, 38 wasps and 16 sawflies (Table 1).
Economic assessments
Economic assessments showed that the average net income from FAP fields was 1% higher in Settat and 2% higher in Sidi Slimane, because coriander and canola seed provide higher income per area than wheat (Tables 2; Supplementary Table 2). However, statistically the average seeds production (main crop and MHEP) of FAP fields was not significantly higher than that of control fields (t Stat = 0.06, P two-tail = 0.95) (Supplementary Table 3). Also, the average income was not significantly higher from FAP vs control fields (t Stat = 0.30, P two-tail = 0.76) (Supplementary Table 4).
Discussion
Our study confirms the value of crop diversification in pollinator-independent crops for insect conservation (Bonsignore and Vacante, 2012; Gayer et al., 2021; Griffiths-Lee et al., 2023; Middleton et al., 2021; Norris et al., 2018; Ostandie et al., 2021; Settele et al., 2019; Wan et al., 2020). MHEP reduced pests in FAP fields, and provided food resources for flower visitors. In contrast to trials with pollinator dependent crops (Bencharki et al., 2022; Christmann et al., 2017; 2021,b), FAP did not trigger higher income in wheat fields. Overall, it appears that seeding MHEP strips within wheat fields is a strategy to conserve and sustain pollinators. Sustain pollinator enhanced diversity could benefit future crops, provide resiliency in case of crop change in the course of climate change, as well as other regional wildlife (Christmann, 2020a).
FAP effects on pest control
Our study found a significant decrease in pest abundance and diversity in FAP fields probably due to habitat (food and shelter) provided by MHEP for natural enemies of pests compared to control fields (100% wheat and no MHEP). Flower strips can help reduce pest populations (Tschumi et al., 2016) by enhancing biodiversity that supports natural enemies and helps alternative prey (Aviron et al., 2009; Harrison et al., 2019). In our case, we did not find any difference in natural enemies’ abundance and diversity because the latter requires time to install and stabilize prey suppression over time (Jonsson et al., 2017). However, we found a significant difference in pests between FAP and control fields. This could be due to the presence of other animals or insects that were not captured by our sampling methods, such as insectivorous birds or ground-dwelling predators. Those animals or insects may play a larger role in pest control (Beaumelle et al., 2021; Nyffeler et al., 2018) or due to composition of surrounding landscape effects, for instance, habitat diversification practices such as hedgerows (Perez-Alvarez et al., 2019).
Plants like coriander (Apiaceae family) whose open flowers make nectar easily accessible to many predatory insects like parasitic wasps and hoverflies, improve ecosystem services such as pest control (Campbell et al., 2012; Wood et al., 2015). Plants such as coriander attract other beneficial invertebrates including predators (Tschumi et al., 2016). By promoting natural enemies and implementing habitat management, it is possible to potentially increase or at least maintain yield with a reduced level of pesticide inputs (Letourneau et al., 2015). Choosing appropriate MHEP may depend on the categories of natural enemies they attract, and their blooming time in relation to potential pest impacts (Wäckers et al., 2007). Understanding the relative attractiveness of flowering plants to natural enemies against pests is important in successful adoption of these practices (Wäckers and van Rijn, 2012). Specific wheat pests in Morocco are Hessian Fly, sunn pest, Cereal leaf beetle, Wheat stem sawfly, Russian wheat aphid, Greenbug, Bird Cherry-Oat Aphid, English grain aphid and orange wheat blossom midge (Tadesse et al., 2021). In the present study, canola and coriander attracted some natural enemies reducing pests.
Natural enemy density is associated with predation rates (Boetzl et al., 2020). The greater the diversity of plants in crop areas, the greater the expected diversity and abundance of natural enemies (Wan et al., 2020). Some plants are highly attractive to particular natural enemies due to the quality of nectar (Tschumi et al., 2016). However, Albrecht et al. (2020) found no correlation between wildflower diversity and pest control service but did find a positive correlation between flower diversity and pollination service. MHEP can play those two roles by providing floral resources to natural enemies and pollinators (Christmann et al., 2021b).
