
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 13 March 2025
Sec. Evolutionary Developmental Biology
Volume 13 - 2025 | https://doi.org/10.3389/fevo.2025.1548208
Phenotypic plasticity, the ability of a genotype to produce different phenotypes in response to environmental conditions, plays a crucial role in adaptation and evolution and can occur during development or adulthood. Sea urchin larvae exhibit developmental plasticity by adjusting their arm length in response to food availability. In this study, we investigated the phenotypic responses of three sea urchin species: Paracentrotus lividus and Arbacia lixula from the Mediterranean Sea, and Strongylocentrotus purpuratus from the Pacific Ocean. In all species, we observed that larvae reared under a 12h light:12h dark cycle exhibited phenotypic responses to food availability. However, the response was suppressed in larvae reared under constant darkness, suggesting that light has a role in mediating this phenotypic plasticity. Moreover, larvae grown in constant darkness were generally smaller than those exposed to light, with the magnitude of this effect varying among species, indicating that light exposure influences not only plasticity but also baseline growth rates. These findings underscore the utility of sea urchins as a model for studying ecological and evolutionary processes shaping phenotypic responses and suggest that light has an important impact on development and growth in sea urchins.
All organisms have evolved strategies to adapt to their environment, which, therefore, contribute to phenotypic variation beyond merely selecting the fittest. Phenotypic plasticity refers to the property of a genotype to produce different phenotypes in response to the environmental conditions. This can occur during adulthood; an example is the sequential hermaphroditism observed in clownfish (Fricke and Fricke, 1977). Plasticity can also occur during development; in this case, it is better described as developmental plasticity; an example is butterflies developing different wing patterns when growing at different temperatures (Brakefield et al., 1996). A character can be either plastic or robust (where an invariant phenotype is produced despite environmental variability) and can switch between plasticity and robustness (or canalization) under selection pressure, contributing to adaptive evolution (Beldade et al., 2011; Paaby and Testa, 2018; Pfennig, 2021).
Sea urchins provide an example of developmental plasticity. Most sea urchins undergo indirect development with a planktotrophic larva stage: the pluteus. These plutei develop longer arms when food is scarce to enhance feeding efficiency (Hart and Strathmann, 1994), while they develop shorter arms when food is abundant to preserve maternal storage (Byrne et al., 2008; Adams et al., 2011). Intriguingly, this response is not simply a case of starvation-induced arm elongation but rather a food-induced inhibition of arm growth mediated by a dopamine signaling (Adams et al., 2011; Kalachev, 2020). Specifically, Adams et al. (2011) demonstrated that dopamine signaling regulates the phenotypic response by suppressing arm growth in response to food availability and suggested that the maximum arm length is predetermined and food availability triggers a neuroendocrine mechanism that constrains arm elongation to save maternal storage when food is abundant. Furthermore, it has been suggested that this regulatory pathway is initiated during embryogenesis, before embryos reach the larval stage (pre-feeding stages) (Miner, 2007). Dopaminergic neurons have been identified in many sea urchin species and have been localized at the base or in the post oral arms, and in the mouth lower-lip (Bisgrove and Burke, 1987; Adams et al., 2011; Slota and McClay, 2018; Paganos et al., 2021; Chen and Adams, 2022) whereas dopamine receptors are expressed in the ciliary band, apical plate, and skeletal cells in S. purpuratus (Paganos, 2021). Interestingly, the number of dopaminergic neurons varies not only between species but also within the same species. A recent study on Mesocentrotus nudus and Paracentrotus lividus revealed that larvae of the same age exhibiting a two- to four-fold difference in the number of dopaminergic neurons (Obukhova et al., 2024). Therese differences were correlated with individual differences in the swimming behavior. Phenotypic plasticity in sea urchins is well-documented across various species, including M. nudus and Strongylocentrotus intermedius (Kalachev et al., 2018), with evidence suggesting it is more common in temperate species than in tropical ones (McAlister, 2008; Soars et al., 2009).
Here we investigate the impact of light on the phenotypic response to food availability across three sea urchin species. We quantified phenotypic plasticity in two species (Paracentrotus lividus and Arbacia lixula) collected from the Mediterranean Sea and one (Strongylocentrotus purpuratus) from the Pacific Ocean and found that the phenotypic plasticity is conserved among all three. Subsequently, to investigate the regulatory function of light, we compared the phenotype of fed and starved larvae reared under constant darkness and found that the phenotypic response is suppressed in all three species. Finally, we compared the phenotype of larvae reared with and without light and found that in general, larvae reared without light are smaller than larvae reared under light exposure, but the amplitude of this response (the difference between a larva reared under constant darkness and under light exposure) depends on the species observed. These data highlight the intricate balance between genotype, environment, and phenotype, showing that sea urchins are an excellent system for studying the ecological and evolutionary processes driving phenotypic responses. Additionally, it underscores the importance of light and other factors, such as thyroid hormones (Heyland et al., 2006; Milonas et al., 2010; Taylor and Heyland, 2018; Yaguchi and Yaguchi, 2021; Cocurullo et al., 2023b), in influencing their development and growth.
Adult Strongylocentrotus purpuratus were obtained from Patrick Leahy (Kerckhoff Marine Laboratory, California Institute of Technology, Pasadena, CA, USA) and maintained in circulating seawater at Stazione Zoologica Anton Dohrn in Naples at 15°C. Adult Paracentrotus lividus and Arbacia lixula were collected from the Gulf of Naples and maintained in circulating seawater at Stazione Zoologica Anton Dohrn (Naples, Italy) at 18°C.
S. purpuratus gametes were obtained by vigorous shaking, and the eggs were collected by placing the female upside down on a small glass beaker filled with filtered Mediterranean seawater diluted 9:1 (FSW 9:1) to recapitulate the osmolarity of their oceanic habitat. Sperm was collected dry and stored at 4°C until use. Eggs were fertilized by adding a few drops of sperm diluted 1:10,000 in FSW 9:1. Cultures were reared according to the experimental design.
P. lividus and A. lixula gametes were collected by injection of 0.5–1 mL of 0.5 M KCl into the coelomic cavity. Gametes were then collected as for S. purpuratus but using undiluted filtered Mediterranean seawater (FSW). Cultures were then reared according to the experimental design.
This experiment was designed to evaluate how light exposure impacts the phenotypic response to food availability and the connection with the dopaminergic system. A schematic representation can be found in Figure 1. To interfere with the dopamine production, we used the α-Methyl-DL-tyrosine methyl ester hydrochloride, which inhibits Tyrosine Hydroxylase (TH Inh) at a 10 nM final concentration. S. purpuratus larvae treated with 10 nM of TH Inh or Dimethyl sulfoxide (DMSO) as a negative control were reared under a 12h light:12h dark cycle (LD) or in constant darkness (DD) conditions. Drugs and food (5,000 cells per mL of D. tertiolecta) were added at the gastrula stage, according to Adams et al. (2011). Zygotes were obtained by crossing a male and a female; subsequently, they were counted and transferred in polystyrene plates (35x15mm) at a density of 25 zygotes per mL; each well contained 4 mL of FSW 9:1 at the final volume. Wells were then assigned to the different experimental conditions; the one to be exposed to the LD and was placed in the incubator at 15°C, while the ones to be kept in DD was placed in a light-tight cardboard box within the same incubator. Light exposure was obtained using an LED Radion lamp (https://ecotechmarine.com/radion). White LEDs only were used to illuminate the plates at an intensity of 1.4 μmol photons m-2 s-1, mimicking the light conditions of the Panasonic MIR-154 Cooled Incubators in use at Stazione Zoologica. At 48 hours post fertilization (hpf), gastrula stage, DMSO or TH Inh and microalgae were administered to the cultures following the scheme illustrated in Figure 1. For the DD condition, TH Inh and food were administered in the dark using a red LED to illuminate the work area. At 6-day post fertilization (dpf), larvae were collected adding a drop of PFA 8% in FSW. The collection time was carefully chosen to ensure it fell within the window when the phenotypic response is elicited and measurable. The larvae were then collected from the bottom of the well and transferred in 1.5 mL tubes, fixed for 10 minutes in PFA 4% in FSW, and washed with PBS having a pH>9 to preserve spicules.
