
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
POLICY AND PRACTICE REVIEWS article
Front. Ecol. Evol., 19 March 2025
Sec. Conservation and Restoration Ecology
Volume 13 - 2025 | https://doi.org/10.3389/fevo.2025.1523537
This article is part of the Research TopicProtected Area Management and Large and Medium-Sized Mammal ConservationView all 7 articles
 Basanta Kumar Das1*
Basanta Kumar Das1* Dibakar Bhakta1
Dibakar Bhakta1 Canciyal Johnson1
Canciyal Johnson1 Thangjam Nirupada Chanu1
Thangjam Nirupada Chanu1 Mitesh Ramteke1
Mitesh Ramteke1 Suraj Kumar Chauhan1
Suraj Kumar Chauhan1 Archisman Ray1
Archisman Ray1 Saurav Nandy1
Saurav Nandy1 Arghya Kunui1
Arghya Kunui1 Shreya Roy1
Shreya Roy1 Trupti Rani Mohanty1
Trupti Rani Mohanty1 Nitish Kumar Tiwari1
Nitish Kumar Tiwari1 Naba Kumar Acharjya1
Naba Kumar Acharjya1 Karmveer Singh1
Karmveer Singh1 Deependra Singh1
Deependra Singh1 Aritriya Jana1
Aritriya Jana1 Atul Kumar1
Atul Kumar1 Tania Kayal1
Tania Kayal1 Sandeep Kumar Behera2
Sandeep Kumar Behera2The Ganges River dolphin (GRD), Platanista gangetica, is one of the most endangered cetaceans in the world and is seriously in danger from dams and barrages, restricted river flows, bycatch, pollution, etc. The GRD is a freshwater dolphin, commonly known as "susu", one of the four freshwater cetacean species in the world. The GRD primarily inhabits freshwater and estuarine zones, never venturing into the sea. The present study (2022–23) conducted a seasonal survey at the Bhagirathi–Hooghly River systems of West Bengal to investigate the abundance, habitat use, and potential threats in the lower stretches of the River Ganga. During the survey we recorded 303 dolphins with higher numbers of individuals in dry season (0.47 dolphins/linear km) than in the wet season (0.29 dolphins/linear km). The study also confirmed that freshwater dolphins primarily inhabit river confluences, or tributary junctions, and river meanderings with abundant prey-fish. The rapidly declining Ganga River dolphin faces numerous potential threats, including aquatic pollution, habitat destruction, net entanglements, overfishing with destructive fishing gear, agricultural and industrial effluents, vessel collisions, sand mining, and a lack of awareness about dolphin conservation. No, or regulated, fishing in dolphin hotspot locations, and ensuring as well as maintaining enough dry season flows, are likely to help preserve dolphin numbers and reduce competition for fish with fishermen.
Globally, rivers and estuaries in Asia and South America are home to three species of freshwater dolphins: Lipotes vexillifer, Inia geoffrensis, and Platanista gangetica. These are among the six species of freshwater dolphins. The Chinese River dolphin, Lipotes vexillifer, is considered functionally extinct (Turvey et al., 2007). The two remaining species are Platanista gangetica, which includes two subspecies: Platanista gangetica minor (the Indus River dolphin) and Platanista gangetica gangetica (GRD). Inia geoffrensis is the name of the Amazon River dolphin. The remaining three species, the tucuxi (Sotalia fluviatilis) in South America, the Irrawaddy dolphin (Orcaella brevirostris) in Asia, and the finless porpoise (Neophocaena phocaenoides) in Asia, inhabit both freshwater and marine environments.
Early 1980s research estimated the number of GRDs in its distribution range to be between 5,000 and 6,000 (Jones, 1982; Reeves et al., 1993; Wakid, 2005). A few individuals survive in Karnali River in Nepal and perhaps the Sapta Kosi River. The Ganga River and its tributaries, such as the Yamuna, Chambal, Ghaghra, Gandak, Rapti, Narayani, and Kosi, are home to dolphins (Smith and Reeves, 2000; Smith et al., 2001; Braulik and Smith, 2017). Among all cetacean species, river dolphins are among the least known and most threatened (Hamilton et al., 2001).
The Indian government designated it as the “National Aquatic Animal” in 2009 (Reeves, 2009), but it currently only protects a limited portion of the Ganga River. The only one devoted to ensuring the GRD Sanctuary receives protection is the Vikramshila GRD Sanctuary, which is located close to Bhagalpur (Roy et al., 2013). The International Union for the Conservation of Nature’s (IUCN) Red List (Smith and Braulik, 2009) lists the subspecies as Endangered, with a reduced historical distribution range and projected population size declines due to increasing threats (IUCN, 1996). It is also included in Schedule I of the Indian Wildlife (Protection) Act 1972, Appendix I of the Convention on International Trade in Endangered Species (CITES), and Appendix II of the Convention on Migratory Species (CMS). The Government of India formally recognized the species as a National Aquatic Animal in May 2010, after it had gained recognition in October 2009. According to reports, the dolphin population has declined by one-third in just four generations (Paudel and Koprowski, 2020).
The causes of habitat fragmentation include the construction of dams and barrages, hunting, poaching, excessive and illegal fishing, unintentional catches in gillnets, mining for sand and stones, excessive water extraction, and the spread of agricultural activities along the banks (Sinha et al., 2000; Sinha and Sharma, 2003; Bashir et al., 2010). This poses the greatest immediate and direct threat to the subspecies, according to Mohan et al. (1997). Prakash et al. (2023a) studied the changes in bacterioplankton and zooplankton communities in response to Covid-19 lockdown at dolphin sighting in the river Ganga and observed that rotifers were dominant during pre-Covid periods, while crustacean species were dominant during the Covid-19 lockdown.
However, immediate conservation efforts are necessary to save the species population within its distribution ranges. Hon’ble Prime Minister Shri Narendra Modi has initiated a very timely and important step towards ensuring a future for dolphins in the country by announcing “Project Dolphin” on 15th August 2020. The Project Dolphin aims to include both river dolphins and marine dolphins in its conservation program. The purpose of this project is to empower stakeholders to participate in dolphin conservation and to address current conservation issues. This entails a multifaceted strategy through science-based conservation that involves the departments of forests, fisheries, and other stakeholders, as well as fishermen (Kolipakam et al., 2022a). Dolphins frequently inhabit long stretches of deep water in meanderings, confluences, and mid-channel sand bars (Smith and Reeves, 2000). The main habitats of dolphins are distinguished by an eddy counter-current system created by a point bar generated by silt deposits in the main river flow. Where eddies form, under sandbars and bridges, river dolphins are also visible (Sinha and Sharma, 2003). The Ganges River serves as a critical habitat for the GRD and represents a significant historical, economic, and cultural symbol of India. It provides diverse ecosystem services, encompassing industrial, provisioning, regulatory, and cultural functions. The introduction of COVID-19 has led to a notable improvement in air and water quality due to reduced human interference in aquatic ecosystems. The micro- to mesoplanktonic community data could help describe where dolphins come to the surface, improve current water quality monitoring efforts, and make it easier to do epidemiological studies on people who use Ganga water for recreation (Prakash et al., 2023b).
After the Farakka Barrage became operational in 1975, the GRD population in the main Ganga waterway split into two subpopulations. They can now be found in five subpopulations in India: Farakka to the Brahmaputra population and Ganga Sagar, Bijnor-Narora, Narora-Kanpur Barrage, and Kanpur Barrage-Farakka.
The GRD population has been the subject of numerous studies covering a wide range of topics, such as habitat features (Khanal et al., 2016), feeding and foraging habits (Kelkar et al., 2018), flow regimes (Choudhary et al., 2012; Khanal et al., 2016), potential threats (Dey et al., 2019), and so on. However, very little is known about the state of the lower Ganga RGD population (Mitra and Chowdhury, 2018). The current study looked at dolphin abundance and potential risks to their habitat in the Bhagirathi-Hooghly River basins in West Bengal, India.