Parasitoid wasps, in general known for their effectiveness in controlling pest populations especially of weevils (Lundin et al., 2012; Campbell et al., 2012; Wood et al., 2015) made up the largest percentage of predators also in FAP fields (Table 2). Green lacewings were more abundant in Sidi Slimane. They mainly prey on soft bodied insects such as aphids, thrips, mites, moths and lepidopteran eggs (Alcalá-Herrera et al., 2022; Sarwar and Salman, 2016). In Settat region, the major natural enemies in our trials were seven-spot ladybird beetles. They are the main natural enemies of English grain aphids (Sitobion avenae) (Alhmedi et al., 2009). In FAP fields, spider densities were lower compared to those in control fields. The low densities of thrips and spiders observed in the Settat trials may be attributed to the increased abundance of Minute pirate bugs. These generalist predators are known to feed on spiders, thrips, and insect eggs found on herbaceous plants (Bonsignore and Vacante, 2012). Likely due to the presence of seven-spot ladybird beetles, the population density of aphids was lower in the trial of Settat compared to Sidi Slimane region, probably because more than 50% of Coccinellidae family prey on aphids (Bonsignore and Vacante, 2012). Overall, the low pest densities of aphids and cereal leaf beetle in both regions in FAP fields may have been camouflaged by the potential effects of FAP.
With no significant increase of natural enemies in wheat production, it is likely that natural enemies attracted by MHEP did not shift to the main crop. Similar to previous studies (Kral-O’Brien et al., 2022; Ranjitha et al., 2019), coriander and canola attracted a lot of flower visitors and natural enemies, thus we can consider them as good plants for conserving insects in pollinator-independent crops. It is possible that various MHEP complement one another by supporting a greater diversity of agriculturally beneficial insects. The addition of another MHEP such as clover (Trifolium incarnatum), which supports spider abundance in oats (Avena sativa) (Boetzl et al., 2023), could complement the habitat resources provided by canola and coriander. In the control fields, there was a higher abundance and diversity of pests than FAP fields, which could indicate that MHEP in FAP fields played a role as the trap plants, similar to beans in maize crop (Bi et al., 2023) and in wheat fields based on intercropping systems (Liu et al., 2022). The selection of plants as trap crops should be appropriate for pest reduction in field crops (Cook et al., 2007). Higher plant diversity in the main crop could reduce the abundance and diversity of pests by helping to sustain natural enemy populations (Harrison et al., 2019). This aligns with our results, as we observed a higher abundance and diversity of pests only in control fields.
Overall, our study showed that planting strips of MHEP every 100 m is a new strategy to conserve the abundance and diversity of flower visitors and natural enemies in pollinator-independent crops. Additionally, FAP reduced pests in the main crop, which could indicate that the MHEP act as trap plants. However, FAP may need to be applied consecutively across years to demonstrate a more impactful effect on pest control, and it might lead to the spillover of natural enemies from MHEP to the main crop, thereby affecting crop production as well.
FAP effects on flower visitor conservation
Even if Kral-O’Brien et al. (2022) highlighted that pollinators could benefit from pollinator-independent crops e.g., from grasses that provide abundant pollen, we did not observe any pollinator in the main crop (i.e. on wheat), but only on MEHP (i.e. coriander and canola). This behavior of collecting pollen from anemophilous plants is indeed quite rare (Michez et al., 2019). On the opposite, coriander and canola are well-known for their attractiveness to a diversity of flower visitors (Christmann et al., 2021b; El-Abdouni et al., 2022). Most flower visitors recorded were short tongued wild bees (Andrenidae family) and honey bees Apis mellifera. The open floral nectary of coriander (Azpiazu et al., 2020) makes nectar easily accessible (Nemeth and Szekely, 2000). Thus, seeding coriander would be positive for supporting short-tongued bees, as well as predator wasps and flies. Canola is known for its attractiveness to managed bees, because the flowers offer a high amount of crude protein to support bee nutrition (Kral-O’Brien et al., 2022). It is evident that flower visitors can be promoted by seeding strips of MHEP within wheat and without insecticides applications. Since wild bee populations have been found to be higher on organic farms (Gayer et al., 2021) due to permanent ban of chemical pesticides, the FAP approach might be providing similar benefits for more conventional agricultural systems.
Increasing floral resources diversity and rotation can have a positive impact by improving the health of managed and wild bees (O’Brien et al., 2021; Palmer et al., 2009; Sentil et al., 2022b). FAP is known for hosting a high abundance and diversity of flower visitors in small fields (Bencharki et al., 2022; Christmann et al., 2021a; b; El-Abdouni et al., 2022; Sentil et al., 2022a) and enhancing production of pollinator dependent crops (Bencharki et al., 2022; Christmann et al., 2017; 2021a; b), and confirmed concerning large fields (Bencharki et al., 2024). High flower diversity leads to an enhanced abundance of pollinators from field (Boetzl et al., 2023) to landscape scale (Kleijn et al., 2018). The effect was stronger in the semi-arid region, and adding floral resources can have implications on the landscape scale because the region is scarce of floral resources. FAP provided 65 days of floral resources in association with a crop that does not need flower visitors, this could contribute to the conservation of regional biodiversity. By connecting patches MHEP might also facilitate local or regional migration of flower visitors in regions with large cereal monocultures.
