
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 26 February 2025
Sec. Conservation and Restoration Ecology
Volume 13 - 2025 | https://doi.org/10.3389/fevo.2025.1513949
This article is part of the Research Topic Diagnostic Tools and Research Applications to Combat Wildlife Trade Issues View all 4 articles
Globally, in order to solve a crime during a legal procedure, the presentation of palpable proof at court is the main tool. The contribution of necrophagous insects to this issue has recently increased as these invertebrates appear to comprise one of the major modern methods for the reinforcement of the judiciary system. This scientific discipline known as forensic entomology is a field of criminalistics/criminology that aims to use the results gathered from the study of insects collected at a crime scene in order to solve crimes involving wildlife or other animals/humans. In court, questions such as the estimation of the time of death, the cause of death, the movement of the corpse after death, and neglect of elderly are recurrent during a criminal investigation. In order to gather data that can be exploited to answer the aforementioned questions, we conducted, from March 18 to June 12, 2023, an experiment on carcasses of rats (Rattus norvegicus, Berkenhout, 1769, var. Wistar) within the University of Yaounde 1 campus. These were exposed to an open-air environment inside a wooden cage for protection in the bush behind Amphitheater 502 of the Faculty of Science of the University of Yaounde 1. The aim of this research work was to census necrophagous arthropod fauna that can always be exploited as harmless witnesses at the crime scene in order to determine the time of death, the cause of death, and the movement of the carcass after the crime. A total of 2,345 arthropod fauna belonging to three classes (Arachnida, Myriapoda, and Hexapoda), 16 orders (Acari, Araneida, Chilopoda, Diplopoda, Diptera, Coleoptera, Lepidoptera, Dermaptera, Hemiptera, Hymenoptera, Dictyoptera, Collembola, Homoptera, Orthoptera, Psocoptera, and Thysanura), 37 families, 14 genera, and 27 species were included in the census. This cadaveric fauna, referred to as “silent crime scene witnesses,” constitutes many trophic guilds such as predators, necrophagous and omnivorous, with 541, 1,289, and 294 insects, respectively. The aforementioned leading guilds were secondarily followed by saprophagous, opportunists, parasitoids, hematophagous, and accidental host, with 122, 85, 8, 3, and 2 individuals, respectively. The present preliminary forensic entomology research work obtained a high biodiversity of necrophagous arthropod fauna from the study site, which showed the presence of several trophic guilds that can potentially be used as accurate tools during faunal criminal inquiries within the Central African sub-region.
Nature is subject to damage due to anthropic activity around the world. This damage in nature affects both vegetals and animals, both wild and domestic. The management of wild animals is a crucial issue as people often use them for food, for illicit trafficking, for decoration, for trade, and for guarding/safekeeping, while some others are killed only for fun or for commercial purposes.
In the case of murder, the determination of the cause/time of death is one of the most important pieces of information during the inquiry (Feugang Youmessi et al., 2021; Feugang Youmessi, 2023, 2024). This exercise can be performed both by the legist medical doctor within 3 days after the death or by an entomologist. After this period of time, the estimation of the time/cause of death is given over only to an entomologist specialized in forensics. Forensic entomology is the use of insects and other arthropods in solving a crime (Naman et al., 2024; Thummela et al., 2024; Zongo et al., 2023) or the study of insects in a legal context (Gelderman et al., 2021). In the case of a wildlife crime scene, forensic entomologists use wild animal carrion insect communities to obtain pieces of evidence in the case of murder, accident, or poaching as these insects are natural silent witnesses to the crime scene (Catts and Goff, 1992; Braet et al., 2012). It is assumed that larvae that are found feeding on a corpse might have ingested, incorporated, and bioaccumulated chemical metabolites of poison such as barbiturates, cocaine, amphetamine, and other toxins from the cadaver into their own tissues (Catts and Goff, 1992). The insect tissues can then be analyzed to detect these substances. This analysis is more important when the corpse is at an advanced stage of decay, when there is no more blood, or when it is no longer possible to use the common toxicological methods of isolating the drugs. This offers insects as physical evidence during legal procedures after the use of poaching worldwide, except in Africa in general and in the Central African sub-region in particular, where publications in this domain are poor/scarce or are simply non-existent. The main families generally captured on corpses include Calliphoridae, Muscidae, Sarcophagidae, Sepsidae, Staphylinidae, Histeridae, Cleridae, Dermestidae, and Trogidae.