To see the sightings and abundance of dolphins, the present study was conducted from downstream of Farakka Barrage to Kakdwip (Bhagirathi-Hooghly distributaries) of the lower stretch of the Ganga. The Bhagirathi-Hooghly Rivers are the two main distributaries of the river Ganga in India (Figure 1). River Ganga bifurcates into Bhagarathi and Padma and flows up to Tribenighat in the name of Bhagirathi (323 km, approx.) and then changes into the tidal zone with the name Hooghly River and ultimately meets the Bay of Bengal. For the estimation of the dolphin population, direct survey methods were followed as suggested by the panel of experts (Perrin and Brownell, 1989). For the direct counting of dolphins, both boat-based and land-based survey methods were followed.
This method involved simple calculations of the number of dolphins per measured area and was used to assess the sighting abundance of the dolphin population by counting the dolphins during the survey and assuming that every individual was spotted. With an inflatable rubber (25 hp)/country boat, the surveys were conducted throughout the pre-monsoon (February–May), post-monsoon (October–January), and monsoon (June–September), which are regarded as the dry and wet seasons, between 8:00 and noon and 15:00 and 17:00 during the day.
Wherever boat-based survey was done, boat maintained a steady speed of 6–8 km/h to make sure it didn’t miss any GRD sightings. Using the boat-based line-transect method, three trained observers (left, right, and front) were stationed at the front of the boat to search for sightings of GRD. The observers were outfitted with binoculars (NIKON 8 × 42), a GPS (GARMIN eTrex 30), a depth sounder (HONDEX PS7), and a range finder (HAWKE Endurance LRF-1000) (Smith and Reeves, 2000; Kreb and Budiono, 2005; Smith et al., 2006).
The study has not differentiated any sexes-adults, sub-adults, juveniles, or calves. A single dolphin count was considered, despite their size and sex. At every point, observations of dolphins and their respective probable anthropogenic threats were recorded. According to the methods described by Tosha et al. (2024), secondary data on dolphin populations and human-made threats were collected from the local fishermen, who spend a lot of time on fishing activities. The study sites were selected based on earlier studies, literature review, fishers’ community interaction, and river characteristics (such as confluences, deeper pools and channels, high fishing zones, etc.). Along with the dolphin population estimates, observations on impending threats to the dolphins and fish species abundance have also been recorded. The survey was conducted once in monsoon (wet seasons), and once in dry seasons. We conducted a Student “t” test to identify any significant differences in the dolphin population between the dry and wet seasons.
Platanista gangetica is recorded from Farakka Barrage to Naya char (Tengra char), just above Kakdwip, according to a comprehensive seasonal dolphin study undertaken in 2022–2023. The survey estimated up to 303 dolphins and dry seasons were found to have a higher abundance than wet seasons. Dolphin populations showed a significant difference between the dry and wet seasons, p<0.001 (p = 0.00037). They are normally seen in groups of 3-7 dolphins, but they can be seen in larger groups (as many as 14-17 dolphins have been reported from Balagarh locations near the confluences of the Bhagirathi and Churni at different periods) near the confluence of rivers and channels with the main river. The highest number of dolphin sightings were at the confluence of rivers and channels, as well as in the ferry ghat.
In the study area, mainly small groups (3-5 in number) of dolphins were recorded; however, at confluence sites such as Katwa (confluences of the Ajoy-Bhagirathi River), Nabadwip (Bhagirathi-Jalangi River confluence), Sabuj Dweep (Hooghly-Behula River confluence), Balagarh (Confluence of the Bhagirathi-Churni River), Kulpi (Hooghly-Haldi River confluence), etc., as many as 5–17 individuals of dolphin, were recorded. Supplementary Table S1 contains the status of dolphin populations, geographical coordinates, abundance of important fish species, impacts, and possible threats.
The study also collected fish samples, both through direct experimental fishing (gill nets with 10–60 mm mesh sizes, bag nets with 5–10 mm cod end mesh sizes, etc.) and from fishermen’s catch, which was primarily captured using various multi-gears. Most of the fish species (>75%) were small fish, with a few exceptions of larger fish (Tenulosa ilisha, Rita rita, Arius spp., sciaenids (primarily Otoithoides pama, Chrysochir aurea, etc.), Pangasisus pangasius, etc.).
Gupta (1986) conducted the first investigation on the dolphin population in the Bhagirathi-Hooghly River systems, West Bengal, based on 1978 research and recorded only nine dolphins. Though the study was mostly based on assumption, as he could only count five dolphins in the Farakka barrage area, the numbers are significantly higher, according to locals. Sinha (1997) conducted a detailed investigation of the dolphin population in the Bhagirathi-Hooghly River basins and reported as many as 151 individuals. The present estimated a total of 37 dolphins in the Farakka Feeder canal, whereas Sinha (1997) reported as many as 20 in the same canal. The increased population of dolphins in the Feeder canal could be attributed to breeding and/or migration from nearby areas.
This study confirmed that river confluences, which are generally regarded as high fish assemblage areas due to favourable hydro-biological conditions and adequate depth (Choudhary et al., 2012), were also identified as favourable dolphin microhabitats (Biswas and Boruah, 2000). Ferry ghats, where human activities are more common and food supplies are accessible for the fish in the surrounding area, are also frequent sites of dolphin sightings (Sinha, 1997).
In the Bhagirathi-Hooghly River basins, the present study findings suggested 0.29–0.47 dolphins per linear km of river length in wet, and dry seasons, respectively. Mohan et al. (1997) estimated 0.44 GRDs per km of Brahmaputra River stretches, but Basir et al. (2010) estimated 0.52 GRDs per km of river length in the upper Ganga (a 28 km stretch of Bihar). The Lohit River in eastern Assam had as few as 0.23 individuals per km (Wakid, 2005), and the Vikramshila Gangetic Dolphin Sanctuary had as many as 1.8 individuals per km (Choudhary et al., 2006). The complete fishing prohibition in the protected regions may account for the higher encounter rate at Vikramshila Gangetic Dolphin Sanctuary. For the study period 2004–2012, Sinha and Kannan (2014) revealed that dolphin group sizes ranged from 1 to 15, with an encounter rate of 0.9–1.6 dolphins/km. A comparative GRDs encounter rate studied by other researchers is provided in Table 1.
According to Sinha and Kannan (2014) investigation, small fish accounted for the bulk (74.0%) of the capture composition at the Vikramshila Gangetic Dolphin Sanctuary. Regulation of fishing intensity in dolphin hotspots helps assure the availability of prey for dolphins. Dolphin numbers, according to our data, are increasing in comparison to previous reports. The substantial decrease in large predatory fish that compete with dolphins for fish prey may be one of the reasons for river dolphin persistence in overexploited systems like the lower reaches of the Ganga.
Furthermore, Sinha (1997) observed a single river dolphin near the mouth of the Hooghly at Kakdwip ferry station. However, during the present study we did not record any river dolphins at Kakdwip or surrounding sites, which may be attributed to the gradual increase in salinity in the area as well as excessive silt deposition. There have been reports of Orcaella brevirostris sightings in Kakdwip fishing areas. Mitra and Chowdhury (2018) also stated that GRDs have disappeared from the Indian Sundarbans due to human and geo-climatic factors, despite historic evidence revealing the presence of GRDs in both the Indian and Bangladesh parts of the Sundarbans (Anderson, 1879).
Anderson first reported the GRD distribution in the 1870s (1879), and at that time, the dolphin’s distribution encompassed a large network of interconnecting river systems across southern Nepal, Bangladesh, and India. From the foothills of the Himalayas to the Sundarbans delta, the Ganga and Brahmaputra River systems, as well as other big and medium-sized tributaries, comprised their dispersion.
Dolphins are currently found in the Ganga and its tributaries, which include the Yamuna, Chambal, Ghaghra, Gandak, Rapti, Narayani, and Kosi Rivers (Sinha et al., 2000). Due to an increase in several human risks, its historical range and population size have declined (Smith et al., 2006). Less than 2000 individuals were identified in India during a survey by WWF-India and its partners that covered the whole distribution range in the Ganges and Brahmaputra River systems, which is approximately 6000 km (WWF, 2017). In the Brahmaputra valley, it stretches into the rivers Tista, Adadhar, Champamat, Manas, Bhareli, Subhansiri, Dihang, Dibang, Lohit, Disang, Dikho, and Kulsi. Dolphin populations are generally larger around river confluences or within the same river’s branches (Sinha, 1997). It flows south, passing through the major tributaries of the Hugh and Meghna rivers before arriving at the mouth of the Ganga River at low tide (Rice, 1998). In portions of their upstream habitat in Nepal and India, Gangetic dolphins have been exterminated; in the areas where they still exist, their populations have decreased and become fewer in number (Wakid, 2009; Sinha and Kannan, 2014).