FAP effects on net income
Due to the decrease in abundance and diversity of pests in FAP fields, yield and income could be increased for farmers, but this increase was not statically significant in the present study. Östman et al. (2003) demonstrated the barley production increased by decreasing of pests’ abundance. FAP trials in pollinator dependent crops found income increases due to lower pest abundance and higher diversity and abundance of flower visitors (Christmann et al., 2017; 2021a; b). However, the farmers still benefited in wheat field from the additional income of coriander and canola strips. MHEP also do not cause opportunity costs, which are caused by wildflowers strips (WFS), making MHEP more farmer-friendly (Christmann et al., 2021b). Furthermore, one year is likely not sufficient to show potential income differences, particularly since it might take time for the increased presence of natural enemies to reduce cereal aphid populations (Östman et al., 2003; Rusch et al., 2013). In addition, results may differ from year to year due to high fluctuations in both pest and predator species. Nevertheless, this study shows that FAP contributes to the conservation of flower visitors and reduced pests by seeding MHEP without subsidies in contrast to WFS. On the other hand, MHEP offer a valuable alternative for farmers (Christmann et al., 2021a; Azpiazu et al., 2020) and a habitat for pollinators and pest predators associated with pollinator-dependent crops (Christmann and Aw-Hassan, 2012). In previous studies, FAP was implemented among smallholders, where 25% of the field was planted with MHEP (Christmann et al., 2017; 2021a; b). In the present study, only 1.5% of wheat fields (in narrow 1 m wide strips) were used for habitat enhancement, this area may have been too small to provide both economic and biodiversity benefit. In contrast to the MHEP in this study, WFS are generally planted in 3-6 m width strips in large fields (often the width of a tractor to ease maintenance) (Feltham et al., 2015).
Conclusion
The FAP approach, which utilizes different MHEP, is known for harboring a higher diversity and abundance of natural enemies and flower visitors. This leads to a reduction in pests’ diversity and abundance in FAP fields compared to control fields. Such a positive impact on pest control can potentially improve income. In this study we can conclude that FAP approach shows benefits also in large fields with one of the main pollinator independent crops, wheat. Incorporating MHEP in cereals production is a way to contribute to conservation of flower visitors in pollinator independent monocultures, benefiting future crop rotations and semi-natural land around. Additional future research is recommended to assess a wider range of MHEP plants in cereal production and to optimize the width and field percentage of MHEP strips in different landscape contexts. Since the study was conducted only in one year, the results may change over time. Income differences and natural enemies’ effect on pest control, especially cereal aphids, may become apparent after years of using the approach. Thus, long-term studies are recommended to validate these findings across various environmental conditions, assess a broader range of MHEP species, and optimize MHEP strip designs in different landscapes.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
YB: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. DM: Conceptualization, Data curation, Resources, Supervision, Writing – review & editing. MS: Investigation, Writing – review & editing. OI: Formal Analysis, Writing – review & editing. AA: Investigation, Writing – review & editing. AS: Investigation, Writing – review & editing. PR: Data curation, Resources, Writing – review & editing. SC: Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work is part of an ICARDA project funded by the German Federal Ministry for the Environment, Nature Protection and Nuclear Safety (BMU) within the International Climate Initiative (IKI, 17_IV_065).