The aim of this research work was to census and identify these arthropod fauna, particularly the cadaver insects that are generally neglected in wildlife crime scenes but are also useful alongside the other items collected by people during inquiries.
This experiment was conducted on the campus of the University of Yaounde 1, where two carcasses of rats (Rattus norvegicus Berkenhout, 1769, var. Wistar), each weighing approximately 350 g, were used as models in the research experiment. The carcasses were exposed inside one wooden cage (150 cm × 150 cm × 150 cm) into a bush opposite Amphitheater 502 of the Faculty of Science (11°33′01″ E–3°51′35″ N; altitude, 720 m), from March 18 to June 12, 2023. The climate of this area is described as “Yaoundean type,” which is characterized by four distinct seasons of unequal duration: a long dry season from mid-November to mid-March, a short rainy season from mid-March to mid-June, a short dry season from mid-June to mid-August, and a long rainy season from mid-August to the end of October. The average annual rainfall fluctuates between 1,600 and 2,000 mm, while the average annual temperature varies between 22°C and 42°C (Kengne and Atangana, 2010; Abessolo et al., 2015). The vegetation/landscape of this part of the campus is dominated mainly by the presence of Elaeis guineensis Jacq, 1763, Musa sp., Ipomea batatas Lam, 1793, and Zea mays Linné, 1753.
The 3-month-old rats (R. norvegicus Berkenhout, 1769, var. Wistar) were brought to the site, weighed, and euthanized by a veterinarian according to ethical rules of the management of animals. Immediately, the rat carcasses were placed inside the cage, the sampling protocols of which followed those of Carvalho et al. (2004). The ambient air temperature was registered every day with a thermo-hygrometer placed inside the cage. The sampling of flying insects was undertaken three times daily during the first postmortem week using a combined method of mosquito nets, hand picking with flexible forceps, and a pitfall trap once daily until the disappearance of all soft tissue (the skeletonized stage). Subsequently, the insect samples were stored in 70% ethanol for identification using a binocular stereomicroscope according to the identification key of Delvare and Alberlenc (1989); Kurahashi and Kirk-Spriggs (2006); Whitworth (2010); Irish et al. (2014), and Rochefort et al. (2015).
Observations of the physical modifications that occurred during the corpse decay yielded five distinct alteration stages: the fresh stage, which lasted for 2 days (days 1 and 2); the bloated stage, from day 3 to day 4; the putrefied stage, from day 5 to day 9; the dried stage, which lasted from day 10 to day 61; and the last stage, called the skeletonized stage, which started from day 62 to the end of the decomposition of the carcasses (Table 1). This finding on the decay process segmentation is in parallel with the recognition made by Dekeirsschieter et al. (2012); Shaalan et al. (2017); de A Azevedo et al. (2018); Gelderman et al. (2021), and Zongo et al. (2023), although the names of the stages varied. However, Schieweck et al. (2024) and Taleb et al. (2017) identified three and four steps, respectively. This inconsistency may be understood as a result of the geographic variations of the abiotic parameters linked to each experimental site, as the process of corpse alteration is typically related to environmental factors.