GRDs inhabit the Ganges-Brahmaputra-Meghna (GBM) and Karnaphuli-Sangu River systems in Nepal, India, Bangladesh, and possibly Bhutan. They can be found from the deltas to as far up the rivers as is navigable (Sinha et al., 2000). Furthermore, river dolphins can be observed looking for eddy countercurrents beneath sandbars, around bends in the river, and in streams that converge. Ganges dolphins scatter locally during the monsoon season to floodplains and tributaries, and then during the dry winter months they return to bigger river channels (Sinha and Sharma, 2003). At a height of 250 meters above mean sea level, dolphins were observed in River Narayani, Dev Ghat, Nepal (Kasuya and Haque, 1972). According to Sinha and Kannan (2014), there are 3607 Ganges dolphins in the rivers of the GBM Basin, with 3025 of them in India, 532 in Bangladesh, and perhaps 50 in Nepal. Table 2 provides a summary of the population status of GRD in the rivers Brahmaputra and Ganga and their tributaries. Supplementary Table S2 provided worldwide other freshwater dolphins population size, distribution, treats, etc.
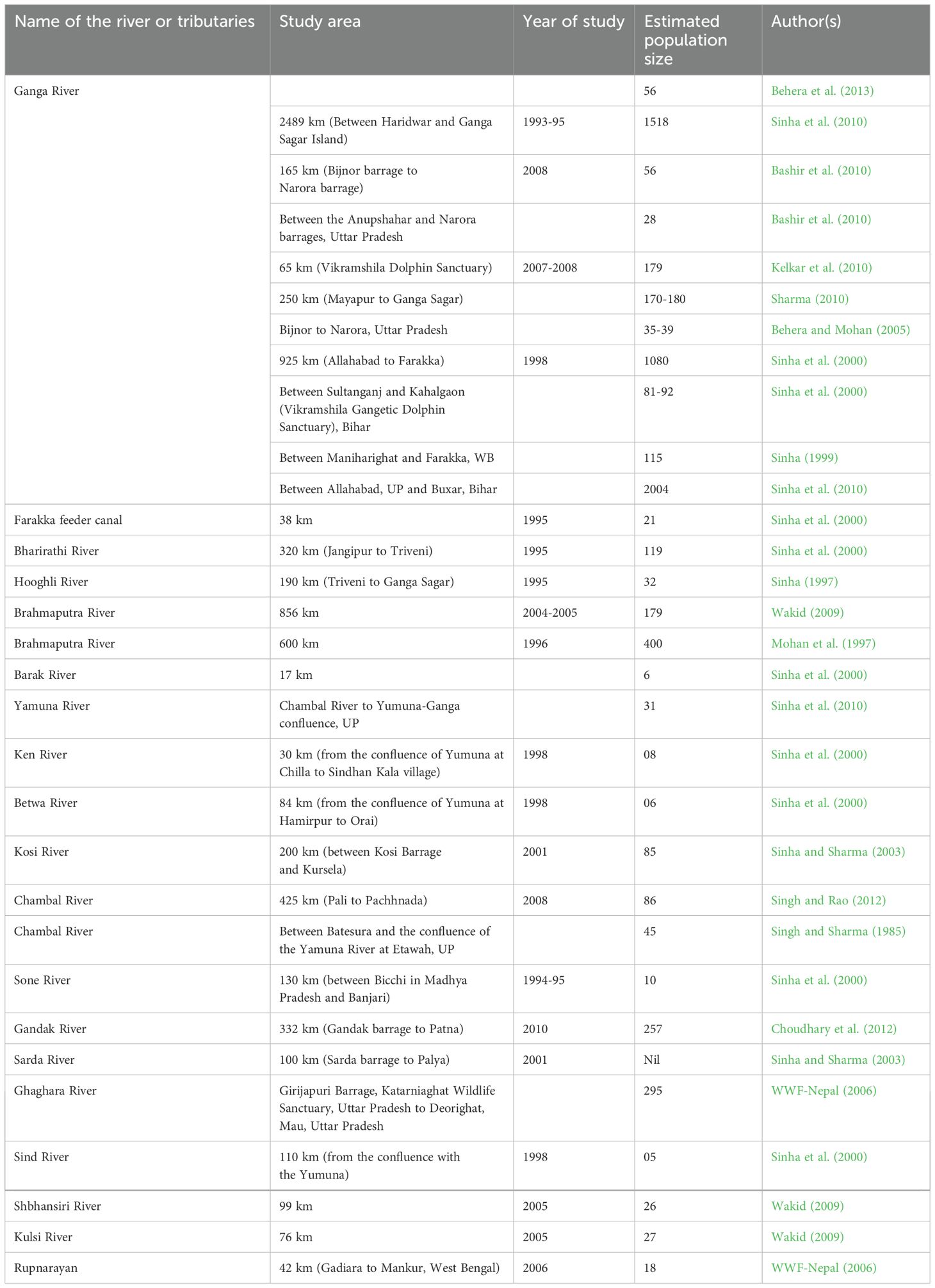
Table 2. Population status of Platanista gangetica in the rivers Brahmaputra and Ganga and their tributaries.
Human activity and a range of other threats, including unintentional capture, dam and barrage construction, vessels collisions, industrial and agricultural pollution, mining, noise pollution from vessel movement, deforestation, which causes significant siltation, and competing demands for freshwater for irrigation, are the main causes of the GRD endangered status. Rajan et al. (2023a) observed that the effects of Covid-19 led to improvements in the flow regime, water quality, and overall restoration of the river Ganga. Zooplankton community structure is the most prevalent indicator of aquatic ecosystem restoration. The study indicates that during the lockdown, dolphin sighting locations at the selected sites migrated towards the riverbank, while the zooplankton community structure transitioned to favour Cladocera without impacting overall species richness. Prakash et al. (2023b) investigated ecosystem variability along the salinity gradients of the Hooghly River estuary. They found that changes in the concentrations of zooplankton, phytoplankton, and bacterioplankton over time and space clearly show how complex and changing the estuary is.
Most of the riparian inhabitants of the lower Gangetic areas rely heavily on the rivers for their livelihoods, and fishing is one of their primary sources of income. The human population explosion has inflated this. GRDs compete fiercely with people for resources such as fish and freshwater. They favour the same environments as fishermen, who both looks for the same fish (Choudhury et al., 2019). As a result, accidental killings occur in fishing gear. A broad variety of fishing gears are employed throughout the year in the lower stretch of the Ganga, some of which are selective for a certain species, but most of them are multi-species gears. Bag nets (stationary), drift gill nets, seine nets, set-barrier nets, purse nets, trawl nets, cast nets, lift lets, hooks and lines, various types of traps, and so on are often used fishing gear (Mitra et al., 1997; Bhakta and Das, 2021). Because most of these fishing gears possess smaller mesh sizes and are not particular to any fish species, or size. As a result, these gears catch any fish that come in their path, even dolphins, resulting in the decline of fish species. The current investigation further revealed the incidental killing of dolphins by monofilament gill nets in heavily fished areas such as Godakhali and Falta of the Hooghly River (Figure 2). Accidental killing of dolphins as by-catch by various fishing gear has also been reported to be one of the most serious threats to GRDs in the Brahmaputra River (Bordoloi and Saharia, 2021).

Figure 2. Incidental killing of dolphin by monofilament gill at Godakhali (photo source: local fishermen).
The current study found no evidence of dolphins being killed directly for food, fuel, or any other reason, especially in the lower Ganga. Though it is believed to have declined in most areas, there are still occasional cases of direct or intentional killing of Ganges dolphins for their meat and oil, which is used as a fish attractant, in the upper reaches of the Brahmaputra River in Assam (Mohan et al., 1997) and the Ganges near Patna (Sinha, 2002; Sinha and Kannan, 2014). Local fishermen said that they understand dolphins’ fragility in the riverine system and that capture or death is illegal. Further, Tosha et al. (2024) mentioned that traditional local fishers of the river Ganges balance their livelihoods as well as conserve the dolphins.