Acknowledgments
The authors wish to thank the following for their support: Jakub Straka (University of Prague, Czechia) (Sphecodes and Nomada), Andreas Müller (Institute of Agricultural Sciences, Zürich, Switzerland) (Osmiini), Achik Dorchin (University of Mons, Belgium) (Eucera), Holger Dathe (Humboldt Universität, Berlin, Germany) and Romain Le Divelec (University of Mons, Belgium) (Hylaeus), Thomas Wood (University of Mons, Belgium) (Andrena), Pierre Rasmont (University of Mons, Belgium) (Anthophora), Simone Flaminio (University of Mons, Belgium) (Halictini (Lasioglossum, Halictus and Seladonia) and Nomioides). Pests and natural enemies were identified by the third author listed above. Hoverflies were identified by the sixth author. We would like to thank Mohamed Kabli for his help with field sampling and data. We are indebted to several reviewers who contributed to improve the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1551190/full#supplementary-material
References
Abudulai M., Nboyine J. A., Quandahor P., Seidu A., Traore F. (2010). Agricultural intensification causes decline in insect biodiversity. IntechOpen 34, 57–67. doi: 10.5772/intechopen.101360
Aizen M. A., Aguiar S., Biesmeijer J. C., Garibaldi L. A., Inouye D. W., Jung C., et al. (2019). Global agricultural productivity is threatened by increasing pollinator dependence without a parallel increase in crop diversification. Global Change Biol. 25, 3516–3527. doi: 10.1111/gcb.14736
Albrecht M., Kleijn D., Williams N. M., Tschumi M., Blaauw B. R., Bommarco R., et al. (2020). The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: a quantitative synthesis. Ecol. Lett. 23, 1488–1498. doi: 10.1111/ele.13576
Alcalá-Herrera R., Cotes B., Agustí N., Tasin M., Porcel M. (2022). Using flower strips to promote green lacewings to control cabbage insect pests. J. Pest Sci. 95, 669–683. doi: 10.1007/s10340-021-01419-7
Alhmedi A., Haubruge E., Francis F. (2009). Effect of stinging nettle habitats on aphidophagous predators and parasitoids in wheat and green pea fields with special attention to the invader Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Entomo. Sci. 12, 349–358. doi: 10.1111/j.1479-8298.2009.00342.x
Aviron S., Nitsch H., Jeanneret P., Buholzer S., Luka H., Pfiffner L., et al. (2009). Ecological cross compliance promotes farmland biodiversity in Switzerland. Front. Ecol. Environ. 7, 247–252. doi: 10.1890/070197
Azpiazu C., Medina P., Adán Á., Sánchez-Ramos I., del Estal P., Fereres A., et al. (2020). The role of annual flowering plant strips on a melon crop in central Spain. Influence on pollinators and crop. Insects 11, 21. doi: 10.3390/INSECTS11010066
Beaumelle L., Auriol A., Grasset M., Pavy A., Thiéry D., Rusch A. (2021). Benefits of increased cover crop diversity for predators and biological pest control depend on the landscape context. Ecol. Solutions. Evid. 2, 1–12. doi: 10.1002/2688-8319.12086
Bencharki Y., Christmann S., Lhomme P., Ihsane O., Sentil A., El-Abdouni I., et al. (2022). Farming with Alternative Pollinators’ approach supports diverse and abundant pollinator community in melon fields in a semi-arid landscape Research. Renew. Agric. Food Syst., 1–34. doi: 10.1017/S1742170522000394
Bencharki Y., Michez D., Ihsane O., Sara R., Aw-Hassan A., Smaili M. C., et al. (2024). Farming with alternative pollinators “ provides benefits also in large-scale fields. Acta Oecol. 122, 1–12. doi: 10.1016/j.actao.2023.103978
Benitez-Cardoza C. G., Popineau Y., Gueguen J. (2007). Expression of thioredoxin-fusion proteins of α-gliadin, γ-gliadin and Low Molecular weight glutenin, from wheat endosperm and their domains in enterobacteria. Am. J. Infect. Dis. 3, 84–91. doi: 10.3844/ajidsp.2007.84.91
Bi S., Wang Y., Xu T., Hu B., Wang Z., Hu F., et al. (2023). Application potential of push-pull cropping of maize and beans to fall armyworm (Spodoptera frugiperda) management in China. Res. Square., 1–19. doi: 10.21203/rs.3.rs-2745649/v1
Blaauw B. R., Isaacs R. (2014). Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J. Appl. Ecol. 51, 890–898. doi: 10.1111/1365-2664.12257
Blaauw B. R., Isaacs R. (2015). Wildflower plantings enhance the abundance of natural enemies and their services in adjacent blueberry fields. Biol. Control. 91, 94–103. doi: 10.1016/j.biocontrol.2015.08.003
Boetzl F. A., Douhan Sundahl A., Friberg H., Viketoft M., Bergkvist G., Lundin O. (2023). Undersowing oats with clovers supports pollinators and suppresses arable weeds without reducing yields. J. Appl. Ecol. 60, 614–623. doi: 10.1111/1365-2664.14361