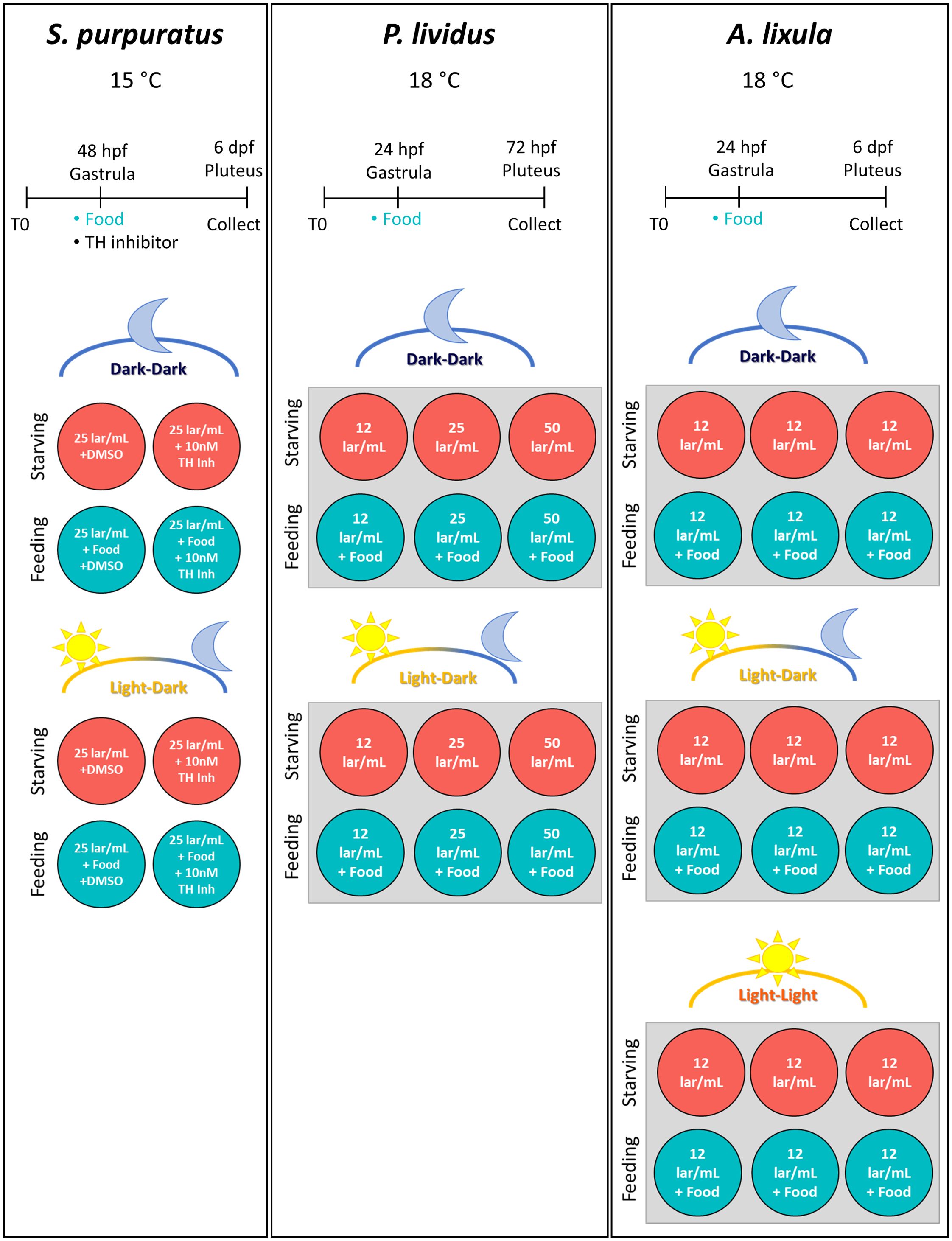
Figure 1. Experimental setup. This figure illustrates the experimental setup and timeline for the three sea urchin species used in this study: S. purpuratus, P. lividus, and A. lixula. For each species, panels show the temperature at which cultures were reared, the experimental timeline, and a schematic representation of the conditions used. For S. purpuratus: food (D. tertiolecta, 5000 cells/mL), DMSO (negative control), and TH inhibitor (10 nM) were administered at the gastrula stage (48 hpf). Larvae were sampled for imaging at various days post-fertilization (dpf). Two sets of four plates were used: one under a light-dark cycle (LD) and the other in constant darkness (DD). Wells were divided into feeding and starving conditions, with control and inhibitor treatments. Each well contained a larval concentration of 25 larvae/mL. For P. lividus: food (D. tertiolecta, 10,000 cells/mL) was provided at the gastrula stage (24 hpf), and larvae were collected at 72 hpf. Two 6-well plates were prepared: one under LD conditions and the other under DD conditions. Each well contained filtered seawater with three larval concentrations (12, 25, and 50 larvae/mL). Wells were split between feeding and starving conditions across increasing larval densities. For A. lixula: food (D. tertiolecta, 10,000 cells/mL) was administered at the gastrula stage (24 hpf), with larvae sampled at 6 dpf during the pluteus stage. Three 6-well plates were set up: one under LD, one under DD, and one in constant light (LL). Each well contained a larval concentration of 12 larvae/mL, with wells divided between feeding and starving conditions.
This experiment was designed to evaluate whether P. lividus larvae exhibit a phenotypic response to food availability and whether light is required for this response. Additionally, we investigated how larval concentration influences arm growth in response to food availability. The experimental setup is illustrated in Figure 1. Zygotes were obtained by crossing a male and a female and subsequently they were transferred in polystyrene 6-well plates; each well contained 4 mL of FSW at the final volume. 50, 100, or 200 zygotes were added to each well, resulting in final larval concentrations of about 12, 25, and 50 larvae/mL (lar/ml), respectively. Two identical plates were prepared as illustrated in Figure 1, and one was placed in a light-tight cardboard box to keep the larvae in constant darkness (DD). The two plates were then placed in a walk-in room at 18°C equipped with a wide-spectrum fluorescent lamp. The light-dark cycle was set to 12h light:12h dark (LD). The LD plate received about 1.4 μmol photons m-2 s-1 light intensity. At 24 hpf (gastrula stage), half of the wells received 10,000 cells/mL of D. tertiolecta. As for S. purpuratus, food in the DD condition was provided under a source of red light. Larvae were collected at 72 hpf adding a drop of PFA 8% in FSW. The collection time was adapted to ensure it fell within the window when the phenotypic response is elicited and measurable. The larvae were then collected from the bottom of the well and transferred in 1.5 mL tubes, fixed for 10 minutes in PFA 4% in FSW, and washed with PBS having a pH>9 to preserve spicules.
This experiment was designed to evaluate whether A. lixula has a plastic response to food availability and if this response is affected by a lack of light or by exposure to constant light. The experimental setup is illustrated in Figure 1. Zygotes were obtained by crossing a male and a female and subsequently they were distributed in three 6-well plates filled with FSW, 4 mL final volume, at 12 larvae/mL density. The plates were organized as illustrated in Figure 1. One plate was exposed to the LD cycle, one was kept in DD as previously described, and a third plate was exposed to constant light (LL). The light intensity and source used are the same as described for S. purpuratus. Embryos were reared at 18°C. As previously described, 10,000 cells/mL of D. tertiolecta were provided at 24 hpf. Larvae were collected at 6 dpf adding a drop of PFA 8% in FSW. The collection time was adapted to ensure it fell within the window when the phenotypic response is elicited and measurable. The larvae were then collected from the bottom of the well and transferred in 1.5 mL tubes, fixed for 10 minutes in PFA 4% in FSW, and washed with PBS having a pH>9 to preserve spicules.
In order to quantify changes in arm length, we measured the lengths of skeletal rods (Figure 2), which are proportional to the arm length. Specifically, we measured the spicules that grow beneath the Post Oral (PO) and Anterolateral (ALA) arms (Figure 2, in magenta and yellow, respectively). Additionally, we measured the length of Body Rods (BR) (Figure 2, in cyan). BR were chosen because their lengths were reported to not change in response to food availability (Adams et al., 2011). In order to place the spicules on one focal plane, larvae were mounted on a glass slide (26x76 mm). A coverslip with play dough applied to the corners was gently placed on top and squashed by gently pressing on the corners. The samples were then imaged using a ZEISS Axio Imager.M1 Microscope equipped with a Differential Inference Contrast (DIC) prism mounted on a 20x objective. The sea urchin skeleton is birefringent, exhibiting a color gradient when imaged using a polarized light source, making it easier to visualize. The images were analyzed using the ZEN 3.1 software (blue edition) or ImageJ v1.52. The measures were then transferred on a spread sheet and analyzed as described in the following section.