Within their range, GRDs need sufficient water flow and quality; these are the essential elements of a suitable habitat that the animals need to maintain their physical well-being, mobility, and ability to effectively forage for food (Sinha and Kannan, 2014). According to several studies (Braulik et al., 2014), habitat fragmentation poses the greatest danger to South Asian GRD populations. This is a significant threat to the Indus River dolphin in the Indus River basin (Braulik et al., 2014) and one of the main causes of the extinction of the Chinese river dolphin from the Yangtze River (Turvey et al., 2007). The Ganges dolphin population has been impacted by the Farakka Barrage.
Since the Farakka barrage has lowered and regulated water flow, water depth downstream has dropped in numerous places and sand bars have restricted dolphin migration. According to Reeves and Leatherwood (1994), barrages diminish or eliminate downstream water discharge in many wild rivers, regenerate floodplains, and contribute to meandering. As water discharges from Farakka barrage with minimal sediment load, fewer dolphins (0.3 dolphin/linear km) have been reported from Farakka barrage to Katwa at Bhagirathi River, reducing the physiographic and hydrologic complexity of that area (Sinha, 1997). The presence of more dolphins and aquatic life downstream of Katwa indicates that the Bhagirathi River has greater hydrophysiographic complexity (Sinha, 1997). Dams and barrages may affect prey downstream due to variations in sediment transport and flow rate (Reeves and Leatherwood, 1994). The dolphin from the Dhaleswari River, a tributary of the Barak River in Assam, has gone extinct because of the construction of a sluice gate, the water’s diversion into an artificial channel (now called the Katakhal River), and the consequent decrease in flow, depth, and width.
The Indus River dolphin (Platanista gangetica minor) loses at least half of its historical range due to dam development, and most of its yearly flow is redirected into canals in Pakistan. The baiji (Lipotes vexillifer), which is already endangered, was negatively impacted by the construction of large dams along the Yangtze River system, including the Ghezouba Dam and the Three Gorges project (Reeves and Leatherwood, 1994). The freshwater habitats of the boto or Amazon River dolphin (Inia geoffrensis) are directly impacted by deforestation, which leads to widespread extinctions of fauna and plants (Mintzer et al., 2013).
In terms of habitat destruction, sand mining was discovered to be a frequent practice along various parts of the Bhagirathi-Hooghly River system. Sand mining has a direct impact on dolphin populations as well as disrupting the ecology of river systems and destroying counter-currents (Sinha et al., 2010). Sand mining has profound effects on dolphins since the presence of a counter-current is required for adequate dolphin habitat (Choudhury et al., 2019). Sand mining has been identified as the largest hazard to dolphins in the river Kulsi (Wakid and Braulik, 2009).
Furthermore, deforestation has resulted in the siltation of riverbeds, diminishing their depth and counter-currents, and destroying appropriate dolphin habitats (Choudhury et al., 2019). Water pollution, according to Smith and Reeves (2000), has an impact on aquatic ecosystems by diminishing productivity, depleting dissolved oxygen, raising biochemical oxygen demand, and ultimately leading to a loss in aquatic biodiversity and prey fish. Rajan et al. (2023b) examined Hooghly River Estuary surface water microplastic contamination over a period. Surface water samples from the Hooghly River Estuary were contaminated with microplastics, and city stations had the highest concentrations. Microplastics are also more likely to enter the biotic compartment directly or indirectly depending on grazers’ eating patterns and foraging strategies, including dolphins (Rajan et al., 2023c; Yadav et al., 2024).
Kannan et al. (1997) state that siltation, river resource extraction (such as stone mining), noise pollution, chemical pollution in rivers from both point and non-point sources, and habitat loss or degradation due to unsustainable abstraction of river water for irrigation and other uses are the main threats to the Ganges dolphin throughout its entire distribution range.
The lower estuarine part of the Ganga, commonly known as the Hooghly estuary, is one of the most diverse and productive estuarine systems in the world (Bhakta et al., 2020 and 2022). There are 172 fish species recorded from the Hooghly-Matlah estuary, with 73 in the freshwater zone and 99 in the saline zone (Ayyappan et al., 2019). The estuary system’s yearly average fish and prawn production increased from 3204 t in 1960–63 to 61194 t in 1998–99 and 117639 t in 2010–11 (CIFRI, 2021; Ayyappan et al., 2019). Because of the estuary zone’s high potential, many fisher populations engaged in fishing activities, and most of the time, such types of fishing activities were thought to be damaging. The Hooghly-Matlah estuary systems are also a potential source of wild prawns and fish seeds for brackish water aquaculture stocking materials. Local fishers (mostly women) collect prawn post-larvae (Penaeus monodon, P. indicus, etc.) as well as fish seeds (Chelon parsia, Planiliza tade, Lates calcarifer, Mystus gulio, etc.), which not only reduces the potential production of those targeted species but also hinders overall fish production (Bhakta and Das, 2021). Dolphins eat primarily fish and prawns, and they tend to congregate near fish-rich areas. Intense fishing by fishermen located near ideal dolphin habitats using a variety of nets has also had a direct impact on the dolphin population due to a lack of food because dolphins serve as top predators feeding on a variety of fish.
Intense overfishing by different types of gill nets was also found to be a potential threat to the dolphin population of the Barak River, Assam, as it directly affects the dolphin population through a shortage of fish food (Mazumder et al., 2014; Bordoloi and Saharia, 2021). Reduced numbers of large fish caught, a decline in selective fishing, and a decrease in mesh size are indicators of overfishing (Shin et al., 2005).
Motorized boats, trawlers, barge trafficking, and manually powered boats are popular in the Bhagirathi-Hooghly River system, which is primarily used for fishing, transportation, sand mining, and other purposes. The accidental killing of dolphins by vessel collisions has also been reported at the lower stretch of the Ganga (Figure 3). Underwater noise from vessels, as stated by Dey et al. (2019), can harm endangered GRD, which are “nearly blind” and rely exclusively on high-frequency echolocation sounds to understand their environment. They also stated that, in addition to vessel noise, and vessel movement, shallow water depth harmed dolphin movement in the middle stretch of the Ganga River. Motorboats were first used for transportation in the 1980s, and according to Sinha (1997), they produce a loud, high-frequency noise that impedes dolphin navigation. Human involvement and high river traffic have been found to have detrimental correlations with dolphin presence in riverine systems (Bashir et al., 2013).

Figure 3. Accidental killing of river dolphins with the collision of vessels at Falta (photo source: local fishermen).
Dolphin motions have been shown to have a significant impact on the path taken by motorboats, manually propelled boats, and massive bamboo rafts as they traverse the rivers in the Barak River system (Choudhury et al., 2019). Moreover, dolphins are known to avoid high-noise environments, and these areas have been connected to changes in dolphin behavior, including surfacing rate (Kelkar, 2017). Due to their heavy reliance on bottom species, dolphins are especially vulnerable to the negative effects of river dredging on river ecology, as it eliminates bottom fauna (Chaudhury et al., 2019). Moreover, damming, and extensive waterway construction, along with the dredging that went along with it, were some of the main causes of the Chinese river dolphin’s disappearance (Turvey et al., 2007). In addition, the dolphin gives birth to a single calf following a 9-10-month gestation period when it reaches reproductive maturity at the age of 10 (Kasuya and Haque, 1972). Consequently, the dolphin breeds slowly, which hinders population expansion even more.
Measures to control overfishing and reduce bycatch yield socioeconomic benefits for local fisherfolk by promoting the sustainability of fish stocks over the long term. The establishment of dolphin-watching tours generates alternative revenue sources for local communities. Additionally, restoring river health enhances water quality, which is advantageous for agricultural, and household uses. Numerous studies indicate that community participation in conservation improves skills and generates employment via programs such as river patrols and citizen science initiatives. The preservation of the Gangetic Dolphin contributes to cultural identity and creates opportunities for educational outreach, thus promoting pride and awareness among local communities (Tosha et al., 2024).
