- Institute of Organismic and Molecular Evolution (iomE), Faculty of Biology, Johannes-Gutenberg-University, Mainz, Germany
Introduction: The carrot family (Apiaceae) is characterized by umbels with umbellets. Traditionally, these umbels are interpreted as inflorescences. Ontogenetic studies, however, indicate that they do not originate from inflorescence meristems but from flower-like floral unit meristems. These meristems repeatedly fractionate sub-meristems, which give rise to umbellets and flowers. Ferula species usually form double racemes with umbels with umbellets. Few species of the genus, previously grouped in the genus Dorema, however, present “panicles with simple umbels”.
Methods: Aiming to identify the developmental processes resulting in the different inflorescence appearance, we investigate inflorescence development in Ferula hezarlalehzarica (double racemes with umbels with umbellets) and Ferula aucheri (panicles with simple umbels).
Results: Both species are andromonoecious (perfect and staminate flowers) and produce huge yellow inflorescences. SEM studies confirm that they share the same developmental patterns. Their development starts with an inflorescence meristem segregating umbel meristems. These pass through two steps of fractionation generating first umbellet meristems and then flower meristems. F. aucheri differs from F. hezarlalehzarica by i) producing several lateral inflorescences apart from one terminal one and ii) extremely elongating the umbel receptacles, thereby separating the umbellets from each other. The unusual branches with simple umbels thus prove to be homologous to umbels with umbellets. Furthermore, F. aucheri shows some intermediate inflorescences with umbellets intermixed with umbels. Considering that umbels and umbellets only differ in one step of fractionation, we interpret this mixture as developmental lability.
Discussion: The study shows that meristem conditions define the character of the umbels as floral units and that developmental processes like fractionation, expansion, and elongation shape their outer appearance. It illustrates that inflorescences can be easily misinterpreted if only adult branching systems are investigated.
Introduction
The carrot family (Apiaceae) is characterized by predominantly white or yellow umbels, which are attributed to the previous family name Umbelliferae (Goebel, 1931; Troll and Heidenhain, 1951; Froebe, 1964). These umbels are unique among angiosperms, as they are not composed of flowers but of umbellets with flowers.
For decades, the apiacean umbel was regarded as a flowering branch system and classified according to its adult appearance (e.g., Rickett, 1944; Stebbins, 1974; Troll, 1964, 1969; Weberling, 1989). It was interpreted as a truncate inflorescence (i.e., a terminal umbellet is lacking) derived from a pleiothyrse (Froebe, 1964, 1971, 1979; Weberling, 1989). The uniformity of the umbels and their unchallenged classification as condensed racemes superseded further morphological and developmental studies.
Recently, it has been recognized that apart from inflorescences (originating from inflorescence meristems), multiflowered units exist that develop from flower-like meristems (Claßen-Bockhoff and Bull-Hereñu, 2013). These units, called floral units, show flower-like developmental patterns. Their determinate meristems (floral unit meristems, FUMs) lack apical activity and instead expand, generating space for a centripetal or centrifugal subdivision into sub-meristems (Claßen-Bockhoff, 2016; Claßen-Bockhoff et al., 2025). This process, known as fractionation (Claßen-Bockhoff and Bull-Hereñu, 2013; Owens et al., 2016), is controlled by local auxin maxima and proceeds until the entire surface of the FUM is used (Runions et al., 2014; Zhang et al., 2021).
The umbel with umbellets has been found to result from such a fractal-like, repeated subdivision (Bull-Hereñu and Claßen-Bockhoff, 2010; Baczyński et al., 2022; Claßen-Bockhoff et al., 2023; Bagherzadeh Homaee and Ajani, 2024). The umbel meristem considerably expands and remains “naked” before it splits into sub-meristems, i.e., umbellet meristems (first fractionation); these again subdivide into sub-meristems and produce flower meristems (second fractionation). The flower meristems fractionate a third time, initiating floral organ primordia. Each step of fractionation is associated with meristem expansion, providing space for the growing subunits.
In the genus Ferula (as well as in other Apiaceae), umbels are arranged in huge inflorescences. However, some species (previously included in the genus Dorema; Puchałka et al., 2023) present “simple umbels” instead of umbels “arranged in a panicle-like inflorescence” (Pimenov, 1988). Ajani and Claßen-Bockhoff (2021) investigated architecture, pollination, and breeding system of Ferula aucheri (Boiss.) Piwczyński, Spalik, M. Panahi & Puchalka (syn. Dorema aucheri Boiss.) and concluded from preliminary ontogenetic investigations that the “simple umbels” could be homologous to umbellets and each of the long “branches” homologous to an extended umbel (Ajani and Claβen-Bockhoff, 2012). If this is true, the “racemose branches” should originate from determinate FUMs and not from indeterminate inflorescence meristems (IMs).
In the present paper, we test this assumption by comparing the inflorescence development of Ferula hezarlalehzarica Ajani (umbels with umbellets) with that of F. aucheri. We expect that the umbels originate from an IM and the umbellets from a FUM. We aim to identify the developmental processes causing the divergence among inflorescence architecture in the two Ferula species, thereby getting a deeper insight into the evolution of flowering shoot systems in Apiaceae-Apioideae.
Material and methods
Collection and preparation of the study samples
Plant material of both species was collected in April 2013 from a locality close to Rayen waterfall, Kerman province, SE Iran (29°32′54.8″N; 57°17′55.1″E, 2,971 m a.s.l.). Vouchers are deposited in the Herbarium of Mainz University (MJG).
Inflorescences were morphologically examined at the locality. For the developmental investigation, different developmental stages of inflorescences and flowers were picked from ca. 10 individuals and fixed with 70% EtOH. The buds were dissected and dehydrated in graded ethanol-acetone series through 80%, 90%, and absolute ethanol, and then a 1:1 ethanol-acetone stage, followed by 100% acetone, each time for approximately 30 minutes. The samples were dried using the Critical Point Dryer Bal-Tec CPD 030 and CO2 as an exchange agent. The specimens were mounted on aluminum stubs and then covered with gold using the sputter coater Bal-Tec SCD005. All stages were conducted according to the manufacturers’ protocols. The probes were analyzed using the scanning electron microscope XL-30 ESEM (Philips).
Although flower development has been already published in a previous paper (Ajani et al., 2016), we include some pictures in the present paper to illustrate the similarity between the fractionation processes in umbels, umbellets, and flower meristems.
Terminology and abbreviations
In the present paper, we follow the concept and terminology presented in Claßen-Bockhoff and Bull-Hereñu (2013), Claßen-Bockhoff (2016), Baczyński et al. (2022), Claßen-Bockhoff et al. (2023), and Claßen-Bockhoff (2024). As our approach differs from traditional inflorescence morphology, we explain the most important abbreviations and terms used in the paper:
Abbreviations:
● FM: flower meristem.
● FSS: flowering shoot system.
● FUM: floral unit meristem.
● IM: inflorescence meristem. tIM, terminal IM; aIM, axillary IM.
● SAM: shoot apical meristem. aSAM, axillary SAM.
● UbM: umbellet meristem.
● UmM: umbel meristem.
Terminology:
● Determinate meristem: A meristem that has lost the capability of open growth. It fractionates completely without leaving an active meristematic rest. FMs and FUMs are indeterminate.
● Floral unit: A multiflowered unit that originates from a flower-like floral unit meristem (FUM). It looks like an inflorescence but differs from this in its developmental processes.
● FUM: A determinate meristem fractionating sub-meristems, which then pass into flower meristems. Although the FUM appears to be an intermediate between an FM and an IM, it has in fact its own specific identity: it starts like a flower with a determinate fractionating meristem and ends up developing like an inflorescence with flower production.
● Flowering shoot system (FSS): A seasonal growth unit of the vegetative shoot system that originates from a vegetative meristem and produces one to several inflorescences, floral units, or solitary flowers.
● Flower meristem (FM): A determinate meristem fractionating floral organ primordia.
● Fractionation: The process of subdividing a determinate meristem into sub-meristems, thereby using the meristem completely. It characterizes FMs and FUMs.
● Indeterminate meristem: A meristem that has the capability of open growth. It continuously produces (“segregates”) lateral appendages and remains active at its tip. SAMs and IMs are indeterminate.
● Inflorescence: A multiflowered part of the FSS that originates from an inflorescence meristem.
● Inflorescence meristem (IM): A reproductive meristem that segregates lateral reproductive meristems until it ceases growth or passes into a terminal reproductive unit. It resembles a SAM in its (initial) indeterminate nature and the process of segregation and differs from it in its limited apical activity, the immediate development of axillary meristems, and the exclusive formation of reproductive units.
● Multiflowered units: Inflorescences and floral units are traditionally summarized under the term “inflorescences”. Floral units differ from inflorescences in their meristematic origin and flower-like developmental processes.
● Segregation: The process of continuously separating lateral meristem parts from an active indeterminate meristem. It characterizes SAMs and the initial stages of IMs.
● Shoot apical meristem (SAM): A vegetative meristem that gives rise to the vegetative plant body. It is indeterminate and produces leaf primordia with axillary meristems. The axillary meristems initially remain inhibited and usually appear in older leaf axils.
● Umbel: Traditionally defined as an inflorescence with petiolate flowers that all arise from a shoot area with inhibited internodes. A compound umbel is an inflorescence with umbels. The umbel stalk is called a ray, the umbellet stalk a raylet, and the flower stalk a peduncle (see Figure 1, ry, rl, pd).
● Umbel with umbellets: The characteristic multiflowered unit of the Apiaceae. This unit is a floral unit and, thus, differs from a compound umbel. Dealing with Apiaceae, we use the term “umbel” equivalent to “umbel with umbellets”.
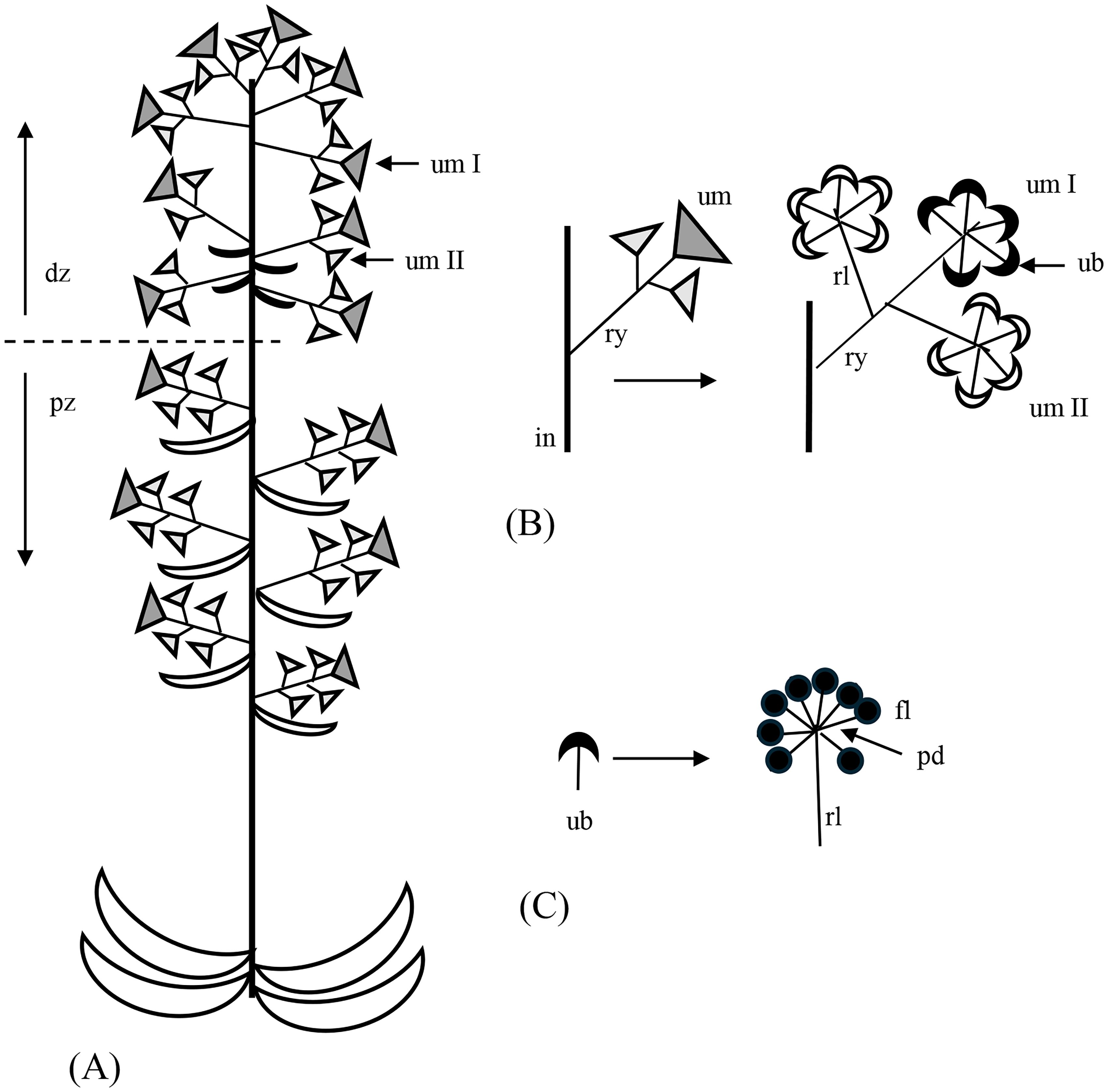
Figure 1. Inflorescence architecture in Ferula hezarlalehzarica. (A) The inflorescence is an extended double raceme with complex umbels (umbels with umbellets) of first and second order (um I and um II, respectively). It is subdivided into a proximal zone (pz) with branches subtended by leaves and separated by internodes, and a distal part (dp) with more closely arranged branches lacking subtending leaves. (B, C) The umbellets (ub) of the first-order umbels (um I) have perfect flowers (filled symbols), and those of the second order (um II) have staminate flowers (open symbols). Bracts are not considered. in, inflorescence shoot; fl, flower; pd, peduncle; rl, raylet (stalk of an umbellet); ry, ray (stalk of an umbel).