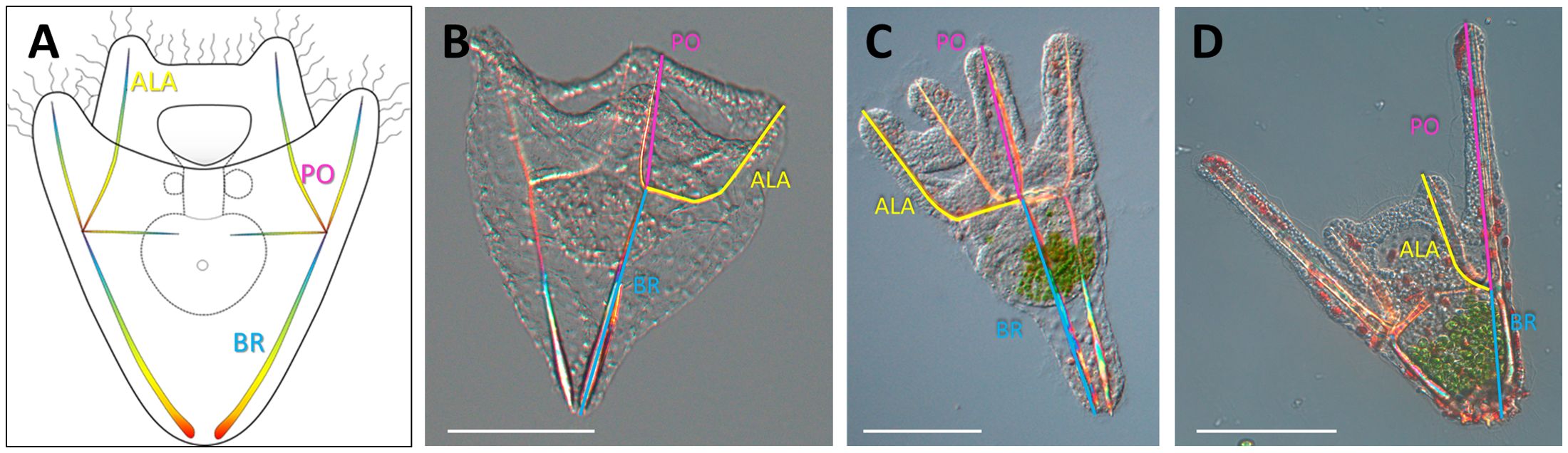
Figure 2. Larval morphology. (A) Schematic representation of a sea urchin larva illustrates the skeletal rods measured in this study. (B) S. purpuratus larva. (C) P. lividus larva. (D) A lixula larva. Larvae were flat mounted between a glass slide and a coverslip and imaged using DIC mounted on a 20x objective. ALA, Anterolateral in yellow; BR, Body Rod in cyan; PO, Post Oral in magenta. Scale bars = 100 µm.
Data analysis and visualization were conducted in R (v4.4.1) using the brms package (https://paulbuerkner.com/brms/), which provides an interface to fit Bayesian generalized (non-)linear multivariate multilevel models using Stan (https://mc-stan.org/) (Bürkner, 2017, 2018; Carpenter et al., 2017; Nalborczyk et al., 2019). Multilevel linear regression models were constructed to estimate the influence of several predictors on larval rod length, assuming a Gaussian distribution, which is a plausible generative model for arm growth. Predictor variables included a scaled version of rod length (L) and larval concentration (C), both transformed using the scale function. Individual variability due to repeated measurements from the same larva was accounted for by including “larva” as a group-level effect (random intercept). To address observed variability in residuals across predictor levels (e.g., smaller variability for BR and larger for PO; greater variability in fed conditions compared to starving conditions), we explicitly modeled the residual standard deviation (sigma) as a function of predictors in the brms formula. Weakly informative priors were employed to guide model estimation, and models were fitted using Markov Chain Monte Carlo (MCMC) sampling. Four chains were run, each consisting of 2,500 iterations following a 1,250-iteration warm-up, to ensure convergence and stable posterior estimates. Model diagnostics included checking effective sample sizes, the Gelman-Rubin diagnostic (Gelman and Rubin, 1992), and trace plots. Leave-one-out cross-validation (LOO) was used to compare alternative plausible models.
Bayesian MCMC models produce joint posterior probability distributions that estimate the probabilities of various effects. To interpret and visualize the model outcomes, we used the marginaleffects package, specifically the avg_predictions function. This function facilitated the calculation of unscaled average predictions across combinations of key variables (e.g., feeding status, light exposure, and rod types). Predicted values (Estimates) and their associated 95% credible intervals (CIs) were visualized using ggplot2. These plots display individual measurements, inferred means, and 95% CIs, representing the interval within which the true rod length is likely to lie with 95% probability, given the data and prior information. The avg_predictions results are included in Supplementary Tables 2–4.
The complete scripts, package versions, and dataset are available in the following GitHub repository: https://github.com/MariaCoc/Urchin_phenotypic_plasticity.
To investigate how light affects phenotypic response to food availability, we measured the phenotypic response to food availability (rod length in fed vs. starved larvae) in S. purpuratus larvae reared under a 12h light:12h dark cycle (LD) and under constant darkness (DD). Given the known role of dopamine in regulating this response (Adams et al., 2011; Kalachev, 2020), we also treated both fed and starved larvae with a TH inhibitor to inhibit dopaminergic signaling, using DMSO as a negative control, specifically exploring its effects under the previously untested DD condition (Figure 1). Pictures from a representative larva for each condition are shown in Figure 3A. First, we compared the phenotypes of fed and starved larvae reared with and without (using DMSO as a negative control) TH Inh in larvae reared under a LD cycle (Figure 3B). Our data confirm the previous observation (Adams et al., 2011) that BR length does not change in response to food availability, while PO rods are shorter in fed larvae (Figure 3B; Supplementary Table 2). Additionally, we observed that ALA rods are longer in starving condition, but this difference is not statistically relevant as the Credible Intervals (CI) slightly overlap. Specifically, Estimate mean for fed larvae is 146.8 with 141.3 lower and 152.5 higher CI limits, while Estimate mean for starved larvae is 159.6 with 151.8 lower and 167 CI higher limits (Supplementary Table 2). The phenotypic response was suppressed by incubating the larvae with 10 nM of TH Inh, inhibiting dopamine production. Rearing the larvae under constant darkness (DD), however, suppressed the phenotypic plasticity response in the control larvae too. Specifically, starved larvae reared in DD had significantly smaller PO rods (Estimate of 86.8, 82.6 lower and 91 higher CI limits) than starved larvae reared under the LD cycle (Estimate of 114.4, 108.3 lower and 120.6 higher CI limits). To investigate whether the observed difference between fed larvae reared in DD and LD conditions is linked to a difference in feeding, we calculated the percentage of larvae having food in the gut. In this scenario, fed larvae reared in LD should have a higher percentage of larvae having food in the gut. This was not the case, as we found no relevant difference (Supplementary Figure 1).

Figure 3. Impact of light on S. purpuratus phenotypic plasticity. Response to food availability was evaluated in control (DMSO) and treated (TH_inh_10nM) specimens reared under constant darkness (DD) and a light-dark cycle (LD). (A) A representative pluteus for each condition was chosen to show the general larval morphology. Little food was observed in the guts of fed larvae. Scale bar = 50 µm. (B) Rods length from larvae reared with and without food is directly compared. Each panel groups data by skeletal rod (from left to right: BR, PO, ALA) and light regime (from top down: DD, LD). Within each panel, skeletal lengths from fed and starved larvae are directly compared in control and treated larvae to show the phenotypic response to food availability and the effects of inhibiting dopaminergic signaling. (C) The data shown in A are differentially plotted to directly compare skeletal rod length from larvae reared with and without an LD cycle. Each panel groups data by skeletal rod (from left to right: BR, PO, ALA) and feeding regime (from top down: fed, starved). Within each panel, skeletal lengths from larvae reared in DD and LD are directly compared in control and treated larvae to show the impact of light regime and dopamine signaling inhibition on the rod length. The background dots represent the raw data (individual measures) and have been colored according to the feeding or light regimes. Overlaid in black are the mean and the 95% Credible Intervals. DD=constant darkness, LD 12h light:12h dark cycle. ALA, Anterolateral; BR, Body Rod; PO; Post Oral.
Furthermore, to assess the impact of light on the larval growth, we directly compared spicule length from larvae reared in DD versus LD conditions (Figure 3C; Supplementary Table 2). Control larvae grew significantly longer PO and ALA under a LD cycle, while BR remained unchanged. In fed larvae, however, only PO arms were significantly longer in LD, and the amplitude of this difference was reduced. Overall, these data suggest that larvae reared in constant darkness are significantly smaller compared to larvae reared under a light-dark cycle. It may be hypothesized that the growth-stimulating effect of light is due to different microalgae growth rates, e.g., larvae reared in LD have more food and eat more, therefore grow more. But this scenario seems unlikely since the differential DD vs. LD growth was greater in the starved larvae. The LD vs. DD comparison performed on larvae treated with the TH inhibitor further complicates the picture as larvae reared in LD look to be smaller and the difference between rods from DD vs. LD is reduced. These data suggest a complex connection between the signaling pathways controlling phenotypic response to food and light.