Restrictions on fishing and other riverine activities can result in immediate economic losses for communities dependent on these practices. The unequal distribution of conservation benefits can intensify socioeconomic inequalities, especially among marginalized groups (Kelkar et al., 2010). The local fisherfolk participated in the establishment of the Vikramshila Dolphin Sanctuary, which facilitated dolphin monitoring and generated alternative income sources through ecotourism. The establishment of the sanctuary led to increased dolphin populations and improved livelihoods for the community. Future research must prioritize the quantification of socio-economic impacts, and the refinement of strategies aimed at maximizing ecological and community benefits.
Dolphin habitats are very important, as evidenced by the prevalence of sightings in river segments with relatively uncommon physiographic and hydrologic characteristics and the aggregate nature of consumption patterns that depend on specific reach conditions. On the other hand, the variable elements of a single river reach are only a picture of the variety of interrelated processes and events that make up the greater river basin environment (Smith et al., 1994). Therefore, the maintenance of habitat site conditions and basin-wide resource management should be the main goals of conservation efforts. For successful approaches to aid in the recovery of depleted populations and ensure that healthy populations with high-quality habitats stay secure, they must be multifaceted, adaptive, and adapted to specific local or regional conditions (Aggarwal et al., 2020). The Bhagirathi-Hooghly River dolphin population can be saved for a long time if the following conservation strategies are implemented:
• The banning of detrimental fishing methods as well as the development and promotion of alternative fishing methods
• Reduce unintentional death by promoting rescue and release and providing enough knowledge to local fishermen through skill development programs
• Managing planned dolphin-focused tourism, which has the potential to provide a steady source of income for fishermen and communities
• Restoring and conserving habitat through law enforcement
• Investigating the biological aspects of Ganges dolphins and lowering river pollution levels
The Ganges and Indus River dolphins, known as “susu” and “bhulan”, respectively, were once classified as different species but are now recognized as subspecies of Platanista gangetica. One of the most serious threats to coastal and riverine habitats is human population growth. As a result, estuaries are frequently converted into industrial harbours; wetlands are drained for agricultural or tourism purposes; and coastal waterways are contaminated with a variety of pollutants. Priorities for conservation must be established since India is one of the only countries where GRD still have healthy populations. The dolphins require sufficient protection. By placing it in Schedule I of the Wildlife (Protection) Act (1972), which prohibits hunting, the Indian government has given the dolphin the strongest legal protection since the act’s establishment.” To protecting dolphins in the Bhagirathi-Hooghly River systems, the following suggestions (Figure 4) have been made:
• Law enforcement to prevent the by-catch and incidental catch of dolphins
• Ensuring adequate water flow and water quality
• Dolphin surveys are conducted regularly
Time series monitoring of dolphin surveys should be conducted on the entire distribution ranges in the Ganga basin to determine the status so that appropriate management plans can be implemented.
The survival of the dolphins is at risk because of this change in gear usage, which has reduced the amount of prey and by-catch. Therefore, the establishment of community fisheries in water bodies connected to rivers is necessary to improve community well-being. In the long run, good use and management of water bodies connected to rivers, such as wetlands, will raise people’s socioeconomic status and lessen their need for rivers.
Fisheries that are becoming less abundant may not be as stressed by alternative revenue streams like cooperatively managed aquaculture or ecotourism. By enabling fishermen to use their innate ecological knowledge and fishing skills, these eco-management programs can help them financially. Recognizing and protecting fishermen’s tenure rights is necessary to provide an economic safety net against the depleting resource base and prevent their marginalization. For those who depend on protected areas for their resources, programs such as the National Rural Job Guarantee Scheme and the Pradhan Mantri Matsya Sampada Yojana (PMMSY) can help establish alternative job opportunities. Tosha et al. (2024) recommended the well-being of the fishers’ community of the River Ganga in relation to dolphin conservation, as follows:
• Local fishers reliant on the Ganga River should participate in the planning of any development initiatives
• Fishers must be cognisant of natural resources, including fish, and the subsequent impacts of exotic species
• The application of harmful chemicals in agricultural fields adjacent to riverbeds, along with the mass killing of fish, necessitates stringent monitoring
• The wetlands associated with the Ganga River require restoration to conserve small fish species, and the conversion of these wetlands to agricultural land should be prohibited
Toxic substances such as heavy metals, organochlorines, etc. have been found in dolphin and other animal tissues. There is an urgent need to prevent such harmful substances from entering river systems. To address the ecological or other challenges facing all higher vertebrate species and undertake conservation and management initiatives, scientific information must be collected and maintained.
River ranching of fish species is one of the managerial techniques to preserve the native germplasm; it involves artificially breeding wild fish stocks with nursery management and releasing the fish in the same environment. GRDs eat mostly fish and shrimp or prawns, according to data from multiple sources (Anderson, 1879; Choudhary et al., 2006). The diet consumed by dolphins was found to be dominated by small prey items (20–30 cm) with low body depth rather than fish species (Kelkar et al., 2018). According to reports, smaller dolphins consume between 0.6 and 1.8 kg of fish per day, whereas larger dolphins consume between 1.3 and 3 kg (Gihr et al., 1972). Intake is highest during the rainy season and lowest during the winter. In a 28 km stretch of the upper Ganges River between the Narora Barrage and the Anupshahar Bridge (Bashir et al., 2010), studied the abundance and prey availability assessment of GRDs. They reported 16 fish species with a length of 3.5–20 cm as the most preferred fish prey for the dolphins. Only fish in good physical condition were consumed; weak or damaged fish were not. Additionally, Gangetic dolphins are said to regularly consume river clupeids (Choudhary et al., 2006).
According to Anderson (1879) and Mohan et al. (1997), habitat degradation and fish stock depletion are the two major risks facing GRDs today. Due to ongoing ecological degradation throughout the entire basin, the Ganga River’s native fish species composition has drastically decreased recently and altered more in favour of minor carp and other species (Vass et al., 2010). The most effective method for restoring decreased fish populations in natural environments is thought to be river-ranching with indigenous fish species (Das et al., 2020). To increase the IMC populations (Labeo catla, Labeo rohita, Cirrhinus mrigala, and Labeo calbasu) while preserving the native germplasm, about 30 lakh fingerlings were ranched at various sites along the Ganga River from 2017 to 2020 by ICAR-CIFRI under the “NMCG” flagship programs (Das et al., 2020), and till May 2023, the ranched fish numbers reached 87 lakhs (CIFRI Website, 2023) (Figure 5). To lower the likelihood of natural death, the seeds of these species were raised to a size of more than 100 mm before ranching.

Figure 5. River ranching is a regular practice by ICAR-CIFRI as a part of fish stock enhancement under the NMCG flagship program (photo source: ICAR-CIFRI).
Indigenous fish species harvested from rivers not only improve fish stocks and the livelihood of the river’s dependent fish population but also provide prey for dolphins, which ultimately helps to lessen conflicts between fishermen and dolphins.
Wherever possible, satellite transmitters should be used to tag GRDs, which will provide vital information about their habitats, movements, and behaviour that will aid in long-term conservation. Additionally, dolphins are prevented from becoming bycatch due to electronic pingers that are linked to fishing nets. Such electronic pingers, which may be affixed to the canal gate pillars, perturb dolphins, which, in concept, causes dolphins to avoid these pinger locations and thereby avoid the risky canals.
Recently, WWF India successfully conducted demonstrations of these electronic pingers on local fishermen’s nets at lower portions of the Ganges River. If the trial is a success, it may help protect dolphins from the dangers of entanglements in fishing nets and associated bycatch. To better understand their movements, behaviours, and habitats, WWF safely fitted three Indus River dolphins with satellite transmitters at the beginning of 2022. Kolipakam et al. (2022b) demonstrated that the use of pingers in fishing nets was particularly efficient in minimizing net entanglement of GRDs in the Guwahati region of the river Brahmaputra. They also stated that visual examination revealed that the attachment of pingers in the nets reduced non-calf encounters by 52% and calf encounters by 9%.
The WWF (2023) established community fishery management zones and, with the assistance of partners, deployed the pinger device in the Indonesian river Mahakam to increase the use of sustainable fishing methods and prevent dolphins from accidentally being caught in nets and other equipment. The pingers prevent dolphins from unintentionally becoming tangled in fishing nets by being fastened to them. They also assist local fishermen by preserving their nets and frequently increasing fish catches. These initiatives, together with continued river guard oversight, assist in preserving present population numbers and fostering constructive neighbourhood relations.