Results
Both Ferula species form huge, approximately 2 m tall, flowering shoot systems with thousands of bright yellow flowers. The flowers are grouped in umbels, but the arrangement of these units varies considerably between the species.
Ferula hezarlalehzarica
F. hezarlalehzarica has a single terminal inflorescence with laterally arranged umbels of the first-order branch (Figure 1A, um I; Figure 2A). Below these umbels, two to four umbels of second order appear (Figure 1A, um II; Figures 2F, G, J, II). As a terminal umbel is lacking, the entire inflorescence is open; it represents a double (or compound) raceme with umbels. Perfect flowers are only produced by the first-order umbels, whereas those of the second order are staminate (Figures 1B, C, 2I, J, light gray). All flowers of the first-order umbels bloom simultaneously, resulting in mass flowering (Figure 2A). They first release pollen (“male” stage) and then spread their styles in the receptive (“female”) stage (Figures 2G, H). After this receptive phase, all flowers of the second-order umbels present pollen (Figures 2I) and fade after anthesis (Figure 2J). As a result, the flowering sequence is duodichogamous; i.e., the phase of pollen reception follows and precedes a phase of pollen donation.

Figure 2. Ferula hezarlalehzarica. (A) Flowering plant in the natural environment, approximately 2 m tall. (B) Leaf rosette with elongating inflorescence shoot. (C) Inflorescence bud before inflorescence elongation. Flowers are already well-developed but still condensed and covered by leaf sheaths (removed). (D) Inflorescence after internode elongation. The developing umbels are protected by enlarged leaf sheaths. (E) Prefloral stages. Elongation of rays separates umbels from each other. (F) First-order umbels (I) start blooming, whereas second-order umbels (II) are still sessile and in bud stage. (G–I) When the first-order umbel is in the pollen receptive stage (upper circle in G; H), the second-order umbels start to release pollen (lower circle in G; I). (J) First-order umbels are fruiting, while the second-order umbels are fading.
Inflorescence architecture
F. hezarlalehzarica is a perennial herb hibernating with a long rhizome. Seasonal growth starts with the formation of a large leaf rosette. The inflorescence originates from the SAM in the center of the rosette (Figure 2B). If one removes the ensheathing leaves from the young shoot, it becomes evident that the young inflorescence already exists (Figure 2C). It consists of approximately 15 umbels of first order pressed against the stem by the leaf sheaths. All umbels are in the same developing stage presenting well-developed flowers, which are not yet separated by internodes. However, a weak developmental divergence can be recognized: the proximal branches remain smaller than the middle ones and develop with delay (Figure 2D). The distal branches produce fewer umbels of second order than the remaining ones, lack bracts, clump together, and appear to develop with delay.
Inflorescence growth continues with the elongation of the main shoot (Figure 2D). The first-order branches are spirally arranged according to Fibonacci systems and form a proximal and a distal zone. In the proximal zone, well-separated first-order branches with more than two second-orderbranches arise from the axils of large sheaths (Figure 1A, pz; Figure 2A). In the distal zone (dz), subtending bracts become smaller and finally are absent (Figure 2F). The umbels and umbellets are closely aggregated (Figure 2E). When the stalks of the umbellets (called raylets) elongate, the receptacle of the umbel remains short, providing the umbel-like shape. Shortly before anthesis, the internodes of the main shoot and the umbel stalks elongate (Figure 2F). Then, the raylets and peduncles elongate and present the flowers to the pollinators (Figure 2G). In this stage, the second-order umbels are still in the bud stage (Figure 2E).
Inflorescence development
Inflorescence development starts from the SAM in the center of the leaf rosette (Figure 3A). The SAM itself is flat and bulges when it passes into the inflorescence meristem (Figures 3B, C, IM). This meristem segregates leaf primordia and axillary meristems, which pass into umbel meristems (UmMs; Figure 3D). The UmMs immediately start to fractionate umbellet meristems (UbMs; Figure 3D). Below the UmM, two transversally placed bract primordia (bp) are formed; their axillary meristems will give rise to second-order umbels.
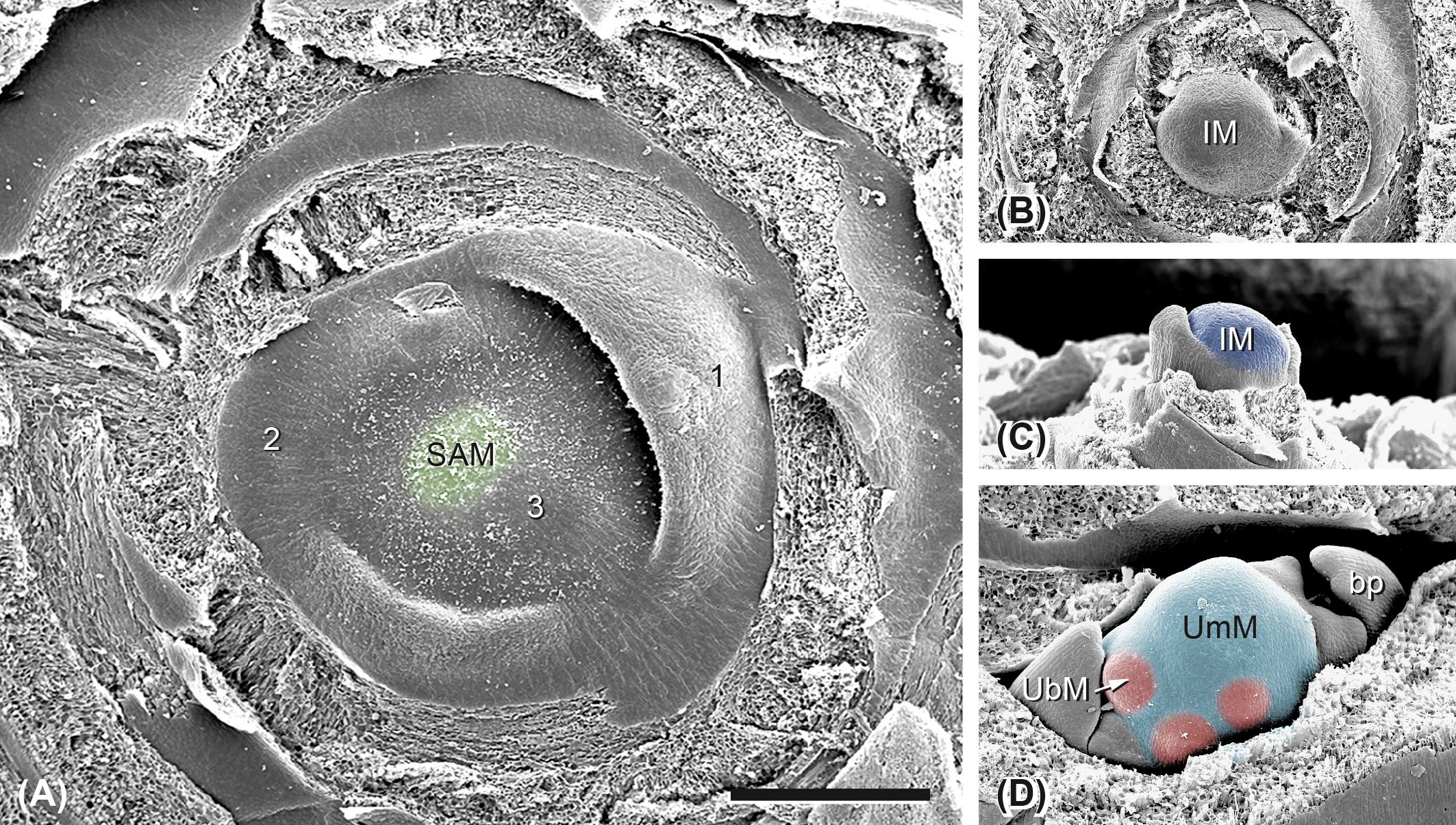
Figure 3. Ferula hezarlalehzarica: transition from the vegetative to the reproductive state. (A) The flat shoot apical meristem (SAM; green) is ensheathed by rosette leaves. 1–3, the three youngest leaf primordia. (B, C) The SAM passes into a globular inflorescence meristem (IM; dark blue). Top (B) and side views (C). (D) Umbel meristem (UmM, light blue) arising from the IM immediately starts to fractionate umbellet meristems (UbMs, red). bp, one of the two transversal bract primordia. Bar in panel A, 500 µm. All pictures in the same scale.
When the bud is approximately 8 mm in length, all umbel meristems of the first order are already present (Figures 4A, B, UmM). The proximal UmMs are subtended by large leaf sheaths (Figure 4B), whereas the distal ones are densely aggregated and lack subtending bracts (Figures 4A, 5A). The UmMs fractionate into umbellet meristems (Figure 4A, UbM). These spring up simultaneously and completely fill the available space. UmMs enlarge and provide space for the development of the UbMs (Figure 4C), which repeat the fractionation process. In the distal zone, UbMs develop almost simultaneously (Figures 4A, C, 5A); in the proximal zone, where the leaf sheaths mechanically press the UmMs against the shoot, they appear in a basipetal sequence (Figures 5B–E). The gradient is still recognizable when the UbMs start to fractionate flower meristems (Figures 5C, D, FM). This third step of fractionation shows a centripetal pattern (Figures 5D, G).