The sea urchin species P. lividus collected from the Mediterranean Sea develops much faster than the Pacific Ocean species S. purpuratus, and it reaches the early larva stage at 48 hpf compared to the 72 hpf of S. purpuratus. Moreover, P. lividus larvae grow much longer post oral and anterolateral arms. Here we investigated whether P. lividus shows the phenotypic response to food availability found in other sea urchin species. Moreover, we tested different larval densities (i.e., larval concentration) according to the scheme reported in Figure 1, since it is common knowledge that larvae reared at high density do not develop properly. In our preliminary tests, we found that a phenotypic response to food availability could be observed at 72 hpf (data not shown). Therefore, we chose this stage for further investigations. Pictures from a representative larva for each condition are shown in Figure 4A. We found that also in the P. lividus larvae growing under a LD cycle developed shorter PO rods when food was available (Figure 4B; Supplementary Table 3), but this response was expressed only at lower larval concentrations. Specifically, when rearing larvae at 12 larvae/mL PO from fed larvae Estimate was 143.4 (with 133.2 lower and 153.9 higher CI limits), and PO from starved larvae Estimate was 197.8 (with 184.1 lower and 211.9 higher CI limits). On the contrary, at 50 larvae/mL PO from fed larvae Estimate was 169.9 (with 163.1 lower and 176.9 higher CI limits), and PO from starved larvae Estimate was 182.1 (with 172.4 lower and 191.9 higher CI limits). Intriguingly, PO rods were differentially affected by larval concentration in fed and starved larvae. PO arm length decreased in starved larvae and increased in fed ones proportionally to the increasing larval concentration, thus suggesting that competition for food could suppress the short arm response to food availability. In contrast, constant darkness suppresses the phenotypic response in PO arms. On the other hand, BR and ALA remained relatively constant at increasing larval concentrations and did not show differences between fed and starved larvae.
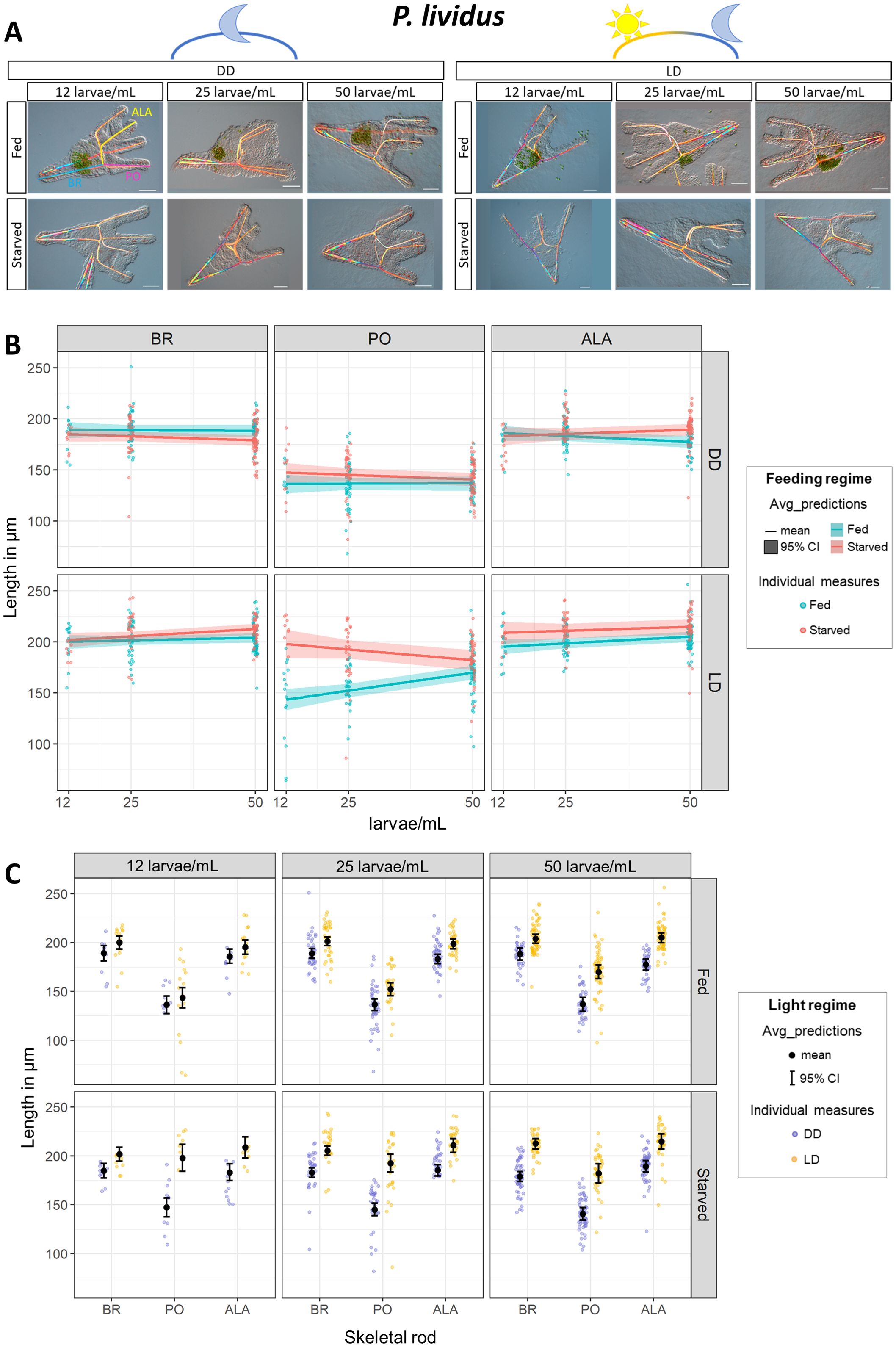
Figure 4. Impact of light on P. lividus phenotypic plasticity. Response to food availability was evaluated under increasing larval concentrations (12, 25, and 50 larvae/mL, on the x axis) in constant darkness (DD) and a light-dark cycle (LD). (A) A representative pluteus for each condition was chosen to show the general larval morphology. Scale bar = 50 µm. (B) Rods length from larvae reared with and without food are directly compared. Each panel groups data by skeletal rod (from left to right: BR, PO, ALA) and light regime (from top down: DD, LD). Within each panel, skeletal lengths from fed and starved larvae are directly compared and plotted against the larval concentration (on the x-axis) to show the phenotypic response to food availability and the effects of increasing larval concentration. (C) The data shown in A are differentially plotted to directly compare skeletal rod length from larvae reared with and without a LD cycle. Each panel groups data by larval concentration (from left to right: 12, 25, 50 larvae/mL) and feeding regime (from top down: Fed, Starved). Within each panel, skeletal lengths (on the x-axis: BR, PO, ALA) from larvae reared in DD and LD are directly compared to show the impact of the light regime on each individual rod length. The background dots represent the raw data (individual measures) and have been colored according to the feeding or light regimes. In A, the ribbons represent the 95% Credible Intervals while the middle line shows the mean. In B, overlaid in black are the mean and the 95% Credible Intervals. DD=constant darkness, LD 12h light:12h dark cycle. ALA, Anterolateral; BR, Body Rod; PO, Post Oral.
Subsequently, we directly compared skeletal rods developed under DD or LD (Figure 4C; Supplementary Table 3). We grouped the data according to larval concentration (12, 25, and 50 larvae/mL) and whether they were fed or starved. Intriguingly, all skeletal rods (BR, PO, and ALA) from starved larvae grew significantly longer when larvae were reared with a LD cycle, while they were significantly shorter when larvae were reared in DD. The difference between skeletal rods from larvae reared in LD vs. DD was reduced when larvae were fed, and no significant difference was observed at the lowest density (12 larvae/mL).