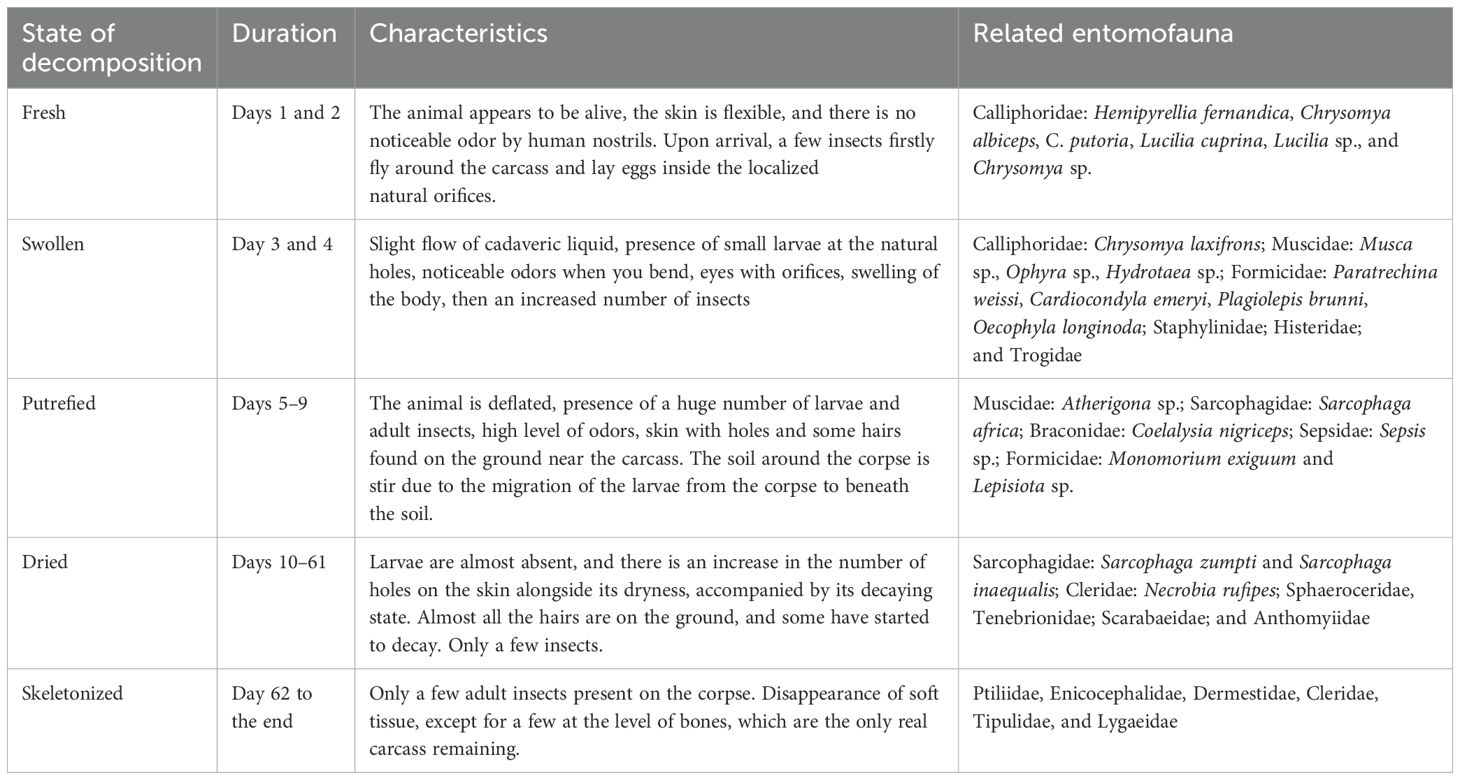
Table 1. Decaying states, duration, physical modifications of the carcasses, and related necroentomofauna.
Table 1 provides a summary of the specific insects according to the state of decay. The fresh state was dominated by the family Calliphoridae (Hemipyrellia fernandica and Chrysomya albiceps), while the bloated state was dominated by Calliphoridae (Chrysomya laxifrons), Muscidae (Musca sp., Ophyra sp., and Hydrotaea sp.), Formicidae (Paratrechina weissi, Cardiocondyla emeryi, Plagiolepis brunni, and Oecophylla longinoda), and the first appearance of Staphylinidae, Histeridae, and Trogidae. The putrefaction phase was dominated by the families Muscidae (Atherigona sp.), Sarcophagidae (Sarcophaga africa), Braconidae (Coelalysia nigriceps), Sepsidae (Sepsis sp.), and Formicidae (Monomorium exiguum and Lepisiota sp.). The dried stage was colonized by the families Sphaeroceridae, Tenebrionidae, Scarabaeidae, Anthomyiidae, Sarcophagidae (Sarcophaga zumpti and Sarcophaga inaequalis), and Cleridae (Necrobia rufipes), while the skeletonization phase was characterized by the families Ptiliidae, Enicocephalidae, Tipulidae, Dermestidae, Cleridae, and Lygaeidae. This relation of the necroentomofauna to the state of the decay process can be used to estimate the time of death, the cause of death, and the place of death. The high diversity of cadaver-related fauna is in line with the results obtained by Dao et al. (2018); Feugang Youmessi et al. (2021); Irish et al. (2014); Koffi et al. (2017), and Rochefort et al. (2015) in the Ivory Coast, in Cameroon, and in South Africa.