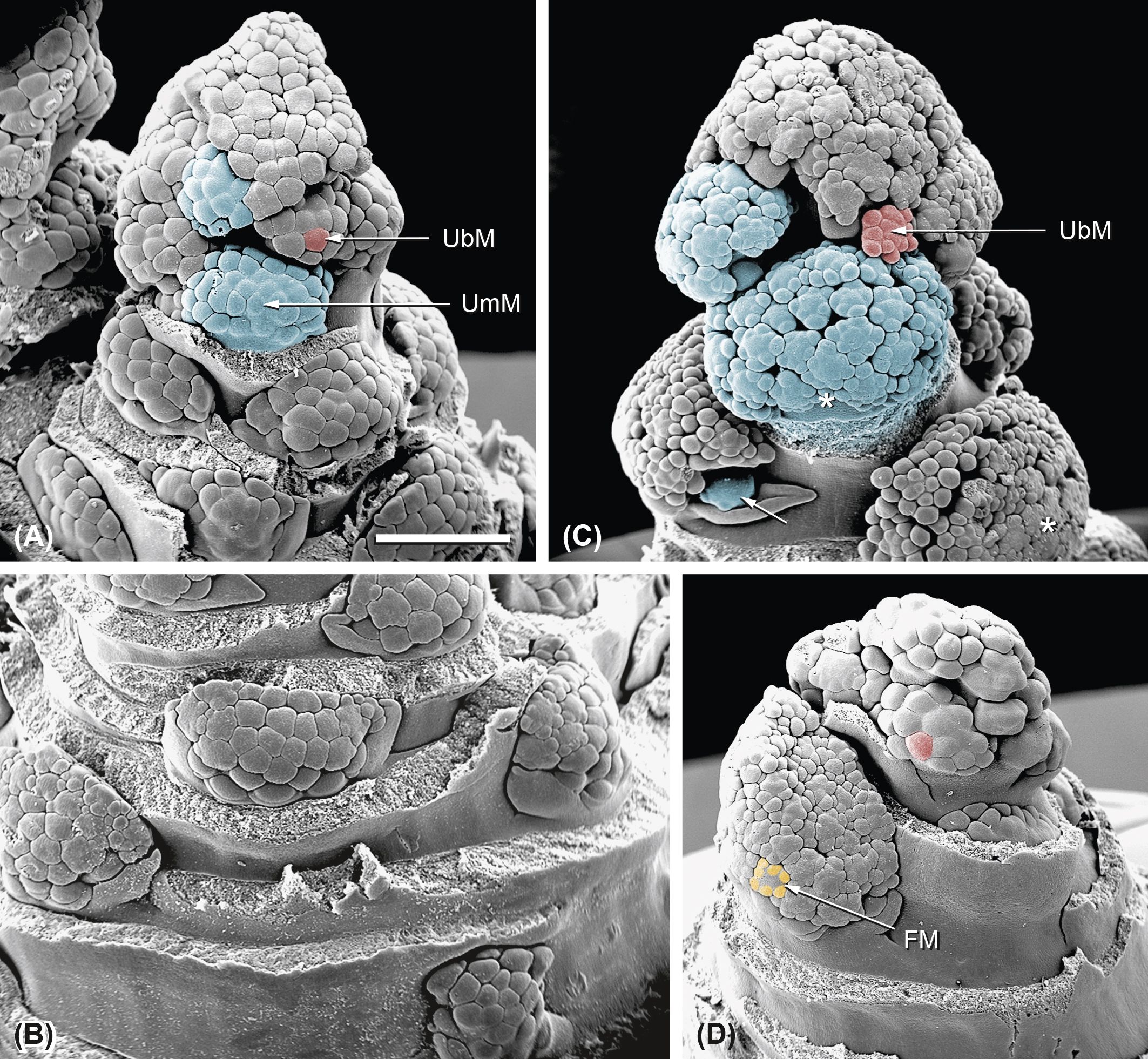
Figure 4. Ferula hezarlalehzarica: inflorescence development. (A, B) Distal (A) and proximal (B) parts of a young inflorescence. The proximal umbel meristems (UmM) are subtended by leaves (removed) and pressed against the stem; the distal ones are closely packed and hardly distinguishable from each other. All umbel meristems are in the same developmental stage: they have passed through the first step of fractionation being completely subdivided into umbellet meristems (UbMs). (C) UmMs expand and enter the second step of fractionation: the umbellet meristems (UbMs) fractionate flower meristems. The proximal UmM shows impressions of the subtending bract (asterisks), while the distal ones approach the umbel shape. The arrow indicates an UmM of second order. (D) Example of a developing inflorescence showing an acropetal gradient in UmM formation: the distal UmMs fractionate UbMs (red), while the UmM below this group already fractionates flower meristems (FMs; yellow). Bar in panel A, 1 mm. All pictures in the same scale.
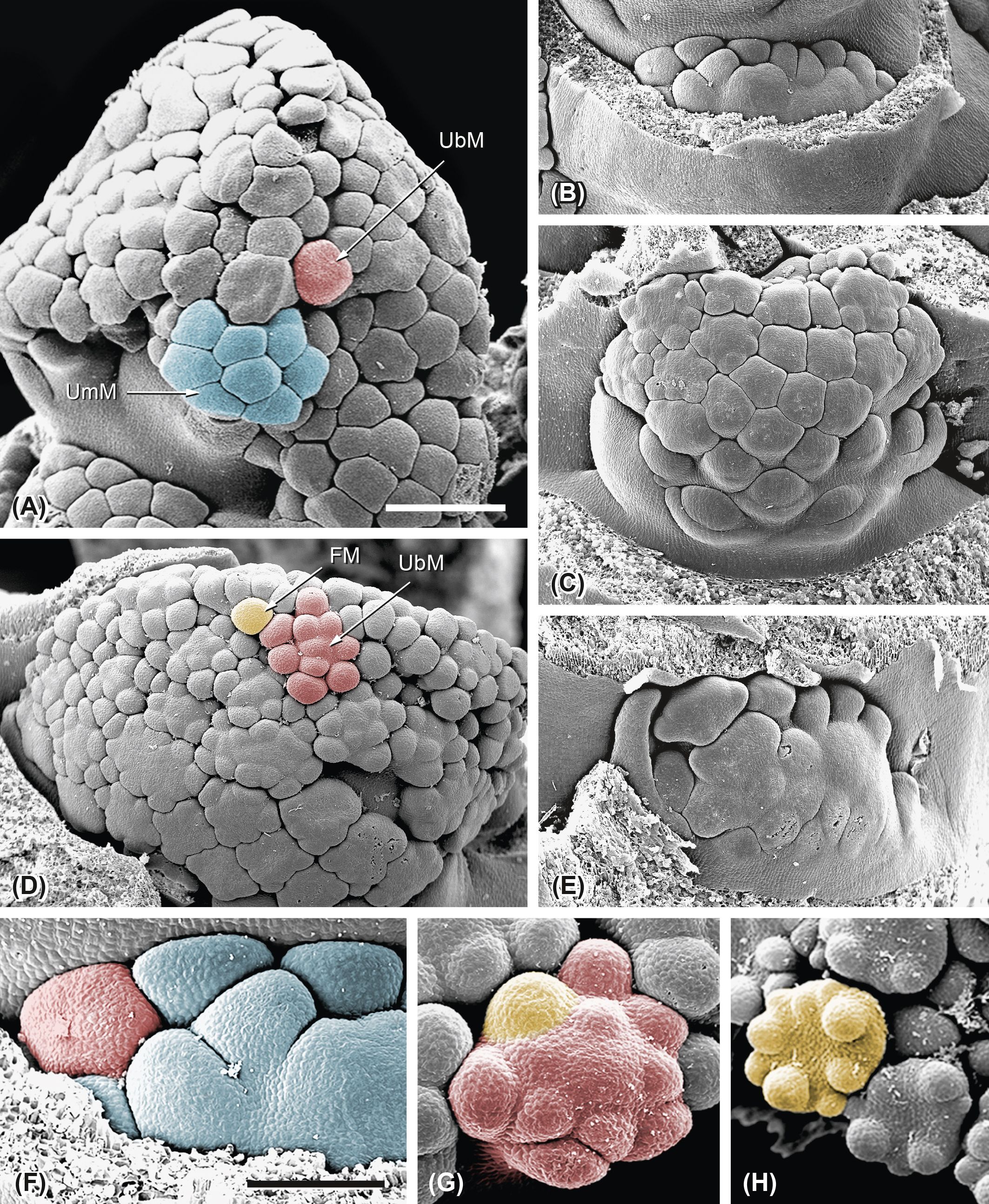
Figure 5. Ferula hezarlalehzarica. Repeated fractionation and mechanical pressure. (A) Distal zone of a developing inflorescence with first-order umbels (UmM) fractionating umbellet meristems (UbMs). (B–E) First-order UmMs show different degrees of mechanical pressure during the fractionation process. The UmMs are pressed against the stem and develop in basipetal sequence. Whereas some UmMs show a regular fractionation pattern (C), others split irregularly (E). (F–H) Repeated fractionation. (F) First step: the UmM (blue) fractions UbMs (red). (G) Second step: the UbM fractions FMs (yellow). (H) Third step: the FM fractions floral organ primordia. Bar in panel A, 500 µm. Same scale as in panels (A–E). Bar in panel (F), 200 µm. Same scale as in panels (F–H).
In the beginning, there is no developmental gradient along the inflorescence shoot (Figures 4A, B). With ongoing growth, the distal zone remains delayed compared to the proximal one: the distal UmMs are still fractionating UbMs, when the proximal ones already split into flower meristems (Figure 4D, FM).
After further expansion of the UmMs, flower development starts (Figures 5D, G). Floral organ primordia are initiated as an outcome of the third step of fractionation. First, stamen primordia arise in a spiral sequence, followed by the first petal primordia (Figures 6B, C, st, pe). The FMs expand, and sepal primordia appear (Figure 6D, se). If the development of the FMs stops at this stage, the fully grown flowers will be staminate (Figure 6E); if it continues, they will be perfect. In this case, the inferior ovary with two carpel tips is formed (Figure 6F, ca). Young flower buds are densely crowded, and it is hard to recognize the individual umbellets (Figure 6A). Below the first-order umbels, second-order umbels, already initiated when the first-order umbels start to fractionate UbMs (Figure 4C, arrow), develop in the axils of bracts.
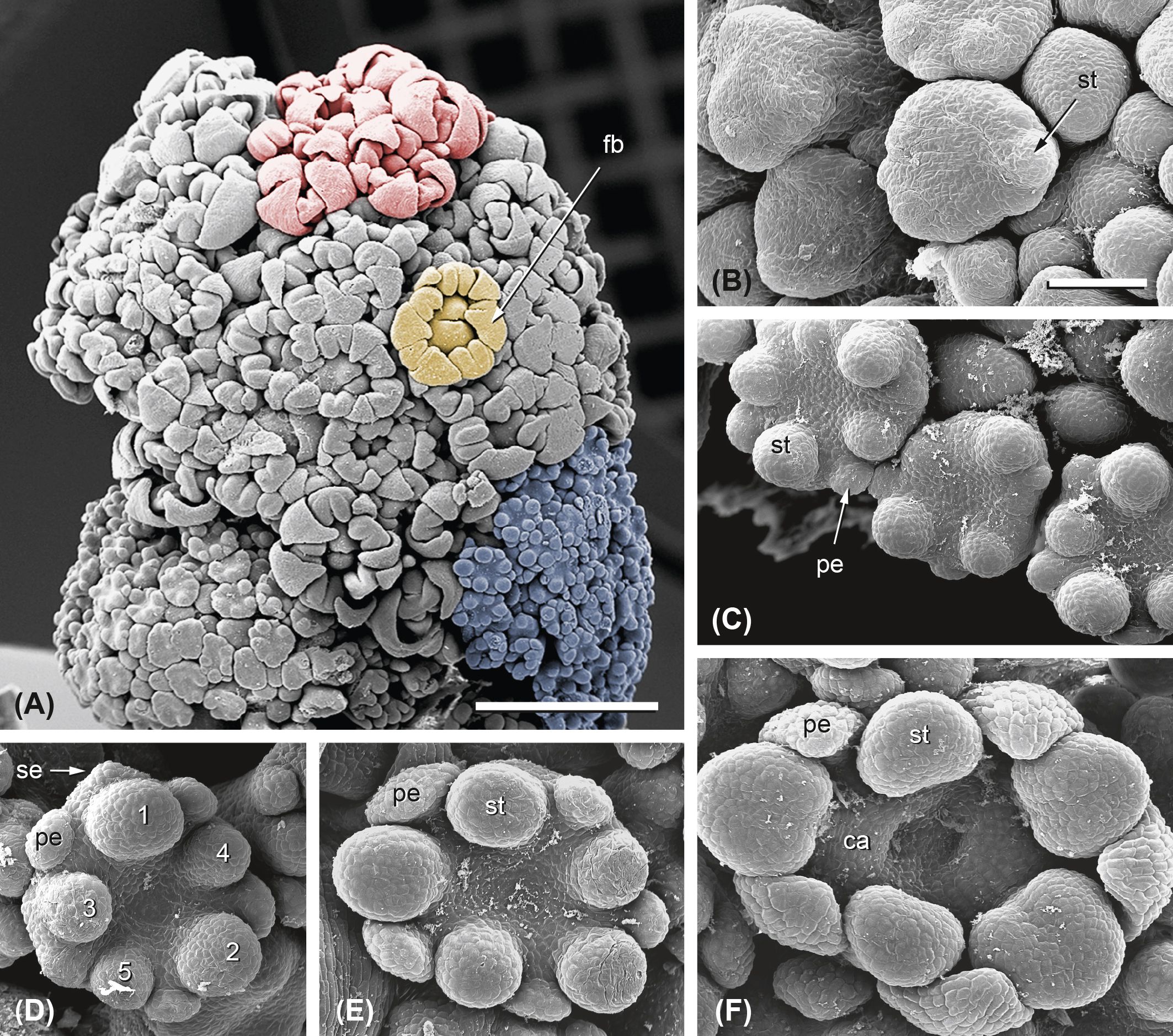
Figure 6. Ferula hezarlalehzarica. Flower development. (A) Single first-order umbel in the prefloral stage showing almost completed flower buds (fb; yellow). These have passed through the third step of fractionation, giving rise to floral organs. The umbellets (one marked in red) are so densely crowded that they are hardly recognizable. Below the first-order umbel, two second-order umbels (one marked in light blue) appear in the flower formation phase. (B–D) Flower development starts with spirally initiated stamen primordia (st, 1–5) followed by petal (pe) and sepal primordia (se). (E) Staminate flowers lack a gynoecium. (F) The gynoecium of perfect flowers is inferior; its formation starts with the elevation of the stamen, petal, and sepal primordia and continues with carpel formation (ca). Bar in panel (A), 1 mm. Bar in panel (B), 100 µm. Same scale as in panels (B–F).