Locals should be made aware of the rules in existence and encouraged to release any dolphins entangled in their nets as soon as possible. This is especially true of fishermen. A focused and comprehensive education and awareness campaign aimed at the various socioeconomic strata in the basin area ought to be initiated to promote a deeper comprehension of the importance and function of these species in the functioning of the river ecosystem (Figure 6). Training of local fishermen or volunteers on the importance of dolphins in aquatic ecosystems, as well as a skill-oriented training program on rescuing dolphins that have become entangled in fishing nets, collided with fishing boats or other vessels, or become stranded in shallow water, including canals, etc. The rescue of a dolphin calf by local fishermen in the Katwa section of the Ganga after it became trapped in shallow waters is another example of such awareness and training programs (Figure 7).

Figure 6. Awareness programs on dolphin conservation along with hilsa and fish conservation are being practised at the whole stretches of the Ganga (photo source: ICAR-CIFRI).

Figure 7. Releasing of dolphin calf stranded in the shallow waters by the local fishers (photo source: local fishermen from Katwa).
Local fishermen may play a crucial role in dolphin conservation and sanctuary management if they are given incentives to monitor and control their fishing operations. Dolphins and fisheries can both be saved through sustained restoration efforts. Instead of eradicating local fisheries, river dolphin conservation may help to restore them. Table 3 provides a summary table on potential threats and recommendations for the GRDs.
The GRD population has drastically decreased because of pollution, increased traffic in water bodies, river flow control, and fragmentation. Several barrages, dams, and irrigation canal water diversions have drastically changed natural flow patterns, especially during the low-flow dry season. As top predators in their river habitats, Ganges dolphins maintain the food chain and the equilibrium of the freshwater ecology in the river. It is believed that the survival of fish and crustaceans depends on their healthy population.
Disturbances and changes in flow will impact the accessibility, migration patterns, and behaviour of dolphins. The Ganga basin must legally enforce appropriate flow releases and biologically significant river flows for both policy and execution purposes. Therefore, we should prioritise protecting the shallow and deepwater habitats of dolphins in their most suitable locations. Along with education and awareness among locals and fishers, community involvement in conservation efforts is also desperately needed.
Written informed consent was obtained from the individual(s) for the publication of any identifiable images or data included in this article.
BD: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. DB: Data curation, Formal analysis, Visualization, Writing – original draft. CJ: Formal analysis, Writing – review & editing. TC: Data curation, Writing – review & editing. MR: Formal analysis, Writing – review & editing. SC: Data curation, Writing – review & editing. AR: Data curation, Writing – review & editing. SN: Data curation, Writing – review & editing. ArK: Data curation, Writing – review & editing. SR: Writing – review & editing. TM: Writing – review & editing. NT: Writing – review & editing. NA: Data curation, Writing – review & editing. KS: Data curation, Writing – review & editing. DS: Data curation, Writing – review & editing. AJ: Data curation, Writing – review & editing. AtK: Data curation, Writing – review & editing. TK: Visualization, Writing – review & editing. SB: Funding acquisition, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. The National Mission for Clean Ganga (NMCG), Ministry of Jal Shakti, Government of India funded the study. The authors gratefully acknowledge the financial support offered by the National Mission for Clean Ganga (NMCG), Ministry of Jal Shakti, Govt. of India.
The authors are also grateful to all the local fishermen, who aided in experimental fishing and shared their catch along with vital information.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1523537/full#supplementary-material
Aggarwal D., Kumar N., Dutta V. (2020). Impact on endangered Gangetic dolphins due to construction of waterways on the river Ganga, India: an overview. Environ. Sustainability 3, 123–138. doi: 10.1007/s42398-020-00104-2
Alam S. M. I., Hossain M. M., Baki M. A., Bhouiyan N. A. (2015). Status of ganges dolphin, platanista gangetica gangetica (Roxburg) in the river buriganga, dhaka. Bangladesh J. Zoology 43 (1), 109–120.
Alam S. M. I., Sarker N. J. (2012). Status and distribution of the gangetic dolphin, platanista gangetica gangetica (Roxburg) in river buriganga during 2003-2004 and its conservation. Bangladesh J. Zoology 40 (1), 21–31.
Anderson J. (1879). Anatomical and zoological researches comparison an account of the zoological results of the two expeditions to Western Yannan in 1868 and 1875 and a monograph of the two Cetacean genera, Platanista and Orcella Vol. 1 (London). doi: 10.5962/bhl.title.50434
Ayyappan S., Moza U., Gopalakrishnan A., Meenakumari B., Jena J. K., Pandey A. K. (2019). “Handbook of fisheries and aquaculture,” in Directorate of knowledge management in agriculture (ICAR, New Delhi), 1116.
Bashir T., Khan A., Behera S. K., Gautam P. (2013). Time dependent activity pattern of Ganges River Dolphin Platanista gangetica gangetica and its response to human presence in Upper Ganges River, India. Mamm. Study 38 , 9–17. doi: 10.3106/041.038.0110
Bashir T., Khan A., Gautam P., Behera S. K. (2010). Abundance and prey availability assessment of Ganges river dolphin (Platanista gangetica gangetica) in a stretch of upper Ganges River, India. Aquat. Mammals 36, 19. doi: 10.1578/AM.36.1.2010.19
Behera S., Mohan S. (2005). Conservation of ganges river dolphin in upper ganga river (New Delhi: WWF-India).
Behera S. K., Singh H., Sagar V. (2013). Status of Ganges River dolphin (Platanista gangetica gangetica) in the Ganga River basin, India: A review. Aquat. Ecosystem Health Manage. 16, 425–432. doi: 10.1080/14634988.2013.845069
Bhakta D., Das S. K. (2021). Sciaenid species diversity and associated gears at in Hooghly-Matlah Estuary of West Bengal, India. J. Mar. Biol. Ass. India 63, 56–61. doi: 10.6024/jmbai.2021.63.1.2230-08
Bhakta D., Das S. K., Das B. K., Nagesh T. S., Behera B. K. (2022). Fishery and population dynamics of Otolithoides pama (Hamilton 1822) from hooghly-matlah estuary of west bengal, India. Aquat. Ecosystem Health Manage. 25, 36–43. doi: 10.14321/aehm.025.02.36
Bhakta D., Das S. K., Das B. K., Nagesh T. S., Samanta R. (2020). Growth, mortality and exploitation status of Otolithoides pama (Hamilton 1822) from hooghly-matlah estuary of west bengal, India. Regional Stud. Mar. Sci. 39, 101451. doi: 10.1016/j.rsma.2020.101451
Biswas S. P., Boruah S. (2000). Ecology of river dolphin (Platanista gangetica) in the Upper Brahmaputra. Hydrobiology 430, 97–111. doi: 10.1023/A:1004077215276
Bordoloi B., Saharia S. (2021). Current status of the endangered ganges river dolphin (Platanista gangetica), the aquatic megafauna in the brahmaputra river system. Curr. World Environ. 16, 600. doi: 10.12944/CWE
Braulik G. T., Arshad M., Noureen U., Northridge S. P. (2014). Habitat Fragmentation and Species Extirpation in Freshwater Ecosystems; causes of range decline ofthe Indus River Dolphin (Platanista gangetica minor). PloS One 9 , e101657. doi: 10.1371/journal.pone.0101657
Braulik G. T., Smith B. D. (2017). Platanista gangetica. IUCN Red List Threatened Species, e.T41758A50383612. doi: 10.2305/IUCN.UK.20173.RLTS.T41758A50383612.en
Choudhary S., Dey S., Dey S., Sagar V., Naird T., Kelkar N. (2012). River dolphin distribution in regulated river systems: implications for dry-season flow regimes in the Gangetic basin. Aquat. Conservation: Mar. Freshw. Ecosyst. 22, 11–25. doi: 10.1002/aqc.1240
Choudhary S. K., Smith B. D., Dey S., Dey S., Prakash S. (2006). Conservation and biomonitoring in the vikramshila gangetic dolphin sanctuary, bihar, India. Oryx 40, 189–197. doi: 10.1017/S0030605306000664
Choudhury N. B., Mazumder M. K., Chakravarty H., Choudhury A. S., Boro F., Choudhury I. B. (2019). The endangered ganges river dolphin heads towards local extinction in the barak river system of assam, india: A plea for conservation. Mamm. Biol. 95 (1), 102–111.