Finally, we quantified phenotypic response to food availability in another sea urchin species harvested from the Mediterranean Sea: A. lixula. In our previous experiments with S. purpuratus and P. lividus, we found that rearing larvae in constant darkness (DD) suppressed the phenotypic response to food availability. However, it remained unclear whether this effect was due to disrupted circadian clock entrainment or simply the absence of light exposure. To address this, we included a constant light (LL) (Figure 1) free-running condition in the A. lixula experiment. This allowed us to determine whether the phenotypic response to food occurs under continuous light, which would suggest that light exposure alone is sufficient. Conversely, if the response were inhibited under LL, it would indicate that proper circadian entrainment is required. We reared A. lixula larvae at 18°C, as we do for P. lividus. Even though A. lixula reached the pluteus stage around 48 hpf, we could not observe phenotypic plasticity at 72 hpf (as for P. lividus), but we could at 6 dpf (Supplementary Figure 2). Pictures from a representative larva for each condition are shown in Figure 5A. As for S. purpuratus and P. lividus, first we focused on comparing individual rods (BR, PO, and ALA) in fed vs. starved larvae reared under DD, LD, and LL light conditions (Figure 5B; Supplementary Table 4). Intriguingly, in A. lixula, both PO and ALA were significantly shorter in fed larvae compared to the starved ones in LD condition, while BR did not change in response to food availability (Figure 5B; Supplementary Table 4). On the contrary, both in DD and LL conditions, the phenotypic response to food availability was inhibited.
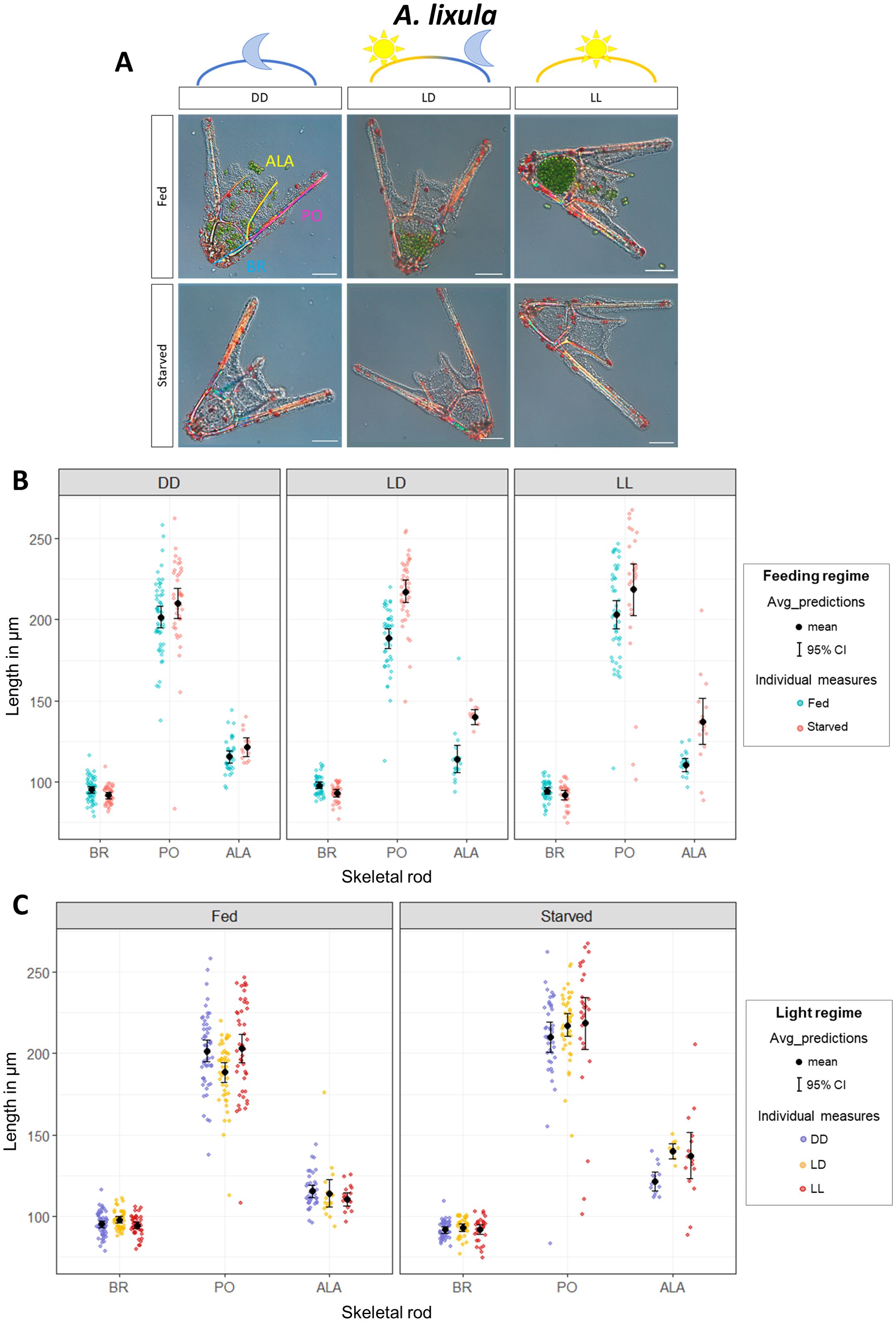
Figure 5. Impact of light on A lixula phenotypic plasticity. Response to food availability evaluated by rod length in fed and starved larvae for each rod type (BR, PO, ALA) in larvae reared in DD, LD, and LL conditions. (A) A representative pluteus for each condition was chosen to show the general larval morphology. Scale bar = 50 µm. (B) Rods length from larvae reared with and without food are directly compared. Each panel groups data by light regime (from left to right: DD, LD, LL). Within each panel, skeletal lengths (on the x-axis: BR, PO, ALA) from larvae reared with (Fed) and without (Starved) food are directly compared to evaluate the phenotypic response to food availability under the different light regimes. (C) The data shown in A are differentially plotted to directly compare skeletal rod length from larvae reared under different light regimes. Each panel groups data by feeding regimes (from left to right: Fed, Starved). Within each panel, skeletal lengths (on the x-axis: BR, PO, ALA) from larvae reared in DD, LD, and LL are directly compared to show the impact of the light regime on the rod length. The background dots represent the raw data (individual measures) and have been colored according to the feeding or light regimes. Overlaid in black are the mean and the 95% Credible Intervals. DD, constant darkness; LD 12h light:12h dark cycle, LL, constant light. ALA, Anterolateral; BR, Body Rod; PO, Post Oral.
A major difference with the previous species is found in the response of A. lixula fed and starved larvae to the light regime (Figure 5C; Supplementary Table 4). In general, there was no significant difference between rods from larvae reared under DD, LD, or LL; moreover, this did not change despite larvae being reared with or without food. The only significant differences observed were in starved larvae which developed significantly longer ALA in LD compared to the DD, and in fed larvae which developed significantly shorter PO in LD compared to DD.
Phenotypic plasticity is a key adaptive mechanism that enables organisms to respond to environmental variability. Sea urchins are broadly distributed across diverse marine habitats, from temperate to tropical waters, providing a fascinating opportunity to investigate how environmental conditions have shaped their phenotypic responses. For example, there is evidence suggesting that phenotypic plasticity is more prevalent in temperate species than in tropical ones (McAlister, 2008; Soars et al., 2009).
Arm development and growth in sea urchin larvae is finely regulated by a complex network of internal and external signaling. Therefore, observing robust phenotypic response to food availability could be challenging. Based on the evidence collected from literature and from this work, we can hypothesize that at the early larva stage, signals from maternal storage and food availability in the environment prevail, guiding the development toward the reduction of arm growth when food is abundant. Subsequently, when larvae begin to feed and digest, nutrition levels become more important, and fed larvae grow significantly longer arms than starved ones because they have more energies. This signaling has been suggested to rely on thyroid hormones ingested with the algae (Heyland and Hodin, 2004; Holzer et al., 2017). As a consequence, the window in which it is possible to observe phenotypic plasticity in sea urchin larvae is quite short, in agreement with Sewell et al. (2004). This might also explains why McAlister (2008) concluded that the species he observed did not show phenotypic plasticity. In his data, Echinometra vanbrunti (from Pacific Ocean) displayed the expected plasticity with longer arms in low food conditions only at 4 days post-fertilization (dpf), while Eucidaris thouarsi (from Pacific Ocean) exhibited longer arms in starvation only between 3 and 4 dpf.