Altogether, inflorescence development in F. hezarlalehzarica is characterized by laterally initiated UmMs passing through repeated steps of fractionation before FMs are formed. The first step happens almost simultaneously, only influenced by mechanical pressure. The second step runs centripetally, whereas flower organ formation (third step) shows a divergent pattern (Figures 5F–H).
Ferula aucheri
Plants of F. aucheri differ from those of F. hezarlalehzarica in the appearance of their flowering shoot systems (Figures 7A, 8A). These are characterized by a terminal inflorescence with perfect flowers (Figure 7A, ti) and lateral inflorescences with staminate ones (li). Contrary to F. hezarlalehzarica, individuals of F. aucheri are monocarpic. Their leaf rosettes persist for approximately 4 years and produce axillary buds (Figure 8B, arrows). As in F. hezarlalehzarica, the flowering sequence is duodichogamous. It starts with the pollen release phase, followed by the pollen receptive phase. When the perfect flowers of the terminal inflorescence enter the fruiting phase, the staminate flowers of the lateral inflorescences release pollen.
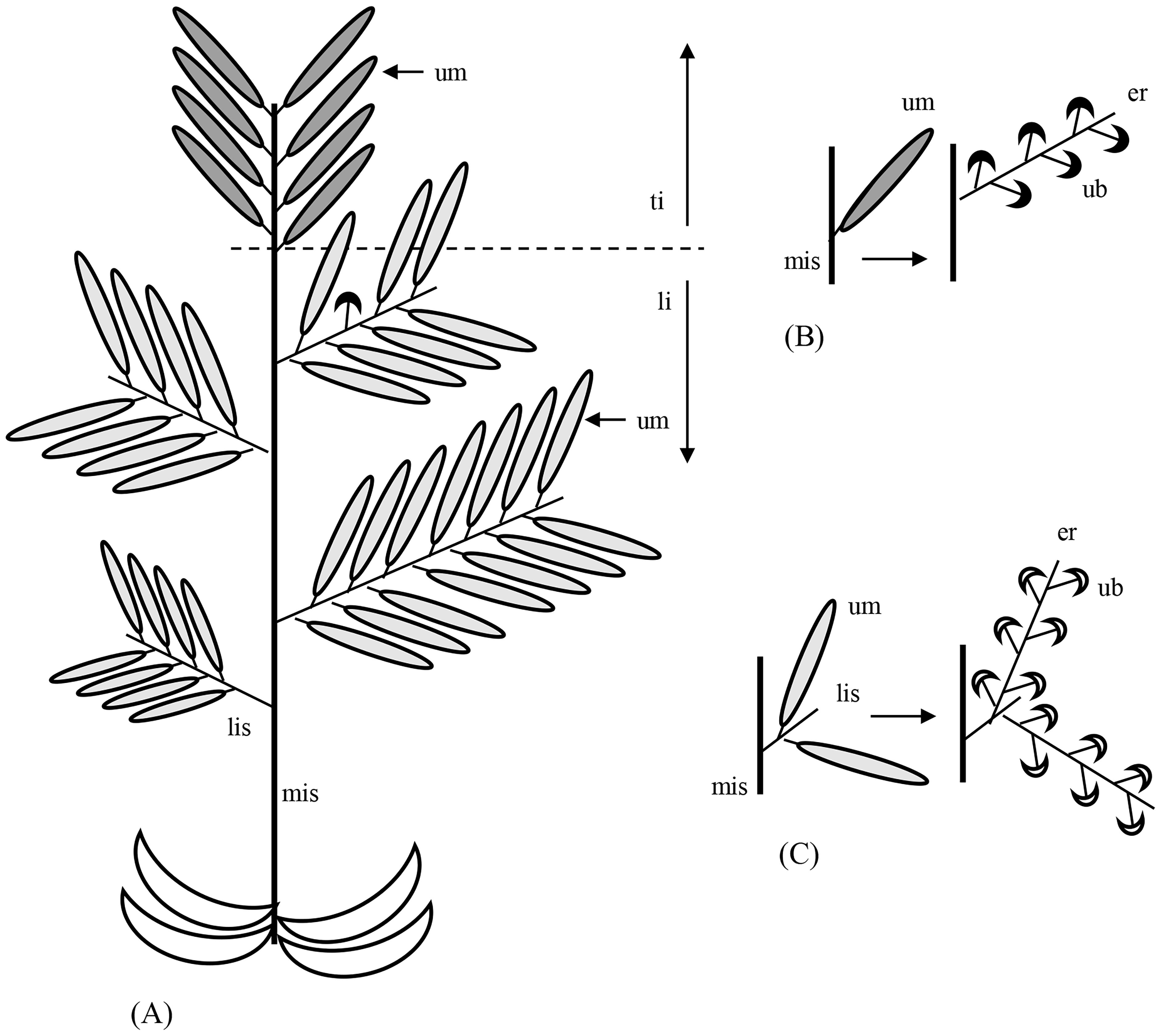
Figure 7. Inflorescence architecture in Ferula aucheri. For clarity, bracts are not shown. (A) The flowering shoot system consists of a terminal inflorescence (ti) and several lateral inflorescences (li). Each inflorescence looks like an extended double raceme with simple umbels. However, the lateral “racemes” are equivalent to complex umbels (um) with elongated receptacles, and the simple umbels correspond to umbellets. lis, lateral inflorescence shoot; mis, main inflorescence shoot. (B) Detail of the terminal inflorescence. Each umbel has many umbellets separated from each other by the elongated umbel receptacle (er). Umbellets (ub) form predominantly perfect flowers (filled symbol). (C) Detail of a lateral inflorescence. The lateral inflorescence repeats the organization of the terminal inflorescence except that their umbellets only produce staminate flowers (open symbols).

Figure 8. Ferula aucheri. (A) Habitus of a flowering plant in its natural environment, approximately 2 m in height. (B) View of the developing flowering shoot system: the terminal inflorescence already forms umbel meristems; the meristems of the lateral inflorescences appear in the axils of leaf sheaths (arrows). (C) Bud stage of a flowering shoot system with all inflorescences developing. The lateral inflorescences repeat the terminal inflorescence with delay. (D, E) Bud of the flowering shoot system developing from the center of the rosette leaves. (D) Elongation of the flowering shoot covered by leaf sheaths. (E) The same plant as in panel D with removed leaves. (F) Image from panel E showing the developing inflorescences. UmM, umbel meristem already fractionated into umbellet meristems.
Architecture of the flowering shoot systems
When the plant enters the reproductive phase, the flowering shoot elongates (Figure 8D). It is covered by the sheaths of the innermost rosette leaves. After removal of the sheaths, one can see that apart from the terminal inflorescence, several lateral inflorescences are developing (Figures 8C, E, F). These originate from axillary meristems below the terminal IM and develop with delay (Figures 8C, F).
The terminal inflorescence is open and looks like a double raceme (Figures 7A, 8A). However, its more than 40 “branches” reveal as umbels of the first order whose receptacles are extremely elongated (approximately 45 cm), mimicking racemes (Figures 7B, C, 9). The inflorescence is, thus, a simple raceme with extended umbels. Each umbel forms approximately 30 umbellets (“simple umbels”), each with 7–10 mainly perfect flowers (Figures 9A–D). They are arranged solitarily, in pairs or loose clusters.

Figure 9. Ferula aucheri. (A–D) Umbellet developing in the terminal inflorescence. (A) Flowers in bud stage. The elongated receptacle (er) of the umbel separates the umbellets (ub) from each other. Umbellets are laterally arranged and bractless. (B) Flowers in the pollen donating (“male”) phase. (C) Flowers in the receptive (“female”) stage. (D) Fruits, among them single staminate flowers (arrows). (E–G) Lateral inflorescences. Each inflorescence bears many laterally arranged umbels. The receptacles of these umbels are elongated and separate the umbellets. (E) Umbels (um) in bud stage are arranged along the inflorescence shoot (in). (F) Flowering umbels: all flowers are staminate. (G) Intermediate inflorescence: flowering perfect umbellets intermixed with umbels in the prefloral stage (see Figures 7A, 13A).
The four to five lateral inflorescences (branches of first order) repeat the branching pattern of the terminal one. They reach a length of 1 m (Figure 8A) and produce approximately 35 extended umbels of second order resembling the first-order ones in the number and arrangement of umbellets and flowers (Figures 9E–G). However, all flowers are staminate. The uppermost lateral inflorescences often diverge from the remaining ones, forming intermediate branches. These are heteromorphic: single umbellets with perfect flowers appear intermixed with elongated umbels with staminate umbellets (Figures 7A, 9G). The perfect umbellets start flowering when the elongated umbels are still in the bud stage, and they are fruiting when the staminate flowers release pollen.
Inflorescence development
As in F. hezarlalehzarica, the base of the leaf rosette in F. aucheri is broad (4–5 mm), and the SAM is small (c. 300 µm) and flat (Figure 10A, SAM). However, in contrast to the previous species, F. aucheri shows lateral meristems in the axils of the rosette leaves (Figure 10A, stars). When the plant passes into the reproductive stage, the SAM considerably enlarges and forms the terminal inflorescence meristem (Figure 10B, tIM). This starts to segregate UmMs in centripetal order. The outermost meristems are subtended by bracts; the inner ones lack bracts (Figures 10B–D, 11A).
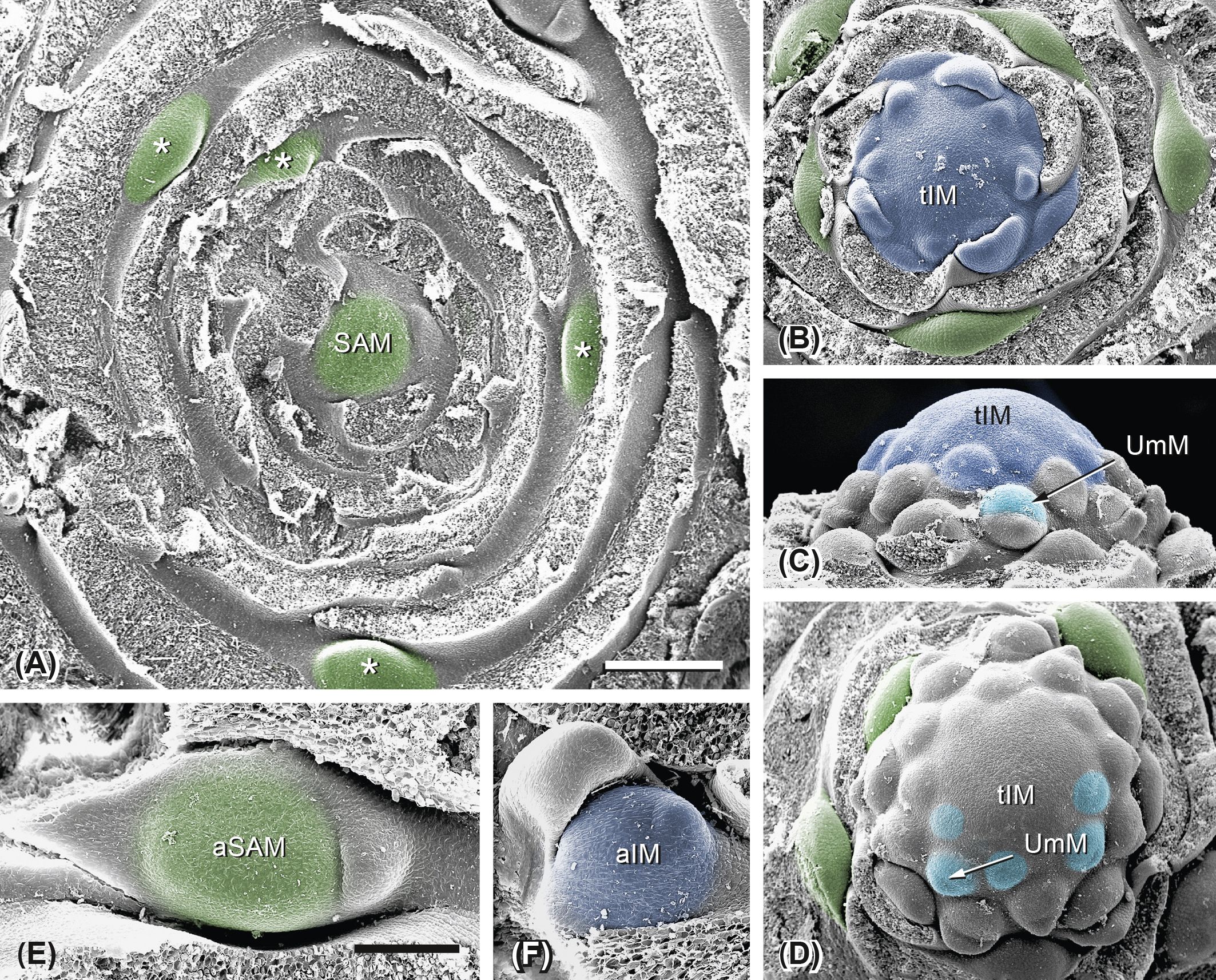
Figure 10. Ferula aucheri. Transition from the vegetative to the reproductive state. (A) The flat shoot apical meristem (SAM; green) is ensheathed by rosette leaves. Asterisks indicate axillary meristems that will give rise to lateral inflorescences. (B) The SAM passes into the terminal inflorescence meristem (tIM; dark blue) and segregates bracts in a spiral Fibonacci order. Axillary meristems are still in the vegetative stage. (C) Umbel meristems (UmM; light blue) appear in the bract axils. (D) The tIM becomes dome-shaped and continues segregation. (E) Axillary meristem (aSAM) with two leaves. (F) Axillary inflorescence meristem (aIM). Bar in panel A, 500 µm. Same scale as in panels (A–D). Bar in panel (E), 200 µm. Same scale as in panels (E, F).