Boetzl F. A., Konle A., Krauss J. (2020). Aphid cards – Useful model for assessing predation rates or bias prone nonsense? J. Appl. Entomol. 144, 74–80. doi: 10.1111/jen.12692
Bommarco R., Kleijn D., Potts S. G. (2013). Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 28, 230–238. doi: 10.1016/j.tree.2012.10.012
Bonsignore C. P., Vacante V. (2012). Integrated control of citrus pests in the Mediterranean Region. Natural Enemies., 66–87. doi: 10.2174/978160805294311201010066
Campbell A., Biesmeijer J., Varma V., Wäckers F. L. (2012). Realising multiple ecosystem services based on the response of three beneficial insect groups to floral traits and trait diversity. Basic. Appl. Ecol. 13, 363–370. doi: 10.1016/j.baae.2012.04.003
Campbell A., Wilby A., Sutton P., Wa¨ckers F. L. (2017b). Do sown fl ower strips boost wild pollinator abundance and pollination services in a spring- fl owering crop? A case study from UK cider apple orchards. Agric. Ecosyst. Environ. 239, 20–29. doi: 10.1016/j.agee.2017.01.005
Campbell A., Wilby A., Sutton P., Wackers F. (2017a). Getting more power from your flowers: multi-functional flower strips enhance pollinators and pest control agents in apple orchards. Insects 8, 1–18. doi: 10.3390/insects8030101
Christmann S. (2019). Under which conditions would a wide support be likely for a Multilateral Environmental Agreement for pollinator protection? Environ. Sci. Policy 91, 1–5. doi: 10.1016/j.envsci.2018.10.004
Christmann S. (2020a). Climate change enforces to look beyond the plant – the example of pollinators. Curr. Opin. Plant Biol. 56, 162–167. doi: 10.1016/j.pbi.2019.11.001
Christmann S. (2020b). Pollinator protection strategies must be feasible for all nations. Nat. Ecol. Evol. 4, 896–897. doi: 10.1038/s41559-020-1210-x
Christmann S., Aw-Hassan A. A. (2012). Farming with alternative pollinators (FAP)-An overlooked win-win-strategy for climate change adaptation. Agricult. Ecosyst. Environ. 161, 161–164. doi: 10.1016/j.agee.2012.07.030
Christmann S., Aw-Hassan A., Güler Y., Sarisu H. C., Bernard M., Smaili M. C., et al. (2021b). Two enabling factors for farmer-driven pollinator protection in low- and middle-income countries. Int. J. Agric. Sustainabil. 20, 54–67. doi: 10.1080/14735903.2021.1916254
Christmann S., Aw-Hassan A., Rajabov T., Khamraev A. S., Tsivelikas A. (2017). Farming with alternative pollinators increases yields and incomes of cucumber and sour cherry. Agron. Sustain. Dev. 37, 1–8. doi: 10.1007/s13593-017-0433-y
Christmann S., Bencharki Y., Anougmar S., Rasmont P., Smaili M. C., Tsivelikas A., et al. (2021a). Farming with Alternative Pollinators benefits pollinators, natural enemies, and yields, and offers transformative change to agriculture. Nature-Sci. Rep. 11, 1–10. doi: 10.1038/s41598-021-97695-5
Clough Y., Barkmann J., Juhrbandt J., Kessler M., Wanger T. C., Anshary A., et al. (2011). Combining high biodiversity with high yields in tropical agroforests. Proc. Natl. Acad. Sci. United. States America 108, 8311–8316. doi: 10.1073/pnas.1016799108
Cole L. J., Kleijn D., Dicks L. V., Stout J. C., Potts S. G., Albrecht M., et al. (2020). A critical analysis of the potential for EU Common Agricultural Policy measures to support wild pollinators on farmland. J. Appl. Ecol. 57, 118–154. doi: 10.4324/9780203009864-14
Cook S. M., Khan Z. R., Pickett J. A. (2007). The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 52, 375–400. doi: 10.1146/annurev.ento.52.110405.091407
Defra (2015). National pollinator strategy 2014 to 2024: implementation plan. Available online at: https://www.gov.uk/government/publications/national-pollinator-strategy-2014-to-2024-implementation-plan.
Defra (2016). National pollinator strategy: progress report 2016. Available online at: www.nationalarchives.gov.uk/doc/open-government-licence/version/3/oremailPSI@nationalarchives.gsi.gov.uk.
Deguines N., Jono C., Baude M., Henry M., Julliard R., Fontaine C. (2014). Large-scale trade-off between agricultural intensification and crop pollination services. Front. Ecol. Environ. 12, 212–217. doi: 10.1890/130054
El-Abdouni I., Lhomme P., Christmann S., Dorchin A., Sentil A., Pauly A., et al. (2022). Diversity and relative abundance of insect pollinators in moroccan agroecosystems. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.866581
FAO (2021). FAOSTAT. Available online at: https://www.fao.org/faostat/en/compare.