Similar to Geissenberger et al. (2024); de A Azevedo et al. (2018), and Ourrad et al. (2022), this fauna belongs to several trophic guilds such as necrophages, predators, omnivores, saprophages, opportunists, parasitoids, hematophages, and accidental hosts, among which necrophages are the most important insects used for forensic entomology during crime scene investigations on wildlife animals/domestic animals.
The predictable succession patterns of silent witnesses of the crime scene, called corpse feeders in the present research, are consistent with the patterns obtained around the world, even though the taxonomic aggregation/assemblage of the two main taxa (Diptera and Coleoptera) were different. This difference of the families in these orders can be explained by the divergence of the environmental characteristics, such as the climate (temperature, relative humidity, wind speed, and pluviometry, among others), the sampling artifacts, and the size of the biological model used, as emphasized by Geissenberger et al. (2024); Thümmela et al. (2024), and Naman et al. (2024).
We recorded a total of 2,345 insects, shared between 3 classes, 13 orders, 37 families, 14 genera, and 27 species (Table 2). Other researchers captured more corpse feeders: de A Azevedo et al. (2018) gathered 10,559 individuals, while Taleb et al. (2017) sampled 8,817 individuals. This variation in number could be the consequence of several scales related to abiotic variables and to interspecific competition within the necroentomofauna assemblages/communities, as well as to specific disturbances as co-inhabiting species are in permanent competition. Each degradation level was endorsed by some specific cadaveric fauna, as reported by Feugang Youmessi (2014) and Schieweck et al. (2024). Hence, the first and second postmortem days can be dated with the presence of H. fernandica, C. albiceps, Chrysomya putoria, Lucilia cuprina, Lucilia sp., and Chrysomya sp. (Calliphoridae). Days 3 and 4 can be dated with the presence of Chrysomya laxifrons (Calliphoridae), Musca sp., Ophyra sp., Hydrotaea sp. (Muscidae), P. weissi, C. emeryi, P. brunni, O. longinoda (Formicidae), Staphylinidae, Histeridae, and Trogidae. From day 5 to day 9, species such as Atherigona sp. (Muscidae), S. africa (Sarcophagidae), C. nigriceps (Braconidae), Sepsis sp. (Sepsidae), M. exiguum, and Lepisiota sp. (Formicidae) can be found. Species such as S. zumpti, S. inaequalis (Sarcophagidae), N. rufipes (Cleridae), Sphaeroceridae, Tenebrionidae, Scarabaeidae, and Anthomyiidae comprised the fauna found from day 10 to postmortem day 61, while day 62 to the end of the decomposition are characterized by the presence of Ptiliidae, Enicocephalidae, Dermestidae, Cleridae, Tipulidae, and Lygaeidae. This postmortem estimation is in line with the results observed by Dao et al. (2018); Feugang Youmessi (2023, 2024), Koffi et al. (2017); Rochefort et al. (2015), and Zongo et al. (2023) in the Ivory Coast, in Cameroon, in South Africa, and in Burkina Faso.
Among the cadaveric organisms, Diptera (Calliphoridae) was the dominant family, followed by Coleoptera or beetles, corroborating the results of other experiments in Cameroon (Fantio et al., 2022; Feugang Youmessi et al., 2021; Feugang Youmessi, 2023; Feugang Youmessi and Djonga, 2024; Djonga, 2024), Africa (Koffi et al., 2017; Zongo et al., 2023; Naman et al., 2024; Taleb et al., 2017; Yapo et al., 2017), and worldwide (de A Azevedo et al., 2018; Amendt et al., 2004; Anderson, 2020; Lutz et al., 2017, 2019; Maisonhaute and Forbes, 2020; Ourrad et al., 2022).
Similar to Geissenberger et al. (2024); de A Azevedo et al. (2018), and Ouiza et al. (op. cit.), this fauna belongs to several trophic guilds such as necrophagous, predators, omnivorous, saprophagous, opportunists, parasitoids, hematophagous and accidentals, among which the necrophages are the most important insects in forensic entomology.