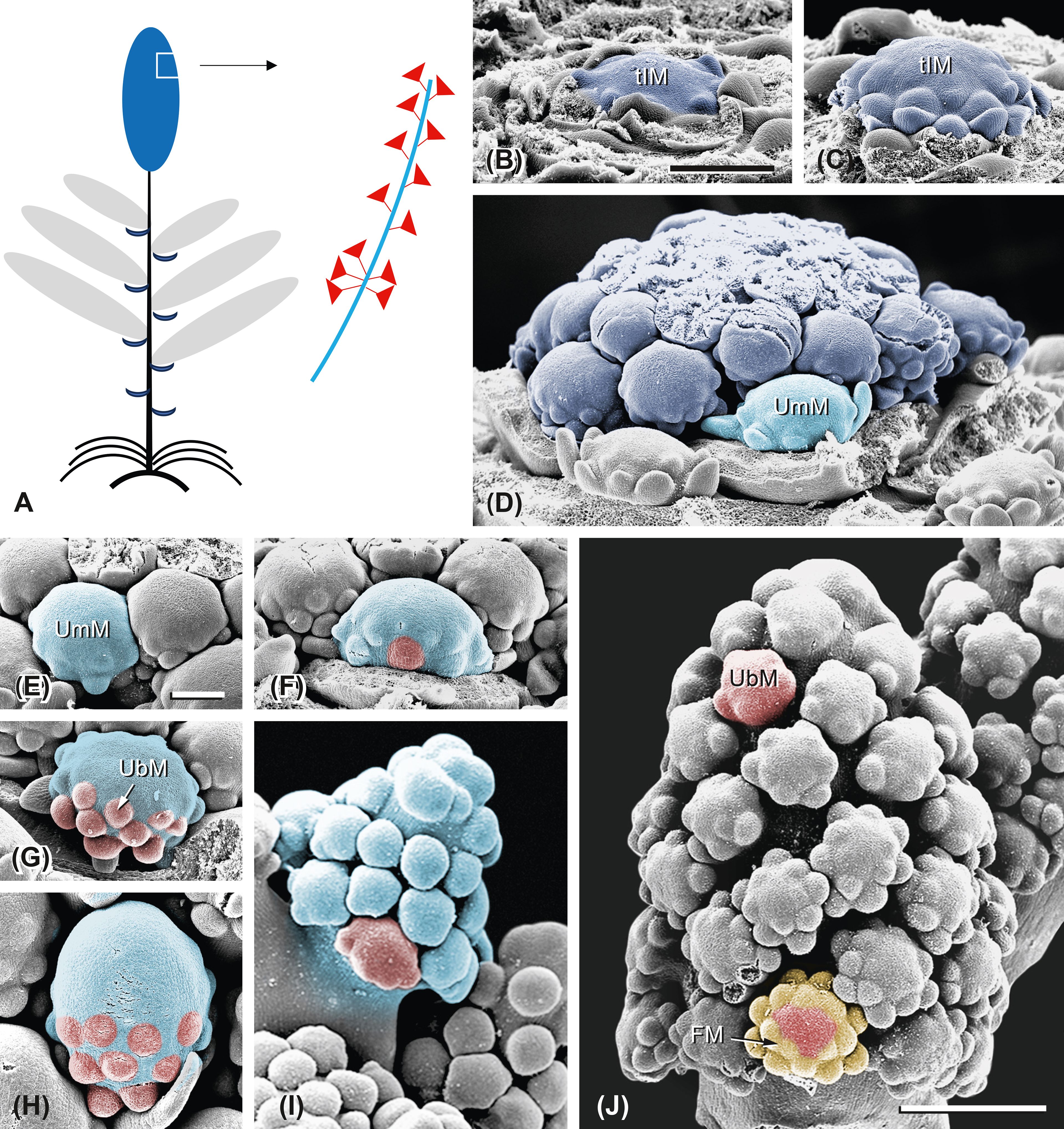
Figure 11. Ferula aucheri. Development of the terminal inflorescence. (A) Scheme of the flowering shoot system. Dark blue, terminal inflorescence. The detail shows an elongated umbel (light blue) with umbellets (red). (B–D) The terminal inflorescence meristem (tIM) is broad and flat. It enlarges and initiates the first umbel meristems (UmMs) subtended by bracts; the subsequent UmMs are bractless. (E–I) Umbel development. The UmM (light blue) is dome-shaped and naked before it starts to fractionate umbellet meristems (UbMs, red) in centripetal order. It expands and elongates while fractionating and is finally completely used by UbMs. (J) The umbellet meristems show a weak developmental gradient in centripetal direction; the proximal ones already fractionate flower meristems (FMs; yellow) in centripetal order. Note the similarity between the fractionation of the UbM (F) and the FM (J). Bar in panel (B), 500 µm. Same scale as in panels (B–D). Bar in panel (E), 200 µm. Same scale as in panels (E–I). Bar in panel (J), 500 µm.
During further development, the tIM enlarges but does not elongate; in consequence, the UmMs are densely aggregated (Figures 11B–D). The latter expand, become hemispheric, and fractionate UbMs in centripetal order (Figures 11E–G). While doing so, they elongate, providing space for the developing UbMs (Figures 11H, I). The UbMs completely use the UmM except for its tip, which remains open, not forming a terminal umbellet (Figures 11I, J). The UbMs expand and fractionate flower meristems in centripetal order (Figure 11J, FM). The comparison between a fractionating UmM (Figure 11F) and a fractioning UbM (Figure 11J) illustrates the similarity between these meristems when the latter repeats the same processes of expansion and fractionation.
The axillary units develop with delay (Figure 12H). In the vegetative state, they have an elliptic shape and form two transversal leaf primordia (Figures 10A, E, aSAM). When the tIM starts initiating UmMs, they are still in the meristematic state (Figures 10B, D). Then, they bulge, enlarge, and produce bract primordia (Figures 10F, 12A, B). The axillary IM segregates UmMs in acropetal sequence (Figure 12C). The first UmMs are subtended by bracts; the succeeding ones show different stages of reduced bracts, which are finally completely absent (Figures 12C, D, sb). The IM elongates during UmM initiation and shows a clear acropetal gradient. When the proximal UmMs start fractionating UbMs, the ones in the middle zone are still naked and globular; they sometimes have a drop-like shape, indicating the presence of bract primordia (Figure 12E). The tip of the inflorescence remains open; a terminal umbel is lacking (Figures 12E, F). The acropetal gradient decreases with further development. The UmMs fractionate UbMs and are finally in the same developmental stage (Figures 12F, G). They are still densely aggregated, as the main shoot of the inflorescence has not been much elongated until then.
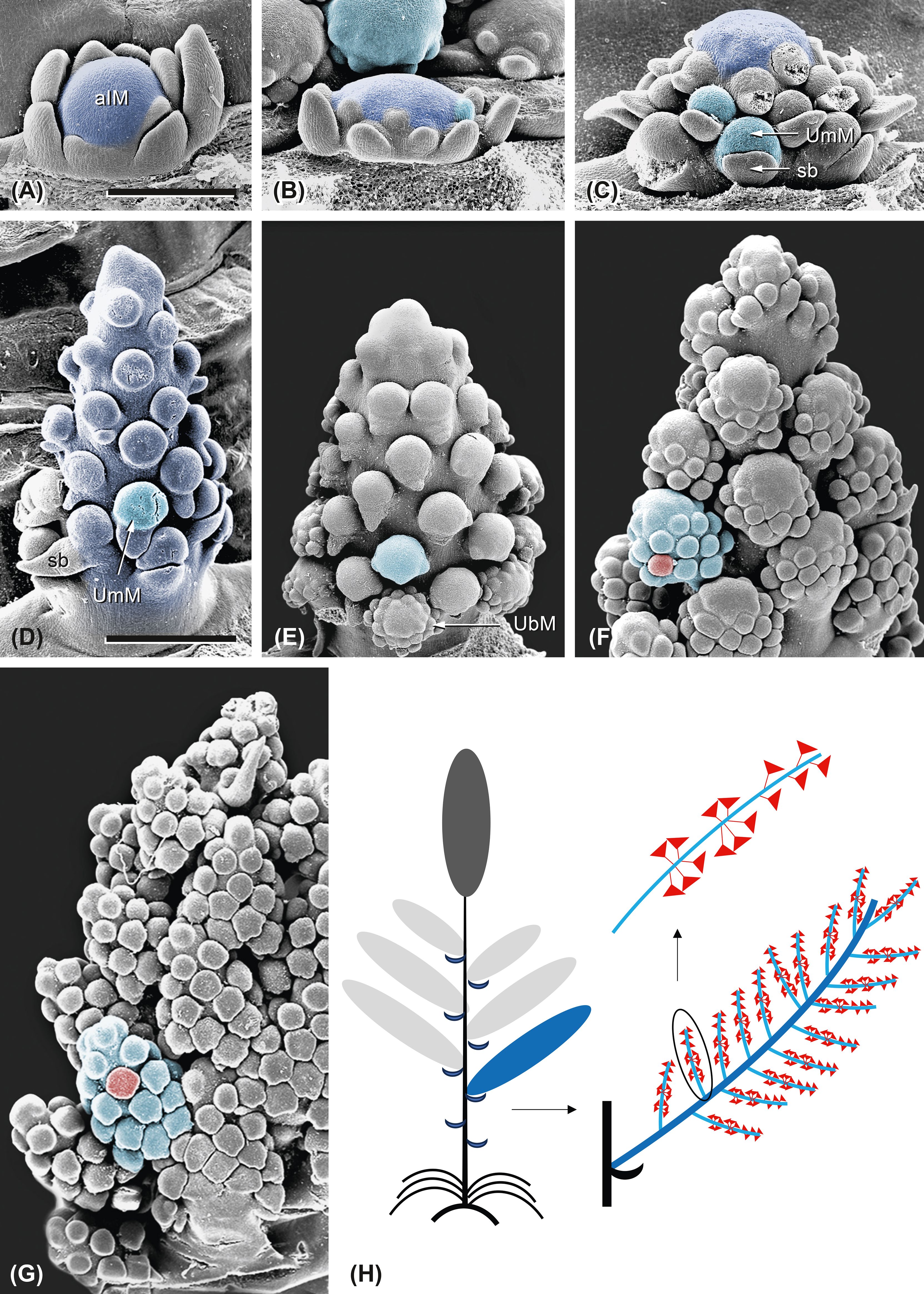
Figure 12. Ferula aucheri. Development of a lateral inflorescence. (A, B) The axillary inflorescence meristem (aIM; dark blue) is dome-shaped in the beginning. (C) It enlarges and segregates umbel meristems (UmMs; light blue) in acropetal order. Sb, subtending bract. (D) The inflorescence elongates and separates the UmMs from each other. The proximal UmMs are subtended by bracts, whereas the distal ones are bractless. (E) The proximal UmMs start to fractionate umbellet meristems (UbMs). (F) The umbel receptacles elongate while fractionating UbMs in centripetal order. (G) UmMs are densely aggregated at the inflorescence axis; each of them presents UbMs arranged along the elongated receptacles. (H) Scheme of the flowering shoot system. The large image shows one lateral inflorescence (dark blue) with umbels. The small image shows one elongated umbel (light blue) with umbellets (red). Bar in panel (A), 500 µm. Same scale as in panels (A–C). Bar in panel (D), 1 mm. Same scale as in panels (D–G).