Feltham H., Park K., Minderman J., Goulson D. (2015). Experimental evidence that wildflower strips increase pollinator visits to crops. Ecol. Evol. 5, 3523–3530. doi: 10.1002/ece3.1444
Ganser D., Mayr B., Albrecht M., Knop E. (2018). Wildflower strips enhance pollination in adjacent strawberry crops at the small scale. Ecol. Evol. 8, 11775–11784. doi: 10.1002/ece3.4631
Gayer C., Berger J., Dieterich M., Gallé R., Reidl K., Witty R., et al. (2021). Flowering fields, organic farming and edge habitats promote diversity of plants and arthropods on arable land. J. Appl. Ecol. 58, 1155–1166. doi: 10.1111/1365-2664.13851
Ghisbain G., Rosa P., Bogusch P., Flaminio S., Le Divelec R., Dorchin A., et al. (2023). The new annotated checklist of the wild bees of Europe (Hymenoptera:Anthophila). Zootaxa 5327, 1–147. doi: 10.11646/zootaxa.5327.1.1
Giri B., Varma A. (2020). “Soil health,” in Soil Health Analysis. Eds. Giri B., Varma A. (India: Springer). doi: 10.1007/978-3-030-44364-1
Griffiths-Lee J., Davenport B., Foster B., Nicholls E., Goulson D. (2023). Sown wildflowers between vines increase beneficial insect abundance and richness in a British vineyard. Agric. For. Entomol. 25, 139–151. doi: 10.1111/afe.12538
Gurr G. M., Lu Z., Zheng X., Xu H., Zhu P., Chen G., et al. (2016). Multi-country evidence that crop diversification promotes ecological intensification of agriculture. Nat. Plants 2, 1–4. doi: 10.1038/NPLANTS.2016.14
Gurr G. M., Wratten S. D., Landis D. A., You M. (2017). Habitat management to suppress pest populations: progress and prospects. Annu. Rev. Entomol. 62, 91–109. doi: 10.1146/annurev-ento-031616-035050
Harrison R. D., Thierfelder C., Baudron F., Chinwada P., Midega C., Schaffner U., et al. (2019). Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith)management: Providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manage. 243, 318–330. doi: 10.1016/j.jenvman.2019.05.011
Jonsson M., Kaartinen R., Straub C. S. (2017). Relationships between natural enemy diversity and biological control. Curr. Opin. Insect Sci. 20, 1–6. doi: 10.1016/j.cois.2017.01.001
Kleijn D., Bommarco R., Fijen T. P. M., Garibaldi L. A., Potts S. G., van der Putten W. H. (2019). Ecological intensification: bridging the gap between science and practice. Trends Ecol. Evol. 34, 154–166. doi: 10.1016/j.tree.2018.11.002
Kleijn D., Linders T. E. W., Stip A., Biesmeijer J. C., Wäckers F. L., Bukovinszky T. (2018). Methods Ecol. Evol. 9), 1727–1738. doi: 10.1111/2041-210X.13017
Kral-O’Brien K. C., Hovick T. J., Harmon J. P. (2022). Quid pro quo? A review on bee utilization of pollinator-independent crops. Ann. Entomo. Soc. America 115, 1–9. doi: 10.1093/aesa/saab029
Lasway J. V., Peters M. K., Njovu H. K., Eardley C., Pauly A., Steffan-Dewenter I. (2022). Agricultural intensification with seasonal fallow land promotes high bee diversity in Afrotropical drylands. J. Appl. Ecol. 59, 3014–3026. doi: 10.1111/1365-2664.14296
Lécuyer L., Alard D., Calla S., Coolsaet B., Fickel T., Heinsoo K., et al. (2022). Conflicts between agriculture and biodiversity conservation in Europe: Looking to the future by learning from the past. Adv. Ecol. Res. 65, 3–56. doi: 10.1016/bs.aecr.2021.10.005
Letourneau D. K., Jedlicka J. A., Bothwell S. G., Moreno C. R., Letourneau D. K., Jedlicka J. A., et al. (2015). Terrestrial Ecosystems All use subject to JSTOR Terms and Conditions Effects of Natural Terrestrial Ecosystems Enemy on the Suppression Biodiversity in of Arthropod Herbivores. Annual Rev. Ecol. Syst. 40, 573–592. doi: 10.1146/annurev.ecolsys.l
Lhomme P., Michez D., Christmann S., Scheuchl E., El-Abdouni I., Hamroud L., et al. (2020). The wild bees (Hymenoptera: Apoidea) of Morocco. Zootaxa 4892, 1–159. doi: 10.11646/zootaxa.4892.1.1
Liu H., Cheng Y., Wang Q., Liu X., Fu Y., Zhang Y., et al. (2022). Push–pull plants in wheat intercropping system to manage Spodoptera frugiperda. J. Pest Sci. 96, 1579–1593. doi: 10.1007/s10340-022-01547-8
Lundin O., Rundlöf M., Smith H. G., Bommarco R. (2012). Towards integrated pest management in red clover seed production. J. Econ. Entomol. 105, 1620–1628. doi: 10.1603/EC12179
Michener C. D. (2007). The bees of the world. Am. Sci. 78, 115–125. doi: 10.1016/0047-2484(91)90057-3
Michez D., Rasmont P., Terzo M., Vereecken N. J. (2019). Bees of europe Vol. 1 (Paris, France: NAP Editions), ISBN: ISBN: 978-2-913688-34-6.