In this study, we compared the phenotypic responses of three sea urchin species: P. lividus and A. lixula, both collected from the Gulf of Naples in the Mediterranean Sea, and S. purpuratus, collected from the coast of California (Pacific Ocean). The results are summarized in Figure 6. Although P. lividus and A. lixula share their habitat in the Mediterranean Sea, P. lividus is phylogenetically closer to S. purpuratus (diverging approximately 80 million years ago, MYA) than to A. lixula which diverged much earlier [around 210 MYA (Koch et al., 2022)]. This phylogenetic framework provides a unique opportunity to explore how adaptive responses to environmental variability have evolved across distinct evolutionary lineages. By comparing the phenotypic plasticity and examining the molecular mechanisms underlying these responses in the three species, we can gain insights into the evolutionary pressures that shaped differences in developmental strategies and environmental sensing. For instance, Chen and Adams (2022) investigated the evolution of phenotypic plasticity in sea urchins with a focus on its link to neurosensory systems. They identified the origin of pre-feeding phenotypic plasticity at the base of regular sea urchins. While the neurosensory foundation was ancestral in echinoids, phenotypic plasticity as a functional trait emerged later, likely influenced by pleiotropic effects. Their findings indicate a pattern of gains and losses in plasticity associated with changes in sensory abilities. Our study, therefore, underscores the importance of reconstructing the sensory and neurosecretory systems across different sea urchin species to better understand how phenotypic plasticity has evolved. Investigating these systems can reveal how neurosensory capabilities have both facilitated and constrained the development of adaptive plastic responses throughout the evolution of echinoids.
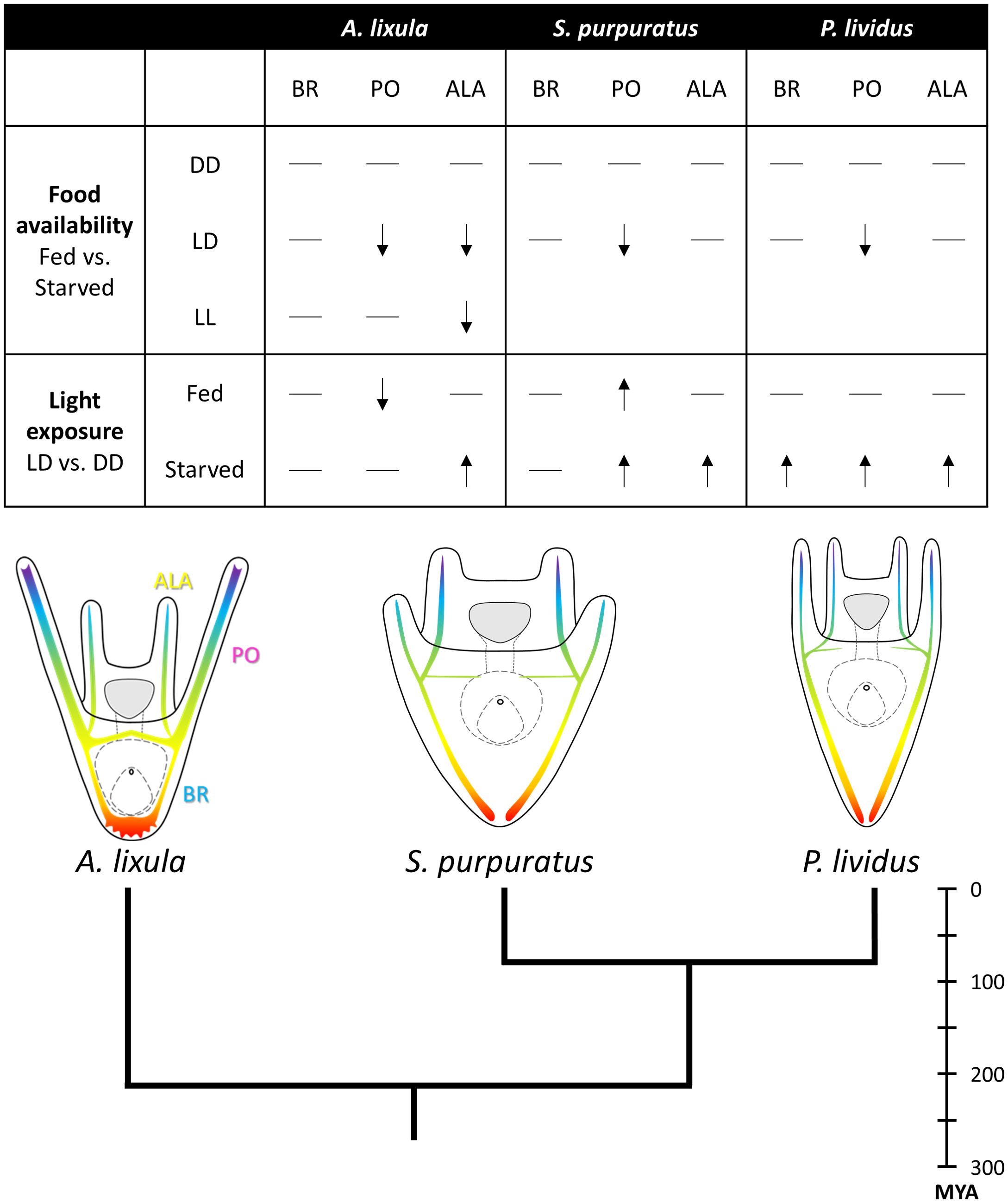
Figure 6. Summary scheme. The figure highlights key findings and concepts investigated in this work. The table summarizes, for the three sea urchin species investigated, the impact of food availability and light exposure on each individual skeletal rod. From top down, it shows the phenotypic response observed in fed larvae compared to starved ones when larvae are reared in DD, LD, or LL light regimes. - indicates that no difference was observed. ↓ indicates that the skeletal rod from fed larvae showed significantly reduced length compared to starved ones. Subsequently, the table summarizes the length of skeletal rods measured in larvae reared in LD compared to DD when larvae were reared with (fed) or without (starved) food. - indicates that no difference was observed. ↑ indicates that skeletal rod from larvae reared in LD were significantly longer compared to larvae reared in DD. ↓ indicates that skeletal rods from larvae reared in LD were significantly shorter compared to larvae reared in DD. On the bottom, a tree illustrates the phylogenetic relationships and evolutionary distances among the sea urchin species investigated. A schematic drawing shows the larval morphology of each species at the investigated time point. Larval schematic representations are not in scale. Evolutionary distances deduced from (Koch et al., 2022). MYA, Million years ago.
Here we found that also the two temperate sea urchin species, P. lividus and A. lixula, collected from the Gulf of Naples in the Mediterranean Sea, exhibit a phenotypic response to food availability. Specifically, their larvae develop shorter PO arms when fed (Figure 6). This supports the hypothesis that phenotypic plasticity is a widespread and evolutionarily conserved trait among sea urchins, particularly in temperate environments where resource variability is common (McAlister, 2008; Soars et al., 2009). Additionally, given that sea urchin larvae at the investigated stages possess a second set of arms located above the mouth (the ALA), we explored the hypothesis that ALA rod growth also responds to food availability. Our data revealed that ALA growth showed a clear response to food availability only in A. lixula, suggesting that the signaling mechanisms regulating ALA and PO arm growth in response to food might differ. Intriguingly, previous research in P. lividus has shown that ALA and PO spicules are in distinct regulatory states (Tarsis et al., 2022).
Additionally, we investigated the effect of light deprivation on the phenotypic response to food availability by rearing fed and starved larvae under both a 12h light:12h dark cycle (LD) and in constant darkness (DD). Our data showed that larvae from all three sea urchin species reared in constant darkness did not exhibit phenotypic plasticity, suggesting that light plays a crucial role in mediating this response. Furthermore, in A. lixula, we tested whether this response is driven solely by light exposure or if it requires proper circadian clock entrainment. To test this, we reared A. lixula larvae under a constant light exposure (LL). This condition suppressed the phenotypic response to food availability in the PO and reduced such response in the ALA, suggesting that the circadian cycle entrainment might be necessary for such response. Intriguingly, the S. purpuratus genome encodes nearly all canonical clock genes known in protostomes and deuterostomes, with the notable exception of period. Petrone (2016) further suggested that the larval apical organ and gut might play a central role in the circadian system, as they co-express Sp-dcry and Sp-tim. On the other hand, nothing is known about the circadian system in the other species investigated here. Therefore, additional experiments are necessary to assess the hypothesized connection between the circadian clock and the response lo food availability and evaluate if this connection exists also in other species.
Furthermore, we directly compared spicule length from larvae reared in different light regimes. Overall, we found that exposure to a LD cycle enhanced development in sea urchin larvae, in agreement with what was shown by Milonas et al. (2010). Additionally, we show that the magnitude of this response varies depending on the species, the specific skeletal rod being measured, and the larvae’s feeding status. Interestingly, both S. purpuratus and P. lividus larvae exhibited significant growth under a LD cycle in the absence of food, suggesting that light exposure might play a role in modulating their developmental response beyond just nutritional input. In contrast, A. lixula larvae did not show such pronounced growth differences under the same conditions, indicating potential species-specific variations in how light exposure influences developmental processes. This divergence in response may reflect differences in the underlying regulatory mechanisms or adaptations to their distinct evolutionary histories.