CIFRI Website (2023). Available at: http://www.cifri.res.in/art644.html.
Das B. K., Swain H. S., Ramteke M. H., Meena D. K., Sahoo A. K., Manna R. K., et al. (2020). Ranching in river Ganga: A protocol of practices for indigenous carps germplasm enhancement and conservation in river ranching in river Ganga: A protocol of practices for indigenous carps germplasm enhancement and conservation in river. J. Inland Fisheries Soc. India 52, 106–109. doi: 10.47780/jifsi.52.1.2020.10655
Dey M., Krishnaswamy J., Morisaka T., Kelkar N. (2019). Interacting effects of vessel noise and shallow river depth elevate metabolic stress in Ganges river dolphins. Sci. Rep. 9, 1–13. doi: 10.1038/s41598-019-51664-1
Gihr M., Kraus C., Pilleri G. (1972). Meteorological influences on the daily feeding rate of the Indus dolphin, Platanista indi, in captivity. Investigations Cetacea 4, 33–43.
Gupta P. D. (1986). “the Gangetic dolphin, Platinista gangetica (Lebeck 1801),” in Wildlife wealth of India (Resources and managements). Ed. Majupuria T. C. (Teepress Service, L.P. Bangkok), 553–562.
Hamilton H., Caballero S., Collins A. G., Brownell R. L. Jr (2001). Evolution of river dolphins. Proc. R Soc. Lond Ser. B Biol. Sci. 268, 549–556. doi: 10.1098/rspb.2000.1385
Jones S. (1982). The present status of the Gangetic susu Platanista gangetica (Roxburgh), with comments on the Indus susu P. minor Owen. FAO Advisory Committee on Marine Resources Research Working Party on Marine Mammals. FAO Fish Set 5, 97–115.
Kannan K., Senthilkumar K., Sinha R. K. (1997). Sources and accumulation of butyltin compounds in Ganges river dolphin, Platanista gangetica. Appl. Organometallic Chem. 11, 223–230. doi: 10.1002/(SICI)1099-0739(199703)11:3<223::AID-AOC543>3.0.CO;2-U
Kasuya T., Haque A. K. M. A. (1972). Some information on the distribution and seasonal movement of the Ganges dolphin. Sci. Rep. 24, 109–115.
Kelkar N. (2017). A River Dolphin’s Ear-view of India’s Waterways development plans. Sanctuary Asia XXXVII 2, 58–61.
Kelkar N., Dey S., Deshpande K., Choudhary S. K., Dey S., Morisaka T. (2018). Foraging and feeding ecology of Platanista: an integrative review. Mammal Rev. 48, 194–208. doi: 10.1111/mam.2018.48.issue-3
Kelkar N., Krishnaswamy J., Choudhary S., Sutaria D. (2010). Coexistence of fisheries with river dolphin conservation. Conser. Bio. 10, 1523–1739. doi: 10.1111/j.1523-1739.2010.01467.x
Khanal G., Suryawanshi K. R., Awasthi K. D., Dhakal M., Subedi N., Nath D., et al. (2016). Irrigation demands aggravate fishing threats to river dolphins in Nepal. Biol. Conserv. 1, 386–393. doi: 10.1016/j.biocon.2016.10.026
Kibria M. M., Siam M. H., Owaresat J. K., Khan A. R., Al Asek A., Nahian S. M. A. (2023). Current status of ganges river dolphin (Platanista gangetica) in halda river, chittagong, Bangladesh. Asian J. Conserv. Biol. 12, 27–34. doi: 10.53562/ajcb.77516
Kolipakam V., Dobriyal P., Badola S., Sah R., Das G. C., Kanwar G., et al. (2022a). A comprehensive action plan for Project Dolphin, 2022–2047. Available online at: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.riverdolphins.org/wp-content/uploads/2024/07/Project-Dolphin_CAP.pdf (Accessed 14 March 2025).
Kolipakam V., Jacob M., Gayathri A., Deori S., Sarma H., Tasfia S. T., et al. (2022b). Pingers are effective in reducing net entanglement of river dolphins. Sci. Rep. 12, 9382. doi: 10.1038/s41598-022-12670-y
Kreb D., Budiono (2005). Conservation management of small core areas: Key to survival of critically endangered population of Irrawaddy River dolphin Orcaella brevirostris in Indonesia. Oryx 39, 178–188. doi: 10.1017/S0030605305000426
Mazumder F., Boro M. K., Barbhuiya B., Singha U. (2014). A study of the winter congregation sites of the Gangetic River Dolphin in southern Assam, India, with reference to conservation. Global Ecol. Conserv. 2, 359–366. doi: 10.1016/j.gecco.2014.09.004
Mintzer V. J., Martin A. R., da Silva V. M., Barbour A. B., Lorenzen K., Frazer T. K. (2013). Effect of illegal harvest on apparent survival of Amazon River dolphins (Inia geoffrensis). Biol. Conserv. 158, 280–286. doi: 10.1016/j.biocon.2012.10.006
Mitra P. M., Karmakar H. C., Sinha M., Ghosh A., Saigal B. N. (1997). Fisheries of the Hooghly-Matlah estuarine system-An appraisal. ICAR-CIFRI Bull No. 67. ICAR-CIFRI, 49 p.
Mitra S., Chowdhury M. R. (2018). Possible range decline of Ganges River Dolphin Platanista gangetica (Mammalia: Cetartiodactyla: Platanistidae) in Indian Sundarban. J. Threatened Taxa 10, 12738–12748. doi: 10.11609/jott.3746.10.13.12738-12748
Mohan R. S. L., Dey S. C., Bairagi S. P., Roy S. (1997). On a survey of Ganges River dolphins Platanista gangetica of Brahmaputra River, Assam. J. Bombay Natural History Soc. 94, 483–495.
Paudel S., Koprowski J. L. (2020). Factors affecting the persistence of endangered ganges river dolphins (Platanista gangetica gangetica). Ecol. Evol. 10 (6), 3138–3148.
Perrin W. F., Brownell R. L. Jr. (1989). “Report of the workshop,” in Biology and conservation of the river dolphins. Eds. Perrin W. F., Brownell R. L., Zhou K., Liu J. (Gland , Switzerland: Occasional papers of the IUCN Species Survival Commission No. 3. IUCN), 1–21.
Prakash D., Dhanker R., Kumar R. (2023a). Changes in bacterioplankton and zooplankton communities in response to Covid-19 forced lockdown at dolphin surfacing sites in the River Ganga. Aquat. Ecosystem Health Manage. 26, 9–19. doi: 10.14321/aehm.026.01.09
Prakash D., Tiwary C. B., Kumar R. (2023b). Ecosystem variability along the estuarine salinity gradient: A case study of Hooghly River Estuary, West Bengal, India. J. Mar. Sci. Eng. 11, 88. doi: 10.3390/jmse11010088
Rajan K., Khudsar F. A., Kumar R. (2023a). COVID-19 lockdown affects zooplankton community structure in dolphin appearing site of the River Ganga at Patna. Aquat. Ecosystem Health Manage. 26, 20–31. doi: 10.14321/aehm.026.01.20
Rajan K., Khudsar F. A., Kumar R. (2023b). Spatio-temporal patterns of microplastic contamination in surface waters of Hooghly River Estuary: Causes and consequences. Regional Stud. Mar. Sci. 65, 103111. doi: 10.1016/j.rsma.2023.103111
Rajan K., Khudsar F. A., Kumar R. (2023c). Urbanization and population resources affect microplastic concentration in surface water of the River Ganga. J. Hazardous Materials Adv. 11, 100342. doi: 10.1016/j.hazadv.2023.100342
Reeves R. R. (2009). Ganges dolphin declared national aquatic animal by government of India: A great step forward. ZOOS’ PRINT. 11, 1.
Reeves R. R., Leatherwood S., Mohan R. S. L. (1993). “Report from a seminar on the conservation of river dolphins of the Indian subcontinent. 18-19 aug.1992, new delhi, India,” in Bath avon. Whale and dolphin conservation society(United Kingdom).