This research work highlights the importance of the use of some invertebrates, particularly insects, in solving crimes related to murder, accidents, and illicit trafficking or poaching affairs in court or during preliminary inquiries. Observation of the physical modifications that occurred during the decay process yielded five stages of decomposition, namely, the fresh stage (days 1 and 2), the bloated stage (days 3 and 4), the putrefied stage (days 5 to 9), the dried stage (days 10 to 61), and the skeletonized stage (day 62 toward the end). The potential strong candidates that can be used as proof in solving crimes are the Diptera families including Calliphoridae [Chrysomya albiceps (Wiedemann, 1819), Chrysomya laxifrons (Villeneuve, 1814), Chrysomya putoria (Wiedemann, 1830), Hemipyrellia fernandica (Manquart, 1855), L. cuprina (Wiedemann, 1830), Lucilia sp. (R-D, 1830), Hemipyrellia sp. (Towsend, 1918), and Chrysomya sp. (R-D, 1830)]; Sarcophagidae [Sarcophaga africa (Wiedemann, 1824), Sarcophaga inaequalis (Austein, 1909), and Sarcophaga sp. (Meigen, 1826)]; and some Coleoptera. This research work must be replicated in order to isolate the necrophagous species that could be used for the determination of the values of the quantity of energy use by each to complete its life cycle, called the accumulated degree day (ADD) or the accumulated degree hour (ADH). These values will help in the estimation of the time elapsed since death (postmortem interval) and, therefore, the date of the crime.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The animal studies were approved by University of Yaoundé 1 ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
FF: Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The author is grateful to the colleagues from the zoology laboratory of the faculty of science of the University of Yaounde 1 for the convivial atmosphere among us.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abessolo S. A., Joseph A. A., Mesmin T., Martin S. M. (2015). Analyse des prélèvements annuels à la gare de Yaoundé de 1895 à 2006. Soil Management. 11 (2), 183–194.
Amendt J., Krettek R., Zehner R. (2004). Forensic entomology. Naturwissenschaften 91, 51–65. doi: 10.1007/s00114-003-0493-5
Anderson G. S. (2020). “Factors that influence insect succession on carrion,” in Forensic entomology: The utility of arthropods in legal investigations, 3rd ed. Eds. Byrd J. H., Tomberlin J. K. (CRC Press, Taylor & Francis, Boca Raton (FL), 103–139.
Braet Y., van Cornelis A., Francis Dupont F. Y. (2012). Coelalysia nigriceps (Szépligeti 1911) reared during a forensic study in Cameroon, with remarks on synonymy and biology (Hymenoptera, Braconidae, Alysiinae). Bull. la Société entomologique France 117, 381–389. doi: 10.3406/bsef.2012.29313
Carvalho L. M. L., Thyssen P. J., Goff M. L., Linhares A. X. (2004). Observations on the succession patterns of necrophagous insects on a pig carcass in an urban area of South Eastern Brazil. J. Forensic Med. Toxicology. 51, 33–39.
Catts E. P., Goff M. L. (1992). Forensic entomology in criminal investigations. Annu. Rev. Entomology 37, 253–272. doi: 10.1146/annurev.en.37.010192.001345
Dao H., Aboua L. R.N., Koffi A. F. (2018). Influence of the ecological zone on the necrophagous insects’ activities involved in the process of decomposition of Pigs Carcasses (Sus scrofa domesticus L.) exposed to the open air in the Sub-Sudanese zone of Côte d’Ivoire. Int. J. entomology Res. 3, 07–16.
de A Azevedo W. T., de Carvalho R. P., de Figueiredo A. L., Ross S. D., Lessa C. S. S., da Rocha Fortes R., et al. (2018). Calliphoridae (Diptera) associated with Rattus rattus carcasses in the Tijuca National Park, Rio de Janeiro, Brazil. J. Med. Entomol. 1–8. doi: 10.1093/jme/tjy013
Dekeirsschieter J., Verheggen F., Frederickx C., Marlet C., Lognay G., Haubruge E. (2012). Comment les insectes communiquent-ils au sein de l’«écosystème-cadavre»? L’écologie chimique des insectes nécrophages et nécrophiles. Entomologie faunistique-Faunistic entomology. 65, 3–13.