A plausible hypothesis is that light might promote algal growth, thereby indirectly influencing arm elongation. However, our observation that starved larvae still exhibited enhanced arm growth under a LD cycle refutes this idea, pointing instead to a direct light-mediated effect on larval development. Supporting this, recent studies have uncovered potential mechanisms underlying this response. Yaguchi and Yaguchi (2021); Yaguchi et al., 2024 showed that strong photoirradiation induces pyloric and anal sphincters opening in Hemicentrotus pulcherrimus, and they suggest that this mechanism is involved in regulating digestive functions in response to light. Their data suggest that the pyloric opening response is mediated by a Go-Opsin expressed at the sides of the larval apical organ, followed by activation of a signaling cascade involving serotonin and nitric oxide. Noteworthy, S. purpuratus expresses a similar Go-Opsin (Opsin3.2) in corresponding cells at the sides of the apical organ (Valero-Gracia et al., 2016; Valencia et al., 2021). Previous work shows that these Go-Opsin3.2 cells in S. purpuratus are neurosecretory and produce a TRH-type neuropeptide (Cocurullo et al., 2023a; Petrone, 2016), which is required for arm elongation (Cocurullo, 2022; Wood, 2020). Interestingly, P. lividus does not express Go-Opsin3.2 at the early larval stage but expresses a different Go-Opsin, Opsin3.1, which appears to be localized within the larval apical organ (Cocurullo et al., 2024). These data provide evidence for a mechanism controlling arm development and growth in response to food and light exposure. This pathway may have been rewired during the evolution of sea urchins, but further studies are necessary to disentangle the signaling pathways involved in the response to food and light, and to understand how these cues are integrated. Additionally, characterizing the sensory/neurosecretory nervous system in different species is essential for understanding how phenotypic plasticity has evolved in sea urchins, as highlighted by Chen and Adams (2022). A final note concerns the type of light used in our experiments. As described in the methods, we used light conditions provided by incubators commonly used in laboratories for growing marine animals. While this is the standard approach, these light conditions are unnatural in both intensity and spectrum. Given the important regulatory role of light in development, future studies should aim to provide more natural light conditions.
Another important aspect to take into account is represented by the maternal storage accumulated in the eggs. There are studies attempting to correlating egg size and storage with growth and amplitude of the phenotypic response (Moran and Allen, 2007; Byrne et al., 2008; McAlister and Moran, 2013) that reach different conclusions. For example, Reitzel and Heyland (2007) concluded that plasticity is reduced when eggs are richly provisioned because there is less dependence on exogenous food, in agreement also with (McAlister and Moran, 2013). In contrast, Kalachev et al. (2018) found that Mesocentrotus nudus has bigger eggs, which are thought to have richer maternal storage, and more significant plasticity compared to Strongylocentrotus intermedius. Nonetheless these data are limited by the fact that they focus on difference in egg sizes and maternal storage among different species, while we lack information on the correlation between egg size and storage within the same species. In our experience, this could be a very important aspect to take into account. In fact, we could observe phenotypic plasticity in P. lividus larvae in late autumn (November), but we mostly failed observing the expected phenotype during the P. lividus reproductive season in the Gulf of Naples (Spring) (Riedl, 1983).
Additionally, the quality of food provided to the larvae may play a key role. In our experience changing the Dunaliella strains resulted in larvae growing much longer arms than starved (data not shown). This likely depends on the different content of nutrients and thyroid hormones in the two microalgae strains.
This study highlights the intricate interplay between environmental cues and developmental plasticity in sea urchin larvae. We demonstrated that light exposure significantly influences the phenotypic response to food availability in three sea urchin species: P. lividus, A. lixula, and S. purpuratus. Notably, the suppression of phenotypic plasticity under constant darkness across all species suggests a critical role of light in regulating this process. Moreover, our results indicate that light also modulates growth rates. The observed interspecies differences in response to light regimes, especially in A. lixula, imply that distinct evolutionary pressures have shaped the regulatory mechanisms underlying plasticity in these species. This finding underscores the need to further investigate how local environmental conditions and phylogenetic history influence adaptive strategies.
Future research could explore seasonal and geographic variability in these responses, providing insights into the role of maternal provisioning and the potential influence of intraspecific competition. Additionally, characterizing the sensory and neurosecretory systems across various species will be crucial to uncovering the molecular and cellular bases of phenotypic plasticity in sea urchins. By integrating ecological, evolutionary, and molecular perspectives, this work advances our understanding of how sea urchin larvae adapt to fluctuating environmental conditions, offering a comprehensive framework for future studies on developmental plasticity.
The complete scripts, package versions, and dataset are available in the following GitHub repository: https://github.com/MariaCoc/Urchin_phenotypic_plasticity.
The manuscript presents research on animals that do not require ethical approval for their study.
MC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JK: Formal analysis, Methodology, Writing – review & editing. MA: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. MC was supported by SZN PhD fellowships for the Open University and by the Human Frontiers Science Program grant number (RGP0002/2019). JK was supported by the Human Frontiers Science Program grant number (RGP0002/2019).
The authors would like to thank Davide Caramiello (SZN) for animal maintenance and for providing the microalgae used to feed the larvae. Moreover, we would like to thank Dr. Rossella Annunziata for her valuable and stimulating discussions, as well as for reading and providing insightful comments on the preliminary version of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors acknowledge that this manuscript was prepared with the assistance of ChatGPT to enhance the clarity of the writing and to generate visualizations. Recognizing the limitations of this tool, such as potential inaccuracies in content generation, we used it strictly for language editing and plot creation. All scientific content, data analysis, and interpretations are based entirely on the authors’ original research and expertise.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1548208/full#supplementary-material
Adams D. K., Sewell M. A., Angerer R. C., Angerer L. M. (2011). Rapid adaptation to food availability by a dopamine-mediated morphogenetic response. Nat. Commun. 2, 592. doi: 10.1038/ncomms1603
Beldade P., Mateus A. R. A., Keller R. A. (2011). Evolution and molecular mechanisms of adaptive developmental plasticity. Mol. Ecol. 20, 1347–1363. doi: 10.1111/j.1365-294X.2011.05016.x
Bisgrove B. W., Burke R. D. (1987). Development of the nervous system of the pluteus larva of Strongylocentrotus droebachiensis. Cell Tissue Res. 248, 335–343. doi: 10.1007/BF00218200
Brakefield P. M., Gates J., Keys D., Kesbeke F., Wijngaarden P. J., Monteiro A., et al. (1996). Development plasticity and evolution of butterfly eyespot patterns. Nature 384, 236–242. doi: 10.1038/384236a0
Bürkner P. C. (2017). brms: An R package for Bayesian multilevel models using Stan. J. Stat. Software 80, 1–28. doi: 10.18637/jss.v080.i01
Bürkner P.-C. (2018). Estimating Multivariate Models with brms. Available online at: https://cran.r-project.org/web/packages/brms/vignettes/brms_multivariate.html (Accessed November 12, 2024).