Middleton E. G., Macrae I. V., Philips C. R. (2021). Floral plantings in large-scale commercial agroecosystems support both pollinators and arthropod predators. Insects 12, 1–18. doi: 10.3390/insects12020091
Ministry of Agriculture, N. and F. Q (2018). NL Pollinator Strategy “Bed & Breakfast for Bees”. Netherlands: Minister of Agriculture, Nature and Food Quality.
Nemeth G., Szekely E. (2000). Floral biology of medicinal plants I. Apiaceae species. Hungary: University of Debrecen Electronic Archive.
Norris S. L., Blackshaw R. P., Critchley C. N. R., Dunn R. M., Smith K. E., Williams J., et al. (2018). Intercropping flowering plants in maize systems increases pollinator diversity. Agric. For. Entomol. 20, 246–254. doi: 10.1111/afe.12251
Nyffeler M., Şekercioğlu Ç.H., Whelan C. J. (2018). Insectivorous birds consume an estimated 400–500 million tons of prey annually. Sci. Nat. 105, 1–13. doi: 10.1007/s00114-018-1571-z
O’Brien P., Kral-O’Brien K., Hatfield J. L. (2021). Agronomic approach to understanding climate change and food security. Agron. J. 113, 4616–4626. doi: 10.1002/agj2.20693
Ollerton J., Winfree R., Tarrant S. (2011). How many flowering plants are pollinated by animals? Oikos 120, 321–326. doi: 10.1111/j.1600-0706.2010.18644.x
Ostandie N., Giffard B., Bonnard O., Joubard B., Cervera S. R., Thiéry D., et al. (2021). Multi − community effects of organic and conventional farming practices in vineyards. Sci. Rep. 11, 1–10. doi: 10.1038/s41598-021-91095-5
Östman Ö., Ekbom B., Bengtsson J. (2003). Yield increase attributable to aphid predation by ground-living polyphagous natural enemies in spring barley in Sweden. Ecol. Econ. 45, 149–158. doi: 10.1016/S0921-8009(03)00007-7
Palmer R. G., Perez P. T., Ortiz-Perez E., Maalouf F., Suso M. J. (2009). The role of crop-pollinator relationships in breeding for pollinator-friendly legumes: From a breeding perspective. Euphytica 170, 35–52. doi: 10.1007/s10681-009-9953-0
Perez-Alvarez R., Nault B. A., Poveda K. (2019). Effectiveness of augmentative biological control depends on landscape context. Sci. Rep. 9, 1–15. doi: 10.1038/s41598-019-45041-1
Potts S. G., Vera I.-F., Ngo H. T., Biesmeijer J. C., Breeze T. D., Dicks A. G., et al. (2016). IPBES 2016. Summary for policymakers of the assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on pollinators, pollination and food production. Population. Dev. Rev. 45, 519–529. doi: 10.1111/padr.12283
Ranjitha M., Koteswara S. R., Rajesh A., Reddi Shekhar M., Revanasidda (2019). Insect pollinator fauna of coriander (Coriandrum sativum L.) ecosystem. J. Entomol. Zool. Stud. 7, 1609–1616.
Raven P. H., Wagner D. L. (2021). Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. United. States America 118, 1–6. doi: 10.1073/PNAS.2002548117
Reverté S., Milicici M., Acanski J., Andric A., Aracil A., Aubert M., et al. (2023). National records of 3000 European bee and hoverfly species: A contribution to pollinator conservation. Insect Conserv. Diversity, 1–18. doi: 10.1111/icad.12680
Rundlöf M., Lundin O., Bommarco R. (2018). Annual flower strips support pollinators and potentially enhance red clover seed yield. Ecol. Evol., 1–12. doi: 10.1002/ece3.4330
Rusch A., Bommarco R., Jonsson M., Smith H. G., Ekbom B. (2013). Flow and stability of natural pest control services depend on complexity and crop rotation at the landscape scale. J. Appl. Ecol. 50, 345–354. doi: 10.1111/1365-2664.12055
Sahib S., Driauach O., Belqat B. (2020). New data on the hoverflies of Morocco (Diptera, Syrphidae) with faunistic and bibliographical inventories. ZooKeys 2020, 59–103. doi: 10.3897/zookeys.971.49416
Sardiñas H. S., Kremen C. (2015). Pollination services from field-scale agricultural diversification may be context-dependent. Agricult. Ecosyst. Environ. 207, 17–25. doi: 10.1016/j.agee.2015.03.020
Sarwar M., Salman M. (2016). From Production to field application methodology of generalist predator green lacewing, chrysoperla carnea [Stephens] (Neuroptera: chrysopidae). J. Zool. Stud. 1, 35–40.