Delvare G., Alberlenc H. P. (1989). “Les insectes d’Afrique et d’Amérique,” in Clés pour la reconnaissance des familles (CIRAD, France), 297.
Djonga G. (2024). Nécroentomofaune des rats et Évaluation de leur taux de parasitoïdisme sur le campus de l’Université de Yaoundé 1 pendant la saison sèche. Mémoire Master Faculté Des. Sciences Université Yaoundé 1, 54.
Fantio R. M., Kenne E. L., Kenne Toukem A. s., Tsekane S. J., Tuekam-Kowa P. S., Kayoum Y. A., et al. (2022). Biodiversity, abundance of flies (Diptera. Brachycera) attacted by fresh flesh and identification of medical or Forensic important species in Douala (Cameroon). Am. J. Entomology. 6, 49–715.
Feugang Youmessi F. D. (2014). Etudes des indicateurs entomologiques utilisables lors des enquêtes criminelles: cas des Arthropodes de Rattus norvegicus (Berkenhout 1769) Muridé souche Wistar à Yaoundé (Thèse de Doctorat Ph/D, Université de Yaoundé 1, Cameroun), 177.
Feugang Youmessi F. D. (2023). Comparison between insects gathered on a death corpse from the study site and insects obtained by rearing larvae within the laboratory under natural environmental conditions. Forensic Res. Criminology Int. J. 11, 126–132. doi: 10.15406/frcij.2023.11.00381
Feugang Youmessi F. D. (2024). Field succession study of necrophagous arthropod fauna of Rat (Rattus norvegicus Linnaeus 1769, var wistar) carrion exposed on natural environment at Nsimalen neighborhood, Yaounde-Cameroon. Int. J. Advanced Res. Med. 6, 98–106. doi: 10.22271/27069567.2024.v6.i3b.576
Feugang Youmessi F. D., Djonga G. (2024). Necrophagous entomofauna and evaluation of his parasitoidism level during the dry season within the campus of the University of Yaounde 1. Int. J. Forensic Med. 6, 110–115. doi: 10.22271/27069567.2024.v6.i3b.576
Feugang Youmessi F. D., Nwane P. B., Djiéto-lordon C., Braet Y., Villet M. H., Bilong Bilong C. F. (2021). Blowflies reared in laboratory conditions from maggots collected on Rat (Rattus norvegicus Berkenhout 1769, var Wistar) carrions in Yaoundé (Cameroon, Central Africa). Asian J. Biol. 12, 33–43. doi: 10.9734/AJOB/2021/v12i230160
Geissenberger J., Amendt J., Klampfer J., Thuemmel L., Jakob L., Monticelli F. C., et al. (2024). Morphological changes and protein degradation during the decomposition process of pig cadavers placed outdoors or in tents-a pilot study. Forensic science Med. pathology.
Gelderman T., Stigter E., Krap t., Amendt J., Duijst w. (2021). The time of death in Dutch court; using the Daubert criteria to evaluate methods to estimate the PMI used in court. Legal Med. 53, 1–12. doi: 10.1016/j.legalmed.2021.101970
Irish S., Lindsay T., Wyatt N. (2014). Key to adult of Afrotropical species of the genus Chrysomya (Robineau-Desvoidy) (Diptera-Calliphoridae). Afr. entomology. 22, 297–306. doi: 10.4001/003.022.0210
Kengne F., Atangana P. (2010). Une diversité du milieu biophysique exceptionnelle Dans Le Cameroun: Autopsie d’une exception plurielle en Afrique. Ed. Kengne F. (L’Harmattan) 25–44.
Koffi A. F., Aboua L. R. N., Dao H., Djojo M., Koffi-Tebele D. E. J., Kpama-Yapo C. (2017). Process of colonization by necrophagous insects of a pig corpse (Sus scrofa domesticus L.) exposed at open air, in the Southern Forest Zone of Ivory Coast. Int. J. Curr. Res. Acad. review. 5, 103–114.