The intermediate branches (Figure 9G) vary in the number and position of the umbellets intermixed with staminate umbels (Figure 13A). In contrast to the UmMs, which are subtended by thin bracts (Figure 13C), these umbellets lack subtending bracts. They develop faster than the staminate umbels (Figures 13B, C) already presenting completely developed flowers when the UbMs of the staminate umbels still fractionate flower meristems (Figure 13B, fl, FM). Sporadically, proliferating umbellets appear, presenting flowers on two levels (as indicated in Figure 13A).
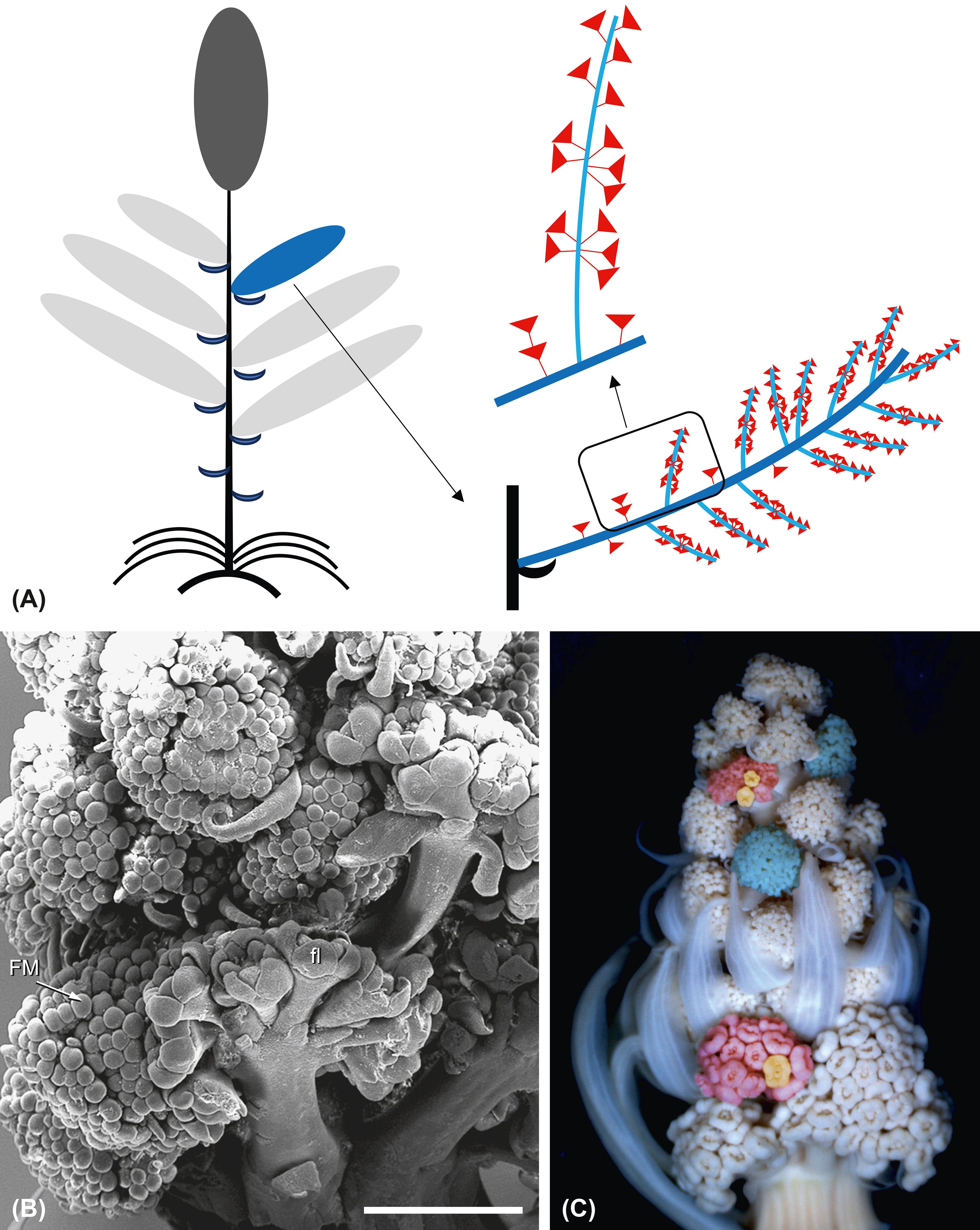
Figure 13. Ferula aucheri. Intermediate inflorescences. (A) Scheme of the flowering shoot system. The large image shows the architecture of an intermediate inflorescence. Elongated umbels with staminate flowers are intermixed with perfect, occasionally proliferating umbellets. The small image shows one elongated umbel with umbellets. (B) SEM picture showing developing UmMs intermixed with UbMs. The perfect flowers (fl) of the latter are already completed, whereas the staminate flowers (FM) are still in the meristematic stage. Bar, 1 mm. (C) Young intermediate inflorescence with umbellets (red) and umbels. Bud size, 7 mm.
The last step of inflorescence development includes the processes of elongation (internodes, rayless, and peduncles) and flower formation. In the early stages, the inflorescence shoots and umbel receptacles are weakly elongated, only providing space for the developing UmMs and UbMs (Figures 14A, E). The latter are densely aggregated and hardly recognizable as individual units. In the next stage of development, when the FMs are initiating floral organ primordia, raylets elongate and involucral bracts appear (Figure 14B, inv, rl; Figure 14C). Elongation of the peduncles follows (Figure 14D, pd). Only then will the internodes of the inflorescence shoot elongate (Figures 9A, E, 14F), while shortly before anthesis, umbel receptacles, raylets, and peduncles extend to their final length.
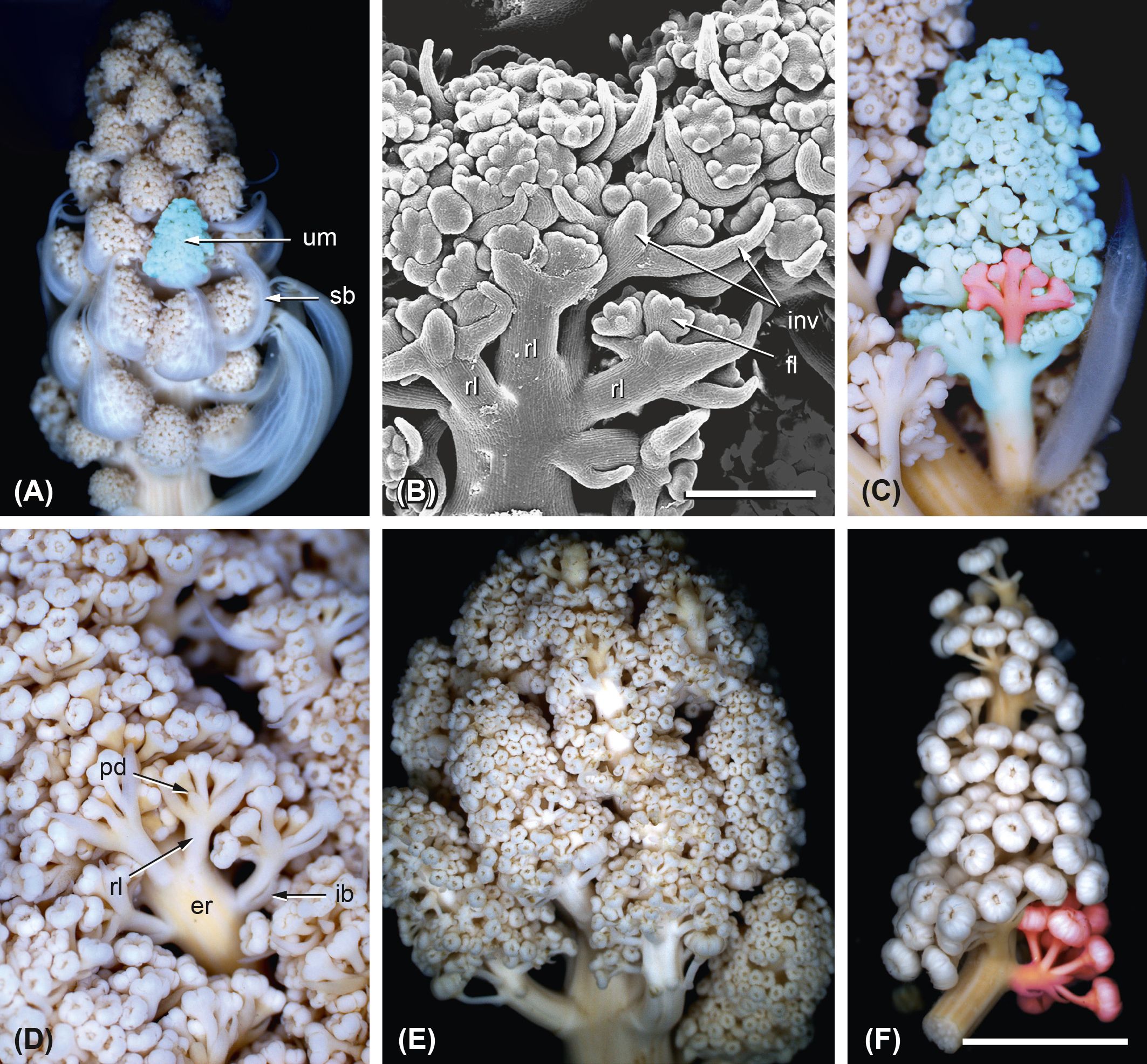
Figure 14. Ferula aucheri. Elongation processes and bract formation. (A–D) Lateral inflorescences. (A) Inflorescence with laterally arranged umbels (um) subtended by bracts (sb). Bud size, 5 mm. (B) Image of a young umbel with elongated raylets (rl), involucral bracts (inv), and developing flowers (fl). Bar, 500 µm. (C) Young umbel with umbellets (one marked in red) arranged along the elongated umbel receptacle; raylets and peduncles start elongation. Bud size, 8 mm. (D) Image of a young umbel showing the elongation of pedicels (pd) and raylets (rl). Involucral bracts (ib) subtend the proximal umbellets. er, elongated receptacle of the umbel. Bar, 500 µm. (E) Terminal inflorescence with elongated umbels arranged along the inflorescence shoot. Bar, 5 mm. (F) A young, not yet fully elongated umbel with umbellets. Flowers in bud stage. Bar, 5 mm.
Flower development is very similar to that in F. hezarlalehzarica. The UbMs fractionate FMs, the outermost being subtended by involucral bracts (Figure 14A, inv). Then, the FMs almost simultaneously fractionate stamen primordia, followed by petal and finally sepal primordia. Staminate flowers stop their development in this stage, whereas perfect flowers form bicarpellate inferior ovaries.
Discussion
The most important result of the present study is the finding that the striking “panicle with simple umbels” in F. aucheri is a group of racemes bearing umbels with umbellets. Apioid umbels usually develop from a FUM (Claßen-Bockhoff and Bull-Hereñu, 2013), pass through repeated steps of meristem expansion and fractionation, and finally reach the characteristic adult stage of an umbel with umbellets (Baczyński et al., 2022; Claßen-Bockhoff et al., 2023; Bagherzadeh Homaee and Ajani, 2024). F. aucheri differs from this general pattern in only a single developmental step, i.e., the elongation of the umbel receptacle.
Racemose inflorescences in FerulaApiaceae usually form several to many umbels per flowering shoot system. The umbels appear solitarily arranged in monopodial or sympodial shoot systems (Reuther and Claßen-Bockhoff, 2010) or are part of an inflorescence as seen in Ferula. In this case, a direct comparison of inflorescence and umbel development is possible.
Flowering shoot systems arise from SAMs, and reproductive shoot systems from IMs. Both meristems have a central zone whose ongoing cell division activity is controlled by a genetic feedback loop, maintaining open growth (Kwiatkowska, 2008 and references herein). SAMs continuously segregate lateral meristem parts, which pass into leaf primordia, each with a residual meristem at the adaxial side. IMs differ from SAMs in having a limited activity. They cease growth and pass into a terminal FM (closed inflorescence) or parenchyma cells (open inflorescence). Furthermore, the residual meristems in the leaf axils develop without delay and only produce flowers or partial inflorescences (Claßen-Bockhoff and Bull-Hereñu, 2013).