Rice D. W. (1998). “Marine mammals of the world. Systematic and distribution,” in Marin. Mamm. Soc (Special Publication, 1231).
Roy S. P., Roy R., Prabhakar A. K., Pandey A., Kumar R., Tseng L. C. (2013). Spatio-temporal distribution and community structure of zooplankton in the Gangetic Dolphin Sanctuar. Aquat. Ecosystem Health Manage. 16, 374–384. doi: 10.1080/14634988.2013.857564
Sharma G. (2010). Current status of susu (Platanista gangetica gangetica, Roxburgh 1801) in river hooghly in west bengal, India. Rec Zool Surv India 110, 61–69. doi: 10.26515/rzsi/v110/i1/2010/158962
Sharma R. K., Mathur R., Sharma S. (1995). Status and distribution of fauna in national chambal sanctuary, madhya pradesh. Indian Forester 121, 912–916.
Shin Y.-J., Rochet M.-J., Jennings S., Field J. G., Gislason H. (2005). Using size-based indicators to evaluate the ecosystem effects of fishing. ICES J. Mar. Sci. 62, 384–396. doi: 10.1016/j.icesjms.2005.01.004
Sinha R. K. (1999). “The ganges river dolphin-a tool for baseline assessment of biological diversity in river ganges, india,” in Final technical report, patna university. tech. rep. no. 1/99.
Singh H., Rao R. J. (2012). Sighting frequency and Group composition of Ganges river dolphin (Platanista gangetica gangetica) in National Chambal Sanctuary, India. Zoo’s Print. 8, 18–23.
Singh L. A. K., Sharma R. K. (1985). Gangetic dolphin, Platanista gangetica: observations on the habits and distribution pattern in National Chambal Sanctuary. J. Bombay Nat. Hist Soc. 82, 648–653.
Sinha R. K. (1997). Status and conservation of Ganges River dolphin in Bhagirathi-Hooghly River systems in India. Int. J. Ecol. Environ. Sci. 23, 343–355.
Sinha R. K. (2002). An alternative to dolphin oil as a fish attractant in the Ganges River system: conservation of the Ganges River dolphin. Biol. Conserv. 107, 253–257. doi: 10.1016/S0006-3207(02)00058-7
Sinha R. K., Kannan K. (2014). Ganges River dolphin: An overview of biology, ecology, and conservation status in India. Ambio 43, 1029–1046. doi: 10.1007/s13280-014-0534-7
Sinha R. K., Sharma G. (2003). Current status of the Ganges river dolphin, Platanista gangetica in the rivers Kosi and Son, Bihar, India. J. Bombay Nat. Hist. Soc 100, 27–37.
Sinha R. K., Smith B. D., Sharma G., Prasad K., Choudhary B. C., Sapkota K., et al. (2000). “Status and distribution of the Ganges susu (Platanista gangetica) in the Ganges River system of India and Nepal,” in Biology and conservation of freshwater cetaceans in Asia. IUCN, Gland. Eds. Reeves R. R., Smith B. D., Kasuya T., 152.
Sinha R. K., Verma S. K., Lalji S. (2010). “Population status and conservation of the Ganges River dolphin (Platanista gangetica gangetica) in the Indian subcontinent,” in Biology, evolution, and conservation of river dolphins within south america and asia. Eds. Ruiz-Garcia M., Shostell J. M. (Nova Science Publishers, Inc, New York, USA).
Smith B. D., Ahmed B., Ali M. E., Braulik G. (2001). Status of the Ganges River dolphin or shushuk Platanista gangetica in Kaptai Lake and the southern rivers of Bangladesh. ryx 35, 61–72. doi: 10.1046/j.1365-3008.2001.00153.x
Smith B. D., Braulik G. T. (2009). “Susu and Bhulan: Platanista gangetica gangetica and P. g. minor,” in Encyclopedia of marine mammals (Cambridge, Massachusetts, London, and San Diego: Academic Press), 1135–1139.
Smith B. D., Braulik G., Strindberg S., Ahmed B., Mansur R. (2006). Abundance of Irrawaddy dolphins (Orcaella brevirostris) and Ganges river dolphins (Platanista gangetica gangetica) estimated using concurrent counts made by independent teams in waterways of the Sundarban mangrove forest in Bangladesh. Mar. Mamm. Sci. 22, 527–547. doi: 10.1111/j.1748-7692.2006.00041.x
Smith B. D., Haque A., Hossain M. S., Khan A. (1998). River Dolphins in Bangladesh: conservation and the effects of water development. Environ. Manage. 22, 323–335. doi: 10.1007/s002679900108
Smith B. D., Reeves R. R. (2000). “Survey methods for population assessment of Asian river dolphins,” in Biology and conservation of freshwater cetaceans in asia, vol. .23 . Eds. Reeves R. R., Smith B. D., Kasuya T. (Occasional Papers of the IUCN Species Survival Commission IUCN, Gland, Switzerland), 97–115.
Smith B. D., Sinha R. K., Regmi U., Sapkota K. (1994). Status of ganges river dolphins (Platanista gangetica) in the karnali, narayani and saptakosi rivers of nepal and india. Mar. Mamm. Scien. 10, 68–75.
Tosha M., Kumar R., Kumar D. (2024). Guardians of the river: Fishers balancing livelihood and dolphin conservation. Newsletter No. 36, April-June. Natl. Mission Clean Ganga Ministry Jal Shakti Govt. India., 27–32.
Turvey S. T., Pitman R. L., Taylor B. L., Barlow J., Akamatsu T., Barrett L. A., et al. (2007). First human-caused extinction of a cetacean species? Biol. Lett. 3 , 537–540. doi: 10.1098/rsbl.2007.0292
Vass K. K., Mondal S. K., Samanta S., Suresh V. R., Katiha P. K. (2010). The environment and fishery status of the River Ganges. Aquat. Ecosystem Health Manage. 13, 385–394. doi: 10.1080/14634988.2010.530139
Wakid A. (2005). Status and distribution of newly documented residential Gangetic Dolphin (Platanista gangetica gangetica) population in eastern Assam. J. Bombay Natural History Soc. 102, 158–161.
Wakid A. (2009). Status and distribution of the endangered Gangetic dolphin (Platanista gangetica gangetica) in the Brahmaputra River within India. Curr. Scien. 97, 1143–1151.
Wakid A., Braulik G. (2009). Protection of endangered Ganges River dolphin in Brahmaputra River, Assam, India. (Gland, Switzerland: Sir Peter Scott Fund of IUCN).
WWF (2017). Knowledge Hub, Ganges River dolphin. Available online at: https://wwf.panda.org/knowledge_hub/endanegeredspecies/cetaceans/about/riverdolphins/gangers_riverdolphin/.
WWF (2023). Available online at: https://www.worldwildlife.org/stories/innovation-in-river-dolphin-conservation.
WWF-Nepal (2006). “Conservation and management of river dolphins in Asia,” in Proceedings of the regional meeting on conservation and management of river dolphins(Kathmandu, Nepal: WWF Nepal Program).
Keywords: abundance, Ganges River dolphins, Bhagirathi–Hooghly River systems, threats, recommendations
Citation: Das BK, Bhakta D, Johnson C, Chanu TN, Ramteke M, Chauhan SK, Ray A, Nandy S, Kunui A, Roy S, Mohanty TR, Tiwari NK, Acharjya NK, Singh K, Singh D, Jana A, Kumar A, Kayal T and Behera SK (2025) Status of Ganges River dolphin Platanista gangetica (Lebeck, 1801) in the lower stretch of the Ganga River, India, with emphasis on threats, conservation, and recommendations. Front. Ecol. Evol. 13:1523537. doi: 10.3389/fevo.2025.1523537
Received: 06 November 2024; Accepted: 19 February 2025;
Published: 19 March 2025.
Edited by:
Fernanda Michalski, Universidade Federal do Amapá, BrazilCopyright © 2025 Das, Bhakta, Johnson, Chanu, Ramteke, Chauhan, Ray, Nandy, Kunui, Roy, Mohanty, Tiwari, Acharjya, Singh, Singh, Jana, Kumar, Kayal and Behera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Basanta Kumar Das, YmFzYW50YWt1bWFyZEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.