Kurahashi H., Kirk-Spriggs (2006). The calliphoridae of Namibia (Diptera: Oestroidea). Zootaxa 1322, 1–131. doi: 10.11646/zootaxa.1322.1.1
Lutz L., Kirstin W. A., Martin V. H., Mfon E., Krzysztof S. (2017). Species identification of adult African blowflies (Diptera/Calliphopridae) of forensic importance. Int. J. Legal Med. 13, 414–425. doi: 10.1007/s00414-01761654-y
Lutz L., Moreau G., Czuprynski S. (2019). An empirical comparison of decomposition and fly colonization of concealed carcasses in the Old and New World. Int. J. Legal Med. 133, 1593–1602. doi: 10.1007/s00414-019-02089-y
Maisonhaute J. É., Forbes S. L. (2020). Decomposition process and arthropod succession on pig carcasses in Quebec (Canada). Can. Soc. Forensic Sci. J. doi: 10.1080/00085030.2020.1820799
Naman K. J., Ubachukwu P., Agwu J. E., Kamani J. (2024). Molecular identification of blowfly species (Diptera: Calliphoridae) and beetles (Coleopterans) of forensic importance associated with pig (Sus scrofa) carrion in Nigeria. Int. J. Trop. Insect Science. doi: 10.1007/s42690-024-01269-7
Ourrad O., Ahmed D. S.-A., Sadou S.-A., Bouzrarf K., Belqat B. (2022). Diversity of flies (Diptera: Brchycera) in breeding farms in the Kabylia region (North-central Algeria), and identification of some myiaseginic species. Biodiversities 23, 2276–2284. doi: 10.13057/biodiv/d230505
Rochefort S., Giroux M., Savage J., Wheeler T. A. (2015). Key to forensically important Piophilidae (Diptera) in the neartic Region. Can. J. Arthropod Identification. 25, 1–37.
Schieweck A., Schulz N., Amendt J., Birngruber C., Holz F. (2024). Catch me if you can-emission patterns of human bodies in relation to postmortem changes. Int. J. Legal Med. 138, 1603–1620. doi: 10.1007/s00414-024-03194-3
Shaalan E. A., El-Moaty Z. A., Abdelsalam S., Anderson G. S. (2017). A preliminary study of insect succession in Al-Ahsaa Oasis in the Eastern Region of the Kingdom of Saoudi Arabia. J. Forensic Sci. 62, 239–243. doi: 10.1111/jfo.2017.62.issue-1
Taleb M., Tail G., Açikgöz H. N. (2017). Ecological roles of cadaveric fauna in relation with decomposition stages. UKECEK, 399–410.
Thümmela L., Degoutriea C., Fonseca-Mu˜nozc A., Amendt J. (2024). Developmental differences in spatially distinct populations of the forensically relevant blow fly Lucilia sericata – About the comparability of developmental studies (and case work application). Forensic Sci. Int. doi: 10.1016/j.forsciint.2024.111972
Whitworth T. (2010). Keys to the genera and species of blow flies (Diptera: Calliphoridae) of West Indies and description of new species of Lucilia Robineau-desvoidy. Zootaxa 2663, 1–35. doi: 10.11646/zootaxa.2663.1.1
Yapo E. Y. C., Aboua N. L. R., Koffi F. A., Dao H. (2017). Some biological parameters of Lucilia sericata M. (Diptera: Calliphoridae) necrophagous insect breeding on pig (Sus scrofa domesticus L.) and Beef’s 5Bos indicus) liver at the National Center of Floristic, Guinean Zone of Ivory Coast. Int. J. Curr. Res. Acad. Rev. 5, 68–76. doi: 10.20546/ijcrar.2017.501.008
Keywords: Cameroon, forensic entomology, arthropod fauna, necrophagous, trophic guilds and parasitoid
Citation: Feugang Youmessi FD (2025) Forensic entomology in Cameroon (Central African sub-region): the use of arthropod fauna of rat (Rattus norvegicus, Berkenhout, 1769, var. Wistar) carcasses as silent crime scene witnesses. Front. Ecol. Evol. 13:1513949. doi: 10.3389/fevo.2025.1513949
Received: 21 October 2024; Accepted: 28 January 2025;
Published: 26 February 2025.
Edited by:
Arame Ndiaye, TRACE Wildlife Forensics Network, SenegalReviewed by:
Hassane Dao, Jean Lorougnon Guédé University, Côte d’IvoireCopyright © 2025 Feugang Youmessi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francis Dupont Feugang Youmessi, ZmZldWdhbmd5b3VtZXNzaUB5YWhvby5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.