The outer appearance of inflorescences is largely based on the underlying branching pattern and the formation of internodes (e.g., Weberling, 1989). The latter originate from tissue zones between two consecutive leaves by cell expansion, diffuse cell division activity, or intercalary meristems. Their length and thickness shape the relative proportions of an inflorescence and cause diverse inflorescence forms, among them racemes, umbels, or heads.
Inflorescence development in the two Ferula species investigated corresponds to the general pattern of racemose inflorescence development (e.g., Sokoloff et al., 2006; Bull-Hereñu and Claßen-Bockhoff, 2011b). The IMs segregate bracts with axillary meristems, which immediately pass into UmMs. In F. hezarlalehzarica, the umbel meristems arise almost simultaneously, while the terminal and lateral inflorescences in F. aucheri show the characteristic acropetal development expected in racemose inflorescences. After umbel formation, the internodes elongate and present the umbels to pollinators.
Repeated meristem expansion and fractionation in umbel development
In contrast to IMs, FMs and flower-like FUMs are determinate, lacking the central zone and open growth (Bull-Hereñu and Claßen-Bockhoff, 2011a; Kwiatkowska, 2008; Tucker and Grimes, 1999). They do not segregate lateral parts and, correspondingly, lack nodes and internodes (Claßen-Bockhoff, 2016). Instead, they considerably expand, forming a naked stage in the beginning and generating new space during development. They are completely used in the process of fractionation. This means that the meristem splits into floral organs in the case of FMs and into sub-meristems in the case of FUMs. Based on the concept of directed auxin flow (Reinhardt et al., 2003), the process of fractionation runs autonomously densely, filling all available space (Claßen-Bockhoff and Meyer, 2016; Douady and Couder, 1996; Runions et al., 2014).
Umbel development in the two Ferula species is characterized by repeated fractionation. UmMs enlarge and rapidly split into umbellet meristems. The outermost umbellets are subtended by bracts, which correspond to involucral bracts; the inner ones are bractless, as is common in apioid umbels (Froebe, 1964).
In F. hezarlalehzarica, UmMs expand and almost simultaneously split into UbMs. The sub-meristems tend to fill the available space completely but are obviously hindered in their development by mechanical pressure caused by the close sheathing of the leaf bases. Mechanical pressure is a common side effect in young developmental stages (Bull–Hereñu et al., 2022) and is often transitory (Naghiloo, 2020). This is also evident in F. hezarlalehzarica not showing any positional effects on the adult stage.
In F. aucheri, UmMs elongate while fractionating UbMs in centripetal sequence. Thereby, the developing umbels receive a raceme-like shape resembling that of developing inflorescences. Nevertheless, we interpret the UmM as a floral unit meristem because it starts with a naked stage, expands while fractionating, and is used completely.
UbMs repeat the processes of expansion and fractionation, initiating FMs. These, again, expand and fractionate floral organ primordia. The two Ferula species are, thus, further examples within the Apiaceae-Apioideae that show alternating processes of expansion and fractionation (Claßen-Bockhoff and Bull-Hereñu, 2013; Baczyński et al., 2022; Claßen-Bockhoff et al., 2023; Bagherzadeh Homaee and Ajani, 2024). These processes are not known from IMs but from FMs, indicating the close relationship between FUMs and FMs.
The role of elongation in shaping the umbel appearance
Inflorescence development has been largely disregarded in the past because inflorescences are traditionally classified based on their branching pattern (e.g., Rickett, 1944; Troll, 1964, 1969; Stebbins, 1974; Weberling, 1989; Judd et al., 1999). Therefore, the present knowledge about FUMs remains limited. Flower-like meristems have been documented in approximately 10 angiosperm families (Harris, 1995; Claßen-Bockhoff and Bull-Hereñu, 2013; Claßen-Bockhoff and Arndt, 2018; Claßen-Bockhoff and Frankenhäuser, 2020), but there may be many more. Whereas most of the examples need a single step of fractionation to form head-like clusters with sessile flowers, Apiaceae pass through two steps of fractionation to form an umbel with stalked umbellets with stalked flowers. The elongation of raylets and peduncles is so far only known from inflorescences and has certainly contributed to the view that “umbels can be derived from racemes whose internodes remain undeveloped” (Goebel, 1931: 167; Froebe, 1964). However, based on ontogeny, raylets and peduncles are no internodes but just stalks elevating umbellets and flowers and presenting them to pollinators. The processes of stalk elongation usually start late in ontogeny and characterize the complex umbels in the Apiaceae.
A unique feature, only found in some Ferula species, is the elongation of the umbel receptacle. In F. aucheri, this elongation starts early in ontogeny, already with the fractionation of UbMs. It provides space for umbellet development and can be interpreted as a manifestation of the overall elongation tendency in apioid umbels.
Receptacle elongations are rare among angiosperms but occur in a few flowers and floral units when they pass into the fruiting stage. Examples are the elongated flower receptacles in Magnolia and Myosurus (Weberling, 1989) and the elongated receptacle of the complex head in Gundelia tournefortii (Claßen-Bockhoff et al., 1989). However, receptacle elongation in the flowering stage fulfilling the same function as an inflorescence branch appears to be unique in F. aucheri (and some of its closest relatives formerly included in the genus Dorema; Pimenov, 1988; Puchałka et al., 2023). The umbels mimic racemes with simple umbellets, and are themselves laterally arranged on inflorescence shoots with elongated internodes.
Korovin (1939, 1947) and Pimenov (1988) used the unique inflorescence structure of some Ferula species to support the maintenance of the genus Dorema Don. Their view, however, came into conflict with phylogenetic findings, indicating that these species did not belong to a monophyletic group but nested within the Ferula alliance (Puchałka et al., 2023). Our study explains that the inflorescences are not so different as they seem to be: if we focus on architecture and not on differential elongation processes, it is evident that the basic schemes in the two Ferula species are indeterminate racemes and double racemes, with complex umbels (Figure 15). In F. hezarlalehzarica, the flowers of the first-order umbels are perfect, and those of the second-order umbels staminate. Although it looks similar in F. aucheri, two different hierarchical levels must be considered here: referring to the whole flowering shoot system, the staminate umbels are also of second order, but referring to the single lateral inflorescence, they are of first order. The example shows that it is important to identify the respective reference systems when comparing branched shoot systems.
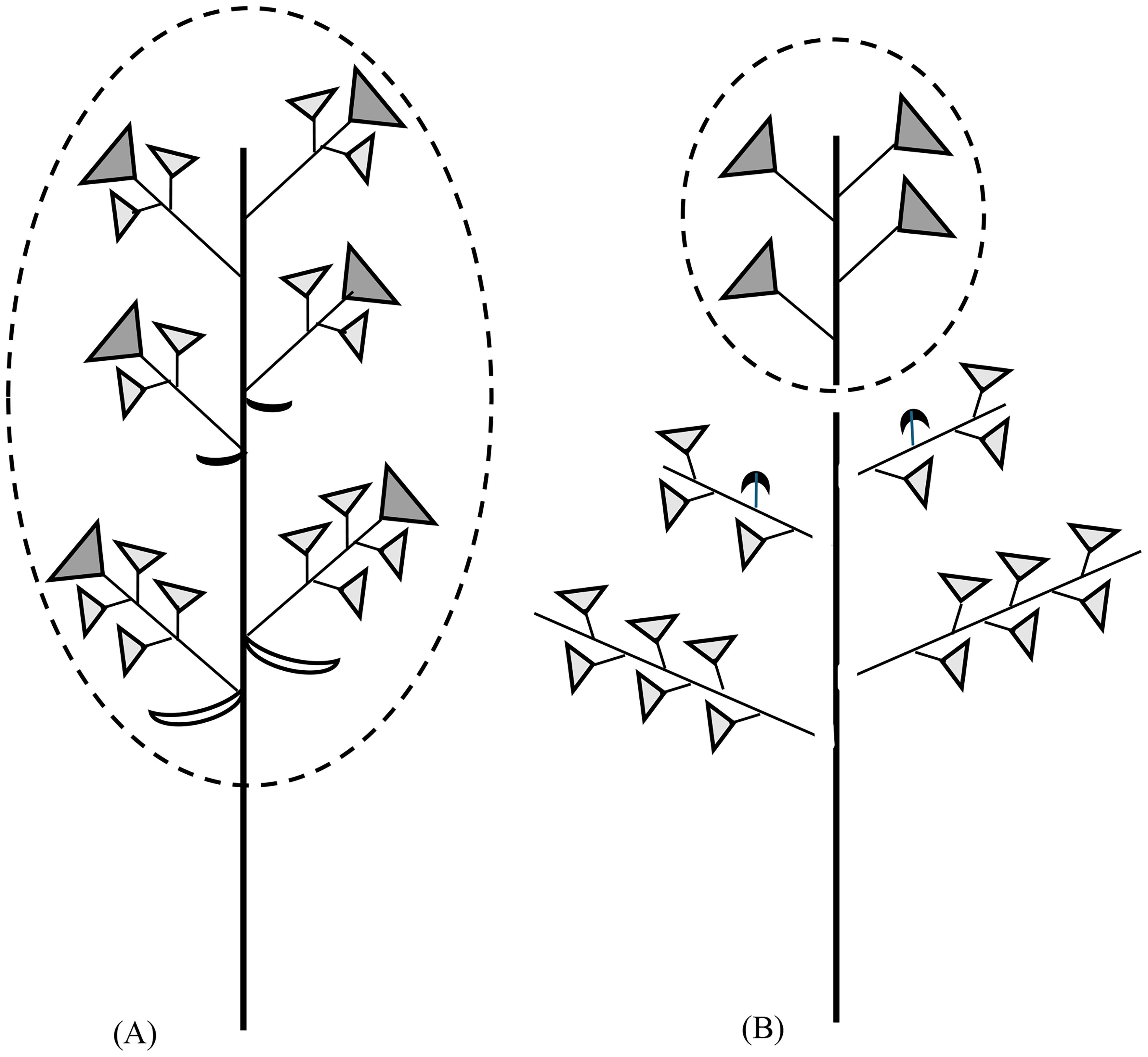
Figure 15. Architecture of the flowering shoot system in Ferula. Leaves and most bracts are not drawn. (A) Ferula hezarlalehzarica. (B) Ferula aucheri. The elongated umbels are shown with the umbel symbol. The black symbol in the intermediate inflorescences represents the umbellets with perfect flowers (see Figure 1C). The terminal and lateral inflorescences are graphically separated from each other to indicate their origin from separate meristems. It is evident that the entire inflorescence of F. hezarlalehzarica corresponds to the terminal inflorescence of F. aucheri (dotted circles). Inflorescences are open racemes in both species, compound in F. hezarlalehzarica and simple in F. aucheri. Both species are andromonoecious and form perfect (dark gray) and staminate umbels (light gray) but differ in their flower morph distribution.
Intermediate inflorescences: fractionation lability and proliferation
Intermediate inflorescences originate from IMs segregating bracts and axillary units. These units develop into umbels with elongated receptacles presenting staminate umbellets. In some positions, however, the umbels are replaced by umbellets or even proliferating umbellets. These umbellets produce perfect flowers and bloom earlier than the staminate flowers of the neighboring umbels, thus arousing the impression that part of the morphogenetic potential of the terminal inflorescence may have been transferred to the uppermost lateral inflorescences.
The replacement of an umbel with umbellets by a single umbellet can be explained by the suppression of the second step of fractionation. A FUM fractionating once forms a simple umbel, and a FUM fractionating twice forms an umbel with umbellets. Similar lability in the number of fractionation steps is also found in naturally occurring malformations, i.e., proliferating umbels and umbellets (Penzig, 1890), or regular variants, as seen in Ferula caspica M.Bieb., Ferula dubjanskyi Korovin, and Ferula feruloides (Steud.) Korovin (Puchałka et al., 2023). It is possible that a combination of receptacle elongation and fractionation lability causes the different forms of proliferation. Given that the occasionally appearing umbellets on the lateral inflorescences lack the second step of fractionation, it is comprehensible that they flower earlier than the umbels, which must pass through a second step of fractionation before they come to flower. However, it remains unclear why these umbellets produce perfect flowers. A morphogenetic correlation between position, degree of fractionation, and flower morph configuration is not known so far.