Sentil A., Lhomme P., Michez D., Reverté S., Rasmont P., Christmann S. (2021). Farming with Alternative Pollinators” approach increases pollinator abundance and diversity in faba bean fields. J. Insect Conserv. doi: 10.1007/s10841-021-00351-6
Sentil A., Reverté S., Lhomme P., Bencharki Y., Rasmont P., Christmann S., et al. (2022a). Wild vegetation and ‘farming with alternative pollinators’ approach support pollinator diversity in farmland. J. Appl. Entomol., 1–14. doi: 10.1111/jen.13060
Sentil A., Wood T. J., Lhomme P., Hamroud L., El Abdouni I., Ihsane O., et al. (2022b). Impact of the “Farming with alternative pollinators” Approach on crop pollinator pollen diet. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.824474
Settele J., Joachim H. Spangenberg K. L. H., Kühn I., Klotz S., Arida G., Burkhard B., et al. (2019). Rice Ecosystem Services in South-East Asia: The LEGATO Project, Its Approaches and Main Results with a Focus on Biocontrol Services. doi: 10.1007/978-3-319-96229-0
Stout J. (2020). Science and policy for pollinator protection: The All-Ireland Pollinator Plan Ecological interactions Human society Prof. Jane Stout. ReNature. (Ireland: Trinity College Dublin).
Tadesse W., Harris M., Herrera L. C., Kehel Z., El Bouhssini M. (2021). “Wheat breeding for resistance to major insect pests learning objectives,” in Agricultural Research & Technology: Open Access Journal. (US: Juniper). 25, 1–4. doi: 10.19080/ARTOAJ.2021.25.556321
Tschumi M., Albrecht M., Collatz J., Dubsky V., Entling M. H., Najar-Rodriguez A. J., et al. (2016). Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 53, 1169–1176. doi: 10.1111/1365-2664.12653
Verovnik R., Beretta S., Rowlings M. (2018). Contribution to the knowledge of the spring butterfly fauna of the southern anti-atlas region, Morocco (Lepidoptera: Papilionoidea). SHILAP. Rev. Lepidopterol. 46, 81–90. doi: 10.57065/shilap.839
Version 4.4.0; R Development Core Team (2023). A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing).
Wäckers F. L., Romeis J., Van Rijn P. (2007). Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 52, 301–323. doi: 10.1146/annurev.ento.52.110405.091352
Wäckers F. L., van Rijn P. C. J. (2012). “Pick and mix: Selecting flowering plants to meet the requirements of target biological control insects,” in Biodiversity and Insect Pests: Key Issues for Sustainable Management. Ed. Gurr G. M., et al (John Wiley & Sons, Ltd, Chichester), 139–165.
Wan N., Zheng X., Fu L., Kiær L. P., Zhang Z., Chaplin-kramer R., et al. (2020). Global synthesis of effects of plant species diversity on trophic groups and interactions. Nat. Plants. doi: 10.1038/s41477-020-0654-y
Wood T. J. (2023). Bee species newly recorded for the Moroccan fauna, including two new species of Ammobatoides and Thyreus (Hymenoptera: Anthophila). Annales. la. Société. Entomol. France. (N.S.). 0, 1–27. doi: 10.1080/00379271.2023.2215216
Keywords: Coriandrum sativum, marketable habitat enhancement plants, pest control, flower visitors, crop production, conservation biocontrol
Citation: Bencharki Y, Michez D, Smaili MC, Ihsane O, Aw-Hassan A, Ssymank A, Rasmont P and Christmann S (2025) Beyond biodiversity: does “Farming with Alternative Pollinators” also boost farmers’ income in wheat (Triticum aestivum L.) fields? a case study in Morocco. Front. Ecol. Evol. 13:1551190. doi: 10.3389/fevo.2025.1551190
Received: 24 December 2024; Accepted: 20 February 2025;
Published: 13 March 2025.
Edited by:
Lei Xu, Chinese Academy of Fishery Sciences (CAFS), ChinaReviewed by:
Pablo Torres-Lima, Metropolitan Autonomous University, MexicoLydia Ndinelao Horn, University of Namibia, Namibia
Copyright © 2025 Bencharki, Michez, Smaili, Ihsane, Aw-Hassan, Ssymank, Rasmont and Christmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youssef Bencharki, eS5iZW5jaGFya2lAY2dpYXIub3Jn
 Youssef Bencharki
Youssef Bencharki Denis Michez
Denis Michez Moulay Chrif Smaili3
Moulay Chrif Smaili3 Pierre Rasmont
Pierre Rasmont