Byrne M., Sewell M. A., Prowse T. A. A. (2008). Nutritional ecology of sea urchin larvae: Influence of endogenous and exogenous nutrition on echinopluteal growth and phenotypic plasticity in Tripneustes gratilla. Funct. Ecol. 22, 643–648. doi: 10.1111/j.1365-2435.2008.01427.x
Carpenter B., Gelman A., Hoffman M. D., Lee D., Goodrich B., Betancourt M., et al. (2017). Stan: A probabilistic programming language. J. Stat. Software 76, 1–32. doi: 10.18637/jss.v076.i01
Chen E. Y., Adams D. K. (2022). The evolution of neurosensation provides opportunities and constraints for phenotypic plasticity. Sci. Rep. 1–11. doi: 10.1038/s41598-022-15583-y
Cocurullo M. (2022). Evolution of the TRH Neuropeptide Pathway and its Growth Regulation Function in Echinoderms. Ph.D thesis. Milton Keynes, UK: Open Univ. doi: 10.21954/ou.ro.00014231
Cocurullo M., Paganos P., Annunziata R., Voronov D., Arnone M. I. M. I. (2023a). Single-cell transcriptomic analysis reveals the molecular profile of go-opsin photoreceptor cells in sea urchin larvae. Cells 12, 2134. doi: 10.3390/cells12172134
Cocurullo M., Paganos P., Benvenuto G., Arnone M. I. (2024). Characterization of thyrotropin-releasing hormone producing neurons in sea urchin, from larva to juvenile. Front. Neurosci. 18. doi: 10.3389/fnins.2024.1378520
Cocurullo M., Paganos P., Wood N. J. N. J., Arnone M. I. M. I., Oliveri P. (2023b). Molecular and cellular characterization of the TH pathway in the sea urchin strongylocentrotus purpuratus. Cells 12, 272. doi: 10.3390/CELLS12020272/S1
Fricke H., Fricke S. (1977). Monogamy and sex change by aggressive dominance in coral reef fish. Nature 266, 830–832. doi: 10.1038/266830a0
Gelman A., Rubin D. B. (1992). Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472. doi: 10.1214/ss/1177011136
Hart M. W., Strathmann R. R. (1994). Functional consequences of phenotypic plasticity in echinoid larvae. Biol. Bull. 186, 291–299. doi: 10.2307/1542275
Heyland A., Hodin J. (2004). Heterochronic developmental shift caused by thyroid hormone in larval sand dollars and its implications for phenotypic plasticity and the evolution of nonfeeding development. Evol. (N. Y). 58, 524–538. doi: 10.1111/j.0014-3820.2004.tb01676.x
Heyland A., Price D. A., Bodnarova-Buganova M., Moroz L. L. (2006). Thyroid hormone metabolism and peroxidase function in two non-chordate animals. J. Exp. Zool. Part B Mol. Dev. Evol. 306, 551–566. doi: 10.1002/jez.b.21113
Holzer G., Roux N., Laudet V. (2017). Evolution of ligands, receptors and metabolizing enzymes of thyroid signaling. Mol. Cell. Endocrinol. 459, 5–13. doi: 10.1016/j.mce.2017.03.021
Kalachev A. V. (2020). Effect of dopamine on early larvae of sea urchins, Mesocentrotus nudus and Strongylocentrotus intermedius. J. Exp. Zool. Part B Mol. Dev. Evol. 334, 373–380. doi: 10.1002/JEZ.B.23001
Kalachev A. V., Yurchenko O. V., Osten V. G. (2018). Phenotypic plasticity in pre-feeding larvae of sea urchins, Mesocentrotus nudus and Strongylocentrotus intermedius. Invertebr. Zool. 15, 420–433. doi: 10.15298/invertzool.15.4.09
Koch N. M., Thompson J. R., Hiley A. S., McCowin M. F., Frances Armstrong A., Coppard S. E., et al. (2022). Phylogenomic analyses of echinoid diversification prompt a re-evaluation of their fossil record. Elife 11, e72460. doi: 10.7554/eLife.72460
McAlister J. S. (2008). Evolutionary responses to environmental heterogeneity in Central American echinoid larvae: Plastic versus constant phenotypes. Evol. (N. Y). 62, 1358–1372. doi: 10.1111/j.1558-5646.2008.00368.x
McAlister J. S., Moran A. L. (2013). Effects of variation in egg energy and exogenous food on larval development in congeneric sea urchins. Mar. Ecol. Prog. Ser. 490, 155–167. doi: 10.3354/meps10420
Milonas L., Pernet B., Bingham B. L. (2010). Light influences feeding and growth of echinoplutei. Mar. Ecol. Prog. Ser. 404, 69–78. doi: 10.3354/meps08488
Miner B. G. (2007). Larval feeding structure plasticity during pre-feeding stages of echinoids: Not all species respond to the same cues. J. Exp. Mar. Bio. Ecol. 343, 158–165. doi: 10.1016/j.jembe.2006.11.001
Moran A. L., Allen J. D. (2007). How does metabolic rate scale with egg size? An experimental test with sea urchin embryos. Biol. Bull. 212, 143–150. doi: 10.2307/25066591
Nalborczyk L., Batailler C., Loevenbruck H., Vilain A., Bürkner P. C. (2019). An introduction to bayesian multilevel models using brms: A case study of gender effects on vowel variability in standard Indonesian. J. Speech Lang. Hear. Res. 62, 1225–1242. doi: 10.1044/2018_JSLHR-S-18-0006
Obukhova A. L., Khabarova M. Y., Semenova M. N., Starunov V. V., Voronezhskaya E. E., Ivashkin E. G. (2024). Spontaneous intersibling polymorphism in the development of dopaminergic neuroendocrine cells in sea urchin larvae: impacts on the expansion of marine benthic species. Front. Neurosci. 18. doi: 10.3389/FNINS.2024.1348999/BIBTEX
Paaby A. B., Testa N. D. (2018). “Developmental plasticity and evolution,” in Evolutionary developmental biology. (Cham: Springer International Publishing), 1–14. doi: 10.1007/978-3-319-33038-9_110-1
Paganos P. (2021). Cell type diversity during sea urchin development: A single-cell approach to reveal different neuronal types and their evolution (Milton Keynes: The Open University).
Paganos P., Voronov D., Musser J., Arendt D., Arnone M. I. (2021). Single cell rna sequencing of the strongylocentrotus purpuratus larva reveals the blueprint of major cell types and nervous system of a nonchordate deuterostome. Elife 10, 1–29. doi: 10.7554/eLife.70416
Petrone L. (2016). Circadian clock and light input system in the sea urchin larva. Ph.D thesis. London: University College London. Available at: http://discovery.ucl.ac.uk/id/eprint/1503707.
Pfennig D. W. (2021). Phenotypic plasticity & Evolution. 1st Editio. Ed. Pfennig D. W. (Boca Raton: CRC Press). doi: 10.1201/9780429343001
Reitzel A. M., Heyland A. (2007). Reduction in morphological plasticity in echinoid larvae: Relationship of plasticity with maternal investment and food availability. Evol. Ecol. Res. 9, 109–121. doi: 10.1002/dvdy.474
Sewell M. A., Cameron M. J., McArdle B. H. (2004). Developmental plasticity in larval development in the echinometrid sea urchin Evechinus chloroticus with varying food ration. J. Exp. Mar. Bio. Ecol. 309, 219–237. doi: 10.1016/j.jembe.2004.03.016
Slota L. A., McClay D. R. (2018). Identification of neural transcription factors required for the differentiation of three neuronal subtypes in the sea urchin embryo. Dev. Biol. 435, 138–149. doi: 10.1016/j.ydbio.2017.12.015
Soars N., Prowse T., Byrne M. (2009). Overview of phenotypic plasticity in echinoid larvae, ‘Echinopluteus transversus’ type vs. typical echinoplutei. Mar. Ecol. Prog. Ser. 383, 113–125. doi: 10.3354/meps07848
Tarsis K., Gildor T., Morgulis M., Ben-Tabou-de-Leon S. (2022). Distinct regulatory states control the elongation of individual skeletal rods in the sea urchin embryo. Dev. Dyn. 251, 1322–1339. doi: 10.1002/DVDY.474
Taylor E., Heyland A. (2018). Thyroid Hormones Accelerate Initiation of Skeletogenesis via MAPK (ERK1/2) in Larval Sea Urchins (Strongylocentrotus purpuratus). Front. Endocrinol. (Lausanne). 9. doi: 10.3389/fendo.2018.00439
Valencia J. E., Feuda R., Mellott D. O., Burke R. D., Peter I. S. (2021). Ciliary photoreceptors in sea urchin larvae indicate pan-deuterostome cell type conservation. BMC Biol. 19, 1–15. doi: 10.1186/S12915-021-01194-Y/FIGURES/6
Valero-Gracia A., Petrone L., Oliveri P., Nilsson D.-E., Arnone M. I. (2016). Non-directional photoreceptors in the pluteus of strongylocentrotus purpuratus. Front. Ecol. Evol. 4. doi: 10.3389/FEVO.2016.00127
Wood N. J. (2020). Investigating the roles of neuropeptides in the development of the sea urchin, Strongylocentrotus purpuratus. Ph.D thesis. London: University College London. Available online at: https://discovery.ucl.ac.uk/id/eprint/10117909/ (Accessed December 18, 2023).
Yaguchi J., Sakai K., Horiuchi A., Yamamoto T., Yamashita T., Yaguchi S. (2024). Light-modulated neural control of sphincter regulation in the evolution of through-gut. Nat. Commun. 15, 8881. doi: 10.1038/s41467-024-53203-7
Keywords: phenotypic plasticity, developmental plasticity, sea urchin, sensory nervous system, light, food
Citation: Cocurullo M, Kirwan JD and Arnone MI (2025) Phenotypic response to food availability in sea urchin larvae and impact of light during development and growth. Front. Ecol. Evol. 13:1548208. doi: 10.3389/fevo.2025.1548208
Received: 19 December 2024; Accepted: 19 February 2025;
Published: 13 March 2025.
Edited by:
Shunsuke Yaguchi, University of Tsukuba, JapanReviewed by:
Pedro Martinez, University of Barcelona, SpainCopyright © 2025 Cocurullo, Kirwan and Arnone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Ina Arnone, bWlhcm5vbmVAc3puLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.