Patterns of flower morph distribution in andromonoecious Apiaceae
Apiaceae-Apioideae are mainly perennial herbs bearing a terminal umbel and repeating this module on lateral branches of consecutive orders (Troll and Heidenhain, 1951). Plants are predominantly andromonoecious; i.e., they produce perfect and staminate flowers within the same plant (Bell, 1971; Schlessman, 2010). Usually, both flower morphs appear in a single umbel, but with increasing umbel order, the percentage of staminate flowers increases, finally resulting in purely staminate umbels (Reuther and Claßen-Bockhoff, 2010). Depending on the presence vs. absence of a terminal flower in the umbellets and the outer vs. inner position of perfect flowers in an umbellet, five different patterns of flower morph distribution in solitary Apioid umbels have been described (Claßen-Bockhoff, 2024).
In Ferula, the umbels are arranged in inflorescences, providing further patterns of flower morph distribution: F. hezarlalehzarica forms double racemes with perfect umbels of first order and staminate umbels of second order. In contrast, F. aucheri forms a terminal raceme with perfect umbels and lateral racemes with staminate umbels (and intermediate forms). This is possible because axillary meristems are stimulated to flower when the terminal inflorescence is developing. In F. hezarlalehzarica, no axillary meristems enrich the terminal inflorescence. This difference between the species may be due to their life cycles: F. hezarlalehzarica is perennial and able to bloom and produce seeds for many years. In contrast, F. aucheri is a monocarpic species that accumulates nutrients over several years before it passes into the flowering stage. It has only a single chance to flower and set seeds. The high number of flowers and seeds most likely evolved under high selection pressure, guaranteeing survival in the high mountainous steppe vegetation (Ajani and Claßen-Bockhoff, 2021).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
RC-B: Funding acquisition, Methodology, Resources, Supervision, Visualization, Writing – review & editing. YA: Data curation, Funding acquisition, Investigation, Methodology, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was financially supported by the Friedericke-Wagner-Stiftung (Mainz) and the German Academic Exchange Service (DAAD).
Acknowledgments
We thank Maria Geyer (Wiesbaden) for preparing the SEM plates and Juliana del Ottra (Sao Paulo) for preparing the photo plates. We also thank Margarita V. Remizowa for editing the paper and the three reviewers Andrey Sinjushin, Rolf Rutishauser, and Alexei Oskolski for their helpful comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ajani Y., Bull-Hereñu K., Claßen-Bockhoff R. (2016). Patterns of flower development in Apiaceae-Apioideae. Flora 221, 38–45. doi: 10.1016/j.flora.2016.02.004
Ajani Y., Claβen-Bockhoff R. (2012). “Inflorescence architecture and sex distribution in the endemic Apiaceae Dorema aucheri Boiss. from Iran,” in 21st Intern. Symp. Biodiversity and Evolutionary Biology, Mainz, 85. Inst. Spez. Bot. Bot Gart (Mainz). Available at: https://www.deutsche-botanische-gesellschaft.de/fileadmin/user_upload/pdf_2012/2012_symposium_program_Sec_Biodiv_evolut.pdf.
Ajani Y., Claßen-Bockhoff R. (2021). The unique inflorescence structure of Dorema aucheri (Apiaceae): An adaptation to the arid environment. J. Arid. Environ. 184, 104194. doi: 10.1016/j.jaridenv.2020.104194
Baczyński J., Celep F., Spalik K., Claßen-Bockhoff R. (2022). Floral pseudanthia in Apiaceae: the unique interplay of meristem conditions, gene expression and morphogenetic gradients. EvoDevo 13, 19. doi: 10.1186/s13227-022-00204-6
Bagherzadeh Homaee M., Ajani Y. (2024). The ontogeny of flowers and inflorescences in the andromonoecious annual Iranian endemic Dicyclophora persica (Apiaceae). Nord. J. Bot. 8, e04198. doi: 10.1111/njb.04198
Bell C. R. (1971). Breeding systems and floral biology of the Umbelliferae, or evidence for specialization in unspecialized flowers. Bot. J. Linn. Soc 64, Suppl. 1, 93–108.
Bull–Hereñu K., Dos Santos P., Toni J. F. G., El Ottra J. H. L., Thaowetsuwan P., Jeiter J., et al. (2022). Mechanical forces in floral development. Plants 11, 661. doi: 10.3390/plants11050661
Bull-Hereñu K., Claßen-Bockhoff R. (2010). Developmental conditions for terminal flower production in apioid umbellets. Plant Div. Evol. 128, 221–232. doi: 10.1127/1869-6155/2010/0128-0010
Bull-Hereñu K., Claßen-Bockhoff R. (2011a). Open and closed inflorescences: more than simple opposites. J. Exp. Bot. 62, 79–88. doi: 10.1093/jxb/erq262
Bull-Hereñu K., Claßen-Bockhoff R. (2011b). Ontogenetic course and spatial constraints in the appearance and disappearance of the terminal flower in inflorescences. Int. J. Plant Sci. 172, 471–498. doi: 10.1086/658922
Claßen-Bockhoff R. (2016). The shoot concept of the flower – still up to date? Flora 221, 46–53. doi: 10.1016/j.flora.2015.11.012
Claßen-Bockhoff R. (2024). Die Pflanze: Morphologie, Entwicklung und Evolution von Vielfalt [The Plant: Morphology, Development and Evolution of Diversity] (Heidelberg, Berlin: Springer-Spektrum).
Claßen-Bockhoff R., Arndt M. (2018). Flower-like heads from flower-like meristems: pseudanthium development in Davidia involucrata (Nyssaceae). J. Plant Res. 131, 443–458. doi: 10.1007/s10265-018-1029-6
Claßen-Bockhoff R., Baczyński J., Hanke V., Henkes S. S., Ferdinand N. (2025). Are capitula inflorescences? A reassessment based on determinate meristem conditions and ray flower development. Ann. Bot. under resubmission.
Claßen-Bockhoff R., Bull-Hereñu K. (2013). Towards an ontogenetic understanding of inflorescence diversity. Ann. Bot. 112, 1523–1542. doi: 10.1093/aob/mct009
Claßen-Bockhoff R., Celep F., Ajani Y., Frenken L., Reuther K., Doğan M. (2023). Dark-centered umbels in Apiaceae: diversity, development, and evolution. AoB. Plants 15, plad065. doi: 10.1093/aobpla/plad065
Claßen-Bockhoff R., Frankenhäuser H. (2020). The ‚male flower’ of Ricinus communis (Euphorbiaceae) interpreted as a multi-flowered unit. Front. Cell. Dev. Biol. 8. doi: 10.3389/fcell.2020.00313
Claßen-Bockhoff R., Froebe H. A., Langerbeins D. (1989). Die Infloreszenzstruktur von Gundelia tournefortii L. (Asteraceae). Flora 182, 463–479.
Claßen-Bockhoff R., Meyer C. (2016). Space matters: meristem expansion triggers corona formation in Passiflora. Ann. Bot. 117, 227–290. doi: 10.1093/aob/mcv177
Douady S., Couder Y. (1996). Phyllotaxis as a dynamic self organizing process. Parts I-III. J. Theor. Biol. 139, 178–312. doi: 10.1006/jtbi.1996.0024
Froebe H. A. (1964). Die Blütenstande der Saniculoideen (Umbelliferae). Eine vergleichend-morpho1ogische und entwicklungsgeschichtliche Untersuchung. Beitr. BioI. Pflanzen. 40, 325–388.
Froebe H. A. (1971). Inflorescence structure and evolution in Umbelliferae. Bot. J. Linn. Soc. 64, Suppl. 1, 157–176.
Froebe H. A. (1979). Die infloreszenzen der hydrocotyloideen (Apiaceen). Trop. Subtrop. Pflanzenwelt. 29, 462–658.
Harris E. M. (1995). Inflorescence and floral ontogeny in Asteraceae: A synthesis of historical and current concepts. Bot. Rev. 61, 93–278. doi: 10.1007/BF02887192
Judd W. S., Campbell C. S., Kellogg E. A., Stevens P. F. (1999). Plant systematics, a phylogenetic approach (Sunderland, MA: Sinauer).
Korovin E. P. (1939). Lignes principales de la systematique du genre Ferula (Tourn.) L. Bull. Soc. Imp. Nat. Moscou. 48, .5–15.
Korovin E. P. (1947). Generis Ferula (Tourn.) L. monographia illustrata. Acad. Sci. UzRSS, Tashkent.
Kwiatkowska D. (2008). Flowering and apical meristem growth dynamics. J. Exp. Bot. 59, 187–201. doi: 10.1093/jxb/erm290
Naghiloo S. (2020). Patterns of symmetry expression in Angiosperms: Developmental and evolutionary lability. Front. Ecol. Evol. 8. doi: 10.3389/fevo.2020.00104
Owens A., Cieslak M., Hart J., Classen-Bockhoff R., Prusinkiewicz P. (2016). Modeling dense inflorescences. ACM Trans. Graph. 35, 4. doi: 10.1145/2897824.2925982. Article 136 (July 2016).
Pimenov M. (1988). Monographic revision of the genus Dorema D. Don (Umbelliferae). Bull. Soc Imp. Nat. Moscou. 93, 76–90.
Puchałka R., Spalik K., Trzeciak P., Banasiak L., Piwczyńsk M. (2023). Phylogenetic position of Dorema within Ferula (Apiaceae). Plant Syst. Evol. 309, 10. doi: 10.1007/s00606-023-01857-z
Reinhardt D., Pesce E. R., Stieger P., Mandel T., Baltensperger K., Bennett M., et al. (2003). Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260. doi: 10.1038/nature02081
Reuther K., Claßen-Bockhoff R. (2010). Diversity behind uniformity - inflorescence architecture and flowering sequence in Apiaceae-Apioideae. Plant Div. Evol. 128, 181–220. doi: 10.1127/1869-6155/2010/0128-0009
Rickett H. W. (1944). The classification of inflorescences. Bot. Rev. 10, 187–231. doi: 10.1007/BF02861094
Runions A., Smith R. S., Prusinkiewicz P. (2014). “Computational models of auxin-driven development,” in Auxin and its role in plant development. Eds. Zazimalová E., Petrasek J., Benková E. (Springer, Berlin), 315–357. doi: 10.1007/978-3-7091-1526-8
Schlessman M. A. (2010). Major events in the evolution of sexual systems in Apiales: ancestral andromonoecy abandoned. Plant Div. Evol. 128, 233~245. doi: 10.1127/1869-6155/2010/0128-0011
Sokoloff D., Rudall P. J., Remizowa M. (2006). Flower-like terminal structures in racemose inflorescences: a tool in morphogenetic and evolutionary research. J. Exp. Bot. 57, 3517–3530. doi: 10.1093/jxb/erl126
Troll W. (1964). Die Infloreszenzen: Typologie und Stellung im Aufbau des Vegetationskörpers. Vol. I (Jena: Fischer).
Troll W. (1969). Die Infloreszenzen: Typologie und Stellung im Aufbau des Vegetationskörpers. Vol. II/1 (Jena: Fischer).
Troll W., Heidenhain B. (1951). Beiträge zur Kenntnis racemöser Infloreszenzformen. Akad. Wiss. Lit. Abh. Math.-Nat. Kl. 5, 141–179.
Tucker S. C., Grimes J. (1999). The inflorescence: introduction. Bot. Rev. 65, 303–316. doi: 10.1007/BF02857752
Weberling F. (1989). Morphology of Flowers and Inflorescences (Cambridge: Cambridge University Press).
Keywords: andromonoecy, fractionation, floral unit meristem, inflorescence architecture, inflorescence development, mechanical pressure, meristem expansion, receptacle elongation
Citation: Claßen-Bockhoff R and Ajani Y (2025) Repeated fractionation and umbel receptacle elongation explain the apparent “panicle with simple umbels” in Ferula species (Apiaceae). Front. Ecol. Evol. 13:1550679. doi: 10.3389/fevo.2025.1550679
Received: 23 December 2024; Accepted: 10 February 2025;
Published: 05 March 2025.
Edited by:
Margarita V. Remizowa, Lomonosov Moscow State University, RussiaReviewed by:
Andrey Sinjushin, Institute of Field and Vegetable Crops, SerbiaRolf Rutishauser, University of Zurich, Switzerland
Alexei Oskolski, University of Johannesburg, South Africa
Copyright © 2025 Claßen-Bockhoff and Ajani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Regine Claßen-Bockhoff, Y2xhc3NlbmJAdW5pLW1haW56LmRl
†Present address: Yousef Ajani, Department of Botany, Research Institute of Forests and Rangelands, Agricultural Research Education and Extension Organization (AREEO), Tehran, Iran
 Regine Claßen-Bockhoff
Regine Claßen-Bockhoff Yousef Ajani†
Yousef Ajani†